Abstract
Aim:
Decisions regarding adjuvant chemotherapy for early breast cancer are complex. Ki67 is increasingly used, in conjunction with conventional prognostic markers, to help decide the use of adjuvant chemotherapy for early breast cancer. Ki67 has been proposed as an economical alternative to Oncotype DX recurrence score (RS), which is a validated prognostic marker for disease recurrence and predictive marker for benefit from chemotherapy. This study aimed to determine, in patients where conventional prognostic markers did not provide a clear recommendation for adjuvant chemotherapy, whether Ki67 could be a substitute for RS.
Methods:
We reviewed all cases of luminal type node negative early breast cancer (T1–2, N0–1mi, M0, ER positive, HER2 negative) referred for Oncotype DX testing by the multidisciplinary team at an Australian tertiary private hospital from 14th December 2006 to 31st December 2013, when conventional prognostic markers did not provide a clear recommendation for adjuvant chemotherapy. RS was correlated with Ki67, along with other conventional prognostic markers including tumour size, grade, mitotic rate and lymphovascular invasion. Spearman’s rank order correlation coefficient and Pearson product-moment correlation coefficient (r) were used for ordinal and continuous variables respectively.
Results:
A total of 58 patients were analysed, median Ki67 was 15% (range 2–50%), the median RS was 16 (range 3–65). There was no positive correlation between Ki67 and RS (r=0.01, p=0.93). No single conventional prognostic marker was shown to significantly correlate with RS, including tumour size (r=−0.02, p=0.88), grade (r=0.10, p=0.44), mitotic rate (r=−0.07, p=0.69) and lymphovascular invasion (r=−0.12, p=0.39).
Conclusion:
Ki67 and conventional prognostic markers do not correlate with Oncotype DX RS. In the setting where conventional prognostic markers do not show a clear indication for or against adjuvant chemotherapy as determined by consensus in a multidisciplinary team, Ki67 is not a substitute for Oncotype DX testing. RS may provide additional information to aid decision making for adjuvant chemotherapy.
Keywords: Early breast cancer, Ki67, Oncotype DX
Introduction
Adjuvant endocrine therapy for patients with early stage oestrogen receptor positive breast cancer has been shown to significantly reduce breast cancer mortality, and consequently in practice the majority of these patients receive endocrine therapy.1, 2 Decisions regarding adjuvant chemotherapy however, are complex, with the benefits of therapy weighed against the potential risks and harms of treatment.3 A number of conventional prognostic markers are routinely used to help with the decision including tumour size, grade, mitotic rate and lymphovascular invasion.
Oncotype Dx is a 21-gene expression assay that has been developed for patients with hormone receptor positive breast cancers, which uses an algorithm to provide a recurrence score (RS). It has been validated to prognosticate the risk of distant recurrence in patients treated with endocrine therapy, and predict the magnitude of benefit for the addition of chemotherapy.4–7 However, due to the cost of the assay and some additional turnaround time, it is not routinely performed for all patients in Australia and most parts of the world. For patients in whom the decision for adjuvant chemotherapy is unclear based on conventional prognostic markers, clinicians or multidisciplinary teams including ours at the Sydney Adventist Hospital may recommend the use of the Oncotype Dx assay to assist with clinical decision-making.
Ki67 protein expression by immunohistochemistry is a proliferation marker which has been increasingly used by clinicians to help with decision making on adjuvant chemotherapy.8 In our centre, Ki67 was considered in conjunction with conventional prognostic markers in decisions on adjuvant chemotherapy, however controversy regarding its use was recognised. We sought to determine whether Ki67 may be an economical alternative to Oncotype Dx RS to guide adjuvant chemotherapy recommendations in the particular setting where conventional prognostic markers have failed to yield a clear answer.
Patients and Methods
We conducted a retrospective, single centre study at the Sydney Adventist Hospital, a tertiary private hospital in Sydney, Australia. We retrieved clinical data from consecutive patients with luminal type (A and B), node-negative, oestrogen-receptor positive, Her2 negative early breast cancer who were referred for Oncotype DX testing by the Breast Cancer Multidisciplinary Team from 14th December 2006 to 31st December 2013, when conventional prognostic markers did not provide a clear recommendation for adjuvant chemotherapy. This involved a consensus decision between surgeons, medical oncologists and radiation oncologists based on tumour Nottingham grade, size, mitotic rate and presence of lymphovascular invasion. This was assessed on an individual case-by-case basis, however was consistent with published guidelines.3 The Adventist HealthCare Limited Ethics Committee, a registered Human Research Ethics Committee (EC00141), approved the study.
