Abstract
Background
Initiation of renal replacement therapy often results from a combination of kidney function deterioration and symptoms related to chronic kidney disease (CKD) progression. We investigated the association between kidney function decline and symptom development in patients with advanced CKD.
Methods
In the European Quality study on treatment in advanced CKD (EQUAL study), a European prospective cohort study, patients with advanced CKD aged ≥65 years and a kidney function that dropped <20 mL/min/1.73 m2 were followed for 1 year. Linear mixed-effects models were used to assess the association between kidney function decline and symptom development. The sum score for symptom number ranged from 0 to 33 and for overall symptom severity from 0 to 165, using the Dialysis Symptom Index.
Results
At least one kidney function estimate with symptom number or overall symptom severity was available for 1109 and 1019 patients, respectively. The mean (95% confidence interval) annual kidney function decline was 1.70 (1.32; 2.08) mL/min/1.73 m2. The mean overall increase in symptom number and severity was 0.73 (0.28; 1.19) and 2.93 (1.34; 4.52) per year, respectively. A cross-sectional association between the level of kidney function and symptoms was lacking. Furthermore, kidney function at cohort entry was not associated with symptom development. However, each mL/min/1.73 m2 of annual kidney function decline was associated with an extra annual increase of 0.23 (0.07; 0.39) in the number of symptoms and 0.87 (0.35; 1.40) in overall symptom severity.
Conclusions
A faster kidney function decline was associated with a steeper increase in both symptom number and severity. Considering the modest association, our results seem to suggest that repeated thorough assessment of symptom development during outpatient clinic visits, in addition to the monitoring of kidney function decline, is important for clinical decision-making.
Keywords: chronic kidney disease, clinical epidemiology, kidney function, kidney function decline, symptoms
INTRODUCTION
Patients with advanced-stage chronic kidney disease (CKD) suffer from a wide range of symptoms. A growing body of evidence exists that CKD symptom burden is negatively correlated with health-related quality of life, and positively correlated with increased morbidity and mortality rates [1, 2]. Previous studies in people with Stages 4–5 CKD show that poor mobility and weakness are experienced by more than two-thirds of the patients, while poor appetite, pain and itching are reported in ∼60% [3]. In terms of number of symptoms and severity, patients with CKD Stage 5, managed conservatively, experienced a symptom burden similar to that of an advanced cancer population [4]. In general, the more prevalent symptoms were rated as more burdensome. However, the symptom pain was an exception, for which a disproportionately greater severity was reported [4]. Patients rate symptoms as one of the most important aspects of their kidney disease. One of the main reasons behind this is the severity of symptoms they experience [5]. Healthcare providers and patients also believe that symptoms should be one of the main focuses of CKD research [6, 7].
In a medical speciality like rheumatology, decision-making often involves evaluation of symptom burden. As an example, the disease activity score, including symptoms, is used in decision-making regarding treatment initiation but also to evaluate the effect of treatment. Also, in clinical nephrology, there is a fundamental knowledge that symptom evaluation is important. Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend the initiation of renal replacement therapy (RRT) when symptoms are present, which is often although not invariably in the glomerular filtration rate (GFR) range between 5 and 10 mL/min/m2 [8]. From a clinical point of view, it could be expected that symptoms increase while kidney function deteriorates in patients with CKD. Surprisingly, however, evidence for this association is lacking. This is important, as in general there is a lack of association between kidney function and symptoms in cross-sectional studies [3, 9, 10]. The interplay between kidney function and symptoms remains unclear for the question when to start dialysis, as also illustrated by the Initiating Dialysis Early And Late (IDEAL) study, where patients were randomized to an early versus late start dialysis based upon estimated GFR (eGFR) [11]. In this study, physical symptoms played an important role in deciding if and when to initiate dialysis. A large proportion of patients randomized to the late starting group started earlier due to the presence of uraemic symptoms. Thus, even though symptom burden was demonstrated to play a major role in the decision-making for dialysis initiation in the IDEAL study, the longitudinal association between change in kidney function and change in symptoms over time in patients with advanced CKD was never empirically investigated.
To fill this gap, we aimed to study the association between kidney function decline and symptom development (i.e. symptom number and severity) over time in patients with advanced CKD. To replicate findings of existing literature, we also studied the cross-sectional association between the level of kidney function and symptoms at baseline, and to expand on this, we explored the association between the level of kidney function and symptom development.
MATERIALS AND METHODS
Study design and population
The European Quality study on treatment in advanced CKD (EQUAL study) is an ongoing prospective cohort study in patients with advanced CKD in Germany, Italy, Poland, Sweden, UK and the Netherlands. Approval was obtained from the medical ethical committees or corresponding institutional review boards (as appropriate) for all participating centres. All included patients gave their written informed consent. A full description of the EQUAL study has been published elsewhere [12]. In short, patients of ≥65 years were included with an incident eGFR drop to or <20 mL/min/1.73 m2 in the last 6 months. Patients were eligible when followed in a nephrology clinic and were excluded when the eGFR drop was the result of an acute event or when a history of RRT (i.e. start of dialysis or kidney transplantation) was present. Identified patients who met the eligibility criteria were consecutively approached. Patients were followed until kidney transplantation, death, moving to a centre not participating in the EQUAL study, refusal of further participation, loss to follow-up or end of follow-up, whichever came first. For the current analyses, the follow-up time would end at the first occurrence of January 2018 or the initiation of dialysis. Follow-up data at cohort entry, after 6 and 12 months of follow-up were used from patients recruited between March 2012 and January 2018 and who filled out at least the symptom part of the patient questionnaire.
