Abstract
Background
Sodium zirconium cyclosilicate (SZC) binds potassium and ammonium in the gastrointestinal tract. In addition to serum potassium reduction, Phase 2 trial data have shown increased serum bicarbonate with SZC, which may be clinically beneficial because maintaining serum bicarbonate ≥22 mmol/L preserves kidney function. This exploratory analysis examined serum bicarbonate and urea, and urine pH data from three SZC randomized, placebo-controlled Phase 3 studies among patients with hyperkalaemia [ZS-003 (n = 753), HARMONIZE (n = 258) and HARMONIZE-Global (n = 267)].
Methods
In all studies, patients received ≤10 g SZC 3 times daily (TID) for 48 h to correct hyperkalaemia, followed by randomization to maintenance therapy with SZC once daily (QD) versus placebo for ≤29 days among those achieving normokalaemia.
Results
Significant dose-dependent mean serum bicarbonate increases from baseline of 0.3 to 1.5 mmol/L occurred within 48 h of SZC TID in ZS-003 (all P < 0.05), which occurred regardless of chronic kidney disease (CKD) stage. Similar acute increases in HARMONIZE and HARMONIZE-Global were maintained over 29 days. With highest SZC maintenance doses, patient proportions with serum bicarbonate <22 mmol/L fell from 39.4% at baseline to 4.9% at 29 days (P = 0.005) in HARMONIZE and from 87.9% to 70.1%, (P = 0.006) in HARMONIZE-Global. Path analyses demonstrated that serum urea decreases (but not serum potassium or urine pH changes) were associated with SZC effects on serum bicarbonate.
Conclusions
SZC increased serum bicarbonate concentrations and reduced patient proportions with serum bicarbonate <22 mmol/L, likely due to SZC-binding of gastrointestinal ammonium. These SZC-induced serum bicarbonate increases occurred regardless of CKD stage and were sustained during ongoing maintenance therapy.
Keywords: hyperkalaemia, potassium, serum bicarbonate, serum urea, sodium zirconium cyclosilicate
KEY LEARNING POINTS
What is already known about this subject?
Patients requiring treatment for hyperkalaemia often have associated chronic kidney disease (CKD) with or without metabolic acidosis.
Sodium zirconium cyclosilicate (SZC) is an orally-administered, selective potassium (K+)-binder approved for the treatment of hyperkalaemia in adults that entraps K+ throughout the gastrointestinal tract in exchange for hydrogen and sodium ions.
In addition to serum K+ reduction, Phase 2 clinical trial data have shown that SZC also increases serum bicarbonate and decreases serum urea, which may be due to SZC trapping ammonium ions, which are similar in size to K+ ions.
What this study adds?
In Phase 3 placebo-controlled trials among patients receiving treatment for hyperkalaemia, SZC consistently and dose-dependently increased serum bicarbonate concentrations, reduced the proportions of patients with serum bicarbonate <22 mmol/L, decreased serum urea concentrations and increased urine pH.
SZC increased mean serum bicarbonate regardless of CKD level by ∼2 mmol/L versus placebo during initial 48-h treatment with 10 g TID and by 2–3 mmol/L during ongoing maintenance therapy with 10 g once daily (QD) over 1 month.
Path analysis showed that the serum bicarbonate increase with SZC was associated with a decrease in serum urea rather than with changes in serum K+ or urine pH, supporting the hypothesis that SZC traps ammonium in the gastrointestinal tract independently of its effect on K+ binding.
What impact this may have on practice or policy?
Given that maintaining serum bicarbonate ≥22 mmol/L is a major goal for the treatment of CKD and that metabolic acidosis in turn is associated with kidney disease progression, the increase in serum bicarbonate with SZC is of potential clinical significance.
INTRODUCTION
Sodium zirconium cyclosilicate (SZC; formerly ZS-9), chemical formula Na∼1.5H∼0.5ZrSi3O9•2–3H2O, forms a 3D framework around a central 3 Å pore containing [ZrO6]2− anions and sodium and hydrogen cations [1]. SZC was developed as an orally-administered, non-absorbable, inorganic, selective potassium (K+)-binding agent that entraps K+ throughout the gastrointestinal tract in exchange for hydrogen and sodium ions [2, 3]. SZC rapidly normalizes serum K+ (3.5–5.0 mmol/L) within 48 h with 3 times daily (TID) administration [4–6] and is associated with maintenance of normokalaemia for up to 1 year with continued once-daily (QD) administration, without requiring significant changes in renin–angiotensin–aldosterone system inhibitor (RAASi) therapy [7]. These effects have been consistently observed among patients with comorbidities such as chronic kidney disease (CKD) [4, 5].
In addition to serum K+ reduction, previous Phase 2 [6] and open-label [7–9] clinical trial data have shown that SZC increases serum bicarbonate and decreases serum urea. These changes may be due to SZC trapping of ammonium ions, which are of a similar size to K+ ions [6, 10, 11], or may also be caused by correction of hyperkalaemia-induced decrease in renal ammoniagenesis [12, 13].
Given that maintaining serum bicarbonate levels ≥22 mmol/L preserves kidney function [14, 15] and reduces metabolic bone disease [16, 17] and mortality [15] in randomized-controlled open-label trials, the SZC-induced increase in serum bicarbonate may be clinically beneficial. Thus, we conducted a confirmatory post hoc analysis of serum bicarbonate, urea, and urine pH using placebo-controlled data from three randomized Phase 3 SZC studies among patients with hyperkalaemia [4, 5, 18]. Across study populations, we evaluated SZC effects: on serum bicarbonate (overall and by levels of CKD severity); on proportions of patients with serum bicarbonate <22 mmol/L; and on serum urea and urine pH. Using path analyses, we explored whether SZC increases serum bicarbonate directly or indirectly via SZC-mediated changes in serum K+ (the primary pharmacodynamic action of SZC), serum urea (a proxy measure for the ammonium trapping hypothesis) or urine pH (a proxy measure for the renal ammoniagenesis hypothesis).
MATERIALS AND METHODS
Patients and study designs
This post hoc analysis used data from three Phase 3 randomized multicentre placebo-controlled trials. The complete study designs and primary results of these studies (ZS-003, NCT01737697 [5]; HARMONIZE, NCT02088073 [4]; and HARMONIZE-Global, NCT02875834 [18]) are published elsewhere. Eligible patients were adults with serum K+ levels between 5.0 and 6.5 mmol/L (ZS-003) or with point-of-care whole blood i-STAT K+ ≥5.1 mmol/L (HARMONIZE and HARMONIZE-Global). Patients were excluded if they were on dialysis or had diabetic ketoacidosis, or a cardiac arrhythmia requiring immediate treatment. Patients with serum K+ >6.5 mmol/L were excluded from ZS-003. Patients receiving sodium polystyrene sulphonate were excluded from HARMONIZE, while those who had received organic polymer resins or phosphate binders within 1 week of enrolment were excluded from ZS-003 and HARMONIZE-Global. Neither specific estimated glomerular filtration (eGFR) thresholds, nor severity of diabetes or cardiac failure, determined patient inclusion or exclusion from the studies.
Study treatments
Patients enrolled in ZS-003 were randomized twice: once at correction phase (CP) entry and again at maintenance phase (MP) entry [5]. Eligible patients were initially randomized to receive double-blind treatment with SZC 1.25, 2.5, 5, 10 g or placebo TID for 48 h. Patients on SZC and whose serum K+ was 3.5–4.9 mmol/L at the end of the CP (48 h) were rerandomized (1:1) to continue their initially assigned SZC dose QD or to receive placebo during Days 3–15 (MP). Patients who had received placebo during the CP were randomized to receive SZC 1.25 g or 2.5 g QD during the MP.
All patients enrolled in HARMONIZE [4] and HARMONIZE-Global [18] received open-label SZC 10 g TID for 48 h and those who achieved normokalaemia at the end of the CP were randomized to receive SZC 5, 10 (both studies) or 15 g (HARMONIZE only) or placebo QD during the 28-day MP.
