Abstract
Current treatments for major depressive disorder are either less effective for older adults (i.e. pharmacotherapy) or are challenging to extend to community settings (i.e. psychotherapy). To improve and extend mental health treatment for older adults, our team has expanded a previously developed streamlined talk-therapy model to incorporate a technology package that includes patient-reported outcome questions (sent via SMS) and a smartwatch. The goal of this pilot study was to assess and improve the usability, usefulness, and acceptability of the technology package. We completed a pilot feasibility and usability assessment with 15 older adults. Participants demonstrated the feasibility of use of the intervention, successfully completing 99% of their assigned tasks during the pilot. Findings were used to address usability barriers in preparation for future clinical trials. Our results highlight the importance completing usability assessment and involving older adults in the intervention design process when incorporating technology into care.
Introduction
The population of older adults (those over the age of 65), is growing more rapidly than any other age group in the world. The population of the “oldest old,” those over the age 85, is expected to grow 351% by 2050.1 With aging populations also comes increased incidence of chronic conditions, such as arthritis, diabetes, hypertension, and heart disease.2 The leading cause of disability in older adults, however, is major depressive disorder (MDD).3 MDD advances brain and epigenetic age, and increases morbidity and mortality.4 MDD has also been linked to an increased risk of frailty, diabetes, stroke, cognitive impairment, cardiac diseases, and arterial disease, not to mention related suffering and increased cost of care.5-10
Despite increased medical need and treatment among older adults, late-life depression (LLD) is undertreated.11,12 As with other chronic conditions, disparities in the incidence and treatment of LLD exist based on gender, race, and ethnicity,13,14 which is problematic because the older adult population is projected to become increasingly racially and ethnically diverse over time.1 Pharmacotherapy for MDD has significantly poorer effectiveness for older adults than younger adults and has been demonstrated to help less than half of LDD cases.15,16 Previous studies have also demonstrated older adults encounter increased challenges in adhering to pharmacotherapy regimens based on cognitive limitations (e.g. learning, working memory) and structural challenges (e.g. obtaining their medications from the pharmacy).17,18 Older adults also have a greater risk of depression relapse, although this risk can be mitigated with pharmacotherapy and psychotherapy.19,20 Psychotherapy, specifically problem-solving therapy, has demonstrated efficacy in improving symptoms of LDD and reducing functional disability.21,22 However, psychotherapies are complex, and many community-based therapists (e.g. social workers, care managers) are not qualified to deliver these treatments, limiting the scalability of psychotherapy in its current form.23
The center grant (ALACRITY) associated with this work focuses on streamlining and simplifying LDD treatments and improving their scalability for community-based settings.24 In earlier work, the team developed a streamlined, stepped therapy grounded in neurobiological constructs but that focuses on simple, efficacious behavioral strategies to enhance scalability of treatments that can be effectively delivered by community-based therapists (Engage). The streamlined therapy has been effectively taught to community-based therapists and has been demonstrated to be non- inferior to the gold standard of psychotherapy for LLD, problem-solving therapy.25,26
A recent review on the treatment of LLD highlighted key areas for future research, including: building patient- centered, culturally sensitive treatments; determining if/how to involve technology in LLD treatment; involving community health workers; and developing treatment models scalable across settings, particularly those situated in the community.13 The ALACRITY Center has addressed each of the aforementioned aims by devising Engage, a community care model embedded in senior centers.25,26 In addition to talk therapy, the team has expanded Engage to include a technology-facilitated platform (Engage-M). Engage-M incorporates patient-reported outcomes collected via text message with a commercial smartwatch and a mobile phone-based activity tracking application. The design was based on a previous concept that increased activity among older adult patients with ischemic heart disease.27 The intervention can be used in conjunction with counseling sessions with a licensed clinical social worker in order to improve access to treatment by allowing social workers to treat more patients, increase number of touch-points with the patient, and facilitate patient engagement.
Given the known barriers to use of health information technology by older adults,28 we considered it essential to assess and improve usability and feasibility of the technology-facilitated intervention package before moving ahead to assess the efficacy of the intervention on treating LLD. Constructs such as usability and usefulness have been demonstrated to predict eventual use of a technology.29 Commercial technologies are also not typically optimized for older adults.30 In addition, persons in underserved groups (e.g. older adults, racial minorities, ethnic minorities) who would benefit most from community-based care, struggle most in overcoming issues related to usability.30 Therefore, an initial usability study of the technological intervention was a critical step prior to clinical assessment. Here, we report our findings of a usability and one-week pilot feasibility study, in which community-dwelling older adults used the Engage-M platform in their daily lives. Our goal was to assess and improve the perceived usability, usefulness, and acceptability of a text-message and smartwatch-based intervention for older adults. In addition, our findings have implications for future mHealth and wearable based interventions for older adults.