Histopathologic examination
The primary lesion, axillary lymph node status and various biomarkers were evaluated following surgical resection. This was based on standard pathology reporting guidelines for breast cancer in Australia.9 Haematoxylin and eosin stained, formalin-fixed, paraffin-embedded tumour sections were examined to confirm pathological staging (T1–2, N0–1mi), tumour size, grade, mitotic rate and the presence of lymphovascular invasion. Oestrogen receptor (ER) status was evaluated using immunohistochemical staining of breast specimens and considered positive if there was moderate to strong nuclear staining in ≥1% of the tumour cells. Her2 status of the tumour was evaluated using immunohistochemical staining and confirmed with fluorescence in-situ hybrodization (FISH), chromogenic in-situ hybridisation (CISH) or silver-enhanced in situ hybridisation (SISH) testing. Her2 was considered negative if the HER2/CEP17 ratio <2 and gene copy number <4 signals/cell.10 Ki67 was evaluated using immunohistochemical staining with rapid semiquantitative assessment (SQA) on single stained sections, by two independent pathologists in the areas of strongest expression or maximum proliferative activity to result in a percentage Ki67 index. Axillary nodal status was confirmed on histopathology based on sentinel lymph node biopsy. Clinical or radiological staging was used to confirm non-distant metastatic disease.
Oncotype DX
Patients were referred for Oncotype DX testing (Genomic Health, Redwood City, CA, USA) a 21-gene panel assay, after reviewing conventional prognostic markers and consensus decision in the Breast Cancer Multidisciplinary Team meeting with informed patient consent. RS was grouped into low-risk (RS<18), intermediate-risk (RS 18–30) or high-risk groups (RS≥31) using the original validated cut-offs.4
Statistical analysis
RS was correlated with Ki67, along with other conventional prognostic markers including tumour size, grade, mitotic rate and lymphovascular invasion using univariate and multivariate analysis. RS and Ki67 were analysed as continuous variables. Grade 3 was not included in the univariate and multivariate analysis due to an outlier patient causing a violation of the assumption of normality of the error terms. Spearman’s rank order correlation coefficient and Pearson product-moment correlation coefficient (r) were used for ordinal and continuous variables respectively.