All concomitant medications remained constant during ZS-003 [5], including diuretics, RAASi and glucose-lowering therapies. Use of concomitant medications was recorded in the HARMONIZE and HARMONIZE-Global studies [4, 18]. No dietary restrictions were imposed on patients in any of the studies.
Measurement of serum bicarbonate, urea and urine pH
Serum bicarbonate, urea and urine pH were measured in centralized local laboratories for all studies. In ZS-003 [5], samples for clinical chemistry analysis were collected on study Days 1 and 3 of the CP and on Days 9, 15 and 21 (end of study) of the MP. In the HARMONIZE and HARMONIZE-Global studies [4, 18], samples for clinical chemistry analysis were collected on Days 1 and 3 of the CP and on Days 1, 15, 29 and 35 (end of study) of the MP.
Statistical analysis
Changes in serum bicarbonate, urea and urine pH
Acute effects of SZC on serum bicarbonate, urea and urine pH were assessed using randomized placebo-controlled intention-to-treat (ITT) data from the 48-h CP of ZS-003. Longer-term effects of SZC on serum bicarbonate, urea and urine pH were assessed using randomized placebo-controlled ITT data during the 28-day MP of HARMONIZE and HARMONIZE-Global. Serum bicarbonate, urea and urine pH levels during each time period by SZC dose, and by SZC dose and baseline CKD level (Stages 1 and 2, eGFR ≥60 mL/min/1.73m2, versus Stage 3, eGFR ≥30 and <60 mL/min/1.73m2, versus Stages 4 and 5, eGFR <30 mL/min/1.73 m2 [19]) or baseline bicarbonate level (<22 mmol/L versus ≥22 mmol/L) were presented graphically using descriptive means and associated 95% confidence intervals (CIs). The statistical significance of continuous measures was assessed using t-tests or analyses of variance (ANOVA, for analyses by baseline CKD or bicarbonate level) for acute changes and using mixed models for longer term changes. For proportions, statistical significance was assessed using Fisher’s exact tests. For further details see Supplementary data, statistical methods.
Mechanistic associations
To explore whether SZC increases serum bicarbonate directly or indirectly via SZC-mediated changes in serum K+, serum urea or urine pH, we employed path analyses using the latent variable analysis (lavaan) package version 0.6-3 in R version 3.5.3 [20]. We chose serum urea as a proxy measure to test the gastrointestinal ammonium trapping hypothesis and urine pH as a proxy measure for the renal ammoniagenesis hypothesis in the absence of measures of faecal and urinary ammonium, respectively (see ‘Discussion’ section for further rationale). Acute associations were assessed in ZS-003 at 48 h and longer term associations were assessed in HARMONIZE and HARMONIZE-Global at Day 15 (the time point representing the first measurement post-randomization to active treatment versus placebo and thus the point of maximal change). For these mechanistic analyses, we analysed patients who completed treatment during the CP (ZS-003) and MP (HARMONIZE and HARMONIZE-Global). While ITT analyses are appropriate for conservative estimates of treatment effect, when analysing hypothesized biological mechanisms of action, it is important to conduct these analyses among patients remaining on treatment to avoid the confounding effects of rebound changes on treatment discontinuation.
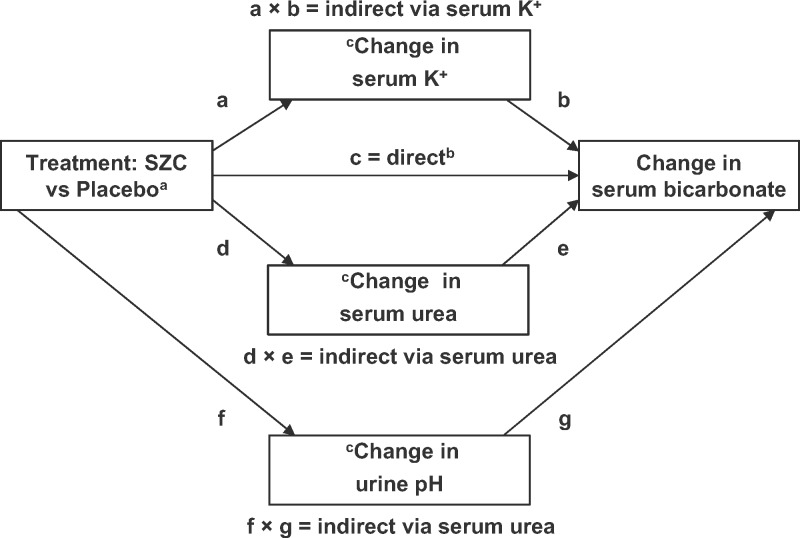
A structural equation path analysis model was specified as illustrated in Figure 1. If the regression path coefficient (c) was significant and the products of both indirect path coefficients, (a × b) and (d × e), were not significant, then this could represent either a direct effect of treatment on change in serum bicarbonate or an indirect effect by unknown/unmeasured mechanisms. If the product of the indirect path coefficients (a × b) was significant and both (c) and the product of the indirect path coefficients (d × e) were not significant, then this would signify full mediation by change in serum K+. If the product of the indirect path coefficients (d × e) was significant and both (c) and the product of the indirect path coefficients (a × b) were not significant, then this would signify full mediation by change in serum urea. If both direct and indirect path coefficients were significant, then this would signify partial mediation [21]. A similar logic is followed to test the path via urine pH (f and g). All covariances between the error terms for changes in serum K+ and serum urea and urine pH were also specified (not shown in Figure 1). Assuming normally distributed change variables, the estimator used was full information maximum likelihood, which is robust under the assumption that data were missing at random. Standardized regression coefficients were calculated. Standard errors were calculated using 1000 bootstrap replications, which provides reliable assessments of the significance of indirect paths, for which the distribution of the product of the two coefficients cannot be assumed to be normal [22]. Model fit indices included the comparative fit index (CFI) and the root mean square error of approximation (RMSEA). Values for CFI >0.95 and RMSEA <0.08 (with a P-value >0.05) defined models that gave an adequate fit to the data.
FIGURE 1.
Structural equation path analysis model. aCategorical ordered independent variables (exogenous in structural equation modelling terminology) can be treated as numeric (http://lavaan.ugent.be/tutorial/cat.html). Thus, for example, in the ZS-003 study, placebo = 0, SZC 1.25 g TID = 3.75, SZC 2.5 g TID = 7.5, SZC 5 g TID = 15 and SZC 10 g TID = 30. bDirect or indirect effect via unmeasured mechanisms. cCovariances between the error terms for changes in serum K+ and urea, and urine pH were also specified (curved arrows not shown).
All P-values reported in this post hoc analysis are nominal and are unadjusted for multiple comparisons.
Role of the funder
The decision to conduct this analysis and submit the manuscript was initiated by author investigators. AstraZeneca provided funding for statistical analyses, which were directed by the authors. The authors received no remuneration for writing the manuscript.
RESULTS
Study participants
In ZS-003, of 754 patients randomized to the CP, 753 (99.9%) were included in the ITT analyses and 736 (97.7%) in the path analysis. In HARMONIZE, of 237 patients randomized to the MP, 231 (97.5%) were included in the ITT analyses and 208 (87.8%) in the path analysis. In HARMONIZE-Global, of 248 patients randomized to the MP, 248 (100%) were included in the ITT analyses and 214 (86.3%) in the path analysis. The main reasons for study withdrawal were hyperkalaemia, withdrawal of consent or adverse events (Supplementary data, Figure S1).
Age and sex distributions were balanced across the three studies (Table 1), but some differences were evident for other demographic and baseline characteristics. ZS-003 and HARMONIZE comprised mainly white or African American participants, whereas HARMONIZE-Global comprised largely Asian participants. The distribution of hyperkalaemia was shifted towards higher levels in HARMONIZE and HARMONIZE-Global compared with ZS-003 because both HARMONIZE inclusion/exclusion criteria did not specify an upper limit for serum K+ concentrations. Mean serum bicarbonate was lower, mean serum urea was higher, and the frequency of baseline serum bicarbonate <22 mmol/L and CKD stages 4/5 were higher in HARMONIZE-Global versus the other studies. A higher proportion of patients in HARMONIZE-Global were receiving RAASi therapy and loop diuretics versus the other studies. The proportions of patients taking sodium bicarbonate therapy at baseline were 7.2% in ZS-003, 7.4% in HARMONIZE and 16.1% in HARMONIZE-Global. No patients commenced a new prescription for sodium bicarbonate during the course of the HARMONIZE study and only one patient did so in the each of the ZS-003 and HARMONIZE-Global studies. These patients were not excluded from analysis.
