Abstract
Background
Individuals receiving behavioral weight loss treatment frequently fail to adhere to prescribed dietary and self-monitoring instructions, resulting in weight loss clinicians often needing to assess and intervene in these important weight control behaviors. A significant obstacle to improving adherence is that clinicians and clients sometimes disagree on the degree to which clients are actually adherent. However, prior research has not examined how clinicians and clients differ in their perceptions of client adherence to weight control behaviors, nor the implications for treatment outcomes.
Purpose
In the context of a 6-month weight-loss treatment, we examined differences between participants and clinicians when rating adherence to weight control behaviors (dietary self-monitoring; limiting calorie intake) and evaluated the hypothesis that rating one’s own adherence more highly than one’s clinician would predict less weight loss during treatment.
Methods
Using clinician and participant-reported measures of self-monitoring and calorie intake adherence, each assessed using a single item with a 7- or 8-point scale, we characterized discrepancies between participant and clinician adherence and examined associations with percent weight change over 6 months using linear mixed-effects models.
Results
Results indicated that ratings of adherence were higher when reported by participants and supported the hypothesis that participants who provided higher adherence ratings relative to their clinicians lost less weight during treatment (p < 0.001).
Conclusions
These findings suggest that participants in weight loss treatment frequently appraise their own adherence more highly than their clinicians and that participants who do so to a greater degree tend to lose less weight.
Keywords: Weight loss, Adherence, Discrepancies, Self-monitoring, Calorie intake
Implications.
Practice: Clients who appraise their own adherence to weight control behaviors more highly than their clinicians may be less successful during behavioral weight loss treatment; thus, clinicians may wish to intervene with these clients early during treatment to ensure that they are evaluating their own adherence levels accurately.
Policy: Policymakers should consider supporting initiatives to routinely include self-report measures of perceived intervention adherence as part of behavioral weight-loss treatment and train clinicians to regularly monitor participant scores on these measures.
Research: Future research should investigate variables that may explain the effect of client-clinician adherence rating discrepancies on weight outcomes – for example, quality of the therapeutic alliance, intrinsic motivation or self-monitoring ability.
INTRODUCTION
Most Americans have overweight or obesity [1], and although gold-standard, high-intensity behavioral weight loss (BWL) treatments can produce clinically significant weight losses (i.e., 5%–8% in 1 year) [2], these losses are on average lower than is recommended for addressing health risks [3]. A major reason that the outcomes of BWL treatment are suboptimal is that individuals do not adhere to prescribed negative energy balance diets. Indeed, prior research has indicated that individuals attempting weight loss showing low levels of adherence to daily calorie prescriptions (including self-monitoring of diet) have poor weight loss outcomes [4–7], with higher self-reported levels of adherence generally predicting weight loss success [5,8–10].
Anecdotally, however, clinicians and clients sometimes disagree on the extent to which clients adhere to weight control prescriptions; for example, a client may believe themselves to be sufficiently recording and monitoring calorie intake, whereas the client’s clinician may believe otherwise based on a review of the client’s recent weight and self-monitoring data. Such discrepancies between clinician and client perceptions of the client’s intervention adherence could be problematic for several reasons. Firstly, despite the weight loss benefits associated with having a high level of self-efficacy regarding one’s ability to control weight [11,12], believing that one is adhering well despite one’s clinician’s opinions to the contrary could indicate that an individual is not appraising their own adherence accurately. Indeed, calorie intake is frequently underreported among individuals seeking weight loss, especially among those with high BMI [13], and self-monitoring adherence is often overreported [14–17]. Secondly, disagreement between clinicians and clients concerning the client’s typical patterns of behavior could also indicate the presence of problems in the therapeutic relationship—for example, a client’s reluctance to be honest with a clinician or a clinician’s failure to establish a strong rapport with a client—which could in turn result in poor long-term weight outcomes [18,19]. These disagreements could potentially also reflect biases on the part of the clinician, for example, a tendency to discredit a particular client’s accounts of their own behavior. In addition, suboptimal adherence to program guidelines (e.g., exceeding calorie targets) could potentially evoke feelings of shame that could decrease the likelihood that clients will openly share their eating patterns with their clinicians and contribute to a further decline in adherence [20,21].
Prior research has not investigated the degree to which client perceptions of their own adherence differ from the perceptions of their weight loss clinicians, or whether clients tend to provide higher or lower adherence ratings than their clinicians. Prior research has also not examined associations between these discrepancies and weight loss outcomes. Thus, using clinician and participant-completed measures of self-monitoring and reported calorie intake adherence collected weekly as part of a 6-month behavioral weight loss treatment, the aims of the present study were to (a) characterize measures of the participant and clinician-reported intervention adherence, as well as their relations with weight outcomes and degree of interrelation; (b) investigate the extent to which participants typically differed from clinicians in appraising participant levels of adherence, and (c) determine whether average discrepancies between participant and clinician adherence ratings predicted weight losses during treatment. Our hypotheses were that (i) higher levels of intervention adherence as reported by both clinicians and participants would predict improved weight loss outcomes and would be strongly intercorrelated; (ii) that on average, participants would provide higher ratings of intervention adherence than their clinicians, and (iii) that when controlling for a clinician’s average rating of adherence for each participant, higher participant-reported adherence ratings relative to clinicians would predict less weight loss.
