Abstract
Multiple organ dysfunction syndrome (MODS) is one of the major causes of death and long-term impairment in critically ill patients. MODS is a complex, heterogeneous syndrome consisting of different phenotypes, which has limited the development of MODS-specific therapies and prognostic models. We used an unsupervised learning approach to derive novel phenotypes of MODS based on the type and severity of six individual organ dysfunctions. In a large, multi-center cohort of pediatric, young and middle-aged adults admitted to three different intensive care units, we uncovered and characterized three distinct data-driven phenotypes of MODS which were reproducible across age groups, where independently associated with outcomes and had unique predictors of in-hospital mortality.
Introduction
Multiple organ dysfunction syndrome (MODS) is one of the major causes of mortality and morbidity in critically ill patients.1 MODS is defined as a dysfunction of two or more major organ systems and can be developed after many types of insults, such as sepsis, major surgery, or trauma. MODS is a complex, heterogeneous syndrome consisting of different subgroups of patients with different phenotypes, which has limited the development of MODS-specific therapies and prognostic models. Following the principle of precision medicine, there is a need to characterize the phenotypes of MODS in order to develop better prognostic tools and targeted therapeutic strategies.2
Unsupervised machine learning methods are designed to uncover natural groupings in data and offer a compelling opportunity to uncover phenotypes using clinical data.2-4 The idea behind data-driven phenotyping is that patients who share similar clinical characteristics are likely to also share a similar underlying pathobiology.5,6 Uncovering these phenotype-specific biological disturbances could then lead to the development of targeted treatments and prognostic models for those phenotypes.7
In this study, we aimed to uncover and characterize clinically-meaningful phenotypes of MODS based on the patterns of organ dysfunction in the early phase of critical illness using a data- and knowledge-driven approach based on consensus clustering. Furthermore, we hypothesized that biologically-robust phenotypes of MODS would be preserved across different cohorts and age groups, so we used data from two cohorts of critically ill pediatric patients and a cohort of young and middle-age critically ill adults to test our hypothesis.
Related work
Consensus clustering has provided promising results in clinical studies aiming at developing data-driven strategies for precision medicine in the acute care setting. Seymour et al.8 used consensus clustering to derive clinical phenotypes of sepsis and identified four relevant phenotypes that demonstrated heterogeneity of treatment effects. Consensus clustering has also been used to identify subgroups for intensive care unit (ICU) patients that reflect patients’ shared needs and clinical trajectories.9 This method has also been extensively used in genomic studies, for example to identify subclones and determine cell types from single-cell RNA sequencing,10,11 or to derive gene expression–based colorectal cancer classifications and elucidate the intrinsic subtypes of the disease.12
Methods
Design
This was multi-center retrospective observational study using electronic health record (EHR) data. We measured the degree of organ dysfunction using the sequential organ failure assessment (SOFA) score in patients admitted to an adult ICU and the pediatric SOFA (pSOFA) score in patients admitted to two pediatric ICUs.1,13,14 The six organ system subscores (i.e. respiratory, coagulation, hepatic, cardiovascular, neurologic, and renal) of both SOFA and pSOFA are scaled from 0 (no dysfunction) to 4 (severe dysfunction). The pSOFA score is identical to the adult SOFA score, except it is age-adjusted for the renal and cardiovascular components and includes the SpO2/FiO2 ratio in the respiratory subscore to account for the lower availability of arterial blood gases in pediatric patients.13 The six subscores were measured in 24-hour periods for the first 72 hours of stay in all patients and the highest score achieved was used as the clinical feature for clustering. The 72-hour time window was chosen as a proxy for the early phase of critical illness and because a large portion of organ dysfunctions tend to peak within the first days after ICU admission.1,13
We included pediatric patients (<18 years old) as well as young and middle-aged adults (<55 years old) to test the hypothesis that biologically-robust phenotypes of MODS would be preserved across age groups. We excluded old adults to limit the influence of advance age-related comorbidities, chronic organ dysfunctions, and the high baseline mortality in that population. Information from pediatric ICU patients from two children’s hospitals in Chicago and adult ICU patients from a general hospital in Boston were used. Data from the pediatric ICUs was extracted from two local EHR databases for the years between 2010 and 2016 and the adult ICU data was extracted from the Medical Information Mart for Intensive Care III (MIMIC-III) database for the years between 2001 and 2012.14 Clinical data was extracted for the first 7 days of stay or until death or discharge from the ICU (whichever comes first) for the first ICU encounter that happened in each hospital admission. Additionally, demographic characteristics and outcome data were extracted.
