Abstract
Weight loss outcomes among young adults in technology-based programs have been equivocal. The purpose of this study was to deliver digital weight loss treatments to young adults and examine the 6, 12, and 18 month effects on weight loss. Young adults with overweight/obesity (N = 459; 23.3 ± 4.4 years) were recruited from two university sites and randomly assigned to receive through Facebook and text messaging either personalized (TAILORED; n = 150) or generic (TARGETED; n = 152) weight loss information, messages, and feedback or general healthy body content (e.g., body image, sleep; CONTROL; n = 157). The study was powered to detect a 2.1-kg difference at all time points with the primary outcome being 18 months. There was no overall effect of treatment group on 6, 12, or 18 month weight loss (ps = NS). However, at 6 months, those in TAILORED who were highly engaged (completing >66%) lost more weight compared to CONTROL (−2.32 kg [95% confidence intervals: −3.90, −0.74]; p = .004), with the trend continuing at 12 months. A significant baseline body mass index (BMI) by treatment group interaction (p = .004) was observed at 6 months. Among participants in the lowest baseline BMI category (25–27.5 kg/m2), those in TAILORED lost 2.27 kg (−3.86, −0.68) more, and those in TARGETED lost 1.72 kg (−3.16, −0.29) more than CONTROL after adjusting for covariates. Among participants with a BMI between 27.5 and 30 kg/m2, those in TAILORED lost 2.20 kg (−3.90, −0.51) more than participants in TARGETED. Results did not persist over time with no treatment interaction at 12 or 18 months. Initial body weight should be considered when recommending weight loss treatments for young adults. More intensive interventions or stepped care approaches may be needed for young adults with obesity.
Keywords: Young adults, Weight loss, Digital interventions, Tailoring, Obesity
Few large-scale weight loss studies have specifically focused on young adults. Young adults have not been as successful in weight loss clinical trials as their older adult counterparts. This study is an 18 month randomized controlled trial comparing two digital weight loss programs adapted for young adults (one with personalized material and the other with generic material) to a control group. There were no differences in weight loss between the groups at any time point when looking at the entire sample. However, we found that initial body mass index (BMI) interacted with treatment group such that those young adults in the lowest BMI category who received one of the two digital weight loss programs lost more weight than the control group. We also found that of participants who were highly engaged (specifically completing at least 66% of the materials), those in the personalized group lost more weight than the control group at 6 and 12 months, along with a similar advantage over those receiving the generic materials at 12 months. The findings indicate that young adults with obesity (BMI >30) may need a stepped care or precision medicine approach that involves greater intensity and contact.
Implications.
Practice: Initial body weight should be taken into consideration when recommending the type and nature of weight loss treatments for young adults. Lower intensity or lower “touch” treatments, such as those delivered via digital channels, may be of sufficient intensity for young adults with overweight. Young adults with obesity (body mass index [BMI] >30) may need a stepped care or precision medicine approach that involves greater intensity and contact.
Policy: Screening based on BMI should be included as part of primary care and university prematriculation physical examinations with young adults being offered tailored treatment recommendations based on weight status.
Research: Further research is needed to better understand the behavioral and psychosocial factors that predict engagement in weight loss programs, as well as the optimization of precision or stepped care approaches that could be more effective for young adults with obesity.
INTRODUCTION
The prevalence of obesity (body mass index [BMI] ≥30 kg/m2) among young adults (aged 20–39 years) living in the USA is currently estimated at 40% among this age group [1]. Obesity in young adulthood tracks into middle age and older age, thereby increasing the lifetime burden of chronic diseases, such as metabolic syndrome, Type 2 diabetes, and cardiovascular disease [2, 3]. The transition from adolescence to early adulthood may be a particularly vulnerable time for excessive weight gain as major life changes and time-consuming obligations often disrupt the ability to be physically active or to eat healthy, and unhealthy behaviors adopted at this time become more difficult to alter as people get older [4, 5]. Young adulthood, therefore, is an opportune time for mitigating the risk of excessive weight gain and associated cardiometabolic disease.
Young adults have not been as successful in weight loss programs as have their older counterparts [6]. The EARLY trials were a consortium of research trials funded by the National Institutes of Health to address obesity among young adults aged 18–35 [7]. Of the seven funded trials, only three focused on weight loss [8–10] and reported significant 6 month weight loss differences between the intervention and the control groups of −1.33 kg (Project SMART [10]); −1.92 kg (CITY Trial [8]); and −3.5 kg (Standard) and −5.9 kg (Enhanced; IDEA Trial [9]). The interventions listed above included group and individual in-person contact [8, 9], as well as health educator telephone contacts [10]. In contrast, the intervention delivered solely via a cellphone app in the CITY trial [8] did not result in significantly different weight loss compared with the control group (−0.87 kg vs. −1.14 kg at 6 months). Technology-based programs may appeal to young adults given the uptake in this population segment [11] and may address barriers such as time, cost, and preference for the receipt of information [12, 13], with trials indicating success of technology-based weight loss programs for college students (e.g., [14, 15]). However, examining digital interventions for delivering weight loss treatments specifically to young adults remains a largely underdeveloped area.
While technology can allow for scalable interventions, a key element of effective interventions is how to deliver persuasive messages using digital channels. Health communication messaging strategies delivered through these channels can be classified as targeted (generic based on an audience characteristic) or tailored (personalized based on individual characteristics or assessment) [16, 17]. Targeted messages are developed with a “one-size-fits-all” approach and are designed to be appealing to a broad segment. Tailored messaging involves a level of personalization to deliver messaging relevant to one specific individual [18]. Tailored interventions have been shown to outperform nontailored [19], potentially due to the salience of the message, greater perceived relevance, and enhanced motivation to process the message or behavior change [20]. However, there are considerations associated with delivering targeted or tailored interventions, such as the ability to reach a broad audience, development and implementation cost, and participant time and burden [21].
The use of technology for delivering health information to young adults presents novel opportunities to disseminate evidence-based weight programs that have been adapted to a digital platform [22]. Unfortunately, there is a paucity of information on the effectiveness of digital strategies for weight loss in this age group, as well as for whom these interventions might be most effective. Commercially available platforms, such as a Facebook, can be used to deliver intervention content. However, the amount of tailoring and personalization needed with these digitally delivered interventions is not clear and may vary by participant characteristics like age, sex, or by weight status itself. Thus, the purpose of this study was to determine the differential effects over 18 months of either a tailored or targeted weight loss intervention compared with a general health education control condition, with all three arms being delivered digitally via Facebook and SMS text messaging. The primary hypothesis was that both treatment interventions would be successful in reducing body weight, with the tailored treatment resulting in greater weight loss (5%) compared with the targeted (2.5%) or control conditions due to its greater specificity and personalization. The study was powered to detect a 2.1-kg difference at all time points with the primary outcome being 18 months.
