Abstract
Background:
The aim of this study was to investigate the effect of pretransplant culture on the survival of pancreatic islet grafts, and to determine the biological characteristics of isolated islets during pretransplant culture.
Methods:
The survival of islets from Wistar rats, transplanted to diabetic C57BL/B6 mice, was compared between fresh islets and cultured islets. A comprehensive gene expression analysis was employed to investigate biological processes during pretransplant culture, and in vitro validation studies were performed.
Results:
Survival of cultured xenografts was significantly prolonged as compared to that of fresh islets (fresh: 12.5 ± 1.9 days, 1-day cultured: 16.0 ± 1.3 days (p= 0.017), 3-day cultured: 17.0 ± 2.6 days (p= 0.014)). Comprehensive gene expression analysis identified significant upregulation of annotated functions associated with inflammation in cultured groups. Six proinflammatory genes, including heme oxygenase 1 (HO-1) and IL-6, were significantly upregulated during culture. Validation studies revealed significantly higher levels of IL-6 in the supernatant of cultured islets and HO-1 in the cultured islets when compared with fresh islets.
Conclusion:
Transplantation of cultured islets induced significant but minimal prolongation of graft survival in xenogeneic combinations. Comprehensive analysis of gene expression in cultured islets showed biological processes associated with proinflammation during culture.
Keywords: islet transplantation, heme oxygenase 1, IL-6, culture, gene expression
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune disease in which the immune system destroys insulin-secreting beta (β) cells, resulting in insulin deficiency. Over 100,000 individuals in Japan, comprising approximately 0.1% of the national population, are currently diagnosed with insulin-dependent diabetes mellitus1). β-cell replacement by transplantation of pancreas or islet cells has been a promising therapy for T1DM patients with unawareness of severe hypoglycemia, despite adequate insulin therapy. The procedure of pancreatic islet transplant is superior to that of solid pancreatic transplant as it is minimally invasive. The results of several short-term clinical trials of islet transplantation have been promising. However, multiple donors were required to achieve sustained insulin independence in one recipient, and the function of implanted islets decreased with time after transplantation2,3). Impairment of islet engraftment might be attributed to loss of islets during their isolation4,5), instant blood-mediated inflammatory response (IBMIR) with thrombosis, and host immune response after transplantation6).
Advances in immunosuppression therapy have facilitated nationwide islet transplantation, and contributory factors for islet graft survival have also been reported. A previous study on the primary efficacy of islet transplantation and its safety outcomes in recipients from the Collaborative Islet Transplant Registry demonstrated that factors predicting insulin independence were recipient age, islet culture (> 6 h), stimulation index >1.5, and immunosuppression therapy7). A brief period of pretransplant islet culture is commonly adopted in clinical transplantation because islet culture prior to transplantation provides flexibility for the evaluation of isolated islets8). Additionally, culturing islets prior to transplantation allows preconditioning of islets or recipients, which improves the outcomes of islet transplantation. A recent preclinical study indicated the necessity of islet culture prior to transplantation to prepare apoptotic donor leukocytes for the induction of tolerance9).
Biologically, cultured islets are reportedly less immunogenic in most experimental studies10). However, loss of islets during culture is detrimental and affects the transplantation outcome11). Therefore, whether cultured islets are superior to fresh islets in islet transplantation remains unclear.
The present study investigated the effects of islet culture on graft survival and the changes in the biological characteristics of islets during preculture.
Materials and Methods
Animals
Islet donors were male, 8- to 11-week-old Wistar (Jcl) rats weighing 250-320 g (CLEA Japan, Inc. Tokyo, Japan). Diabetes was induced in male, 8- to 9-week-old C57BL/6 (H-2b) mice (B6 mice; Nihon Clea, Inc., Shizuoka, Japan) by a single intraperitoneal (i.p.) injection of streptozotocin (STZ; 250 mg/kg body weight; Sigma-Aldrich, St Louis, MO, USA). The rats and mice were housed in cages at a controlled temperature and with a suitable light/dark cycle. Mice with two consecutive non-fasting blood glucose levels of > 400 mg/dL served as recipients. All animals were anesthetized using isoflurane inhalation. The Ethics Review Committee for Animal Experimentation of Fukushima Medical University approved this study. All procedures in the experiment were performed according to the guidelines of the National Council’s Guide for the Care and Use of Laboratory Animals.
