Abstract
Background
Microhematuria is common in immunoglobulin A nephropathy (IgAN). However, current prognostication is based on proteinuria and mesangial hypercellularity, endocapillary hypercellularity, segmental sclerosis, tubulointerstitial fibrosis and crescent (MEST-C) scores.
Methods
In this retrospective study, we evaluated whether MEST-C score components are associated with the presence of microhematuria at biopsy and whether the degree of microhematuria during follow-up is associated with change in estimated glomerular filtration rate (eGFR), after adjusting for clinical and histological parameters. We identified 125 patients with biopsy-proven IgAN and MEST-C scoring who were not on immunosuppressive therapy at biopsy. Microhematuria was defined as ≥3 red blood cells (RBCs)/high-power field (hpf).
Results
Of the 125 patients, 97 had microhematuria at baseline and were more likely to have M1, E1 and C ≥ 1 lesions (P < 0.05 for all) compared with patients without microhematuria. Of the 125 patients, 72 had follow-up data available. An increase in the degree of microhematuria was significantly associated with an eGFR decline of −0.81 mL/min/1.73 m2 [95% confidence interval (CI) −1.44 to −0.19, P = 0.01], after adjusting for follow-up time, proteinuria and T score. Severe microhematuria (≥21 RBCs/hpf) was associated with an even larger decline in eGFR (−3.99 mL/min/1.73 m2; 95% CI −6.9411 to −1.0552, P = 0.008), after similar adjustments.
Conclusion
Degree of microhematuria during follow-up is an independent predictor of eGFR decline after adjusting for clinical and histological parameters. Therefore, monitoring the degree of microhematuria as well as proteinuria is important when evaluating patients with IgAN. Additional studies using improvement in microhematuria as a primary surrogate outcome are needed.
Keywords: IgA nephropathy, MEST-C score, microhematuria, proteinuria
INTRODUCTION
Immunoglobulin A nephropathy (IgAN) is the most common type of glomerulonephritis worldwide with up to 50% of affected patients progressing to end-stage renal disease (ESRD) in their lifetime [1]. Immunosu ppressive therapy is typically reserved for patients who are considered to be at high risk of progression based on their clinical and histological parameters. However, identifying this group of patients remains an ongoing clinical challenge. The most predictive recognized clinical parameter is proteinuria >1 g/24 h, which has been associated with poor renal outcomes in numerous studies [2–5]. However, there is no reliable method to determine if this proteinuria is due to active reversible glomerular inflammation that may be responsive to immunosuppressive therapy, or to an irreversible scarring process.
Since microhematuria is a common finding in IgAN, determining whether it is a marker of active inflammation would be of significant clinical utility that could help guide therapy. However, data regarding microscopic hematuria and renal outcomes are conflicting [6–8]. The Oxford classification of IgAN, which includes mesangial hypercellularity (M), endocapillary hypercellularity (E), segmental sclerosis (S), tubulointerstitial fibrosis (T) and crescents (C) (MEST-C), has been shown to be independently associated with renal outcomes [9–11]. The association between MEST-C score and microhematuria at the time of renal biopsy has not yet been elucidated. In addition, whether or not hematuria levels during follow-up could be an independent risk factor for estimated glomerular filtration rate (eGFR) decline and progression to ESRD irrespective of other clinical and histological (MEST-C) parameters is unclear. Thus far, immunosuppressive therapy has been reserved for patients with significant proteinuria without taking into account the degree of hematuria, at the start of immunosuppressive therapy [12–14].
In this study, we sought to investigate two key concepts. First, are the components of the MEST-C score associated with the presence of microhematuria at the time of renal biopsy? Second, is the degree of microhematuria during follow-up an independent risk factor for adverse renal outcomes (progression to ESRD or decline in eGFR) after adjusting for clinical and histological parameters? To investigate this, we identified a cohort of patients with biopsy-proven IgAN who were not on immunosuppression at the time of biopsy and collected their clinical and laboratory parameters throughout their disease course.