Data processing
The subscores of the six organ systems were calculated based on the worst value for each variable in each 24-hour period. The variables included: PaO2/FiO2 ratio or SpO2/FiO2 ratio for the respiratory subscore, platelet count for the coagulation subscore, total bilirubin for the hepatic subscore, mean arterial pressure or catecholamine infusions for the cardiovascular subscore, Glasgow Coma Score (GCS) for the neurologic subscore, and creatinine for the renal subscore. For the renal criteria, we didn’t include the urine output since this data was considered unreliable in the EHR datasets. Missing values, such as absent laboratory test results or observations like a GCS, were assumed to be normal and a score of zero was assigned to those components, as it is common in organ dysfunction score studies.13,15,16 This follows the assumption that laboratory test that were not obtained or observations that are not recorded are deemed of low clinical importance by the clinicians taking care of the patients. Similarly, non-recorded interventions, such as absent mechanical ventilation parameters or vasoactive infusions were assumed to not have occurred. In the particular case of organ dysfunction scores, prior studies have demonstrated that performing multiple imputation as opposed to assuming normal values for missing values does not meaningfully change the results.15 MODS was defined as two or more organ systems with a SOFA/pSOFA subscore ≥2, which is similar to the criteria used in previous MODS studies and SOFA cutoffs used for denoting organ dysfunction and failure.17,18 We used the maximum values of each of the six subscores in the first 72 hours as features for consensus clustering.
Hierarchical and Consensus Clustering
Hierarchical clustering analysis (HCA) is a classic clustering algorithm.19 HCA uses a vertex adjacency matrix which contains N × N nodes as input with distance measures, such as Pearson correlation, where N represents the number of items being clustered, for example, patients in a clinical dataset. HCA has two main types of approaches: agglomerative approach (AA) and divisive approach (DA). AA is a bottom-up approach which first measures the distance of any pair of items (or patients in our example), makes every item a single cluster, then merges the two closet clusters and updates the distance matrix repeatedly until clusters meet pre-specified stop criteria. DA is a similar, but top-down approach. The time complexity of HCA is T(KN2) where K represents the number of clusters and N represents the number of items being clustered. Owing to this time-consuming feature, HCA works better on relatively smaller datasets.20 HCA has been successfully applied to ICU settings where it has been used to visualize high dimensional or complex multivariate data and identifies hidden physiologic patterns for critically ill patients,21 or identify novel phenotypes of asthma.22 However, HCA has some drawbacks, for example, this algorithm is heuristic and searches for a splitting or merging decision at each iteration which lead to the optimized clustering goals unclear and small changes in the data use can shift the splitting or clustering.23 Some of these limitations may be overcome by using HCA in a consensus clustering framework.24
Consensus clustering is an ensemble of clustering iterations with set of sub-sampled items (or patients in our case) in which the goal is to ascertain how often a pair of items are clustered together, which is consistent with a higher consensus and more robust clustering. The framework for consensus clustering is as follows: we are given a set of items D= {x1, x2, …, xN} and a set of clusters ={π1, π2, π3, …, πn}of the items in D.25 Each clustering is a mapping of items from D to {1, ..., nπi}, where nπi indicates the number of clusters in πi. The process utilizes an inner-loop clustering algorithm to partition sub-sampled items.25 In order to calculate the proportion of times that two items in the set D are assigned to the same clusters πi among all the times when both of them were sampled, a consensus value (CV) is generated for each pair of items. The CV ranges from 0-1, with smaller values indicating weaker consensus, and vice versa. If the CV of a pair approximates 1, this means both of the items in the pair are always assigned to the same clustering πi among all the times that they were both sampled.
In this study, we used a consensus agglomerative hierarchical clustering algorithm with Pearson correlation as the distance measure, with 100 iterations and 80% random sub-sampling to generate all the item pairings and determine the CV of each pair. The optimal number of clusters for each cohort was determined using both a combination of cluster size and characteristics of the cumulative distribution function (CDF) curve, as performed in previous studies.8,25 The range of clusters tested was 2 to 10.