Methods
Intervention description
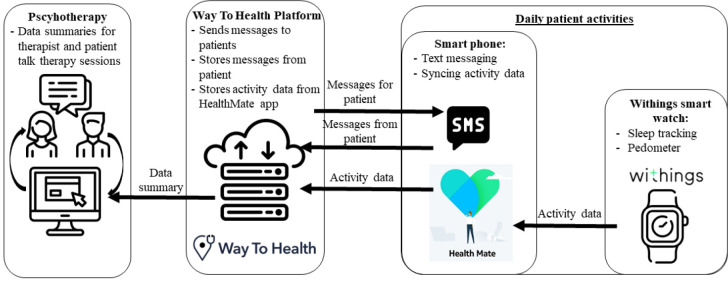
The Engage-M intervention involves patients reporting outcomes twice a day via text message and tracking their activities using a smartwatch (Withings Steel HR; https://www.withings.com/us/en/steel-hr). See Figure 1. The technology platform we partnered with, Way To Health, facilitates sending and receiving text messages and receiving patient activity data. Way To Health is a technology platform that facilitates communication with patients through SMS-messaging and integration with wearable devices to store activity data.31 Their system uses branching logic to have adapted, individually tailored exchanges with patients. For Engage-M, Way To Health sent four SMS questions in the morning, and one to three questions in the evening, with automated prompts if participants did not respond or sent erroneous responses (e.g. answering with letters to a question requiring numerical input). The platform stored patient responses and step and sleep data. The data from patient messages was populated into a clinician-facing interface that allowed community therapists to review how the patient was feeling during the week and incorporate this information into the patient’s therapy.
Figure 1.
Format and structure of Engage-M technology package, and how it can be used to facilitate psychotherapy sessions.
In addition, Way To Health connects with HealthMate, the mobile phone application that interfaces with the smartwatch activity tracker. Through HealthMate, the patient’s activity data, in this case sleep and step data, is populated in the therapist’s interface in Way To Health.
Similar to previous interventions developed by our team, the patient-facing portion of the technological intervention was based on behavioral economics-based reward systems aimed to increase adherence to treatment and monitoring procedures.25 In this case, patients can move between levels (e.g. bronze, silver, gold) based on completing activities related to their mental health and well-being in a gamified manner.27 In other words, patients would work with their therapist to select an enjoyable activity (such as walking, telephoning a friend, or going to a movie), and then receive points if they complete it each week. In an upcoming clinical trial, patients will have the opportunity to receive cash prizes for making it to the highest level.
Study design and Sample
We assessed the usability, usefulness, and acceptability of the Engage-M intervention using semi-structured interviews, questionnaires, and system use data. All participants were recruited from a single senior center in the Northeastern United States. Senior center members were eligible for the study if they were over 49 years old, had the ability to speak and read English, and if they owned a smartphone. Those meeting inclusion criteria and agreeing to participate first completed an initial interview where they received an introduction to the intervention and provided feedback. Because time constraints prohibited purposive sampling for MDD, all participants instead completed a PHQ-9 and were asked if they had experience with talk therapy (see Table 2). Participants then participated in the intervention for a one-week trial period. Following the trial period, participants completed a follow-up interview in which they discussed their perceptions of the intervention. This study was approved by the Weill Cornell Medicine Institutional Review Board, and participants provided written informed consent.
Table 2.
Summary of study participant characteristics.
