Abstract
Purpose
Slc26a4−/− mice exhibit severer defects in the development of the cochlea and develop deafness, while the underlying mechanisms responsible for these effects remain unclear. Our study was to investigate the potential mechanism linking SLC26A4 deficiency to hearing loss.
Materials and Methods
RNA sequencing was applied to analyze the differential gene expression of the stria vascularis (SV) from wildtype and Slc26a4−/− mice. GO and KEGG pathway analysis were performed. Quantitative RT-PCR was applied to validate the expression of candidate genes affected by Slc26a4. ELISA and immunofluorescence technique were used to detect the homocysteine (Hcy) level in serum, brain, and SV, respectively.
Results
183 upregulated genes and 63 downregulated genes were identified in the SV associated with Slc26a4 depletion. Transcriptomic profiling revealed that Slc26a4 deficiency significantly affected the expression of genes associated with cell adhesion, transmembrane transport, and the biogenesis of multicellular organisms. The SV from Slc26a4−/− mice exhibited a higher expression of Bhmt mRNAs, as well as altered homocysteine (Hcy) metabolism.
Conclusions
The altered expression of Bhmt results in a dramatic change in multiple biochemical reactions and a disruption of nutrient homeostasis in the endolymph which may contribute to hearing loss of Slc26a4 knockout mouse.
1. Introduction
Pendred syndrome, characterized by deafness with enlargement of aqueduct and goiter, is caused by mutations of SLC26A4, one of the most prevalent causes of hereditary hearing loss globally [1]. A large-scale study showed mutations of SLC26A4 that occur in approximately 5-10% sensorineural hearing loss children among a variety of different ethnic populations [2]. The onset of hearing loss in patients with Pendred syndrome is variable. Some patients may have hearing loss at birth, while others suffer fluctuating, or progressive hearing loss during their childhood, which may provide a window of opportunity for treatment [3].
To investigate the underlying mechanism of the Pendred syndrome, researchers have created a mouse model for study. The Slc26a4 knockout mice suffer severe hearing loss and malformation of cochlea at birth. Previous studies looking at the Slc26a4 knockout mice have found that pendrin, encoded by the Slc26a4 gene, locates in root cells of the outer sulcus, cells overlying the spiral prominence, and spindle-shaped cells of the stria vascularis in cochlea. Meanwhile, three main changes occur in the inner ears of Slc26a4 mutant mouse. (1) A lack of pendrin causes the acidification of the endolymph [4]. Pendrin is an anion exchange protein, similar to Cl− and HCO3- exchangers that participate in fluid regulation of the endolymph [5]. In the absence of pendrin, HCO3- secretion into the endolymph is affected, which causes the endolymph to become acidic and leads to inhibition of Ca2+ reabsorption [4, 6]. (2) The scala media is enlarged due to the dysfunction of the endolymphatic sac. The cochlear lumen is formed by the balance of the fluid secretion in the vestibular labyrinth and fluid absorption in the endolymphatic sac, from embryonic day (E) 13.5 and 14.5 [7]. Thus, dysfunction of fluid absorption in the endolymphatic sac, due to Slc26a4 mutation, results in an enlarged volume of the scala media [8, 9]. (3) The expression of Kcnj10 is reduced, and this inwardly rectifying K+ channel subunit, located in the intermediate cells of the stria vascularis (SV), is responsible for the establishment of the endolymphatic potential (EP) [10, 11]. Wangemann et al. have reported an absence of Kcnj10 in the intermediate cells of Slc26a4 mutant mice at 1-4 months of age, and this lack of channel protein causes a decrease in the EP, which may directly lead to deafness [12]. Among these three changes, the pathology in the SV is the initial and predominant cause of the hearing loss associated with Slc26a4−/− mice. However, how Slc26a4 deficiency affects morphology and function of SV remains unknown.
In this study, we used RNA sequencing methodology to compare differences in the transcriptomes in the SV from wildtype and Slc26a4 mutant mice. We found that Slc26a4 deficiency significantly affected SV genes required for multicellular organism biogenesis. Furthermore, the SV from Slc26a4−/− mice exhibited higher expression of Bhmt mRNAs, as well as a dramatic reduction of Hcy, suggesting abnormality of Hcy metabolism as a novel mechanism by which SLC26A4 affects hearing.
2. Materials and Methods
2.1. Animals
Slc26a4 knockout mice were obtained from the Jackson Laboratory, and Calca knockout mice were from Cyagen Co. LTD. All animals were maintained in heterozygotes in SPF circumstances and adapted to the experimental circumstances for a week before the experiments. All experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Shanghai Jiao Tong University Affiliated Sixth People's Hospital [Permit number: SYXK 2016-0020].
2.2. RNA-seq
Wildtype and Slc26a4−/− mice were sacrificed at P14. The stria vascularis were isolated from inner ear and then lysed in Trizol (Invitrogen). Total RNA was isolated according to the manufacturer's instructions. All samples were sequenced on an Illumina HiSeq2500 platform at 15 million 100-bp single reads per sample. After quality control of the sequencing libraries, reads were trimmed and mapped against the Ensembl genome annotation and the human genome assembly (hg19/GRCh38) using Tophat2. Reads mapping to ribosomal RNAs or the mitochondrial genome were removed.
2.3. Quantitative Real-Time RT-PCR
Total RNA from wildtype and Slc26a4−/− stria vascularis was extracted with TRIzol reagent according to the manufacturer's instructions (Invitrogen). One microgram of total RNA was reverse transcribed using the ReverTra Ace® qPCR RT Kit (Toyobo, FSQ-101) according to the manufacturer's instructions. A SYBR RT-PCR kit (Toyobo, QPK-212) was used for quantitative real-time PCR analysis. The relative mRNA expression of different genes was calculated by comparison with the control gene Gapdh (encoding GAPDH) using the 2-△△Ct method (Table 1).
Table 1.