Isolation of pancreatic islets and culture
Islets were isolated using a method similar to that reported previously12). In brief, pancreatic islets were isolated from Wistar rats using stationary collagenase digestion (Liberase; 0.23 mg/mL; Roche, Basal, Switzerland), followed by centrifugation using a discontinuous gradient of Ficoll (Sigma Chemical Co, St. Louis, MO). The crude number of islets was converted into a standard number of islet equivalents (IEQ; diameter standardized to 150 μm). The crude islets obtained after the second Ficoll separation were divided into 3 groups depending on pretransplant culture time as follows: (1) 30 min (fresh-islets), (2) 24 h (d1-islets), and (3) 72 h (d3-islets). The islets were cultured in complete medium (RPMI 1640 with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 2 mM L-glutamine (MP Biomedicals, Santa Ana, CA, USA), 100 U/mL penicillin (Life Technologies, Carlsbad, CA, USA), 100 mg/mL streptomycin sulfate (Life Technologies), and 0.5 mg/mL amphotericin B) supplemented with 10% fetal bovine serum (FBS) at 37℃ in a humidified atmosphere containing 5% CO2 and 95% air.
Percent islets recovery
Following isolation and culture, islets were harvested and counted to determine islet yield. Aliquots from respective samples were stained with dithizone (Sigma Aldrich Canada Co., Oakville, ON, CA) and counted in triplets. The percentage of islet recovery was determined by the ratio of total islets harvested post-culture relative to the number of islets harvested immediately post-isolation. In brief, approximately 200 islets were seeded into a 6-well culture plate (Corning Coster Co, Cambridge, MA, USA). After 30 min, 24 h, and 72 h, rat islets were counted using an optical graticule (n = 6 in each group). The crude number of islets was converted to the standard number of IEQ.
Glucose-stimulated insulin secretion
Insulin secretory capacities in response to low (3.3 mM) and high (20 mM) glucose concentrations were evaluated. Briefly, 3 sets of 30 IEQ islets were placed in a 12-well Transwell microplate in RPMI 1640 containing 3.3 mM glucose and 0.1% fetal calf serum at 37℃ for 60 min in an atmosphere containing 5% CO2/95% air for stabilization. After this preincubation, the Transwell was placed in a second well in RPMI 1640 containing 3.3 mM glucose and 0.1% FBS and incubated at 37℃ for 60 min. The Transwell was then placed in the third well with RPMI 1640 containing 20 mM glucose and 0.1% FBS for 60 min. The supernatants of the second and third wells were collected immediately. The insulin content of the supernatant was measured using an ultrasensitive rat insulin enzyme-linked immunosorbent assay (ELISA) kit (Morinaga, Kanagawa, Japan). The stimulation index was calculated by dividing the insulin secretion at high glucose concentrations by that at the low glucose concentration.
Islet transplantation
Following isolation and culture, 200 islets were implanted in the left renal subcapsular space of STZ-induced diabetic B6 mice. Non-fasting blood glucose levels of each mouse were measured daily to monitor islet graft survival. Graft rejection was defined as the day in which the blood glucose level exceeded 350 mg/dL on 2 consecutive days.
RNA purification, amplification, and microarray analysis
Three hundred islets were manually harvested with the aid of a microscope. They were immediately stored at −80℃. Total islet RNA was purified using the RNeasy Mini Kit (Qiagen N.V., Venlo, The Netherlands) according to the manufacturer’s protocol. RNA quantity and purity were measured using a NanoDrop 1000 spectrophotometer and an Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, CA, USA). Samples with an RNA integrity number > 7.0 and concentrations > 33 ng/μL were used for amplifications. Each total RNA sample was reverse-transcribed into double-stranded complementary DNA (cDNA), which was used in in vitro transcription reactions with the Ambion WT Expression Kit (Thermo Fisher Scientific, Inc.) to amplify antisense mRNA (complementary RNA [cRNA]). The cRNA was labeled and fragmented using the Affymetrix GeneChip WT Terminal Labeling Kit (Affymetrix, Santa Clara, CA, USA). The labeled cDNA from each islet sample was hybridized to a single GeneChip Rat Gene 1.0 ST Array (Affymetrix, Santa Clara, CA, USA). This array can detect >30,000 transcripts of known rat genes and potentially expressed sequences. The hybridized arrays were scanned using the Gene Chip Scanner 3000 7G (Thermo Fisher Scientific, Inc.) to generate images of fluorescence intensity. Image data were quantified using GeneChip Command Console Software (Affymetrix).