Table 1.
Patient demographics and characteristics at correction phase baseline (ITT populations)
| Study | ZS-003 | HARMONIZE | HARMONIZE-Global |
|---|---|---|---|
| (n = 753) | (n = 258) | (n = 267) | |
| Age (years), mean (SD) | 66 (12) | 64 (13) | 68 (11) |
| Male, n (%) | 448 (59.5) | 149 (57.8) | 171 (64.0) |
| Race, n (%) | |||
| Asian | 12 (1.6) | 4 (1.6) | 227 (85.0) |
| Black/African American | 86 (11.4) | 36 (14.0) | 0 |
| White | 643 (85.4) | 214 (82.9) | 40 (15.0) |
| Other | 12 (1.6) | 4 (1.6) | 0 |
| Ethnicity, n (%) | |||
| Hispanic | 231 (30.7) | 105 (40.7) | 2 (0.7) |
| Non-Hispanic | 522 (69.3) | 153 (59.3) | 265 (99.3) |
| Serum K+ (mmol/L), mean (SD) | 5.3 (0.4) | 5.6 (0.4) | 5.7 (0.5) |
| Serum bicarbonate (mmol/L), mean (SD) | 23.0 (3.4) | 22.7 (4.2) | 17.4 (3.9) |
| Serum bicarbonate <22 mmol/L, n (%) | 233 (31.4) | 99 (39.4) | 233 (87.9) |
| Serum urea (mmol/L), mean (SD) | 12.6 (6.6) | 13.0 (7.9) | 15.7 (7.1) |
| CKD | eGFR,an (%) | |||
| Stage 5 | <15 mL/min/1.73 m2 | 56 (7.4) | 32 (12.4) | 58 (21.7) |
| Stage 4 | 15 to <30 mL/min/1.73 m2 | 209 (27.8) | 61 (23.6) | 101 (37.8) |
| Stage 3 | 30 to <60 mL/min/1.73 m2 | 296 (39.3) | 86 (33.3) | 88 (33.0) |
| Stage 2 | 60 to <90 mL/min/1.73 m2 | 141 (18.7) | 49 (19.0) | 15 (5.6) |
| Stage 1 | ≥90 mL/min/1.73 m2 | 42 (5.6) | 23 (8.9) | 3 (1.1) |
| Missing | 9 (1.2) | 7 (2.7) | 2 (0.7) |
| Comorbidity history,bn (%) | |||
| CKD | 450 (59.8) | 157 (60.9) | 209 (78.3) |
| Diabetes | 468 (62.2) | 169 (65.5) | 172 (64.4) |
| Heart failure | 75 (10.0) | 29 (11.2) | 50 (18.7) |
| Hyperkalaemia | 300 (39.8) | 77 (29.8) | 40 (15.0) |
| Hypertension | 653 (86.7) | 229 (88.8) | 225 (84.3) |
| Concomitant medications, n (%) | |||
| RAASi therapy | 500 (66.4) | 176 (68.2) | 204 (76.4) |
| ACE inhibitors | 332 (44.1) | 126 (48.8) | 26 (9.7) |
| ARBs | 178 (23.6) | 55 (21.3) | 178 (66.7) |
| MRAs | 46 (6.1) | 11 (4.3) | 15 (5.6) |
| Renin inhibitors | 3 (0.4) | 0 | 1 (0.4) |
| Diureticsc | 252 (33.5) | 93 (36.0) | 86 (32.2) |
| Thiazide | 42 (16.7) | 15 (16.1) | 12 (14.0) |
| Loop | 199 (79.0) | 74 (79.6) | 75 (87.2) |
| Other | 23 (9.1) | 10 (10.8) | 6 (7.0) |
| Sodium bicarbonate use | 54 (7.2) | 19 (7.4) | 43 (16.1) |
As per KDIGO 2017 updated guidelines (Kidney International Supplements, 2017. 7: 1–59).
Diabetes, heart failure and CKD were defined by SMQ Narrow, and hyperkalaemia was defined by terms of “hyperkalaemia” or “blood potassium increased” in medical history, and hypertension was defined by term of “hypertension” in medical history. MedDRA version 15.1E was used.
Subjects using multiple diuretics were counted multiple times in different classes; aldosterone antagonists (ATC code C03DA) were excluded, since they were counted within the RAASi group.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ATC, Anatomical Therapeutic Chemical Classification System; KDIGO, kidney disease improving global outcomes; MedDRA, medical dictionary for regulatory activities; MRA, mineralocorticoid receptor antagonist; SMQ, standardized MedDRA query.
Effects of SZC on serum bicarbonate
Acute effects
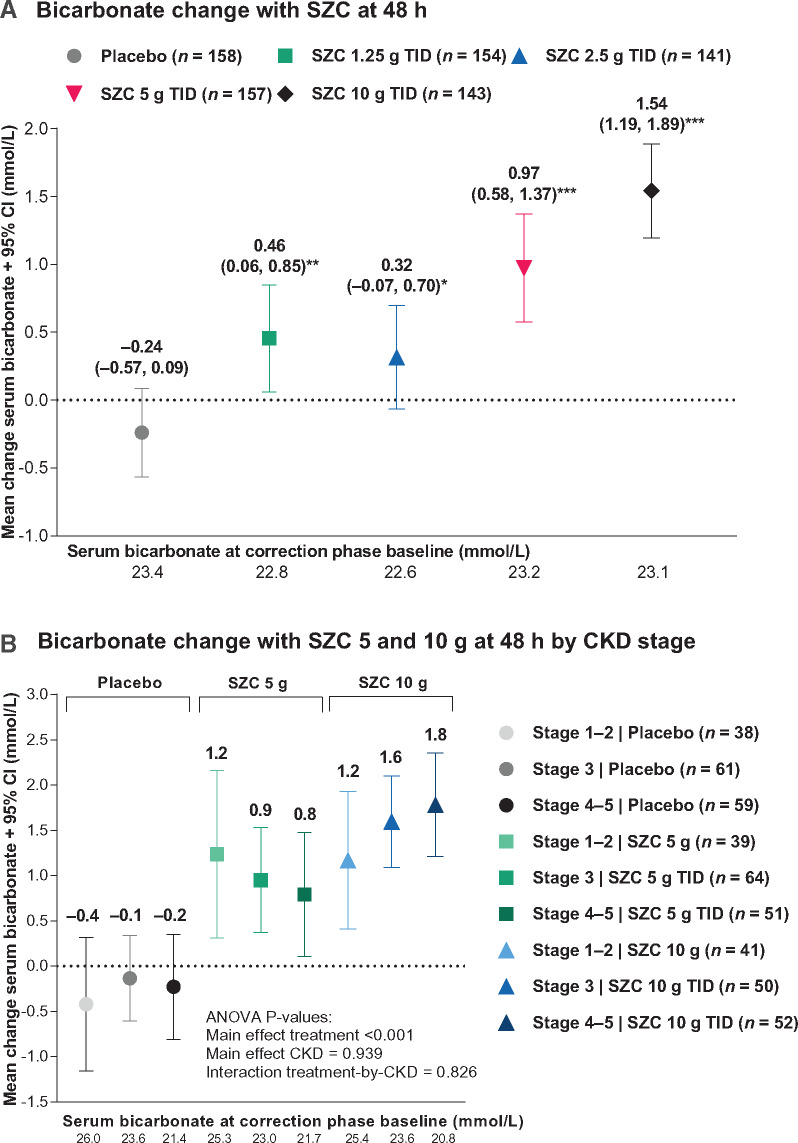
Rapid dose-dependent serum bicarbonate increases occurred within 48 h following SZC administration versus placebo in ZS-003 (Figure 2A; P < 0.05 versus placebo for all doses; change with SZC 10 g TID versus placebo = 1.8 mmol/L). A corresponding reduction in proportion of patients with serum bicarbonate <22 mmol/L occurred with the SZC 10 g TID dose [Table 2; odds ratio, 0.51 (95% CI 0.29, 0.90), P = 0.025]. Serum bicarbonate significantly increased with SZC doses 5 g TID and 10 g TID versus placebo within all CKD levels (all P < 0.02, Supplementary data, Table S1), whereas no differences between CKD levels within each placebo versus SZC dose comparison were apparent (all P > 0.5, Supplementary data, Table S1; Figure 2B; P-value for treatment-by-CKD interaction = 0.826). While the magnitude of serum bicarbonate changes appeared greater among patients with baseline serum bicarbonate <22 mmol/L, this was driven by a significant main effect of baseline serum bicarbonate level (P < 0.001) (Supplementary data, Figure S2; P-value for treatment-by-baseline bicarbonate level interaction = 0.762). Thus, serum bicarbonate significantly increased with SZC doses 5 g TID and 10 g TID versus placebo regardless of baseline serum bicarbonate level (all P < 0.001, Supplementary data, Table S2), whereas no differences between baseline serum bicarbonate levels within each placebo versus SZC dose comparison were apparent (all P > 0.5, Supplementary data, Table S2). Median (range) serum bicarbonate concentration at 48 h was 26 mmol/L (21–33) among patients with baseline serum bicarbonate level ≥22 mmol/L and treated with SZC 10 g TID.