To evaluate the reproducibility of the clusters, we run three independent consensus clusterings, one for each of the three sites (2 pediatric ICUs, 1 adult ICU), and compared the resulting clusters for similarities in the organ dysfunction patterns.
Phenotype Characterization and Validation
Clusters with similar patterns across the three cohorts were considered valid and were characterized as MODS phenotypes for further analysis. We used a clinical knowledge-driven, pragmatic, rule-based approach to characterize the phenotypes based on the maximum organ dysfunction of the most reproducible and characteristic organ systems across each similar cluster in the three cohorts.
To validate the robustness of the phenotypes across age groups, we merged the pediatric cohorts and compared them to the adult cohort in terms of relative demographic and clinical characteristics, associated laboratory test values, and outcomes (in-hospital mortality and persistent MODS on day 7).
Additionally, we tested whether phenotype membership was independently associated with in-hospital mortality after adjusting for confounders using logistic regression. Confounders included age, severity of illness on admission, history of cancer, and presence of multiple comorbidities, which were included in a multivariable regression model in which in-hospital mortality was the dependent variable and the phenotype membership and the confounders were the independent variables. Comorbidities were based on the Elixhauser Index for adult ICU patients and Feudtner’s classification in pediatric ICU patients.26,27 The severity of illness on admission was based on the Simplified Acute Physiology Score-II (SAPS II) score in adults and the Pediatric Risk of Mortality-III (PRISM III) score in pediatric patients.
Finally, to determine if the features associated with outcomes (in-hospital mortality and persistent MODS at 7 days) were different across phenotypes, we fitted two multivariable logistic regression models for each phenotype including type and severity of organ dysfunction, age, history of cancer and comorbidities as candidate predictors of outcome.
Statistical analysis
Data was managed and analyzed using PostgreSQL version 10.9 and R version 3.4.4 (R Foundation for Statistical Computing). Categorical variables were compared using chi-square test and multivariable analysis using logistic regression. The statistical significance threshold was set at less than .05 for 2-sided tests.
Results
There were 10,617 patients with MODS in the three cohorts: 3,180 in the first pediatric ICU cohort, 2,117 in the second pediatric ICU cohort, and 5,320 in the adult ICU cohort. Pediatric MODS patients had a mortality of 8.5%, and 22% had persistent MODS at day 7. Young and middle-aged adult MODS patients had a mortality of 14.9%, and 32.1% had persistent MODS at day 7.
After consensus clustering, the average consensus values (CV) were high in the three cohorts for the number of cluster selected based on the CDF and cluster size: Pediatric cohort 1 (4 clusters) = median CV was 0.974 (interquartile range [IQR]: 0.92- 0.975); pediatric cohort 2 (4 clusters) = 0.985 (0.984- 0.986); adult cohort (3 clusters) = 0.987 (0.987- 0.988). Consensus clustering uncovered three reproducible clusters across the three cohorts (Table 1). Based on the type and severity of organ dysfunction for each of these clusters, three phenotypes of MODS were clinically characterized using the following rules based on the SOFA/pSOFA subscores:
Table 1.