METHODS
Participants
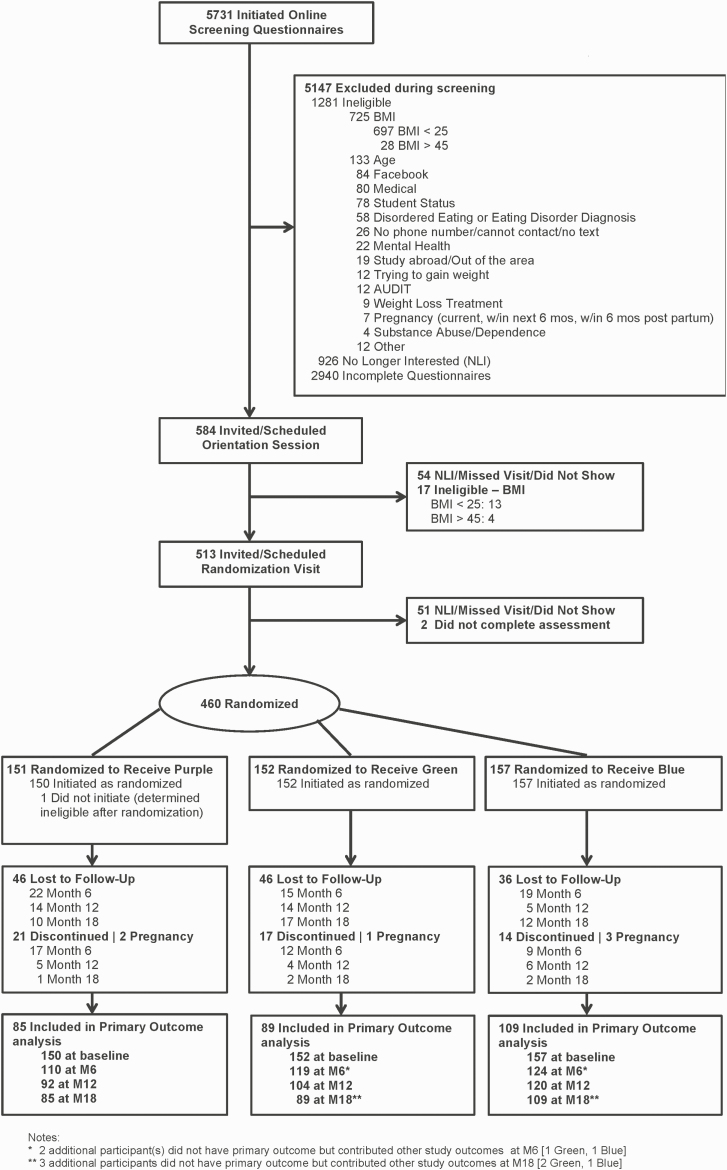
Participants were recruited from the George Washington University and the University of Massachusetts Boston by mass e-mailings, flyers, and in-person events [23] between 2015 and 2018. Eligible participants had a measured BMI between 25 and 45 kg/m2, were between 18 and 35 years of age and a university student (undergraduate or graduate) in the Washington, DC, or Boston areas, and had no known contraindications for participating in weight loss and physical activity. Furthermore, they were active on Facebook (indicated by a log-in within the prior month) with regular text message access and were fluent in English. Additional information on study design and inclusion/exclusion criteria has been described elsewhere [24]. The recruitment strategies yielded 5,731 respondents, with 584 being invited for an orientation session, 513 eligible for randomization, and 460 randomly assigned to one of three treatment arms (Fig. 1). One participant was determined to be ineligible following randomization and did not initiate treatment, leaving an analysis sample of 459. The study was registered on clinicaltrials.gov (NCT02342912). Study participants provided written informed consent and all study procedures were approved by the institutional review boards of both universities.
Fig 1.
Consort flow diagram for 18 months.
Measures
Sociodemographic characteristics of the participants were obtained via self-report at baseline. Physical activity, dietary, and cardiometabolic assessments were also collected [24]. Weight and height were measured at baseline and again at 6, 12, and 18 months using a digital scale (Seca Model 769) and standard portable stadiometer. All measures were taken in duplicate after participants removed bulky outer clothing and shoes. Weight was recorded to the nearest 0.2 kg and height was recorded to the nearest 0.1 cm. During the majority of the trial, weight was measured in a fasted state at baseline, Month 6 ,and Month 18 due to accompanied blood draws. However, at Month 12, weight was measured in a postprandial state for some participants. Body mass index then was calculated as kilograms per square meter. For those participants unable to attend a clinic visit due to moving from the area (M06: n = 2, M12: n = 5, M18: n = 11), a virtual weight visit was conducted via live or prerecorded video call in which the participant calibrated his/her scale with a known weight and then self-weighed.
Procedures and overview of interventions
Following baseline clinic-measured height and weight, a block randomization, stratified by study site and BMI group (<30 or ≥30 kg/m2), was implemented via the REDCap [25] randomization function. Participants were randomly assigned to one of three digitally delivered intervention groups. Two of the interventions (TARGETED and TAILORED) were weight loss focused and included content adapted from the Diabetes Prevention Program (DPP) for online delivery to young adults. Participants in both of the weight loss interventions were given calorie, weight loss [26], and physical activity goals at each of the assessment visits. The initial weight loss goal was 7%, with a calorie goal range from 1,200 to 2,000 depending on initial body weight, and recommendation to build up to 250 min of moderate-to-vigorous physical activity per week. Participants also were given self-monitoring tips and a list of apps for tracking. The third intervention (CONTROL) included wellness content related to healthy body weight in young adults (e.g., sleep, stress, and body image). All three interventions included a Facebook delivery component, text messaging, and weekly reports and were delivered over an 18 month period. All participants were sent a “friend” request and were enrolled in a private Facebook group corresponding to their group assignment. Participants were also given Facebook privacy settings recommendations. There were a total of 38 content delivery cycles (weekly during the first 6 months, biweekly for Months 7–9, monthly for Months 10–12, and bimonthly for Months 13–18); all intervention components were delivered during these cycles. For more details on these procedures, see [removed to ensure blinded review] [24].
