Abstract
Around 2·5 million deaths and more than 110 million COVID-19 cases have been reported globally. Although it initially appeared that HIV infection was not a risk factor for COVID-19 or more severe disease, more recent large studies suggest that people living with HIV (particularly with low CD4 cell counts or untreated HIV infection) might have a more severe clinical course than those who are HIV-negative. Moreover, the COVID-19 pandemic has disrupted HIV prevention and treatment services worldwide, creating huge challenges to the continuity of essential activities. We have reviewed the most relevant features of COVID-19 in people living with HIV and highlighted topics where further research is required.
Introduction
On Dec 31, 2019, a pneumonia cluster of 44 patients was reported by Chinese authorities.1 Since then, SARS-CoV-2, the novel coronavirus that causes COVID-19, has caused a devastating pandemic with over 2·5 million deaths reported globally.2 According to the estimates of the Johns Hopkins University tool,2 there are more than 110 million confirmed cases of COVID-19 to date, with the most affected countries being the USA, India, and Brazil, followed by several countries in Europe. The highest number of deaths has been reported in the USA, Brazil, and Mexico. With the exception of South Africa, the African continent has been affected less severely, although underdiagnosis and under-reporting cannot be excluded.2
At present, more than 38 million people worldwide are living with HIV, approximately 25 million of those in sub-Saharan Africa. Although 26 million people living with HIV are estimated to be receiving antiretroviral therapy (ART), most of those not receiving ART, and those who are immunosuppressed, live in sub-Saharan Africa.3 There has not yet been a clear geographical overlap of the COVID-19 and HIV pandemics, which is fortunate because evidence suggests poor clinical outcomes in people living with HIV who become infected with SARS-CoV-2, particularly in those who are immunosuppressed or not receiving ART.4, 5, 6 However, the direct clinical effects of COVID-19 should not only be considered at the individual level but also at the populational level. It has been estimated that disruptions to HIV prevention and treatment services might have caused an excess of HIV/AIDS mortality in 2020 of around 400 000 people.7
Current evidence indicates that people living with HIV represent around 1·0% (95% CI 0·0–3·0) of total hospitalised COVID-19 cases,8, 9, 10 whereas SARS-CoV-2 infection prevalence in people living with HIV is between 0·68–1·8%, similar to the SARS-CoV-2 prevalence (0·6–0·8%) reported in the general population.11, 12, 13 In people living with HIV and with symptoms following SARS-CoV-2, 66·5% had mild symptoms, 21·7% reported severe symptoms, and 11·8% needed critical care.11, 12 However, asymptomatic infection rates in people living with HIV are most likely underestimated.11 The epidemiology of COVID-19 in people living with HIV and the overlap between the two pandemics might be affected in the future by SARS-CoV-2 vaccination, depending on the vaccine coverage, vaccination priorities for people living with HIV, and the responses of this population to immunisation by the range of available vaccines. Despite an increasingly consolidated body of evidence on COVID-19 in the general population, the interaction between SARS-CoV-2 and HIV infection is still unclear and data are, at times, conflicting.11, 14
Pathogenesis
Immune status in people living with HIV and SARS-CoV-2 response
A favourable clinical course of COVID-19 has been associated with appropriate class-I interferon responses and timely production of neutralising antibodies and specific cell-mediated immunity.15 On the contrary, low or delayed immune responses allow viral dissemination and are associated with hyperinflammatory states, or cytokine storms, leading to massive pneumonia, respiratory failure, and death.16 However, much remains to be learned regarding SARS-CoV-2 immunity,17 and reports suggest that, in patients with severe COVID-19 infection, sustained interferon responses in combination with IL-1 and tumour necrosis factor production in lung monocytes could exacerbate the cytokine storm.18 Therefore, the effect of immunodeficiency in chronic HIV infection on the appropriate immune response to COVID-19 might be a matter of concern or might offer potential protection against severe forms of disease.
Immune response to SARS-CoV-2 mediated by interferon in people living with HIV
Interferon is the first barrier to infection, which SARS-CoV-2 is sensitive to in vitro. In response, SARS-CoV-2 has developed several mechanisms to counteract the interferon response.19 Accordingly, poor COVID-19 prognosis has been associated with decreased interferon response,20 frequently observed in older patients, genetic defects in interferon-associated pathways, and anti-interferon antibody generation.20, 21 HIV infection triggers interferon responses in acute infection, contributing to HIV-replication control and transmitted/founder virus selection.22 However, in chronic phases of HIV infection, persistently high interferon concentrations and interferon-stimulated gene activation might correlate with disease progression, low CD4 counts, and long-term immunoactivation. These factors could suggest a detrimental role of chronic interferon signalling in HIV infection, normalised by ART.23
HIV-infected response: adaptive immunity and potential gaps against SARS-CoV-2
Early generation of robust functional IgG antibodies against the SARS-CoV-2 spike protein is associated with survival in severe COVID-19 infection.24 In addition, broad, strong CD4 and CD8 memory cell responses are observed in recovered patients, suggesting that coordinated antigen-specific B-cell and T-cell responses provide protective immunity against severe COVID-19 infection and death.24
In people living with HIV, active replication of HIV is associated with T-cell activation, increased CD8 T cells, inflammation, and lymphocytic exhaustion.25 These parameters are normalised by ART, particularly when treated early. Profound defects in B-cell function have been reported—eg, polyclonal activation, absence of and dysfunction of memory B cells, and defective follicular helper T-cell activity.26 Some of these defects are not fully recovered after ART, and long-term antibody production and decreased responses to neoantigens or recall antigens can persist in patients with HIV, representing a potential threat to vaccine response. These defects in T-cell and B-cell functions can potentially result in severe clinical course and poor COVID-19 prognosis in patients with HIV, particularly when low CD4 counts and viral replication persist despite ART. Two studies have shown that the concentration and duration of IgG, IgM, and neutralising antibodies in people living with HIV after SARS-CoV-2 infection are similar to the population that are HIV-negative,27, 28 although a third study showed a lower rate of neutralising antibodies in people living with HIV.29
Proinflammatory status in people living with HIV
Patients with COVID-19 and severe clinical course develop dysfunctional immune responses characterised by profound lymphopenia (including both low CD4 and CD8 T-cell counts), increased cytokines and chemokines, massive natural killer cell and lymphocytic activation, and subsequent exhaustion. At the pulmonary level, macrophage activation and endothelial damage lead to cellular recruitment, increased inflammation, and activation of complement and coagulation pathways, leading to aggravated viral pneumonia, respiratory failure, systemic injury, and death. This cytokine storm is also present with lower intensity in acute HIV infection in which strong immune-system activation is triggered by high viraemia.25, 30 ART restores interferon, cytokine, and chemokine concentrations, although some patients present low-level persistent activation and inflammatory markers. Although evidence is scarce, restoration of interferon concentrations might be beneficial for responses to SARS-CoV-2.
