Abstract
Background
MicroRNAs (miRNAs) play an important role in the pathogenesis of chronic thromboembolic pulmonary hypertension (CTEPH). However, the potential correlation between miRNA expression and the severity of CTEPH remains unclear. Our previous study indicated that miRNAs hsa-let-7b-3p, hsa-miR-17-5p, hsa-miR-106b-5p, hsa-miR-3202, hsa-miR-665, and hsa-miR-93-5p are closely involved in CTEPH. This study assessed the associations between the expression levels of these miRNAs and clinical parameters in CTEPH patients.
Methods
A total of eight CTEPH patients and eight healthy adults as a reference group were included, and clinical data including total protein (TP), albumin (Alb), lactate dehydrogenase (LDH), hydroxybutyrate dehydrogenase (HBDH), uric acid (UA), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were collected. Right heart catheterization was conducted to obtain hemodynamic data including cardiac index (CI). The expression levels of let-7b-3p, miR-17-5p, miR-106b-5p, miR-3202, miR-665, and miR-93-5p were measured by quantitative real-time PCR (qPCR). Correlation analysis was applied to estimate the associations between miRNA expression levels and clinical parameters in CTEPH patients.
Results
Serum TP and Alb levels were decreased, while LDH, HBDH, and UA levels were increased in CTEPH patients compared with the reference group (P < 0.05). miR-3202 and miR-665 were upregulated, whereas let-7b-3p, miR-17-5p, miR-106b-5p, and miR-93-5p were downregulated in CTEPH patients relative to the reference group (P < 0.05). miR-93-5p expression was positively correlated with NT-proBNP level and negatively correlated with CI (P < 0.05). Moreover, let-7b-3p tended to be positively correlated with mean pulmonary arterial pressure.
Conclusions
miR-93-5p expression was associated with the severity of CTEPH and could act as a potential predictor of high-risk CTEPH.
1. Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH), which manifests as progressive exertional dyspnea and right ventricular failure, is caused by pulmonary artery (PA) and pulmonary arteriole obstruction with proximal thromboembolism or distal vascular remodeling [1, 2]. The occurrence of CTEPH in pulmonary embolism survivors is approximately 3% [3]. However, severe CTEPH is associated with a high mortality rate of 24–67%, which increases over time [4]. A strategy for identifying high-risk CTEPH patients is therefore critical for improving the treatment and prognosis of these patients. However, predictive biomarkers for the severity and clinical outcomes of CTEPH are lacking.
Blood-derived biomarkers reflecting disease progression of CTEPH may provide potential predictive values. MicroRNAs (miRNAs) play critical roles in a variety of biological processes via the regulation of gene expression through mRNA inhibition or degradation at the posttranscriptional level [5]. Peripheral blood miRNAs are easy to collect and quantify, and they have become attractive circulating biomarkers for multiple diseases [6, 7]. For example, miRNAs have recently been identified as diagnostic and prognostic biomarkers for cardiovascular diseases including pulmonary hypertension (PH) [8]. miR-204 reflects PH severity, and miR-214-3p was confirmed to be a regulator of the hypoxia-induced proliferation and migration of pulmonary artery smooth muscle cells [9, 10]. Furthermore, miR-759 [11] and miR-22 [12] were reported as important indicators in the pathogenesis of CTEPH. In our previous study, we identified several critical miRNAs involved in the progression of CTEPH, including hsa-let-7b-3p, hsa-miR-17-5p, hsa-miR-106b-5p, hsa-miR-3202, hsa-miR-665, and hsa-miR-93-5p [13], indicating that these miRNAs might be useful in the diagnosis and treatment of CTEPH. Nevertheless, in-depth studies of the predictive values of these miRNAs remain insufficient. miR-223, miR-127, and miR-146a were shown to be positively associated with cardiovascular events independently of N-terminal pro-B-type natriuretic peptide (NT-proBNP) level and Framingham risk score even in asymptomatic individuals [14]. miR-424 (322) is upregulated in PH patients and linked with more serious symptoms and hemodynamics [15]. Taken together, research to date indicates that miRNAs may be correlated with clinical parameters reflecting disease severity in CTEPH patients and may serve as potential prognostic biomarkers.
The current study is aimed at detecting associations between the expression levels of miRNAs, including let-7b-3p, miR-17-5p, miR-106b-5p, miR-3202, miR-665, and miR-93-5p, and key clinical parameters in CTEPH patients. The predictive value of these miRNAs for the severity of CTEPH was determined.