Primer used in this study.
| Target | Forward (5'to3') | Reversed (5'to 3') |
|---|---|---|
| Slc26a7 | GCATGATGAAACCTCGCAACA | TTCATTGCAGTTGCCGTTGG |
| Ace2 | CACTCCTGCCACACCACGTT | TGGTCTTTAGGTCAAGTTTACAGCC |
| Gpr176 | GGTGTTATGGTCAACTTGCCG | AGAGCATCGTATAGATCCACCAG |
| Lrrc8d | ATGTTTACCCTTGCGGAAGTTG | CATCAGCATAACGACTGCCAG |
| Padi1 | TGTGTGCGTGGTAGGTGTG | TCGAGGGATCGTAGACCATGT |
| Arrb1 | AAGGGACACGAGTGTTCAAGA | CCCGCTTTCCCAGGTAGAC |
| Alox15 | CAGGGATCGGAGTACACGTT | GATTGTGCCATCCTTCCAGT |
| Bmht | CTGGGGAAGTGGTTTGGACA | GCCGGAAGCTATTCGCAGAT |
| Bhmt2 | CTCCAGAAGCAGTGGTAGAACATC | CATCAGCTCCCGCTCTCAAG |
| Ahcy | TCGAAGTGTCCAATGTTACAGAC | CTTGGCCGGCACTTTGAG |
| Cbs | GCAGCGCTGTGTGGTCATC | GTCACTCAGGAACTTGGACATGTAGT |
2.4. Immunostaining
Wildtype and Slc26a4−/− mice were sacrificed at P14. The cochleae were isolated from temporal bone, fixed by 4% PFA, dehydrated by 30% sucrose, and cut into frozen sections. The section was then permeabilized with 0.1% Triton X-100 in PBS and blocked with 1% BSA in PBS. The samples were incubated with anti-Hcy (PAD984Ge01, Clonecloud) at 4°C overnight and then secondary antibody or DAPI at room temperature for 30 min. The stria vascularis were dissected from lateral wall and its actin structure were shown by phalloidin staining. Samples were examined and the figures were acquired with an LSM 710 confocal laser-scanning microscope (Carl Zeiss, Oberkochen, Germany) at 20x or 63x magnification.
2.5. Enzyme-Linked Immunosorbent Assay
The blood plasma and brain homogenate from wildtype and Slc26a4−/− mice were prepared and persevered at -20°C until analysis. Hcy level was measured using ELISA Kit for Homocysteine (CED984Ge, Clone-Cloud) according to the manufacture's instruction. Absorbance was assessed by an ELISA microplate reader at 450 nm and the results were expressed in pg/ml.
2.6. ABR Threshold Testing
The hearing condition of mice was detected by auditory brain stem response (ABR) thresholds. The mice used for ABR testing were fully anesthetized by a mixture of ketamine and xylazine. The initial dose of ketamine and xylazine was 85 and 15 mg/kg, respectively. The stimulus generation and biosignal acquisition parameters were similar to those used in a previous study [13]. Auditory stimuli were presented from a speaker 10 cm ahead of the mouse's head, needle electrodes were positioned in the vertex of the head and the reference, and grounding electrodes were positioned posterior to the external auditory canals. The tone bursts were generated by TDT RP2.1 Real-time signal processor, (10-ms duration with 0.5-ms rise/fall time presented at 21.1/s intervals) at 4, 8, 16, 24, and 32 kHz with SigGen software. The biological signals picked up by the electrodes were led to a RA4PA preamplifier from Tucker-Davis Technologies (TDT System III; Alachua, FL, USA). The evoked ABR responses were amplified 20x by a PA4 preamplifier (TDT) and averaged 1000 times. The intensity of tone bursts started at 90 dB SPL and declined by a step of 10 dB, except around threshold where it was 5 dB. The threshold was determined based on visibility and reproducibility of negative–positive waves. Threshold was defined as the lowest sound level that produces a reproducible response.
2.7. Statistical Analysis
The results are represented as the mean ± s.e.m., and statistical significance between groups was determined using an unpaired t-test or the Mann–Whitney U test. The GraphPad Prism software 8.0 was used for all analyses, and a ∗p < 0.05 was considered statistically significant.
3. Results
3.1. Dramatic Morphological Changes in the Stria Vascularis from Slc26a4−/− Mice
Slc26a4 −/− mice typically have larger inner ears due to the dysfunction of the endolymphatic sac. Compared to those from Slc26a4+/+ mice, the cochlear lumen of the inner ears, isolated from 6-week-old Slc26a4−/− mice, is significantly enlarged. Furthermore, the SV can be visualized through the pigmentation of the intermediate cells [14], and the lateral wall appears darker and more obvious in Slc26a4+/+ mice when compared to Slc26a4−/− (Figure 1(a)).
Figure 1.
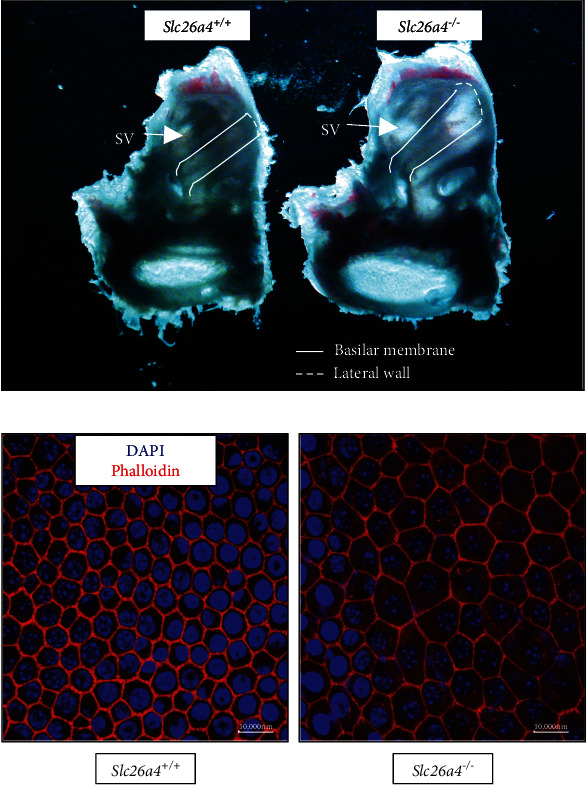
The dramatic morphological changes occurred in stria vascularis from Slc26a4−/− mice. (a) The scala media, formed by basilar membrane, lateral wall, and vestibular membrane, is significantly enlarged in Slc26a4−/− mice. SV: stria vascularis. The outline of stria vascularis, which is visualized through pigmentation in intermediate cells, is clearer in Slc26a4+/+ mice. (b) Marginal cells in stria vascularis from wildtype or Slc26a4−/− mice were coated on coverlips and sent for phalloidin staining.