METHODS
Parent Study
We conducted a secondary analysis of data collected from 468 individuals enrolled in a two-stage sequential multiple assignment randomized trial (SMART). The purpose of the parent study was to evaluate the optimal timing (3 vs. 7 weeks) to intervene with suboptimal responders to standard behavioral weight loss treatment (SBT), and to assess the relative efficacy of two alternate treatments, acceptance-based treatment (ABT) or portion-controlled meals (PCM), for individuals categorized as suboptimal responders. All individuals received 20 treatment sessions over 6 months from a weight loss clinician (Master’s-level or higher) and were then re-randomized to receive either ABT or PCM from this same clinician if classified as a suboptimal responder at Week 3 (<2.5% weight loss) or Week 7 (<5.0%). Of note, 47 participants (10.0%) experienced a change of clinician on at least one occasion during the study; thus, all statistical analyses examining associations between adherence measures and weight outcomes were repeated controlling for whether or not a participant experienced a clinician change, and as results were equivalent, they are not reported. Participants were provided with an option to track their calorie intake either on paper or digitally. Participants who reported being comfortable with smartphone technology were encouraged to track digitally and were allowed to use any mobile application dedicated to calorie tracking that they preferred, although the MyFitnessPal app was recommended. To estimate the calorie content of foods and beverages consumed, participants who opted to self-monitor digitally relied on the contents of their chosen mobile application’s dedicated nutrition database, whereas participants who opted to use paper records were provided with a book containing estimates of calorie content for a wide range of foods and beverages [22]. At the start of the intervention (Session 1), 83.0% of participants opted to use a mobile application to track calorie intake, and this proportion increased to 91.0% by the end of the intervention (Session 20). Participants were not given an option to provide more than one form of self-monitoring record. All participants received training in how to estimate calorie content based on portion sizes and limit intake, regardless of the self-monitoring method used. In addition, participants were expected to self-monitor calorie intake and limit their intake based on a pre-specified target (calorie goal) on all seven days of each week. Participants were assigned a calorie goal at baseline of either 1200, 1500 or 1800 kcal/day based on body weight, to induce 2–3 lbs of weight loss per week. Other details about randomization, recruitment and blinding procedures, and intervention content are described in a protocol paper that has been published previously [23]. The study was approved by the Institutional Review Board.
Participants
Adherence and weight data from all 468 participants were available for analysis. Participants were enrolled between May 2015 and September 2017 and met the following inclusion criteria: (a) 21–70 years old; (b) BMI ≥30.0 and ≤45 kg/m2; and (c) able to participate for 18 months. Exclusion criteria were as follows: (i) pregnant, breastfeeding or planning a pregnancy; (ii) currently involved in a weight loss program or diet intervention; (iii) reporting dietary restrictions (e.g., gluten-free); and (iv) presence of insulin-dependent diabetes. Enrolled participants were 76.3% female, 81.2% White, 11.8% African-American, 2.1% Asian, 0.4% American Indian, 1.1% belonging to another race and 3.4% identifying with multiple races. 5.1% of the participants reported Hispanic ancestry.
Measures
Weight was assessed twice prior to each treatment session, and also at the 6-month assessment, using a digital scale (Seca 876 Flat Scale). Participants were weighed in light clothing without shoes, and measurements differing by 0.2 kg or more were repeated for a third time. Weight data for each participant were averaged within sessions. Measures of each participant’s adherence to daily self-monitoring and calorie intake goals over the previous week were completed by participants immediately prior to each treatment session, after weighing and before the delivery of session content. These same measures were also completed by clinicians at the end of each session, based on a review of the participant’s printed digital or paper self-monitoring records. Participants completed both adherence measures without the clinician present, and the clinician collected both measures prior to beginning the session. Both participants and clinicians rated dietary self-monitoring adherence using a seven-point Likert scale (1–7) where higher scores reflect higher adherence to dietary self-monitoring (1 = no information recorded; 7 = all foods/meals recorded with detailed information recorded on 7 days of the week), and rated daily reported calorie intake adherence using an eight-point Likert scale (0–7) indicating the number of days over the previous week that the clinician or participant judged that the participant had met their assigned daily calorie goal (i.e., a score of 7 would indicate that the clinician or participant believed that the participant met the goal on all 7 days during the past week). For dietary self-monitoring adherence, clinicians were instructed to rate based on whether records were complete (i.e., whether meals/snacks appeared to be missing) rather than on whether daily calorie totals appeared to be accurate. For daily reported calorie intake adherence, clinicians were instructed to provide a rating of 0 if the participant did not turn in a self-monitoring record for that week; thus, a clinician-reported calorie intake adherence score of 0 could reflect either that no self-monitoring occurred or that the clinician judged that the participant did not achieve their calorie goal on any day during the previous week. This instruction was made based on an assumption that a lack of self-monitoring likely also reflects poor adherence to dietary guidelines, given robust associations between self-monitoring adherence and weight outcomes [16,24,25].
Statistical Analyses
All analyses were performed using R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria). Cumulative percent weight change (PWC), where more negative values indicate more weight lost, was calculated for each participant at each treatment session and the 6-month assessment visit as cumulative percentage of body weight lost since baseline. Associations between mean levels of intervention adherence (self-monitoring; calorie intake) and percent weight loss at 6 months were examined using Spearman correlations, as all adherence measures were negatively skewed. Associations were also examined between mean clinician and participant adherence ratings, also using Spearman correlations. To evaluate differences between clinician and participant adherence ratings, we calculated a mean discrepancy score for each participant on each adherence measure, with clinician scores subtracted from participant scores (thus, a positive discrepancy score would indicate that the participant rated their own adherence more highly than the clinician on average), and evaluated whether these scores significantly differed from zero using one-sample t-tests.