Intervention descriptions
TARGETED intervention (n = 152).
Each week, content was posted to Facebook on 5 days. Postings included: (a) a didactic video (range: 1:30–7:42 min) summarizing the adapted DPP-lesson topic for the week with handouts to accompany the didactic video; (b) a peer-led video (range: 0:29–13:09 min), which depicted a prescripted situation of young adults modeling a key behavioral skill or problem-solving message; (c) a poll or discussion; (d) wrap-up and live moderated session; and (e) a reminder to review the weekly report. Sample didactic lesson topics included: Tip the Calorie Balance; Jumpstarting Your Activity Plan; and Ways to Stay Motivated. The private Facebook groups were monitored by study staff to validate participation (e.g., “liking” a participant’s post) and for inappropriate postings. Additionally, participants received text messaging each day. The purpose of the text messaging was to reinforce the self-monitoring and provide tips about monitoring (e.g., “Set up an announcement on ur computer asking if u self-monitored today, that way u can’t miss it”) and high-risk weight related behaviors, such as prolonged sitting, late night snacking, portion size (e.g., “U might feel the urge to late night eat bc ur bored! Call a friend to chat to keep u occupied”; Table 1 for the timing and nature of the text messages). For the Targeted group, self-monitoring questions were generic (i.e., What did you monitor [weight, calories, and physical activity minutes]?), and high-risk behavior tips were randomly selected from a pregenerated list. At the end of each content week, participants received a report summarizing the topic for the week. For further detail on the intervention and sample, content see [removed to ensure blinded review] [24].
Table 1.
Text message type and frequency by treatment group
| SMS message type | Targeted | Tailored | Control | |
|---|---|---|---|---|
| Tips related to monitoring | 2 texts per week | 2 texts per week | 2 texts per week | |
| Prompts related to self-monitoring | Query regarding which behaviors were monitored | 1 text per week (W, C, P) | 2 texts per week (W, C, P) | 1 text per week (M, E, B) |
| Prompt for data to drive tailored messaging | – | 6 texts per week AM reminder (n = 3/week)/ PM prompt for data (n = 3/week) | – | |
| Feedback on the number of days successfully submitted monitoring data | 1 text per week | |||
| Tips to address high-risk weight loss behaviors | 2 texts per week (general) | 2 texts per week (personalized) | 2 texts per week (general) | |
| Reminder to review Facebook content | 2 texts per week | 2 texts per week | 2 texts per week | |
Total number of days per week receiving a text for all groups was 7. The total number of outgoing texts per week was 7 for targeted and control and 15 for tailored.
B, body image; C, calories; E, energy; M, mind; P, physical activity minutes; W, weight.
TAILORED intervention (n = 157).
The Facebook component was the same as that in the TARGETED intervention group. Similar to the TARGETED group, text messaging was centered on self-monitoring and high-risk eating behaviors. The TAILORED group received text messaging with specific prompts to report weight, calorie, and minutes of physical activity. This information was used to generate a personalized feedback report that included individual weight and physical activity progress. Tips on high-risk behaviors for the TAILORED group were delivered based on participant’s own selection of behaviors they anticipated to be most challenging. The TAILORED intervention provided personalized, specific feedback that was delivered via a weekly personal report. At the completion of each content week, participants received a report that included a summary of the weekly topic, as well as their personalized, specific feedback on their progress.
CONTROL (n = 157).
Participants in the CONTROL group received general healthy body content on three branded topic areas, mind, body, and energy. Sample weekly topics included Technology and Your Sleep, Building Body Attitude, and Signs of Stress. The content was educational rather than focused on specific behavior change. The Facebook delivery and content structure were the same as that for the TARGETED intervention group and, similar to the TARGETED group, text messaging was centered on generic self-monitoring and tips. At the end of each content week, participants received a report summarizing the topic for the week.
Intervention fidelity for all three groups was determined by monitoring participant engagement with the three main components of program delivery: (a) accessing the Facebook group (i.e., seeing, liking/loving, or commenting on a Facebook post); (b) responding to text messages; and (c) viewing weekly reports. Participants were contacted for nonengagement at prespecified intervals. A weekly engagement score was assigned that ranged from 0 to 3, depending upon the number of components a participant completed. A total engagement score, range 0–114, was calculated for all 38 content weeks. Retention strategies were employed, which included incentives for attending study measurement visits and additional incentives (e.g., travel vouchers and raffle prizes).
Statistical analysis plan
Univariate descriptive statistics are presented as mean ± standard deviation [SD], frequencies (%), or median [interquartile range] for nonnormally distributed data. Tests of group differences, including interactions, were modeled through constrained longitudinal data analysis [27]. This method is similar to applying an analysis of covariance at each time point while controlling for baseline values but is typically better [28] in accounting for missing data under the commonly applied “missing at random” assumption. In addition to constraining baseline weight as equal across the three groups, age, height, sex, and study site were included as covariates. Potential biases due to missing outcome data were assessed visually by comparing the longitudinal curves for those lost to follow-up after 6 and 12 months with those having complete outcome data through 18 months. Influential cases were identified through the examination of the distribution of the following regression-based influence statistics: (a) the restricted likelihood distance; (b) Cook’s D; and (c) the PRESS statistic.
To examine whether intervention effects would vary by initial body weight status, the longitudinal mixed-model regression described above was repeated by removing height and adding a third-order interaction term of baseline BMI × Study group × Follow-up visit in an exploratory analysis. Statistically significant interaction results were followed by the Johnson–Neyman technique [29], which provided an estimate of the cut point of baseline BMI where treatment effects become statistically significant. Based on the observed distribution of engagement scores and a literature review, high engagement was defined as the top tertile of scores, corresponding to engaging with 66% of the content.
The current trial was powered to detect a mean difference in weight loss of 2.1 kg between any two groups at any of the three follow-up times with the primary outcome being 18 months. A prepost correlation of r = .91 was assumed along with a within-group SD of 12 kg, and a desired statistical power of β = 0.80. Analyses were conducted in SAS 9.4 (with STAT/STAT version 14.3) and R, with the primary outcome at an alpha level (two-sided) of α = 0.016 based on a Bonferroni adjustment for three paired comparisons for the primary hypothesis. For secondary exploratory outcomes, p-values <.05 were considered statistically significant without adjustments made for multiple tests.