FIGURE 2.
Serum bicarbonate levels for the ZS-003 study during the randomized treatment CP (ITT population): (A) mean change (95% CI) from baseline at 48 h; and (B) mean change (95% CI) from baseline at 48 h by CKD stage among patients treated with SZC 5 or 10 g TID. In (A), P-values versus placebo *<0.05, **<0.01 and ***<0.001 were derived from two-sample two-sided t-tests. In (B), the P-values were derived from Type III ANOVA tests of the main effects and interaction between and treatment (placebo versus all SZC doses) and CKD stage.
Table 2.
Proportions of patients with serum bicarbonate <22 mmol/L in the correction phase ITT populations of ZS-003 (n = 753), HARMONIZE (n = 258) and HARMONIZE-Global (n = 267); and the maintenance phase ITT populations of HARMONIZE (n = 237) and HARMONIZE-Global (n = 248)
| ZS-003 | Placebo | SZC 1.25 g TID | SZC 2.5 g TID | SZC 5 g TID | SZC 10 g TID |
|---|---|---|---|---|---|
| CP baseline | |||||
| n | 157 | 150 | 138 | 153 | 143 |
| <22 mmol/L, n (%) | 44 (28.0) | 48 (32.0) | 50 (36.2) | 52 (34.0) | 39 (27.3) |
| CP 48 h | |||||
| n | 154 | 147 | 132 | 149 | 139 |
| <22 mmol/L, n (%) | 43 (27.9) | 45 (30.6) | 46 (34.8) | 33 (22.1) | 23 (16.5) |
| Odds ratio (95% CI) versus PBO | 1.14 (0.69–1.87) | 1.38 (0.84–2.28) | 0.73 (0.43–1.29) | 0.51 (0.29–0.90) | |
| P-valuea | 0.615 | 0.249 | 0.289 | 0.025 | |
| HARMONIZE | Placebo | SZC 5 g QD | SZC 10 g QD | SZC 15 g QD | SZC 10 g TID |
| CP baseline | |||||
| n | 251 | ||||
| <22 mmol/L, n (%) | 99 (39.4) | ||||
| CP 48 h | |||||
| n | 250 | ||||
| <22 mmol/L, n (%) | 56 (22.4) | ||||
| MP Day 15 | |||||
| n | 79 | 43 | 44 | 52 | |
| <22 mmol/L, n (%) | 27 (34.2) | 11 (25.6) | 7 (15.9) | 7 (13.5) | |
| Odds ratio (95% CI) versus PBO | 0.66 (0.29–1.52) | 0.36 (0.14–0.93) | 0.30 (0.12–0.75) | ||
| P-valuea | 0.414 | 0.036 | 0.009 | ||
| MP Day 29 | |||||
| n | 72 | 37 | 37 | 42 | |
| <22 mmol/L, n (%) | 19 (26.4) | 10 (27.0) | 8 (21.6) | 2 (4.8) | |
| Odds ratio (95% CI) versus PBO | 1.03 (0.42–2.53) | 0.77 (0.30–1.97) | 0.14 (0.03–0.63) | ||
| P-valuea | 1.000 | 0.646 | 0.005 | ||
| HARMONIZE-Global | Placebo | SZC 5 g QD | SZC 10 g QD | SZC 10 g TID | |
| CP baseline | |||||
| n | 265 | ||||
| <22 mmol/L, n (%) | 233 (87.9) | ||||
| CP 48 h | |||||
| n | 255 | ||||
| <22 mmol/L, n (%) | 201 (78.8) | ||||
| MP Day 15 | |||||
| n | 48 | 89 | 92 | ||
| <22 mmol/L, n (%) | 43 (89.6) | 76 (85.4) | 64 (69.6) | ||
| Odds ratio (95% CI) versus PBO | 0.68 (0.23–2.04) | 0.27 (0.10–0.74) | |||
| P-valuea | 0.601 | 0.011 | |||
| MP Day 29 | |||||
| n | 40 | 83 | 87 | ||
| <22 mmol/L, n (%) | 37 (92.5) | 71 (85.5) | 61 (70.1) | ||
| Odds ratio (95% CI) versus PBO | 0.48 (0.13–1.81) | 0.19 (0.05–0.67) | |||
| P-valuea | 0.381 | 0.006 | |||
P-value derived from Fisher’s exact test.
PBO, placebo.
Maintenance of effects
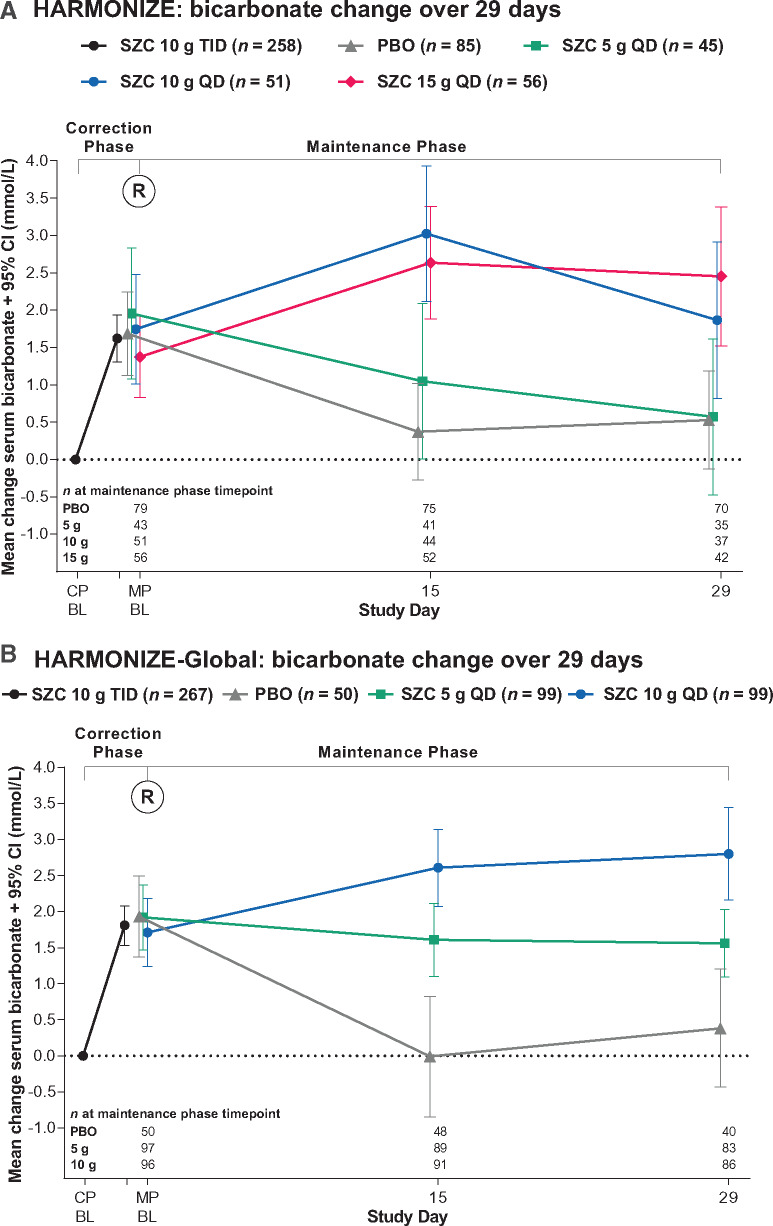
Serum bicarbonate increases during the CPs were sustained during the MPs with SZC doses of 10 g QD or more in HARMONIZE (Figure 3A) and 5 g QD or more in HARMONIZE-Global (Figure 3B); the mixed model derived Days 15–29 least squares (LS) mean changes from baseline significantly increased versus placebo for the SZC 10 g QD and 15 g QD doses in HARMONIZE (Table 3), and for the SZC 5 g QD and 10 g QD doses in HARMONIZE-Global (Table 3). Among patients randomized to receive placebo and SZC 5 g in HARMONIZE and patients randomized to receive placebo in HARMONIZE-Global during the MPs, serum bicarbonate returned to pre-treatment levels (Figure 3A and B). Similarly, the increase in serum bicarbonate during the CP of ZS-003 among patients first randomized to SZC 10 g TID was continued during the MP after rerandomization to SZC 10 g QD, whereas among those rerandomized to placebo, serum bicarbonate returned to pre-treatment levels (Supplementary data, Figure S3).