Results
Patient and tumour characteristics
The patient and tumour characteristics are shown in Table 1. The median age was 54 years (range 35–70 years). The median Ki67 was 15% (range 1.5–45%, mean 16%) and the median RS was 16 (range 3–65, mean 19). A total of 32 of 58 (55%) of patients were given a low risk RS, 23 of 58 (40%) were given an intermediate risk RS, and 3 of 58 (5%) were given a high risk RS.
Table 1 –
Patient and tumour characteristics
| Characteristic | n (%) |
|---|---|
| Number of patients | 58 |
| Age | |
| <40 years | 1 (1.7) |
| 40–49 years | 21 (36.2) |
| 50–59 years | 23 (39.7) |
| ≥60 years | 13 (22.4) |
| Tumour size (cm) | |
| ≤1 | 16 (27.6) |
| 1.1–2.0 | 38 (65.5) |
| 2.1–4.0 | 4 (6.9) |
| >4.0 | 0 (0) |
| Grade | |
| 1 | 8 (13.8) |
| 2 | 44 (75.9) |
| 3 | 6 (10.3) |
| Mitoses (/10hpf) | |
| <5 | 18 (31.1) |
| 5–10 | 30 (51.7) |
| >10 | 10 (17.2) |
| Lymphovascular invasion | |
| Absent | 45 (77.6) |
| Present | 13 (22.4) |
| Ki67 (%) | |
| <10 | 12 (20.7) |
| 11–20 | 32 (55.2) |
| >20 | 14 (24.1) |
| Oncotype Dx recurrence score | |
| Low (<18) | 32 (55.2) |
| Intermediate (18–30) | 23 (39.6) |
| High (≥31) | 3 (5.2) |
Correlation between Oncotype Dx RS and Ki67
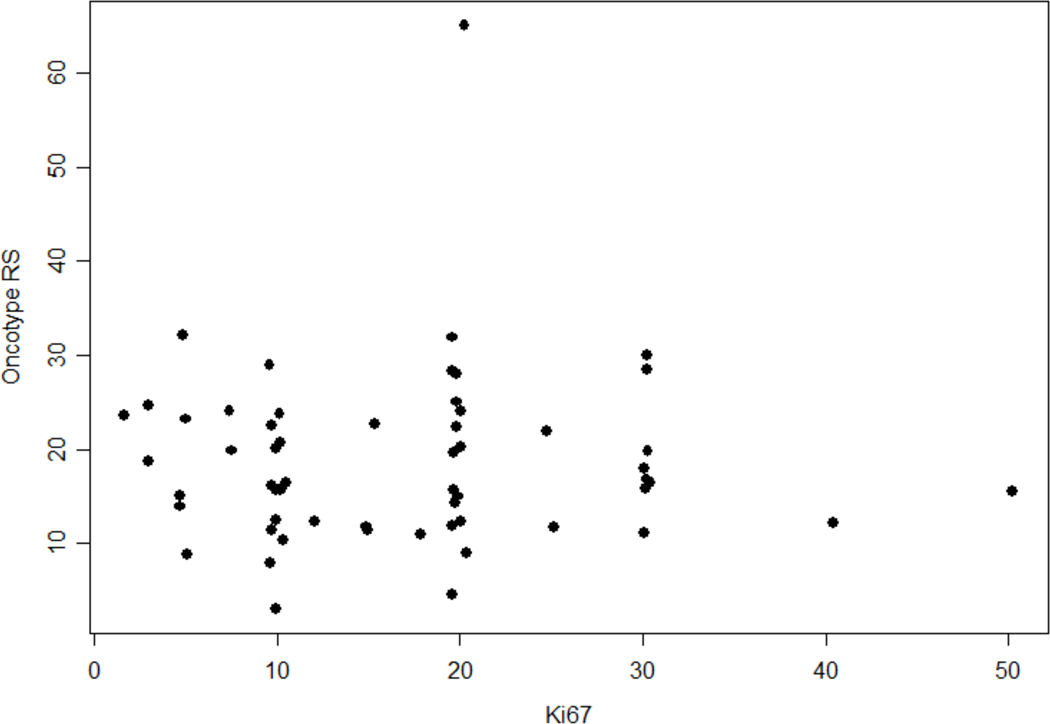
There was no positive correlation between Ki67 and RS (r=0.01, p=0.93). A scatter plot of the Oncotype RS and Ki67 is shown in Figure 1.
Figure 1 –
Scatter Plot of Oncotype RS versus Ki67
Correlation between Oncotype Dx RS and other prognostic markers
No single conventional prognostic marker was shown to significantly correlate with RS, including tumour size (r=−0.02, p=0.88), grade (r=0.10, p=0.44), mitotic rate (r=−0.07, p=0.69) and lymphovascular invasion (r=−0.12, p=0.39). On multivariate analysis, there were no variables that were significant predictors of Oncotype RS (F-value=0.76, p=0.63) as shown in Table 2. Similarly on univariate analysis, none of the predictor variables were significant as shown in Table 3.