MATERIALS AND METHODS
Study population
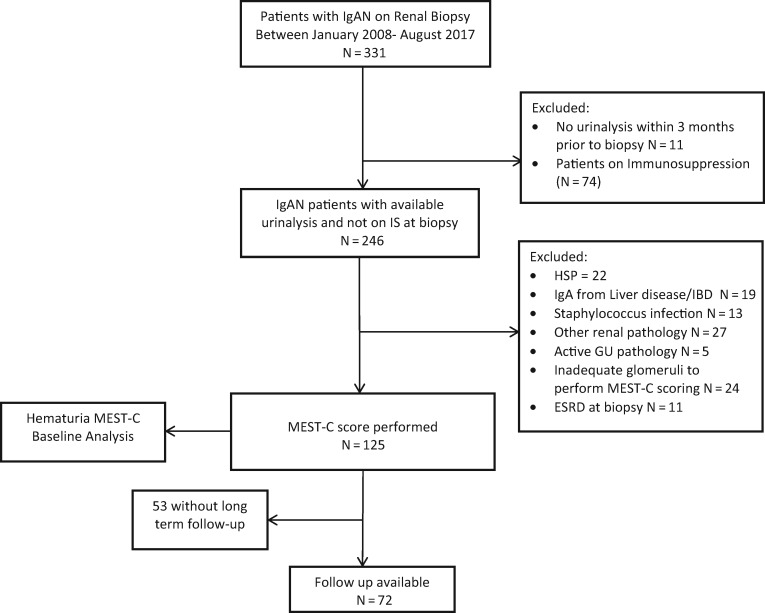
The study was approved by the Mayo Clinic Institutional Review Board. We retrospectively identified all adult patients who underwent a native kidney biopsy at the Mayo Clinic from 1 January 2008 to 31 August 2017 and had a diagnosis of IgAN (defined as predominant mesangial IgA staining), from the pathology files. We applied inclusion and exclusion criteria as outlined in Figure 1.
FIGURE 1.
Study design. IS, immunosuppression; IBD, inflammatory bowel disease; GU, genitourinary.
Urine studies and renal biopsy evaluation
Renal biopsies were evaluated by a renal pathologist who was blinded to the presence or absence of hematuria. Biopsies were classified according to the updated Oxford classification of IgAN [11]. M1 was defined as an M score of >0.5, E and S were scored as absent (0) or present (1), T was scored according to the estimated percentage of interstitial fibrosis and tubular atrophy in the cortex: T0 (≤25% of cortex), T1 (26–50%) and T2 (>50%). A crescent was defined as extra capillary proliferation of more than two cell layers of any size. We included only cellular or fibrocellular crescents. Crescent was scored as C0 (no crescents), C1 (crescents in more than zero but less than one-fourth of glomeruli) and C2 (crescents in one-fourth or more of glomeruli) [10]. Biopsies had to have eight or more glomeruli in order to be included in the study.
All patients had urinalysis performed at Mayo Clinic Health System Renal Testing Laboratories using a previously published methodology [15]. Briefly, for manual microscopy, urine was centrifuged at 2000g. The sediment was then re-suspended after supernatant removal and 25 µL was placed on a slide. The following components were then documented: number of red blood cells (RBCs) and white blood cells (WBCs)/high-power field (hpf), fat, crystals, renal epithelial and transitional cells and granular, RBC, WBC and hyaline casts. Dysmorphic RBCs were evaluated using phase-contrast microscopy at ×400 if there were ≥3 RBCs/hpf present. Degree of hematuria was reported as 0, <3, 3–10, 11–20, 21–30, 31–40, 41–50, 51–100 or >100 RBCs/hpf [15].
For the purpose of this study, microhematuria was considered severe if there were ≥21 RBCs/hpf, moderate if there were between 3 and 20 RBCs/hpf and absent if there were <3 RBCs/hpf. Baseline hematuria was defined as positive if hematuria was ≥3 RBCs/hpf using the urinalysis prior to the biopsy. Follow-up hematuria was defined as being present if the patient had a median hematuria of ≥3 RBCs/hpf during follow-up visits prior to ESRD and otherwise was considered absent during follow-up. Proteinuria was determined via estimated 24-h proteinuria.
Data collection and follow-up
The following parameters were collected at baseline for each patient: age, gender, race, serum creatinine, eGFR (Chronic Kidney Disease Epidemiology Collaboration equation), degree of hematuria, estimated 24-h proteinuria, systolic and diastolic blood pressure and treatment with renin–angiotensin system blockade (RASB). For patients who were seen during follow-up, we collected serum creatinine, eGFR, estimated 24-h proteinuria, hematuria, and the presence and type of immunosuppression or RASB at each assessment prior to ESRD.
Statistical analyses
All data are presented as mean ± standard deviation (SD) or n (%). P-values comparing baseline characteristics with baseline and follow-up hematuria were derived using the equal variance t-test for continuous measures and the Chi-square test for categorical measures. M, E, S, T and C variables were considered as binary variables with the categories 0 versus 1 in all analyses except for T and C (0 versus ≥1). P-values comparing change in eGFR per year with median hematuria during follow-up were derived using linear regression. Predicted eGFR decline rate per year was calculated by taking the difference of the first and last eGFR for each patient and dividing by follow-up time, and then modeled by each patient’s median degree of hematuria during follow-up (Table 2).
Table 2.
Estimates for rate of eGFR change per year by median degree of hematuria throughout follow-up
| Median degree of hematuria throughout follow-upa | eGFR decline rate (SE)b |
|---|---|
| 0 | 5.80 (2.05) |
| 1 to ≤3 | 2.10 (1.44) |
| 3–10 | −1.60 (1.14) |
| 11–20 | −5.30 (1.38) |
| 21–30 | −9.00 (1.96) |
| 31–40 | −12.70 (2.67) |
| 41–50 | −16.40 (3.43) |
| 51–100 | −20.10 (4.22) |
| >100 | −23.80 (5.01) |
Median degree of hematuria throughout follow-up is in RBCs/hpf.
eGFR rate of change is in mL/min/1.73 m2/year. SE, standard error.