RESULTS
Demographic and anthropometric baseline characteristics were similar across the three study groups (Table 2). Of the 459 eligible randomized participants, 77%, 69%, and 62% had primary outcome data at the 6, 12, and 18 month visits, respectively (Fig. 1). Retention rates were similar for the three groups: 57%, 59%, and 69% for TAILORED, TARGETED, and CONTROL at 18 months, respectively.
Table 2.
Study participant characteristics by study group
| All | Tailored | Targeted | Control | |||||
|---|---|---|---|---|---|---|---|---|
| N | N | N | N | |||||
| Demographics | ||||||||
| Age (years) | 459 | 23.3 ± 4.4 | 150 | 23.4 ± 4.4 | 152 | 23.5 ± 4.4 | 157 | 22.9 ± 4.4 |
| Sex | ||||||||
| % Male | 98 (21.4%) | 26 (17.3%) | 33 (21.7%) | 39 (24.8%) | ||||
| % Female | 361 (78.6%) | 124 (82.7%) | 119 (78.3%) | 118 (75.2%) | ||||
| Race/ethnicity | ||||||||
| % African American | 91 (19.8%) | 33 (22.0%) | 36 (23.7%) | 22 (14.0%) | ||||
| % Non-Hispanic White | 225 (49.0%) | 70 (46.7%) | 76 (50.0%) | 79 (50.3%) | ||||
| % Asian/Hawaiian Pac. Is. | 43 (9.4%) | 13 (8.7%) | 13 (8.6%) | 17 (10.8%) | ||||
| % Hispanic (any race) | 62 (13.5%) | 23 (15.3%) | 20 (13.2%) | 19 (12.1%) | ||||
| % Multiracial/unknown/ refused | 38 (8.3%) | 11 (7.3%) | 7 (4.6%) | 20 (12.7%) | ||||
| Academic year | ||||||||
| % Undergraduate | 274 (59.7%) | 89 (59.3%) | 87 (57.2%) | 98 (62.4%) | ||||
| % Graduate | 185 (40.3%) | 61 (40.7%) | 65 (42.8%) | 59 (37.6%) | ||||
| School | ||||||||
| % GWU | 234 (51.0%) | 77 (51.3%) | 76 (50.0%) | 81 (51.6%) | ||||
| % UMB | 225 (49.0%) | 73 (48.7%) | 76 (50.0%) | 76 (48.4%) | ||||
| Anthropometrics | ||||||||
| BMI (kg/m2) | 459 | 31.2 ± 4.4 | 150 | 31.6 ± 4.6 | 152 | 31.1 ± 4.4 | 157 | 31.0 ± 4.3 |
| BMI category | ||||||||
| % <30 | 225 (49.0%) | 72 (48.0%) | 75 (49.3%) | 78 (49.7%) | ||||
| % ≥30 | 234 (51.0%) | 78 (52.0%) | 77 (50.7%) | 79 (50.3%) | ||||
| MVPA per day | 408 | 44.5 ± 23.8 | 137 | 44.3 ± 20.3 | 138 | 42.0 ± 24.0 | 133 | 47.3 ± 26.7 |
| MVPA per day | ||||||||
| % <150 | 113 (24.6%) | 27 (18.0%) | 42 (27.6%) | 44 (28.0%) | ||||
| % 150 to <300 | 153 (33.3%) | 57 (38.0%) | 50 (32.9%) | 46 (29.3%) | ||||
| % ≥300 | 193 (42.0%) | 66 (44.0%) | 60 (39.5%) | 67 (42.7%) | ||||
| Total caloric intake | 452 | 1,827.2 ± 696.1 | 148 | 1,883.9 ± 625.7 | 149 | 1,757.1 ± 675.3 | 155 | 1,840.5 ± 774.0 |
BMI, Body Mass Index; GWU, The George Washington University; MVPA, moderate-to-vigorous physical activity; UMB, University of Massachusetts Boston.
A descriptive analysis of baseline demographic variables, weight, and BMI revealed that participants without any follow-up data (baseline visit only) were more likely to be younger (p = .006), undergraduates (p = .008), and underactive (<150 min/week of moderate-to-vigorous intensity physical activity; p = .015) compared with those who had data at any time point. For each of the three study groups, mean weight trends were compared descriptively between participants who dropped out at different times. These trends were similar for the TAILORED and CONTROL groups, but TARGETED participants who dropped out after 6 months showed an average weight gain during the 0–6 month period compared to a weight loss among those who remain in the study. Influence statistics generated through mixed-model regression analyses identified two values for body weight that were potentially influential on model coefficients. When those values were deleted from the primary analysis on body weight, the findings were not substantively different. Therefore, these two values were retained in the reported analyses.
Main effects
Full sample
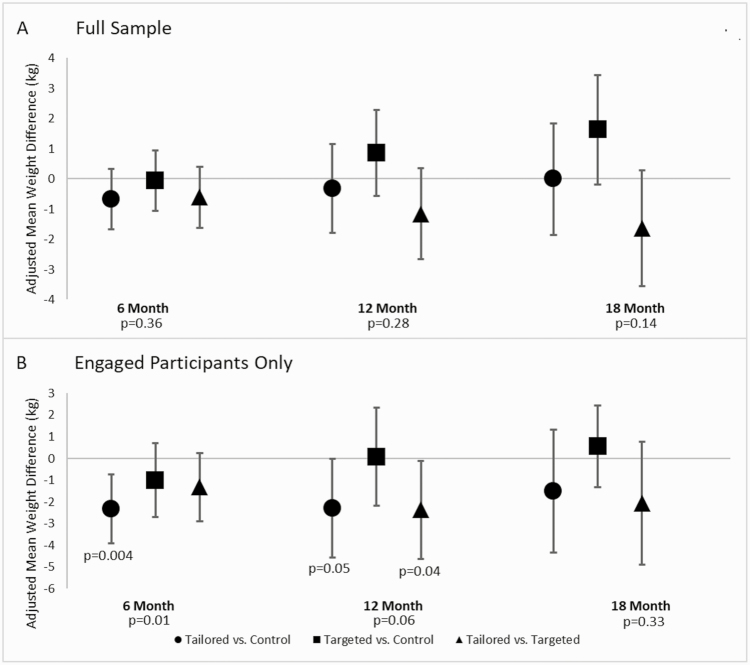
Unadjusted change in body weight is displayed in Supplementary Table 1. Adjusted trends in body weight over the follow-up by study group are displayed in Fig. 2A and Supplementary Table 2. As indicated, the TAILORED group showed a slightly steeper decline in weight between baseline and 6 months compared with the other two groups: −0.06 kg (95% confidence intervals [CIs]: [−1.05, 0.92]) versus TARGETED and −0.67 kg (95% CIs [−1.68, 0.34]) versus CONTROL; however, these differences were not statistically significant. By 18 months, any apparent advantage in weight loss for the TAILORED group disappeared.