2. Materials and Methods
2.1. Patient Recruitment
Eight CTEPH patients who were admitted at Beijing Chao-Yang Hospital from March to December 2017 and eight control subjects as a reference group were included. CTEPH was diagnosed based on the International Guidelines of Pulmonary Hypertension (2015 ERS/ESC guidelines for pulmonary hypertension) [16]. Briefly, CTEPH was defined by the following: (1) a mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg and a pulmonary arterial wedge pressure (PAWP) ≤ 15 mmHg verified by right heart catheterization (RHC) and (2) chronic thrombosis with mismatched perfusion defects demonstrated by computed tomographic angiography or ventilation/perfusion scanning. In addition, the diagnostic criteria were met by right floating catheter, pulmonary ventilator-perfusion imaging, and/or spiral computed tomography pulmonary angiography. The exclusion criteria included hypertension, diabetes, coronary heart disease, cerebrovascular disease, malignancy, or other circulatory diseases, such as PH.
In the reference group, healthy adults who underwent routine physical examination were enrolled. The reference group had no history of heart, pulmonary, cancer, or thrombosis-related diseases. Additionally, no abnormality on laboratory measurements including routine blood tests, routine urine tests, biochemical tests, carcinoembryonic antigen and alpha fetoprotein levels, and chest radiographs was observed.
All patients were included on the date of diagnosis and had not been given PH-related treatment previously. Peripheral blood samples were obtained on the second day to ensure that CTEPH-related treatment had no effect on the expression of miRNAs.
2.2. Clinical Data Collection
Hemorheological and biochemical indexes reflecting the severity of CTEPH were collected. For CTEPH and control groups, blood parameters including hemoglobin (Hb) level, hematocrit (HCT) level, and platelet (Plt) count were tested. Additionally, biochemical indicators including total protein (TP) level, albumin (Alb) level, albumin : globulin (A : G) ratio, aspartate transaminase (AST) level, alanine transaminase (ALT) level, lactate dehydrogenase (LDH) level, hydroxybutyrate dehydrogenase (HBDH) level, total bilirubin (TBIL) level, direct bilirubin (DBIL) level, blood urea nitrogen (BUN) level, creatinine (Cr) level, and uric acid (UA) level were detected. For the CTEPH group, a number of specific clinical indicators were also collected, including World Health Organization function classification (WHO FC), medical history, 6-minute walk distance (6MWD), and NT-proBNP level.
2.3. RHC
RHC was performed for all CTEPH patients on the day of admission. The hemodynamic data were detected using a Philips monitoring system (Shenzhen Goldway Industrial Inc., China), and a Swan-Ganz catheter was used for the assessment of pressure in the right ventricle and pulmonary artery via the jugular vein. The mPAP and mixed venous oxygen saturation (SvO2) were recorded, and PAWP was calculated to exclude postcapillary pulmonary hypertension. Cardiac output (CO) was detected using thermodilution, and the average cardiac index (CI) of triplicate measurements was calculated. Pulmonary vascular resistance (PVR) was calculated using the following formula: PVR = (mPAP–PAWP)/CO.
2.4. Quantitative Analysis of miRNA Expression
Peripheral blood samples were collected from CTEPH patients and the reference subjects. Total RNA was extracted by RNA prep Pure Blood Kit (Tiangen Biotech Co., Ltd., China) following the manufacturer's protocol. Then, RNA was purified using the mirVana™ miRNA Isolation Kit (AM1561, Ambion, USA). The concentration and quality of RNA were detected by a Nanodrop ND1000 spectrophotometer (Thermo Fisher Scientific, USA) and an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). The primer sequences for let-7b-3p, miR-106b-5p, miR-17-5p, miR-3202, miR-665, and miR-93-5p were designed using the Primer Premier 6.0 software (Premier Software Inc., USA) and produced by Sangon Biotech Co., Ltd (Shanghai, China) (Table S1). After conducting the reverse transcription reaction, quantitative real-time PCR (qPCR) analysis was performed using the SYBR Green master mix kit (Applied Biosystems, USA). The 20 μL amplification reaction mixture contained the following: 1 μL forward primer, 1 μL reverse primer, 8 μL cDNA template (at a constant concentration), and 10 μL SYBR Premix Ex Taq (2×). The reaction program was as follows: 50°C for 3 min; 95°C for 3 min; 95°C for 10 s, 40 cycles at 60°C for 30 s; melt curve 60–95°C, with an increment of 0.5°C for 10 s plate read. U6 was used as the internal reference gene. All experiments were repeated three times to ensure accuracy.
2.5. Ethics Statement
This study was approved by the Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University. All procedures in studies involving human participants were performed in accordance with the ethics standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethics standards. The need for written informed consent was waived, because the research involved no risk to the subjects and the waiver did not adversely affect the rights and welfare of the subjects.