Overall, full viral suppression with ART ensures near-complete immune recovery in people living with HIV. Therefore, for well controlled infections in people with well controlled HIV infection, SARS-CoV-2 infection should be managed as in the HIV-negative population. However, persistent viral replication, low CD4 counts, and increased concentrations of inflammatory markers have been described in a subgroup of patients treated with ART, a scenario potentially leading to severe COVID-19 disease progression. Additionally, defective B-cell functioning, not completely recovered by ART, might contribute to decreased COVID-19-vaccine response. Moreover, increased rates of cancer, neurological disease, and cardiovascular disease have been described in people living with HIV, adding potential risk factors to COVID-19 disease progression.31, 32 There is also a progressive increase in the median age of people living with HIV, particularly in high-income countries,33 and the natural immune-senescence process should be also considered in older people living with HIV, which might also impair immune responses.34
Advantages and disadvantages of immune HIV status in tackling COVID-19
People living with HIV with CD4 counts less than 200 cells per μL, unsuppressed HIV RNA, or opportunistic illnesses in the preceding 6 months, have been considered an at-risk population since the COVID-19 pandemic began.35 As declining CD4 cells are associated with COVID-19 severity, people living with HIV with low CD4 cells might face a higher risk of severe COVID-19 infection.36, 37 Similarly, untreated HIV infections might worsen the immunological effect of COVID-19 infection.38 At present, effective ART is universally recommended and immunological recovery is expected in most patients with HIV infection,39 but COVID-19 might occur in people living with HIV unaware of their HIV status. In the largest published cohorts, the potentially higher risk for poorer COVID-19-related outcomes in people living with HIV with lower CD4 cell counts40, 41 might be driven by concomitant comorbidities,42 more common in people living with HIV than in uninfected people,43 the prevalence of which is inversely proportional to CD4 counts in people living with HIV.44
Virological and immunological issues of SARS-CoV-2 in people living with HIV
Nasopharyngeal and oropharyngeal samples are most widely used for laboratory diagnosis of COVID-19.45 SARS-CoV-2 can also be detected in other respiratory and non-respiratory samples including in plasma, stool, and urine.46 In general, no differences in SARS-CoV-2 viral load have been observed between people that are HIV-negative and people living with HIV. However, in specific scenarios—eg, in advanced disease, low CD4 cell counts, or uncontrolled HIV replication—people living with HIV might show prolonged SARS-CoV-2 shedding, which has been described in other immunosuppressed populations, and might eventually promote the emergence of SARS-CoV-2 variants.47, 48 As described in other acute infections49, 50 and vaccination,51 acute COVID-19 infection might lead to transient increases in HIV RNA due to overall T-cell activation and mobilisation of HIV reservoirs.52 No major consequences on progression in people living with HIV receiving ART are expected, but there is an absence of data for long-term follow-up.52, 53
Standard confirmation of acute SARS-CoV-2 infection relies on detecting unique viral sequences by nucleic acid amplification tests, such as real-time RT-PCR. The assay targets include regions on the E, RdRP, N, and S genes of SARS-CoV-2. Optimal diagnostics consist of nucleic acid amplification test assay with at least two independent targets on the SARS-CoV-2 genome. Given the extensive SARS-CoV-2 transmission, a simple algorithm might be adopted with one single discriminatory target.54 No data describe nucleic acid amplification tests in people living with HIV compared with individuals that are HIV-negative. Coinfections are common in immunocompromised patients (eg, people living with HIV) and might be associated with greater morbidity and mortality than in immunocompetent individuals. The use of multiplex PCR against multiple common human respiratory pathogens in parallel might facilitate single-test differential diagnosis.55
Antibody testing is dependent on host immune responses to infection; hence antibody responses to infection and vaccination might be expected to be impaired in immunosuppressed people living with HIV, despite the low number of studies on antibody responses in people living with HIV. Knowledge gaps include the duration of detectable IgG and total antibodies, the relationship of seropositivity to infectious virus shedding during convalescence, and factors affecting antibody response (eg, age, comorbid medical conditions, and immunocompromised status, including HIV infection).56
Clinical manifestations and disease progression
Since the first reported series of COVID-19 in people living with HIV,8 a stream of single-centre or multicentre case-series have been published.13, 37, 40, 41, 57, 58, 59, 60, 61, 62, 63 These series report epidemiological features, clinical presentation, and outcomes in people living with HIV (table 1 ). Globally, the initial series showed no clear evidence for higher COVID-19 infection rates or different disease course in people living with HIV. However, these series were limited by small sample sizes. Most studies reported a younger age population compared with hospitalised patients who are HIV-negative and had COVID-19 but reported similar rates of comorbidity. The patients had a good overall immunological status; however, with a high proportion of these patients receiving ART, evaluations of clinical course in more immunosuppressed patients and in those not taking ART were hampered (table 1).
Table 1.
Main epidemiological and clinical results of studies (published until Nov 1, 2020) reporting at least 50 cases of people living with HIV and COVID-19 infections
| Country | Study design | Number of participants | Age (years) | Sex (men) | Confirmed SARS-CoV-2 infection | CD4 count (cells per μl) | Undetectable HIV viral load | Prevalence of comorbidities | |
|---|---|---|---|---|---|---|---|---|---|
| Inciarte et al (2020)62 | Spain | Single-centre retrospective cohort | 53 | 44 | 81% | 79·2% | 618 | 96·2% | At least 1 comorbidity (43%)* |
| Vizcarra et al (2020)61 | Spain | Single-centre retrospective cohort | 51 | 53·3 | 84% | 69% | 565 | 98% | Liver disease (47%), hypertension (35%), cardiovascular disease (27%)† |
| Sigel et al (2020)40 | US | Multicentre case-control study | 88 | 61 | 75% | 100% | 44% higher than 500 | 81% | Hypertension (38%), diabetes (27%), chronic kidney disease (22%) |
| Ho et al (2021)37 | US | Muticentre retrospective cohort | 93 | 58 | 72% | 100% | 554 | 83·8% | Hypertension (52·7%), diabetes (34·4%), respiratory disease (26·9%) |
| Etienne et al (2020)63 | France | single-centre prospective cohort | 54 | 54 | 61·1% | 70·3% | 583 | 96·2% | Cardiovascular disease (46·3%), hypertension (29·6%), respiratory disease (9·3%)‡ |
| Dandachi et al (2020)41 | US and Spain | COVID-19 in people living with HIV registry | 286 | 51·1 | 74·1% | 100% | 531 | 88·7% | Hypertension (46·5%), obesity (32·3%), diabetes (21·3%) |
| Boulle et al (2020)4 | South Africa | Population cohort study | 2895 | 20–39 (57% of participants) | 21% | 100% | 24% higher than 200 | NR (60% viral load more than 1000 copies) | NR |
| Miyashita et al (2021)64 | US | Muticentre retrospective cohort | 161 | 51–65 (51% of participants) | 78% | NR | NR | NR | Hypertension (46%), dyslipidaemia (34%), diabetes (29%) |
| Del Amo et al (2020)65 | Spain | Muticentre retrospective cohort | 236 | 50–59 (42% of participants) | 75% | 100% | NR | NR (100% on ART) | NR |
| Geretti et al (2020)5 | UK | Muticentre prospective cohort | 122 | 56 | 66·1% | 90·5% | NR | NR (91·8% on ART) | Chronic kidney disease (18·1), cardiovascular disease (17·1%), obesity (17%)§ |
| Cabello et al (2021)57 | Spain | Muticentre retrospective cohort | 63 | 46 | 88·9% | 49·2% | 605 | NR (96·8% on ART) | Hypertension (19%), obesity (13%), cardiovascular disease (12·7%) |
ART=antiretroviral therapy. NR=not reported.