To examine associations between discrepancy scores and weight change during treatment, two separate linear mixed-effects models were constructed including self-monitoring (Model 1) and reported calorie intake (Model 2) discrepancy scores as predictors of cumulative percent weight change at each treatment session (approximately weekly) over the 6-month intervention period. As linear mixed-effects models are robust to unbalanced and missing within-subject data [26], session-level weight data were not imputed. We constructed three-level models including mean discrepancy score and session number (1–20) as subject-level (Level 2) predictors of session-level (Level 1) cumulative percent weight change, with subjects nested within clinicians (Level 3). Responder status (effect-coded such that 0 = categorized as a suboptimal responder at Week 3 or Week 7, and 1 = categorized as a responder at both timepoints) and mean clinician adherence ratings were also controlled for by including them as predictors in both models, as both variables were found to be associated with discrepancy scores. All predictors were standardized to have a variance of one and mean of zero, and cross-level interactions were included between discrepancy score and session number to examine associations with weight change over time. Residual plots were visually inspected to ensure normality, homoscedasticity and absence of outliers, and we confirmed that multicollinearity was not a concern (all variance inflation factors < 2.0). Both Models 1 and 2 allowed for slopes and intercepts for discrepancy scores to vary randomly as well as covary between both subjects and clinicians. Both models used restricted maximum likelihood estimation (REML), used an unstructured covariance matrix and were fit using the R packages lme4 [27] and lmerTest [28] (see Appendix 1 for R model specification, and Appendix 2 for random-effects variance estimates). Exploratory follow-up analyses investigated (a) whether linear mixed-effects model results were consistent across participant subgroups and (b) whether changes to discrepancy score variables from the first half (sessions 1–10) to the second half (sessions 11–20) of treatment were predictive of weight change over 6 months. For exploratory follow-up analyses, identical model specifications were used to Models 1 and 2.
RESULTS
Mean levels of adherence to self-monitoring were 5.28 (SD = 1.47) as rated by clinicians and 5.74 (SD = 1.29) as rated by participants. Consistent with the hypothesis that higher levels of intervention adherence would predict greater weight loss, adherence to self-monitoring was significantly negatively correlated with 6-month percent weight change (i.e., higher adherence predicted greater weight loss) for both clinicians and participants (rclinician = –0.48, p < 0.001; rparticipant = –0.39, p < 0.001). Mean reported calorie intake adherence was 4.10 (SD = 1.74) as rated by clinicians and 5.09 (SD = 1.38) as rated by participants, and reported calorie intake adherence measures were also significantly negatively correlated with 6-month percent weight change (rclinician = –0.50, p < 0.001; rparticipant = –0.32, p < 0.001). Strong correlations (r’s > 0.70) were observed between clinician and participant adherence measures (Table 1), indicating clinicians and participants were generally concordant in their appraisals of the participant’s adherence. Consistent with the hypothesis that participants would rate their own adherence more favorably than clinicians, mean discrepancy scores were positive and significantly different from zero for both self-monitoring (M = 0.47, SD = 0.72, 75.0% > 0, t(462) = 13.91, p < 0.001) and reported calorie intake (M = 0.99, SD = 1.15, 85.0% > 0, t(462) = 18.50, p < 0.001). Mean discrepancy scores by clinician ranged from 0.12–0.76 for self-monitoring and 0.32–1.52 for reported calorie intake. Of note, mean discrepancies were less positive among individuals categorized as responders than among individuals who were categorized as non-responders at any point during the intervention for both self-monitoring (t(438.87) = –1.97, d = 0.18, p = 0.049) and reported calorie intake (t(433.01) = –3.59, d = 0.33, p < 0.001) (see Appendix 3 for discrepancy scores by participant subgroup). In addition, discrepancy scores appeared to be more influenced by clinician than participant adherence ratings, being negatively correlated with clinician adherence ratings for both self-monitoring (r = –0.49, p < 0.001) and reported calorie intake (r = –0.63, p < 0.001) but uncorrelated with participant adherence ratings for either measure (r’s < 0.05).
Table 1 |.