Data collection and variable definitions
In the EQUAL study, patients are followed while receiving routine medical care as provided by the nephrology clinics. Data were collected and entered into a web-based clinical record form, developed for this specific purpose. Collected information included patients’ demographics, primary kidney disease, comorbid condition, ethnicity, medication, diet, physical examination and laboratory data. Physical examinations and collection of laboratory data were performed according to standard protocols and procedures following the routine care at the local participating sites. For uniformity of data, all participating centres completed a questionnaire capturing details on local laboratory methods, units of measurement and reference ranges. Subsequently, all data were recalculated into one uniform unit of choice. Kidney function was estimated according to the four-variable Modification of Diet in Renal Disease (MDRD) formula, taking into account age, sex, race and serum creatinine [13]. See Supplementary data, Table S1, for detailed variable descriptions of primary kidney disease, educational level, diabetes mellitus and psychiatric disease.
Data on lifestyle, marital status, and symptom number and severity were obtained through self-administered paper questionnaires. The list of symptoms (Supplementary data, Table S1) was composed of the original validated Dialysis Symptom Index (DSI) complemented by items assessing the following symptoms: bleeding, loss of weight and loss of strength [14]. These symptoms were added based on the expert opinion of nephrologists collaborating on the EQUAL study. Furthermore, these symptoms were added at the bottom of the original DSI, and thus did not influence the validity of the questionnaire. Patients responded about whether these symptoms were present in the past month. In total, 33 symptoms were assessed, thus the total sum score for symptom number ranged from 0 to 33 symptoms. Additionally, for each symptom scored ‘present’, patients also rated symptom severity (how much burden they experienced) on a 5-point Likert scale ranging from 1 ‘not at all’ to 5 ‘very much’ burdensome. An overall symptom severity sum score ranging from 0 to 165 was generated, assigning a score of zero for symptoms that were absent [15].
Statistical analyses
Baseline characteristics were presented as mean with standard deviation (SD) for normally distributed continuous variables, as median with interquartile range (IQR) for skewed continuous variables and as frequencies with percentages for categorical variables.
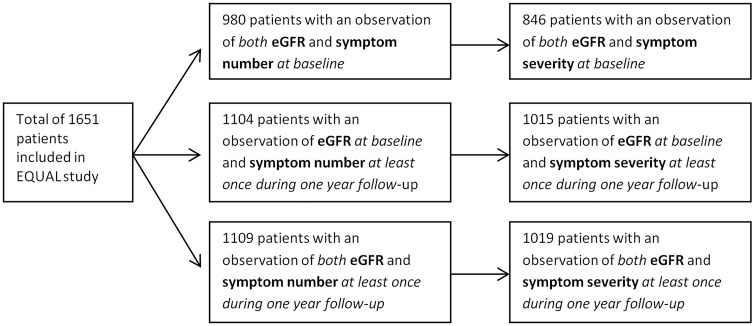
For the main analyses, patients were included when at least one observation of both kidney function and symptom score was available. For the cross-sectional analysis, this applied at baseline and for the longitudinal analysis, this applied for one observation in the 1 year of follow-up. Using linear mixed models only one observation is needed [16]. As a result, different patient numbers were used in the analyses (see Figure 1).
FIGURE 1.
Flowchart of patient inclusion for the present analyses, based on data availability.
We performed three main analyses. First, linear regression analysis was performed to estimate the cross-sectional association between the level of eGFR at baseline and both the number and severity of symptoms at baseline to replicate findings of existing studies.
Secondly, to investigate the association between the level of eGFR at baseline and the development in symptom number and severity over time, we used linear mixed-effects models where patients were included as random intercepts and reported coefficient for the interaction between a continuous time and the level of eGFR at baseline [16].
Thirdly, the longitudinal association between eGFR decline and the development of symptom burden (either the number or severity of symptoms) over time was also estimated using linear mixed-effects models. Regression coefficients for the additional change in symptom burden with one unit change in GFR were obtained as outcome by modelling trajectories of kidney function and symptoms simultaneously, thereby allowing within and between individual variations using the fixed and random-effects model. Correlations and SEs were estimated using the delta method [17].
Multiple imputation was used to minimize the risk of bias due to missing data [18]. Estimates and SEs were calculated in each imputation set and pooled into one overall estimate and SE according to Rubin’s rules [19, 20]. All confounders were assumed to be missing at random, for which multiple imputation using a fully conditional specification with 10 repetitions is a valid technique and reduces bias compared with complete case analysis [21, 22]. Exposure and outcome variables were not imputed. In the multiple imputation model, we included all potential confounders, exposure and outcome variables. Non-normally distributed variables were transformed to approximate normality before imputation and then the imputed values were transformed back to the original scale [21].
All aforementioned analyses were adjusted for age, sex, ethnicity, country of residence, educational level, diabetes mellitus, cerebrovascular disease, myocardial infarction, hypertension, malignancy, psychiatric disease, body mass index (BMI), primary kidney disease, haemoglobin and proteinuria. For all analyses, the baseline confounders were used to adjust for confounding. In all aforementioned analyses, causal interpretations should be avoided [23].
For the purpose of illustration, mean trajectories of kidney function decline and development in number and severity of symptoms are plotted in figures using estimated marginal means obtained from linear mixed models with a random intercept for each patient, including time as categorical variable at baseline, after 6 and 12 months of follow-up.