Comorbidity details not described in study.
63% chance of having at least one comorbidity.
55·6% chance of having at least one comorbidity.
74·6% chance of having at least one comorbidity.
As with individuals that are HIV-negative, the risk of severe COVID-19 illness in these series was reported to increase with age, affected by sex (men in particular), and by certain chronic medical problems, such as arterial hypertension, cardiovascular disease, chronic lung disease, obesity, and diabetes. Many of these comorbidities are more prevalent among people living with HIV at any given age, particularly in high-income countries. Not surprisingly, a more severe outcome was described in people living with HIV who also had three or more comorbidities (table 1).41 However, larger cohort studies were published soon after, some suggesting poorer outcomes for individuals with HIV compared with the smaller series,64 but other studies did not report these outcomes.65 A cohort study from the UK5 and, in particular, a study from South Africa4 found a higher risk of poor outcome (which resulted in admission to the intensive care unit [ICU] or mortality, or both) in coinfected patients compared with individuals that are HIV-negative. The South African study was much larger and from a country with high HIV seroprevalence; most of the patients who were infected with SARS-CoV-2 were women, and people living with HIV were much younger with more pronounced immunodeficiency. Consequently, this study was probably capable of detecting an HIV-infection effect unnoticed in smaller studies.4 In a large New York study,6 previous HIV diagnosis was associated with higher rates of severe disease requiring hospitalisation, and the risk of hospitalisation (but not death) increased with progression of HIV disease stage. The only significant factor associated with in-hospital mortality among hospitalised patients living with HIV was age, with those aged 40 years or older being three to four times more likely to die in hospital.6 Recent cohort studies indicated increased age and different comorbidities (such as diabetes and renal insufficiency) and low CD4 cell count as risk factors for hospitalisation in people living with HIV infected with SARS-CoV-2 in the USA.66, 67, 68
In studies comparing people living with HIV with individuals that are HIV-negative, clinical COVID-19 presentation was no different to typical reports in the general population. Fever, cough, fatigue, and dyspnoea were consistently the most frequently reported signs and symptoms in most of these series. Fever and cough were sometimes significantly more frequent in people living with HIV than in those who are HIV-negative.41, 61, 62 Headache, myalgia, and odynophagia (or sore throat) were also very prevalent and rates of anosmia or dysgeusia seem to be similar in people living with HIV compared with HIV-negative individuals. Radiological findings of people living with HIV were also similar to HIV-negative patients, presenting mostly with patchy bilateral alveolo-interstitial infiltrates. Among laboratory abnormalities, as expected, lymphopenia was more frequent in people living with HIV,5, 37 CD4 and CD8 T-cell counts could be very low during the disease36 as a result of COVID-19-induced lymphopenia, and patients with poor outcome (ie, ICU admission, mechanical ventilation, and death) had higher concentrations of inflammatory markers.37
Nevertheless, drawing definite conclusions from these studies is difficult; some studies aggregated confirmed and suspected cases,5, 61, 62, 63 others focused only on hospitalised patients,5, 40 and some reported no relevant clinical data. The possibility of HIV infection and late presentation as a differential diagnosis of COVID-19 must always be considered. The most relevant differential diagnosis with COVID-19 is pneumonia caused by Pneumocystis jirovecii,69 but chest x-ray infiltrates in COVID-19 are frequently more peripheral than central; the opposite occurs with pneumonia caused by P jirovecii. Nevertheless, P jirovecii should always be excluded when CD4 cell counts are low. Likewise, COVID-19 causes a deep lymphopenia, and therefore, patients with severe disease can have very low CD4 and CD8 T-cell counts,36 thus increasing the risk of pneumonia caused by P jirovecii. Although, reports of high frequency of pneumonia caused by P jirovecii resulting from COVID-19-induced lymphopenia are scarce. Finally, concomitant diagnoses should be considered, such as COVID-19 and tuberculosis,70 pneumonia caused by P jirovecii,69, 71 or cryptococcosis.
No clear evidence indicates that HIV infections prolong or spread SARS-CoV-2 infections; hence monitoring SARS-CoV-2 shedding durations has not been recommended for people living with HIV. However, as in other immunocompromised individuals,47, 48 it is reasonable to consider a longer viral shedding in severely immunosuppressed people living with HIV. No specific differences exist regarding infection prevention and control measures for people living with HIV, and those measures indicated for immunosuppressed patients, in general, should be followed.
Prognostic factors for ICU admission and death
For series reporting only hospitalisations, ICU admission for people living with HIV ranged between 17% and 33%.5, 40 In instances when outpatients were included, overall rates of ICU admission ranged between 3% and 22%.57, 64 As previously explained, severe illness increases with age and multimorbidities, and is increased in men,11, 12 with multimorbidities being reported in nearly two-thirds of patients coinfected with HIV and SARS-CoV-2.12 The risk of mortality in hospitalised patients in the UK showed an adjusted hazard ratio of 1·69 (95% CI 1·15–2·48; p=0·008),5 whereas in the UK, in primary care alone, after adjustment for age, sex, deprivation, ethnicity, smoking and obesity, the adjusted hazard ratio was 2·59 (1·74–3·84; p<0·0001). Although, most deceased people with HIV had other comorbidities.9 Similar results were found in Western Cape, South Africa, where after adjusting for other risk factors, HIV increased the risk of death in patients with COVID-19 by a factor of 2·14 (1·70–2·70).4 However, global mortality varied considerably across studies, depending on the design. Mortality was as high as 24% in the UK series (only hospitalisations)5 and as low as 2% in the French series,63 and 3·6% in the South African cohort study4 with a higher number of patients and the inclusion of outpatients (table 2 ).
Table 2.