Functional analysis of differentially expressed genes (DEGs)
Normalization and filtering of microarray data were performed using Gene Spring GX software version 12.5 (Agilent Technologies, Santa Clara, CA, USA). DEGs were defined as those whose levels changed > 2-fold or ≤ 2-fold (log2) compared to the respective control. Ingenuity Pathway Analysis (Qiagen, Redwood City, CA, USA; qiagen.com/ingenuity) was used to identify the biological processes that were differentially affected in cultured or fresh islets. This tool provides information about functional categories such as diseases, molecular and cellular functions, and physiological and developmental functions of genes obtained from microarray analysis. To determine the biological functions most closely associated with pretransplant culture, an activation z-score of >2 or ≤2, and a Pvalue of <0.05 were used. The activation z-score assesses the match between observed and predicted upregulation or downregulation patterns that indicate significant differential biological activity of cultured islets compared to that of fresh islets. The Pvalue calculated using the Fisher exact test indicates the statistical significance of the association of a biological function with a set of DEGs.
Western blotting
After each culture period, islet samples (about 300 islets per culture condition) were centrifuged. The supernatant was discarded and the pellet containing the islets was frozen in liquid nitrogen and stored at −80℃ until use. The frozen pellet was dissolved in a lysis buffer mixture (100 mM Tris-HCl, pH 7.5, 159 mM NaCl, 15% glycerol, 5 mM EDTA, 1% Triton X-100 [Sigma-Aldrich Co, St Louis, Mo]), containing protease inhibitor cocktail (Roche Diagnostics Co, Mannheim, Germany), phosphatase inhibitor cocktail (Sigma-Aldrich Co), and loading buffer (66 mM Tris-HCl, 10% glycerol, 2% SDS, 100 mM DL-dithiothreitol, 0.001% bromophenol blue), and boiled for 7 min. The sample was centrifuged for 1 min at 15,000 rpm at 4℃, and the supernatant was subjected to 5% to 20% polyacrylamide gel electrophoresis. Equal volumes of the supernatant were loaded and electrophoresed at 90 V for 5 min and then at 150 V for 1 h. The resolved proteins were transferred to a nitrocellulose membrane (Amersham Biosciences Co, Chalfont St Giles, Buckinghamshire, UK) at 25 V for 16 h. The membranes were blocked with 7% nonfat dried milk in PBS containing 0.1% Tween-20 for 30 min at room temperature and then incubated with the mouse monoclonal antibody against heme oxygenase 1 (HO-1) (Abcam Cambridge, UK; 4 μg/mL) and β-actin (Millipore Co, Billerica, Mass; dilution 1: 1,000) in blocking buffer for 1 h at room temperature. The membrane was washed twice with PBS containing 0.1% Tween-20 for 10 min each and then incubated for 1 hour at room temperature with anti-mouse IgG-peroxidase conjugate (GE Healthcare Co.; dilution 1: 2,000). After washing for another 30 min, the blot signals were developed using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Inc.) and LAS-1000 apparatus (Fujifilm Co, Tokyo, Japan). Quantification was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). For statistical analysis, the signal intensities were normalized to that of β-actin which was used as an internal control.
Immunoassay of cytokines in islet culture supernatants
Aliquots of pancreatic islet preparations (240 islets/mL) were cultured in 3 mL of RPMI 1640 medium in a 3.5-cm dish (Sumilon, Sumitomo Bakelite Co., Utsunomiya, Japan) in a CO2 incubator. Supernatants of the cultured islets were collected after 24 h and 72 h of culture and immediately stored at −80℃. The levels of interleukin 6 (IL-6) were determined using a Quantikine ELISA kit (R&D Systems, Minneapolis, MN, USA). Each reaction was performed in duplicate. The lower limits of detection density were measured at 450 nm using a microplate reader (Multiskan GO, Thermo Scientific, Yokohama, Japan).
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM). Islet yield ratio and HO-1 levels in cultured islets were compared using Student’s t-test. Protein expression ratios were compared, as appropriate, using Student’s t-test or one-way analysis of variance (ANOVA) with Bonferroni adjustment. Graft survival curves were calculated using the Kaplan-Meier method and compared using the log-rank test. Statistical significance was defined as P< 0.05. All statistical analyses were performed using Graph-Pad PRISM 5.0 software (GraphPad, La Jolla, CA, USA).