Characteristics of clusters by age group
| Critically Ill Children Cohort 1 | ||||
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
| Total, No. (%) | 2,162 (68) | 583 (18) | 203 (6) | 232 (7) |
| Max. pSOFA subscores, median (IQR) | ||||
| Respiratory | 3 (3-4) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| Coagulation | 0 (0-1) | 3 (2-4) | 1 (0-2) | 0 (0-0) |
| Hepatic | 0 (0-0) | 0 (0-2) | 0 (0-0) | 0 (0-2) |
| Cardiovascular | 1 (1-2) | 1 (1-3) | 1 (1-2) | 2 (1-3) |
| Neurologic | 3 (2-3) | 2 (1-2) | 2 (1-2) | 3 (2-3) |
| Renal | 0 (0-0) | 0 (0-0) | 4 (3-4) | 0 (0-0) |
| Critically Ill Children Cohort 2 | ||||
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
| Total, No. (%) | 1,811 (86) | 113 (5) | 133 (6) | 60 (3) |
| Max. pSOFA subscores, median (IQR) | ||||
| Respiratory | 3 (2-4) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| Coagulation | 0 (0-1) | 3 (2-3) | 0 (0-2) | 0 (0-0) |
| Hepatic | 0 (0-0) | 2 (0-2) | 0 (0-0) | 2 (2-3) |
| Cardiovascular | 1 (1-1) | 1 (1-3) | 1 (0-1) | 1 (1-1) |
| Neurologic | 4 (3-4) | 0 (0-1) | 1 (0-2) | 2 (1-4) |
| Renal | 0 (0-0) | 0 (0-0) | 3 (2-4) | 1 (0-2) |
| Critically Ill Young and Middle-Aged Adult Cohort | ||||
| Cluster 1 | Cluster 2 | Cluster 3 | - | |
| Total, No. (%) | 4,635 (87) | 361 (7) | 324 (6) | - |
| Max. SOFA subscores, median (IQR) | ||||
| Respiratory | 3 (2-3) | 0 (0-0) | 0 (0-0) | - |
| Coagulation | 1 (0-2) | 2 (2-3) | 2 (0-2) | - |
| Hepatic | 0 (0-1) | 2 (2-3) | 0 (0-2) | - |
| Cardiovascular | 1 (1-3) | 1 (1-3) | 1 (1-2) | - |
| Neurologic | 4 (3-4) | 0 (0-1) | 0 (0-1) | - |
| Renal | 0 (0-1) | 0 (0-1) | 4 (2-4) | - |
Abbreviations: SOFA, Sequential Organ Failure Assessment; pSOFA, pediatric Sequential Organ Failure Assessment; IQR, interquartile range.
If patients had maximum respiratory subscore of ≥3, a maximum neurologic subscore of ≥3, and maximum cardiovascular score of ≥1, then we classified those patients as Severe Hypoxemia and Shock Phenotype.
If patients had maximum coagulation subscore of ≥3, and maximum cardiovascular score of ≥1, then we classified those patients as Thrombocytopenia and Shock Phenotype.
If patients had maximum renal subscore of 4 and maximum cardiovascular score of ≥1, then we classified those patients as Renal Dysfunction and Shock Phenotype.
If patients didn’t meet any of the above criteria, then we classified those patients as Other MODS.
In total, 56.7% of pediatric MODS patients and 51.6% of adult MODS patients were classified into one of the three phenotypes. The mortality was higher in the patients who met criteria for one of these phenotypes compared to the Other MODS patients (12.5% vs. 3.3% in pediatric ICU patients and 20.4% vs. 9% in adult ICU patients, p<0.001). Table 2 presents the demographics, clinical characteristics, laboratory values, and outcomes of the three phenotypes stratified into pediatric vs. adult patients. In general, the three clusters had similar distribution, relative characteristics and outcomes across the two different age groups (Table 2 and Fig. 1).
Table 2.
Characteristics of the three phenotypes by age group
| Severe Hypoxemia and Shock Phenotype | Thrombocytopenia and Shock Phenotype | Renal Dysfunction and Shock Phenotype | ||||
| Pediatric | Adult | Pediatric | Adult | Pediatric | Adult | |
| Total, No. (%) | 2,335 (44) | 2,157 (41) | 536 (10) | 330 (6) | 131 (2) | 274 (5) |