| N | % | ||
| Gender | |||
| Female | 8 | 53.3 | |
| Male | 7 | 46.6 | |
| Age* | |||
| 50-59 | 1 | 7.1 | |
| 60-69 | 10 | 71.4 | |
| 70-79 | 2 | 14.2 | |
| 80+ | 1 | 7.1 | |
| Race | |||
| White | 2 | 13.3 | |
| Black | 1 | 6.7 | |
| Asian | 11 | 73.3 | |
| Other | 1 | 6.7 | |
| Ethnicity | |||
| Not Hispanic | 14 | 93.3 | |
| Hispanic | 1 | 6.7 | |
| Education | |||
| High school (no degree) | 1 | 6.7 | |
| High school degree | 5 | 33.3 | |
| Some college or | 5 | 33.3 | |
| Associate’s degree | |||
| Bachelor’s degree | 4 | 26.7 | |
| Experience with | |||
| talk therapy | |||
| Yes | 2 | 13.3 | |
| No | 13 | 86.7 | |
| PHQ-9 ca | tegory | ||
| None-minimal | 8 | 53.3 | |
| Mild | 5 | 33.3 | |
| Moderate** | 2 | 13.3 | |
| Moderately severe | 0 | 0 | |
| Severe | 0 | 0 | |
N = 14, one participant elected not to answer
Treatment, consideration of counseling or pharmacotherapy recommended
Data collection
Our team included experts in usability assessment, user-centered design, and clinical psychology and psychiatry. Three research assistants and a PhD-level user-centered design researcher (NCB) completed in-person data collection (i.e. interviews). All of the research assistants had previous experience in a community-based setting completing interviews with older adults. Prior to data collection, the team of research assistants underwent a series of hands-on, immersive training sessions with the PhD-level researcher who designed the study.
Initial interview:
During the initial interview, participants first received an overview of the intervention, provided informed consent (as applicable), and were enrolled in the pilot intervention. As noted in the intervention description, the intervention is based on reward exposure linked to a daily planned activity. For the purposes of the usability study, we had participants imagine that their planned activity was to go for a walk although we made it clear to them it was their choice about whether or not they did this.
Participants then completed a cognitive walkthrough where they received each of the text messages included in the intervention and were asked to “think-aloud” regarding how they thought they should respond to each message. Cognitive walkthroughs are an established usability assessment technique in which users verbalize their thought process or “think-aloud” which can help identify design components that are unintuitive or challenging to understand.32 During the cognitive walkthrough, study team members also asked participants structured questions to assess comprehension of the messages (e.g. “how do you think you should respond to this message if at all?”). Following the cognitive walkthrough portion of the interview, participants completed a series of questionnaires, including:
- Unified Theory of Technology Acceptance , and Use of Technology (UTAUT2) assessment adapted for assessment of healthcare wearable devices and mobile health technology29,33
- Demographics survey
- PHQ-9 – a validated instrument for assessing major depressive disorders34,35
Study team members then completed the technology setup, gave participants their study provided smartwatch, and gave a brief tutorial for using the smartwatch and mobile health application. The study team member also scheduled a time with the participant for the follow-up interview. Prior to conclusion of the study, participants had an opportunity to ask questions and resolve any doubts. Lastly, the study team member utilized teach-back to ensure the participants could repeat their study tasks in their own words.36
One-week pilot period:
Participants were asked to use the smartwatch, respond to morning mood and evening activity questions, and sync their smartwatch data with their mobile device by opening the HealthMate application once per day. Throughout the pilot period, the W2H system stored data related to participant responses to text messages and whether the participant synced their watch with the mobile device application.
Follow-up interview:
After participants completed the one-week pilot, they met in-person with a study team member for a follow-up interview. Participants provided feedback related to their experience using the smartwatch and responding to study messaging. The study team members also discussed perceived helpfulness of the intervention, willingness to utilize similar interventions, and willingness to recommend the intervention to a fried/family member to support emotional health and quality of life. At the conclusion of the interview, the participants completed the UTAUT2 a second time.
Rapid data analysis and iterative intervention re-design
Table 1 provides a summary of what study phase data was collected in, brief description of the data, and an analysis summary related to how results are presented.
Table 1.
Description of the data collected in each study phase including an analysis summary.
| Collection phase | Description | Analysis summary |
| Demographics PHQ-9 |
Descriptive statistics Descriptive statistics |
|
| Initial | UTAUT2 | Not presented here |
| interview | Audio recording of cognitive walkthrough | - Transcribed by professional service - “Think-aloud” feedback used to summarize common errors and issues |
| Intervention | Morning mood question responses Evening activity questions responses |
Descriptive statistics of completion, errant responses Descriptive statistics of completion, errant responses |
| pilot | Steps activity | Descriptive statistics of sync completion |
| Sleep activity | Descriptive statistics of sync completion | |
| Follow-up | UTAUT2 Audio recording regarding |
Not presented here - Transcribed by professional service |
| interview | intervention feedback | - Transcripts iteratively reviewed to identify potential areas for improvement of the intervention |
A single investigator from our team (NCB), who has extensive experience conducting and analyzing data from usability studies, completed the aggregation of descriptive statistics and review of the interview transcripts. Data collection, analysis, and intervention re-design occurred in a highly abbreviated time-frame to meet a 6-week deadline for launching the subsequent RCT study.37 Rapid development approaches have become increasingly in the development of modern technologies and have demonstrated success in creating user-oriented products.38 Given the rapid cycle development process, a formal, inductive qualitative analysis was not appropriate. Review of the both the initial and follow up interview transcripts focused pragmatically on common problems and areas improvement of the intervention. The initial interview transcripts were iteratively reviewed to determine common sources of error or confusion (Table 3). Review of the final interview transcripts elicited common barriers to use of the technology package and recommendations for improvement.