Summary of outcomes in studies reporting on more than 50 people living with HIV who have been infected with COVID-19
| Hospitalisation | Intensive care unit admission | Mechanical ventilation | Mortality rate | Other relevant results and conclusions | |
|---|---|---|---|---|---|
| Inciarte et al (2020)62 | 49% | 8% | 4% | 4% | No HIV or ART role identified as prognostic factor |
| Vizcarra et al (2020)61 | 55% | 12% | 9·8% | 4% | No differences in COVID-19 presentation due to HIV status |
| Sigel et al (2020)40 | NA* | 17% | 18% | 21% | Smoking and comorbidities more frequent in people living with HIV than in people who are HIV-negative, but both groups had similar outcomes |
| Ho et al (2021)37 | NA* | 26·4% | 20·8% | 26·4% | Higher inflammatory markers in people living with HIV with poor outcome |
| Etienne et al (2020)63 | NR | 9·3% | NR | 2% | Sub-Saharan African ethnicity and metabolic disorders associated with critical outcome; CD4 cell count not related |
| Dandachi et al (2020)41 | 57·3% | 28·7% | 22·6% | 16·5% | CD4 counts of less than 200 cells per μL was associated with intensive care unit admission, mechanical ventilation, or death |
| Boulle et al (2020)4 | 20·75% | NR | NR | 3·6% | Higher mortality in people living with HIV compared with people who are HIV negative |
| Miyashita et al (2021)64 | NR | 22% | 12% | 14% | Poor outcomes related to comorbidities |
| Del Amo et al (2020)65 | 64% | 6·35% | NR | 8·5% | Incidence of COVID-19 not higher than in the general population; tenofovir might be protective |
| Geretti et al (2020)5 | NA* | 33% | 16·4% | 24% | After adjusting for age and other variables, higher mortality seen in people living with HIV |
| Cabello et al (2021)57 | 32·3% | 3·2% | 3·2% | 3·2% | Prognosis related to age and comorbidities |
ART=antiretroviral therapy. NR=not reported. NA=not applicable.
Studies included only hospitalised patients.
Prognosis, according to HIV status and CD4 cell count, is difficult to evaluate because most studies from Europe and the USA on levels of immunity reported an overall high CD4 cell count.37, 41, 61, 62, 63 In these virologically suppressed patients with good immune status, poor outcomes remained relating to comorbidities and age (as in the general population). Other studies, with larger cohorts did not provide CD4 T-cell count. Nevertheless, in the South African cohort study (with a larger number of patients, but also with the most immunosuppressed population), HIV was an independent prognosis factor for poor outcome, suggesting people living with HIV with low CD4 counts have a more severe COVID-19 clinical course than those who are HIV-negative.4 Dandachi and colleagues41 reported poor outcomes for patients with CD4 count of less than 200 cells per μL. A 2021 cohort study from the UK (CD4 data were not provided) and another multicentre cohort study also found people living with HIV to be at higher risk of poor outcome.9, 72 In a case-control study from Spain, people living with HIV had higher mortality (9·8% vs 3·4% compared with HIV-negative individuals), related mostly to the presence of comorbidities but not to virological or immunological factors, or ART use.73 In other studies, a poorer outcome was not noticed.74, 75, 76
The potential relationship of antiretroviral drugs to improve or worsen outcomes remains controversial. Although Del Amo and colleagues65 reported lower COVID-19 incidence in patients taking tenofovir, a selection bias might have existed, because most patients with comorbidities do not take tenofovir. Furthermore, Boulle and colleagues4 reported the use of tenofovir being associated with reduced mortality compared with other therapies. In a Spanish study, the use of tenofovir–emtricitabine was associated with lower seropositivity against SARS-CoV-2, suggesting a lower infection rate.77 Although no other published study reported any antiretroviral drug to be protective or associated with poor outcomes in people living with HIV and COVID-19, there are ongoing studies that are researching this.
Drug therapies
Antiretroviral drugs with potential anti-SARS-CoV-2 activity
Some antiretroviral drugs have been investigated for their potential action against SARS-CoV-2. Data suggest tenofovir might be active in vitro65, 78 due to its tight union with a critical SARS-CoV-2 lifecycle enzyme, the RNA-dependent RNA polymerase.79 However, conflicting results have emerged from clinical trials and observational studies investigating the anti-SARS-CoV-2 activity of the enzyme in treatment and prevention.65, 78 Lopinavir–ritonavir and darunavir, both protease inhibitors, have also been evaluated. In-vitro activity of darunavir was identified but no anti-SARS-CoV-2 activity at clinically relevant concentrations (EC50 >100 μM) was shown. Lopinavir–ritonavir showed no efficacy in reducing mortality and mechanical ventilation in hospitalised patients in the RECOVERY study (<1% of people living with HIV) and in the large Solidarity trial.80, 81 Trials with maraviroc and cenicriviroc (CCR5 inhibitors) are ongoing (NCT04441385 and NCT04500418).
State-of-the-art COVID-19 treatment
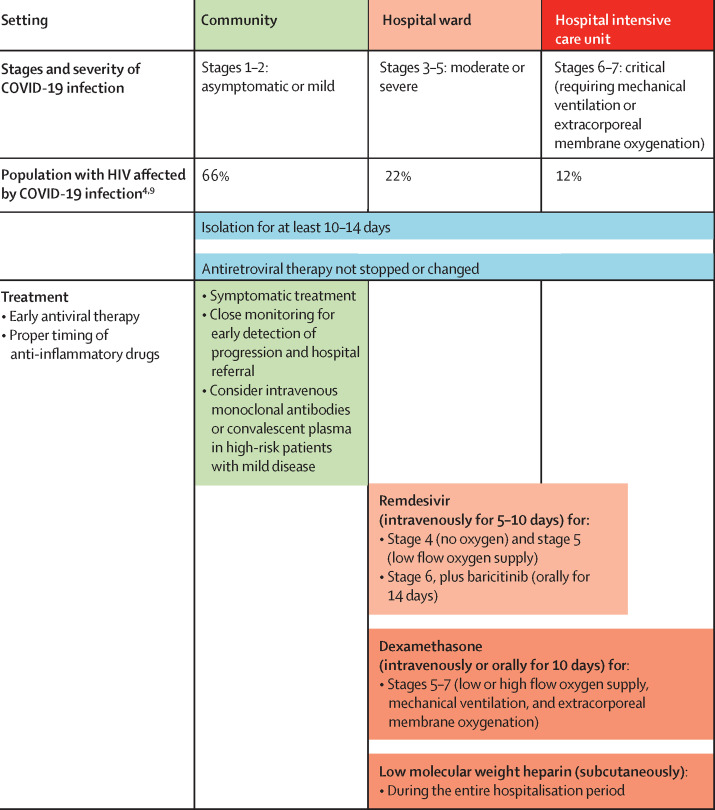
Remdesivir (a viral RNA-dependent RNA polymerase inhibitor) in monotherapy or combined with baricitinib (a selective JAK inhibitor with dual antiviral and anti-inflammatory activity) improved time to recovery compared with standard of care or placebo among patients with stages 4–6 on the US National Institutes of Health severity scale (figure 1 ).81, 82, 83, 84, 85, 86 However, the Solidarity trial, which analysed stages 5 and 6, did not find improvement in survival.81 This finding has led to contradictory positions held by the US Food and Drug Administration and the European Medicines Agency, which approved remdesivir use, and by WHO, which does not recommend its use. Convalescent plasma and some specific neutralising antibody administration in mild disease in the community has shown to prevent progression and hospital admission compared with placebo.87, 88, 89, 90 However, data are scarce on all antivirals used by people living with HIV. Many off-label antimicrobial agents, such as ivermectin or sofosbuvir, are under evaluation.