| Male, No. (%) | 1,310 (56) | 1396 (64.7) | 301 (56) | 200 (60.6) | 92 (70) | 163 (59.5) |
| Age in years, median (IQR) | 2.5 (0.6-9.9) | 46.2 (38.2-51.2) | 11.3 (3.6-16.1) | 47.2 (40.4-51.3) | 10.3 (2.5-15.8) | 46.9 (39.3-50.7) |
| Race/ethnicity, No. (%) | ||||||
| White Non-Hispanic | 695 (29.8) | 1414 (65.6) | 215 (40.1) | 242 (73.3) | 43 (32.8) | 138 (50.4) |
| Black Non-Hispanic | 892 (38.2) | 188 (8.7) | 70 (13.1) | 19 (5.8) | 46 (35.1) | 94 (34.3) |
| Hispanic | 520 (22.3) | 125 (5.8) | 194 (36.2) | 16 (4.9) | 31 (23.6) | 21 (7.6) |
| Other | 228 (9.8) | 430 (20) | 57 (10.6) | 53 (16) | 11 (8.4) | 21 (7.7) |
| Comorbidity, No. (%) | ||||||
| Chronic Cardiovascular | 652 (27.9) | 853 (39.6) | 183 (34.1) | 95 (28.8) | 47 (35.9) | 144 (52.6) |
| Chronic Respiratory | 502 (21.5) | 390 (18.1) | 54 (10.1) | 33 (10) | 11 (8.4) | 37 (13.5) |
| Chronic Renal | 73 (3.1) | 179 (8.3) | 20 (3.7) | 45 (13.6) | 131 (100) | 213 (77.7) |
| Cancer | 182 (7.8) | 106 (4.9) | 246 (45.9) | 47 (14.2) | 12 (9.2) | 8 (2.9) |
| Multiple Comorbidities | 1,296 (55.5) | 523 (24.3) | 383 (71.5) | 114 (34.6) | 108 (82.4) | 165 (60.2) |
| Infection, No. (%) | 1,303 (55.8) | 1102 (51.1) | 383 (71.5) | 222 (67.3) | 65 (50) | 134 (48.9) |
| Max. SOFA/pSOFA subscores in the first 72h, median (IQR) | ||||||
| Respiratory | 3 (3-4) | 3 (3-4) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-1) |
| Coagulation | 0 (0-0) | 1 (0-2) | 3 (3-3) | 3 (3-3) | 0 (0-0) | 0 (0-2) |
| Hepatic | 0 (0-0) | 0 (0-1) | 0 (0-1) | 2 (1-3) | 0 (0-0) | 0 (0-0) |
| Cardiovascular | 1 (1-3) | 1 (1-4) | 1 (1-3) | 1 (1-1) | 1 (1-2) | 1 (1-3) |
| Neurologic | 3 (3-4) | 4 (3-4) | 1 (0-2) | 2 (1-3) | 1 (0-2) | 2 (1-3) |
| Renal | 0 (0-1) | 0 (0-1) | 1 (0-2) | 1 (0-2) | 4 (4-4) | 4 (4-4) |
| Laboratory test results in the first 72h, median (IQR) | ||||||
| Max. WBC, K/uL | 13.5 (9.4-18.9) | 15.9 (11.4-21.3) | 5.9 (0.9-12.9) | 8.3 (4.7-13.3) | 13.2 (9.8-18.1) | 13.5 (9.5-18.3) |
| Min. Lymphocytes, K/ | uL 1.2 (0.6-2) | 1 (0.5-1.6) | 0.3 (0.1-0.8) | 0.6 (0.3-1) | 0.9 (0.5-1.9) | 1 (0.5-1.3) |
| Min. Hemoglobin, g/d | L 8.8 (7.3-10.4) | 9.2 (8-10.8) | 7.5 (6.5-8.7) | 8.2 (7.1-9.2) | 8.3 (7.5-9.9) | 8.9 (8-10.2) |
| Max. INR | 1.4 (1.2-1.9) | 1.4 (1.2-1.9) | 1.5 (1.2-2) | 1.8 (1.4-2.5) | 1.2 (1-1.3) | 1.5 (1.2-2.1) |
| Max. PTT, sec. | 39 (32-53) | 36 (29-57) | 41 (32-57) | 42 (33-57) | 27 (32-44) | 36 (31-57) |
| Max. BUN, mg/dL | 13 (9-20) | 21 (14-34) | 15 (10-24) | 29 (17-54) | 59 (46-78) | 65 (47-86) |
| Max. ALT, U/L | 31 (17-95) | 46 (25-120) | 46 (23-114) | 53 (29-99) | 28 (15-148) | 40 (22-92) |
| Max. Lactate, mmol/L | 2.2 (1.3-4.5) | 3.1 (1.9-5.5) | 2 (1.2-3.6) | 3.1 (1.9-5.2) | 1.5 (1.1-2.8) | 2.2 (1.5-3.3) |
| Hospital LOS in days, median (IQR) | 13 (6-24) | 13 (7-23) | 10 (5-18) | 10 (6-22) | 10 (7-17) | 9 (6-16) |
| In-hospital mortality, No. (%) | 315 (13.5) | 443 (20.5) | 56 (10.4) | 87 (26.4) | 3 (2.3) | 32 (11.7) |
| MODS on day7, No. (%) | 1,025 (44) | 1,144 (53) | 139 (25.9) | 67 (20.3) | 19 (10.2) | 45 (16.4) |
Abbreviations: SOFA, Sequential Organ Failure Assessment; pSOFA, pediatric Sequential Organ Failure Assessment; IQR, interquartile range; WBC, White blood cells; INR, International Normalized Ratio; PTT, partial thromboplastin time; BUN, blood urea nitrogen; ALT, Alanine aminotransferase; LOS, length of stay; MODS, multiple organ dysfunction syndrome.