Sensitivity analyses
Several pre-planned sensitivity analyses were performed to assess the robustness of our main results. Analyses were repeated using eGFR based on the CKD Epidemiology Collaboration (CKD-EPI) equation instead of the MDRD. The cross-sectional association between kidney function and symptoms was also assessed after 6 and 12 months of follow-up, to allow for more variability in eGFR. Furthermore, the longitudinal analyses regarding the association between kidney function level and symptom development, and the association between the kidney function and symptom trajectories were repeated using a two-stage approach in linear regression analysis [24]. First, we calculated the individual linear regression slopes of change in symptoms and kidney function per patient. In the second stage, we correlated either the baseline eGFR or individual eGFR declines with the calculated individual slopes of either symptom number or overall symptom severity in a linear regression model. Finally, analyses were repeated for 13 uraemia- or disease-related symptoms (see Supplementary data, Table S1). These 13 symptoms are an adapted list of symptoms based on symptoms reported by the KDOQI guidelines and reported as most prevalent, frequent or severe in advanced kidney failure in literature [3, 9, 15, 25–29].
Analyses using linear mixed-effects models were performed using the SAS statistical package (version 9.4, SAS Institute, Cary, NC, USA). All other analyses were performed using SPSS 23.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics
For the present analyses, a total of 1109 patients were included with at least one observation of symptom number and eGFR-MDRD, and 1019 patients were included with at least one observation of overall symptom severity and eGFR-MDRD. Median (IQR) follow-up time was 0.98 (0.64–1.03) year. The baseline characteristics of both patient groups are presented in Table 1. The mean (SD) baseline eGFR was 18.9 (5.4) and 18.8 (5.3) mL/min/1.73 m2 in those patients with scores on either the number or overall severity of symptoms available, respectively. The median (IQR) age was 75.9 (70.5–80.8) and 75.7 (70.2–80.5) years for patients with symptom number and symptom severity scores available, respectively. The symptoms muscle soreness, difficulty concentrating, constipation and decreased appetite increased the most in terms of reported symptom presence over 1-year follow-up period in our study population (see Supplementary data, Figure S1). The symptom severity increased the most for the symptoms difficulty in becoming sexually aroused, muscle soreness, difficulty concentrating and decreased interest (see Supplementary data, Figure S2).
Table 1.
Baseline characteristics in patients with at least two visits with eGFR-MDRD and overall symptom score available during first year of pre-dialysis
| Symptom number and eGFR-MDRD available for at least one visit during 1-year pre-dialysis (n = 1109)a | Symptom severity and eGFR-MDRD available for at least one visit during 1-year pre-dialysis (n = 1019)b | |
|---|---|---|
| Sex, male | 764 (68.9) | 698 (68.5) |
| Age, years | 75.9 (70.5–80.8) | 75.7 (70.2–80.5) |
| Ethnicity | ||
| Caucasian | 1087 (98.4) | 1000 (98.4) |
| Black | 6 (0.5) | 6 (0.6) |
| Other | 12 (1.1) | 10 (1.0) |
| Primary kidney disease | ||
| Glomerular disease | 106 (9.6) | 99 (9.7) |
| Tubulo-interstitial disease | 95 (8.6) | 89 (8.7) |
| Diabetes mellitus | 214 (19.3) | 187 (18.4) |
| Hypertension | 385 (34.7) | 361 (35.4) |
| Other/unknown | 309 (27.9) | 283 (27.8) |
| Educational levelc | ||
| No | 27 (2.5) | 24 (2.4) |
| Low | 308 (28.8) | 266 (27.0) |
| Intermediate | 544 (50.9) | 510 (51.8) |
| High | 154 (14.4) | 151 (15.3) |
| Other | 36 (3.4) | 34 (3.5) |
| Marital status, married or living together | 714 (66.0) | 662 (66.6) |
| Diabetes mellitus, yesd | 449 (41.3) | 404 (40.4) |
| Hypertension, yese | 991 (92.2) | 919 (92.6) |
| Cerebrovascular disease, yes | 168 (15.5) | 152 (15.3) |
| Myocardial infarction, yes | 202 (18.5) | 185 (18.5) |
| Malignancy, yes | 228 (21.2) | 210 (21.1) |
| Psychiatric disease, yes | 86 (7.9) | 75 (7.5) |
| BMI, kg/m² | 28.2 (±5.3) | 28.2 (±5.3) |
| eGFR baseline, mL/min/1.73 m² | 18.9 (±5.4) | 18.8 (±5.3) |
| Serum albumin, g/L | 37.6 (±5.9) | 37.6 (±5.8) |
| Haemoglobin, mmol/L | 7.2 (±0.9) | 7.2 (±0.9) |
| Proteinuria, g/24 h | 1.5 (0.5–5.0) | 1.5 (0.5–5.4) |
Values are given as frequency (percentage), mean (±SD) or median (IQR), as appropriate.
Missings: 0.4% ethnicity, 0.9% educational level, 2.5% marital status, 1.9% diabetes, 3.1% hypertension, 2.4% cerebrovascular disease, 1.8% myocardial infarction, 2.8% malignancy, 2.3% psychiatric disease, 6.6% BMI, 9.8% albumin, 2.1% haemoglobin, 71.8% proteinuria.
Missings: 0.3% ethnicity, 2.5% marital status, 3.3% educational level, 1.9% diabetes, 2.6% hypertension, 2.3% cerebrovascular disease, 1.8% myocardial infarction, 2.4% malignancy, 2.2% psychiatric disease, 6.8% BMI, 9.7% albumin, 2.1% haemoglobin, 71.9% proteinuria.
Defined as: low, no education or primary school only; intermediate, primary and secondary school; high, academic education.