Results
Islet yield and glucose-stimulated insulin secretion before transplantation
To evaluate islet yield equivalents after culture, the IEQ of isolated islets from rat pancreas was compared between d1-islets and d3-islets. The reduction rates of d1-islets and d3-islets compared to the rate of fresh islets were 13% and 24% (fresh-islets vs d1-islets; p= 0.004; fresh-islets vs d3-islets; p< 0.0005), respectively (Figure 1A). To assess the islet quality in each group in vitro, rat islets were evaluated using the stimulation index. The stimulation index was not significantly different between the three groups (Figure 1B).
Figure 1.
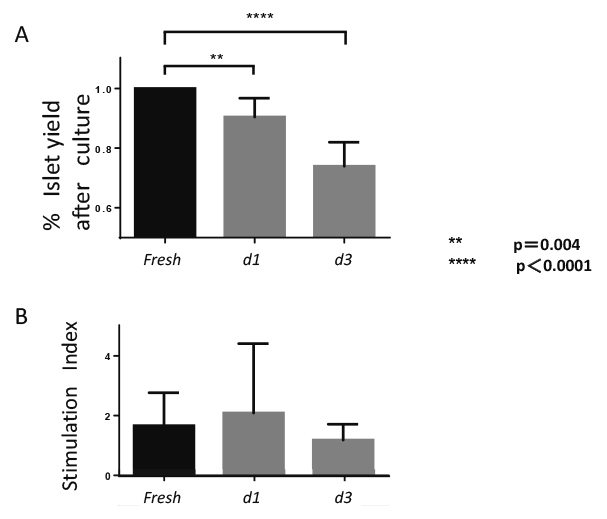
Islets recovery and stimulation index after culture
(A) Percentage of islet equivalents (IEQ) recovery of cultured islets compared to that of fresh islets (fresh-islets). Recovery rate of IEQ was measured in each group (n = 6, d1- and d3-islets).
(B) Stimulation index of cultured islets. The supernatants were collected and insulin levels were determined using a commercially available ELISA kit. The stimulation index was not significantly different between the three groups (each group, n = 6).
In vivo function of fresh or cultured islets
Non-fasting blood glucose levels in animals receiving cultured fresh, d1-, and d3-islets are shown in Figure 2A. Blood glucose levels in all diabetic mice were normalized two days after transplantation. Graft survival of d1-islets (16.0 ± 1.3 days) and d3-islets (17.0 ± 2.6 days) was significantly longer than that of fresh islets (12.5 ± 1.9 days); however, all the transplanted islets were rejected by day 24 of transplantation. (Figure 2B, fresh islets vs. d1-islets, p= 0.017; fresh-islets vs d3-islets, p= 0.014).
Figure 2.

Blood glucose levels of diabetic mice with transplantation of islet rat xenografts
(A) Rat islets (n = 300) were transplanted under the kidney capsule of diabetic mice. The levels of blood glucose were measured in each group of mice.
(B) The engraftment periods of fresh-, d1-, and d3-islets compared by Kaplan-Meier method. The engraftment period of the cultured islets was significantly increased compared to that of the fresh islets.
Comprehensive analysis of gene expression in fresh and cultured islets
Compared to fresh islets, the expression of genes whose functions fall under categories of cellular movement, cellular growth and proliferation, and cell death and survival were enhanced in d3-islets (Table 1). Among the top 10 significantly upregulated genes, 6 (SERPINA3, MMP13, HMOX1 (HO-1), FGG, BCAT1, and IL-6) were proinflammatory genes (Table 2).
Table 1.
Significant alteration of annotated molecular and cellular functions in cultured islets.
| Category | Disease/Bio-function | #genes in dataset | overlap p-value | Activation & z-score |
|---|---|---|---|---|
| Cellular movement | cell movement | 166 | 1.02E-43 | 4.647 |
| migration of cells | 153 | 3.44E-40 | 4.456 | |
| leukocyte migration | 71 | 1.6E-23 | 2.652 | |
| cell movement of phagocytes | 50 | 1.9E-21 | 1.394 | |
| cell movement of myeloid cells | 48 | 5.58E-21 | 1.41 | |
| Cellular growth and proliferation | proliferation of cells | 214 | 5.08E-37 | 3.089 |
| proliferation of connective tissue cells | 57 | 3.17E-24 | 1.811 | |
| generation of cells | 104 | 4.48E-20 | 1.635 | |
| stimulation of cells | 44 | 1.5E-17 | 2.819 | |
| endothelial cell development | 38 | 3.16E-17 | 0.297 | |
| Cell death and survival | necrosis | 148 | 1.11E-33 | 1.783 |
| cell death | 184 | 2.9E-28 | 1.078 | |
| apoptosis | 157 | 7.26E-27 | 0.724 | |
| cell survival | 76 | 2.49E-15 | 3.775 | |
| cell viability | 65 | 2.86E-15 | 3.282 |
Differentially expressed genes were analyzed with Ingenuity Pathway Analysis software to indentify the most enriched biological functions in cultured islets. The top 3 of disease and bio-function in each annotated category, sorted according to P value, are shown with the number of genes and z-score. A disease and bio-function with the z-score of higher than 2 indicated the upregulated status of the gene expression. Expression levels of numerous genes involved function such as “cellular movement” were upregulated most significantly in cultured islets during culture period prior to transplantation.
