Abstract
Background
Large international differences exist in access to renal replacement therapy (RRT) modalities and comprehensive conservative management (CCM) for patients with end-stage kidney disease (ESKD), suggesting that some patients are not receiving the most appropriate treatment. Previous studies mainly focused on barriers reported by patients or medical barriers (e.g. comorbidities) reported by nephrologists. An overview of the non-medical barriers reported by nephrologists when providing the most appropriate form of RRT (other than conventional in-centre haemodialysis) or CCM is lacking.
Methods
We searched in EMBASE and PubMed for original articles with a cross-sectional design (surveys, interviews or focus groups) published between January 2010 and September 2018. We included studies in which nephrologists reported barriers when providing RRT or CCM to adult patients with ESKD. We used the barriers and facilitators survey by Peters et al. [Ruimte Voor Verandering? Knelpunten en Mogelijkheden Voor Verbeteringen in de Patiëntenzorg. Nijmegen: Afdeling Kwaliteit van zorg (WOK), 2003] as preliminary framework to create our own model and performed meta-ethnographic analysis of non-medical barriers in text, tables and figures.
Results
Of the 5973 articles screened, 16 articles were included using surveys (n = 10), interviews (n = 5) and focus groups (n = 1). We categorized the barriers into three levels: patient level (e.g. attitude, role perception, motivation, knowledge and socio-cultural background), level of the healthcare professional (e.g. fears and concerns, working style, communication skills) and level of the healthcare system (e.g. financial barriers, supportive staff and practice organization).
Conclusions
Our systematic review has identified a number of modifiable, non-medical barriers that could be targeted by, for example, education and optimizing financing structure to improve access to RRT modalities and CCM.
Keywords: CAPD, chronic haemodialysis, ESKD, kidney transplantation, peritoneal dialysis
INTRODUCTION
Large international differences exist in the access to renal replacement therapy (RRT) modalities and comprehensive conservative management (CCM) for patients with end-stage kidney disease (ESKD) [1]. In 2016, the number of prevalent patients with ESKD treated by dialysis varied between 112 per million population (p.m.p.) in Bangladesh and 3251 p.m.p. in Taiwan [2]. Most patients received conventional thrice weekly in-centre haemodialysis as in many countries home-based dialysis modalities [home haemodialysis (HHD) and peritoneal dialysis (PD)] are not available. The number of patients living with a functioning kidney transplant varied between 25 p.m.p. in South Africa and 693 p.m.p. in Portugal [2]. Exact numbers on the prevalence of CCM are lacking. In a survey in 2010 in 11 European countries, nephrologists estimated that ∼15% of their patients with ESKD received CCM [3].
Currently not all patients with ESKD receive the most appropriate treatment with respect to clinical and psychosocial outcomes and patient preference [4]. Kidney transplantation (Tx) is associated with the greatest longevity, highest quality of life and lowest costs [5–7]. However, several patients are unsuitable for this treatment due to, for example medical contraindications. In this case, other forms of RRT or CCM could be more appropriate [8–10].
Various barriers have been described for specific RRT modalities or CCM. Many studies have described barriers experienced by patients such as demographic barriers, medical barriers, psychosocial barriers and socioeconomic barriers [11–13]. In contrast, a few studies have described barriers experienced by nephrologists. These studies usually focused on medical barriers such as comorbidity and medical contraindications [14–17].
An overview of non-medical barriers experienced by nephrologists is lacking. Such an overview may identify modifiable barriers that could be targeted by interventions to improve access to all RRT modalities and CCM. If access is improved, then more patients may receive the treatment that is most appropriate for them.
The aim of this systematic review was to provide an overview of non-medical barriers experienced by nephrologists when providing the most appropriate form of RRT or CCM to adults with ESKD.
MATERIALS AND METHODS
Search strategy
We systematically searched EMBASE and Medline via Ovid. Medical Subject Headings (MeSH) terms, text words and synonyms for nephrologist were combined with terms relating to RRT (haemodialysis, PD, Tx) and CCM and synonyms for barriers or subheadings related to barriers (e.g. resource allocation, ethics, organization and administration). References in all included articles were reviewed but this did not result in extra articles to be included. A detailed search strategy is provided in the Supplementary Methods.
Eligibility criteria
We included original peer-reviewed articles published between January 2010 and September 2018. We restricted to this time period as barriers may have changed over time, and to keep the number of abstracts manageable. We included studies with a quantitative or qualitative cross-sectional study design (survey, interviews or focus groups). The article needed to describe non-medical barriers (outcome) reported by nephrologists (population) when providing other than conventional in-centre haemodialysis [thus non-conventional haemodialysis (NCHD), HHD or PD], Tx or CCM to adult patients with ESKD.
We defined non-medical barriers as barriers not related to medical contraindications or comorbidity. The barriers must be experienced after referral of the patient to a nephrologist and before initiation of RRT, therefore excluding problems with referral to nephrology care or problems with the treatment itself (e.g. ultrafiltration failure or transplant rejection).
To avoid misinterpretation of the qualitative research findings, we only included articles in English.
Studies on barriers for nephrologists and other healthcare professionals (e.g. nurses) or patients were only included if barriers for nephrologists were described separately. If the study failed to meet inclusion criteria, then we noted the primary reason for exclusion (in order of priority: publication type, study design, population or outcome).
Study selection
Duplicates were removed. Two authors (R.W.d.J. and V.S.S.) independently reviewed all retrieved abstracts using Rayyan software [18]. Disagreement between the reviewers was resolved by discussion. Any article that was judged relevant on the basis of its title or abstract was retrieved in full-text form. Full texts were reviewed by the first author (R.W.d.J.) to assess eligibility for this study. In case of doubt, the article was discussed between R.W.d.J. and V.S.S.
Data extraction and data synthesis
The following data were collected from the included articles by one author (R.W.d.J.) using a standardized data extraction form: name of first author, journal, year of publication, country where research was undertaken, study method (survey, focus group or interviews), treatments discussed, sample size, response rate for surveys, any information on age and/or gender of the participants, qualitative methodology and analysis technique (qualitative studies) or question type (e.g. dichotomous, categorical) for quantitative studies.
One author (R.W.d.J.) repeatedly read the results reported in both qualitative and quantitative articles (in text, tables and figures) to extract barriers (defined as ‘circumstance or obstacle that may prevent the provision of RRT or CCM’). As we did not use the original transcripts of the qualitative studies, meta-ethnography was used to identify barriers [19].
Due to heterogeneity in questions and answers, the option to perform a meta‐analysis of the quantitative data was not appropriate. We therefore decided to analyse the results from quantitative studies in a qualitative manner. Thus results from quantitative articles were coded in the same way and were collected regardless of the degree of importance of the barrier in the original article.