Table 2 –
Multivariate analysis of Oncotype RS with prognostic markers†
| Parameter | Coefficient Estimate (95% CI) | p-value |
|---|---|---|
| Intercept | 12.24 (−2.1 – 26.61) | 0.10 |
| Ki67 | −0.06 (−0.26 – 0.14) | 0.58 |
| Age | 0.14 (−0.08 – 0.37) | 0.21 |
| Size | −0.31 (−0.63 – 0.01) | 0.06 |
| Grade 1 | 0.40 (−8.49 – 9.29) | 0.93 |
| Grade 2 | 2.01 (−5.02 – 9.03) | 0.58 |
| Mitosis | 0.14 (−0.39 – 0.67) | 0.61 |
| LVI group 1 | 3.28 (−1.48 – 8.05) | 0.18 |
One patient was removed from the statistical analysis due to a violation of the assumption of normality of the error terms
Table 3 –
Univariate analysis of Oncotype RS with prognostic markers†
| Univariate Parameter | p-value for Overall F-test | Coefficient Estimate (95% CI) | p-value |
|---|---|---|---|
| Ki67 | 0.83 | −0.02 (−0.20 – 0.16) | 0.83 |
| Age | 0.63 | 0.05 (−0.16 – 0.27) | 0.63 |
| Size | 0.21 | −0.19 (−0.48 – 0.10) | 0.20 |
| Grade 1 | 0.55 | −1.18 (−8.61 – 6.26) | 0.76 |
| Grade 2 | 1.54 (−4.61 – 7.69) | 0.62 | |
| Mitosis | 0.65 | 0.09 (−0.31 – 0.49) | 0.65 |
| LVI group 1 | 0.50 | 1.49 (−2.78 – 5.75) | 0.49 |
One patient was removed from the statistical analysis due to a violation of the assumption of normality of the error terms
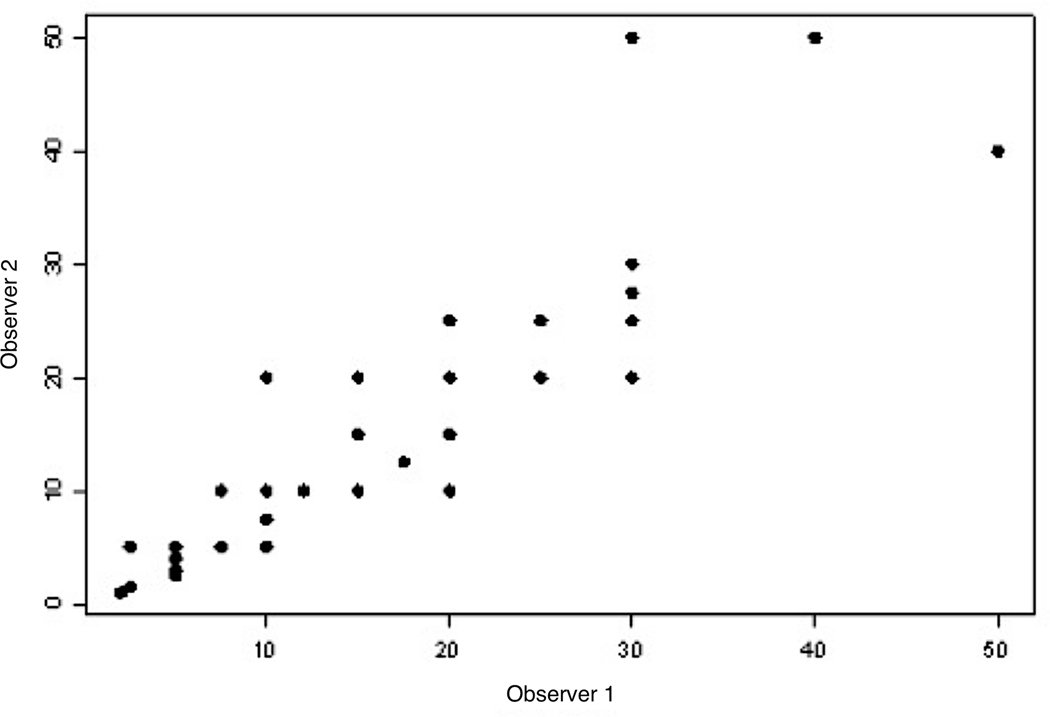
Concordance between Ki67 rating
There was significant correlation between scores from the two pathologists (r=0.86, p=<0.0001), with the scatter plot shown in Figure 2.
Figure 2 –
Scatter Plot of Ki67 between Rater 1 and Rater 2
Discussion
Our study demonstrated that Ki67 could not be used as a substitute for the Oncotype DX RS when conventional prognostic markers do not provide a clear recommendation for adjuvant chemotherapy. In this group of patients, determined by consensus from a multidisciplinary meeting to have unclear additional benefit from adjuvant chemotherapy, Oncotype DX RS was used to aid clinical decision-making. Additionally there were no other conventional prognostic markers that correlated with the RS either alone or in combination. This suggests that in this highly selected subset of patients, the RS yielded a result that could not be determined from Ki67 or conventional prognostic markers, further indicating its independent value in assisting clinical decision making on the use of adjuvant chemotherapy.