Defined as the presence of diabetes mellitus as primary kidney disease or a history of diabetes mellitus, both Type I and Type II.
Defined as either the presence of hypertension as primary kidney disease or a history of hypertension.
Baseline characteristics of patients with no observations of both eGFR-MDRD and overall symptom score during the first year of pre-dialysis care are shown in Supplementary data, Table S2. The baseline characteristics of included and excluded patients were comparable, though included patients comprised a slightly higher percentage of males than excluded patients. In the total EQUAL study population of 1651 patients, 205 patients initiated dialysis and 168 patients dropped out during the first year of follow-up, and 239 patients did not reach the end of the first-year follow-up period.
Cross-sectional association of kidney function and symptoms at baseline
At cohort entry, there was no cross-sectional association between the level of kidney function and the number of symptoms (Table 2). Furthermore, we found no association between the level of kidney function and overall severity of symptoms at baseline.
Table 2.
Cross-sectional effect per unit lower eGFR-MDRD on symptom number and severity at baseline
| Symptom number (n = 980) | Symptom severity (n = 846) | |
|---|---|---|
| Unadjusted | −0.01 (−0.08; 0.07) | −0.06 (−0.34; 0.23) |
| Adjusteda | 0.004 (−0.07; 0.08) | 0.06 (−0.22; 0.34) |
Adjusted for: age, sex, ethnicity, country of residence, educational level, diabetes mellitus, cerebrovascular disease, myocardial infarction, hypertension, malignancy, psychiatric disease, BMI, primary kidney disease, haemoglobin and proteinuria at each specific time point (baseline, 6 or 12 months after cohort entry).
Association of kidney function at baseline and symptom development
No association was found between the level of kidney function at cohort entry and the development of symptoms over time. This applied to both the number and overall severity of symptoms in the unadjusted and adjusted analysis (Table 3).
Table 3.
Effect per unit lower eGFR-MDRD at baseline on annual change in symptom number and severity
| Symptom number (n = 1104) | Symptom severity (n = 1015) | |
|---|---|---|
| Mean annual increase (95% CI) | 0.76 (0.30; 1.21) | 3.00 (1.41; 4.59)* |
| Extra increase per unita lower kidney function at baseline | ||
| Unadjusted | 0.02 (−0.08; 0.11) | −0.03 (−0.37; 0.30) |
| Adjustedb | 0.08 (−0.01; 0.17) | 0.21 (−0.13; 0.55) |
One unit is 1 mL/min/1.73 m2.
Adjusted for: age, sex, ethnicity, country of residence, educational level, diabetes mellitus, cerebrovascular disease, myocardial infarction, hypertension, malignancy, psychiatric disease, BMI, primary kidney disease, haemoglobin and proteinuria at baseline.
P < 0.05.
Association of kidney function decline and symptom development
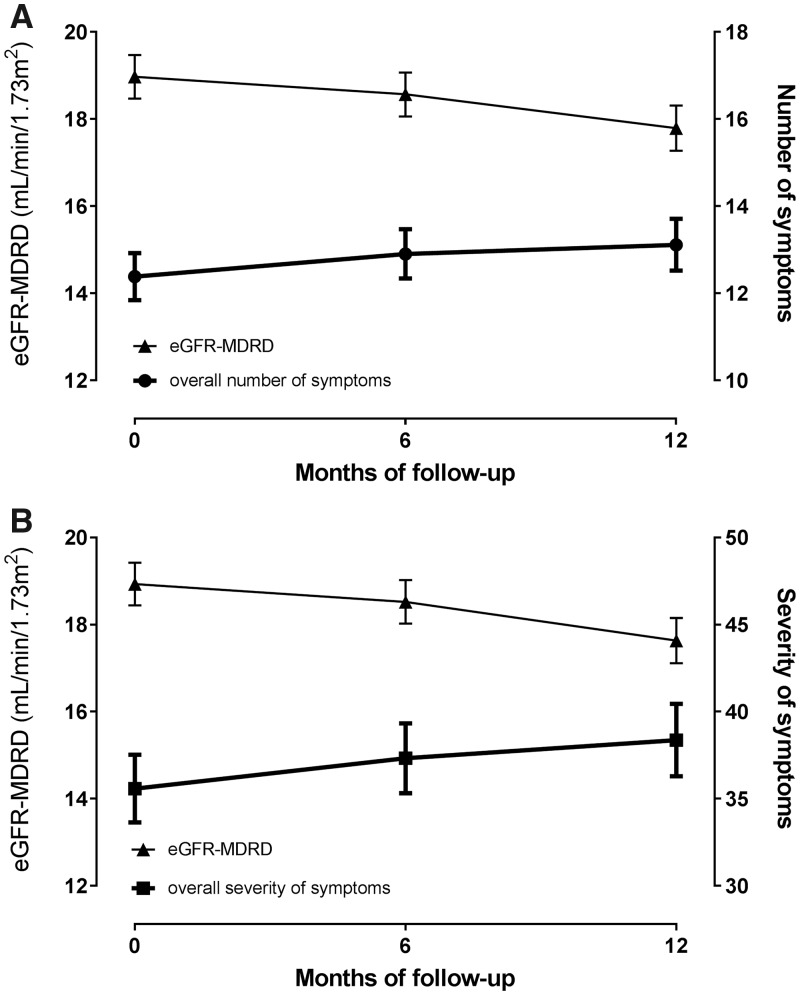
The trajectories of kidney function decline and development of both the number and severity of symptoms over time are presented in Figure 2. The mean [95% confidence interval (CI)] annual kidney function decline was 1.63 (1.26; 2.00) mL/min/1.73 m2. The mean (95% CI) annual increase in the number of symptoms was 0.73 (0.28; 1.19). Each unit (=1 mL/min/1.73 m2) annual decline of kidney function was associated with an adjusted extra annual increase in number of symptoms with 0.23 (0.07; 0.39) point (Table 4). Besides, the mean increase in overall symptom severity was 2.93 (1.34; 4.52) points per year. Thereby, the symptoms difficulty concentrating, restless legs and decreased appetite increased most severely over time. Each unit of annual kidney function decline was associated with an adjusted extra annual increase in overall symptom severity with 0.87 (0.35–1.40) point (Table 4). In other words, a faster kidney function decline was associated with a steeper increase in both the number of symptoms and the overall severity of symptoms per year in patients with advanced CKD. These numbers correspond to 32% and 30% of the mean annual increase of 0.73 in symptom number and 2.93 in overall symptom severity, respectively. Figure 3 illustrates the impact of one additional unit decline of kidney function on the development of overall symptom severity in an average patient.