Table 2.
Top 10 up-regurated genes in cultured islets.
| Day 0→1 | Day 0→3 | |||||
|---|---|---|---|---|---|---|
| Symbol | Entrez Gene Name | Fold
Change |
Symbol | Entrez Gene Name | Fold
Change |
|
| SERPINA3 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 3 | 84.543 | SERPINA3 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 3 | 100.774 | |
| MMP13 | matrix metallopeptidase 13 (collagenase 3) | 83.013 | FGG | fibrinogen gamma chain | 44.117 | |
| HMOX1 | heme oxygenase (decycling) 1 | 39.944 | MMP13 | matrix metallopeptidase 13 (collagenase 3) | 32.904 | |
| FGG | fibrinogen gamma chain | 34.698 | HMOX1 | heme oxygenase (decycling) 1 | 28.581 | |
| BCAT1 | branched chain amino-acid transaminase 1, cytosolic | 17.276 | VCAN | versican | 19.919 | |
| TPH1 | tryptophan hydroxylase 1 | 16.743 | POSTN | periostin, osteoblast specific factor | 19.315 | |
| LIF | leukemia inhibitory factor | 13.264 | IL6 | interleukin 6 (interferon, beta 2) | 18.704 | |
| IL6 | interleukin 6 (interferon, beta 2) | 13.136 | C9 | complement component 9 | 17.212 | |
| MT1H | metallothionein 1H | 12.784 | BCAT1 | branched chain amino-acid transaminase 1, cytosolic | 15.658 | |
| CCL13 | chemokine (C-C motif) ligand 13 | 10.937 | HPX | hemopexin | 14.35 | |
Top 10 genes, analyzed with Ingenuity Pathway Analysis software in cultured islets, were sorted according to fold change. The 6 genes, SERPINA3, MMP13, HMOX-1(HO-1), FGG, BCAT1, and IL-6, were upregulated in common under two condittions.
HO-1 protein expression in rat islets
On the basis of gene expression analysis, HO-1 protein expression in cultured islets was validated by Western blotting (Figure 3). The expression of HO-1 protein was significantly higher in d1- and d3-islets (fresh-islets vs d1-islets; p<0.0001; fresh-islets vs d3-islets; p= 0.047) than in fresh islets.
Figure 3.
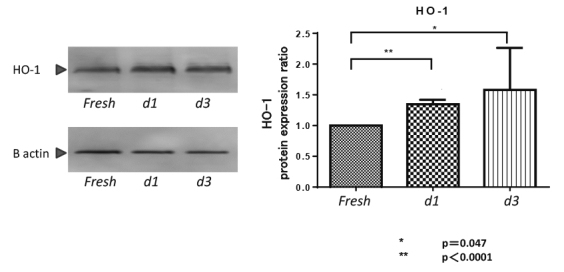
Heme oxygenase 1 (HO-1) protein expression in cultured islets.
Western blotting for HO-1 and β-actin expression in cultured islets. The expression of HO-1 protein was significantly higher in d1- and d3-islets than in fresh-islets.
IL-6 release from cultured islets
The IL-6 levels in islet culture supernatants, as determined by ELISA, were measured to validate the results of the comprehensive gene expression analysis. IL-6 release from d1-islets and d3-islets was confirmed (Figure 4).
Figure 4.