Figure 1.
Therapeutic management of COVID-19 in patients with HIV in January, 2021
Adaptive COVID-19 Treatment Trial scores on the ordinal scale: 1=not hospitalised, no limitations of activities; 2=not hospitalised, limitation of activities, home oxygen requirement, or both; 3=hospitalised, not requiring supplemental oxygen and no longer requiring ongoing medical care (used if hospitalisation was extended for infection control reasons); 4=hospitalised, not requiring supplemental oxygen but requiring ongoing medical care (COVID-19-related or other medical conditions); 5=hospitalised, requiring any supplemental oxygen; 6=hospitalised, requiring non-invasive ventilation or use of high-flow oxygen devices; 7=hospitalised, receiving invasive mechanical ventilation or extracorporeal membrane oxygenation; and 8=death. Asymptomatic or presymptomatic infections=individuals positive for SARS-CoV-2 with a virological test (ie, a nucleic acid amplification test or an antigen test), but with no symptoms consistent with COVID-19. Mild illness=individuals with any of the various signs and symptoms of COVID-19 (eg, fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhoea, and loss of taste and smell) but without shortness of breath, dyspnoea, or abnormal chest imaging. Moderate illness=individuals with evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation of 94% or higher in room air at sea level. Severe illness=individuals who have oxygen saturation of less than 94% in room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen of less than 300 mm Hg, respiratory frequency of more than 30 breaths per min, or lung infiltrates of more than 50%. Critical illness=individuals who have respiratory failure, septic shock, or multiple organ dysfunction. Remdesivir is approved by the regulatory agencies (ie, US Food and Drug Administration and European Medicines Agency) but is currently not recommended by WHO for COVID-19 treatment (regardless of HIV status).
Steroids, other anti-inflammatory drugs, and anticoagulants
Anti-inflammatory drugs, such as systemic corticosteroids, IL-6 inhibitors (eg, tocilizumab, siltuximab, and sarilumab), IL-1 inhibitors (eg, anakinra), colchicine, and baricitinib (JAK inhibitor), might act in virus-driven hyperinflammation and cytokine storms occurring in severe COVID-19 infection and many of these drugs are under investigation.91 Dexamethasone (at low doses of 6 mg per day for up to 10 days, or equivalent doses of hydrocortisone or methylprednisolone) is the only drug that reduces mortality in patients requiring oxygen therapy.92 To date, no data indicate that anti-inflammatory drug use increases the risk of reactivation of opportunistic infections in people living with HIV or that drug use should differ from the general population. Prophylactic anticoagulation is generally recommended in all hospitalised patients with COVID-19 and should be used similarly in people living with HIV.93
Relevant drug–drug interactions are available for many anticoagulants and systemic steroids when pharmacokinetic enhancers (eg, ritonavir and cobicistat) are provided in ART regimens. Dose adaptation or changes in the concomitant medication might be necessary. With immunomodulatory drugs (eg, tocilizumab), drug–drug interactions are less relevant, but evidence is scarce. Online resources, such as the University of Liverpool's interaction checker, can be consulted.
Monitoring HIV infection in outpatient and care facilities
Disruption in health-care services has affected the prevention and care of various illnesses, including HIV. In the short term, there have been decreases in testing and diagnoses of new cases of HIV infection and other transmittable infections; and limitations in laboratory monitoring, clinical visits, and access to ART might have resulted in suboptimal care of HIV infection and other comorbidities in people living with HIV.94, 95, 96 The real long-term consequences are not completely known, and time will be necessary for accurate evaluations. Infrastructure for HIV consultations has been adapted to the constraints of the pandemic in most institutions.
In-person visits have been the rule for monitoring HIV infection. Before the COVID-19 pandemic, telehealth interventions had been used satisfactorily but were restricted, at least, partly due to major technical issues and costs. The COVID-19 pandemic has brought a renewed need for remote care. Simple telephone calls and email messages replaced in-person visits at first. The Spanish HIV association, GeSIDA, has established a minimum set of recommendations for telehealth for patients with HIV.97 More sophisticated systems (eg, videoconferencing) have developed during the COVID-19 pandemic, and this option, alongside in-person visits, will most likely remain in the future for stable and non-complicated cases. Consultation for post-exposure prophylaxis or symptomatic sexually transmitted infections need to be maintained by in-person visits, as well as the initiation of ART for newly diagnosed individuals. In the follow-up of people living with HIV, approaches to screening asymptomatic SARS-CoV-2 infection, isolation, and quarantine rules should be the same as in the general population.
People with drug addictions are particularly susceptible to COVID-19, due to poorer social health determinants. Social isolation might increase the risks of addiction and deaths caused by drug overdose. People using substances might have reduced access to harm reduction and treatment services.98 There might be disruptions in illicit drug supplies, affecting availability and cost, and increasing the risk of drug adulteration.99 Individuals with addictions have been at higher risk of multimorbidity and mortality during the COVID-19 pandemic.100 Some centres have ensured a sufficient quantity of take-home doses for patients on methadone maintenance treatment to maximise their adherence during COVID-19-related lockdowns.101
The COVID-19 pandemic has affected mental health, particularly in patients with pre-existing psychiatric disorders.102 The consequences were substantially higher among people living with HIV, in racial and ethnic minorities, immigrants, transgender people, sex workers, and socioeconomically disadvantaged groups.103 Women were especially affected in some studies.104, 105 Other studies (but not all) have described substantial reductions in sex with non-steady partners during the first weeks of lockdown among men who have sex with men (MSM).106, 107, 108, 109 These studies describe MSM as indicating fewer sexual partners or fewer opportunities for having sex. Reduced sexual activity with sexual partners external to the household is more likely to result in reduced sexual transmitted infections among HIV-positive MSM.106 A Belgian study described MSM who had sex with non-steady partners were significantly more likely to be HIV positive, to use pre-exposure prophylaxis (PrEP), or to have engaged in sexual practices such as group sex, chemsex, and sex work, before the first national lockdown, compared with their counterparts.106
Lockdown restrictions and reducing in-person visits during the COVID-19 pandemic might have interrupted both pre-exposure prophylaxis for adults at high-risk of HIV infection and antiretroviral treatment for people living with HIV. Hence outreach programmes for treatment and prevention of HIV have become increasingly relevant and should be rapidly implemented in case of further waves. Developing remote pharmacy visits alongside the widespread use of commercial home delivery services ensured continued ART access for people living with HIV. As with remote medical visits, there were technical and legal obstacles to overcome. The Spanish Society of Hospital Pharmacy released a document on telepharmacy and medication home delivery.110
SARS-CoV-2 vaccines and HIV infection
All licensed, virally effective vaccines are based on the induction of neutralising antibodies directed against viral envelope surface proteins and the spike receptor binding domain. Although cellular responses are probably important in establishing a protective response, vaccines that are unable to induce antibodies that block infection are no longer considered for clinical development.111, 112 This principle has targeted the SARS-CoV-2 spike protein in all COVID-19 vaccines,113 even if some prototypes also hope to induce cellular immunity. HIV preventive vaccine research is focusing on the induction of broadly neutralising antibodies.114, 115 Many HIV-preventive prototypes under development (eg, RNA, adenovirus vectors, or trimeric envelopes) have been translated to the coronavirus field, enabling the rapid results observed in SARS-CoV-2 vaccinology.113
Few people living with HIV have been included in reported phase 3 vaccine trials, so the readiness of their immune systems remains unknown.116 Predictions and potential concerns are based on two sets of data: the immune damage to B-cell compartments and antibody generation caused by HIV infection that could potentially decrease humoral responses to neoantigens (eg, the SARS-CoV-2 spike protein); and the scarce response of people living with HIV to other vaccines, particularly in patients with low CD4 T-cell count. People living with HIV display lower or delayed responses to several vaccines, including pneumococcal, influenza and hepatitis B vaccines.117 Accordingly, specific recommendations have been generated, including repeated vaccine doses or vaccination with particular prototypes.118, 119 Studies analysing the response of patients with HIV to COVID-19 vaccines are needed to define the potential advantage of some prototypes or the need for additional vaccine doses to achieve full protective immunity.