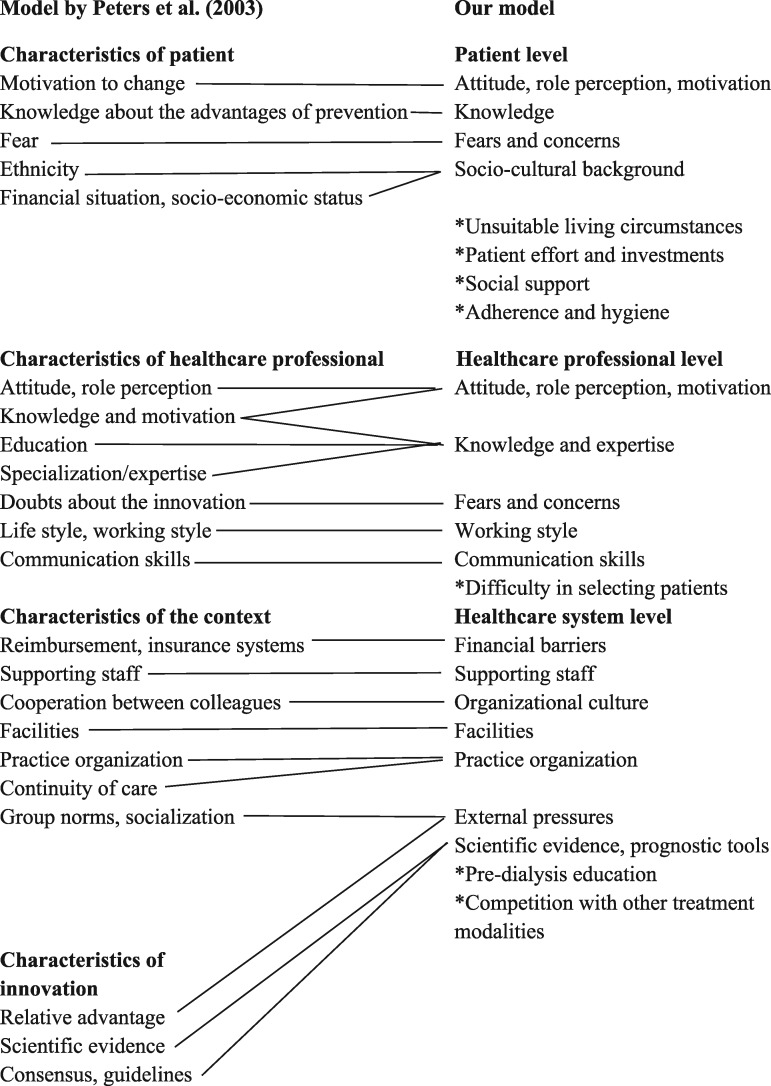
The barriers were coded using a coding frame based on the barriers and facilitators assessment instrument (a priori framework) [20]. Among many other models for implementation, this framework consists of structural, organizational, individual provider and patient, and innovation-related barriers [21]. Developed after literature study and consensus procedure, this instrument originally assesses barriers for implementation of preventive healthcare. We merged two categories to create a model with three categories: barriers on the patient level, healthcare professional level and healthcare system level. Next we used the factors from the literature review by Peters et al. [22] and our own data to create subcategories (Figure 1). The categorization of the data was repeatedly deliberated between R.W.d.J. and V.S.S. to refine the model. During this process, a minority of the data were recoded or placed in another subcategory.
FIGURE 1.
Modification of barriers and facilitators instrument into our model. Subcategories added to the model are marked with an asterisk. Several characteristics from the model of Peters et al. [22] were not used. Characteristics of patient not used: age, gender, marital status, health status, new/known patient, number of patient contacts, previous experiences, responsibility. Characteristics of healthcare professional not used: age, experience, gender, involvement, knowledge of medical background, lack of time, quality of doctor–patient relationship, quality of screening. Characteristics of the context not used: attention from the media, information and administration systems, laws/regulations, opening hours of practice, practice building, practice population, size of practice, type of practice/healthcare organization. Characteristics of innovation not used: applicability, attractiveness, clear definition, compatibility, complexity, cost–effectiveness, didactive benefit, discomfort for patient, image, observability, specificity/flexibility, time investment, tryability, visible results.
Quality assessment
One author (R.W.d.J.) assessed the study quality for the quantitative and qualitative studies, using, respectively, the method of Greenhalgh et al. and the Critical Appraisal Skills Programme Qualitative checklist [23, 24].
RESULTS
Included studies
Of the 5973 abstracts screened, 77 articles were reviewed in full-text format. Sixteen articles met the inclusion criteria [25–40] (Figure 2).
FIGURE 2.
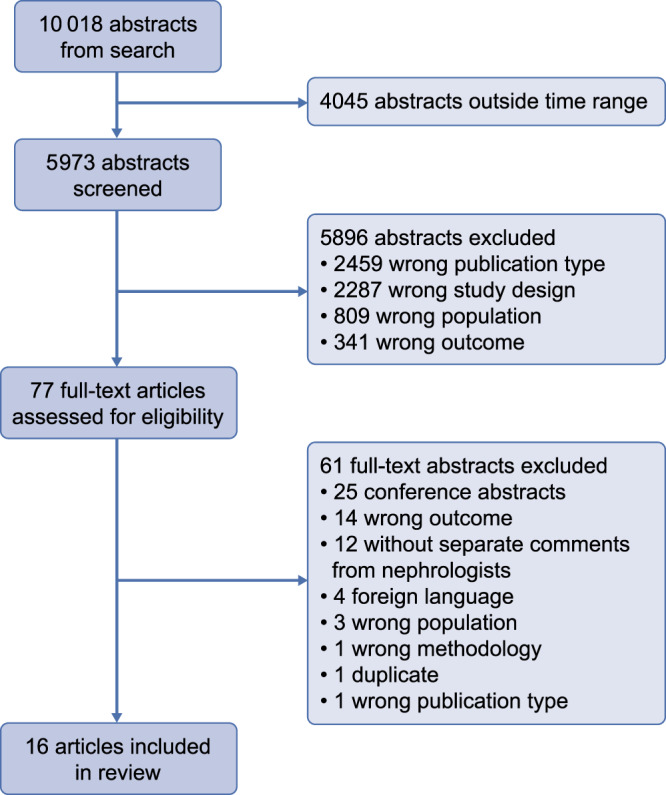
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Table 1 presents the characteristics of the included articles. Most studies were conducted in developed countries and two surveys were conducted internationally [25, 32]. Methodology used consisted of surveys (n = 10), interviews (n = 5) and focus groups (n = 1). The studies provided information on non-medical barriers for NCHD (n = 2), HHD (n = 2), PD (n = 4), both PD and HHD (n = 3), Tx (n = 2) and CCM (n = 3). Sample sizes varied from 16 to 286 nephrologists. Most participants, from studies providing information about gender (n = 9), were male. Various qualitative methodologies and analyses and questions about barriers (e.g. yes–no, scale, top-5) were used. Response rate on surveys varied between 2.7% and 80.8%.
Table 1.
Characteristics of included articles
| References | Country | Methodology | Modalities discussed | No. of adult nephrologistsa | Gender | Age | Qualitative methodology and qualitative data analysis as reported in the original articleb | |
|---|---|---|---|---|---|---|---|---|
| Qualitative studies | ||||||||
| Combes et al. [26] | UK | Interview | HHD, PD | 29 | NR | NR | NR | |
| Ghahramani et al. [29] | USA | Focus group | Kidney transplantation | 16 | 60% male | Median age 58 years | NR | |
| Thematic content analysis | ||||||||
| Grubbs et al. [30] | UK, USA | Interview | CCM | 59 | 76% male | ≤45 years 58% | Narrative and thematic analysis | |
| 46–65 years 34% | Constant comparative analysis | |||||||
| ≥66 years 9% | ||||||||
| Hanson et al. [31] | Australia, New Zealand | Interview | Living kidney donor transplantation | 41 | 80% male | 30–39 years 15% | Grounded theory | |
| 40–49 years 37% | Thematic analysis | |||||||
| 50–59 years 32% | ||||||||
| >60 years 7% | ||||||||
| Ladin et al. [33] | USA | Interview | CCM | 35 | 80% male | NR | NR | |
| Thematic and narrative analysis | ||||||||
| Tong et al. [38] | Italy, Portugal, France, Germany, Sweden, Argentina | Interview | HHD | 28 | 69% malec | 20–29 years 2% | Grounded theory | |
| 30–39 years 14% | Thematic analysis | |||||||
| 40–49 years 41% | ||||||||
| 50–59 years 31% | ||||||||
| 60–69 years 12%c | ||||||||
| References | Country | Methodology | Modalities discussed | N of adult nephrologistsa | Gender | Age | Response rate | Barriers |
|---|---|---|---|---|---|---|---|---|
| Quantitative studies | ||||||||
| Allen et al. [25] | Internationald | Survey | NCHDe | 259 | NR | NR | 15.6% | Indicate per statement (n = 4) if barrier or not (dichotomous) |
| Provide other barriers (open question) | ||||||||
| Dahlan et al. [27] | Saudi Arabia | Survey | PD | 124 | 90% male | 30–39 years 14.5% | 62.9% | Indicate per statement (n = 10) if major role or minor role (dichotomous) |
| 40–49 years 33.0% | ||||||||
| 50–59 years 39.5% | ||||||||
| >60 years 3.0% | ||||||||
| Desmet et al. [28] | Belgium | Survey | PD | 97 | NR | NR | 80.8% | Select 3 most important of 12 statements |
| Jayanti et al. [32] | Internationalf | Survey | HHD | 272 | NR | 35–44 years 22.4% | NR | Indicate per statement (n = 6) if barrier or not (dichotomous) |
| 45–54 years 35.7% | ||||||||
| 55–64 years 29% | ||||||||
| Ludlow et al. [34] | Australia | Survey | HHD, PD | 44 | NR | NR | 26% | Indicate per statement (n = 18) if agree/neutral/disagree (categorical) |
| Provide other barriers (open question) | ||||||||
| Parvez et al. [35] | USA | Survey | CCM | 265 | 71% male | 20–29 years 1.0% | 2.7% | Indicate per statement (n = 8) if (strongly) agree/neutral/(strongly) disagree (categorical) |
| 30–39 years 29.7% | ||||||||
| 40–49 years 30.8% | ||||||||
| 50–59 years 19.4% | ||||||||
| 60–69 years 16.0% | ||||||||
| ≥70 years 3.4% | ||||||||
| Savla et al. [36] | Bangladesh | Survey | PD | 43 | NR | NR | NR | Select five most important of unknown number of statements |
| Thumfart et al. [37] | Germany | Survey | NCHDd | 286 | NR | <45 years 23% | 13.9% | Indicate per statement (n = 3) if agree/uncertain/disagree (categorical) |
| 45–54 years 44% | Indicate per statement (n = 4) if barrier or not (dichotomous) | |||||||
| >55 years 33% | ||||||||
| Walker and Marshall [39] | New Zealand | Survey | PD | 30 | NR | NR | 48% | Indicate importance per statement (n = 8) on 3-point scale (categorical) |
| Walker et al. [40] | New Zealand | Survey | HHD PD | 49 | NR | NR | 70% | Indicate importance per factor (n = 15) on 7-point scale (categorical) |
N/A, not applicable; NR, not reported.