Concordance (Spearman correlations) between clinician and participant intervention adherence measures
| Clinician-rated dietary self-monitoring adherence | Participant-rated dietary self-monitoring adherence | Clinician-rated reported calorie intake adherence | Participant-rated reported calorie intake adherence | |
|---|---|---|---|---|
| Clinician-rated dietary self-monitoring adherence | – | |||
| Participant-rated dietary self-monitoring adherence | 0.85*** | – | ||
| Clinician-rated reported calorie intake adherence | 0.79*** | 0.69*** | – | |
| Participant-rated reported calorie intake adherence | 0.50*** | 0.57*** | 0.76*** | – |
*p < 0.05, **p < 0.01, ***p < 0.001
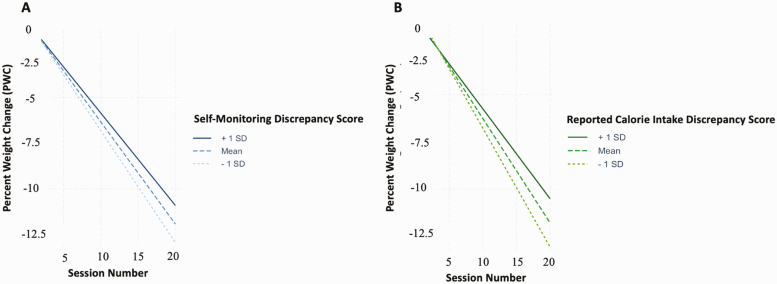
The results of Models 1 and 2 indicated that higher mean discrepancy scores were predictive of less weight loss for both self-monitoring (b = 0.30, SE = 0.02, df = 6937.89, t = 13.08, p < 0.001) and reported calorie intake (b = 0.34, SE = 0.02, df = 6905.08, t = 19.08, p < 0.001) (Table 2), consistent with the hypothesis that higher ratings of adherence relative to one’s own clinician would predict less weight loss during treatment. Model-estimated percent weight change by session as a function of mean discrepancy scores are presented in Fig. 1. Exploratory analyses indicated that these patterns were generally consistent across study subgroups (see Appendix 4). A follow-up analysis of simple slopes (R package interactions [29]) confirmed linear mixed-effects model results, indicating that whereas model-predicted 6-month weight losses evaluated at 1 standard deviation above the mean discrepancy score were 10.2% (95% CI [9.8%, 10.4%]) and 9.6% (95% CI [9.2%, 9.8%]) for self-monitoring and reported calorie intake, respectively, model-predicted weight losses evaluated at 1 standard deviation below the mean were 12.2% (95% CI [12.0%, 12.4%]) and 12.6% (95% CI [12.4%, 12.8%]) for self-monitoring and reported calorie intake, respectively. Exploratory follow-up analyses indicated that greater increases in discrepancy scores from the first to the second half of treatment were associated with less weight loss (see Appendix 5 for detailed results). Taken together, these findings indicate that participants typically appraised their own levels of adherence more highly than their clinicians, and that individuals who rated their adherence more favorably than their clinicians tended to lose less weight during treatment.
Table 2 |.
Results of linear mixed-effects models examining associations between discrepancy scores and percent weight change (PWC) during the 6-month intervention period
| Model 1 (self-monitoring) | Model 2 (reported calorie intake) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | df | t | p | Estimate | SE | df | t | p | |
| Intercept | 0.68 | 0.32 | 138.59 | 2.15 | 0.03* | 0.52 | 0.33 | 3.60 | 1.60 | 0.19 |
| Session number | –0.40 | 0.01 | 6935.09 | –29.80 | <0.001*** | –0.43 | 0.01 | 6934.93 | –56.22 | <0.001*** |
| Discrepancy score | –0.28 | 0.58 | 160.06 | –0.48 | 0.63 | –0.45 | 0.60 | 4.58 | –0.75 | 0.49 |
| Responder status | –3.11 | 0.20 | 486.14 | –15.71 | <0.001*** | –3.03 | 0.20 | 493.23 | –15.31 | <0.001*** |
| Clinician adherence Rating | –0.49 | 0.04 | 7161.76 | –12.91 | <0.001*** | –0.47 | 0.04 | 7117.99 | –12.33 | <0.001*** |
| Discrepancy score × session number | 0.30 | 0.02 | 6937.89 | 13.08 | <0.001*** | 0.34 | 0.02 | 6905.10 | 19.08 | <0.001*** |
*p < 0.05, **p < 0.01, ***p < 0.001. Subject and clinician were included as random effects. All models included a random slope and intercept for discrepancy score, used an unstructured covariance matrix and restricted maximum likelihood (REML) estimation. Satterwhaite approximations were used to estimate degrees of freedom and p-values. Session number ranged from 1 to 20 and responder status was effect-coded (0 = non-responder at either week 3 or week 7, 1 = responder). For Model 1, AIC = 29602.32, BIC = 29692.02. For Model 2, AIC = 29397.48, BIC = 29487.17.
Fig. 1.
Model-estimated percent weight change by session as a function of discrepancy score for both self-monitoring adherence (Model 1; A) and reported calorie intake adherence (Model 2; B). For both measures, change to weight over time was estimated at 1 standard deviation above the mean discrepancy score (1.19 for self-monitoring adherence; 2.14 for reported calorie intake adherence) as well as at the mean discrepancy score (0.47 for self-monitoring adherence; 0.99 for reported calorie intake adherence) and 1 standard deviation below the mean discrepancy score (–0.25 for self-monitoring adherence; –0.16 for reported calorie intake adherence).
DISCUSSION
The aims of the present study were to (a) examine associations between clinician and participant-reported measures of intervention adherence with weight loss outcomes during treatment, as well as interrelations among these measures, (b) characterize discrepancies between participants and clinicians on adherence measures, and (c) examine associations between adherence discrepancies and percent weight change during behavioral weight loss treatment. In line with our hypotheses, higher levels of adherence as reported by both participants and clinicians were associated with greater weight loss, whereas higher participant ratings of adherence relative to clinicians were associated with less weight loss. In addition, participants generally rated their own intervention adherence levels more favorably than their clinicians, and participants whose adherence ratings increased relative to their clinician’s ratings from the first to the second half of treatment also tended to lose less weight.