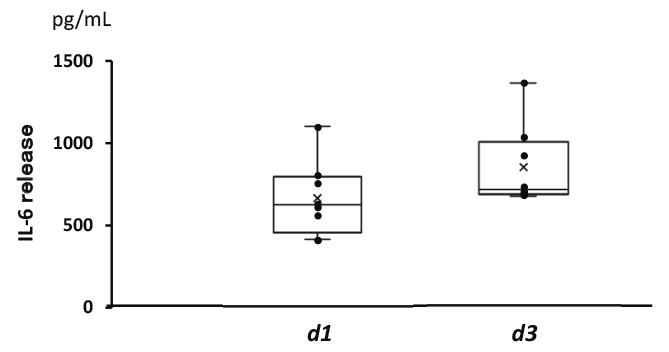
Levels of IL-6 in islet culture medium
IL-6 levels in islet culture supernatants were measured using an ELISA kit. IL-6 release from d1- and d3-islets was confirmed.
Discussion
The present comprehensive study on the biological characteristics of islets during pretransplant short-term culture revealed significant upregulation of gene clusters annotated as cellular movement, cellular growth, and cell death and survival. The results of the gene expression analysis confirmed the secretion of IL-6 from the islets as well as the expression of HO-1 protein in the cultured islets. In vitro assessment of islet viability revealed no significant difference in glucose-stimulated insulin release between fresh islets and cultured islets, whereas IEQ decreased in cultured islets. Statistically significant prolongation of the survival time was observed in the cultured islets compared to fresh islets. Still, the difference was minimal as all the cultured islets failed by day 24 after transplantation.
Islet transplantation is a characteristically multistep process that involves distention of the pancreas, digestion using collagenase, and purification. During each step, the islets can be damaged by hypoxia, warm ischemia, activated proteolytic enzymes released from acinar cells, mechanical stress, or oxidative stress13-16). The stresses encountered during isolation detrimentally affect β cell physiology during subsequent culture and graft survival. In the present study, upregulation of proinflammatory gene clusters was confirmed in cultured islets, which could be partly triggered by the cell stress induced during their isolation. These findings are consistent with previous observations of the induction of proinflammatory cytokines and danger signals, including tumor necrosis factor-alpha17), monocyte chemoattractant protein-118), and tissue factor19) induced during islet isolation. These inductions result in the loss of a significant number of isolated islets due to apoptosis and necrosis4,5), which hinders islet engraftment following transplantation10,11). A strategy targeting these cytokines has been suggested to enhance graft survival20). Conversely, Marzorati et al. reported that cultured islets displayed significantly decreased MCP-1 release and tissue factor production21), suggesting that culture conditions can modulate the proinflammatory state of islets. Additionally, Ihm SH et al. demonstrated that culturing islets did not affect their functional recovery from the cell stress induced by non-physiological stimuli during isolation22). The collective findings highlight the continuing debate regarding the short-term culture of islets following transplantation.
Presently, there was no significant difference in glucose-stimulated insulin release between fresh and cultured islets, whereas the IEQ was decreased in cultured islets compared to that in fresh islets (Figure 1). Additionally, there were no differences between the fresh islets and the cultured islets in terms of functional activity to normalize blood sugar levels after transplantation in the diabetes recipients (Figure 2B). These findings indicated that functional islet yield after 3 days of culture might not be less than that of fresh-islets. Regarding graft survival, a statistically significant prolongation of graft survival was observed in cultured islets compared to fresh islets, but the impact on graft survival was minimal. A previous study reported the superiority of cultured islets for engraftment in a rat-to-rat allotransplantation model10). In contrast, another in vivo study using nude mice reported no superiority of human cultured islets compared to fresh islets23). A previous study showed that culture prior to transplantation reduced immunogenicity of allogenic islets, which might be part of the mechanism of the prolonged survival of the cultured islets in the current study24). Given that our graft survival results were derived from a concordant rat-to-mouse xenotransplantation model, the effect of preculture on immunogenicity against xenogeneic islets needs to be carefully understood. Therefore, further studies to evaluate the immune response against allogenic or xenogeneic cultured islets using immunological assays are warranted.
The concept of islet culture prior to transplantation has changed in the past several decades. The Edmonton Protocol recommends the use of islets that are freshly prepared immediately after isolation to minimize the risk of islet injury during culture25). However, short-term culture prior to transplantation is usually employed in clinical islet transplantation to allow sufficient time for functional assessment and recipient preparation26). Neither the optimal culture period nor islet culture conditions have been fully evaluated in clinical islet transplantation.
This lack of information prompted the present comprehensive evaluation of biological processes dominant in islets during culturing. The findings of the present study did not reveal a critical mechanism supporting the superiority of cultured islets. However, upregulation of proinflammatory gene clusters was maintained, even in d3-islets, suggesting the need for pretreatment to lessen inflammation in the graft during culture.