FIGURE 3.
Serum bicarbonate mean change (95% CI) from baseline values over time during the open-label CP and maintenance randomized-treatment phase (ITT populations) for (A) the HARMONIZE study and (B) the HARMONIZE-Global study. BL, baseline; PBO, placebo; R, randomization.
Table 3.
Mixed model results for Days 15–29 mean changes from baseline in serum bicarbonate, urea and urine pH in the maintenance phase ITT populations of HARMONIZE (n = 237) and HARMONIZE-Global (n = 248)
| Placebo | SZC 5 g | SZC 10 g | SZC 15 g | |
|---|---|---|---|---|
| HARMONIZE | n = 85 | n = 45 | n = 51 | n = 56 |
| Serum bicarbonate (mmol/L) | ||||
| n | 75 | 41 | 47 | 52 |
| LS mean change (95% CI) | 0.34 (−0.19, 0.86) | 0.73 (0.07, 1.39) | 2.21 (1.56, 2.86) | 2.64 (2.05, 3.23) |
| Difference versus placebo | 0.40 (−0.38, 1.17) | 1.87 (1.11, 2.63) | 2.30 (1.57, 3.04) | |
| P-value | 0.316 | <0.001 | <0.001 | |
| Serum urea (mmol/L) | ||||
| n | 75 | 41 | 47 | 52 |
| LS mean change (95% CI) | 0.05 (−0.66, 0.76) | 0.23 (−0.67, 1.12) | −0.98 (−1.86, −0.10) | −0.74 (−1.54, 0.06) |
| Difference versus placebo | 0.18 (−0.87, 1.23) | −1.03 (−2.06, −0.00) | −0.79 (−1.78, 0.21) | |
| P-value | 0.740 | 0.0495 | 0.121 | |
| Urine pH | ||||
| n | 76 | 40 | 47 | 52 |
| LS mean change (95% CI) | −0.15 (−0.24, −0.05) | −0.15 (−0.27, −0.03) | 0.00 (−0.12, 0.13) | 0.10 (−0.02, 0.21) |
| Difference versus placebo | −0.00 (−0.15, 0.14) | 0.15 (0.01, 0.29) | 0.24 (0.10, 0.38) | |
| P-value | 0.978 | 0.039 | 0.001 | |
| Placebo | SZC 5 g | SZC 10 g | ||
| HARMONIZE-Global | n = 50 | n = 99 | n = 99 | |
| Serum bicarbonate (mmol/L) | ||||
| n | 48 | 90 | 91 | |
| LS mean change (95% CI) | 0.25 (−0.39, 0.88) | 1.61 (1.09, 2.14) | 2.71 (2.17, 3.25) | |
| Difference versus placebo | 1.36 (0.71, 2.02) | 2.46 (1.81, 3.11) | ||
| P-value | <0.001 | <0.001 | ||
| Serum urea (mmol/L) | ||||
| n | 48 | 92 | 92 | |
| LS mean change (95% CI) | 0.58 (−0.38, 1.54) | −0.83 (−1.62, −0.05) | −2.24 (−3.05, −1.44) | |
| Difference versus placebo | −1.41 (−2.40, −0.43) | −2.82 (−3.80, −1.84) | ||
| P-value | 0.005 | <0.001 | ||
| Urine pH | ||||
| n | 48 | 90 | 92 | |
| LS mean change (95% CI) | −0.19 (−0.29, −0.08) | −0.02 (−0.11, 0.07) | 0.18 (0.09, 0.27) | |
| Difference versus placebo | 0.17 (0.06, 0.28) | 0.36 (0.25, 0.47) | ||
| P-value | 0.004 | <0.001 | ||
Results derived from a mixed model with change from baseline in serum bicarbonate, serum urea or urine pH as response and fixed effects of treatment group; visit; treatment-by-visit interaction; baseline bicarbonate, urea or urine pH values (CP and MP); baseline eGFR; age category (<55, 55–64, ≥65 years); and baseline RAASi, CKD, heart failure and diabetes statuses.
Reductions in proportions of patients with serum bicarbonate <22 mmol/L during the CP continued to fall during the MP with an SZC dose of 15 g in HARMONIZE [Table 2; odds ratio, 0.30 (95% CI 0.12–0.75), P = 0.009 at 15 days, and 0.14 (95% CI 0.03–0.63), P = 0.005 at 29 days] and were preserved during the MP with an SZC dose of 10 g in HARMONIZE-Global [Table 2; odds ratio, 0.27 (95% CI 0.10–0.74), P = 0.011 at 15 days, and 0.19 (95% CI 0.05–0.67), P = 0.006 at 29 days].
Among patients with CKD Stages 3 and 4–5, serum bicarbonate increases were maintained versus placebo in HARMONIZE with SZC 10 g and 15 g doses (Supplementary data, Figure S4A; Supplementary data, Table S3) and in HARMONIZE-Global studies with SZC 5 g and 10 g doses (Supplementary data, Figure S4B; Supplementary data, Table S4).
In HARMONIZE with SZC 5 g and 10 g doses, increases in serum bicarbonate were maintained to a greater extent among patients with baseline serum bicarbonate <22 mmol/L versus ≥22 mmol/L (Supplementary data, Figure S5; Supplementary data, Table S5). In HARMONIZE-Global, there were too few patients with baseline serum bicarbonate ≥22 mmol/L to conduct such analyses.
Effects of SZC on serum urea
Acute effects
Dose-dependent decreases in serum urea occurred within 48 h following SZC administration versus placebo in ZS-003 (Supplementary data, Figure S6; P < 0.05 versus placebo for SZC 5 g TID and 10 g TID doses).
Maintenance of effects
Serum urea decreases from baseline during the CPs were maintained during the MPs with an SZC dose of 15 g QD in HARMONIZE (Supplementary data, Figure S7A) and 5 g and 10 g QD in HARMONIZE-Global (Supplementary data, Figure S7B); the mixed model derived 15- to 29-day LS mean changes from baseline were significantly decreased versus placebo for the SZC 10 g QD dose in HARMONIZE (Table 3) and for the SZC 5 g and 10 g QD doses in HARMONIZE-Global (Table 3). Among patients randomized to receive placebo and SZC doses of 5 g in HARMONIZE and patients randomized to receive placebo in HARMONIZE-Global during the MPs, serum urea returned to pre-treatment levels (Supplementary data, Figure S7A and S7B).