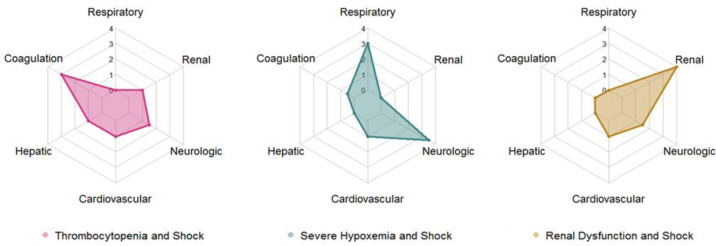
Figure 1.
Radar charts of the three MODS phenotypes based on the maximum organ dysfunction subscores achieved in the first 72 hours of critical illness
The Severe Hypoxemia and Shock Phenotype had the highest proportion of patients, occurred in a relatively younger subset in each age group and these patients had a lower rate of multiple comorbidities. Patients in this phenotype had higher white blood cell count and lactate levels in the first 72 hours when compared with the other two phenotypes. Finally, patients in this phenotype had high in-hospital mortality, the highest rate of persistent MODS at 7 days, and the longest hospital lengths of stay.
The Thrombocytopenia and Shock Phenotype had the second highest proportion of patients and occurred in a relatively older subset of patients in each age group, who also had a higher rate of cancer diagnosis and suspected or confirmed infections. Interestingly, Black Non-Hispanic patients were underrepresented in this phenotype in both younger and older patients. Patients in this phenotype had higher rates of lymphopenia, relative anemia, and mild-to-moderate hepatobiliary dysfunction depending on the age group compared to the other phenotypes. Finally, patients in this phenotype had similar in-hospital mortality but lower rates of persistent MODS at 7 days and shorter length of stay than the Severe Hypoxemia and Shock Phenotype.
The Renal Dysfunction and Shock Phenotype had the lowest incidence and it appears to represent an acute worsening of chronic comorbidities, as most of these patients have pre-existing kidney and cardiovascular disease. These patients had the lowest in-hospital mortality and lowest rates of persistent MODS at 7 days of the three phenotypes.
After adjusting for age, severity of illness on admission, the presence of cancer, and multiple comorbidities, adult ICU patients with the Severe Hypoxemia and Shock Phenotype and the Thrombocytopenia and Shock Phenotype had higher odds of in-hospital mortality compared to the Other MODS patients (adjusted odds ratios [aOR] of 1.5 and 2, respectively, both p <0.001). Adult ICU patients with the Renal Dysfunction and Shock Phenotype had similar in- hospital mortality as Other MODS patients after adjusting for confounders (p=0.07). After adjusting for the same confounders, pediatric ICU patients with the Severe Hypoxemia and Shock Phenotype had higher odds of in-hospital mortality compared to the Other MODS patients (aOR of 1.6, p = 0.002). PICU patients with the Thrombocytopenia and Shock Phenotype and the Renal Dysfunction and Shock Phenotype had similar in-hospital mortality as Other MODS patients after adjusting for confounders.
Table 3 presents the results of the multivariable analysis of predictors of in-hospital mortality for the two highest risk MODS phenotypes, the Severe Hypoxemia and Shock Phenotype and the Thrombocytopenia and Shock Phenotype. Due to the lower mortality rate and risk of overfitting, patients with the Renal Dysfunction and Shock Phenotype were not included in this analysis. For the Severe Hypoxemia and Shock Phenotype, in both adult and pediatric ICU patients the predictors of in-hospital mortality were: the severity of the respiratory, cardiovascular and neurologic dysfunction, and the presence of cancer comorbidity. Additionally, in adult ICU patients the severity of hepatic dysfunction was also a predictor of mortality. For the Thrombocytopenia and Shock Phenotype, in both adult and pediatric ICU patients the predictors of in-hospital mortality were: presence of cancer comorbidity and the severity of the hepatic dysfunction.