Fig 2.
Study main effects for the full sample and highly engaged participants. *p < .05 for the pairwise comparison. Panel A shows adjusted mean weight differences for the full sample. No statistically significant differences were found between the treatment groups. Panel B shows adjusted mean weight differences among the engaged participants (66%+ engagement). Among this highly engaged subset, the TAILORED group lost more weight than the CONTROL group at 6 and 12 months, along with a similar advantage over the TARGETED group at 12 months. By 18-months, these differences were no longer significant.
Highly engaged participants only
We then repeated the longitudinal analysis of main effects among only the study participants who showed evidence of substantial engagement (defined as accomplishing at least 66% of the study intervention components [n = 146]). Among the subset of participants with high engagement at 6 (n = 137), 12 (n = 127), and 18 months (n = 114), global p-values for treatment group differences were p = .01 at 6 months, p = .06 at 12 months, and p = .33 at 18 months. Among this subset of participants with high engagement, the TAILORED group lost significantly more weight compared with the CONTROL group at 6 months (−2.32 kg, 95% CIs [−3.90, −0.74]; p = .004). At 12 months, there was a similar advantage for the TAILORED group versus CONTROL (−2.28 kg, 95% CIs [−4.55, −0.01]; p = .05), as well as an advantage for the TAILORED group over the TARGETED group (−2.36, 95% CIs [−4.61, −0.10]; p = .04). These between-group differences disappeared by 18 months (Fig. 2B and Supplementary Table 2).
Effect modification by baseline BMI
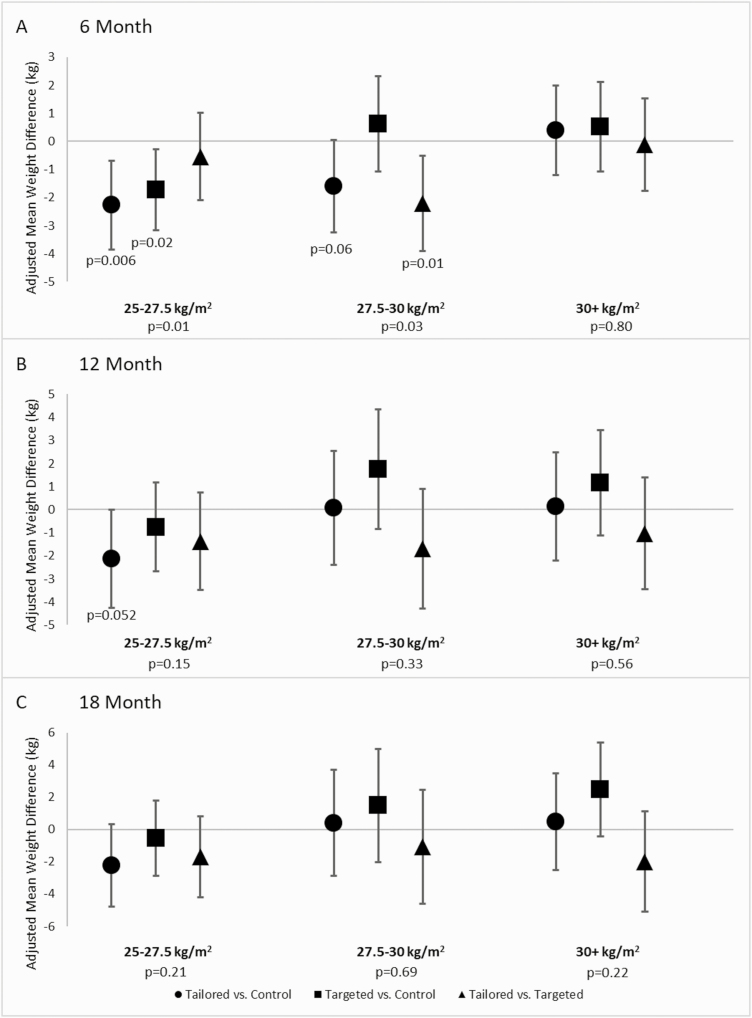
We observed a statistically significant interaction between baseline BMI and study group at 6 months (p = .005) but not at 12 (p = .343) or 18 (p = .059) months. We then used the Johnson–Neyman technique on the 6 month data to identify a cut point of baseline BMI that may have given a weight loss advantage to one study group over another. This technique identified that when baseline BMI is 30.1 kg/m2 or greater, neither treatment group was superior to the CONTROL condition. To illustrate this interaction, the study sample was divided according to a BMI <30 or BMI ≥30 kg/m2, and then those with a BMI <30 kg/m2 were divided again into two similarly sized groups. As indicated in Fig. 3 and Supplementary Table 2 among those with the lowest BMI (25 to <27.5 kg/m2) (n = 84), both the TAILORED (−2.27 kg, 95% CIs [−3.86, −0.68]; p = .006) and the TARGETED groups (−1.72 kg, 95% CIs [−3.16, –0.29]; p = .02) experienced greater weight losses at 6 months compared with the CONTROL group. Among those with a baseline BMI between 27.5 and <30 kg/m2 (n = 87), only the TAILORED intervention appeared superior to the TARGETED intervention for weight loss (−2.20 kg, 95% CIs [−3.90, −0.51]; p = .01). Among those with a BMI >30 kg/m2 (n = 183), weight loss at 6 months was similar for the three study groups. Although the global interaction tests at 12 and 18 months did not reach statistical significance, among those in the lowest baseline BMI category, there was a trend for greater weight loss or attenuated weight gain in the TAILORED group compared with the other two groups.
Fig 3.
Adjusted mean weight change by study group according to baseline body mass index (BMI) category. *p < .05 for the pairwise comparison. This figure depicts the interaction between baseline BMI category and treatment group at the three follow-up time points. As indicated in Panel A, at 6 months among those in the lowest BMI category, both the TAILORED and the TARGETED groups experienced greater weight losses compared with the CONTROL group. Similarly, among those in the middle BMI category, the TAILORED intervention participants experienced greater weight losses than the TARGETED. There were no differences found among those with a BMI >30 kg/m2 or at the 12 or 18 month follow-up time points.