2.6. Statistical Analysis
The results are presented as mean ± standard deviation for normally distributed data and as median and interquartile range for nonnormally distributed continuous variables. The data for categorical variables are expressed as percentages. Student's t-test was utilized for comparisons between the CTEPH and normal groups. To evaluate the potential value of miRNAs for estimating the severity of CTEPH, the associations between miRNA expression levels and clinical data were analyzed by Pearson correlation (continuous variables) or Spearman correlation (rank variables). Statistical significance was set at P < 0.05. Statistical analyses were performed with the SPSS 25.0 software (USA).
3. Results
3.1. Demographic and Clinical Data
A total of eight CTEPH patients (four males and four females) and eight healthy adults as a reference group (four males and four females) were recruited for this study. The median ages were 61.00 ± 6.82 years and 56.13 ± 4.49 years for the CTEPH patients and the reference group, respectively.
The CTEPH patients had significantly decreased serum TP (67.15 ± 4.56 vs. 76.79 ± 4.14 g/L, P < 0.05) and Alb (39.80 ± 2.88 vs. 46.09 ± 2.79 g/L, P < 0.05) levels and remarkably increased LDH (243.75 ± 45.96 vs. 180.16 ± 27.30 U/L, P < 0.05), HBDH (214.38 ± 40.20 vs. 153.00 ± 153.00 U/L, P < 0.05), and UA (447.87 ± 111.28 vs. 299.75 ± 76.62 U/L, P < 0.05) levels compared with the reference individuals. There were no significant differences in Hb, HCT, Plt, A : G, AST, ALT, TBIL, DBIL, BUN, and Cr levels between the two groups (all P > 0.05, Table 1).
Table 1.
Demographic and biochemical data of CTEPH patients and the reference group.
| Parameters | Reference group (n = 8) | CTEPH group (n = 8) | P value |
|---|---|---|---|
| Gender, female | 4 | 4 | - |
| Age, years | 56.13 ± 4.49 | 61.00 ± 6.82 | 0.113 |
| Hemoglobin, g/L | 137.88 ± 15.52 | 153.00 ± 18.61 | 0.099 |
| Hematocrit, % | 40.96 ± 3.67 | 42.84 ± 4.11 | 0.352 |
| Platelet, 106/L | 199.25 ± 40.27 | 224.00 ± 45.39 | 0.268 |
| TP, g/L | 76.79 ± 4.14 | 67.15 ± 4.56 | 0.001 |
| Alb, g/L | 46.09 ± 2.79 | 39.80 ± 2.88 | 0.001 |
| A : G | 1.50 ± 0.18 | 1.46 ± 0.09 | 0.603 |
| AST, U/L | 27.38 ± 4.90 | 29.75 ± 18.88 | 0.736 |
| ALT, U/L | 25.12 ± 9.97 | 30.50 ± 24.78 | 0.578 |
| LDH, U/L | 180.16 ± 27.30 | 243.75 ± 45.96 | 0.011 |
| HBDH, U/L | 153.00 ± 153.00 | 214.38 ± 40.20 | 0.006 |
| TBIL, μmol/L | 14.66 ± 4.73 | 19.88 ± 7.70 | 0.130 |
| DBIL, μmol/L | 4.53 ± 1.46 | 7.40 ± 3.67 | 0.071 |
| BUN, mmol/L | 5.14 ± 0.73 | 6.78 ± 2.24 | 0.082 |
| Cr, μmol/L | 69.50 ± 12.81 | 76.80 ± 13.90 | 0.295 |
| UA, U/L | 299.75 ± 76.62 | 447.87 ± 111.28 | 0.008 |
TP: total protein; Alb: albumin; A : G: albumin : globulin; AST: aspartate transaminase; ALT: alanine transaminase; LDH: lactate dehydrogenase; HBDH: hydroxybutyrate dehydrogenase; TBIL: total bilirubin; DBIL: direct bilirubin; BUN: blood urea nitrogen; Cr: creatinine; UA: uric acid.
Among the patients in the CTEPH group, five were classified as WHO FC I-II while three were classified as WHO FC III-IV. For this group, the mean disease duration was 43.88 ± 33.37 months. Also, the mean 6MWD was 390.06 ± 108.06 m, and the median NT-proBNP concentration was 1142.00 (145.42 and 2491.75) picograms per milliliter (Table 2).
Table 2.