The effects of baseline clinical characteristics on incident ESRD were estimated using the Cox proportional hazard model. The outcome of interest was time to ESRD, defined as an eGFR <15 mL/min/1.73 m2 or start of dialysis or renal transplantation, and was censored on last follow-up. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were presented. We used a time-dependent Cox model [16] to explore the effect of hematuria, estimated proteinuria and medication use (RASB and immunosuppression) on ESRD during follow-up. The proportional hazards assumption was checked for all models using Martingale residuals. Generalized estimating equation (GEE) models [17] adjusting for time were used to evaluate the association between the degree of hematuria and eGFR throughout follow-up, where degree of hematuria was considered as an ordinal variable (degrees: 0, <3, 3–10, 11–20, 21–30, 31–40, 41–50, 51–100, >100 RBCs/hpf). Models were separately adjusted for proteinuria, MEST-C score and treatment. Sensitivity analyses were also performed in which hematuria was categorized into moderate/absent (<21 RBCs/hpf) and severe (≥21 RBCs/hpf), as well as after performing a subgroup analysis among patients with baseline hematuria ≥3 RBCs/hpf and estimated 24-h proteinuria > 1 g. P-values <0.05 were considered statistically significant. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA), or R version 3.4.2.
RESULTS
MEST-C score and microhematuria at baseline
Our cohort included 331 patients who had biopsy-proven IgAN. Patients were excluded if they were missing a urinalysis within 3 months prior to biopsy (n = 11), were already on immunosuppression at baseline (n = 74), or had Henoch–Schonlein Purpura (HSP) (n = 22), IgA associated with liver disease (n = 19), staphylococcus infection (n = 13), other renal pathology findings (n = 27), active genitourinary pathology (n = 5), inadequate glomeruli to perform MEST-C scoring (n = 24) or ESRD at biopsy (n = 11), leaving 125 patients with data available for analysis at biopsy (Figure 1). The mean age at diagnosis was 44.8 years (SD 15.7), with 84 (67.2%) males, and 104 (83.2%) of white descent. On renal biopsy, 102 (81.6%) patients had M1, 30 (24%) had E1, 82 (65.6%) had S1, 45 (36.0%) had T ≥ 1 and 34 (27.2%) had C ≥ 1. Of these 125 patients, 97 (78%) had microhematuria at baseline (≥3 RBCs/hpf) and were more likely to have M1, E1 and C ≥ 1 lesions (P < 0.05 for all) but not S or T lesions, compared with 28 patients without microhematuria. All patients who had E1 lesions on biopsy also had microhematuria at baseline. Those with baseline microhematuria also had a higher eGFR at baseline (58.0 mL/min/1.73 m2 versus 43.3 mL/min/1.73 m2, P = 0.019) (Table 1). Among the 28 patients without hematuria at baseline, only 12 had sufficient follow-up data; among these, 3 of 12 (25%) had median hematuria during follow-up.
Table 1.
Association between patient characteristics and hematuria at baseline
| Hematuria at baselinea |
||||
|---|---|---|---|---|
| Baseline variables | Absent (n = 28) | Present (n = 97) | P-value | Total |
| Demographics | ||||
| Age (years), mean (SD) | 45 (16) | 45 (16) | 45 (16) | |
| Gender, n (%) | 0.71 | |||
| Female | 10 (35.7) | 31 (32.0) | 41 (32.8) | |
| Male | 18 (64.3) | 66 (68.0) | 84 (67.2) | |
| White, n (%) | 23 (82.1) | 81 (83.5) | 0.87 | 104 (83.2) |
| Laboratory measures, mean (SD) | ||||
| Serum creatinine (mg/dL) | 2.0 (0.6) | 1.7 (0.8) | 0.072 | 1.7 (0.7) |
| eGFR (mL/min/1.73 m2) | 43 (23) | 58 (31) | 0.019 | 55 (29) |
| Systolic BP (mmHg)b | 124.9 (11.7) | 122.5 (15.1) | 0.44 | 123.0 (14.5) |
| Diastolic BP (mmHg) | 79.5 (10.6) | 76.4 (9.9) | 0.16 | 77.1 (10.1) |
| Estimated proteinuria (g/24 h)c | 2.4 (2.1) | 2.5 (2.9) | 0.88 | 2.5 (2.7) |
| Histology, n (%) | ||||
| M (1 versus 0) | 18 (64.3) | 84 (86.6) | 0.007 | 102 (81.6) |
| E (1 versus 0) | 0 (0.0) | 30 (30.9) | 0.001 | 30 (24.0) |
| S (1 versus 0) | 20 (71.4) | 62 (63.9) | 0.46 | 82 (65.6) |
| T (≥1 versus 0) | 13 (46.4) | 32 (33.0) | 0.19 | 45 (36.0) |
| C (≥1 versus 0) | 3 (10.7) | 31 (32.0) | 0.026 | 34 (27.2) |
Hematuria (≥3 RBCs/hpf = present or <3 RBCs/hpf = absent).