Table 3.
Summary of common errors and confusion related to study messages in initial interview.
| Message Group | Response type | Common errors and confusion |
| Study initiation – description of reward plan |
None | - Did not understand how to gain or lose points - Confusion between “points” (e.g. 100) and levels (e.g. bronze, silver) - Thought they needed to respond to the message - Text very long, required scrolling, especially for those with larger font settings |
| Morning mood questions | Numeric (1-10) | - Flip-flopped scale orientation - Wanted clarification for pain question (i.e. physical vs. mental) |
| Evening activity questions | Numeric (1-10) | - Did not understand the word “accomplishment” |
| Evening activity questions | Yes/No | - Variations of yes/no (e.g. “Y,” “N,” “yes, of course”) - Wanted to include information about their planned activity (e.g. “I walked”) |
| Weekly reward plan updates |
None | - Thought they needed to respond to the message |
| Response reminder | Numeric (1-10), or Yes/No) |
- Wanted to respond affirmatively (e.g. “okay”) instead of answering most recent question - Confused about which question to respond to |
| Error message | Numeric (1-10), or Yes/No) |
- Confused by words “value” and “numeric” |
The information was summarized and reviewed with another team member with expertise in usability evaluation (JSA). The summaries were used to create initial recommendations for updates to the intervention and study conduct for an upcoming RCT. The results were then reviewed with all study investigators to come to a consensus on what updates would ultimately be made to the intervention and study methods (summarized in the Discussion).
Results
Characteristics of Study Participants
A total of 15 participants met inclusion criteria, consented to participate, and completed the study. Two other participants dropped out during the initial interview due to English language and technology barriers. Table 2 presents the characteristics of the convenience sample of study participants obtained from recruitment in a single community senior center.
Initial Interviews
Table 3 summarize participants’ comprehension of the various groups of messages included in the study. The most concerning error pertained to participants who indicated in their think aloud response that they had flipped the orientation of the scale for mood and activity questions. For example, they said they would respond a 10 (“most sad”) to the question about sadness then describe that they responded this number because they felt very happy. The errors that involved responding an out-of-range value (e.g. not “Yes”/”No”, or a number 1-10) were less concerning as the Way To Health platform can trigger an automated error message to prompt the user to enter a valid response.
One common theme was that participants believed they needed to respond to questions that were not designed to require a response (e.g., the welcome message at study initiation, or weekly updates about participant status). Participants also struggled with answering questions about their planned activity and how their planned activity related to their reward plan. We believe that this challenge was because the planned activity was hypothetical. This is of less concern for the future RCT, in which participants will have tailored activities planned with their therapist and documented for them in a physical calendar.
Intervention Pilot
Participants were generally very successful in responding to study messages and syncing their activity data each day. Participants completed 99.0% (104/105) of the morning mood questions and 100.0% (105/105) of the evening activity questions. During the follow-up interview, the participant who missed one morning of mood question sets noted that they were unable to respond because they underwent a surgical procedure that morning. Throughout the pilot, participants sent a total of 6 invalid responses, 3 in response to numeric questions (i.e. 1-10) and 3 in responses to Yes/No questions. The invalid numeric response included two instances of entering the letter “O”, presumably instead of the number “0”, and one instance of entering “0 not sad.” The invalid responses to the Yes/No questions included “Yo”, “Y Es”, and “No” followed by an explanation of why they did not complete the activity. Importantly, in all 6 instances, participants received an error message and were able to send a valid response in their next message. In addition to the invalid responses, participants also responded to messages where a response was not required in 114 instances, equivalent to more than once per participant per day. The vast majority of these messages (94/114; 82.4%) involved some variation of “okay” and “thank you.”
Participants successfully synced their step and sleep activity data 100.0% (105/105) and 95.2% (100/105) days of the pilot intervention, respectively. The participant that noted that they underwent a surgical procedure during the pilot accounted for 60% (3/5) of the days where participants had missing sleep data.