The inner layer of the SV is formed by marginal cells facing the endolymph [10]. Therefore, we used the actin stain phalloidin, to profile the structure of these marginal cells in SV. Indeed, enlargement of marginal cells was seen in Slc26a4−/− mice, while the marginal cells from Slc26a4+/+ mice were normal in both size and organization [15] (Figure 1(b)).
3.2. Transcript Regulation in the Stria Vascularis Caused by Slc26a4 Deletion
To determine the role of SLC26A4 in the function of the cochlea, wildtype and Slc26a4−/− mice were sacrificed and their SV were carefully isolated. Total RNA from pairs of SVs was extracted and then sent for next-generation sequencing and data from each sample generated on average 24-Mb of clean reads, after low-quality filtering, and these clean reads were mapped for reference. Each of the data sets contained 24-Mb reads and a mapping rate of 92–93%. Moreover, we counted the number of identified expressed genes and calculated their proportion and distribution to the total gene number in the database for each sample. The correlation for gene expression levels among the samples is a key criterion to determine whether the experiments are reliable and whether the samples chosen are reasonable, and principal component analysis was performed to assess gene expression levels (Figure 2(a)).
Figure 2.
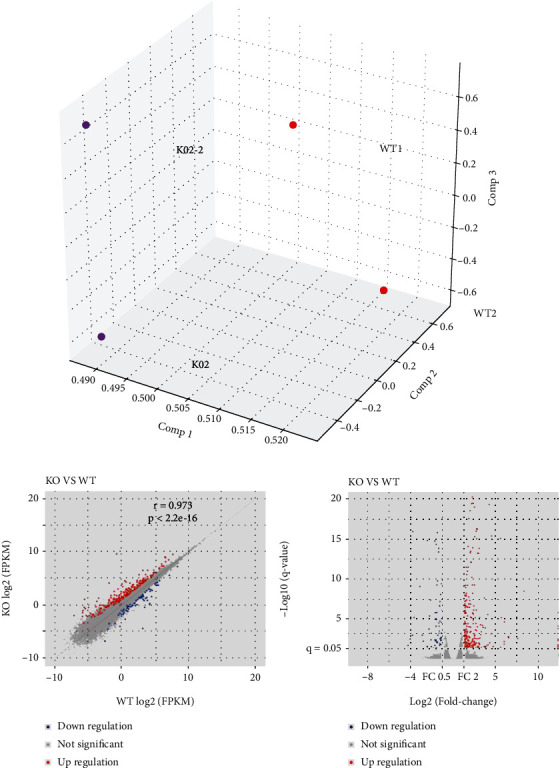
Transcripts regulated in stria vascularis of Slc26a4 deletion. (a) Principal component analysis of RNAs from stria vascularis of wildtype (WT) and Slc26a4−/− (KO) mice. (b, c) Scatter plot (b) and Volcano plot (c) indicate individual RNAs sequenced.
Volcano and Scatter plots showed differentially expressed transcripts for a fold change of >2 in the Slc26a4 deficient group when compared to the wildtype group (183 upregulated genes and 63 downregulated genes) (Figures 2(b) and 2(c)).
3.3. Gene Ontology Analysis of the Differential Genes
To better understand the associated functions of these differentially expressed genes in Slc26a4-mediated cochlear function, gene ontology (GO) analysis was used to perform enrichment analysis and classifications (Figures 3(a) and 3(b)). GO analysis identified enriched biological processes associated with “biological adhesion,” “cellular component organization or biogenesis,” “multicellular organismal processes,” and “developmental processes,” indicating that a strong multicellular organism biogenesis process occurred during the Slc26a4-mediated maintenance of cochlear homeostasis. Furthermore, enriched cellular component terms associated with “membrane,” “organelle,” “extracellular region,” and “membrane-enclosed lumen” were identified implying that diverse cellular components were involved in Slc26a4-mediated cochlear function. Enriched molecular functions were defined as associated with “structural molecule activity,” “catalytic activity,” and “molecular transducer activity,” indicating that formation of organelle structure and intracellular signal transduction were the major molecular functions for Slc26a4.
Figure 3.