Clinical indicators and hemodynamic data of CTEPH patients.
| Parameters | CTEPH group |
|---|---|
| WHO FC (I-II; III-IV) | 5; 3 |
| Disease duration, months | 43.88 ± 33.37 |
| 6MWD, m | 390.06 ± 108.06 |
| NT-proBNP, pg/mL | 1142.00 (145.42, 2491.75) |
| mPAP, mmHg | 54.13 ± 12.43 |
| PAWP, mmHg | 9.6 ± 2.8 |
| SvO2, % | 54.25 ± 9.44 |
| CI, L/(min·m2) | 2.33 ± 0.41 |
| PVR, Wood units | 11.80 ± 2.97 |
WHO FC: World Health Organization function classification; 6MWD: 6-minute walk distance; NT-proBNP: N-terminal pro-B-type natriuretic peptide; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary artery wedge pressure; SvO2: mixed venous oxygen saturation; CI: cardiac index; PVR: pulmonary vascular resistance.
3.2. Hemodynamic Data
All CTEPH patients underwent RHC, and the average values for mPAP, SvO2, CI, and PVR were 54.13 ± 12.43 mmHg, 54.25 ± 9.44%, 2.33 ± 0.41 L/(min·m2), and 11.80 ± 2.97 Wood units, respectively (Table 2).
3.3. Comparison of miRNA Expression Levels in the CTEPH and Reference Groups
qPCR was adopted to detect the expression levels of let-7b-3p, miR-17-5p, miR-106b-5p, miR-3202, miR-665, and miR-93-5p in the peripheral blood samples from both groups. The peripheral blood expression levels of miR-3202 and miR-665 were significantly higher in patients with CTEPH than in the reference group (∗P < 0.05; ∗∗P < 0.01). Conversely, the peripheral blood expression levels of let-7b-3p, miR-17-5p, miR-106b-5p, and miR-93-5p were significantly lower in CTEPH patients than in the reference group (∗P < 0.05; ∗∗P < 0.01; Figure 1).
Figure 1.
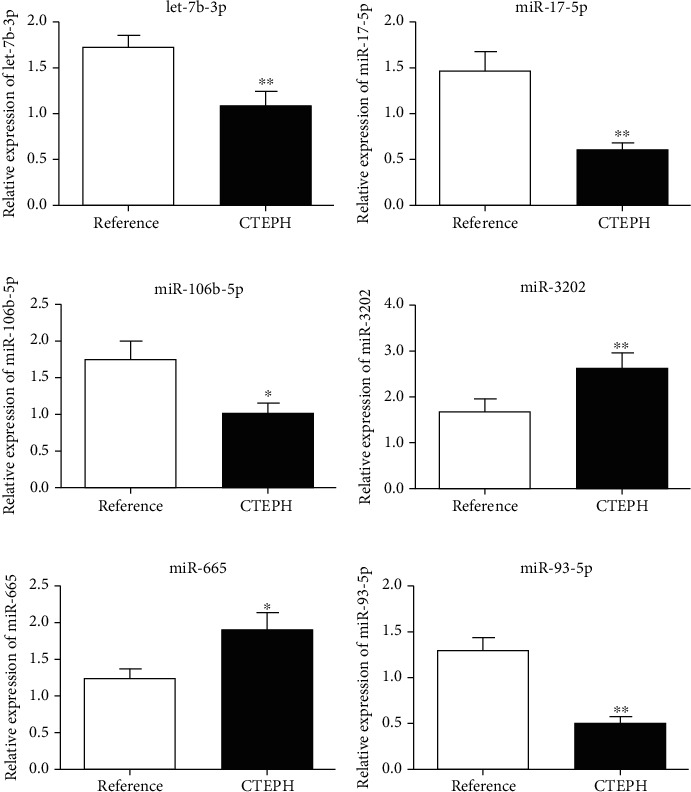
Relative expression of (a) let-7b-3p, (b) miR-17-5p, (c) miR-106b-5p, (d) miR-3202, (e) miR-665, and (f) miR-93-5p in CTEPH patients and the reference group. CTEPH: chronic thromboembolic pulmonary hypertension. ∗P < 0.05; ∗∗P < 0.01.
3.4. Correlations between miRNA Expression and Changes in Clinical Parameters
A positive correlation was observed between miR-93-5p expression and NT-proBNP level (r = 0.831, P < 0.05), while a negative correlation was observed between miR-93-5p expression and CI (r = −0.861, P < 0.05). Additionally, let-7b-3p expression showed a trend toward a positive correlation with mPAP (r = 0.811, P = 0.096). However, no significant correlations were observed between the expression levels of other miRNAs and clinical parameters (Table 3).
Table 3.