DISCUSSION
Digital delivery of interventions can mitigate time-, travel-, and stigma-related barriers to in-person delivery. Despite showing feasibility of delivering an adapted DPP program to young adults via digital channels, results from this trial were not as hypothesized. We observed no main effects of treatment on weight loss at 6, 12, or 18 months; however, we did observe a significant interaction between treatment and baseline BMI at 6 months, indicating that the TAILORED and TARGETED interventions were successful only in those participants with a BMI <30 kg/m2 at the start of the study. Similar findings were noted in a workplace sample with those of achieving 5% weight loss at 1 year having lower-median BMIs compared with those who did not meet the 5% threshold [30]. This effect modification by BMI suggests that a greater intervention dose (e.g., physical activity and/or caloric restriction, more in-depth problem solving) or different modality (e.g., video chat versus text) [31] may have been necessary to induce and sustain meaningful weight loss in those with obesity. This is consistent with other findings that those with BMIs <35 had better weight loss outcomes with self-directed treatment, whereas BMI did not moderate the effect for a counselor-led intervention [32]. Those who have more established obesity may require “precision medicine” [33] (i.e., individualization based on biological, lifestyle, or environmental factors) or “stepped care” [34] (i.e., customization based on weight progress) approaches. It also may be possible that those with obesity had greater outcome expectations for behaviors that lead to weight loss and may have become discouraged when their expectations were not met [35]. This interaction effect was not observed at 12 or 18 months, however, and presumably reflects the difficulties of maintaining weight loss that have been reported previously [36].
Engagement was an important determinant of weight loss success at 6 months among those in the TAILORED group, regardless of weight status. This is similar to the findings from others [37–39], with greater engagement with intervention components being associated with better weight loss outcomes. Indeed, engagement in at least 66% of the personalized interventions resulted in significantly greater weight loss than the CONTROL group at 6 months, with the trend continuing at 12 months but disappearing by 18 months. However, these results may have been due to the earlier dropout of participants who were gaining weight. With content that is delivered remotely, innovative methods are needed to support those who may lose interest in the study content or who are less successful with their weight loss goals. These methods can include gamification, such as badges, competitions, or leaderboards, or engaging video content, such as storylines [40].
The current trial resulted in weight losses comparable to other technology-based weight loss studies among young adults. A systematic review of technology-based weight loss programs among adults aged 18–25 reported a pooled weight loss of about 2.96 kg for those interventions combining dietary, physical activity, and motivational skills training [41]. Studies reporting greater amounts of weight loss [9] used technology to supplement in-person treatment. We delivered the interventions digitally through Facebook and text messaging, thereby offering a potentially scalable and cost-effective method of treatment. Facebook was chosen as the delivery portal for three reasons: (a) at the time of study initiation, it was the most widely used social networking platform among our participants; (b) it permitted delivery of intervention through a channel young adults were already using; and (3) it allowed for both posting of videos and handouts while mimicking the social support provided by in-person weight loss groups. Given that Facebook is losing popularity among the younger generations and the challenges with using an external commercial platform (i.e., changes to security and limitations on the format of postings), future studies may consider different delivery channels. For instance, data published online in 2019 indicate about 90% of young adults use YouTube, which could be used to deliver didactic and video-based content [42].
Weight gain among young adults is a significant concern [43, 44]. National data [44] and those from a longitudinal trial indicate that those adults <25 years of age who were assigned to the control group and received no intervention gained as much as 7.5 kg over a 6 year period [43]. In our study, among those who started in the lowest BMI category and in whom engagement was >66%, percentage weight loss averaged about 2.6%. This small amount of weight loss can be beneficial: the Look AHEAD study reported improvements in cardiometabolic risk factors (i.e., systolic blood pressure, glucose, HbA1c, and triglycerides) beginning at a weight loss threshold of 2% [45]. Thus, even small weight losses should be considered in the context of both mitigating significant weight gain and preventing cardiometabolic disease.
There are a number of strengths to this trial, including the randomized, controlled study design. Body weight was measured via in-person assessment visits or validated by video recording and calibrated scales, and height was reassessed at each clinic visit to account for any changes. Also, the intervention and messages were automated and delivered digitally with the exception of provision of calorie, weight, and physical activity goals at the assessment visits, thereby making this a potentially cost-effective delivery method for future weight loss studies.
We note the limitations to this study that may put our findings in context. First, despite treatment fidelity and retention strategies, primary outcome data were available for only 62% of the sample. Recruiting and retaining young adults in digital interventions presents a number of challenges due to their mobility, school, work and social demands, and life transitions. We attempted to mitigate these issues related to mobility by implementing virtual weight visits. However, retaining young adults in weight loss clinical trials remains challenging as young adults have multiple demands on their time and the health consequences of overweight and obesity may seem distal, which some have termed “the young invincible” [46]. Retention was greater in older students, suggesting that they may have placed more importance on their excess body weight and health. The difficulty in retaining young adults in digital interventions may reflect a population trend in the use of digital programs. For example, among downloaded apps in 2019, 25% were used only once [47]. This difficulty may also reflect the diversification of digital platforms used by young adults. Pew Research Center data from 2019 indicate that 91% of 18–29 year olds reported using YouTube, 79% reported using Facebook, 67% reported using Instagram, and 62% reported using Snapchat, among other popular outlets (e.g., Twitter, Reddit, and WhatsApp) [48]. The use of multiple outlets may dilute engagement with digital interventions delivered through these sources. We also noted that those participants assigned to the TARGETED group who gained weight from baseline to 6 months were more likely to be lost to follow-up. We suspect that there may have been an interaction between the delivery of a content-only intervention along with participant outcome expectation of more weight loss such that as a participant gained weight, they could have become discouraged and dropped out of the study. Both outcome expectations of lower ideal body weight and greater initial weight loss success have been shown to predict dropout [49, 50]. This further substantiates the importance of precision medicine or stepped care approaches for weight loss among those individuals needing more intensive and targeted treatment. While we obtained weight in a fasted state at baseline, Month 6, and Month 18 for most participants, some at Month 12 who were providing a virtual weight may have done so in a nonfasted state. Since this is a randomized trial, we assume any misclassification or measurement error was equal across all treatment groups. The sample was 79% female and 49% non-Hispanic white; the results may not generalize to men or other races or ethnicities not represented in the current sample. Finally, the study sample was recruited from two urban university settings and, therefore, results may not apply to young adults who do not attend college or graduate school or to those with lower educational attainment. While we do not have a measure of socioeconomic status, approximately 66% of the students at one of our recruitment sites are first-generation college students [51].