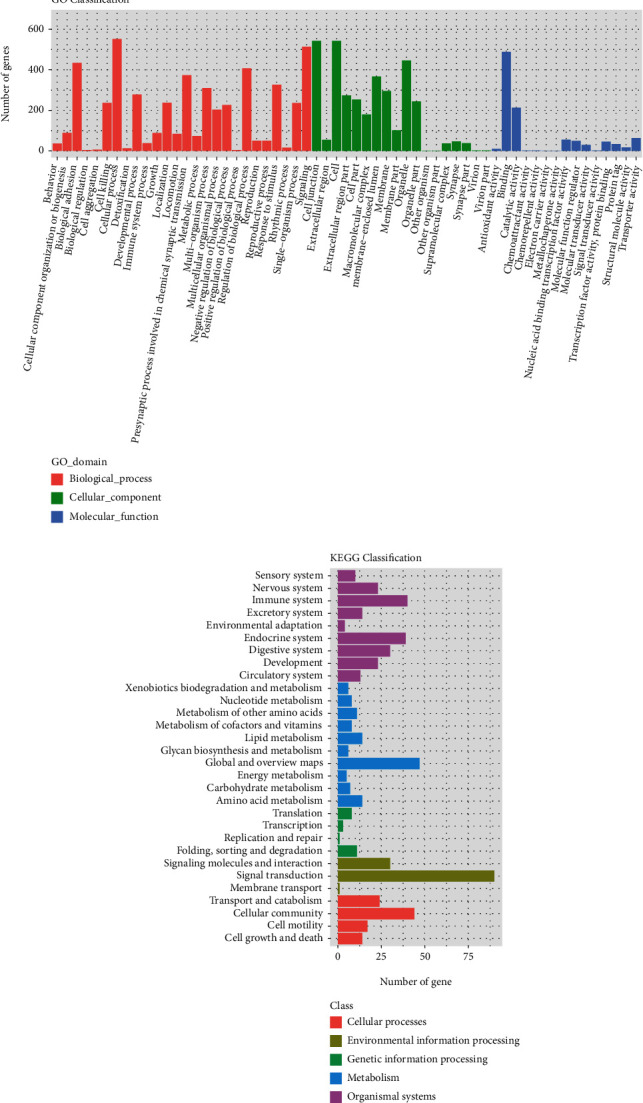
Gene ontology analysis and KEGG analysis of differentiated expressed genes in stria vascularis of Slc26a4 deletion.
3.4. Analysis of Important KEGG Pathways
We next used the differential genes for KEGG pathway enrichment, and the results showed that genes involving in the “ECM-receptor interaction pathway,” “focal adhesion pathway,” “aldosterone-regulated sodium reabsorption pathways,” and “taste transduction pathways” were significantly enriched, indicating that loss of Slc26a4 genes may lead to disrupted transmembrane cell communication and transport in the SV (Figures 3(c) and 3(d)).
Collectively, GO and KEGG pathway analysis suggested an essential role for SLC26A4 in the regulation of extracellular structure formation, cell-to-cell or cell-to-ECM adhesion, and transmembrane transport.
3.5. Validation of Selected Genes
Several important upregulated and downregulated genes are represented as shown in the heatmap (Figures 4(a) and 4(b)). Quantitative RT-PCR results validated the differential expression of selected candidate genes, including Padi1, Arrb1, Ace2, Gpr176, Lrrc8d, Alox15, and Calca (Figure 4(c)).
Figure 4.
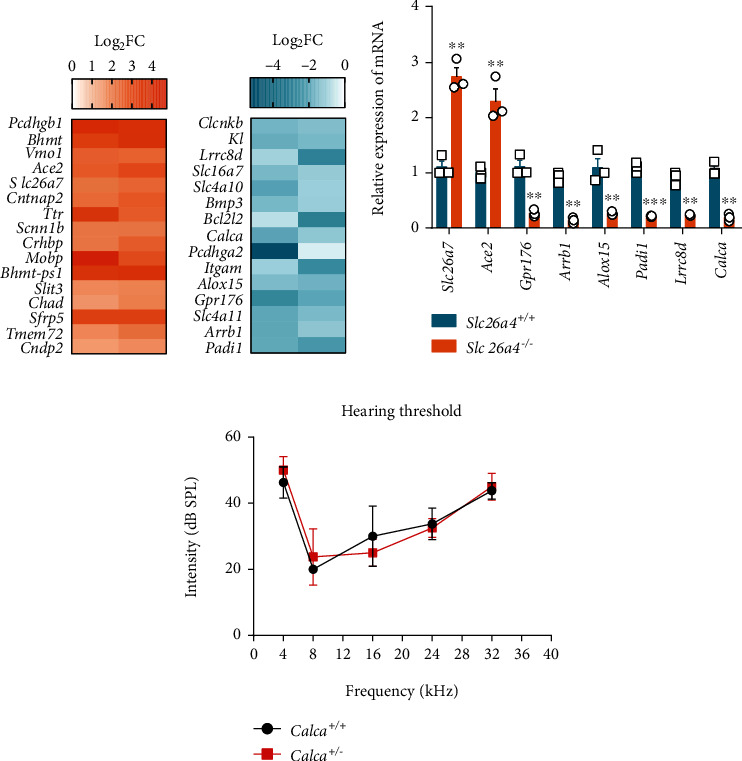
Validation of differentiated expressed genes in stria vascularis of Slc26a4 deletion. (a, b) Heatmap indicated selected upregulated genes (a) and downregulated genes (b) in Slc26a4−/− SVs. (c) Quantitative RT-PCR analysis of expression of indicated genes in Slc26a4−/− SVs. (d) ABR test of wildtype and Calca+/- mice. ∗p < 0.05 and ∗∗p < 0.01 by the unpaired t-test (c). Data are from three independent experiments with biological duplicates in each (c, d; mean ± s.e.m. of n = 3 duplicates).
We noted that one of the important downregulated genes in the SV of Slc26a4 deficient mice was Gpr176, which encodes a G-protein-coupled receptor [16] and has previously been reported to be associated with adult-onset autosomal dominant cerebellar ataxia, with deafness and narcolepsy [17]. Quantitative RT-PCR analysis confirmed the downregulation of Gpr176 in the SV of Slc26a4−/− mice (Figure 4(c)), suggesting a reduction of GPR176-mediated signaling upon Slc26a4 deficiency.
Another important gene found was Calca and therefore, we generated a Calca knockout mouse using CRISPR/Cas9 technology and carefully examine its ABR threshold. The results showed that Calca+/- mice had equivalent hearing to the wildtype controls (Figure 4(d)), suggesting that at least heterozygous deletion of Calca had no significant effect on hearing.