FIGURE 2.
Overall mean (95% CI) trajectories, based on estimated marginal means, of kidney function decline and increase in number of symptoms (A) and mean (95% CI) kidney function decline and development of severity of symptoms over time in advanced CKD patients (B).
Table 4.
Effect per unit decline in eGFR-MDRD (per year) on annual change in symptom number and severity
| Symptom number (n = 1109) | Symptom severity (n = 1019) | |
|---|---|---|
| Mean annual increase (95% CI) | 0.73 (0.28; 1.19)* | 2.93 (1.34; 4.52)* |
| Extra increase per unita decline in kidney function | ||
| Unadjusted | 0.24 (0.08; 0.40)* | 0.88 (0.34; 1.41)* |
| Adjustedb | 0.23 (0.07; 0.39)* | 0.87 (0.35; 1.40)* |
One unit is 1 mL/min/1.73 m2 decline per year.
Adjusted for: age, sex, ethnicity, country of residence, educational level, diabetes mellitus, cerebrovascular disease, myocardial infarction, hypertension, malignancy, psychiatric disease, BMI, primary kidney disease, haemoglobin and proteinuria at baseline.
P < 0.05.
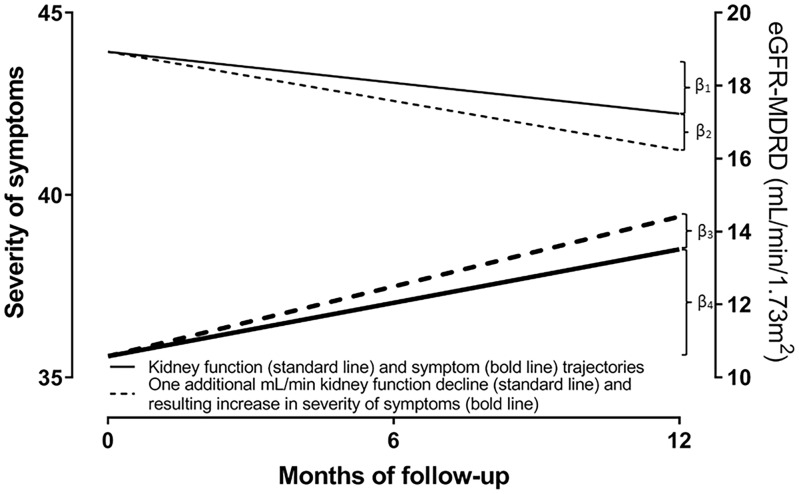
FIGURE 3.
Illustration of the adjusted mean annual slopes of kidney function (β1 = 1.70 mL/min/1.73 m2) and overall symptom severity (β4 = 2.93) in a patient with average covariate values (solid line). Furthermore, we show the impact of one additional mL/min/1.73 m2 kidney function decline (β2 = 1.00 mL/min/1.73 m2) per year on the extra increase of the overall severity of symptoms over time (β3 = 0.87). The additional kidney function decline and resulting increase in symptom severity is represented with the dashed lines; this results in a total decline of kidney function of β1 + β2 (= 2.70 mL/min/1.73 m2) and associates with a total increase in symptoms of β3 + β4 (= 3.80) per year.
Sensitivity analyses
Using the CKD-EPI instead of the MDRD equation yielded comparable results (Supplementary data, Tables S3–S5). After 6 and 12 months of follow-up, there was no cross-sectional association between the level of kidney function and either the number or severity of symptoms (Supplementary data, Table S6). Repeating the longitudinal analyses with linear regression on individual slopes instead of linear mixed-effects models yielded comparable results (Supplementary data, Tables S7 and S8). Also, repeating the analyses in individuals with complete questionnaire data on 13 disease-related symptoms did not materially change the results. Each unit decrease in kidney function decline was significantly associated with a more progressive increase in both number and overall severity of symptoms (Supplementary data, Tables S9–S11). The association between kidney function decline and increase in overall symptom burden was slightly weaker.
DISCUSSION
In our study of older adults with advanced-stage CKD, we found that a faster kidney function decline was associated with a steeper increase in the symptom burden over time in patients with advanced CKD. For each unit (=mL/min/1.73 m2) annual decline of kidney function the increase in number and severity of symptoms steepens, with 0.23 and 0.87 per year. This may seem modest, but corresponds to ∼30% of the mean annual increase in both symptom number and severity. We found neither a cross-sectional association in level of kidney function and symptoms nor an association between baseline kidney function and symptom development during the pre-dialysis phase.