Our finding that higher ratings of adherence for both self-monitoring and calorie intake predicted greater weight loss during treatment is consistent with prior research [5,8,9], and also likely reflects that these two constructs are strongly interrelated, with higher self-monitoring adherence reflecting better adherence to dietary guidelines, as has been suggested by other authors [16,24]. The observation that on average, most participants provided higher ratings of adherence relative to their clinician is consistent with prior studies suggesting that errors when estimating one’s own level of adherence tend to be self-serving, that is, individuals overstate the degree to which they are actually adherent [14–16,30], which may result from either memory recall or social desirability biases [31,32] (e.g., due to a desire to appease one’s clinician or avoid criticism). Although it may be the case that participants in our sample tended to overestimate their own levels of adherence, it is also possible that clinicians tended to underestimate their participants’ levels of adherence. Clinicians in the present study had several forms of data available by which to assess adherence (i.e., weight change over time and self-monitoring records) and were also experienced with behavioral weight loss treatment. However, it remains possible that weight and self-monitoring data do not always provide a complete picture of a participant’s actual efforts to adhere in a given week, which could lead clinicians to mistakenly assume that clients were inadherent. For example, a clinician might exhibit biases towards discrediting a particular client’s accounts of their own behavior, or a participant might have limited calorie intake in line with their assigned daily calorie goal yet still fail to lose weight (e.g., if the goal was not sufficiently low), or, less likely, a participant might have misplaced rather than simply failed to complete self-monitoring records.
The finding that adherence discrepancies were negatively associated with weight loss could have several possible explanations. Potentially, individuals who rate their own adherence more highly than their clinicians generally tend to overestimate the quality of their own adherence, and this tendency results in a failure to sufficiently regulate eating behavior. This interpretation would be consistent with evidence suggesting that over-appraising one’s abilities in a particular behavioral domain can lead to complacency and poor performance [33–35]. However, discrepancies between clinicians and participants on adherence measures might also reflect problems in the therapeutic relationship, for example, poor communication on the part of either the clinician or the client, which could reduce participant engagement in treatment and negatively impact outcomes [18,19]. Some clients may also experience feelings of shame when they fail to meet dietary guidelines, which could further reduce treatment engagement and also contribute to weight gain by encouraging overeating [20]. Future research should attempt to distinguish which, if any of these potential explanations for our results is most accurate, for example by examining process variables or motivational constructs that mediate relationships between discrepancies in adherence reporting and weight loss outcomes.
The current study was limited by the fact that only certain aspects of adherence to weight control (self-monitoring and reported calorie intake) were evaluated, as data that were collected for other aspects of adherence (compliance with daily physical activity goals, completion of assigned homework) were rated on different scales between participants and clinicians and thus could not be used for analyses. Another limitation was that no objective measure of adherence to calorie intake was available and thus estimates of reported calorie intake adherence were based on self-report, which may be unreliable for measuring dietary intake [15,36]. However, because self-reported dietary recall appears to systematically underestimate objectively-measured calorie intake by only about 12%–25% [37,38], we believe that our measure of reported calorie intake adherence still provided valuable information about participants’ relative levels of dietary adherence. Furthermore, discrepancies between clinicians and clients on measures of a participant’s reported calorie intake adherence may still provide valuable clinical information (e.g., regarding the therapeutic relationship) independently of whether participant self-report estimates are objectively accurate. Lastly, as this was a secondary data analysis and all findings were correlational in nature, we were not able to determine whether clinician and participant adherence rating discrepancies were causally related to intervention adherence or merely reflect other factors known to impact these behaviors (e.g., therapeutic alliance, motivation).
Taken in sum, the present findings support that individuals receiving weight-loss treatment often appraise their own levels of adherence to weight control behaviors more favorably than their clinicians, and that such discrepancies may be a negative predictor of outcomes. Future research should thus investigate methods for decreasing the magnitude of these discrepancies by either improving intervention adherence or improving the methods through which adherence is assessed. For example, digital intervention systems such as smartphone applications and wearable self-monitoring devices that provide tailored, in-the-moment feedback or coaching based on continuous monitoring of dietary or behavioral data have shown promise for improving adherence [39,40] and can be used to prompt participants to better adhere to weight control behaviors during daily life [41]. Other technology-based methods such as digital photography could allow for a more objective assessment of adherence by both clients and clinicians [42]. Another possibility is that discrepancies in perceptions of adherence may need to be addressed directly by clinicians in a manner that does not lead clients to feel criticized or invalidated. For example, clinicians may rely on motivational interviewing strategies [43], work to identify and address any ruptures that have occurred in the therapeutic relationship [44], validate a client’s perceptions that adherence is challenging or express genuine concern about the client’s adherence patterns without passing blame. Other strategies for improving adherence have been summarized elsewhere and include greater specificity in assignment-giving, rehearsal of adherence activities and setting up a self-reward system, among others [45]. Thus, our findings highlight potential targets for enhancing intervention adherence, and thus improving weight loss outcomes, among individuals who do not initially respond well to behavioral weight loss treatment.