Follow-up Interviews
Our assessment of participant feedback focused on issues and recommendations for improvement that could be adjusted in an upcoming RCT. One major concern was that messages were too repetitive. Participants also wanted additional follow-up or to know that a human was reading their responses, which will presumably be resolved in future studies where the intervention is used in ongoing therapy. Multiple participants also noted that they would have liked additional training related to how to use the HealthMate app and understand their activity data.
Other issues noted were outside of elements we could control as investigators using commercially available technologies. Some participants noted issues with the watch design, specifically that the viewfinder window which showed activity and phone notifications and the analog clock were too small and hard to see. Others noted that, at times, the data collected in the HealthMate application seemed inaccurate.
Overall, the participants described the intervention as acceptable and easy to use. Many participants described that the intervention helped them become more self-aware about their mood and activity.
Final intervention updates based on study results
Our team utilized the results of the usability study to collaboratively update the Engage-M intervention and the study materials for an upcoming RCT. Changes to the intervention focused on the text messaging as the other study components, the Withings Smartwatch and HealthMate mobile application, involve commercially available technologies that we had little ability to alter.
Morning mood questions, evening activity questions:
We placed the description of the anchors of the scale (e.g. not sad, most sad) immediately following the first mention (example below), for each of the messages. Our rationale for this change was to avoid comprehension errors about the orientation of the scale for questions that required numeric answers. We anticipate that with the initial design the participants may have just seen 0 to 10 and made their own assumption about the directionality without fully reading the message.
| Initial message (example) | Updated message (example) |
| “On a scale of 0 to 10 how sad do you feel today (0=not sad, 10=most sad)? Please type a number. |
“How sad do you feel today? Please type a number from 0 (not sad) to 10 (most sad).” |
We also found the that usability study was critical in detecting misunderstanding in answering mood and activity questions, so we have also added a segment to the RCT study initiation session where patients will think aloud about how they should respond to the messages to similarly catch potential issues.
Based on participant feedback, we also clarified the mood question that asked about pain to refer specifically to “physical pain.”
Out of office message:
During the pilot intervention, many participants responded to messages where no response was required, which commonly included phrases such as “okay” or “thank you”. While these responses do not raise issues, one of the greatest safety concerns related to using this intervention with patients with MDD is that patients will report serious symptoms (e.g. severe depression, suicidal ideation) via text thinking their therapist will see it. To reduce this possibility, we have added an “out of office message” stating, Thank you for your message. We are not monitoring this texting program but if you have any information to share, please discuss with your therapist at your next visit. If you need urgent assistance please go to an emergency room. Receiving this message each time the participant simply sends “okay” or “thanks” may become annoying, so we have configured this message to send adaptively, only when it involves a message other than “okay” or “thank you”.
Study initiation message – description of reward plan:
Participants struggled to comprehend the components of the study initiation message during the initial interview. We also noted that the message required a lot of scrolling, particularly for those who had larger font size settings. To mitigate this issue, we broke the study initiation into four shorter messages that send one at a time at the beginning of the study with a lag in between, so participants have ample time to read the message. In the RCT, therapists also plan to review the reward plan with the patient during study initiation to ensure understanding.
Reducing repetitiveness:
Many participants in the follow-up interview described concerns that the messages were too repetitive and would become boring over time. For the RCT, we created three variations of message wording for activity reminder and weekly reward plan updates that will be varied at random. We chose these message groups because the participant receives them more than once (daily and weekly, respectively) and these messages do not involve eliciting responses from patients where consistent wording may be more critical, as is the case with the morning mood and evening activity questions.
Therapist training and study initiation manual:
In the RCT, therapists (not research assistants) will complete the study initiation. The technology setup process for the intervention is complex due to the number of components involved, so we have conducted multiple trainings with the therapists and created a step-by-step manual with pictures to walk them through the setup process. We have also incorporated additional steps into the therapist manual to ensure the patient leaves the study initiation session equipped with the knowledge necessary to successfully utilize the intervention, including:
Standardized phone setup for those receiving a phone as part of the study - therapists will set up study smartphones with a standard configuration so the buttons/apps the participant needs (calls, messages, HealthMate) are set as shortcuts.
Adaptive phone set up – the therapists have instructions to adjust the font settings on the phone to a size easily readable by the patient. In previous iterations of the study, we provided instructions to patients to complete this step on their own.