One patient without hematuria at baseline missing systolic BP measurement.
Three patients with hematuria at baseline missing estimated proteinuria.
P-values in bold denote significance at the 0.05 level.
BP, blood pressure.
MEST-C score, eGFR and microhematuria during follow-up
Of the 125 patients, 72 had follow-up data available [with a median Interquartile Range (IQR) follow-up time of 3.69 years (2.07, 6.96)]; of these, 60 (83%) had microhematuria at baseline. Of the 72 patients, 46 were classified as having microhematuria present during follow-up. There was no difference in terms of M1, E1 and C ≥ 1 between patients who had median microhematuria during follow-up versus those who did not (Supplementary data, Table S1). The rate of eGFR decline was found to be significantly accelerated with each increase in median degree of hematuria throughout follow-up (−3.70 mL/min/1.73 m2/year, P < 0.001). Estimates for rate of eGFR change per year by median degree of hematuria throughout follow-up are presented in Table 2.
Degree of microhematuria during follow-up and decline in eGFR
Based on results from the GEE models, each increase in the degree of hematuria during follow-up was significantly associated with a decline in eGFR of −1.12 mL/min/1.73 m2 (95% CI −1.68 to −0.56, P = 0.0001), adjusted for follow-up time (Table 3). Results were similar after further adjusting for proteinuria and T score; with each increase in the degree of hematuria associated with an eGFR decline of −0.81 mL/min/1.73 m2 (95% CI −1.44 to −0.18, P = 0.01). We also noted similar results when adjusting for treatment with RASB and immunosuppression (Table 3) and restricting analysis to patients with hematuria and proteinuria >1 g/24 h at baseline (Supplementary data, Table S2).
Table 3.
GEE parameter estimates for the association of degree of hematuria during follow-up with eGFR during follow-up
| Average eGFR decline per degree increase in hematuria |
||||
|---|---|---|---|---|
| Adjusted for | eGFR decline (mL/min/1.73 m2) | 95% Lower | 95% Upper | P-value |
| Time | −1.1192 | −1.6834 | −0.5551 | <0.001 |
| Time + estimated proteinuria | −0.7432 | −1.3567 | −0.1297 | 0.018 |
| Time + M | −1.1248 | −1.7069 | −0.5427 | <0.001 |
| Time + E | −1.0750 | −1.6281 | −0.5220 | <0.001 |
| Time + S | −1.0712 | −1.6287 | −0.5138 | <0.001 |
| Time + T | −1.1346 | −1.7087 | −0.5606 | <0.001 |
| Time + C | −1.1214 | −1.6798 | −0.5630 | <0.001 |
| Time + RASB | −1.1337 | −1.7277 | −0.5396 | <0.001 |
| Time + immunosuppression | −1.1532 | −1.7374 | −0.5690 | <0.001 |
| Time + RASB, immunosuppression | −1.1642 | −1.7734 | −0.5550 | <0.001 |
| Time + estimated proteinuria + T | −0.8122 | −1.4358 | −0.1886 | 0.011 |
n = 390 laboratory measures available between biopsy date and last follow-up or ESRD, among 72 patients.
GEE models using time-dependent predictors were modeled as main effects, and time-independent predictors (MEST-C scores) were considered along with an interaction term for time in order to estimate a within-subjects effect.
P-values in bold denote significance at the 0.05 level.
Among the MEST-C score, T was the only score that was found to be significantly associated with a decline in eGFR during follow-up. At any given time, eGFR in a patient with T1/T2 was estimated to be an average −23.44 mL/min/1.73 m2 (95% CI −35.26 to 11.62, P < 0.001) less compared with a patient with T0, adjusted for follow-up time, hematuria and proteinuria.
In sensitivity analyses considering severe microhematuria (≥21 RBCs/hpf) versus moderate/absent hematuria (<21 RBCs/hpf), severe microhematuria was associated with a significant decline in eGFR of −3.99 mL/min/1.73 m2 (95% CI −6.94 to −1.06, P = 0.008), after adjusting for time, estimated 24-h proteinuria and T score (Table 4).
Table 4.