Trainees, paediatric nephrologists, etc., excluded.
See Bristowe et al. for more information on qualitative research theories in nephrology [92].
Gender and age distribution of all participants (including nurses).
Respondents from USA (26.7%), Europe (21.5%), South America (12.9%), Canada (11.9%), Asia (10.9%), Africa/Middle East (10%) and Australia/New Zealand (6.1%).
Non-conventional haemodialysis includes nocturnal haemodialysis (NHD), short daily haemodialysis (SDHD), long, thrice weekly conventional haemodialysis. NHD and SDHD included both home and in-centre HD.
Respondents from Europe (61.4%), Middle East (9.6%), Asia (8.8%), North America (7.7%), South and Central America (5.9%), Oceania (3.7%) and Africa (2.9%).
Several studies or individual researchers were (partly) supported by a grant from a governmental institute, a dialysis provider or a pharmaceutical company [26, 30, 33, 35–37, 40].
Quality assessment of included studies
Quality assessments of the included studies are provided as supplementary material (Tables S1 and S2). Quality of most studies was moderate to good. Most qualitative studies contained sufficient information on the study design, data collection and data analysis. Information on the relationship between the researcher and participants and ethical issues (e.g. information to participants, confidentiality) was not adequately reported by the majority of included studies. Most quantitative studies contained a clear research aim often related to opinion or attitude of nephrologists. Opinions can be investigated not only with surveys (quantitatively) but also with interviews (qualitatively). Most studies used self-designed surveys with mainly closed-ended questions. These surveys were usually pre-tested on potential participants but were not validated. Not all original surveys could be obtained, which made it impossible to assess formulation and content of the questions. In most studies, complete and clear results were presented.
Barriers to CCM and different RRT modalities
An overview of all non-medical barriers as experienced by the nephrologists is presented in Table 2 separated for NCHD, HHD, PD, transplantation and CCM. Barriers for HHD, PD and CCM were described both in quantitative and qualitative studies, barriers for NCHD were only described in quantitative studies and barriers for transplantation were only described in qualitative studies.
Table 2.
Non-medical barriers to particular treatment modalities as experienced by nephrologists assessed by quantitative and qualitative studies
|
Quantitative studies
|
Qualitative studies |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Barriers | NCHD | HHD | PD | Tx | CCM | NCHD | HHD | PD | Tx | CCM |
| Barriers on the patient level | ||||||||||
| Attitude, role perception, motivation | Allen et al. [25] | Ludlow et al. [34] | Dahlan et al. [27], Desmet et al. [28], Ludlow et al. [34] | No studies available | No studies available | Tong et al. [38] | Ghahramani et al. [29], Hanson et al. [31] | |||
| Knowledge | Walker and Marshall [39] | Ghahramani et al. [29], Hanson et al. [31] | Grubbs et al. [30], Ladin et al. [33] | |||||||
| Fears and concerns | Tong et al. [38] | Ghahramani et al. [29] | ||||||||
| Socio-cultural background | Ludlow et al. [34], Walker et al. [40] | Ludlow et al. [34], Walker et al. [40] | Tong et al. [38] | Ghahramani et al. [29], Hanson et al. [31] | Grubbs et al. [30], Ladin et al. [33] | |||||
| Unsuitable living circumstances | Ludlow et al. [34], Walker et al. [40] | Ludlow et al. [34], Walker and Marshall [39], Walker et al. [40] | Tong et al. [38] | |||||||
| Patient effort and investments | Allen et al. [25] | Ludlow et al. [34] | Ludlow et al. [34] | Ghahramani et al. [29], Hanson et al. [31] | ||||||
| Social support | Walker et al. [40] | Savla et al. [36], Walker and Marshall [39], Walker et al. [40] | Ghahramani et al. [29], Hanson et al. [31] | Ladin et al. [33] | ||||||
| Adherence and hygiene | Walker et al. [40] | Savla et al. [36], Walker et al. [40] | Hanson et al. [31] | |||||||
| Barriers on the level of the healthcare professional | ||||||||||
| Attitude, role perception, motivation | Thumfart et al. [37] | Dahlan et al. [27], Desmet et al. [28] | No studies available | Parvez et al. [35] | No studies available | Hanson et al. [31] | Grubbs et al. [30], Ladin et al. [33] | |||
| Knowledge and expertise | Allen et al. [25], Thumfart et al. [37] | Ludlow et al. [34] | Dahlan et al. [27], Desmet et al. [28], Ludlow et al. [34] | Combes et al. [26] | Combes et al. [26] | Grubbs et al. [30], Ladin et al. [33] | ||||
| Fears and concerns | Dahlan et al. [27], Desmet et al. [28] | Parvez et al. [35] | Tong et al. [38] | Ladin et al. [33] | ||||||
| Working style | Tong et al. [38] | Hanson et al. [31] | Ladin et al. [33] | |||||||
| Difficulty in selecting patients | Parvez et al. [35] | |||||||||
| Communication skills | Combes et al. [26] | Combes et al. [26] | Grubbs et al. [30] | |||||||
| Barriers on the level of the healthcare system | ||||||||||
| Financial barriers | Allen et al. [25], Thumfart et al. [37] | Jayanti et al. [32], Ludlow et al. [34] | Ludlow et al. [34], Savla et al. [36] | No studies available | No studies available | Tong et al. [38] | Hanson et al. [31] | Grubbs et al. [30], Ladin et al. [33] | ||
| Supporting staff | Thumfart et al. [37] | Jayanti et al. [32], Ludlow et al. [34] | Dahlan et al. [27], Desmet et al. [28], Ludlow et al. [34], Savla et al. [36] | Hanson et al. [31] | ||||||
| Competition with other treatment modalities | Jayanti et al. [32] | Desmet et al. [28] | Tong et al. [38] | |||||||
| Organizational culture | Thumfart et al. [37] | Jayanti et al. [32] | Hanson et al. [31] | Ladin et al. [33] | ||||||
| Facilities | Allen et al. [25] | Jayanti et al. [32], Ludlow et al. [34] | Dahlan et al. [27], Ludlow et al. [34] | Combes et al. [26], Tong et al. [38] | Combes et al. [26] | Hanson et al. [31] | ||||
| Practice organization | Ludlow et al. [34] | Desmet et al. [28], Ludlow et al. [34] | Parvez et al. [35] | Combes et al. [26] | Combes et al. [26] | Hanson et al. [31] | Ladin et al. [33] | |||
| External pressure | Hanson et al. [31] | Grubbs et al. [30], Ladin et al. [33] | ||||||||
| Scientific evidence, prognostic tools | Allen et al. [25] | Jayanti et al. [32], Ludlow et al. [34] | Parvez et al. [35] | Grubbs et al. [30], Ladin et al. [33] | ||||||
| Pre-dialysis education | Ludlow et al. [34] | Dahlan et al. [27], Ludlow et al. [34] | Combes et al. [26], Tong et al. [38] | Combes et al. [26] | Ghahramani et al. [29] | |||||
Table 3 contains all themes, description and illustrative quotations (indicated by Q1 till Q26 in the text below).