Correlation analysis results for miRNA expression levels and clinical data.
| Parameters | let-7b-3p | miR-17-5p | miR-106-5p | miR-3202 | miR-665 | miR-93-5p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | r | P value | r | P value | r | P value | |
| NT-proBNP | 0.357 | 0.555 | 0.612 | 0.197 | 0.570 | 0.237 | 0.160 | 0.705 | -0.120 | 0.820 | 0.831 | 0.041# |
| mPAP | 0.811 | 0.096 | 0.577 | 0.230 | 0.258 | 0.621 | 0.295 | 0.478 | -0.076 | 0.887 | 0.435 | 0.389 |
| SvO2 | -0.597 | 0.288 | -0.669 | 0.146 | 0.647 | 0.165 | -0.355 | 0.388 | 0.276 | 0.596 | -0.725 | 0.103 |
| CI | -0.279 | 0.649 | 0.111 | 0.833 | 0.138 | 0.794 | -0.150 | 0.723 | -0.083 | 0.876 | -0.861 | 0.027∗ |
| PVR | 0.675 | 0.211 | 0.524 | 0.286 | 0.255 | 0.625 | 0.070 | 0.869 | -0.402 | 0.429 | 0.682 | 0.135 |
NT-proBNP: N-terminal pro-B-type natriuretic peptide; mPAP: mean pulmonary arterial pressure; SvO2: mixed venous oxygen saturation; CI: cardiac index; PVR: pulmonary vascular resistance. #miR-93-5p and NT-proBNP are significantly positively correlated. ∗miR-93-5p and CI are significantly negatively correlated.
3.5. Comparison of miRNA Expression Levels in CTEPH Patients with Different WHO FCs and Risk Stratifications
CTEPH patients were categorized into a WHO FC II group (n = 5) and a WHO FC III group (n = 3). No significant differences in miRNA expression were observed between the two groups (Table 4). CTEPH patients were further classified into the intermediate-risk or the high-risk groups based on the risk assessment criteria in the 2015 ESC/ERS guidelines [16]. Again, no significant differences in the expression levels of miR-17-5p, miR-665, miR-106-5p, miR-3202, and let-7b-3p were found between the two groups. However, a marginally significant difference in expression was observed for miR-93-5p (0.348 ± 0.156 vs. 0.655 ± 0.133, P = 0.060; Table 5).
Table 4.
Comparison of miRNA expression between CTEPH patients with different WHO FCs.
| miRNA | WHO FC II group (n = 5) | WHO FC III group (n = 3) | P value |
|---|---|---|---|
| miR-17-5p | 0.360 ± 0.064 | 1.066 ± 0.379 | 0.227 |
| miR-665 | 2.357 ± 0.906 | 1.024 ± 0.023 | 0.122 |
| miR-93-5p | 0.437 ± 0.219 | 0.630 ± 0.178 | 0.346 |
| miR-106-5p | 0.500 ± 0.480 | 1.519 ± 0.591 | 0.080 |
| miR-3202 | 2.609 ± 1.684 | 2.556 ± 0.447 | 0.950 |
| let-7b-3p | 0.964 ± 0.694 | 1.296 ± 0.663 | 0.632 |
Table 5.
Comparison of miRNA expression between CTEPH patients with different risk stratifications.
| miRNA | Intermediate-risk CTEPH (n = 4) | High-risk CTEPH (n = 4) | P value |
|---|---|---|---|
| miR-17-5p | 0.357 ± 0.078 | 0.834 ± 0.483 | 0.167 |
| miR-665 | 1.969 ± 0.576 | 1.856 ± 1.440 | 0.905 |
| miR-93-5p | 0.348 ± 0.156 | 0.655 ± 0.133 | 0.060 |
| miR-106-5p | 0.545 ± 0.667 | 1.239 ± 0.740 | 0.329 |
| miR-3202 | 2.282 ± 1.752 | 2.897 ± 0.773 | 0.544 |
| let-7b-3p | 0.964 ± 0.694 | 1.296 ± 0.663 | 0.632 |
4. Discussion
In the current study, we evaluated the relationships between miRNA expression levels and clinical parameters in patients with CTEPH. The severity of CTEPH was assessed according to miRNA expression as well. Our findings indicate that miR-3202 and miR-665 were upregulated, whereas let-7b-3p, miR-17-5p, miR-106b-5p, and miR-93-5p were downregulated in CTEPH. More importantly, miR-93-5p expression was positively correlated with the serum NT-proBNP concentration and negatively correlated with CI.