Effects of SZC on urine pH
Acute effects
An increase in urine pH occurred within 48 h following SZC administration versus placebo in ZS-003 with the SZC 10 g TID dose (Supplementary data, Figures S8; P < 0.001 versus placebo); a small decrease from baseline in the placebo group also occurred.
Maintenance of effects
Urine pH increases from baseline during the CPs were maintained during the MPs with an SZC dose of 15 g QD in HARMONIZE (Supplementary data, Figure S9A) and 5 g and 10 g QD in HARMONIZE-Global (Supplementary data, Figure S9B); the mixed model derived 15- to 29-day LS mean changes from baseline were significantly increased versus placebo for the SZC 10 g and 15 g QD doses in HARMONIZE (Table 3) and for the SZC 5 g and 10 g QD doses in HARMONIZE-Global (Table 3). Among patients randomized to receive placebo or SZC 5 g QD or during the MPs of both studies, urine pH either returned to pre-treatment levels or fell below baseline levels (Supplementary data, Figure S9). Changes in urine pH during the MP with the SZC 10 g dose in HARMONIZE were difficult to interpret because at randomization, this group had a greater reduction in urine pH pre-randomization by chance (Supplementary data, Figure S9A).
Mechanistic associations
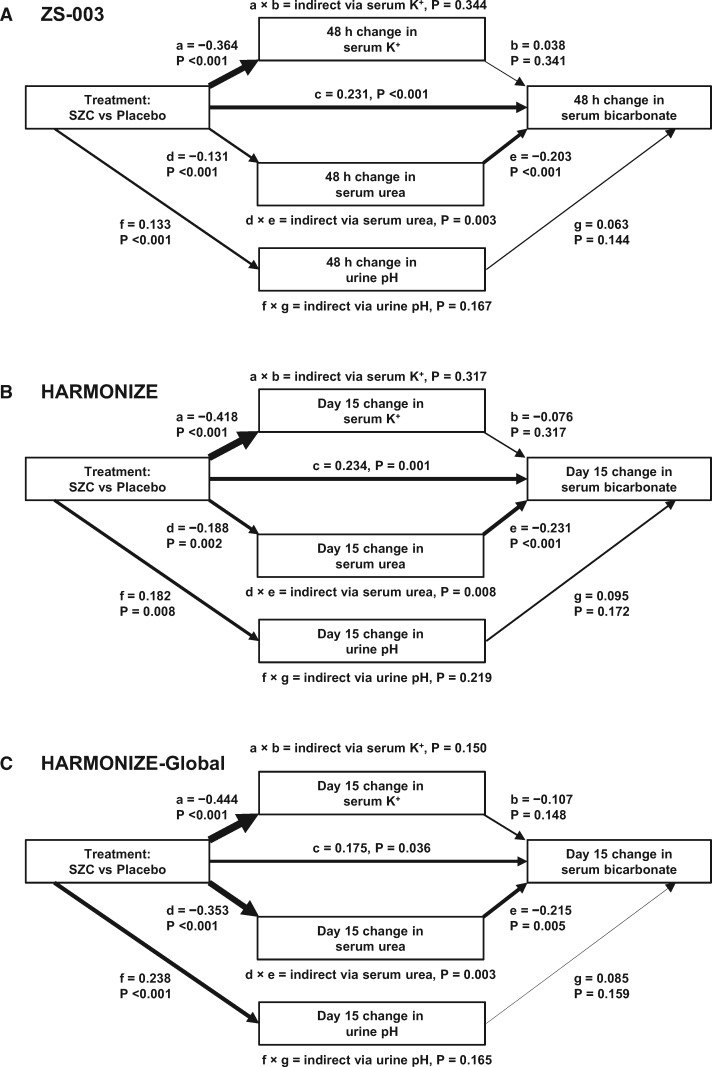
Change variables were normally distributed (Supplementary data, Figure S10), meeting assumptions for path analysis maximum likelihood estimation. Path analyses (Figure 4 and Table 4) showed that SZC significantly and consistently increased serum bicarbonate both directly (Path c) and indirectly via reductions in serum urea (Path d × e). While SZC significantly decreased serum K+ (Path a) and increases urine pH (Path f), the indirect paths via change in serum K+ (Path a × b) and via change in urine pH (f × g) were not significant, indicating that SZC-induced changes in serum K+ and urine pH were not responsible for the observed increase in serum bicarbonate with SZC therapy. Although these coefficients were modest in magnitude, a consistent pattern was evident for both acute changes at 48 h in ZS-003 as well as for later changes at Day 15 in HARMONIZE and HARMONIZE-Global. In HARMONIZE-Global, the path via urea was the dominant path (Figure 4 and Table 4).
FIGURE 4.
Path analyses showing that SZC significantly increases serum bicarbonate both directly (Path c) and indirectly via reductions in serum urea (Path d × e) at (A) 48 h in the ZS-003 study (n = 736), (B) Day 15 in the HARMONIZE study (n = 208) and (C) Day 15 in the HARMONIZE-Global study (n = 214). Although SZC significantly decreases serum K+ (Path a) and increases urine pH (Path f), the indirect paths via change in serum K+ (Path a × b) and via change in urine pH (Path f × g) were not significant, indicating that SZC-induced changes in serum K+ and urine pH are not responsible for the observed increase in serum bicarbonate with SZC therapy. CFI = 1.000 and RMSEA = 0.000 (P > 0.05) for all models. Path line weights are proportional to the magnitude of the standardized β coefficients.
Table 4.
Path analysis standardized β coefficients
| ZS-003 at 48 h (n = 736) |
HARMONIZE at Day 15 (n = 208) |
HARMONIZE-Global at Day 15 (n = 214) |
|||||
|---|---|---|---|---|---|---|---|
| Path | Regression | β (95% CI) | P-value | β (95% CI) | P-value | β (95% CI) | P-value |
| a | Change in serum K+ on treatment | −0.364 (−0.434, −0.294) | <0.001 | −0.418 (−0.531, −0.305) | <0.001 | −0.444 (−0.543, −0.344) | <0.001 |
| Change in serum bicarbonate on: | |||||||
| b | Change in serum K+ | 0.038 (−0.041, 0.118) | 0.341 | −0.076 (−0.226, 0.073) | 0.317 | −0.107 (−0.252, 0.038) | 0.148 |
| c | Treatment | 0.231 (0.161, 0.302) | <0.001 | 0.234 (0.093, 0.374) | 0.001 | 0.175 (0.012, 0.339) | 0.036 |
| e | Change in serum urea | −0.203 (−0.283, −0.123) | <0.001 | −0.231 (−0.344, −0.119) | <0.001 | −0.215 (−0.364, −0.067) | 0.005 |
| g | Change in urine pH | 0.063 (−0.022, 0.148) | 0.144 | 0.095 (−0.041, 0.230) | 0.172 | 0.085 (−0.033, 0.202) | 0.159 |
| d | Change in serum urea on treatment | −0.131 (−0.197, −0.066) | <0.001 | −0.188 (−0.305, −0.071) | 0.002 | −0.353 (−0.472, −0.233) | <0.001 |
| f | Change in urine pH on treatment | 0.133 (0.061, 0.205) | <0.001 | 0.182 (0.048, 0.316) | 0.008 | 0.238 (0.124, 0.351) | <0.001 |
| a × b | Indirect path via change in serum K+ | 0.344 | 0.317 | 0.150 | |||
| d × e | Indirect path via change in serum urea | 0.003 | 0.008 | 0.003 | |||
| f × g | Indirect path via change in urine pH | 0.167 | 0.219 | 0.165 | |||
DISCUSSION
In this post hoc analysis of data from three randomized, placebo-controlled trials, SZC rapidly and dose-dependently increased serum bicarbonate among patients with hyperkalaemia, including those with varying stages of CKD. These increases were maintained over 1 month with SZC 10 g QD in both the HARMONIZE and HARMONIZE-Global studies as well as with SZC 5 g QD in HARMONIZE-Global, likely due to the lower baseline serum bicarbonate values in HARMONIZE-Global. These findings are consistent with other data, in which a sustained increase in serum bicarbonate occurred among a large population of patients with hyperkalaemia (n = 746) treated with open-label SZC for up to 1 year [7]. The increase in serum bicarbonate with SZC therapy is a clear effect that differentiates it from other K+-binding agents, such as sodium polystyrene sulphonate [23] or patiromer [24].