Appendix 1
| For Models 1 and 2, as well as models restricted to individual participant subgroups and models examining changes to discrepancy score from the first to second half of treatment as a predictor of percent weight change, the following R code specification was used (packages lme4, lmerTest):
lmer(PWC ~ SessionNumber*DiscrepancyScore + ResponderStatus + ClinicianAdherence + (DiscrepancyScore | Clinician/Subject), data=dat,na. action=”na.omit”)
| Subject | Clinician | Residual | |
|---|---|---|---|
| ICC | 0.50 | 0.01 | |
| Variance | 11.18 | 0.31 | 11.02 |
APPENDIX 2
Intraclass correlation and variance estimates associated with the random effects of subject and clinician on cumulative percent weight change (PWC) at each session.
Note. ICC and random effect variances were calculated using intercept-only models with no predictors included. ICC represents the proportion of total variance in percent weight change accounted for by differences between subjects and clinicians.
Appendix 3
| Descriptive statistics for discrepancy scores for entire sample as well as by participant subgroups.
| Dietary self-monitoring discrepancy score | Reported calorie intake discrepancy score | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Full sample (N = 468) | 0.47 | 0.72 | 0.99 | 1.15 |
| Responder (N = 209) | 0.39 | 0.63 | 0.76 | 1.01 |
| ABT-3 (N = 70) | 0.54 | 0.70 | 1.04 | 0.99 |
| ABT-7 (N = 57) | 0.41 | 0.53 | 1.09 | 1.01 |
| PCM-3 (N = 70) | 0.50 | 0.78 | 1.26 | 1.34 |
| PCM-7 (N = 62) | 0.48 | 0.63 | 0.98 | 0.93 |
Note. Responder = categorized as a responder for the duration of the intervention (i.e. at both Week 3 and Week 7); ABT-3 = re-randomized based on non-responder status to receive acceptance-based treatment at Week 3, including only data from Week 3 and later; ABT-7 = re-randomized based on non-responder status to receive acceptance-based treatment at Week 7, including only data from Week 7 and later; PCM-3 = re-randomized based on non-responder status to receive portion-controlled meals at Week 3, including data from only Week 3 and later; PCM-7 = re-randomized based on non-responder status to receive portion-controlled meals at Week 7, including data from only Week 7 and later.
Appendix 4
|Results of linear mixed-effects models evaluated within each participant subgroup (responders, individuals re-randomized to receive ABT at Week 3 or 7, individuals re-randomized to receive PCM at Week 3 or 7) examining associations between discrepancy scores and percent weight change (PWC) during the six-month intervention period. For simplicity and brevity, only cross-level interactions between discrepancy score and session number are presented (full model results are available upon request).
| Self-monitoring | Reported calorie intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | df | t | p | Estimate | SE | df | t | p | |
| Responder | 0.23 | 0.04 | 3004.95 | 6.20 | <0.001* | 0.26 | 0.03 | 3030.06 | 8.75 | <0.001* |
| ABT-3 | 0.05 | 0.05 | 874.40 | 1.09 | 0.27 | 0.11 | 0.05 | 879.43 | 2.36 | 0.01* |
| ABT-7 | 0.27 | 0.10 | 576.13 | 2.79 | 0.006* | 0.37 | 0.06 | 577.77 | 6.75 | <0.001* |
| PCM-3 | 0.25 | 0.06 | 989.13 | 4.06 | <0.001* | 0.29 | 0.04 | 996.01 | 6.61 | <0.001* |
| PCM-7 | 0.33 | 0.07 | 682.61 | 4.62 | <0.001* | 0.41 | 0.06 | 680.96 | 6.73 | <0.001* |
*p < 0.05, **p < 0.01, ***p < 0.001. Responder = categorized as a responder for the duration of the intervention (i.e., at both Week 3 and Week 7; N = 209); ABT-3 = re-randomized to receive acceptance-based treatment at Week 3, including only data from Week 3 and later (N = 70); ABT-7 = re-randomized to receive acceptance-based treatment at Week 7, including only data from Week 7 and later (N = 57); PCM-3 = re-randomized to receive portion-controlled meals at Week 3, including data from only Week 3 and later (N = 70); PCM-7 = re-randomized to receive portion-controlled meals at Week 7, including data from only Week 7 and later (N = 62). All models included Subject and Clinician as a random effect, included a random slope and intercept for Discrepancy Score, used an unstructured covariance matrix and restricted maximum likelihood (REML) estimation. Satterwhaite approximations were used to estimate degrees of freedom and p-values. Session Number ranged from 1–20 and Responder Status was effect-coded (0 = non-responder at either week 3 or week 7, 1 = responder).
Appendix 5
Results of linear mixed-effects models examining associations between mean change to discrepancy score (discrepancy change) between the first half of treatment (sessions 1–10) and second half of treatment (sessions 11–20) and percent weight change during the six-month intervention period.