The symptom burden was substantial in our study population, which has been shown previously at baseline [30]. The symptom number at cohort entry is in concordance with observations in literature, reporting an average number of symptoms between 6 and 20 symptoms in patients with CKD [6, 31]. Our symptom severity was somewhat higher than reported by Almutary et al. [25]. Our mean annual increase in number of symptoms was similar to the increase of approximately half a symptom found in the 24–12 months prior to reaching the endpoint dialysis, transplantation or death in the study of de Goeij et al. [9]. We found a mean (95% CI) increase in symptom severity of 2.93 (1.34; 4.52) per year. Our study is the first that examined the increase in symptom severity over time in CKD patients. It is important to distinguish between symptom number and symptom severity in each individual patient [4, 25]. A higher symptom number does not necessarily mean that these patients experience a higher symptom severity. In a previous EQUAL study, we demonstrated that both symptom number and symptom severity influence the patient-reported health-related quality of life [2]. The contribution of symptoms to the quality of life variable was also larger than any other condition (e.g. age and comorbidity) investigated.
The pathophysiological mechanisms underlying the onset of these symptoms and the interplay with kidney function are still not fully understood [32]. It is expected that with disease progression, the subjective manifestation of that condition (i.e. symptoms) will increase. This assumption also seems applicable to the symptom development in patients with advanced CKD: an increased number of symptoms and an increased symptom severity were experienced by patients with a faster kidney function decline. However, this relationship is not as straightforward as it appears. As in previous research that explored the relationship between kidney function and symptoms, we found no cross-sectional association between the level of kidney function and either symptom number or severity [3, 9, 33, 34]. Murphy et al. found no cross-sectional association between eGFR and either symptom number or severity in conservatively managed patients with advanced CKD [3]. Furthermore, de Goeij et al. showed that symptoms and eGFR-MDRD were not correlated in patients with CKD Stages 4 and 5 at four different time points during pre-dialysis care [9]. Apparently, the symptom score varies widely in patients with the same kidney function, considering the absence of these associations, and several possible explanations exist for these differences. First, the timing of symptom onset differs between patients, i.e. at different levels of kidney function [9, 29]. Secondly, literature suggests that, in addition to disease progression itself, social and psychological determinants play an important role in symptom development [32]. In particular, psychological determinants are deemed to be relevant for patients’ experience of symptoms and their perception of symptom burden, for example, illness perceptions and coping strategies [32, 35, 36]. Thus, the lack of cross-sectional associations could be because patients with the same kidney function could report a variety of symptom number and severity due to differences in psychological factors [33–38]. In addition, CKD patients often have several comorbid conditions that would also contribute to the overall symptom burden. All of the above would dilute the true effect of symptoms caused by low kidney function in any cross-sectional investigation. Studying the effect of kidney function loss and symptom development over time makes it easier to disentangle the association with kidney function on symptom burden per se.
To our knowledge, this is the first study that examined the longitudinal association between change in kidney function and change in symptoms over time in patients with advanced CKD. In contrast to our findings, Brown et al. found no association between categories (stable, improved or worsening) of symptoms and stable or decline in eGFR in elderly non-dialysis patients with CKD Stage 5 [39]. However, we investigated the continuous change in kidney function and symptoms. The lack of an association in the study of Brown et al. could be explained by the lack of adjustment for confounding and the loss of information by categorizing the change in symptoms. We extended these findings by showing the impact of a faster kidney function decline on the more progressive increase in symptoms over time in patients with advanced CKD, including adjustment for confounding. In addition, further research on this topic is warranted to unravel the mechanisms underlying the interplay between kidney function decline and symptom development, and the possible role of psychological factors (e.g. illness perceptions) in the onset and development of symptoms. It is important that healthcare professionals continue to focus on supporting patients in finding a way to deal with complaints and symptoms [40].
A major strength is that the EQUAL study is a large European multicentre prospective cohort study of incident patients with advanced CKD of at least 65 years of age. This allowed us to examine the longitudinal association between kidney function decline and symptom development. The study design, with a combination of limited exclusion criteria and the elimination of survivor bias by following patients from a common starting point (defined as incident eGFR ≤20 mL/min/1.73 m2), increases the generalizability of the obtained results to the clinical practice of pre-dialysis care for elderly patients. Limitations include the use of a single eGFR at each time point, possibly not reflecting the variability in eGFR. However, this is common in real-world clinical practice. Furthermore, the current analysis is restricted to the responders with at least one follow-up measurement. However, the baseline characteristics of these responders are similar to the characteristics of excluded patients. Furthermore, comparable results were obtained when confining the analyses to the 13 CKD-related symptoms or individuals with three available measurements of kidney function and symptoms. We should note that the advanced age of the cohort limits the generalizability to the whole non-dialysis patient population with CKD Stages 4 and 5 and results should only be generalized to patients who are least 65 years old. We should acknowledge the possible limitations of the use of eGFR estimated based on serum creatinine, since serum creatinine excretion declines in elderly and is determined by a person’s size and muscle mass. Furthermore, we assigned an equal weight to all symptoms to build a sum score based on the methodology of Abdel-Kader et al. [15]. However, some symptoms could be more burdensome than others, although literature on this is scarce, therefore we were not able to assign different weights to each symptom. Finally, the DSI is the most commonly used symptom questionnaire, although it was developed and validated in dialysis patients. However, the DSI has been used in non-dialysis-dependent patients before [41, 42]. The DSI is used in the EQUAL study, because the EQUAL study captures the pre-dialysis, transition and dialysis phase.