CONCLUSION
Obesity in young adulthood tracks into middle and older age and increases the lifetime burden of chronic disease. Young adulthood is an ideal time for learning weight loss and maintenance behaviors that can set the foundation for better health outcomes later in life. University and community college settings are locations to potentially reach these age groups to provide intervention and skills training. Digital interventions hold promise for delivering weight loss treatments in this age group, particularly for those who begin treatment at a lower body weight and for whom engagement remains high. More intensive interventions, such as precision medicine or stepped care, may be needed for young adults with more severe obesity.
Supplementary Material
Acknowledgments
We would like to recognize the contributions of the study participants. We also acknowledge all of the undergraduate and graduate students who contributed to the project, especially the efforts of Caitlin Bailey, Erika Blankenship, Catherine Cameron, Mira Kahn, Madeline Kirch, Mary Moran, Tamarandobra Ogeh, Heather Ryan, Benjamin Shambon, and Timothy Tsung.
Funding: Research reported in this manuscript was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK100916 to M.A.N. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Conflicts of Interest: The authors declare no conflict of interest..
Author Contributions
conceptualization: LD, LLH, MAN, and JAW; methodology: LD, LLH, MAN, SS, and JAW; formal analysis: SS and AHT; investigation: LD, JF, MM, SM, MAN, GW, and JAW; data curation: JF, MM, SS, AHT; writing–original draft preparation: LD, JF, LLH, MM, MAN, SS, AHT, and JAW; writing–review & editing: all; visualization: AHT and SS; supervision: LD, MAN, GW, and JAW; project administration: LD, JF, MM, MAN, and JAW; funding acquisition: LD, LLH, MAN, SS, JAW.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Study registration: The study was preregistered at ClinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT02342912).
Analytic plan preregistration: While the analysis plan was prespecified by the investigative team, the analysis plan was not formally preregistered in a central repository.
Data availability
Deidentified data from this study are not available in a public archive. Deidentified data that support the findings of this study will be made available (as allowable according to institutional review board standards) 6 months following publication for 5 years to researchers submitting a specific request and data sharing agreement to the corresponding author.
Analytic code availability: Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author.
Material availability: Some of the materials used to conduct the study, including intervention protocols, and examples of intervention content are available in [24].
References
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020;(360):1–8. [PubMed] [Google Scholar]
- 2. Lloyd-Jones DM, Liu K, Colangelo LA, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: The Coronary Artery Risk Development in Young Adults Study. Circulation. 2007;115(8):1004–1011. [DOI] [PubMed] [Google Scholar]
- 3. Loria CM, Liu K, Lewis CE, et al. Early adult risk factor levels and subsequent coronary artery calcification: The CARDIA Study. J Am Coll Cardiol. 2007;49(20):2013–2020. [DOI] [PubMed] [Google Scholar]
- 4. Chen W, Bao W, Begum S, Elkasabany A, Srinivasan SR, Berenson GS. Age-related patterns of the clustering of cardiovascular risk variables of syndrome X from childhood to young adulthood in a population made up of black and white subjects: The Bogalusa Heart Study. Diabetes. 2000;49(6):1042–1048. [DOI] [PubMed] [Google Scholar]
- 5. Alberti KGMM, Eckel Robert H, Grundy Scott M, et al. Harmonizing the metabolic syndrome. Circulation. 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 6. Gokee-LaRose J, Gorin AA, Raynor HA, et al. Are standard behavioral weight loss programs effective for young adults? Int J Obes (Lond). 2009;33(12):1374–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lytle LA, Svetkey LP, Patrick K, et al. The EARLY trials: A consortium of studies targeting weight control in young adults. Transl Behav Med. 2014;4(3):304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Svetkey LP, Batch BC, Lin PH, et al. Cell phone intervention for you (CITY): A randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity (Silver Spring). 2015;23(11):2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jakicic JM, Davis KK, Rogers RJ, et al. Effect of wearable technology combined with a lifestyle intervention on long-term weight loss: The IDEA randomized clinical trial. JAMA. 2016;316(11):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Godino JG, Merchant G, Norman GJ, et al. Using social and mobile tools for weight loss in overweight and obese young adults (Project SMART): A 2 year, parallel-group, randomised, controlled trial. Lancet Diabetes Endocrinol. 2016;4(9):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vogels EA. Millennials stand out for their technology use, but older generations also embrace digital life. Available at https://www.pewresearch.org/fact-tank/2019/09/09/us-generations-technology-use/. Accessibility verified December 2, 2020.