| Self-monitoring | Reported calorie intake | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | df | t | p | Estimate | SE | df | t | p | |
| Intercept | 0.66 | 0.16 | 315.5 | 4.09 | <0.001*** | 0.60 | 0.16 | 307.8 | 3.69 | <0.001*** |
| Session number | –0.53 | 0.005 | 6685 | –104.78 | <0.001*** | –0.53 | 0.005 | 6658 | –95.04 | <0.001*** |
| Discrepancy change | –0.88 | 0.46 | 93.65 | –1.91 | 0.06 | –0.67 | 0.35 | 175.9 | –1.92 | 0.06 |
| Responder status | –3.25 | 0.29 | 429.7 | –14.55 | <0.001*** | –3.24 | 0.22 | 431.9 | –14.47 | <0.001*** |
| Clinician-rated adherence | –0.48 | 0.04 | 6861 | –11.91 | <0.001*** | –0.45 | 0.04 | 6857 | –11.15 | <0.001*** |
| Discrepancy change × session | 0.16 | 0.02 | 6651 | 8.94 | <0.001*** | 0.14 | 0.01 | 6608 | 9.78 | <0.001*** |
* p < 0.05. Discrepancy change scores were computed as the mean difference in discrepancy score from Sessions 1–10 to Sessions 11–20 for each subject and for each adherence measure. Mean change to self-monitoring adherence discrepancy from first to second half of treatment was 0.23 (SD = 0.82); mean change to reported calorie intake discrepancy from first to second half of treatment was 0.54 (SD = 1.18). All models included subject and clinician as a random effect, included a random slope and intercept for Discrepancy Score, used an unstructured covariance matrix and restricted maximum likelihood (REML) estimation. Satterwhaite approximations were used to estimate degrees of freedom and p-values. Session Number ranged from 1 to 20 and responder status was effect-coded (0 = non-responder at either Week 3 or Week 7, 1 = responder). For self-monitoring, AIC = 28543.6, BIC = 28632.73. For reported calorie intake, AIC = 28527.3, BIC = 28616.42.
Funding
This work was funded by grants from the National Cancer Institute (1R01CA188892) and the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK050456; P30DK092924).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards: Evan M. Forman receives royalties from Oxford University Press for a published acceptance-based treatment manual and is a member of the Scientific Advisory Board for Tivity Health. All other authors have no conflicts of interest to report.
Human Rights: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Transparency Statement: The parent trial for this study was pre-registered at ClinicalTrials.gov, identifier NCT02368002 (https://clinicaltrials.gov/ct2/show/NCT02368002). The analysis plan for the study was not formally pre-registered. De-identified data from this study are not available in a public archive. De-identified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the corresponding author. Analytic code used to conduct the analyses presented in this study are not available in a public archive. Some analytic code is presented in the Appendices, and other code may be available by emailing the first author. Materials used to conduct the study are not publicly available, although survey instruments may be available by emailing the first author.
References
- 1. Centers for Disease Control and Prevention. (2020). Adult Obesity Facts. Retrieved from https://www.cdc.gov/obesity/data/adult.html
- 2. Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34(4):841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–1872. [DOI] [PubMed] [Google Scholar]
- 4. Alhassan S, Kim S, Bersamin A, King AC, Gardner CD. Dietary adherence and weight loss success among overweight women: results from the A TO Z weight loss study. Int J Obes (Lond). 2008;32(6):985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarwer DB, Wadden TA, Moore RH, et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis. 2008;4(5):640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robinson AH, Adler S, Stevens HB, Darcy AM, Morton JM, Safer DL. What variables are associated with successful weight loss outcomes for bariatric surgery after 1 year? Surg Obes Relat Dis. 2014;10(4):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kong A, Beresford SAA, Alfano CM, et al. Self-monitoring and eating-related behaviors are associated with 12-month weight loss in postmenopausal overweight-to-obese women. J Acad Nutr Diet. 2012;112(9):1428–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wakayama L, Nameth K, Adler S, Safer DL. Replication and extension of dietary adherence as a predictor of suboptimal weight-loss outcomes in postbariatric patients. Surg Obes Relat Dis. 2019;15(1):91–96. [DOI] [PubMed] [Google Scholar]
- 9. Calugi S, Marchesini G, El Ghoch M, Gavasso I, Dalle Grave R. The association between weight maintenance and session-by-session diet adherence, weight loss and weight-loss satisfaction. Eat Weight Disord. 2020;25(1):127–133. [DOI] [PubMed] [Google Scholar]
- 10. Dubasi SK, Ranjan P, Arora C, et al. Questionnaire to assess adherence to diet and exercise advices for weight management in lifestyle-related diseases. J Family Med Prim Care. 2019;8(2):689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Linde JA, Rothman AJ, Baldwin AS, Jeffery RW. The impact of self-efficacy on behavior change and weight change among overweight participants in a weight loss trial. Health Psychol. 2006;25(3):282–291. [DOI] [PubMed] [Google Scholar]
- 12. Warziski MT, Sereika SM, Styn MA, Music E, Burke LE. Changes in self-efficacy and dietary adherence: the impact on weight loss in the PREFER study. J Behav Med. 2008;31(1):81–92. [DOI] [PubMed] [Google Scholar]
- 13. Carels RA, Harper J, Konrad K. Qualitative perceptions and caloric estimations of healthy and unhealthy foods by behavioral weight loss participants. Appetite. 2006;46(2):199–206. [DOI] [PubMed] [Google Scholar]
- 14. Macdiarmid J, Blundell J. Assessing dietary intake: Who, what and why of under-reporting. Nutr Res Rev. 1998;11(2):231–253. [DOI] [PubMed] [Google Scholar]