Multiple proinflammatory cytokines are secreted from the islets. These potently stimulate the host innate cell-mediated immune response. These molecules may serve as therapeutic targets to enhance islet transplantation27,28). The present study confirmed the upregulation of IL-6 gene expression in the cultured islets and secretion of IL-6 protein from islets during the culture period. IL-6 secreted from islets is considered a proinflammatory cytokine that induces a strong host immune response29). Itoh et al. determined that anti-IL-6R antibody could prevent high mobility group box 1-mediated early loss of transplanted islets30). Additionally, Citro et al. insisted that anti-inflammatory treatment targeting a single proinflammatory axis is insufficient because of the redundancy and promiscuity of chemokine signaling mechanisms28). Short-term culture of isolated islets could provide an opportunity for preconditioning to improve the outcome of islet transplantation. Our previous examination of islets treated with mitomycin-C (MMC) prior to transplantation revealed a decrease in multiple cytokines from MMC-treated islets, including IL-6, which could lead to prolonged engraftment of MMC treated islets without immunosuppression31-33). These findings suggest that preconditioning during islet culture as a strategy against inflammatory responses could be promising for islet transplantation.
To validate the microarray results, we selected HO-1 and IL-6 with higher differences in mRNA levels in cultured islets. In vitro experiments revealed that HO-1 protein expression and IL-6 secretion were increased in d1- and d3-cultured islets. HO-1 is one of isoforms of an enzyme that catalyzes the degradation of heme. The induction of the enzyme is important for cellular protection against both heme- and non-heme-mediated oxidant injury34). Vivot et al. evaluated the expression of HO-1, an antioxidant mediator, in isolated islets and reported that HO-1 activation could decrease the inflammatory status and oxidative stress in islets35). Thus, it is reasonably assumed that elevated HO-1 expression in the isolated islets during culture may act as anti-inflammatory signals on the islets, but this issue needs to be defined in future experiments.
Conclusions
Our comprehensive interpretation of the biological characteristics of islets during short-term culture demonstrated that genes related to proinflammatory mediators were most significantly upregulated. Additionally, we demonstrated that cultured islets before transplantation do not hinder their functional recovery, while the impact on survival prolongation is minimal. The collective findings suggest that an anti-inflammatory approach targeting multiple proinflammatory mediators might be required to enhance islet graft survival.
Acknowledgements
We thank Yukiko Kikuta, Eiko Otomo, Yuiko Watanabe and Hidemi Sasaki for excellent technical support.
Conflict of interest disclosure
The authors declare no conflicts of interest associated with this manuscript.
References
- 1.Gruessner RW, Sutherland DE, Troppmann C, et al. The surgical risk of pancreas transplantation in the cyclosporine era: an overview. J Am Coll Surg, 185(2): 128-144, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro A, Ricordi C, Hering B, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med, 355(13): 1318-1330, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes, 54(7): 2060-2069, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Kin T, Senior P, O’Gorman D, Richer B, Salam A, Shapiro A. Risk factors for islet loss during culture prior to transplantation. Transpl Int, Nov; 21(11): 1029-1035, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Berney T. Islet culture and counter-culture. Commentary on: Effect of short-term culture on functional and stress-related parameters in isolated human islets by Ihm, et al. Transpl Int, 22(5): 531-533, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet, 360(9350): 2039-2045, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care, 35(7): 1436-1445, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marzorati S, Antonioli B, Nano R, et al. Culture medium modulates proinflammatory conditions of human pancreatic islets before transplantation. Am J Transplant, 6(11): 2791-2795, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Singh A, Ramachandran S, Graham ML, et al. Long-term tolerance of islet allografts in nonhuman primates induced by apoptotic donor leukocytes. Nat Commun, 10(1): 3495, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahr H, Hussmann B, Eckhardt T, Bretzel RG. Successful single donor islet allotransplantation in the streptozotocin diabetes rat model. Cell Transplant, 11(6): 513-518, 2002. [PubMed] [Google Scholar]
- 11.Kedinger M, Haffen K, Grenier J, Eloy R. In vitro culture reduces immunogenicity of pancreatic endocrine islets. Nature, 270(5639): 736-738, 1977. [DOI] [PubMed] [Google Scholar]