Association between severe hematuria (≥21 RBCs/hpf) and eGFR during follow-up
| Average eGFR decline for hematuria ≥21 RBCs/hpf versus <21 RBCs/hpf |
||||
|---|---|---|---|---|
| Adjusted for | eGFR decline (mL/min/1.73 m2) | 95% Lower | 95% Upper | P-value |
| Time | −1.3928 | −7.9793 | −2.5198 | <0.001 |
| Time + estimated proteinuria | −3.8229 | −6.7408 | −0.9049 | 0.010 |
| Time + M | −5.2376 | −7.9856 | −2.4896 | <0.001 |
| Time + E | −5.0537 | −7.6789 | −2.4285 | <0.001 |
| Time + S | −5.0082 | −7.6671 | −2.3493 | <0.001 |
| Time + T | −5.2425 | −8.0541 | −2.4310 | <0.001 |
| Time + C | −5.2517 | −7.9126 | −2.5908 | <0.001 |
| Time + RASB | −5.2768 | −8.0925 | −2.4611 | <0.001 |
| Time + immunosuppression | −5.2773 | −8.0307 | −2.5239 | <0.001 |
| Time + RASB, immunosuppression | −5.2995 | −8.1296 | −2.4694 | <0.001 |
| Time + estimated proteinuria + T | −3.9867 | −6.9359 | −1.0375 | 0.008 |
n = 390 laboratory measures available between biopsy date and last follow-up or ESRD, among 72 patients.
Severe hematuria was categorized as severe (≥21) versus not severe (<21).
GEE models using time-dependent predictors were modeled as main effects, and time-independent predictors (MEST-C scores) were considered along with an interaction term for time in order to estimate a within-subjects effect.
P-values in bold denote significance at the 0.05 level.
Baseline characteristics and MEST-C with ESRD
Nine of the 72 patients (12.5%) progressed to ESRD, with six of these nine having both >1 g of proteinuria and microhematuria at baseline. Among the remaining three that progressed to ESRD, two had hematuria at baseline, of which one had <1 g of proteinuria (creatinine 2.0 mg/dL) and one had baseline proteinuria data missing (creatinine 2.3 mg/dL). One patient had neither >1 g of proteinuria nor microhematuria at baseline (creatinine 2.4 mg/dL). There were no deaths prior to ESRD in this cohort. Higher values of serum creatinine (HR 4.50, 95% CI 1.94–10.44, per 1 mg/dL increase), systolic blood pressure (HR 1.73, 95% CI 1.29–2.30, per 10 mmHg increase) and proteinuria (HR 1.38, 95% CI 1.15–1.64, per increase in 0.5 g) at baseline were significantly associated with higher risk of incident ESRD (Table 5). However, neither baseline (Table 5) nor isolated follow-up microhematuria was found to be significantly associated with ESRD (Supplementary data, Table S3). The only biopsy score at baseline found to be predictive of ESRD was T ≥ 1 (HR 12.21, 95% CI 1.52–97.70, P = 0.018) (Table 5).
Table 5.
Association between patient characteristics at baseline and incidence of ESRD
| Baseline variables | HR | 95% Lower | 95% Upper | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, per year | 1.038 | 0.996 | 1.081 | 0.078 |
| Sex (female versus male) | 0.667 | 0.166 | 2.677 | 0.57 |
| Laboratory measures at baseline | ||||
| Serum creatinine, per 1 mg/dL | 4.501 | 1.940 | 10.443 | 0.001 |
| eGFR, per 10 mL/min/1.73 m2 | 0.294 | 0.127 | 0.680 | 0.004 |
| Systolic BP, per 10 mmHg | 1.726 | 1.294 | 2.303 | <0.001 |
| Diastolic BP, per 10 mmHg | 0.928 | 0.480 | 1.796 | 0.83 |
| Estimated proteinuria, per 0.5 g | 1.375 | 1.154 | 1.639 | <0.001 |
| Baseline hematuria, per degreea (ordinal) | 0.853 | 0.639 | 1.140 | 0.28 |
| Hematuria (≥3 RBCs/hpf versus <3 RBCs/hpf) | 1.458 | 0.180 | 11.811 | 0.72 |
| Histology at biopsy | ||||
| M (1 versus 0) | 1.368 | 0.171 | 10.971 | 0.77 |
| E (1 versus 0) | 0.761 | 0.158 | 3.678 | 0.73 |
| S (1 versus 0) | 4.643 | 0.578 | 37.287 | 0.15 |
| T (≥1 versus 0) | 12.208 | 1.525 | 97.707 | 0.018 |
| C (≥1 versus 0) | 0.986 | 0.246 | 3.944 | 0.98 |
| RASB | 1.242 | 0.333 | 4.633 | 0.75 |
Degree of hematuria treated as ordinal variable with the following levels: as 0, <3, 3–10, 11–20, 21–30, 31–40, 41–50, 51–100, >100.
P-values in bold denote significance at the 0.05 level.
BP, blood pressure.
Medication usage and microhematuria during follow-up
Medication usage (immunosuppression and/or RASB) throughout follow-up was reported as the percentage of follow-up visits where each medication was used. Median (IQR) percentage of follow-up visits where immunosuppression was in use was 0 (0–30)% while those where RASB was given was 80 (45–100)%. Both medications were significantly associated with microhematuria when modeled separately as well as together. After adjusting for predicted proteinuria, use of immunosuppression retained significance while RASB was no longer significant (see Supplementary data, Table S4).