- 12. LaRose JG, Leahey TM, Lanoye A, Reading J, Wing RR. A secondary data analysis examining young adults’ performance in an internet weight loss program with financial incentives. Obesity (Silver Spring). 2020;28(6):1062–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bihuniak JD, Bryant T, Kleiman J, et al. Behavioural weight loss treatment preferences of college students with overweight and obesity. Clin Obes. 2020;10(1):e12343. [DOI] [PubMed] [Google Scholar]
- 14. Harvey-Berino J, Pope L, Gold BC, Leonard H, Belliveau C. Undergrad and overweight: an online behavioral weight management program for college students. J Nutr Educ Behav. 2012;44(6):604–608. [DOI] [PubMed] [Google Scholar]
- 15. Napolitano MA, Hayes S, Bennett GG, Ives AK, Foster GD. Using Facebook and text messaging to deliver a weight loss program to college students. Obesity. 2013;21(1):25–31. [DOI] [PubMed] [Google Scholar]
- 16. Noar SM, Harrington NG, Aldrich RS. The role of message tailoring in the development of persuasive health communication messages. Ann Int Commun Assoc. 2009;33(1):73–133. [Google Scholar]
- 17. Kreuter MW, Wray RJ. Tailored and targeted health communication: strategies for enhancing information relevance. Am J Health Behav. 2003;27(suppl 3):S227–S232. [DOI] [PubMed] [Google Scholar]
- 18. Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133(4):673–693. [DOI] [PubMed] [Google Scholar]
- 19. Lustria ML, Noar SM, Cortese J, Van Stee SK, Glueckauf RL, Lee J. A meta-analysis of web-delivered tailored health behavior change interventions. J Health Commun. 2013;18(9):1039–1069. [DOI] [PubMed] [Google Scholar]
- 20. Rimer BK, Kreuter MW. Advancing tailored health communication: A persuasion and message effects perspective. J Commun. 2006;56(S1):S184-S201. [Google Scholar]
- 21. Napolitano MA, Marcus BH. Targeting and tailoring physical activity information using print and information technologies. Exerc Sport Sci Rev. 2002;30(3):122–128. [DOI] [PubMed] [Google Scholar]
- 22. Willmott TJ, Pang B, Rundle-Thiele S, Badejo A. Weight management in young adults: Systematic review of electronic health intervention components and outcomes. J Med Internet Res. 2019;21(2):e10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whiteley JA, Faro JM, Mavredes M, Hayman LL, Napolitano MA. Application of social marketing to recruitment for a digital weight management intervention for young adults. Transl Behav Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Napolitano MA, Whiteley JA, Mavredes MN, et al. Using social media to deliver weight loss programming to young adults: Design and rationale for the Healthy Body Healthy U (HBHU) trial. Contemp Clin Trials. 2017;60:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Diabetes Prevention Program Research Group. The diabetes prevention program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Twisk J, Bosman L, Hoekstra T, Rijnhart J, Welten M, Heymans M. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun. 2018;10:80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coffman CJ, Edelman D, Woolson RF. To condition or not condition? Analysing “change” in longitudinal randomised controlled trials. BMJ Open. 2016;6(12):e013096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson PO, Fay LC. The Johnson-Neyman technique, its theory and application. Psychometrika. 1950;15(4):349–367. [DOI] [PubMed] [Google Scholar]
- 30. Chakkalakal RJ, Connor LR, Rolando LA, et al. Putting the National Diabetes Prevention Program to work: Predictors of achieving weight-loss goals in an employee population. Prev Chronic Dis. 2019;16(E125):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. West DS, Stansbury M, Krukowski RA, Harvey J. Enhancing group-based internet obesity treatment: A pilot RCT comparing video and text-based chat. Obes Sci Pract. 2019;5(6):513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azar KM, Xiao L, Ma J. Baseline obesity status modifies effectiveness of adapted diabetes prevention program lifestyle interventions for weight management in primary care. Biomed Res Int. 2013;2013:191209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Severin R, Sabbahi A, Mahmoud AM, Arena R, Phillips SA. Precision medicine in weight loss and healthy living. Prog Cardiovasc Dis. 2019;62(1):15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jakicic JM, Tate DF, Lang W, et al. Effect of a stepped-care intervention approach on weight loss in adults: A randomized clinical trial. JAMA. 2012;307(24):2617–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomas DM, Kyle TK, Stanford FC. The gap between expectations and reality of exercise-induced weight loss is associated with discouragement. Prev Med. 2015;81:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(suppl 1):222S–225S. [DOI] [PubMed] [Google Scholar]
- 37. Lin PH, Grambow S, Intille S, et al. The association between engagement and weight loss through personal coaching and cell phone interventions in young adults: Randomized controlled trial. JMIR Mhealth Uhealth. 2018;6(10):e10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Power JM, Phelan S, Hatley K, et al. Engagement and weight loss in a web and mobile program for low-income postpartum women: Fit Moms/Mamás Activas. Health Educ Behav. 2019;46(suppl 2):114–123. [DOI] [PubMed] [Google Scholar]
- 39. Harvey J, Krukowski R, Priest J, West D. Log often, lose more: Electronic dietary self-monitoring for weight loss. Obesity (Silver Spring). 2019;27(3):380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Evans WD, Harrington C, Patchen L, et al. Design of a novel digital intervention to promote healthy weight management among postpartum African American women. Contemp Clin Trials Commun. 2019;16:100460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poobalan AS, Aucott LS, Precious E, Crombie IK, Smith WC. Weight loss interventions in young people (18 to 25 year olds): A systematic review. Obes Rev. 2010;11(8):580–592. [DOI] [PubMed] [Google Scholar]
- 42. Pew Research Center. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Available at https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/. Accessibility verified November 20, 2020.
- 43. Wing RR, Espeland MA, Tate DF, et al. ; Study of Novel Approaches to Weight Gain Prevention (SNAP) Research Group . Weight gain over 6 years in young adults: the study of novel approaches to weight gain prevention randomized trial. Obesity (Silver Spring). 2020;28(1):80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dietz WH. Obesity and excessive weight gain in young adults: New targets for prevention. JAMA. 2017;318(3):241–242. [DOI] [PubMed] [Google Scholar]
- 45. Wing RR, Lang W, Wadden TA, et al. ; Look AHEAD Research Group . Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gooding HC, Sheldrick RC, Leslie LK, Shah S, de Ferranti SD, Mackie TI. Adolescent perceptions of cholesterol screening results: “Young Invincibles” or developing adults? J Adolesc Health. 2016;59(2):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Statista. Percentage of mobile apps that have been used only once from 2010 to 2019. Available at https://www.statista.com/statistics/271628/percentage-of-apps-used-once-in-the-us/. Accessibility verified December 1, 2020.
- 48. Pew Research Center. Social media fact sheet. Available at https://www.pewresearch.org/internet/fact-sheet/social-media/. Accessibility verified February 2, 2021.
- 49. Dalle Grave R, Calugi S, Molinari E, et al. ; QUOVADIS Study Group . Weight loss expectations in obese patients and treatment attrition: An observational multicenter study. Obes Res. 2005;13(11):1961–1969. [DOI] [PubMed] [Google Scholar]
- 50. Burgess E, Hassmén P, Pumpa KL. Determinants of adherence to lifestyle intervention in adults with obesity: A systematic review. Clin Obes. 2017;7(3):123–135. [DOI] [PubMed] [Google Scholar]
- 51. University of Massachusetts at Boston. About our students. Available at https://www.umb.edu/academics/vpass/career_services/employers/about_our_students. Accessibility verified December 8, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data from this study are not available in a public archive. Deidentified data that support the findings of this study will be made available (as allowable according to institutional review board standards) 6 months following publication for 5 years to researchers submitting a specific request and data sharing agreement to the corresponding author.
Analytic code availability: Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author.
Material availability: Some of the materials used to conduct the study, including intervention protocols, and examples of intervention content are available in [24].