3.6. Altered Hcy Metabolism in the Stria Vascularis of Slc26a4−/− Mice
We next determined the expression of Bhmt, as well as several other enzymes required for Hcy metabolism including Bhmt2, Ahcy, and Cbs in the SV and brain from Slc26a4−/− mice using quantitative RT-PCR. The results showed that Slc26a4 deficiency significantly increased Bhmt expression in the SV but not the brain, while the expressions of other enzymes involved in Hcy metabolism were similar in both groups (Figures 5(a) and 5(b)). Interestingly, we also observed a significant increase in the Bhmt pseudogene (Bhmt-ps), in the SV of Slc26a4−/− mice but not in brain, which was similar to the levels of Bhmt itself, suggesting that there may be a common regulation of Bhmt and Bhmt-ps in the SV. Thus, we applied immunostaining assays using an anti-Hcy polyclonal antibody to measure levels in the SV from wildtype and Slc26a4−/− mice and found that Hcy levels were dramatically decreased in the SV of Slc26a4−/− mice (Figure 6(a)). However, we did not observe a significant change in serum Hcy levels in these mice when compared to the wild types, based on an anti-Hcy ELISA (Figures 6(b) and 6(c)).
Figure 5.
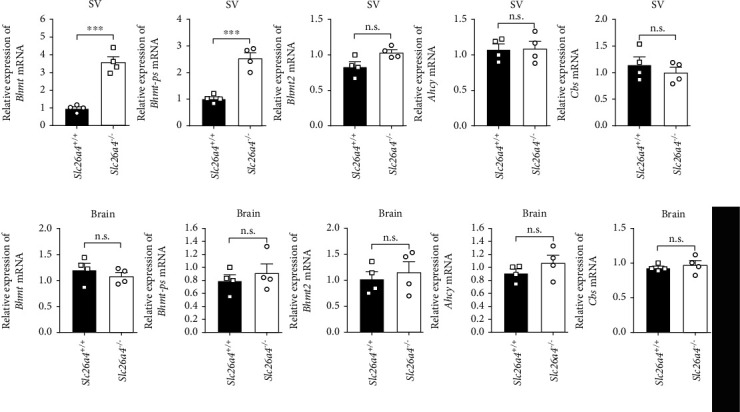
Altered expression of Hcy metabolism enzymes in Slc26a4−/− SVs. (a, b) Quantitative RT-PCR analysis of expression of Hcy metabolism enzymes in Slc26a4−/− SVs (a) or brains (b). n.s.: no significance. ∗p < 0.05 and ∗∗p < 0.01 by the unpaired t-test (c). Data are from three independent experiments with biological duplicates in each (a, b; mean ± s.e.m. of n = 3 duplicates).
Figure 6.
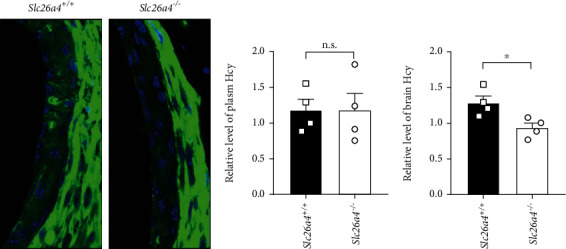
Altered Hcy metabolism in Slc26a4−/− SVs. (a) Stria vascularis from wildtype or Slc26a4−/− mice were coated on coverlips and sent for immunostaining against Hcy. (b, c) Elisa assay of Hcy level in plasma (b) and brain (c) from wildtype or Slc26a4−/− mice. n.s.: no significance. ∗p < 0.05 by the unpaired t-test (b, c). Data are from three independent experiments with biological duplicates in each (a, b, c; mean ± s.e.m. of n = 3 duplicates).
4. Discussion
Hearing loss could be caused by genetic factors, aging, chronic cochlear infections, infectious diseases, ototoxic drugs, and noise exposure [18–24]. Most of the hearing loss is due to the damage of hair cells and spiral ganglion neurons and has been extensively investigated in many previous studies [25–30], while the detailed mechanism of stria vascularis-related hearing loss, such as Pendred syndrome, has not been well known. SLC26A4 affects cochlear function both in humans and mice; however, the mechanism by which SLC26A4 achieves this is unclear [15]. Transcriptome sequencing has been extensively used in many previous studies to investigate the detailed mechanism in the inner ear [31–37]. In our study, we applied a transcriptome sequencing approach to examine the levels of differentially expressed genes in the SV from Slc26a4−/− mice. Using bioinformatic analysis, we identified 183 upregulated genes and 63 downregulated genes in the SV associated with Slc26a4 depletion. Furthermore, the cellular functions of these genes are related to cell communication, extracellular matrix organization, and transmembrane transportation. Therefore, our findings suggest a novel mechanism by which SLC26A4 can affect cochlear function.
Previous studies have shown that macrophage invasion contributes to degeneration of the SV in the Slc26a4−/− mouse model [14, 38]. Consistent with this, we found an additional two well-characterized inflammation regulating genes, Alox15, that encoded arachidonate 15-lipoxygenase [39] and Arrb1, encoding β-arrestin 1 [40, 41], in top downregulated genes in the SV of Slc26a4−/− mice. Quantitative RT-PCR results also showed that the expression levels of Arrb1 and Alox15 were decreased in the SV of these knockout mice. These findings suggest a possible role for tissue inflammation in the degeneration of the SV in the Pendred syndrome mouse model [42, 43].
The Calca gene, encoding calcitonin and alpha-calcitonin gene-related peptide [44], is one of the top downregulated genes found in our study. As reported in a previous study, Calca is expressed in mouse cochlea at an early stage during development [45]. We therefore obtained a Calca mutant mouse to determine whether the low expression of Calca affected hearing. Since Calca plays a critical role in multiorgan development [46, 47], Calca−/− mice, created by CRISPR/Cas9 methodology, were unhealthy and died soon after birth. Therefore, we were forced to generate a Calca+/- heterozygous mouse model to determine hearing. The ABR result showed that there was no difference in the hearing threshold between the wildtype and the Calca+/- animals. No significant hearing loss may be due to the heterozygotic nature of the mice used. Therefore, further research is needed to determine whether more subtle, or hidden hearing loss is present in these mice. Also, a new Calca minus model is needed to further investigate its effect upon hearing.