DISCUSSION
Hematuria is one of the most common findings in IgAN, yet the mainstay of treatment decision-making is based on proteinuria and MEST-C score. Our results show that microhematuria was present in 78% of patients at the time of renal biopsy and was significantly associated with three components of the MEST-C score—specifically M1, E1 and C ≥ 1, the latter two of which typically represent glomerular inflammation and cellular proliferation. This suggests that the presence of hematuria at the time of renal biopsy is a reflection of ongoing inflammation in the kidney.
In our study, we selected patients that were not on immunosuppressive therapy at biopsy. This was due to the fact that the independent predictive value of the MEST-C score does diminish in the presence of immunosuppressive therapy [18], with E lesions being the most affected by this [19, 20]. Indeed, we found that all patients with an E1 lesion had hematuria at the time of biopsy. This further supports speculation that the presence of hematuria could be indicative of active renal inflammation.
The absence of a significant association between hematuria and S or T score at the time of biopsy is also clinically relevant. Segmental sclerosis and tubular atrophy are more reflective of disease stage at biopsy and are associated with lower eGFR and poor renal outcomes [18, 19, 21–24]. This was confirmed by our finding that the only histological marker at baseline associated with both lower eGFR and progression to ESRD was that of T ≥ 1. Patients with higher S and T scores are therefore more likely to have an advanced disease in association with proteinuria in the absence of hematuria, which suggests that this group may not benefit substantially from immunosuppressive therapy.
Importantly, in this study, we found that hematuria was an independent risk factor for eGFR decline over and above follow-up time, proteinuria, MEST-C score and treatment. This decline in eGFR was especially pronounced when evaluating patients who had severe hematuria (≥21 RBCs/hpf).
Indeed, the concept of an association between hematuria and proliferative lesions on renal biopsy is not new, but its clinical importance has been ignored for far too long. In 1983, Bennett and Kincaid-Smith [25] reported on the association between hematuria and proliferative lesions (crescents). The authors concluded that careful assessment of the urine for evidence of hematuria can help define disease activity. However, this study did not compare hematuria with MEST-C score as the Oxford classification was not part of the standard of practice at that time.
Past studies looking at the relationship between hematuria in IgAN and ESRD have been conflicting. This is partly due to the fact that most studies evaluated the association between hematuria at baseline rather than over time [7, 26–30]. A recent study by Sevillano et al. showed that indeed time-averaged (TA) hematuria was predictive of renal survival and progression to ESRD, while resolution of hematuria resulted in improvement in renal survival [8]. In another study of over 1100 Chinese patients with IgAN, TA hematuria during follow-up was the most important risk factor of renal failure [31]. Our study supports and strengthens these prior findings, considering they did not take into account the varying time component of the TA hematuria exposure in the analysis. When we evaluated the association of hematuria during follow-up and incident ESRD, we modeled hematuria as a time-dependent covariate in our analysis, since its value can change over the course of follow-up. We did not find an association between hematuria during follow-up and incident ESRD; however, due to the small number of events in our cohort, our study was not sufficiently powered to detect a small or moderate effect of hematuria on incident ESRD. Similar to the study by Sevillano et al., in our cohort six of nine (67%) patients who progressed to ESRD had persistent hematuria during follow-up in addition to proteinuria >1 g/24 h.
The traditional approach of taking patients with high-grade proteinuria alone (without taking into account the degree of hematuria) will inadvertently result in selecting patients who have proteinuria due to irreversible damage (likely to have histological features of high S and T scores). A recent highly publicized randomized controlled trial (Supportive Versus Immunosuppressive Therapy for the Treatment of Progressive IgA Nephropathy; STOP-IgAN) in which patients with proteinuria >1 g/24 h were randomized to immunosuppressive therapy versus no immunosuppressive therapy after 6 months of conservative treatment did not find a significant improvement in renal outcome in patients who received immunosuppressive therapy compared with those that did not, and instead found a higher rate of adverse events in those that received immunosuppressive treatment compared with those who did not [13]. Even though the majority of patients (up to 74% in the immunosuppressive group) had hematuria at baseline, the degree of hematuria or degree of scarring on renal biopsy was not taken into account as a stratification factor when patients were undergoing randomization. Similarly, a recent publication that sought to identify and validate a prediction model for disease progression in patients with the IgAN using histological and clinical risk factors failed to include hematuria in the model [13]. Including patients who have >1 g/24 h of proteinuria regardless of the presence or absence of hematuria in clinical trials may be masking the benefits of immunosuppressive therapy in the subgroup of IgAN patients with reversible injury.