Table 3.
Themes, description and illustrative quotes
| Subthemes | Description | Quotes |
|---|---|---|
| Barriers on the patient level | ||
| Attitude, role perception, motivation | Patients are attached to professional care, do not want to have responsibility or simply refuse particular treatments | (Q1) The Portuguese don’t like the responsibilities. I think the majority of patients want others to care [for them] [38].—Portuguese nephrologist on HHD |
| Knowledge | Patients have misperceptions about treatments or limited health literacy | |
| Fears and concerns | Patients are afraid of several aspects of the treatment such as undergoing surgery, medication side effects or the dialysis machine | (Q2) The patients that are very afraid of everything in the dialysis room, when an alarm of the machine calls, they get very, very scared so I think that at home they will be very scared, because they would [not] feel safe [38].—Italian nephrologist on HHD |
| Socio-cultural background | Including language barriers religious beliefs, distrust in healthcare professionals, cultural barriers, low socioeconomic level and poor education | (Q3) There are people for instance that practice medicine in a hospital that has been in existence from the 1800s and up until the late 1960s or 1970s, people of African American heritage were not very trusting for a good reason. It’s not that way anymore, but there are people still alive today that remember the 60s and find it very difficult to give their trust in a physician that comes out of that system [30].—American nephrologist on CCM |
| (Q4) I think language barriers do play a role as well. It’s a lot easier to convince someone of the benefits of live donor transplants if you can have a full frame conversation with them… It’s very hard to have a delicate conversation through an interpreter [31].—Nephrologist on transplantation | ||
| Unsuitable living circumstances | Includes inadequate housing, insufficient supply of power and/or water, distant location | (Q5) I think it could be a problem for them to manage themselves if they have little space in their house to put the dialysis machine and especially if they are not the owner of their house… maybe the costs of the electricity or hydraulics [38].—Italian nephrologist on HHD |
| (Q6) It’s very difficult to implement when you have limitations. Because you need the thing that… a lot of regions in Argentina don’t have… good water [38].—Argentinian nephrologist on HHD | ||
| Patient effort and investments | Patients have to invest financial measure, time and efforts for training for home dialysis or travelling to the hospital | |
| Social support | Patients’ lack of support to pursue transplantation or perform home dialysis | (Q7) If they're a professional type person, a good advocate for themselves, then they'll go out and get a donor. Whereas if they haven't gone to university or haven't finished high school then they don't have a social network around them, then actually finding a donor is quite difficult [31].—Nephrologist on transplantation |
| Adherence and hygiene | Patients may be non-adherent or have poor personal hygiene | (Q8) If you think someone's going to be noncompliant but you're not really sure… with a deceased donor you might be a bit more likely to just give it a go. Whereas if it's a live donor, you think about the consequences for their relationship if they don't take the pills and they lose their kidneys [31].—Nephrologist on transplantation |
| Barriers on the level of the healthcare professional | ||
| Attitude, role perception, motivation | Nephrologists’ perception about their role (healing patients, protecting kidney donors), personal opinion on survival versus quality of life, lack of motivation for non-conventional treatments | (Q9) I suspect some of it is—well, in my case it’s about a sense of failure of being unable to help, to heal, and to do the job that I was trained to do to make someone better [30].—English nephrologist on CCM |
| (Q10) I know of a doctor who feels people shouldn’t be transplanted till they have been on dialysis for a period because when they get their transplant they’ll be more compliant [31].—Nephrologist on transplantation | ||
| Knowledge and expertise | Nephrologists received little or no training about non-conventional treatments, nephrologists reported a lack of expertise | (Q11) None [time spent on training about home therapies]. I very rarely get involved with PD peritonitis but that’s about it, nothing else and nothing on home haemodialysis [26].—English nephrologist on HHD and PD |
| Fears and concerns | Nephrologists are afraid of complications of home dialysis | (Q12) I don’t think that security of dialysis at home is the same as in the centres. In the centres we’ve got a lot of protocols… They are all alone [38].—French nephrologist on HHD |
| Working style | Nephrologists report differences in individual ways to handle situations (strictly following regulations, dealing with risks) | (Q13) Some people are very regimented by guidelines, and not necessarily personal patient issues. Sometimes, the longer you've been practicing, the more likely you are to consider you can probably get across a problem as opposed to being more junior; you're less likely to take a risk [31].—Nephrologist on transplantation |
| (Q14) I wouldn’t say that I present [options] neutrally for them to make a decision… because my own bias is that the [patients] that I’m presenting [dialysis] to are generally the ones that I think it would be beneficial [33].—American nephrologist on CCM | ||
| Difficulty in selecting patients | Nephrologists have problems with selecting suitable patients for CCM | |
| Communication skills | Nephrologists have limited skills or lack confidence to discuss treatments | |
| Barriers on the level of the healthcare system | ||
| Financial barriers | Financial incentive to conventional haemodialysis, lack of funding for home adaptation, insufficient reimbursement, cost of supplies | (Q15) Every time they lose a dialysis patient… they lose income [31].—Nephrologist on transplantation |
| (Q16) For home haemodialysis in countries like Argentina, Chile, Uruguay, you have financial and economic limits. It’s very expensive. The very big cost is around the machine, the dialysis machine and the water treatment (…) [38].—Argentinian nephrologist on HHD | ||
| Supporting staff | Lack of adequately trained nurses and surgeons | |
| Competition with other treatment modalities | Financial measures have to be divided between several treatment modalities, conventional centre haemodialysis is widely available | (Q17) We have an in clinic environment more or less every 10 kilometres in capital cities and every 30 kilometres in rural areas… why should you buy additional equipment to comfort people to get treatment at home?… there is just simply no need to do it at home… [38].—German nephrologist on HHD |
| (Q18) We had a priority to set up extended dialysis with a nightshift dialysis… because… the patients wanted that. So that’s what we’ve basically done the last two months and that means also that half of our clinics are not—I mean right now they’re more recruiting for nocturnal dialysis rather than offering them additional options with a home haemodialysis (…) [38].—German nephrologist on HHD | ||
| Organizational culture | Strict rules and procedures may limit uptake whereas supportive culture, cooperation with colleagues and enthusiasm may stimulate uptake | (Q19) The transplant team have a meeting, we’re not invited. They make their decisions and we need to live with them. If we disagree, we either live with it or take our business elsewhere. It usually spurs them on to think harder [31].—Nephrologist on transplantation |
| Facilities | Lack of space, dialysis supplies and training facilities | (Q20) You need to have also good logistics and structure for [home haemodialysis]… [38].—Swedish nephrologist on HHD |
| Practice organization | Insufficient coordination of care, poor communication between dialysis and transplant centres and availability of other services (e.g. psychological support) may limit uptake | (Q21) There's no coordination as a a-stop shop, which there really should be if you're asking people to travel 4, 5, 6 hours down to the city to see them, which they do, and then make them come back repeatedly for different tests [31].—Nephrologist on transplantation |
| (Q22) [Conservative management] does take some collaboration between us and primary doctors and other supports… We as a nephrology division can’t do [conservative management] on our own… without any of those additional services to help out [33].—American nephrologist on CCM | ||
| External pressure | Decision making is influenced by opinions from other nephrologists, other medical specialists or the family of the patient and transplant nephrologists needed to protect their centres interests | (Q23)… If a cardiac surgeon does an open heart [surgery] in an 85-year-old and the patient develops renal failure tomorrow how can I come and say, ‘I don’t want to dialyze this patient because she’s 85,’ or something like that. So, what am I supposed to do at that time [30]?—American nephrologist on CCM |
| (Q24) There is a strong motivation for transplanting hospitals to protect their credibility and maintain their performance, and to some extent this leads to gatekeeping to avoid high-risk patients [31].—Nephrologist on transplantation | ||
| Scientific evidence, prognostic tools | Lack of scientific evidence, no tools to estimate prognosis with or without dialysis | (Q25) We really don’t know who’s going to do well and who doesn’t. So I always err on the side of—at least give them a trial, see how it goes [30].—American nephrologist on CCM |
| Pre-dialysis education | Insufficient pre-dialysis education due to complexity of information, lack of time and lack of staff | (Q26) I think nephrologists don’t talk about it to the patients in most cases. Many patients don’t know that it is a possibility [38].—Portuguese nephrologist on HHD |
Barriers on the patient level
Patient’s attitude, role perception and motivation could limit the care provision by attachment to professionals and concurrent lack of motivation to take responsibility for one’s own treatment (Q1). This attitude could result from a lack of knowledge and limited health literacy or from concerns about particular aspects of the treatment (e.g. surgery, immunosuppressive medication, alarms of the dialysis machine) (Q2).