There is debate on whether people living with HIV should be considered as an at-risk group requiring prioritisation for COVID-19 vaccination. No evidence supports this indication, but patients with comorbidities or those with low CD4 counts (less than 200 cells per μL) might be at higher risk of developing severe COVID-19, and hence should benefit from early COVID-19 vaccination. The European AIDS Clinical Society statement on COVID-19 in people living with HIV also supports the vaccination priority scheme.120 Unfortunately, data on people living with HIV included in approved phase 2 and 3 vaccine trials so far are scarce: in the Moderna vaccine trials, only 0·6% of participants were people living with HIV121 and only 0·5% in the Pfizer trials.122 In the Novavax phase 2b study in South Africa, responses in people living with HIV seem to have been much lower than in individuals that do not have HIV. Although, the final results remain unavailable.123 ART should be continued during vaccination of people living with HIV.
Future trends in SARS-CoV-2 and HIV infections
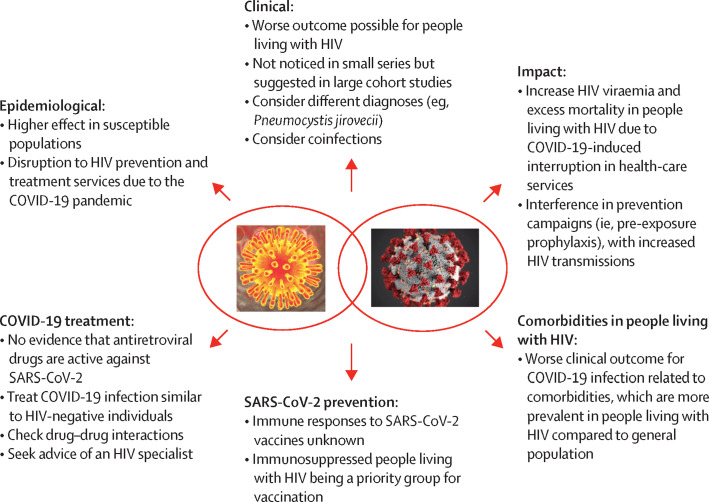
Unfortunately, clearly establishing the interplay between the HIV/AIDS and COVID-19 pandemics will require a higher quality of evidence than is available. Emerging evidence seems to indicate a moderately increased risk of death and severity of COVID-19 in people living with HIV.4, 5, 6, 124 Many aspects of COVID-19 and HIV/AIDS interaction need to be elucidated (figure 2 ). Although data are insufficient to determine whether HIV viral load, CD4 T-cell count, or ART use are associated with COVID-19-related death, there are some signs that low CD4 counts (less than 200 cells per μL) might potentially be associated with poor outcomes. As the data are not unequivocal, a large dataset comparing outcomes in HIV-positive and HIV-negative cohorts with broader geographical representation is required. Among other initiatives, the WHO Global COVID-19 Clinical Platform125 is gathering anonymised and standardised individual-level clinical data from people living with HIV and the general population hospitalised with COVID-19. One ongoing discussion is whether the use of specific antiretroviral drugs might protect against COVID-19. Although evidence on the potential protective role of tenofovir–emtricitabine65 on SARS-CoV-2 acquisition and progression is conflicting, PrEP should continue to be provided to protect against HIV-infection acquisition. Similarly, no evidence suggests people living with HIV should be managed differently in preventing COVID-19 clinical progression. Modifications switching to protease inhibitor-based ART or including any antiretroviral drug increasing protection for COVID-19 are not justified.
Figure 2.
Interaction of the HIV and SARS-CoV-2 pandemics and unanswered questions
When health-care systems are overwhelmed, deaths from manageable conditions, such as HIV, might increase substantially due to disruptions in essential health services. Lockdown measures and stigmatisation have affected the functioning of HIV clinics in many settings, particularly in low-income and middle-income countries. A survey by WHO done between April and June, 2020, showed that 34 of 127 countries reported ART disruptions. In a subsequent update, 12 countries reported ART stock availability of 3 months or less for the major first-line drugs.126 To support countries in reorganising and maintaining access to high-quality essential health services for all, WHO has published updated guidance, including a set of immediate targeted actions that countries should consider.127 WHO also recommends clinically stable people living with HIV to benefit from multiple refills (every 3–6 months) of antiretroviral drugs and other medications, such as opiate substitution and tuberculosis preventative therapies. Considering the social determinants of health, accessibility to health systems and to ART should be a priority in any further waves of the COVID-19 pandemic (panel ).
Panel. General principles of COVID-19 management in people living with HIV.
Studies on therapeutics were mostly done in HIV-negative individuals, and no evidence indicates different therapeutic approaches should be considered in people living with HIV. Some general principles are applicable when treating COVID-19 infection in people living with HIV:
-
•
Treatment, including antiviral, anti-inflammatory, and anticoagulant therapies, should be the same as for HIV-negative individuals (figure 1)
-
•
Antiretroviral therapy (ART) regimens should not be stopped or modified to promote anti-SARS-CoV-2 activity
-
•
Initial ART should be started with regimens that have high barriers to resistance and low drug–drug interactions, and treatment changes should be delayed unless strictly necessary
-
•
Drug–drug interactions should be considered, particularly with boosted protease inhibitors or boosted integrase-strand-transfer inhibitors. Non-boosted integrase-strand-transfer inhibitors (eg, raltegravir, dolutegravir, and bictegravir) have no major drug–drug interactions
-
•
ART dispensary practice should allow for 3–6-month drug supplies, and monitoring might be deferred with good adherence and absent new toxicity or drug–drug interactions
-
•
Consider consulting infectious disease or HIV specialists in complex cases or for questions on ART and drug–drug interactions
-
•
Although data are scarce for pregnant women living with HIV, COVID-19 prevention and treatment should be identical to treatment given to HIV-negative pregnant women
To protect people living with HIV from COVID-19, a vaccine should be promptly available and accessible. Despite initial concerns on whether a vaccine with the adenovirus 5 platform might increase the risk of acquiring HIV,128 no evidence suggests that the vaccines approved might increase the risk of HIV infection or that people with HIV have a different response; although data on this are scarce. Therefore, there is an urgent need to know the response of patients with HIV to different COVID-19 vaccines and whether two doses are enough to achieve full protective immunity.