Table 3.
Multivariable analysis of predictors of in-hospital mortality in the two high-risk phenotypes by age group
| Severe Hypoxemia and Shock Phenotype, aOR (95% CI) | Thrombocytopenia and Shock Phenotype, aOR (95% CI) | |||
| Pediatric | Adult | Pediatric | Adult | |
| Age, years | 1 (1-1) | 1 (0.98-1) | 1 (1-1) | 0.98 (0.9-1) |
| Cancer | 2.4 (1.5-3.8) | 5.9 (3.3-10.5) | 2.8 (1.4-5.6) (1.3-8.3) | 3.3 (1.3-8.3) |
| Multiple Comorbidities | 1.4 (1-1.8) | 0.7 (0.5-1) | 0.9 (0.5-2.1) | 0.9 (0.4-1.9) |
| Max. SOFA/pSOFA subscores in the first 72h | ||||
| Respiratory | 2.3 (1.6-3.5) | 2.1 (1.6-2.9) | 1.6 (1.2-2) | 1.3 (0.9-1.8) |
| Coagulation | 1.1 (1-1.3) | 1.2 (1-1.3) | 1.2 (0.6-2.3) | 2 (0.8-4.8) |
| Hepatic | 0.9 (0.8-1) | 1.5 (1.3-1.7) | 1.4 (1.1-1.8) | 1.5 (1.2-1.9) |
| Cardiovascular | 2.4 (2.1-2.7) | 1.3 (1.2-1.4) | 1.1 (0.8-1.5) | 1.3 (1-1.8) |
| Neurologic | 2.1 (1.5-2.9) | 2 (1.4-2.9) | 1.7 (1.3-2.2) | 1.3 (1-1.6) |
| Renal | 1.1 (1-1.3) | 1.1 (1-1.3) | 1.6 (1.2-2.1) | 1.3 (1-1.6) |
Abbreviations: aOR, adjusted odds ratios; CI, confidence interval; SOFA, Sequential Organ Failure Assessment; pSOFA, pediatric Sequential Organ Failure Assessment. Results in bold denote statistically significant associations.
Additionally, in pediatric ICU patients the severity of respiratory, neurologic, and renal dysfunction were also predictors of mortality.
Discussion
We identified three distinct, reproducible phenotypes of MODS in a large cohort of pediatric patients and young and middle-age adults using consensus clustering. Patients within each phenotypes shared clinical characteristics and outcomes across age groups and each phenotype had distinct predictors of mortality associated. Better understanding and characterizing these phenotypes could help us develop precision medicine strategies for the management of MODS in pediatric and adult ICU patients.2
Prior adult studies using unsupervised learning approaches in patients with sepsis and sepsis-associated MODS have characterized similar phenotypes. Both Knox et al.6 and Seymour et al.8 described four phenotypes of sepsis, and in both cases those phenotypes included a phenotype with predominant hypoxemia and shock, a coagulation and hepatobiliary dysfunction phenotype, and a renal dysfunction phenotype. To our knowledge, this is the first study to demonstrate these phenotypes in all MODS patients and in both pediatric and adult critically ill patients.
There are several limitations in our study. First, this was a retrospective study based on EHR data, so only data obtained during routine clinical care was available. Second, we only included data on organ dysfunction during the first 72 hours and used a pragmatic approach to define the rules for phenotyping, which can potentially introduce bias. Third, we only used data from one adult cohort and two pediatric cohorts. Larger, multi-center studies are needed to confirm our results and determine the clinical utility of this phenotype classification schema.
Conclusion
MODS is one of the major causes of mortality in critically ill patients, and is a complex, heterogeneous syndrome consisting of different phenotypes. We used consensus clustering to derive three novel data- and knowledge-driven phenotypes of MODS in a large, multi-center cohort of pediatric, young and middle-aged adults admitted to three different ICUs. These phenotypes shared similar clinical characteristics, outcomes and predictors of mortality across age groups. Further validation in large, multi-center studies is needed to confirm our results.
Acknowledgements
The authors thank Neethi Pinto, MD of the University of Chicago for her support of this project, we also thank Jiahong Shen of Northwestern University for providing supercomputing platform for the data analysis. This research is partly supported by NIH/NICHD (R21HD096402, Sanchez-Pinto). *Corresponding author.