Characteristics of the socio-cultural background (e.g. distrust, religious or language barriers) often challenged nephrologists when informing patients about the different treatment options for ESKD (Q3–4). The provision of home dialysis modalities was limited by unsuitable living circumstances and distant locality (Q5–6). Patients often had to invest time and financial resources to apply for home dialysis or transplantation. They did not always have caregivers or social support to pursue home dialysis or transplantation (Q7). Finally, nephrologists reported patient adherence and poor hygiene as barriers for home dialysis and transplantation (Q8).
Barriers on the level of the healthcare professional
Nephrologists recognized that their own attitude, role perception and motivation influenced the uptake of NCHD, PD, transplantation and CCM (Q9–10). Nephrologists also reported lack of knowledge, fears and concerns, in particular about home dialysis and CCM (Q11–12). Selection of patients for CCM was hampered by nephrologists’ uncertainty about eligibility. In addition, nephrologists reported lack of skills and confidence to communicate with patients about RRT and CCM. Lastly, nephrologists were sometimes frustrated by the lack of uniformity in working style [e.g. following guidelines and dealing with risks (Q13–14)].
Barriers on the level of the healthcare system
Financial barriers were reported for all RRT modalities and CCM. Additional costs for water and electricity, home adaptation and assistance with home dialysis were often not reimbursed (Q16), and some nephrologists suggested that private doctors may not promote pre-emptive transplantation as they would lose income when a patient was not treated with dialysis first (Q15). Lack of skilled staff (nephrologists, nurses, surgeons, transplant coordinators) was reported as a barrier for all dialysis modalities and transplantation. Several nephrologists reported competition between treatment modalities as conventional haemodialysis was widely available and different forms of non-conventional dialysis had to share financial measure and patient interest (Q17–18). In addition, nephrologists experienced various external pressures: other nephrologists and other specialists were not in favour of certain treatments, pressure from the patient’s family, and several transplant nephrologists mentioned the need to protect their centre's reputation (Q23–24).
Three aspects of the organization of healthcare—organizational culture, facilities and practice organization—also limited the provision of RRT modalities. Strict division between dialysis and transplantation centres prevented efficient communication, knowledge transfer and involvement in each other’s specialization (Q19). Lack of space, supplies and training facilities limited the uptake of non-conventional dialysis forms (Q20). Problems with the coordination of care and cooperation with other healthcare professionals limited the provision of transplantation and CCM (Q21–22).
Moreover, a perceived lack of scientific evidence and lack of prognostic tools limited the uptake of NCHD, HHD, PD and CCM (Q25). Finally, insufficient pre-dialysis education, caused by complexity of information, limited time and lack of staff, was reported as a barrier for home dialysis and transplantation (Q26).
DISCUSSION
This systematic review identified non-medical barriers experienced by nephrologists when providing different RRT modalities (other than conventional in-centre haemodialysis) or CCM to adult patients with ESKD using a modified model of barriers and facilitators [22]. We found barriers on the patient level, on the level of the nephrologist and on the level of the healthcare system for all RRT modalities and CCM. Barriers for HHD and PD largely overlapped.
The importance of these barriers probably varies by country [41]. In some countries, a treatment modality may not be available at all (e.g. HHD in several countries [42] including Hungary [43] or Donation after Circulatory Death (DCD) Tx in Poland [44]), whereas other countries would like to increase the uptake of a certain modality (e.g. Tx in Spain by using uncontrolled DCD kidney donors [45]). Barriers in initiating a treatment modality could be different from those limiting its expansion.
In the following paragraphs, we will discuss our findings and their relationship with other studies grouped by level of barriers.
Barriers on the patient level
All of the patient-related barriers as experienced by nephrologists in this study were confirmed in studies with patients.
In line with our findings (Q1), many patients reported not wanting to perform home dialysis because they did not want to take responsibility for their therapy, had low self-confidence or preferred professional care [46, 47]. Nephrologists perceived a capability willingness gap; they believed that patients were capable of several dialysis-related tasks, but assumed that patients were generally not willing to perform these tasks [48].
The provision of home dialysis was also limited by unsuitable living circumstances (Q5–6), which is confirmed for instance by Canadian patients reporting barriers for PD [46].
Moreover, we found that nephrologists were limited by the patient’s lack of knowledge when providing PD, transplantation or CCM. We believe that lack of knowledge influences the provision of all RRT modalities as a substantial number of ESKD patients reported lack of knowledge and lack of information about treatment options [49–51].
Religion as a socio-cultural barrier was mainly described in studies about transplantation. Both Christian and Muslim patients reported religious beliefs against transplantation [52, 53]. Religious beliefs against organ transplantation are also seen in other religions, e.g. Buddhism [54]. Most studies on the influence of religion do not focus on CCM but on dialysis withdrawal and palliative kidney care [55, 56]. It seems that patients’ modality choice is barely influenced by religious beliefs [57].
In agreement with our findings, patients reported that they needed to invest a lot of time and money to pursue home dialysis or transplantation. Training for home dialysis caused patients to miss work and the reimbursement provided was inadequate, particularly if patients required home modifications [58, 59]. Several patients from developed and developing countries reported that they were unable to receive a transplant because of healthcare access problems (such as insurance issues, no dentist, transportation problems) and financial constraints [60–62].
Finally, patients also reported being limited by a lack of social support when considering transplantation or home dialysis (Q7) [46, 63]. Lack of social support can lead to less favourable evaluation for transplantation, whereas the presence of social support can improve eligibility for home dialysis [64, 65].
Barriers on the level of the healthcare professional
Since we found much information on this subject, we believe that the attitude, role perception and motivation of the nephrologist are important barriers on the level of the healthcare professional. Nephrologists’ enthusiasm about the modality has been associated with a higher uptake and nephrologists with a positive attitude tended to report fewer barriers [66, 67].
Our finding of nephrologists’ lack of knowledge and expertise (Q11) is supported by results of several surveys among both recently graduated and experienced nephrologists [68–70]. Lack of knowledge may be partly caused by lack of scientific evidence (as described below as barrier on the level of healthcare system) and may result in a negative attitude towards the treatment or a difficulty in selecting patients.
Furthermore, in our study, nephrologists reported fears or concerns about complications and safety of home dialysis (Q12). Interestingly, Bouvier et al. described that nephrologists from centres with low prevalence of PD reported more concerns about complications [67]. Safety of home dialysis may be increased by offering assisted home dialysis, performing home visits and remote patient monitoring [71].
Offering CCM was associated with specific barriers: many nephrologists experienced moral concerns, problems with the selection of patients and discomfort about initiating what was expected to be a difficult discussion [30, 33].
Barriers on the level of the healthcare system
In our review, we described many barriers related to the organization of RRT provision, such as lack of staff, financial barriers and insufficient facilities. As the organization of kidney care varies markedly between and within regions of the world [72], the nature and the importance of healthcare system-related barriers likely varies as well. For example, 82% of the low-income countries reported a shortage of nephrologists compared with 42% of the high-income countries [41]. In developed countries, home dialysis therapies are generally cheaper than in-centre haemodialysis, whereas they may be more expensive in developing countries since high nursing costs are a major factor in the overall costs of in-centre haemodialysis in developed countries [73]. Many physicians described perverse economic incentives that resulted in more expensive therapies being promoted [30, 38].
Moreover, we found that nephrologists were limited by external pressures when offering transplantation or CCM (Q23–24). The protection of a transplantation centre’s reputation influenced the decision to offer transplantation, and nephrologists may use ‘cherry picking’ to select the best candidates to uphold the centre’s graft and patient survival rate [74].