Integration of technologies into daily activities – therapists will discuss with patients to ensure they have a place to keep and charge their phone.
Review of patient education booklet – therapists will review newly added information regarding how to use their phone (for those receiving a study phone) and use of the tools related to the intervention (messaging, HealthMate application).
Patient teach-back – the study initiation session will include with the therapist reviewing the activities the patient should complete each day and having the patient repeat the activities in their own words.
Patient education booklet:
Prior to the usability study, team members designed an education booklet regarding information on their condition and strategies for mitigating depressive symptoms. The usability study only included patients who already owned a smartphone, but to improve accessibility of the intervention in the RCT, participants who do not have their own smartphone will be given one. As described above, we have added simple, mostly photo- based materials explaining the key functions patients will need to complete using a smartphone (charging, making phone calls, messaging). The booklet also includes information about the study interventions. The information will first be reviewed with patients by their therapist to provide patients an opportunity to ask questions and ensure they know where these instructions are should they need them throughout the course of the intervention.
Discussion
In this study, we demonstrated the feasibility of older adults using a relatively complex technological intervention involving reporting outcomes twice a day via text message and tracking their activities using a smartwatch. This required careful attention to technology and instructional design. Below, we highlight four lessons for future studies aimed to design useful, usable consumer health technologies, particularly for older adults. For more in-depth guidance on designing for older adults, see works such as Czaja et al. 2019.39
Utilize usability assessment and cognitive walkthroughs to understand what may be challenging or unintuitive.
The intervention changes we made would not have been possible without input from our older adult participants. The think-aloud protocol was particularly helpful in identifying portions of the design that were not intuitive. Our participants provided their thoughts with ease. This method was also relatively easy to teach to a group of research assistants who had no prior experience with usability assessment and little knowledge of semi-structured interviewing methodologies.
Tailor the technology to the tasks and the person.40
Our technology setup process involves making the most frequently used functions (messaging and the HealthMate applications) for the intervention tasks easily accessible. We also plan to work with the participants to ensure device settings (e.g. font size), fit well with their physiological and cognitive needs. When possible, it is beneficial to go through these accommodations with the user, as opposed to giving them an “out-of-the-box” technology and expecting them to determine how to make accommodations.
Take advantage of simplified visual design.
Since the early days of web design, user interface designers have pushed the concept of minimalism, simplifying interfaces “by removing unnecessary elements or content that does not support user tasks”.41 We incorporated this concept by shortening text messages into more digestible segments (see study initiation message) and providing proximal visual cues to reduce confusion in numeric response questions.
Leverage key principles of instructional design.
Ideally, intuitive technologies would not require instructions. However, since technology design is not optimized for older adults and those with less technology experience,30,39 instructional design becomes and important piece of consumer health technology interventions. Czaja et al.’s chapter on instructional design for older adults recommends using Merrill’s phases for effective instruction, including integration, activation, application, and demonstration.42 Therapists and instructional materials help the patient integrate the technologies into their daily lives by discussing where the phone can be charged and daily wearing of the smartwatch. The patient instruction booklet also provides a routine whereby the patient charges their phone each night and answer text messages at the same time each day. The phone instructions activate structures of previously used technologies, for example, by comparing the noise their phone will make when they have a message to a familiar sound, such as a timer. During the initial setup, patients will be asked to apply their knowledge of the messages and describe how they think they should be answered. Lastly, the initial setup concludes with the patient demonstrating their understanding of the activities they need to complete via teach-back.
Limitations
This study contained limitations in the patient characteristics and study time-frame. First, all participants were recruited from a single community center. Our sample contained a greater proportion of Asian participants than would be expected in a sample of the United States adult population.43 The patients were, however, relatively balanced in terms of gender and educational attainment (Table 2). This study was also limited to those who already owned a smartphone and did not specifically target those with MDD. The pilot study also only involved one week of technology use, although the actual intervention involves a 12-week use period.
Many of the limitations of the pilot study will be addressed in an upcoming RCT. Measures to address the aforementioned limitations include:
- Patient recruitment from multiple senior centers
- Providing smartphones to those who do not already have them
- Adding inclusion criteria to include only those indicating moderate (or worse) mental health symptoms as based on a PHQ-9 assessment
- Implementing a 12-week study period
Conclusions
Our pilot study demonstrated the feasibility of older adults using a text-messaging and wearable device-based intervention. Our work provides further proof of concept that these health technologies can be successfully utilized by older adults through careful consideration of technology and instructional design. The findings of our study underscore the importance of usability testing and incorporating representative end users into the intervention design. Our technology and study design process benefited from previously established user-centered design concepts including task-technology fit and minimalism, as well as instructional design principles. Future work should assess the efficacy of our and other consumer health technology interventions in improving health outcomes for older adults.