Our transcriptome sequencing results found that the most distinctive upregulated gene in the SV from Slc26a4−/− mice was Bhmt, which encodes betaine Hcy S methyltransferase, whose activity is required for the transfer of a methyl group from betaine to Hcy, a nonproteinaceous sulfur amino acid [48, 49]. Malfunction of Bhmt leads to hyperhomocysteinemia and several previous epidemiological and experimental studies have revealed a correlation between hyperhomocysteinemia and hearing loss [50]. Previous study showed that Hcy level plays an important part in keeping the integrity of SV, which is the foundation of SV to establish the EP [51]. We consistently observed a dramatic decrease in Hcy from the SV of Slc26a4−/− mice when compared to wildtypes, and recently, nutritional imbalance is emerging as a causative factor associated with hearing loss. Furthermore, Teresa Partearroyo et al. have reported that Bhmt plays a central role in the homeostasis of methionine metabolism in the cochlea and its deficiency in mice causes increased susceptibility to noise-induced hearing loss [52, 53]. Moreover, a decrease in Bhmt expression and an increase of Hcy in the SV is thought to explain the hearing loss phenotype seen in Cx30−/− mice [54]. However, one difference between our findings and previous studies is that in the SV of Slc26a4−/− mice, Bhmt is upregulated, which leads to decreased amounts of Hcy, which is part of the folate and methionine cycles. The elevated expression of Bhmt leads to an increased consumption of Hcy and folate and production of methionine and S-Adenosylmethionine, which serve as the major donors of methyl groups in the synthesis of hormones, nucleotides, and membrane lipids. It is speculated that the altered expression of Bhmt results in a dramatic change in multiple biochemical reactions and a disruption of nutrient homeostasis in the endolymph. However, whether the decrease in Hcy levels accounts for cochlear dysfunction and hearing loss in Pendred syndrome mouse models requires further investigation.
5. Conclusion
Transcriptomic profiling revealed that Slc26a4 deficiency significantly affected the expression of genes associated with cell adhesion, transmembrane transport, and the biogenesis of multicellular organisms. The altered expression of Bhmt results in a dramatic change in multiple biochemical reactions and a disruption of nutrient homeostasis in the endolymph which may contribute to hearing loss of Slc26a4 knockout mouse.
Acknowledgments
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided. This study was supported by the grants from the general program grants of the Natural Science Foundation of China [grant number: 81770998, 82071040].
Contributor Information
Yanmei Feng, Email: feng.yanmei@126.com.
Dongzhen Yu, Email: drdzyu@126.com.
Data Availability
Data are available from the author Wenyue Xue (xuecindy0107@163.com) upon reasonable request and with permission of the Department of Otolaryngology-Head and Neck Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Wenyue Xue and Yuxin Tian have contributed equally to this work.
References
- 1.Zhang Z., Wang J., Li C., Xue W., Xing Y., Liu F. Gene therapy development in hearing research in China. Gene Therapy. 2020;27(7-8):349–359. doi: 10.1038/s41434-020-0177-1. [DOI] [PubMed] [Google Scholar]
- 2.Park H. J., Shaukat S., Liu X. Z., et al. Origins and frequencies of SLC26A4 (PDS) mutations in east and south Asians: global implications for the epidemiology of deafness. Journal of Medical Genetics. 2003;40(4):242–248. doi: 10.1136/jmg.40.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackler R. K., De La Cruz A. The large vestibular aqueduct syndrome. The Laryngoscope. 1989;99(12):1238–1243. doi: 10.1288/00005537-198912000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Wangemann P., Nakaya K., Wu T., et al. Loss of cochlear HCO3- secretion causes deafness via endolymphatic acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. American Journal of Physiology Renal Physiology. 2007;292(5):F1345–F1353. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amlal H., Petrovic S., Xu J., et al. Deletion of the anion exchanger Slc26a4 (pendrin) decreases apical Cl−/HCO3− exchanger activity and impairs bicarbonate secretion in kidney collecting duct. American Journal of Physiology-Cell Physiology. 2010;299(1):C33–C41. doi: 10.1152/ajpcell.00033.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito T., Li X., Kurima K., Choi B. Y., Wangemann P., Griffith A. J. Slc26a4-insufficiency causes fluctuating hearing loss and stria vascularis dysfunction. Neurobiology of Disease. 2014;66:53–65. doi: 10.1016/j.nbd.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Zhou F., Marcus D. C., Wangemann P. Endolymphatic Na(+) and K(+) concentrations during cochlear growth and enlargement in mice lacking Slc26a4/pendrin. PLoS One. 2013;8(5, article e65977) doi: 10.1371/journal.pone.0065977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H. M., Wangemann P. Failure of fluid absorption in the endolymphatic sac initiates cochlear enlargement that leads to deafness in mice lacking pendrin expression. PLoS One. 2010;5(11, article e14041) doi: 10.1371/journal.pone.0014041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madden C., Halsted M., Meinzen-Derr J., et al. The influence of mutations in the SLC26A4 gene on the temporal bone in a population with enlarged vestibular aqueduct. Archives of Otolaryngology – Head & Neck Surgery. 2007;133(2):162–168. doi: 10.1001/archotol.133.2.162. [DOI] [PubMed] [Google Scholar]
- 10.Wangemann P., Itza E. M., Albrecht B., et al. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Medicine. 2004;2(1):p. 30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcus D. C., Wu T., Wangemann P., Kofuji P. KCNJ10 (Kir4.1) potassium channel knockout abolishes endocochlear potential. American Journal of Physiology-Cell Physiology. 2002;282(2):C403–C407. doi: 10.1152/ajpcell.00312.2001. [DOI] [PubMed] [Google Scholar]