CTEPH usually results in progressive exertional dyspnea and right ventricular failure. NT-proBNP is an inactive by-product of proBNP cleavage that occurs under high filling pressures [17]. It remains stable at room temperature and increases to a higher level in pathological conditions [18, 19]. Previous studies suggested that the serum NT-proBNP concentration could reflect the severity of right ventricular function injury, with a higher NT-proBNP level indicating a more severe CTEPH classification [20–22]. In the present study, the associations between miRNA expression levels (let-7b-3p, miR-17-5p, miR-106b-5p, miR-3202, miR-665, and miR-93-5p) and the NT-proBNP concentration was investigated. We observed that the miR-93-5p expression was positively correlated with NT-proBNP concentration, which suggested that miR-93-5p may serve as an indicator of the severity of CTEPH.
Hemodynamic parameters, including mPAP, SvO2, CI, and PVR, represent right ventricular function and thus are important indicators reflecting the severity of CTEPH [23]. Among these parameters, CI is an indicator of cardiac pumping function. A decrease in the CI is accompanied by an increase in right ventricular end-diastolic and pulmonary arterial pressure, as well as a decrease in right ventricular work. In severe CTEPH patients, right ventricular dysfunction leads to a CI decrease [24]. In the present study, miR-93-5p expression was negatively correlated with CI, implying that miR-93-5p may be an alternative biomarker for assessing right ventricular function and the severity of CTEPH.
We speculate that miR-93-5p may affect the severity of CTEPH by interacting with NT-proBNP and influencing the CI via myeloid leukemia/hepatitis C pathways in cancer/HTLV-I infection/axon guidance, because pathway enrichment analysis indicated that the top five pathways for the targets of miR-93-5p are chronic myeloid leukemia, hepatitis C, cancer-related pathways, HTLV-I infection, and axon guidance [13]. Previous studies demonstrated that miR-93-5p is involved in multiple pathophysiological processes, including angiogenesis, autophagy, and inflammation. An in vitro study showed that miR-93-5p regulates the function of human endothelial cells through downregulating epithelial protein loss in neoplasm [25]. Vascular endothelial growth factor and the inflammatory cytokine interleukin-8 were reported to be the major targets of miR-93-5p in neuroblastoma cells [26]. In acute myocardial infarction, miR-93-5p was found to have a cardioprotective effect by suppressing Atg7-mediated autophagy and TLR4/NF-κB-mediated inflammatory responses [27]. Increasing evidence indicates that miR-93-5p may play an important role in the regulation of the cardiovascular system. However, the specific mechanism of miR-93-5p in CTEPH needs to be further clarified.
Furthermore, mPAP is an important pulmonary hemodynamic parameter for identifying and managing CTEPH [28, 29]. It can reflect the severity of mosaic perfusion [30]. Guo et al. previously found that let-7b is downregulated in CTEPH patients. miRNA let-7b affects the migration of pulmonary arterial endothelial cells and smooth muscle cells by altering endothelin-1 and transforming growth factor (beta receptor I) expression [31]. In the present study, let-7b-3p expression appeared to be positively correlated with mPAP, although the association did not reach statistical significance. Accordingly, the role of let-7b in the pathogenesis of CTEPH warrants further investigation.
To our knowledge, this is the first study to investigate the relationship between miRNA expression levels and the clinical characteristics of CTEPH. This work expands the current understanding of the roles of miRNAs in the development of CTEPH. miRNAs are ideal candidates for future studies based on their connection with disease severity indicators (e.g., NT-proBNP and CI). Further research including in-depth functional evaluation and protein-level analysis may provide insight into the genetic and molecular mechanisms of CTEPH.
There are several limitations in this study. First, the sample size was relatively small, which may cause deviation in the results. In the current study, we did not detect miRNA expression differences in CTEPH patients with different WHO FC classifications or risk stratifications, possibly due to the limited sample size. Second, miRNA expression was detected in peripheral blood samples. As CTEPH is a multietiological disease, diverse miRNA sources might provide greater insight into the mechanisms of this disease. Third, the underlying mechanisms of the associations between miRNA expression levels and clinical indices need to be further elucidated. A large-scale study is warranted to verify our findings.
5. Conclusions
In conclusion, our study suggests that miR-93-5p expression correlates with the severity of CTEPH and could serve as a potential predictor of high-risk CTEPH. The impact of miR-93-5p in the pathogenesis of CTEPH is worth further investigation.
Acknowledgments
This study was supported by National Natural Science Foundation of China (Project numbers: 81300044, 31670928, 81871356, 81770253, 81370362, 81871328, and 81900047), Beijing Natural Science Foundation (Project numbers: 7162069 and 7182149), the National Key Research and Development Program of China (Project number: 2016YFC0905600), Beijing Municipal Administration of Hospitals' Youth Programme (Project number: QML20160301), and the Open Foundation from Beijing Key Laboratory of Hypertension Research (2018GXY-KFKT-02).