Other studies have found that RS correlated with a combined oestrogen receptor, progesterone receptor, Ki67 and Her2 immunohistochemical score, the IHC4,11 as well as Ki67 alone or other proliferative markers.12, 13 Allison et al. found that there was strong correlation with low RS for patients with grade 1, strong PR expression and Ki67 ≤10% characteristics, and for high RS with grade 3, low to absent PR expression and Ki67 >10%.14 Equations have even been developed based on conventional prognostic markers that can closely estimate the RS.15 However, these studies have largely looked at patients who were clearly low or high risk based on conventional prognostic markers. These cases correlated strongly with RS which incorporates the genes expressing proliferation and hormonal status, including Ki67 itself. Yet in a study of 109 Israeli patients whose conventional prognostic markers did not provide a clear answer and who were subsequently referred for Oncotype DX, Wolf et al. found no correlation between standard clinicopathologic features and the RS.16 Similarly in our study, it is important to emphasise that patients were selected based on consensus at a multidisplinary meeting to not have clear recommendation for adjuvant chemotherapy by conventional prognostic markers. This may potentially explain the lack of correlation between Ki67 and RS in this group of patients whose conventional prognostic markers gave inconsistent biologic information (e.g. small size, low grade, high mitotic count, lymphovascular invasion), in contrast to previous studies of tumors had conventional prognostic markers that were consistently low risk or high risk. The small sample size in our study may also have contributed. The findings from our study of Australian patients support the growing evidence that in a subset of patients whose conventional prognostic markers fail to provide clear guidance on adjuvant chemotherapy, Oncotype DX provides additional independent data to help with decision making.
There is strong evidence for Ki67 as an additional prognostic marker, and it has also been shown to have predictive value.17–20 Ki67 may be particularly important in identifying Luminal B breast cancer.21 There are limitations of Ki67, particularly a lack of a standardised scoring system resulting in interlaboratory variation,22 different methods of measuring Ki67 and practical issues in implementing these for routine use in laboratories.23 However, its utility as a relatively cost effective and simple immunohistochemical test should be emphasised. Furthermore, there is expert consensus recommending its use to aid in decision making for chemotherapy.24
In our study, there is the inherent bias associated with the selection of patients based on consensus from a multidisciplinary meeting. This relies on the expertise and interpretation from the group of clinicians including surgeons, pathologists, oncologists and radiologists that attended these meetings. This may not reflect practices in other centres, and may be indicative of our specific patient population. However this only highlights the importance of the multidisciplinary team in making decisions on adjuvant chemotherapy in early breast cancer.25 Our study also did not evaluate clinical outcomes, and consequently we are unable to assess the predictive utility of Ki67 or RS. It would be interesting to determine if either Ki67 or RS was predictive of oncologic outcomes in our group of patients. Similarly we did not differentiate between luminal A and B type breast cancer or analyse progesterone receptor positivity, histology and the presence of intratumoural heterogeneity for Ki67, and this represents a potential limitation of the study. Such exploratory subgroup analyses would require greater sample size for any statistical comparisons. Nevertheless large prospective studies have confirmed that the use of multigene expression assays such as Oncotype DX and Mammaprint can have major impacts on adjuvant treatment decision making and affect patient outcomes.7, 26, 27 Although there is moderate concordance and correlation between different assays, such as Oncotype DX and EndoPredict,28 whether Ki67 and other prognostic markers would correlate with other assay scores in selected patients remains to be elucidated.
Conclusion
In this patient population, Ki67 and conventional prognostic markers did not correlate with Oncotype DX RS. In the setting where conventional prognostic markers do not show a clear indication for or against adjuvant chemotherapy as determined by consensus in a multidisciplinary team,, Ki67 is not a substitute for Oncotype DX testing. RS may provide additional information to aid decision making for adjuvant chemotherapy.
Acknowledgement
Bob T. Li is supported by the Comprehensive Cancer Center Core Grant (P30 CA008748) at Memorial Sloan Kettering Cancer Center from the National Institutes of Health, USA.
Footnotes
Disclosures
BTL has received consulting fees from Biosceptre International; all other authors declare no competing interests.