- 15. Lansky D, Brownell KD. Estimates of food quantity and calories: errors in self-report among obese patients. Am J Clin Nutr. 1982;35(4):727–732. [DOI] [PubMed] [Google Scholar]
- 16. Burke LE, Sereika SM, Music E, Warziski M, Styn MA, Stone A. Using instrumented paper diaries to document self-monitoring patterns in weight loss. Contemp Clin Trials. 2008;29(2):182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker RC, Kirschenbaum DS. Self-monitoring may be necessary for successful weight control. Behavior Therapy. 1993;24(3):377–394. [Google Scholar]
- 18. Martin LR, Williams SL, Haskard KB, Dimatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189–199. [PMC free article] [PubMed] [Google Scholar]
- 19. Kelley JM, Kraft-Todd G, Schapira L, Kossowsky J, Riess H. The influence of the patient-clinician relationship on healthcare outcomes: a systematic review and meta-analysis of randomized controlled trials. Plos One. 2014;9(4):e94207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bottera AR, Kambanis PE, De Young KP. The differential associations of shame and guilt with eating disorder behaviors. Eat Behav. 2020;39:101427. [DOI] [PubMed] [Google Scholar]
- 21. Hazzard VM, Loth KA, Hooper L, Becker CB. Food Insecurity and eating disorders: a review of emerging evidence. Curr Psychiatry Rep. 2020;22(12):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borushek A. The Calorie King: Calorie, Fat & Carbohydrate Counter 2013. Family Health Publications; 2013. [Google Scholar]
- 23. Sherwood NE, Butryn ML, Forman EM, et al. The BestFIT trial: A SMART approach to developing individualized weight loss treatments. Contemp Clin Trials. 2016;47:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boutelle KN, Kirschenbaum DS. Further support for consistent self-monitoring as a vital component of successful weight control. Obes Res. 1998;6(3):219–224. [DOI] [PubMed] [Google Scholar]
- 25. Goldstein SP, Goldstein CM, Bond DS, Raynor HA, Wing RR, Thomas JG. Associations between self-monitoring and weight change in behavioral weight loss interventions. Health Psychol. 2019;38(12):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldstein H. Multilevel Statistical Models. Vol. 922. Hoboken, NJ: John Wiley & Sons. 2011. [Google Scholar]
- 27. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:1406.5823. 2014. [Google Scholar]
- 28. Kuznetsova A, Brockhoff PB, Christensen RH. lmerTest package: tests in linear mixed effects models. J Stat Soft. 2017;82(13):1–26. [Google Scholar]
- 29. Long JA. (n.d.). Interactions: comprehensive, user-friendly toolkit for probing interactions. Available at https://interactions.jacob-long.com/. Retrieved 10 July 2020. [Google Scholar]
- 30. Myers RJ, Klesges RC, Eck LH, Hanson CL, Klem ML. Accuracy of self-reports of food intake in obese and normal-weight individuals: effects of obesity on self-reports of dietary intake in adult females. Am J Clin Nutr. 1988;48(5):1248–1251. [DOI] [PubMed] [Google Scholar]
- 31. Flett JAM, Fletcher BD, Riordan BC, Patterson T, Hayne H, Conner TS. The peril of self-reported adherence in digital interventions: a brief example. Internet Interv. 2019;18:100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vancouver JB, Thompson CM, Tischner EC, Putka DJ. Two studies examining the negative effect of self-efficacy on performance. J Appl Psychol. 2002;87(3):506–516. [DOI] [PubMed] [Google Scholar]
- 34. Moores TT, Chang JC-J. Self-efficacy, overconfidence, and the negative effect on subsequent performance: a field study. Inform Manage. 2009;46(2):69–76. [Google Scholar]
- 35. Vancouver JB, Thompson CM, Williams AA. The changing signs in the relationships among self-efficacy, personal goals, and performance. J Appl Psychol. 2001;86(4):605–620. [DOI] [PubMed] [Google Scholar]
- 36. Schoeller DA, Thomas D, Archer E, et al. Self-report-based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr. 2013;97(6):1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freedman LS, Commins JM, Moler JE, et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol. 2014;180(2):172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Subar AF, Freedman LS, Tooze JA, et al. Addressing current criticism regarding the value of self-report dietary data. J Nutr. 2015;145(12):2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Turk MW, Elci OU, Wang J, et al. Self-monitoring as a mediator of weight loss in the SMART randomized clinical trial. Int J Behav Med. 2013;20(4):556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cavero-Redondo I, Martinez-Vizcaino V, Fernandez-Rodriguez R, et al. Effect of behavioral weight management interventions using lifestyle mhealth self-monitoring on weight loss: a systematic review and meta-analysis. Nutrients. 2020;12(7):1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Conroy MB, Yang K, Elci OU, et al. Physical activity self-monitoring and weight loss: 6-month results of the SMART trial. Med Sci Sports Exerc. 2011;43(8):1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin CK, Nicklas T, Gunturk B, Correa JB, Allen HR, Champagne C. Measuring food intake with digital photography. J Hum Nutr Diet. 2014;27 Suppl 1:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Dorsten B. The use of motivational interviewing in weight loss. Curr Diab Rep. 2007;7(5):386–390. [DOI] [PubMed] [Google Scholar]
- 44. Summers RF, Barber JP. Therapeutic alliance as a measurable psychotherapy skill. Acad Psychiatry. 2003;27(3):160–165. [DOI] [PubMed] [Google Scholar]
- 45. Levy RL, Feld AD. Increasing patient adherence to gastroenterology treatment and prevention regimens. Am J Gastroenterol. 1999;94(7):1733–1742. [DOI] [PubMed] [Google Scholar]