We found that nephrologists reported a lack of guidelines, scientific evidence and prognostic tools as barriers to HHD and CCM (Q25). Symptoms, quality of life and survival of patients with Stage 4 chronic kidney disease (CKD) or patients with Stage 5 CKD not undergoing dialysis are currently being investigated in large studies [75–77]. In addition, guidelines about CCM have recently become available [78, 79] although the evidence for their recommendations is solely based on observational studies comparing RRT and CCM. A randomized controlled trial comparing dialysis and CCM was initiated in 2017 (ISRCTN17133653, Prepare for kidney care) [80].
Remarkably, in our study, lack of donor kidneys for transplantation was not reported as a barrier, whereas we believe this is actually a major barrier. The causes for the lack of kidneys are different for living and deceased donors; the number of living donors may be influenced by legislation and financial constraints for potential living donors, whereas the number of deceased donors may be influenced by donor consent system and legislation about the use of different types of donors [81–84]. Increased availability of kidneys could result, among other things, from actively promoting living donation, organizing financial support for living donors, changing legislation and using kidneys fulfilling expanded donor criteria [81, 82, 85, 86]. Changes in legislation, however, may not be the most important driver in changing the hospital setting. Increased funding of more designated staff and infrastructure to facilitate donor procurement may have more of an effect.
Lastly, a substantial number of nephrologists experienced difficulties with pre-dialysis education (Q26) [87]. We believe that this important barrier is associated with other barriers such as patients’ language barriers, nephrologists’ attitude and lack of knowledge, and lack of staff (especially nursing staff). In addition, nephrologists reported lack of time, a large amount of complex information that has to be transferred and a restricted range of teaching methods as causes for difficulties with education [26, 39, 88]. However, the quality and quantity of pre-dialysis education may influence, for instance, home dialysis uptake [89, 90].
Strengths and limitations
This is the first systematic review summarizing non-medical barriers on the level of the patient, healthcare professional and healthcare system as experienced by nephrologists when providing the most appropriate treatment to adults with ESKD. Our study included articles about RRT treatment modalities and CCM to make comparison between modalities possible. Moreover, our findings were reported by nephrologists themselves, while in other reviews, nephrologists were not questioned about barriers. In these reviews, the authors composed a list of barriers themselves based on the literature. The risk of selection bias was reduced by two independent authors performing study selection and frequent discussion on the data, themes and interpretation within the author team.
As most studies were performed in developed countries, our findings may not be generalizable to developing countries. In addition, our results may be influenced by limitations of the study designs and reporting of the included articles. Results from the surveys may be influenced by selection and response bias and we were unable to assess the relative importance of the barriers as this was a qualitative analysis. Though relevant, we were unable to investigate relationships between the different non-medical barriers (e.g. language barriers of the patient and suboptimal communication skills of the healthcare professional may lead to problems with pre-dialysis education) as this analysis may be complex and requires a different study design. Finally, data extraction and quality assessment were performed by one author. However, consultation took place on a regular basis between the first two authors to increase the reliability of the data extraction and quality assessment.
CONCLUSION
Within this systematic review, we found a large number of non-medical barriers experienced by nephrologists for the provision of different RRT modalities (other than conventional in-centre haemodialysis) and CCM to patients with ESKD. Modalities could have similar barriers, and a successful approach of a barrier for one modality may also work if the barrier is experienced for another modality. The nature and importance of these barriers may vary by country, which needs to be investigated in further research [91]. This overview of non-medical barriers may support the development of interventions to target modifiable barriers. Guided by nephrologists’ experiences, interventions could focus on improving education and optimizing the financing structure of healthcare systems. Education could increase knowledge, which may influence attitude and motivation and could reduce fears and concerns of both patients and nephrologists. Financial stimulation could increase the uptake of home dialysis, CCM or transplantation, by for instance reimbursing home modification, employing extra nursing staff and financial compensation for living donors. These kinds of interventions may improve the access to RRT and CCM so that more patients receive the treatment that is most appropriate for them.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contribution of Joost Daams MA, clinical librarian at Amsterdam UMC—location AMC, Amsterdam, the Netherlands.
FUNDING
This publication is part of the Effect of Differing Kidney Disease Treatment Modalities and Organ Donation and Transplantation Practices on Health Expenditure and Patient Outcomes (EDITH) project (Grant Agreement PP-01-2016), which is receiving funding from the European Union. The content of this presentation represents the views of the authors only and is his/her sole responsibility; it cannot be considered to reflect the views of the European Commission or any other body of the European Union. The European Commission does not accept any responsibility for use that may be made of the information it contains. The funders had no role in the study design or the collection, analysis or interpretation of the data or writing the report. The EDITH project is also financially supported by the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA).
AUTHORS’ CONTRIBUTIONS
R.W.d.J., V.S.S. and K.J.J. were responsible for the study design and first draft of the manuscript. R.W.d.J. and V.S.S. were responsible for the literature selection. All authors (R.W.d.J., V.S.S., J.G.H., M.M., Z.A.M. and K.J.J.) were responsible for data interpretation and approved final submission of the manuscript.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part. R.W.d. J., V.S.S. and K.J.J. report grants from European Union and the ERA-EDTA, during the conduct of the study. Z.A.M. reports grants and other from Amgen, other from Sanofi-Genzyme, grants from French Government, grants from Amgen, grants from MSD, grants from Sanofi-Genzyme, grants from GSK, grants from Lilly, grants from FMC, grants and other from Baxter, grants from Outsuka, other from Daiichi, other from Astellas and other from Medice outside the submitted work. J.G.H. and M.M. have nothing to declare.
REFERENCES
- 1. Robinson BM, Akizawa T, Jager KJ. et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016; 388: 294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System. 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 3. van de Luijtgaarden MW, Noordzij M, van Biesen W. et al. Conservative care in Europe—nephrologists' experience with the decision not to start renal replacement therapy. Nephrol Dial Transplant 2013; 28: 2604–2612 [DOI] [PubMed] [Google Scholar]
- 4. Dahlerus C, Quinn M, Messersmith E. et al. Patient perspectives on the choice of dialysis modality: results from the Empowering Patients on Choices for Renal Replacement Therapy (EPOCH-RRT) Study. Am J Kidney Dis 2016; 68: 901–910 [DOI] [PubMed] [Google Scholar]
- 5. Wolfe RA, Ashby VB, Milford EL. et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341: 1725–1730 [DOI] [PubMed] [Google Scholar]
- 6. Cameron JI, Whiteside C, Katz J. et al. Differences in quality of life across renal replacement therapies: a meta-analytic comparison. Am J Kidney Dis 2000; 35: 629–637 [DOI] [PubMed] [Google Scholar]
- 7. Laupacis A, Keown P, Pus N. et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int 1996; 50: 235–242 [DOI] [PubMed] [Google Scholar]
- 8. Mowatt G, Vale L, MacLeod A.. Systematic review of the effectiveness of home versus hospital or satellite unit hemodialysis for people with end-stage renal failure. Int J Technol Assess Health Care 2004; 20: 258–268 [DOI] [PubMed] [Google Scholar]