Figures & Table
References
- 1.Vincent J-L, Moreno R, Takala J, et al. Springer-Verlag; 1996. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. [DOI] [PubMed] [Google Scholar]
- 2.Seymour CW, Gomez H, Chang C-CH, et al. Precision medicine for all? Challenges and opportunities for a precision medicine approach to critical illness. Critical Care. 2017;21(1):257. doi: 10.1186/s13054-017-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchez-Pinto LN, Luo Y, Churpek MM. Big data and data science in critical care. Chest. 2018;154(5):1239–1248. doi: 10.1016/j.chest.2018.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye J. The Role of Health Technology and Informatics in a Global Public Health Emergency: Practices and Implications From the COVID-19 Pandemic. JMIR medical informatics. 2020;8(7):e19866. doi: 10.2196/19866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayhew MB, Petersen BK, Sales AP, Greene JD, Liu VX, Wasson TS. Flexible, cluster-based analysis of the electronic medical record of sepsis with composite mixture models. Journal of biomedical informatics. 2018;78:33–42. doi: 10.1016/j.jbi.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knox DB, Lanspa MJ, Kuttler KG, Brewer SC, Brown SM. Phenotypic clusters within sepsis-associated multiple organ dysfunction syndrome. Intensive care medicine. 2015;41(5):814–822. doi: 10.1007/s00134-015-3764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Sherwood ER. Getting sepsis therapy right. Science. 2015;347(6227):1201–1202. doi: 10.1126/science.aaa8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. Jama. 2019;321(20):2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vranas KC, Jopling JK, Sweeney TE, et al. Identifying Distinct Subgroups of Intensive Care Unit Patients: a Machine Learning Approach. Critical care medicine. 2017;45(10):1607 doi: 10.1097/CCM.0000000000002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grün D, Lyubimova A, Kester L, et al. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature. 2015;525(7568):251–255. doi: 10.1038/nature14966. [DOI] [PubMed] [Google Scholar]
- 11.Kiselev VY, Kirschner K, Schaub MT, et al. SC3: consensus clustering of single-cell RNA-seq data. Nature methods. 2017;14(5):483–486. doi: 10.1038/nmeth.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nature medicine. 2015;21(11):1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA pediatrics. 2017;171(10):e172352–e172352. doi: 10.1001/jamapediatrics.2017.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Scientific data. 2016;3(1):1–9. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. Jama. 2017;317(3):290–300. doi: 10.1001/jama.2016.20328. [DOI] [PubMed] [Google Scholar]
- 16.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) Jama. 2016;315(8):762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno R, Vincent J-L, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Intensive care medicine. 1999;25(7):686–696. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 18.Typpo K, Watson RS, Bennett TD, Farris RW, Spaeder MC, Petersen NJ. Outcomes of Day 1 Multiple Organ Dysfunction Syndrome in the PICU. Pediatric Critical Care Medicine. 2019;20(10):914–922. doi: 10.1097/PCC.0000000000002044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akman O, Comar T, Hrozencik D, Gonzales J. Algebraic and Combinatorial Computational Biology. Elsevier; 2019. Data Clustering and Self-Organizing Maps in Biology; pp. 351–374. [Google Scholar]
- 20.Rao C, Gudivada VN. Computational Analysis and Understanding of Natural Languages: Principles, Methods and Applications. Elsevier; 2018. [Google Scholar]
- 21.Cohen MJ, Grossman AD, Morabito D, Knudson MM, Butte AJ, Manley GT. Identification of complex metabolic states in critically injured patients using bioinformatic cluster analysis. Critical Care. 2010;14(1):R10. doi: 10.1186/cc8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. American journal of respiratory and critical care medicine. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J, Kamber M, Pei J. Data mining concepts and techniques third edition. The Morgan Kaufmann Series in Data Management Systems. 2011. pp. 83–124.
- 24.Monti S, Tamayo P, Mesirov J, Golub T. Consensus clustering: a resampling-based method for class discovery and visualization of gene expression microarray data. Machine learning. 2003;52(1-2):91-118 [Google Scholar]
- 25.Wilkerson MD, Hayes DN. ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics. 2010;26(12):1572–1573. doi: 10.1093/bioinformatics/btq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 27.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106(Supplement 1):205–209. [PubMed] [Google Scholar]