Increased serum bicarbonate with SZC is of potential clinical significance, particularly among patients with CKD. Low serum bicarbonate increases risk of CKD progression, metabolic bone disease and mortality, as well as incident reductions in eGFR among those with preserved kidney function (eGFR >60 mL/min/1.73 m2) [17, 25–28]. In contrast, oral sodium bicarbonate supplementation, with the aim of maintaining serum bicarbonate levels ≥22 mmol/L, preserves kidney function [14, 15, 29–31] and reduces metabolic bone disease [16] and mortality [15] in randomized-controlled open-label trials among patients with metabolic acidosis and CKD. Therefore, the Kidney Disease Improving Global Outcomes guidelines for CKD recommend maintaining serum bicarbonate levels ≥22 mmol/L with oral bicarbonate supplements as needed [17].
The SZC-associated increase in serum bicarbonate could provide adjunctive treatment alongside oral sodium bicarbonate for patients with CKD and acidosis, which could enable sodium bicarbonate dose reductions for some patients. Analyses by baseline bicarbonate level showed that SZC increased serum bicarbonate acutely versus placebo by a similar amount regardless of baseline serum bicarbonate level. However, among patients with baseline serum bicarbonate <22 mmol/L, acute increases in serum bicarbonate were greater than among patients with baseline bicarbonate ≥22 mmol/L. In the longer term, SZC maintained serum bicarbonate increases to a greater extent among patients with baseline serum bicarbonate <22 mmol/L versus ≥22 mmol/L. The net effect of this suggests that SZC does not overcorrect low serum bicarbonate or precipitate alkalosis. In HARMONIZE-Global (baseline serum bicarbonate 17.4 mmol/L and urea 15.7 mmol/L), change from baseline in serum bicarbonate with SZC 10 g QD versus placebo at 29 days (2.5 mmol/L) was similar to that with veverimer (a selective gastrointestinal hydrochloric acid binder) 6 g QD versus placebo at 4 weeks (∼3 mmol/L) among patients with CKD and metabolic acidosis (baseline serum bicarbonate 17.3 mmol/L and urea 13.8 mmol/L) [32]. For an overview of the known safety issues with SZC, namely oedema occurring in 6% of patients receiving SZC 10 g QD in placebo-controlled trials of 28-day duration (n = 1009) and in 8–11% of patients maintained on SZC ≤15 g QD for up to 1 year (n = 746), and hypokalaemia (serum potassium <3.5 mmol/L) in 4% of patients, see associated references [2, 7, 8].
Consistent with serum bicarbonate increases, SZC also reduced frequencies of serum bicarbonate <22 mmol/L that were maintained over time. The proportions of patients receiving SZC with serum bicarbonate <22 mmol/L at Day 29 was lower in HARMONIZE (17.2%) than in HARMONIZE-Global (77.6%) due to the differences in the proportion of patients with serum bicarbonate <22 mmol/L at CP baseline between these studies (39.4% and 87.9%, respectively). Consistent with these reductions in proportions with serum bicarbonate <22 mmol/L, SZC treatment was associated with dose-dependent decreases in serum urea and increases in urine pH in all three studies.
The mechanisms underlying the increase in serum bicarbonate with SZC are not fully understood, but may be due to the binding and removal of ammonium by SZC in the gastrointestinal tract [6, 10, 11], or to augmentation of renal ammoniagenesis through correction of hyperkalaemia [12, 13]. Ammonium ions have similar biophysical properties to K+ ions in aqueous solution [33]; thus, SZC demonstrates significant binding of ammonium ions in addition to K+ in the gastrointestinal tract [3]. Despite the release of hydrogen as one of the SZC exchange ions, paradoxically SZC increases serum bicarbonate and corrects acidosis compared with placebo. This suggests that although SZC exchanges hydrogen and sodium for K+, SZC must mostly exchange sodium for ammonium and not hydrogen for ammonium, which would otherwise release a stronger acid while binding a weaker one. Urea in the gastrointestinal tract is hydrolysed by bacterial ureases to release bicarbonate and ammonium ions, which are absorbed through the gastrointestinal epithelium and enter the liver, where urea is resynthesized [34, 35]. Thus, capture of ammonium by SZC in exchange for sodium ions, with subsequent elimination of ammonium in the faeces, interrupts the ammonium component of this enterohepatic loop. This allows bicarbonate to be reabsorbed while reducing the reabsorption of ammonium, which, in turn, reduces the substrate available for resynthesis of urea in the liver. The overall effect is an increase in serum bicarbonate, reduction in serum urea and net acid loss [10].
An alternative hypothesis for the increase in serum bicarbonate with SZC is that normalization of serum K+ levels may increase renal ammoniagenesis, which is decreased in patients with hyperkalaemia [12]. Ammonia (NH3) produced in the kidney is secreted into the collecting duct and combines with hydrogen ions (H+) where it forms ammonium (), a major route of acid excretion [36]. Renal ammoniagenesis is strongly linked to urinary acid excretion and, in turn, renal tubular bicarbonate production [12, 13].
While one might assume that SZC-associated K+-lowering is linked to serum bicarbonate increases via an increase in renal acid excretion, this is not the case as SZC increases urine pH. Instead, the current analysis strongly suggests that SZC-associated serum bicarbonate increase is due to ammonium trapping in the gastrointestinal tract, supported by the significant association with the path via serum urea reduction but not with the paths via serum K+ reduction or urine pH increase (Figure 4). This significant path via serum urea reduction was consistent across all three studies, and was the dominant path in HARMONIZE-Global, likely due to that study’s higher baseline serum urea level.
This post hoc analysis has several strengths, the most important being the inclusion of a placebo-control group in each study. Use of oral sodium bicarbonate was limited to ∼7% of patients in ZS-003 and HARMONIZE and ∼16% of patients in HARMONIZE-Global at baseline, and remained stable throughout the studies. Furthermore, these observations have clinical implications for the management of patients with CKD, including those with severely impaired renal function (CKD Stages 4 and 5), as ∼40% of patients included in this analysis had advanced CKD at baseline.
Analysis limitations include its retrospective nature and that data available for exploration of the mechanism by which SZC increases serum bicarbonate were proxy measures (i.e. serum urea and urine pH instead of the difficult-to-measure faecal and urine ammonium, respectively). While path analysis is useful for evaluating the strength of causal hypotheses, it does not prove the direction of causality. Because no data were available on the progression of CKD owing to the short study durations, whether SZC-induced serum bicarbonate increases preserve kidney function among patients with CKD awaits further study.
In conclusion, SZC consistently and dose-dependently increased serum bicarbonate concentrations, reduced the proportions of patients with serum bicarbonate <22 mmol/L, decreased serum urea and increased urine pH during treatment of hyperkalaemia. These serum bicarbonate increases occurred regardless of CKD stage, were sustained during ongoing maintenance therapy, and were associated with decreases in serum urea rather than with changes in serum K+ or urine pH, supporting the hypothesis that SZC traps ammonium in the gastrointestinal tract independently to its effect on K+ binding.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
Post hoc statistical analyses were provided by Pharmapace, Inc. (San Diego, CA, USA), which was funded by AstraZeneca. Medical writing support was provided by Sarah Greig, Ph.D., of inScience Communications, Springer Healthcare (Auckland, New Zealand), which was funded by AstraZeneca.
FUNDING
This analysis was funded by AstraZeneca.
AUTHORS’ CONTRIBUTIONS
S.D.R., B.S.S., S.F., S.R.A., J.G.M., C.M.Q. and D.K.P. contributed to the conception or design of the work; S.D.R. and D.K.P. contributed to acquisition of data; J.G.M. contributed to specification and supervision of statistical analyses; S.D.R., B.S.S., E.V.L., S.F., S.R.A., J.G.M., C.M.Q. and D.K.P. contributed to interpretation of data; S.D.R., B.S.S., E.V.L., S.F., S.R.A., J.G.M., C.M.Q. and D.K.P. contributed to drafting/writing the manuscript; S.D.R., B.S.S., E.V.L., S.F., S.R.A., J.G.M., C.M.Q. and D.K.P. contributed to revising the manuscript critically for important intellectual content. All authors had full access to the data, can assert to the reliability of the analyses/findings and approved manuscript submission.