References
- 1.Davies C, Godwin J, Gray R et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378: 771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowsett M, Forbes JF, Bradley R et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015; 386: 1341–52. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 2009; 20: 1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paik S, Shak S, Tang G et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351: 2817–26. [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Tang G, Shak S et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006; 24: 3726–34. [DOI] [PubMed] [Google Scholar]
- 6.Albain KS, Barlow WE, Shak S et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010; 11: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sparano JA, Gray RJ, Makower DF et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med 2015; 373: 2005–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates AS, Winer EP, Goldhirsch A et al. Tailoring therapies--improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015; 26: 1533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Breast and Ovarian Cancer Centre and Australian Cancer Network. The pathology reporting of breast cancer. A guide for pathologists, surgeons, radiologists and oncologists (3rd edition). National Breast and Ovarian Cancer Centre, Surry Hills NSW: 2008. [Google Scholar]
- 10.Wolff AC, Hammond MEH, Hicks DG et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. Journal of Clinical Oncology 2013; 31: 3997–4013. [DOI] [PubMed] [Google Scholar]
- 11.Cuzick J, Dowsett M, Pineda S et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 2011; 29: 4273–8. [DOI] [PubMed] [Google Scholar]
- 12.Sahebjam S, Aloyz R, Pilavdzic D et al. Ki 67 is a major, but not the sole determinant of Oncotype Dx recurrence score. Br J Cancer 2011; 105: 1342–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DJ, Cohen C, Darrow M, Page AJ, Chastain B, Adams AL. Proliferation (Ki-67 and phosphohistone H3) and oncotype DX recurrence score in estrogen receptor-positive breast cancer. Appl Immunohistochem Mol Morphol 2011; 19: 431–6. [DOI] [PubMed] [Google Scholar]
- 14.Allison KH, Kandalaft PL, Sitlani CM, Dintzis SM, Gown AM. Routine pathologic parameters can predict Oncotype DX recurrence scores in subsets of ER positive patients: who does not always need testing? Breast Cancer Res Treat 2012; 131: 413–24. [DOI] [PubMed] [Google Scholar]
- 15.Klein ME, Dabbs DJ, Shuai Y et al. Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol 2013; 26: 658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf I, Ben-Baruch N, Shapira-Frommer R et al. Association between standard clinical and pathologic characteristics and the 21-gene recurrence score in breast cancer patients: a population-based study. Cancer 2008; 112: 731–6. [DOI] [PubMed] [Google Scholar]
- 17.Inwald EC, Klinkhammer-Schalke M, Hofstadter F et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 2013; 139: 539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kontzoglou K, Palla V, Karaolanis G et al. Correlation between Ki67 and breast cancer prognosis. Oncology 2013; 84: 219–25. [DOI] [PubMed] [Google Scholar]
- 19.Jonsdottir K, Assmus J, Slewa A et al. Prognostic value of gene signatures and proliferation in lymph-node-negative breast cancer. PLoS One 2014; 9: e90642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura R, Osako T, Okumura Y, Hayashi M, Arima N. Clinical significance of Ki-67 in neoadjuvant chemotherapy for primary breast cancer as a predictor for chemosensitivity and for prognosis. Breast Cancer 2010; 17: 269–75. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Lopez ME, Garcia-Gomez J, Alves MT, Paradela A, Garcia-Mata J, Garcia-Caballero T. Ki-67 is a prognostic marker for hormone receptor positive tumors. Clin Transl Oncol 2016; 18: 996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010; 11: 174–83. [DOI] [PubMed] [Google Scholar]
- 23.Harvey J, Thomas C, Wood B et al. Practical issues concerning the implementation of Ki-67 proliferative index measurement in breast cancer reporting. Pathology 2015; 47: 13–20. [DOI] [PubMed] [Google Scholar]
- 24.Goldhirsch A, Winer EP, Coates AS et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013; 24: 2206–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Breast Cancer Centre. Multidisciplinary meetings for cancer care: a guide for health service providers. National Breast Cancer Centre, Camperdown NSW: 2005. [Google Scholar]
- 26.de Boer RH, Baker C, Speakman D, Chao CY, Yoshizawa C, Mann GB. The impact of a genomic assay (Oncotype DX) on adjuvant treatment recommendations in early breast cancer. Med J Aust 2013; 199: 205–8. [DOI] [PubMed] [Google Scholar]
- 27.Cardoso F, van’t Veer LJ, Bogaerts J et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med 2016; 375: 717–29. [DOI] [PubMed] [Google Scholar]
- 28.Varga Z, Sinn P, Fritzsche F et al. Comparison of EndoPredict and Oncotype DX test results in hormone receptor positive invasive breast cancer. PLoS One 2013; 8: e58483. [DOI] [PMC free article] [PubMed] [Google Scholar]