Although healthcare providers are aware of the symptom burden in patients with advanced CKD, and evaluation of symptoms is rated as important in the KDIGO guidelines [8], the evidence behind this recommendation is ‘not graded’. This complicates anticipating treatment choices and advising when to initiate dialysis for symptom relief. Our results seem to suggest that repeated thorough assessment of both symptom burden and severity, in addition to the monitoring of kidney disease progression, is important throughout the pre-dialysis period, for instance using Patient-Reported Outcomes Measures (PROMs). Current research such as the Symptom monitoring WIth Feedback Trial (SWIFT) in Australia/New Zealand and OPTimising routine collection of electronic Patient-Reported Outcomes into disease registries (OPT-ePRO) in the UK are investigating the effectiveness of routinely capturing PROMs in renal care. The underlying purpose is to improve symptom control, to reduce symptom number and severity, and to prepare for end-stage kidney disease care. Developing better treatments to reduce symptoms of CKD is also suggested as the main research priority by patients [7]. Future research should focus on which CKD-related symptoms possibly increase the most with kidney function deterioration. Additionally, uraemic signs and symptoms were rated as the most important factor guiding the timing of dialysis initiation in an international survey [43]. The important role of physical symptoms in deciding when to start dialysis was also seen in the IDEAL study [11]. Furthermore, each additional sign or symptom has been shown to be associated with a higher odds for earlier dialysis initiation [odds ratio of 1.16 (95% CI 1.06; 1.28) per symptom] in nursing home residents [44]. For future research, it would be interesting to investigate whether the increase in symptom burden is associated with time to dialysis initiation or hospitalization; a longer follow-up would be needed in order to provide enough events. Ultimately, a clinical decision rule, including kidney function decline and symptom development, may be useful to decide what the optimal timing is for dialysis initiation. Of course, we have to keep in mind that non-specific symptoms could be related to other comorbid conditions or illnesses precipitating early dialysis initiation among some providers.
To conclude, we showed that a faster kidney function decline associates with a more progressive increase in both overall symptom number and severity in patients with advanced CKD. Considering the modest association, our results seem to suggest that repeated thorough assessment of symptom development during outpatient clinic visits, in addition to the monitoring of kidney function decline, is important for clinical decision-making.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all the patients and healthcare professionals participating in the EQUAL study.
FUNDING
Funding was received from the European Renal Association – European Dialysis and Transplant Association (ERA-EDTA), the Swedish Medical Association (SLS), the Stockholm County Council ALF, Njurfonden (Sweden), the Italian Society of Nephrology (SIN-Reni), the Dutch Kidney Foundation (SB 142), the Young Investigators grant in Germany and the National Institute for Health Research (NIHR) in the UK.
AUTHORS’ CONTRIBUTIONS
F.W.D., M.v.D. and C.J.J. contributed to research idea and study design; all authors contributed to data acquisition and interpretation of data; C.J.J. and M.v.D. were responsible for data analysis and statistical analysis; M.v.D. and F.W.D. contributed to supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Amro A, Waldum B, von der Lippe N. et al. Symptom clusters predict mortality among dialysis patients in Norway: a prospective observational cohort study. J Pain Symptom Manage 2015; 49: 27–35 [DOI] [PubMed] [Google Scholar]
- 2. Voskamp PWM, van Diepen M, Evans M. et al. The impact of symptoms on health-related quality of life in elderly pre-dialysis patients: effect and importance in the EQUAL study. Nephrol Dial Transplant 2015; 34: 1707–1715 [DOI] [PubMed] [Google Scholar]
- 3. Murphy EL, Murtagh FE, Carey I. et al. Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: use of a short patient-completed assessment tool. Nephron Clin Pract 2008; 111: c74–c80 [DOI] [PubMed] [Google Scholar]
- 4. Murtagh FE, Addington-Hall JM, Edmonds PM. et al. Symptoms in advanced renal disease: a cross-sectional survey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J Palliat Med 2007; 10: 1266–1276 [DOI] [PubMed] [Google Scholar]
- 5. Urquhart-Secord R, Craig JC, Hemmelgarn B. et al. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. Am J Kidney Dis 2016; 68: 444–454 [DOI] [PubMed] [Google Scholar]
- 6. Almutary H, Bonner A, Douglas C.. Symptom burden in chronic kidney disease: a review of recent literature. J Ren Care 2013; 39: 140–150 [DOI] [PubMed] [Google Scholar]
- 7. Tong A, Sainsbury P, Carter SM. et al. Patients’ priorities for health research: focus group study of patients with chronic kidney disease. Nephrol Dial Transplant 2008; 23: 3206–3214 [DOI] [PubMed] [Google Scholar]
- 8. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 9. de Goeij MC, Ocak G, Rotmans JI. et al. Course of symptoms and health-related quality of life during specialized pre-dialysis care. PLoS One 2014; 9: e93069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rocco MV, Gassman JJ, Wang SR. et al. Cross-sectional study of quality of life and symptoms in chronic renal disease patients: the Modification of Diet in Renal Disease Study. Am J Kidney Dis 1997; 29: 888–896 [DOI] [PubMed] [Google Scholar]
- 11. Cooper BA, Branley P, Bulfone L. et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 2010; 363: 609–619 [DOI] [PubMed] [Google Scholar]