- 12.Ito T., Nishio A., Wangemann P., Griffith A. J. Progressive irreversible hearing loss is caused by stria vascularis degeneration in an Slc26a4-insufficient mouse model of large vestibular aqueduct syndrome. Neuroscience. 2015;310:188–197. doi: 10.1016/j.neuroscience.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H., Xing Y., Zhang Z., et al. Coding-in-noise deficits are not seen in responses to amplitude modulation in subjects with cochlear synaptopathy induced by a single noise exposure. Neuroscience. 2019;400:62–71. doi: 10.1016/j.neuroscience.2018.12.048. [DOI] [PubMed] [Google Scholar]
- 14.Singh R., Wangemann P. Free radical stress-mediated loss of Kcnj10 protein expression in stria vascularis contributes to deafness in Pendred syndrome mouse model. American Journal of Physiology Renal Physiology. 2008;294(1):F139–F148. doi: 10.1152/ajprenal.00433.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royaux I. E., Belyantseva I. A., Wu T., et al. Localization and functional studies of pendrin in the mouse inner ear provide insight about the etiology of deafness in pendred syndrome. Journal of the Association for Research in Otolaryngology. 2003;4(3):394–404. doi: 10.1007/s10162-002-3052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi M., Murai I., Kunisue S., et al. Gpr176 is a Gz-linked orphan G-protein-coupled receptor that sets the pace of circadian behaviour. Nature Communications. 2016;7(1, article 10583) doi: 10.1038/ncomms10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Care4Rare Canada Consortium, Kernohan K. D., Cigana Schenkel L., et al. Identification of a methylation profile for DNMT1-associated autosomal dominant cerebellar ataxia, deafness, and narcolepsy. Clinical Epigenetics. 2016;8(1):p. 91. doi: 10.1186/s13148-016-0254-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Qi J., Chen X., et al. Critical role of spectrin in hearing development and deafness. Science Advances. 2019;5(4, article eaav7803) doi: 10.1126/sciadv.aav7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao S., Cheng C., Wang M., et al. Blebbistatin inhibits neomycin-induced apoptosis in hair cell-like HEI-OC-1 cells and in cochlear hair cells. Frontiers in Cellular Neuroscience. 2020;13:p. 590. doi: 10.3389/fncel.2019.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y., Li W., He Z., et al. Pre-treatment with fasudil prevents neomycin-induced hair cell damage by reducing the accumulation of reactive oxygen species. Frontiers in Molecular Neuroscience. 2019;12:p. 264. doi: 10.3389/fnmol.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S., Zhang Y., Dong Y., et al. Knockdown of Foxg1 in supporting cells increases the trans-differentiation of supporting cells into hair cells in the neonatal mouse cochlea. Cellular and Molecular Life Sciences: CMLS. 2020;77(7):1401–1419. doi: 10.1007/s00018-019-03291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian F., Wang X., Yin Z., et al. The slc4a2b gene is required for hair cell development in zebrafish. Aging. 2020;12(19):18804–18821. doi: 10.18632/aging.103840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H., Qian X., Xu N., et al. Disruption of Atg7-dependent autophagy causes electromotility disturbances, outer hair cell loss, and deafness in mice. Cell Death & Disease. 2020;11(10):p. 913. doi: 10.1038/s41419-020-03110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan F., Chu C., Qi J., et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nature Communications. 2019;10(1):p. 3733. doi: 10.1038/s41467-019-11687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Z. H., Zou S. Y., Li M., et al. The nuclear transcription factor FoxG1 affects the sensitivity of mimetic aging hair cells to inflammation by regulating autophagy pathways. Redox Biology. 2020;28:p. 101364. doi: 10.1016/j.redox.2019.101364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W., Xu X., Fan Z., et al. Wnt signaling activates TP53-induced glycolysis and apoptosis regulator and protects against cisplatin-induced spiral ganglion neuron damage in the mouse cochlea. Antioxidants & Redox Signaling. 2019;30(11):1389–1410. doi: 10.1089/ars.2017.7288. [DOI] [PubMed] [Google Scholar]
- 27.Guo R., Xiao M., Zhao W., et al. 2D Ti3C2TxMXene couples electrical stimulation to promote proliferation and neural differentiation of neural stem cells. Acta Biomaterialia. 2020 doi: 10.1016/j.actbio.2020.12.035. [DOI] [PubMed] [Google Scholar]
- 28.Qi J., Liu Y., Chu C., et al. A cytoskeleton structure revealed by super-resolution fluorescence imaging in inner ear hair cells. Cell Discovery. 2019;5(1):p. 12. doi: 10.1038/s41421-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Z., Guo L., Shu Y., et al. Autophagy protects auditory hair cells against neomycin-induced damage. Autophagy. 2017;13(11):1884–1904. doi: 10.1080/15548627.2017.1359449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L., Chen Y., Qi J., et al. Wnt activation protects against neomycin-induced hair cell damage in the mouse cochlea. Cell Death & Disease. 2016;7(3, article e2136) doi: 10.1038/cddis.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng C., Wang Y., Guo L., et al. Age-related transcriptome changes in Sox2+ supporting cells in the mouse cochlea. Stem Cell Research & Therapy. 2019;10(1):p. 365. doi: 10.1186/s13287-019-1437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang Q., Zhang Y., Chen X., et al. Three-dimensional graphene enhances neural stem cell proliferation through metabolic regulation. Frontiers in Bioengineering and Biotechnology. 2020;7:p. 436. doi: 10.3389/fbioe.2019.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang M., Li J., He L., et al. Transcriptomic profiling of neural stem cell differentiation on graphene substrates. Colloids and surfaces B: Biointerfaces. 2019;182, article 110324 doi: 10.1016/j.colsurfb.2019.06.054. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Guo L., Lu X., et al. Characterization of Lgr6+ cells as an enriched population of hair cell progenitors compared to Lgr5+ cells for hair cell generation in the neonatal mouse cochlea. Frontiers in Molecular Neuroscience. 2018;11:p. 147. doi: 10.3389/fnmol.2018.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You D., Guo L., Li W., et al. Characterization of Wnt and Notch-responsive Lgr5+ hair cell progenitors in the striolar region of the neonatal mouse utricle. Frontiers in Molecular Neuroscience. 2018;11:p. 137. doi: 10.3389/fnmol.2018.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S., Zhang Y., Yu P., et al. Characterization of Lgr5+ progenitor cell transcriptomes after neomycin injury in the neonatal mouse cochlea. Frontiers in Molecular Neuroscience. 2017;10:p. 213. doi: 10.3389/fnmol.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng C., Guo L., Lu L., et al. Characterization of the transcriptomes of Lgr5+ hair cell progenitors and Lgr5- supporting cells in the mouse cochlea. Frontiers in Molecular Neuroscience. 2017;10:p. 122. doi: 10.3389/fnmol.2017.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jabba S. V., Oelke A., Singh R., et al. Macrophage invasion contributes to degeneration of stria vascularis in Pendred syndrome mouse model. BMC Medicine. 2006;4(1):p. 37. doi: 10.1186/1741-7015-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uderhardt S., Herrmann M., Oskolkova O. V., et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36(5):834–846. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Frederick T. J., Miller S. D. Arresting autoimmunity by blocking beta-arrestin 1. Nature Immunology. 2007;8(8):791–792. doi: 10.1038/ni0807-791. [DOI] [PubMed] [Google Scholar]
- 41.Shi Y., Feng Y., Kang J., et al. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nature Immunology. 2007;8(8):817–824. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- 42.Everett L. A., Belyantseva I. A., Noben-Trauth K., et al. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Human Molecular Genetics. 2001;10(2):153–161. doi: 10.1093/hmg/10.2.153. [DOI] [PubMed] [Google Scholar]
- 43.Su Z., Xiong H., Liu Y., et al. Transcriptomic analysis highlights cochlear inflammation associated with age-related hearing loss in C57BL/6 mice using next generation sequencing. PeerJ. 2020;8, article e9737 doi: 10.7717/peerj.9737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoe A., de Jonge E., Klautz R. J., van Dissel J. T., van de Vosse E. Single-nucleotide polymorphisms in the CALCA gene are associated with variation of procalcitonin concentration in patients undergoing cardiac surgery. American Journal of Respiratory and Critical Care Medicine. 2016;194(6):767–769. doi: 10.1164/rccm.201604-0772LE. [DOI] [PubMed] [Google Scholar]
- 45.Wu J. S., Vyas P., Glowatzki E., Fuchs P. A. Opposing expression gradients of calcitonin-related polypeptide alpha (Calca/Cgrpα) and tyrosine hydroxylase (Th) in type II afferent neurons of the mouse cochlea. The Journal of Comparative Neurology. 2018;526(3):425–438. doi: 10.1002/cne.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camacho C. P., Lindsey S. C., Melo M. C., et al. Measurement of calcitonin and calcitonin gene-related peptide mRNA refines the management of patients with medullary thyroid cancer and may replace calcitonin-stimulation tests. Thyroid. 2013;23(3):308–316. doi: 10.1089/thy.2012.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacifico L., Osborn J. F., Natale F., Ferraro F., De Curtis M., Chiesa C. Procalcitonin in pediatrics. Advances in Clinical Chemistry. 2013;59:203–263. doi: 10.1016/B978-0-12-405211-6.00007-3. [DOI] [PubMed] [Google Scholar]
- 48.Yang S. L., Aw S. S., Chang C., Korzh S., Korzh V., Peng J. Depletion of Bhmt elevates sonic hedgehog transcript level and increases β-cell number in zebrafish. Endocrinology. 2011;152(12):4706–4717. doi: 10.1210/en.2011-1306. [DOI] [PubMed] [Google Scholar]
- 49.Evans J. C., Huddler D. P., Jiracek J., et al. Betaine-homocysteine methyltransferase: zinc in a distorted barrel. Structure. 2002;10(9):1159–1171. doi: 10.1016/S0969-2126(02)00796-7. [DOI] [PubMed] [Google Scholar]
- 50.Martínez-Vega R., Partearroyo T., Vallecillo N., Varela-Moreiras G., Pajares M. A., Varela-Nieto I. Long-term omega-3 fatty acid supplementation prevents expression changes in cochlear homocysteine metabolism and ameliorates progressive hearing loss in C57BL/6J mice. The Journal of Nutritional Biochemistry. 2015;26(12):1424–1433. doi: 10.1016/j.jnutbio.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Partearroyo T., Vallecillo N., Pajares M. A., Varela-Moreiras G., Varela-Nieto I. Cochlear homocysteine metabolism at the crossroad of nutrition and sensorineural hearing loss. Frontiers in Molecular Neuroscience. 2017;10:p. 107. doi: 10.3389/fnmol.2017.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martínez-Vega R., Garrido F., Partearroyo T., et al. Folic acid deficiency induces premature hearing loss through mechanisms involving cochlear oxidative stress and impairment of homocysteine metabolism. The FASEB Journal. 2015;29(2):418–432. doi: 10.1096/fj.14-259283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Partearroyo T., Murillo-Cuesta S., Vallecillo N., et al. Betaine‐homocysteineS‐methyltransferase deficiency causes increased susceptibility to noise-induced hearing loss associated with plasma hyperhomocysteinemia. The FASEB Journal. 2019;33(5):5942–5956. doi: 10.1096/fj.201801533R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen-Salmon M., Regnault B., Cayet N., et al. Connexin30 deficiency causes instrastrial fluid-blood barrier disruption within the cochlear stria vascularis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(15):6229–6234. doi: 10.1073/pnas.0605108104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the author Wenyue Xue (xuecindy0107@163.com) upon reasonable request and with permission of the Department of Otolaryngology-Head and Neck Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital.