Acknowledgements
This work was supported by the National Institute for Mental Health (P50MH113838; PI-Alexopoulos, G.S.).
Figures & Table
References
- 1.Nations U. World population ageing Department of Economic and Social Affairs. 2015.
- 2.Statistics NCfH. Older Americans 2016 . US Government Printing Office. Washington DC, USA: 2016. Key Indicators of Well-Being. Federal Interagency Forum on Aging-Related Statistics. [Google Scholar]
- 3.Friedrich M. Depression is the leading cause of disability around the world. Jama. 2017;317(15):1517–1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 4.Han LK, Aghajani M, Clark SL, et al. Epigenetic aging in major depressive disorder. American Journal of Psychiatry. 2018;175(8):774–782. doi: 10.1176/appi.ajp.2018.17060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. Jama. 2011;306(11):1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daskalopoulou M, George J, Walters K, et al. Depression as a risk factor for the initial presentation of twelve cardiac, cerebrovascular, and peripheral arterial diseases: data linkage study of 1.9 million women and men. PloS one. 2016;11(4) doi: 10.1371/journal.pone.0153838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buigues C, Padilla-Sánchez C, Garrido JF, Navarro-Martínez R, Ruiz-Ros V, Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging & mental health. 2015;19(9):762–772. doi: 10.1080/13607863.2014.967174. [DOI] [PubMed] [Google Scholar]
- 8.Penninx BW. Depression and cardiovascular disease: epidemiological evidence on their linking mechanisms. Neuroscience & Biobehavioral Reviews. 2017;74:277–286. doi: 10.1016/j.neubiorev.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Lim J, Oh IK, Han C, et al. Sensitivity of cognitive tests in four cognitive domains in discriminating MDD patients from healthy controls: a meta-analysis. International psychogeriatrics. 2013;25(9):1543–1557. doi: 10.1017/S1041610213000689. [DOI] [PubMed] [Google Scholar]
- 10.Manning KJ, Steffens DC. State of the Science of Neural Systems in Late-Life Depression: Impact on Clinical Presentation and Treatment Outcome. Journal of the American Geriatrics Society. 2018;66:S17–S23. doi: 10.1111/jgs.15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unützer J. Late-Life depression. New England Journal of Medicine. 2007;357(22):2269–2276. doi: 10.1056/NEJMcp073754. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell AJ, Rao S, Vaze A. Do primary care physicians have particular difficulty identifying late-life depression? A meta-analysis stratified by age. Psychother Psychosom. 2010;79(5):285–294. doi: 10.1159/000318295. [DOI] [PubMed] [Google Scholar]
- 13.Hoeft TJ, Hinton L, Liu J, Unützer J. Directions for effectiveness research to improve health services for late-life depression in the United States. The American Journal of Geriatric Psychiatry. 2016;24(1):18–30. doi: 10.1016/j.jagp.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinton L, Zweifach M, Tang L, Unützer J, Oishi S. Gender disparities in the treatment of late-life depression: qualitative and quantitative findings from the IMPACT trial. The American journal of geriatric psychiatry. 2006;14(10):884–892. doi: 10.1097/01.JGP.0000219282.32915.a4. [DOI] [PubMed] [Google Scholar]
- 15.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Focus. 2018;16(4):420–429. doi: 10.1176/appi.focus.16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexopoulos GS. “The Depression–Executive Dysfunction Syndrome of Late Life”: A Specific Target for D3 Agonists? The American Journal of Geriatric Psychiatry. 2001;9(1):22–29. [PubMed] [Google Scholar]
- 17.Campbell NL, Boustani MA, Skopelja EN, Gao S, Unverzagt FW, Murray MD. Medication adherence in older adults with cognitive impairment: a systematic evidence-based review. The American journal of geriatric pharmacotherapy. 2012;10(3):165–177. doi: 10.1016/j.amjopharm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Insel K, Morrow D, Brewer B, Figueredo A. Executive function, working memory, and medication adherence among older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2006;61(2):P102–P107. doi: 10.1093/geronb/61.2.p102. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds CF, Dew MA, Pollock BG, et al. Maintenance treatment of major depression in old age. New England Journal of Medicine. 2006;354(11):1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- 20.Kok RM, Heeren TJ, Nolen WA. Continuing treatment of depression in the elderly: a systematic review and meta-analysis of double-blinded randomized controlled trials with antidepressants. The American journal of geriatric psychiatry. 2011;19(3):249–255. doi: 10.1097/jgp.0b013e3181ec8085. [DOI] [PubMed] [Google Scholar]
- 21.Alexopoulos GS, Sirey JA, Banerjee S, et al. Two interventions for patients with major depression and severe chronic obstructive pulmonary disease: impact on dyspnea-related disability. The American Journal of Geriatric Psychiatry. 2018;26(2):162–171. doi: 10.1016/j.jagp.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexopoulos GS, Raue PJ, Kiosses DN, et al. Problem-Solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Archives of general psychiatry. 2011;68(1):33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Academy of Sciences Psychosocial Interventions for Mental and Substance Use Disorders A Framework for Establishing Evidence-Based Standards. 2015. [PubMed]
- 24.Alexopoulos GS ALACRITY for Late- and Mid-Life Mood Disorders. National Institute for Mental Health. 2017.