In conclusion, although emerging data on COVID-19 epidemiology and physiopathology and its intersection with HIV infection continue to accumulate, knowledge gaps remain and should be a focus for the global research community.
Search strategy and selection criteria
We identified references searching PubMed using a Boolean strategy with the terms: “COVID-19”, “SARS-CoV-2” and “HIV-infection” and “epidemiology”, “clinical manifestations”, “pathogenesis”, “diagnosis”, “prognosis”, “prevention”, “treatment”, “antiretrovirals”, “vaccines”, and “low-, middle- and high-income countries”. We searched for papers from Jan 1 to Nov 1, 2020, including cohort studies, case-series and clinical trials; duplicates were eliminated. This period had 73 094 COVID-19 publications, including 963 (1·32%) that were HIV related. The first case series was published on April 15, 2020. Only publications in English were reviewed and only published data were analysed. Preprint publications were not considered. Given rapid developments, we also considered relevant printed publications between Nov 1, 2020, and Feb 3, 2021, (there were approximately 500 additional publications) and specific relevant data presented in important conferences (ie, the 2021 Conference on Retroviruses and Opportunistic Infections). The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
Declaration of interests
JA has participated in advisory boards and received consulting honoraria or research grants, or both, from Gilead Sciences, Janssen Pharmaceuticals, and ViiV Healthcare, outside the submitted work. EM reports grants and personal fees from Merck Sharp & Dohme and ViiV Healthcare, and personal fees from Gilead Sciences and Janssen Pharmaceuticals, outside the submitted work. JMM reports grants and personal fees from AbbVie, Angelini, ContraFect, Cubist, Genentech, Gilead Sciences, Jansen Pharmaceuticals, Lysovant, Medtronic, Merck Sharp & Dohme, Novartis, Pfizer, and ViiV Healthcare, outside the submitted work. All other authors declare no competing interests. The views and opinions expressed in this Review are those of the authors and do not necessarily reflect the official policy or position of the WHO.
Contributors
JMM contributed to the study conception and design of this paper. All authors contributed to the acquisition, analysis, and interpretation of data. All authors contributed to the drafting of this paper and the revision of this paper for important intellectual content. All authors approved the final version of this paper for publication. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
COVID-19 in HIV Investigators
Juan Ambrosioni, Jose L Blanco, Lorena de la Mora, Felipe Garcia-Alcaide, Ana González-Cordón, Alexis Inciarte, Montserrat Laguno, Lorna Leal, Esteban Martínez-Chamorro, María Martínez-Rebollar, José M Miró, Jhon F Rojas, Berta Torres, Josep Mallolas (HIV/AIDS Unit), Laia Albiac, Daiana L Agüero, Marta Bodro, Celia Cardozo, Mariana Chumbita, Nicol García, Carolina García-Vidal, Marta M Hernández-Meneses, Sabina Herrera, Laura Linares, Antonio Moreno, Laura Morata, Jose A Martínez-Martínez, Pedro Puerta, Verónica Rico, Alex Soriano (Infectious Diseases Service), Mikel Martínez, María del Mar Mosquera, Maria A Marcos, Jordi Vila (Microbiology Service), Montse Tuset, Dolors Soy (Pharmacy Department), Anna Vilella (Preventive Medicine Service), Alex Almuedo, María J Pinazo, Jose Muñoz (IsGlobal).
Contributor Information
COVID-19 in HIV Investigators:
Juan Ambrosioni, Jose L. Blanco, Lorena de la Mora, Felipe Garcia-Alcaide, Ana González-Cordón, Alexis Inciarte, Montserrat Laguno, Lorna Leal, Esteban Martínez-Chamorro, María Martínez-Rebollar, José M Miró, Jhon F. Rojas, Berta Torres, Josep Mallolas, Laia Albiac, Daiana L. Agöero, Marta Bodro, Celia Cardozo, Mariana Chumbita, Nicol García, Carolina García-Vidal, Marta M. Hernández-Meneses, Sabina Herrera, Laura Linares, Antonio Moreno, Laura Morata, Jose A. Martínez-Martínez, Pedro Puerta, Verónica Rico, Alex Soriano, Mikel Martínez, María del Mar Mosquera, Maria A. Marcos, Jordi Vila, Montse Tuset, Dolors Soy, Anna Vilella, Alex Almuedo, María J. Pinazo, and Jose Muñoz
References
- 1.Wuhan City Health Committee Wuhan Municipal Health Commission notified the outbreak of pneumonia (in Chinese) Jan 1, 2020. https://epaper.hubeidaily.net/pc/content/202001/01/content_15040.html
- 2.Johns Hopkins University & Medicine Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html
- 3.UNAIDS . Joint United Nations Programme on HIV/AIDS; Geneva: 2020. UNAIDS data 2020. [PubMed] [Google Scholar]
- 4.Boulle A, Davies M-A, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1198. published online Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geretti AM, Stockdale AJ, Kelly SH, et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) clinical characterization protocol (UK): a prospective observational study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1605. published online Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tesoriero JM, Swain CE, Pierce JL, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friends of the global fight against AIDS, tuberculosis and malaria How COVID-19 is affecting the global response to AIDS, tuberculosis and malaria. March 26, 2021. https://www.theglobalfight.org/covid-aids-tb-malaria
- 8.Blanco JL, Ambrosioni J, Garcia F, et al. COVID-19 in patients with HIV: clinical case series. Lancet HIV. 2020;7:e314–e316. doi: 10.1016/S2352-3018(20)30111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8:e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okoh AK, Sossou C, Dangayach NS, et al. Coronavirus disease 19 in minority populations of Newark, New Jersey. Int J Equity Health. 2020;19:93. doi: 10.1186/s12939-020-01208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper TJ, Woodward BL, Alom S, Harky A. Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS patients: a systematic review. HIV Med. 2020;21:567–577. doi: 10.1111/hiv.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirzaei H, McFarland W, Karamouzian M, Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. 2021;25:85–92. doi: 10.1007/s10461-020-02983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo W, Ming F, Dong Y, et al. A survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China. SSRN. 2020 doi: 10.2139/ssrn.3550029. published online March 13. (preprint). [DOI] [Google Scholar]
- 14.Prabhu S, Poongulali S, Kumarasamy N. Impact of COVID-19 on people living with HIV: a review. J Virus Erad. 2020;6 doi: 10.1016/j.jve.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park A, Iwasaki A. Type I and type III interferons–induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med. 2020;217 doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JS, Shin EC. The type I interferon response in COVID-19: implications for treatment. Nat Rev Immunol. 2020;20:585–586. doi: 10.1038/s41577-020-00429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sa Ribero M, Jouvenet N, Dreux M, Nisole S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle T, Goujon C, Malim MH. HIV-1 and interferons: who's interfering with whom? Nat Rev Microbiol. 2015;13:403–413. doi: 10.1038/nrmicro3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Díez-Fuertes F, De La Torre-Tarazona HE, Calonge E, et al. Transcriptome sequencing of peripheral blood mononuclear cells from elite controller-long term non progressors. Sci Rep. 2019;9 doi: 10.1038/s41598-019-50642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atyeo C, Fischinger S, Zohar T, et al. Distinct early serological signatures track with SARS-CoV-2 survival. Immunity. 2020;53:524. doi: 10.1016/j.immuni.2020.07.020. 32.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenwick C, Joo V, Jacquier P, et al. T-cell exhaustion in HIV infection. Immunol Rev. 2019;292:149–163. doi: 10.1111/imr.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moir S, Fauci AS. B-cell responses to HIV infection. Immunol Rev. 2017;275:33–48. doi: 10.1111/imr.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alrubayyi AS, Gea-Mallorqui E, Touizer E, et al. Characterization of SARS-CoV-2–specific responses in people living with HIV. Virtual Conference on Retroviruses and Opportunistic Infections; San Francisco, CA; March 6–10, 2021 (abstr 262).