Data Availability
The datasets generated and analyzed in the present study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Table S1: primer sequences used for qPCR detection of miR-let-7b-3p, miR-17-5p, miR-106b-5p, miR-3202, miR-665, and miR-93-5p expression.
References
- 1.Yan L., Li X., Liu Z., et al. Research progress on the pathogenesis of CTEPH. Heart Failure Reviews. 2019;24(6):1031–1040. doi: 10.1007/s10741-019-09802-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Yu X., Jin Q., et al. Advances in targeted therapy for chronic thromboembolic pulmonary hypertension. Heart Failure Reviews. 2019;24(6):949–965. doi: 10.1007/s10741-019-09798-x. [DOI] [PubMed] [Google Scholar]
- 3.Dodson M. W., Allen-Brady K., Brown L. M., Elliott C. G., Cannon-Albright L. A. Chronic thromboembolic pulmonary hypertension cases cluster in families. Chest. 2019;155(2):384–390. doi: 10.1016/j.chest.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Delcroix M., Staehler G., Gall H., et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. The European Respiratory Journal. 2018;52(5):p. 1800248. doi: 10.1183/13993003.00248-2018. [DOI] [PubMed] [Google Scholar]
- 5.Vistbakka J., Sumelahti M. L., Lehtimaki T., Elovaara I., Hagman S. Evaluation of serum miR-191-5p, miR-24-3p, miR-128-3p, and miR-376c-3 in multiple sclerosis patients. Acta Neurologica Scandinavica. 2018;138(2):130–136. doi: 10.1111/ane.12921. [DOI] [PubMed] [Google Scholar]
- 6.Schulte C., Karakas M., Zeller T. MicroRNAs in cardiovascular disease - clinical application. Clinical Chemistry and Laboratory Medicine. 2017;55(5):687–704. doi: 10.1515/cclm-2016-0576. [DOI] [PubMed] [Google Scholar]
- 7.Fan H. M., Sun X. Y., Guo W., et al. Differential expression of micro RNA in peripheral blood mononuclear cells as specific biomarker for major depressive disorder patients. Journal of Psychiatric Research. 2014;59:45–52. doi: 10.1016/j.jpsychires.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Guo L. J., Liu J., et al. MicroRNA expression profile of pulmonary artery smooth muscle cells and the effect of let-7d in chronic thromboembolic pulmonary hypertension. Pulmonary Circulation. 2013;3(3):654–664. doi: 10.1086/674310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X., Xiang D., Shu Y., Hu K., Zhang Y., Li Y. MicroRNA-204 as an indicator of severity of pulmonary hypertension in children with congenital heart disease complicated with pulmonary hypertension. Medical Science Monitor. 2019;25:10173–10179. doi: 10.12659/MSM.917662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing X. Q., Li B., Xu S. L., Liu J., Zhang C. F., Yang J. MicroRNA-214-3p regulates hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration by targeting ARHGEF12. Medical Science Monitor. 2019;25:5738–5746. doi: 10.12659/MSM.915709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z., Nakajima T., Tanabe N., et al. Susceptibility to chronic thromboembolic pulmonary hypertension may be conferred by miR-759 via its targeted interaction with polymorphic fibrinogen alpha gene. Human Genetics. 2010;128(4):443–452. doi: 10.1007/s00439-010-0866-8. [DOI] [PubMed] [Google Scholar]
- 12.Opitz I., Kirschner M. B. Molecular research in chronic thromboembolic pulmonary hypertension. International Journal of Molecular Sciences. 2019;20(3):p. 784. doi: 10.3390/ijms20030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miao R., Dong X., Gong J., et al. hsa-miR-106b-5p participates in the development of chronic thromboembolic pulmonary hypertension via targeting matrix metalloproteinase 2. Pulm Circ. 2020;10(3, article 204589402092830) doi: 10.1177/2045894020928300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilbrow A. P., Frampton C. M., Richards A. M., Troughton R. W., Cameron V. A. Elevated levels of circulating microRNA-223, microRNA-127 and microRNA-146a are associated with risk of near future cardiovascular events in asymptomatic individuals. Circulation. 2014;130, article A18066 [Google Scholar]
- 15.Baptista R., Marques C., Catarino S., et al. MicroRNA-424(322) as a new marker of disease progression in pulmonary arterial hypertension and its role in right ventricular hypertrophy by targeting SMURF1. Cardiovascular Research. 2018;114(1):53–64. doi: 10.1093/cvr/cvx187. [DOI] [PubMed] [Google Scholar]