- 12.Saito T, Ohashi K, Utoh R, et al. Reversal of diabetes by the creation of neo-islet tissues into a subcutaneous site using islet cell sheets. Transplantation, 92(11): 1231-1236, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Gotoh M, Maki T, Kiyozumi Y, et al. An improved method for isolation of mouse pancreatic islets. Transplantation, 40: 437-438, 1985. [DOI] [PubMed] [Google Scholar]
- 14.Ricordi C, Gray D, Hering B, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat, 27(3): 185-195, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Bank HL. Rapid assessment of islet viability with acridine orange and propidium iodide. In Vitro Cell Dev Biol, 24(4): 266-273, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Benhamou PY, Oberholzer J, Toso C, et al. Human islet transplantation network for the treatment of Type I diabetes: first data from the Swiss-French GRAGIL consortium (1999-2000). Groupe de Recherche Rhin Rhĵne Alpes Genève pour la transplantation d’Ilots de Langerhans. Diabetologia, 44(7): 859-864, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Hanley S, Liu S, Lipsett M, et al. Tumor necrosis factor-alpha production by human islets leads to postisolation cell death. Transplantation, 82(6): 813-818, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Melzi R, Mercalli A, Sordi V, et al. Role of CCL2/MCP-1 in islet transplantation. Cell Transplant, 19(8): 1031-1046, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet, 360(9350): 2039-2045, 2020. [DOI] [PubMed] [Google Scholar]
- 20.Citro A, Cantarelli E, Pellegrini S, Dugnani E, Piemonti L. Anti-Inflammatory Strategies in Intrahepatic Islet Transplantation: A Comparative Study in Preclinical Models. Transplantation, 102(2): 240-248, 2018. [DOI] [PubMed] [Google Scholar]
- 21.Marzorati S, Antonioli B, Nano R, et al. Culture Medium Modulates Proinflammatory Conditions of Human Pancreatic Islets Before Transplantation. Am J Transplant, 6: 2791-2795, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Ihm SH, Matsumoto I, Zhang HJ, Ansite1 JD, Hering BJ. Effect of short-term culture on functional and stress-related parameters in isolated human islets. Transpl Int, 22(2): 207-216, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi H, Naziruddin B, Jackson A, et al. Fresh islets are more effective for islet transplantation than cultured islets. Cell Transplant, 21(2-3): 517-523, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Malejczyk M, Malejczyk J, Abgarowicz K, Smogorzewski M. Immunogenicity of allogeneic pancreatic islet grafts: the effect of in vitro culture and the site of transplantation. Arch Immuno The Exp (Warsz), 37(1-2): 11-16, 1989. [PubMed] [Google Scholar]
- 25.Shapiro AMJ, Lakey JRT, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med, 343(4): 230-238, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Hering BJ, Kandaswamy R, Ansite JD, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA, 293(7): 830-835, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Merani S, Truong WW, Hancock W, Anderson CC, Shapiro AM. Chemokines and their receptors in islet allograft rejection and as targets for tolerance induction. Cell Transplant, 15(4): 295-309, 2006. [PubMed] [Google Scholar]
- 28.Citro A, Cantarelli E, Piemonti L. Anti-inflammatory strategies to enhance islet engraftment and survival. Curr Diabetes Rep, 13(5): 733-744, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B. Inflammatory response in islet transplantation. Int J Endocrinol, 2014. DOI:10.1155/2014/451035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh T, Nitta T, Nishinakamura H, et al. HMGB1-Mediated Early Loss of Transplanted Islets Is Prevented by Anti-IL-6R Antibody in Mice. Pancreas, 44(1): 166-171, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Gunji T, Saito T, Sato Y, et al. Mitomycin-C treatment followed by culture produces long-term survival of islet xenografts in a rat-to mouse model. Cell Transplant, 17(6): 619-629, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Matsuyama S, Gunji T, Ise K, Sato Y, Saito T, Gotoh M. Permanent acceptance of mitomycin C-treated islet allograft. Transplantation, 76(1): 65-71, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Sato N, Haga J, Anazawa T, et al. Ex vivo Pretreatment of Islets with Mitomycin C: Reduction in Immunogenic Potential of Islets by Suppressing Secretion of Multiple Chemotactic Factors. Cell Transplant, 26(8): 1392-1404, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol, 15(1): 9-19, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Vivot K, Langlois A, Bietiger W, et al. Pro-inflammatory and pro-oxidant status of pancreatic islet in vitro is controlled by TLR-4 and HO-1 pathways. PLoS One, 9(10): e107656. doi:10.1371/journal.pone.0107656, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