- 12. Jager KJ, Ocak G, Drechsler C. et al. The EQUAL study: a European study in chronic kidney disease stage 4 patients. Nephrol Dial Transplant 2012; 27 (Suppl 3): iii27–iii31 [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Bosch JP, Lewis JB. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 14. Weisbord SD, Fried LF, Mor MK. et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol 2007; 2: 960–967 [DOI] [PubMed] [Google Scholar]
- 15. Abdel-Kader K, Unruh ML, Weisbord SD.. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 2009; 4: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Janmaat CJ, van Diepen M, Tsonaka R. et al. Pitfalls of linear regression for estimating slopes over time and how to avoid them by using linear mixed-effects models. Nephrol Dial Transplant 2019; 34: 561–566 [DOI] [PubMed] [Google Scholar]
- 17. Stuart A, Ord K. Chapter 10.6 Standard errors. In: Kendall’s Advanced Theory of Statistics. Distribution Theory, Vol. 1, 5th edn. London: Charles Griffin & Company, 1987, 323–325 [Google Scholar]
- 18. Donders AR, van der Heijden GJ, Stijnen T. et al. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 2006; 59: 1087–1091 [DOI] [PubMed] [Google Scholar]
- 19. Kenward MG, Carpenter J.. Multiple imputation: current perspectives. Stat Methods Med Res 2007; 16: 199–218 [DOI] [PubMed] [Google Scholar]
- 20. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons, 1987 [Google Scholar]
- 21. Sterne JA, White IR, Carlin JB. et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009; 338: b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Janssen KJ, Donders AR, Harrell FE Jr. et al. Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol 2010; 63: 721–727 [DOI] [PubMed] [Google Scholar]
- 23. Fitzmaurice GM, Laird NM, Ware JH.. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, 2004 [Google Scholar]
- 24. Pfister R, Schwarz K, Carson R. et al. Easy methods for extracting individual regression slopes: comparing SPSS, R, and Excel. Tutor Quant Methods Psychol 2013; 9: 72–78 [Google Scholar]
- 25. Almutary H, Bonner A, Douglas C.. Which patients with chronic kidney disease have the greatest symptom burden? A comparative study of advanced CKD stage and dialysis modality. J Ren Care 2016; 42: 73–82 [DOI] [PubMed] [Google Scholar]
- 26. Brown SA, Tyrer FC, Clarke AL. et al. Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J 2017; 10: 788–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cabrera VJ, Hansson J, Kliger AS. et al. Symptom management of the patient with CKD: the role of dialysis. Clin J Am Soc Nephrol 2017; 12: 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Kidney Foundation. KDOQI clinical pracice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 2015; 66: 884–930 [DOI] [PubMed] [Google Scholar]
- 29. Meyer TW, Hostetter TH.. Uremia. N Engl J Med 2007; 357: 1316–1325 [DOI] [PubMed] [Google Scholar]
- 30. van de Luijtgaarden MWM, Caskey FJ, Wanner C. et al. Uraemic symptom burden and clinical condition in women and men of ≥65 years of age with advanced chronic kidney disease: results from the EQUAL study. Nephrol Dial Transplant 2019; 34: 1189–1196 [DOI] [PubMed] [Google Scholar]
- 31. O’Connor NR, Kumar P.. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med 2012; 15: 228–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thong MS, van Dijk S, Noordzij M. et al. Symptom clusters in incident dialysis patients: associations with clinical variables and quality of life. Nephrol Dial Transplant 2008; 24: 225–230 [DOI] [PubMed] [Google Scholar]
- 33. Clarke AL, Yates T, Smith AC. et al. Patient’s perceptions of chronic kidney disease and their association with psychosocial and clinical outcomes: a narrative review. Clin Kidney J 2016; 9: 494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Subramanian L, Quinn M, Zhao J. et al. Coping with kidney disease - qualitative findings from the Empowering Patients on Choices for Renal Replacement Therapy (EPOCH-RRT) study. BMC Nephrol 2017; 18: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meuleman Y, de Goeij MC, Halbesma N. et al. Illness perceptions in patients on predialysis care: associations with time until start of dialysis and decline of kidney function. Psychosom Med 2015; 77: 946–954 [DOI] [PubMed] [Google Scholar]
- 36. Pagels AA, Soderquist BK, Heiwe S.. Differences in illness representations in patients with chronic kidney disease. J Ren Care 2015; 41: 146–155 [DOI] [PubMed] [Google Scholar]
- 37. Leventhal H, Phillips LA, Burns E.. The common-sense model of self-regulation (CSM): a dynamic framework for understanding illness self-management. J Behav Med 2016; 39: 935–946 [DOI] [PubMed] [Google Scholar]
- 38. Pennebaker JW. The Psychology of Physical Symptoms. New York, NY: Springer, 1982 [Google Scholar]
- 39. Brown MA, Collett GK, Josland EA. et al. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol 2015; 10: 260–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schipper K, van der Borg WE, de Jong-Camerik J. et al. Living with moderate to severe renal failure from the perspective of patients. BMC Nephrol 2016; 17: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Almutary H, Bonner A, Douglas C.. Arabic translation, adaptation and modification of the Dialysis Symptom Index for chronic kidney disease stages four and five. BMC Nephrol 2015; 16: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramer S, Germain A, Dohar S. et al. Event-related distress in kidney disease patients. Nephrol Dial Transplant 2012; 27: 299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ledebo I, Kessler M, van Biesen W. et al. Initiation of dialysis-opinions from an international survey: report on the dialysis opinion symposium at the ERA-EDTA congress, 18 September 2000, Nice. Nephrol Dial Transplant 2001; 16: 1132–1138 [DOI] [PubMed] [Google Scholar]
- 44. Kurella Tamura M, O’Hare AM, McCulloch CE. et al. Signs and symptoms associated with earlier dialysis initiation in nursing home residents. Am J Kidney Dis 2010; 56: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.