CONFLICT OF INTEREST STATEMENT
S.D.R. has received travel fees for investigator meetings and honoraria for serving on advisory boards for AstraZeneca, ZS Pharma, Vifor Pharma and Amgen. B.S.S. has received grant support from ZS Pharma and has received consulting and lecture fees from AstraZeneca. E.V.L. is a sub-investigator with Research by Design and has received grant support from ZS Pharma. S.F. has received travel fees and honoraria for serving on advisory boards from AstraZeneca. S.R.A. was involved in the design and development of SZC and has received honoraria for speaking engagements from AstraZeneca. J.G.M. provided statistical consultancy on the design of this post hoc analysis, which was funded by AstraZeneca; he is employed by inScience Communications, Springer Healthcare (Paris, France), which received funding from the sponsor for medical writing support. C.M.Q. is an employee of and holds ownership interest in AstraZeneca, PLC. D.K.P. has received travel fees and honoraria for serving on advisory boards from AstraZeneca and ZS Pharma and has served as an investigator on the SZC clinical trials.
(See related article by Wesson. Sodium zirconium cyclosilicate for hyperkalemia: a collateral acidbase benefit? Nephrol Dial Transplant 2021; 36: 756–760)
REFERENCES
- 1.European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP). Assessment report: Lokelma. International non-proprietary name: sodium zirconium cyclosilicate. Procedure No. EMEA/H/C/004029/0000, 2018. https://www.ema.europa.eu/en/documents/assessment-report/lokelma-epar-public-assessment-report_en.pdf (16 January 2020, date last accessed)
- 2.AstraZeneca. Lokelma™ (Sodium Zirconium Cyclosilicate) for Oral Suspension, 2018. https://www.azpicentral.com/pi.html? product=lokelma&country=us&popup=no (16 January 2020, date last accessed)
- 3. Stavros F, Yang A, Leon A. et al. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One 2014; 9: e114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kosiborod M, Rasmussen HS, Lavin P. et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA 2014; 312: 2223–2233 [DOI] [PubMed] [Google Scholar]
- 5. Packham DK, Rasmussen HS, Lavin PT. et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 2015; 372: 222–231 [DOI] [PubMed] [Google Scholar]
- 6. Ash SR, Singh B, Lavin PT. et al. A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int 2015; 88: 404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spinowitz BS, Fishbane S, Pergola PE. et al. , on behalf of the ZS-005 Study Investigators. Sodium zirconium cyclosilicate in individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol 2019; 14: 798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roger SD, Lavin PT, Lerma EV. et al. Long-term safety and efficacy of sodium zirconium cyclosilicate for hyperkalaemia in patients with mild/moderate versus severe/end-stage chronic kidney disease: comparative results from an open-label, Phase 3 study. Nephrol Dial Transplant 2020; gfz285. doi: 10.1093/ndt/gfz285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roger SD, Spinowitz BS, Lerma EV. et al. Efficacy and safety of sodium zirconium cyclosilicate for treatment of hyperkalemia: an 11-month open-label extension of HARMONIZE. Am J Nephrol 2019; 50: 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCullough PA, Costanzo MR, Silver M. et al. Novel agents for the prevention and management of hyperkalemia. Rev Cardiovasc Med 2015; 16: 140–155 [DOI] [PubMed] [Google Scholar]
- 11. Rafique Z, Peacock WF, LoVecchio F. et al. Sodium zirconium cyclosilicate (ZS-9) for the treatment of hyperkalemia. Expert Opin Pharmacother 2015; 16: 1727–1734 [DOI] [PubMed] [Google Scholar]
- 12. Karet FE. Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol 2009; 20: 251–254 [DOI] [PubMed] [Google Scholar]
- 13. Pourafshar N, Pourafshar S, Soleimani M.. Urine ammonium, metabolic acidosis and progression of chronic kidney disease. Nephron 2018; 138: 222–228 [DOI] [PubMed] [Google Scholar]
- 14. de Brito-Ashurst I, Varagunam M, Raftery MJ. et al. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol 2009; 20: 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Iorio BR, Bellasi A, Raphael KL. et al. , The UBI Study Group. Treatment of metabolic acidosis with sodium bicarbonate delays progression of chronic kidney disease: the UBI Study. J Nephrol 2019; 32: 989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lefebvre A, de Vernejoul MC, Gueris J. et al. Optimal correction of acidosis changes progression of dialysis osteodystrophy. Kidney Int 1989; 36: 1112–1118 [DOI] [PubMed] [Google Scholar]
- 17. Kidney Disease Improving Global Outcomes. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 18. Zannad F, Hsu BG, Maeda Y. et al. Efficacy and safety of sodium zirconium cyclosilicate for hyperkalaemia: the randomized, placebo-controlled HARMONIZE-Global study. ESC Heart Fail 2020; 7: 54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosseel Y. lavaan: an R package for structural equation modeling. J Stat Softw 2012; 48: 1–36 [Google Scholar]
- 21. Rosseel Y. Lavaan (Latent Variable Analysis): Mediation. http://lavaan.ugent.be/tutorial/mediation.html (16 January 2020, date last accessed)
- 22. Hallquist M. Introductory SEM using lavaan: 4 Testing mediation. 15 August 2018. https://psu-psychology.github.io/r-bootcamp-2018/talks/lavaan_tutorial.html#testing-mediation (16 January 2020, date last accessed)
- 23. Parks M, Grady D.. Sodium polystyrene sulfonate for hyperkalemia. JAMA Intern Med 2019; 179: 1023–1024 [DOI] [PubMed] [Google Scholar]
- 24. Weir MR, Bakris GL, Bushinsky DA. et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372: 211–221 [DOI] [PubMed] [Google Scholar]
- 25. Dobre M, Yang W, Chen J. et al. Association of serum bicarbonate with risk of renal and cardiovascular outcomes in CKD: a report from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 2013; 62: 670–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Driver TH, Shlipak MG, Katz R. et al. Low serum bicarbonate and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2014; 64: 534–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldenstein L, Driver TH, Fried LF. et al. Serum bicarbonate concentrations and kidney disease progression in community-living elders: the Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis 2014; 64: 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kovesdy CP, Anderson JE. et al. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant 2008; 24: 1232–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goraya N, Simoni J, Jo CH. et al. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 2014; 86: 1031–1038 [DOI] [PubMed] [Google Scholar]
- 30. Hu MK, Witham MD, Soiza RL.. Oral bicarbonate therapy in non-haemodialysis dependent chronic kidney disease patients: a systematic review and meta-analysis of randomised controlled trials. JCM 2019; 8: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Navaneethan SD, Shao J, Buysse J. et al. Effects of treatment of metabolic acidosis in CKD: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2019; 14: 1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wesson DE, Mathur V, Tangri N. et al. Veverimer versus placebo in patients with metabolic acidosis associated with chronic kidney disease: a multicentre, randomised, double-blind, controlled, phase 3 trial. Lancet 2019; 393: 1417–1427 [DOI] [PubMed] [Google Scholar]
- 33. Weiner ID, Verlander JW.. Ammonia transporters and their role in acid-base balance. Physiol Rev 2017; 97: 465–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vaziri ND, Wong J, Pahl M. et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int 2013; 83: 308–315 [DOI] [PubMed] [Google Scholar]
- 35. Watford M. The urea cycle: teaching intermediary metabolism in a physiological setting. Biochem Mol Biol Educ 2003; 31: 289–297 [Google Scholar]
- 36. Bourgeois S, Bounoure L, Mouro-Chanteloup I. et al. The ammonia transporter RhCG modulates urinary acidification by interacting with the vacuolar proton-ATPases in renal intercalated cells. Kidney Int 2018; 93: 390–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.