- 25.Alexopoulos GS, O’Neil R, Banerjee S, et al. “Engage” therapy: Prediction of change of late-life major depression. Journal of affective disorders. 2017;221:192–197. doi: 10.1016/j.jad.2017.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexopoulos GS, Raue PJ, Gunning F, et al. “Engage” therapy: behavioral activation and improvement of late-life major depression. The American Journal of Geriatric Psychiatry. 2016;24(4):320–326. doi: 10.1016/j.jagp.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chokshi NP, Adusumalli S, Small DS, et al. Loss-framed financial incentives and personalized goal-setting to increase physical activity among ischemic heart disease patients using wearable devices: the ACTIVE REWARD Randomized Trial. Journal of the American Heart Association. 2018;7(12):e009173. doi: 10.1161/JAHA.118.009173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pew Research Center Mobile Fact Sheet. https://www.pewresearch.org/internet/fact-sheet/mobile/ . Published 2019. Accessed 01/22/2020, 2020.
- 29.Venkatesh V, Thong JY, Xu X. Consumer acceptance and use of information technology: extending the unified theory of acceptance and use of technology. MIS quarterly. 2012;36(1) [Google Scholar]
- 30.Taha J, Sharit J, Czaja SJ. Usability of an Electronic Personal Health Record (PHR) Among a Diverse Group of Adults. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. 2014;58(1):619–623. [Google Scholar]
- 31.Asch DA, Volpp KG. On the way to health. LDI Issue Brief. 2012;17(9):1–4. [PubMed] [Google Scholar]
- 32.Blackmon MH, Polson PG, Kitajima M, Lewis C. SIGCHI conference on human factors in computing systems. Minnesota, USA: Minneapolis; 2002. Cognitive walkthrough for the web. [Google Scholar]
- 33.Gao Y, Xiaojun Wang, White L, Li H, Luo Y. An empirical study of wearable technology acceptance in healthcare. Industrial Management & Data Systems. 2015;115(9):1704–1723. [Google Scholar]
- 34.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric annals. 2002;32(9):509–515. [Google Scholar]
- 35.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradke P, Brinker E. Teach-Back gives direction for clarification: Uncover reasons for noncompliance. Patient Education Management. 2011;18(10):111–112. [Google Scholar]
- 37.Gerber A, Van Der Merwe A, Alberts R. Practical implications of rapid development methodologies. 2007.
- 38.Naz R, Khan M. Rapid applications development techniques: A critical review. International Journal of Software Engineering and Its Applications. 2015;9(11):163–176. [Google Scholar]
- 39.Czaja SJ, Boot WR, Charness N, Rogers WA. Designing for older adults: Principles and creative human factors approaches. CRC press; 2019. [Google Scholar]
- 40.Goodhue DL, Thompson RL. Task-Technology fit and individual performance. MIS quarterly. 1995:213–236. [Google Scholar]
- 41.Moran K. The Characteristics of Minimalism in Web Design. Nielsen Norman Group; https://www.nngroup.com/articles/characteristics-minimalism/ . Published 2015. Accessed 3/10/2020. [Google Scholar]
- 42.Merrill MD. First principles of instruction. Educational technology research and development. 2002;50(3):43–59. [Google Scholar]
- 43.U.S. Census Bureau Language Use in the United States. 2011. p. 2011.