Given that our results show that hematuria is associated with E1 and C ≥ 1 scores at baseline, along with the finding of hematuria itself being an independent risk factor for eGFR decline, we propose a new approach in identifying ‘high-risk’ IgA patients. The combination of high-risk features including proteinuria >1 g/day, severe hematuria (≥21 RBCs/hpf) and histological findings of E1 and C ≥ 1 should alert the clinician to suspect a higher chance of renal deterioration over time for that patient. Similar concepts have been explored in the pediatric population. Feng et al. demonstrated that children who had persistent asymptomatic microhematuria and proteinuria were more likely to have adverse renal outcomes compared with those with isolated microhematuria group [26]. The patients with ‘high-risk features’ may have a favorable risk-to-benefit ratio when using immunosuppressive therapy; however, this would need to be explored further in future IgAN trials looking at both hematuria and proteinuria. Since urinalysis is a cheap, non-invasive and widely available test worldwide [6], its application as a biomarker of disease activity would be of significant clinical importance. Indeed, it is already utilized to assess disease activity in other disease entities such as Anti-Neutrophilic Cytoplasmic Autoantibodies (ANCA) associated vasculitis [32]. As such, adding microscopic hematuria to the risk stratification of patients with IgAN would follow a practice that is already in place for management of other forms of glomerulonephritis.
Whether microhematuria is a marker of glomerular inflammation or has direct nephrotoxicity, or both, is not well-established. Heme has been shown to have direct pro-oxidant effects and can cause nephrotoxicity through tubular damage in addition to podocyte injury. As a result, the heme-oxygenase-1 system, which is an innate defense system, has developed to protect the kidney against heme-related injury [33].
Our study has several limitations. This was a retrospective analysis from a tertiary center, which could result in referral bias. In addition, the median follow-up time in our cohort was 3.7 years and it is likely that with longer follow-up we would have had more patients who would have reached ESRD. We therefore lacked power to evaluate the effect of hematuria on development of ESRD in this cohort. There was also heterogeneity in the management of these patients throughout their disease course. We did, however, adjust for treatment when evaluating the association between hematuria and eGFR decline in order to account for this limitation, but the data presented are unsuitable to address therapeutic questions. Our findings are generalizable to patients with IgAN, but not those with other variants such as HSP, or those with IgAN associated with other causes such as liver dysfunction, or those who had additional pathology present on biopsy such as concomitant ANCA vasculitis. We also acknowledge that our cohort primarily consisted of Caucasians (83.2%), while IgAN often affects Asians.
Notable strengths of our study include our choice to select patients who were not on immunosuppression at the time of biopsy, which has a substantial impact on the E1 score in this setting. Furthermore, all urinalysis testing and microscopy were consistently performed within the same health system. In our study, when defining the presence or absence of follow-up hematuria, we calculated median degree of hematuria during the course of follow-up, since this is a robust measure with respect to outliers compared with using averages. Lastly, we were able to collect and evaluate crescent data as part of the MEST-C score for this study.
CONCLUSION
We demonstrate that hematuria during follow-up is an independent predictor of eGFR decline after adjusting for follow-up time, proteinuria, MEST-C score and treatment. These findings suggest that monitoring the degree of hematuria as well as proteinuria levels during follow-up is essential. Additional studies using a decline in hematuria as a primary surrogate outcome are needed.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
FUNDING
This publication was made possible by Clinical and Translational Science Awards (CTSA) Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH. Funding for this publication was provided by the Mayo Clinic Medical Foundation.
AUTHORS’ CONTRIBUTIONS
S.A.B., F.C.F. and L.Z. contributed to the research idea and study design; S.A.B., M.P.A., R.G. and K.S. contributed to data acquisition; S.A.B., M.P.A., K.S., L.E.V., L.C., S.S., R.G., R.J.G., F.C.F. and L.Z. contributed to data analysis/interpretation; L.E.V. was responsible for the statistical analysis. Each author contributed important intellectual content during manuscript drafting or revision.
CONFLICT OF INTEREST STATEMENT
R.J.G. reports personal fees from Omeros, Apellis, Ionis, Chemocentryx and Retrophin, and ‘other’ from Wolters-Kluwer (UpToDate), outside the submitted work. The authors also confirm that neither the manuscript nor any part of it, except in abstract form, has been presented or is being considered for publication elsewhere.