- 16.Galiè N., Humbert M., Vachiery J. L., et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. The European Respiratory Journal. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 17.Calzetta L., Orlandi A., Page C., et al. Brain natriuretic peptide: much more than a biomarker. International Journal of Cardiology. 2016;221:1031–1038. doi: 10.1016/j.ijcard.2016.07.109. [DOI] [PubMed] [Google Scholar]
- 18.Guo M., Cao X., Shen B., et al. The predictive value of NT-pro-brain natriuretic peptide for risk of pneumonia in patients on maintenance hemodialysis. Blood Purification. 2020;49(3):348–355. doi: 10.1159/000504524. [DOI] [PubMed] [Google Scholar]
- 19.Panneer Selvam A., Prasad S. Nanosensor electrical immunoassay for quantitative detection of NT-pro brain natriuretic peptide. Future Cardiology. 2013;9(1):137–147. doi: 10.2217/fca.12.76. [DOI] [PubMed] [Google Scholar]
- 20.Liu J. L., Pang S. The clinical value of serum levels of hot shock protein 70 and NT-pro brain natriuretic peptide in the aged patients with chronic congestive heart failure. Modern Preventive Medicine. 2015;42:2279–2281. [Google Scholar]
- 21.Tesic M., Seferovic J., Trifunovic D., et al. N-terminal pro-brain natriuretic peptide is related with coronary flow velocity reserve and diastolic dysfunction in patients with asymmetric hypertrophic cardiomyopathy. Journal of Cardiology. 2017;70(4):323–328. doi: 10.1016/j.jjcc.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Quintana E., Marrero-Negrin N., Gopar-Gopar S., Rodriguez-Gonzalez F. Right ventricular function and N-terminal pro-brain natriuretic peptide levels in adult patients with simple dextro-transposition of the great arteries. Echocardiography. 2017;34(6):876–880. doi: 10.1111/echo.13526. [DOI] [PubMed] [Google Scholar]
- 23.Hong C., Li J. Y., Chen R. K., et al. Correlation between peripheral venous oxygen saturation and hemodynamic parameters in patients with pulmonary hypertension. Chinese Journal of Tuberculosis and Respiratory Diseases. 2018;41(1):37–40. doi: 10.3760/cma.j.issn.1001-0939.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Darrin W., Nicholas M. Update in echocardiography: chronic thromboembolic pulmonary hypertension. Journal of Pulmonary and Respiratory Medicine. 2015;5 [Google Scholar]
- 25.Liang L., Zhao L., Zan Y., Zhu Q., Ren J., Zhao X. MiR-93-5p enhances growth and angiogenesis capacity of HUVECs by down-regulating EPLIN. Oncotarget. 2017;8(63):107033–107043. doi: 10.18632/oncotarget.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fabbri E., Montagner G., Bianchi N., et al. MicroRNA miR-93-5p regulates expression of IL-8 and VEGF in neuroblastoma SK-N-AS cells. Oncology Reports. 2016;35(5):2866–2872. doi: 10.3892/or.2016.4676. [DOI] [PubMed] [Google Scholar]
- 27.Liu J., Jiang M., Deng S., et al. miR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Molecular Therapy--Nucleic Acids. 2018;11:103–115. doi: 10.1016/j.omtn.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montani D., Günther S., Dorfmüller P., et al. Pulmonary arterial hypertension. Orphanet Journal of Rare Diseases. 2013;8(1):p. 97. doi: 10.1186/1750-1172-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasai H., Matsumura A., Sugiura T., et al. Mean pulmonary artery pressure using echocardiography in chronic thromboembolic pulmonary hypertension. Circulation Journal. 2016;80(5):1259–1264. doi: 10.1253/circj.CJ-15-1080. [DOI] [PubMed] [Google Scholar]
- 30.Leone M. B., Giannotta M., Palazzini M., et al. A new CT-score as index of hemodynamic changes in patients with chronic thromboembolic pulmonary hypertension. La Radiologia Medica. 2017;122(7):495–504. doi: 10.1007/s11547-017-0750-x. [DOI] [PubMed] [Google Scholar]
- 31.Guo L., Yang Y., Liu J., et al. Differentially expressed plasma microRNAs and the potential regulatory function of Let-7b in chronic thromboembolic pulmonary hypertension. PLoS One. 2014;9(6, article e101055) doi: 10.1371/journal.pone.0101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: primer sequences used for qPCR detection of miR-let-7b-3p, miR-17-5p, miR-106b-5p, miR-3202, miR-665, and miR-93-5p expression.
Data Availability Statement
The datasets generated and analyzed in the present study are available from the corresponding author upon reasonable request.