- 9. Pauly RP, Gill JS, Rose CL. et al. Survival among nocturnal home haemodialysis patients compared to kidney transplant recipients. Nephrol Dial Transplant 2009; 24: 2915–2919 [DOI] [PubMed] [Google Scholar]
- 10. Verberne WR, Geers AB, Jellema WT. et al. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin J Am Soc Nephrol 2016; 11: 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lockwood MB, Bidwell JT, Werner DA. et al. Non-biological barriers to referral and the pre-kidney transplant evaluation among African Americans in the United States: a systematic review. Nephrol Nurs J 2016; 43: 225–238 [PubMed] [Google Scholar]
- 12. Navaneethan SD, Singh S.. A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplant 2006; 20: 769–775 [DOI] [PubMed] [Google Scholar]
- 13. Sauvé C, Vandyk AD, Bourbonnais FF.. Continuing nursing education. Exploring the facilitators and barriers to home dialysis: a scoping review. Nephrol Nurs J 2016; 43: 295–308 [PubMed] [Google Scholar]
- 14. Jager KJ, Korevaar JC, Dekker FW. et al. The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in The Netherlands. Am J Kidney Dis 2004; 43: 891–899 [DOI] [PubMed] [Google Scholar]
- 15. Jassal SV, Krishna G, Mallick NP. et al. Attitudes of British Isles nephrologists towards dialysis modality selection: a questionnaire study. Nephrol Dial Transplant 2002; 17: 474–477 [DOI] [PubMed] [Google Scholar]
- 16. Mendelssohn DC, Mullaney SR, Jung B. et al. What do American nephrologists think about dialysis modality selection? Am J Kidney Dis 2001; 37: 22–29 [DOI] [PubMed] [Google Scholar]
- 17. Jung B, Blake PG, Mehta RL. et al. Attitudes of Canadian nephrologists toward dialysis modality selection. Peritoneal Dial Int 1999; 19: 263–268 [PubMed] [Google Scholar]
- 18. Ouzzani M, Hammady H, Fedorowicz Z. et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Noblit G, Hare R.. Meta-Ethnography: Synthesizing Qualitative Studies. Newbury Park, California, USA: Sage Publications, 1988 [Google Scholar]
- 20. Harmsen M, Peters M, Wensing M.. Barriers and Facilitators Assessment instrument - Introduction, Instructions and Instrument. Nijmegen: Centre for Quality of Care Research (WOK), Radboud University Nijmegen Medical Centre, 2005 [Google Scholar]
- 21. Chaudoir SR, Dugan AG, Barr C.. Measuring factors affecting implementation of health innovations: a systematic review of structural, organizational, provider, patient, and innovation level measures. Implement Sci 2013; 8: 22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peters M, Harmsen M, Laurant M. et al. Ruimte Voor Verandering? Knelpunten en Mogelijkheden Voor Verbeteringen in de Patiëntenzorg. Nijmegen: Afdeling Kwaliteit van zorg (WOK; ), 2003 [Google Scholar]
- 23.Critical Appraisal Skills Programme. CASP Qualitative Checklist. https://casp-uk.net/wp-content/uploads/2018/01/CASP-Qualitative-Checklist-2018.pdf (14 August 2017, date last accessed)
- 24. Greenhalgh T, Robert G, Bate P. et al. Diffusion of innovations in health service organisations: a systematic literature review, Hoboken, New Jersey, USA: Wiley-Blackwell. 2005 [Google Scholar]
- 25. Allen N, Schwartz D, Komenda P. et al. International practice patterns and factors associated with non-conventional hemodialysis utilization. BMC Nephrol 2011; 12: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Combes G, Allen K, Sein K. et al. Taking hospital treatments home: a mixed methods case study looking at the barriers and success factors for home dialysis treatment and the influence of a target on uptake rates. Implement Sci 2015; 10: 148–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dahlan R, Qureshi M, Akeely F. et al. Barriers to peritoneal dialysis in Saudi Arabia: nephrologists' perspectives. Perit Dial Int 2016; 36: 564–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Desmet JM, Fernandes V, Des Grottes JM. et al. Perceptive barriers to peritoneal dialysis implementation: an opinion poll among the French-speaking Belgian nephrologists. Clin Kidney J 2013; 6: 358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghahramani N, Sanati-Mehrizy A, Wang C.. Perceptions of patient candidacy for kidney transplant in the United States: A qualitative study comparing rural and urban nephrologists. Exp Clin Transplant 2014; 12: 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grubbs V, Tuot DS, Powe NR. et al. System-level barriers and facilitators for foregoing or withdrawing dialysis: a qualitative study of nephrologists in the United States and England. Am J Kidney Dis 2017; 70: 602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hanson CS, Chadban SJ, Chapman JR. et al. Nephrologists' perspectives on recipient eligibility and access to living kidney donor transplantation. Transplantation 2016; 100: 943–953 [DOI] [PubMed] [Google Scholar]
- 32. Jayanti A, Morris J, Stenvinkel P. et al. Home hemodialysis: beliefs, attitudes, and practice patterns. Hemodial Int 2014; 18: 767–776 [DOI] [PubMed] [Google Scholar]
- 33. Ladin K, Pandya R, Kannam A. et al. Discussing conservative management with older patients with CKD: an interview study of nephrologists. Am J Kidney Dis 2018; 71: 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ludlow MJ, George CR, Hawley CM. et al. How Australian nephrologists view home dialysis: results of a national survey. Nephrology 2011; 16: 446–452 [DOI] [PubMed] [Google Scholar]
- 35. Parvez S, Abdel-Kader K, Pankratz VS. et al. Provider knowledge, attitudes, and practices surrounding conservative management for patients with advanced CKD. Clin J Am Soc Nephrol 2016; 11: 812–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Savla D, Ahmed S, Yeates K. et al. Barriers to increasing use of peritoneal dialysis in Bangladesh: a survey of patients and providers. Perit Dial Int 2017; 37: 234–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thumfart J, Wagner S, Jayanti A. et al. Attitudes of nephrologists towards intensified hemodialysis. Clin Nephrol 2018; 90: 255–261 [DOI] [PubMed] [Google Scholar]
- 38. Tong A, Palmer S, Manns B. et al. Clinician beliefs and attitudes about home haemodialysis: A multinational interview study. BMJ Open 2012; 2: e002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walker RC, Marshall MR.. Increasing the uptake of peritoneal dialysis in New Zealand: A national survey. J Ren Care 2014; 40: 40–48 [DOI] [PubMed] [Google Scholar]
- 40. Walker RC, Marshall R, Howard K. et al. “Who matters most?”: Clinician perspectives of influence and recommendation on home dialysis uptake. Nephrology 2017; 22: 977–984 [DOI] [PubMed] [Google Scholar]
- 41. Lunney M, Alrukhaimi M, Ashuntantang GE. et al. Guidelines, policies, and barriers to kidney care: findings from a global survey. Kidney Int Suppl 2018; 8: 30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kramer A, Pippias M, Noordzij M. et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2015: A summary. Clin Kidney J 2018; 11: 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haris A, Polner K.. Starting with Home Dialysis. 2016. https://bns-hungary.hu/documents/23bns/2016bns_0831_0945.pdf (30 August 2019, date last accessed)
- 44. Stadnik H, Małkiewicz T, Stronka M. et al. Introducing of the first DCD kidney transplantation program in Poland. BioMed Res Int 2019; 2019: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Del Rio F, Andres A, Padilla M. et al. Kidney transplantation from donors after uncontrolled circulatory death: the Spanish experience. Kidney Int 2019; 95: 420–428 [DOI] [PubMed] [Google Scholar]
- 46. Prakash S, Perzynski AT, Austin PC. et al. Neighborhood socioeconomic status and barriers to peritoneal dialysis: a mixed methods study. Clin J Am Soc Nephrol 2013; 8: 1741–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cafazzo JA, Leonard K, Easty AC. et al. Patient-perceived barriers to the adoption of nocturnal home hemodialysis. Clin J Am Soc Nephrol 2009; 4: 784–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yau M, Carver M, Alvarez L. et al. Understanding barriers to home-based and self-care in-center hemodialysis. Hemodialysis Int 2016; 20: 235–241 [DOI] [PubMed] [Google Scholar]
- 49. Finkelstein FO, Story K, Firanek C. et al. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int 2008; 74: 1178–1184 [DOI] [PubMed] [Google Scholar]
- 50. Mehrotra R, Marsh D, Vonesh E. et al. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int 2005; 68: 378–390 [DOI] [PubMed] [Google Scholar]
- 51. Fortnum D, Grennan K, Ludlow M.. Dialysis Consumer Perspectives Survey Two: Complete Dataset Report. Kidney Health Australia, 2015 [Google Scholar]