Supplementary Material
REFERENCES
- 1. Lafayette RA, Canetta PA, Rovin BH. et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol 2017; 28: 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartosik LP, Lajoie G, Sugar L. et al. Predicting progression in IgA nephropathy. Am J Kidney Dis 2001; 38: 728–735 [DOI] [PubMed] [Google Scholar]
- 3. Floege J, Barbour SJ, Cattran DC. et al. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 95: 268–280 [DOI] [PubMed] [Google Scholar]
- 4. Zhao YF, Zhu L, Liu LJ. et al. Measures of urinary protein and albumin in the prediction of progression of IgA nephropathy. Clin J Am Soc Nephrol 2016; 11: 947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moriyama T, Tanaka K, Iwasaki C. et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One 2014; 9: e91756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coppo R, Fervenza FC.. Persistent microscopic hematuria as a risk factor for progression of IgA nephropathy: new floodlight on a nearly forgotten biomarker. J Am Soc Nephrol 2017; 28: 2831–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iwasaki C, Moriyama T, Tanaka K. et al. Effect of hematuria on the outcome of immunoglobulin A nephropathy with proteinuria. J Nephropathol 2016; 5: 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sevillano AM, Gutierrez E, Yuste C. et al. Remission of hematuria improves renal survival in IgA nephropathy. J Am Soc Nephrol 2017; 28: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barbour SJ, Espino-Hernandez G, Reich HN. et al. The MEST score provides earlier risk prediction in IgA nephropathy. Kidney Int 2016; 89: 167–175 [DOI] [PubMed] [Google Scholar]
- 10. Haas M, Verhave JC, Liu ZH. et al. A multicenter study of the predictive value of crescents in IgA nephropathy. J Am Soc Nephrol 2017; 28: 691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Markowitz G. Updated Oxford classification of IgA nephropathy: a new MEST-C score. Nat Rev Nephrol 2017; 13: 385. [DOI] [PubMed] [Google Scholar]
- 12. Glomerulonephritis K. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2012; 2: 209–217 [Google Scholar]
- 13. Rauen T, Eitner F, Fitzner C. et al. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 2015; 373: 2225–2236 [DOI] [PubMed] [Google Scholar]
- 14. Lv J, Zhang H, Wong MG. et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: The TESTING randomized clinical trial. JAMA 2017; 318: 432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamadah AM, Gharaibeh K, Mara KC. et al. Urinalysis for the diagnosis of glomerulonephritis: role of dysmorphic red blood cells. Nephrol Dial Transplant 2018; 33: 1397–1403 [DOI] [PubMed] [Google Scholar]
- 16. Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008; 167: 492–499 [DOI] [PubMed] [Google Scholar]
- 17. Hanley JA, Negassa A, MDd E. et al. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003; 157: 364–375 [DOI] [PubMed] [Google Scholar]
- 18. Coppo R, Troyanov S, Bellur S. et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 2014; 86: 828–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trimarchi H, Barratt J, Cattran DC. et al. Oxford classification of IgA nephropathy 2016: an update from the IgA nephropathy classification working group. Kidney Int 2017; 91: 1014–1021 [DOI] [PubMed] [Google Scholar]
- 20. Shen XH, Liang SS, Chen HM. et al. Reversal of active glomerular lesions after immunosuppressive therapy in patients with IgA nephropathy: a repeat-biopsy based observation. J Nephrol 2015; 28: 441–449 [DOI] [PubMed] [Google Scholar]
- 21.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT. et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76: 534–545 [DOI] [PubMed] [Google Scholar]
- 22. Kang SH, Choi SR, Park HS. et al. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrol Dial Transplant 2012; 27: 252–258 [DOI] [PubMed] [Google Scholar]
- 23. Nasri H, Sajjadieh S, Mardani S. et al. Correlation of immunostaining findings with demographic data and variables of Oxford classification in IgA nephropathy. J Nephropathol 2013; 2: 190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng CH, Le W, Ni Z. et al. A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult Chinese patients. Am J Kidney Dis 2012; 60: 812–820 [DOI] [PubMed] [Google Scholar]
- 25. Bennett WM, Kincaid-Smith P.. Macroscopic hematuria in mesangial IgA nephropathy: correlation with glomerular crescents and renal dysfunction. Kidney Int 1983; 23: 393–400 [DOI] [PubMed] [Google Scholar]
- 26. Feng CY, Xia YH, Wang WJ. et al. Persistent asymptomatic isolated hematuria in children: clinical and histopathological features and prognosis. World J Pediatr 2013; 9: 163–168 [DOI] [PubMed] [Google Scholar]
- 27. Goto M, Wakai K, Kawamura T. et al. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrol Dial Transplant 2009; 24: 3068–3074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le W, Liang S, Chen H. et al. Long-term outcome of IgA nephropathy patients with recurrent macroscopic hematuria. Am J Nephrol 2014; 40: 43–50 [DOI] [PubMed] [Google Scholar]
- 29. Lee H, Kim DK, Oh KH. et al. Mortality of IgA nephropathy patients: a single center experience over 30 years. PLoS One 2012; 7: e51225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka K, Moriyama T, Iwasaki C. et al. Effect of hematuria on the outcome of IgA nephropathy with mild proteinuria. Clin Exp Nephrol 2015; 19: 815–821 [DOI] [PubMed] [Google Scholar]
- 31. Le W, Liang S, Hu Y. et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 2012; 27: 1479–1485 [DOI] [PubMed] [Google Scholar]
- 32. Mukhtyar C, Lee R, Brown D. et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009; 68: 1827–1832 [DOI] [PubMed] [Google Scholar]
- 33. Tracz MJ, Alam J, Nath KA.. Physiology and pathophysiology of heme: implications for kidney disease. J Am Soc Nephrol 2007; 18: 414–420 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.