- 52. Laouad I, Hbali G, Mouhoub R. et al. Knowledge and attitudes of Moroccan hemodialysis patients toward renal transplantation: did we inform our patients enough? Transplant Proc 2011; 43: 445–447 [DOI] [PubMed] [Google Scholar]
- 53. Gordon EJ. Patients' decisions for treatment of end-stage renal disease and their implications for access to transplantation. Soc Sci Med 2001; 53: 971–987 [DOI] [PubMed] [Google Scholar]
- 54. Oliver M, Woywodt A, Ahmed A. et al. Organ donation, transplantation and religion. Nephrol Dial Transplant 2011; 26: 437–444 [DOI] [PubMed] [Google Scholar]
- 55. Elliott BA, Gessert CE, Larson P. et al. Religious beliefs and practices in end-stage renal disease: implications for clinicians. J Pain Symptom Manag 2012; 44: 400–409 [DOI] [PubMed] [Google Scholar]
- 56. Cervantes L, Jones J, Linas S. et al. Qualitative interviews exploring palliative care perspectives of Latinos on dialysis. Clin J Am Soc Nephrol 2017; 12: 788–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chanouzas D, Ng KP, Fallouh B. et al. What influences patient choice of treatment modality at the pre-dialysis stage? Nephrol Dial Transplant 2012; 27: 1542–1547 [DOI] [PubMed] [Google Scholar]
- 58. Hanson CS, Chapman JR, Craig JC. et al. Patient experiences of training and transition to home haemodialysis: a mixed-methods study. Nephrology 2017; 22: 631–641 [DOI] [PubMed] [Google Scholar]
- 59. Walker RC, Howard K, Tong A. et al. The economic considerations of patients and caregivers in choice of dialysis modality. Hemodialysis Int 2016; 20: 634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abdelwahab HH, Shigidi MM, Ibrahim LS. et al. Barriers to kidney transplantation among adult Sudanese patients on maintenance hemodialysis in dialysis units in Khartoum State. Saudi J Kidney Dis Transpl 2013; 24: 1044–1049 [DOI] [PubMed] [Google Scholar]
- 61. Lockwood M, Chon W, Josephson M. et al. Patient-reported barriers to the pre-kidney transplant evaluation in an at-risk population in the United States. Am J Transplant 2017; 17: 631. [DOI] [PubMed] [Google Scholar]
- 62. Kwalimwa J, Mwaura J, Muiva M. et al. Barriers to access of quality renal replacement therapy in end- stage renal disease patients at the Kenyatta national hospital. J Nurs Health Sci 2015; 4: 24–31 [Google Scholar]
- 63. Bailey PK, Ben-Shlomo Y, Tomson CRV. et al. Socioeconomic deprivation and barriers to live-donor kidney transplantation: a qualitative study of deceased-donor kidney transplant recipients. BMJ Open 2016; 6: e010605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ladin K, Emerson J, Berry K. et al. Excluding patients from transplant due to social support: Results from a national survey of transplant providers. Am J Transplant 2019; 19: 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oliver MJ, Garg AX, Blake PG. et al. Impact of contraindications, barriers to self-care and support on incident peritoneal dialysis utilization. Nephrol Dial Transplant 2010; 25: 2737–2744 [DOI] [PubMed] [Google Scholar]
- 66. Castledine CI, Gilg JA, Rogers C. et al. Renal centre characteristics and physician practice patterns associated with home dialysis use. Nephrol Dial Transplant 2013; 28: 2169–2180 [DOI] [PubMed] [Google Scholar]
- 67. Bouvier N, Durand PY, Testa A. et al. Regional discrepancies in peritoneal dialysis utilization in France: the role of the nephrologist's opinion about peritoneal dialysis. Nephrol Dial Transplant 2008; 24: 1293–1297 [DOI] [PubMed] [Google Scholar]
- 68. van Biesen W, van de Luijtgaarden MW, Brown EA. et al. Nephrologists' perceptions regarding dialysis withdrawal and palliative care in Europe: lessons from a European Renal Best Practice survey. Nephrol Dial Transplant 2015; 30: 1951–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beaton TJ, Krishnasamy R, Toussaint ND. et al. Nephrology training in Australia and New Zealand: a survey of outcomes and adequacy. Nephrology 2017; 22: 35–42 [DOI] [PubMed] [Google Scholar]
- 70. Okamoto I, Tonkin-Crine S, Rayner H. et al. Conservative care for ESRD in the United Kingdom: a national survey. Clin J Am Soc Nephrol 2015; 10: 120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Martino F, Adibelli Z, Mason G. et al. Home visit program improves technique survival in peritoneal dialysis. Blood Purif 2014; 37: 286–290 [DOI] [PubMed] [Google Scholar]
- 72. Bello AK, Levin A, Tonelli M. et al. Assessment of global kidney health care status. J Am Med Assoc 2017; 317: 1864–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Just PM, de Charro FT, Tschosik EA. et al. Reimbursement and economic factors influencing dialysis modality choice around the world. Nephrol Dial Transplant 2008; 23: 2365–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tong A, Howard K, Wong G. et al. Nephrologists' perspectives on waitlisting and allocation of deceased donor kidneys for transplant. Am J Kidney Dis 2011; 58: 704–716 [DOI] [PubMed] [Google Scholar]
- 75. Jager KJ, Ocak G, Drechsler C. et al. The EQUAL study: a European study in chronic kidney disease stage 4 patients. Nephrol Dial Transplant 2012; 27: iii27–iii31 [DOI] [PubMed] [Google Scholar]
- 76. Noble HR, Agus A, Brazil K. et al. PAlliative Care in chronic Kidney disease: the PACKS study–quality of life, decision making, costs and impact on carers in people managed without dialysis. BMC Nephrol 2015; 16: 104–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Moranne O, Fafin C, Roche S. et al. Treatment plans and outcomes in elderly patients reaching advanced chronic kidney disease. Nephrol Dial Transplant 2018; 33: 2182–2191 [DOI] [PubMed] [Google Scholar]
- 78. Warwick G, Mooney A, Russon L. et al. Planning, Initiating and Withdrawal of Renal Replacement Therapy. UK Renal Association, 2014 [DOI] [PubMed] [Google Scholar]
- 79. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 80.Prepare for Kidney Care Trial. http://www.bristol.ac.uk/population-health-sciences/projects/prepare-kc-trial/ (20 June 2019, date last accessed)
- 81. Ambagtsheer F, Weimar W.. The EULOD project living organ donation in Europe results and recommendations. Lengerich: Pabst Science Publishers. 2013; 1–184 [Google Scholar]
- 82. Tushla L, Rudow DL, Milton J. et al. Living-donor kidney transplantation: reducing financial barriers to live kidney donation–recommendations from a consensus conference. Clin J Am Soc Nephrol 2015; 10: 1696–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ugur ZB. Does presumed consent save lives? Evidence from Europe. Health Econ 2015; 24: 1560–1572 [DOI] [PubMed] [Google Scholar]
- 84. Dominguez-Gil B, Haase-Kromwijk B, Van Leiden H. et al. Current situation of donation after circulatory death in European countries. Transplant Int 2011; 24: 676–686 [DOI] [PubMed] [Google Scholar]
- 85. Vanholder R, Stel VS, Jager KJ. et al. How to increase kidney transplant activity throughout Europe—an advocacy review by the European Kidney Health Alliance. Nephrol Dial Transplant 2019; 34: 1254–1261 [DOI] [PubMed] [Google Scholar]
- 86. Maggiore U, Oberbauer R, Pascual J. et al. Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol Dial Transplant 2015; 30: 217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schell JO, Patel UD, Steinhauser KE. et al. Discussions of the kidney disease trajectory by elderly patients and nephrologists: a qualitative study. Am J Kidney Dis 2012; 59: 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Combes G, Sein K, Allen K.. How does pre-dialysis education need to change? Findings from a qualitative study with staff and patients. BMC Nephrol 2017; 18: 334–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Little J, Irwin A, Marshall T. et al. Predicting a patient's choice of dialysis modality: Experience in a United Kingdom renal department. Am J Kidney Dis 2001; 37: 981–986 [DOI] [PubMed] [Google Scholar]
- 90. Manns BJ, Taub K, Vanderstraeten C. et al. The impact of education on chronic kidney disease patients' plans to initiate dialysis with self-care dialysis: a randomized trial. Kidney Int 2005; 68: 1777–1783 [DOI] [PubMed] [Google Scholar]
- 91. Jager KJ, Stel VS, Branger P. et al. The effect of differing kidney disease treatment modalities and organ donation and transplantation practices on health expenditure and patient outcomes. Nephrol Dial Transplant 2017; 33: 560–562 [DOI] [PubMed] [Google Scholar]
- 92. Bristowe K, Selman L, Murtagh FE.. Qualitative research methods in renal medicine: an introduction. Nephrol Dial Transplant 2015; 30: 1424–1431 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.