- 28.Pallikkuth S, Sharkey M, Beauchamps L, et al. Persistence of SARS-COV-2–specific AB response in HIV+ individuals on ART. Virtual Conference on Retroviruses and Opportunistic Infections; San Francisco, CA; March 6–10, 2021 (abstr 260).
- 29.Spinelli M, Lynch KA, Yun CA, et al. SARS-CoV-2 seroprevalence and IgG levels are lower among people living with HIV. Virtual Conference on Retroviruses and Opportunistic Infections; San Francisco, CA; March 6–10, 2021 (abstr 627).
- 30.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 32.Collins LF, Sheth AN, Mehta CC, et al. The prevalence and burden of non-AIDS comorbidities among women living with or at-risk for HIV infection in the United States. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa204. published online March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelchen-Matthews A, Ryom L, Borges ÁH, et al. Aging and the evolution of comorbidities among HIV-positive individuals in a European cohort. AIDS. 2018;32:2405–2416. doi: 10.1097/QAD.0000000000001967. [DOI] [PubMed] [Google Scholar]
- 34.Pinti M, Appay V, Campisi J, et al. Aging of the immune system: Focus on inflammation and vaccination. Eur J Immunol. 2016;46:2286–2301. doi: 10.1002/eji.201546178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.British HIV Association Comment from BHIVA and THT on UK government guidance on coronavirus (COVID-19), social distancing to protect vulnerable adults and shielding to protect extremely vulnerable adults. March 23, 2020. https://www.bhiva.org/comment-from-BHIVA-and-THT-on-UK-Government-guidance-on-Coronavirus-COVID-19
- 36.Zhang H, Wu T. CD4+T, CD8+T counts and severe COVID-19: a meta-analysis. J Infect. 2020;81:e82–e84. doi: 10.1016/j.jinf.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ho H, Peluso MJ, Margus C, et al. Clinical outcomes and immunologic characteristics of coronavirus disease 2019 in people with human immunodeficiency virus. J Infect Dis. 2021;223:403–408. doi: 10.1093/infdis/jiaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharov KS. HIV/SARS-CoV-2 co-infection: T cell profile, cytokine dynamics and role of exhausted lymphocytes. Int J Infect Dis. 2021;102:163–169. doi: 10.1016/j.ijid.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambrosioni J, Nicolas D, Agüero F, Manzardo C, Miro JM. HIV treatment outcomes in Europe and North America: what can we learn from the differences? Expert Rev Anti Infect Ther. 2014;12:523–526. doi: 10.1586/14787210.2014.906900. [DOI] [PubMed] [Google Scholar]
- 40.Sigel K, Swartz T, Golden E, et al. Coronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clin Infect Dis. 2020;71:2933–2938. doi: 10.1093/cid/ciaa880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dandachi D, Geiger G, Montgomery MW, et al. Characteristics, Comorbidities, and outcomes in a multicenter registry of patients with human immunodeficiency virus and coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1339. published online Sept 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS. 2020;34:F3–F8. doi: 10.1097/QAD.0000000000002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winston A, De Francesco D, Post F, et al. Comorbidity indices in people with HIV and considerations for coronavirus disease 2019 outcomes. AIDS. 2020;34:1795–1800. doi: 10.1097/QAD.0000000000002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winichakoon P, Chaiwarith R, Liwsrisakun C, et al. Negative nasopharyngeal and oropharyngeal swabs do not rule out COVID-19. J Clin Microbiol. 2020;58:e00297–e00320. doi: 10.1128/JCM.00297-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karataş A, İnkaya AÇ, Demiroğlu H, et al. Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma. Transfus Apheresis Sci. 2020;59 doi: 10.1016/j.transci.2020.102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donovan RM, Bush CE, Markowitz NP, Baxa DM, Saravolatz LD. Changes in virus load markers during AIDS-associated opportunistic diseases in human immunodeficiency virus-infected persons. J Infect Dis. 1996;174:401–403. doi: 10.1093/infdis/174.2.401. [DOI] [PubMed] [Google Scholar]
- 50.Mole L, Ripich S, Margolis D, Holodniy M. The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis. 1997;176:766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 51.Günthard HF, Wong JK, Spina CA, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis. 2000;181:522–531. doi: 10.1086/315260. [DOI] [PubMed] [Google Scholar]
- 52.Peluso MJ, Bakkour S, Busch MP, Deeks SG, Henrich TJ. A high percentage of people with human immunodeficiency virus (HIV) on antiretroviral therapy experience detectable low-level plasma HIV-1 RNA following coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1754. published online Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu R, Yan H, Liu M, et al. Brief report: virologic and immunologic outcomes for HIV patients with coronavirus disease 2019. J Acquir Immune Defic Syndr. 2021;86:213–218. doi: 10.1097/QAI.0000000000002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO . World Health Organization; Geneva: 2020. Laboratory assessment tool for laboratories implementing COVID-19 virus testing, 08 April 2020. [Google Scholar]
- 55.Parčina M, Schneider UV, Visseaux B, Jozić R, Hannet I, Lisby JG. Multicenter evaluation of the QIAstat respiratory panel: a new rapid highly multiplexed PCR based assay for diagnosis of acute respiratory tract infections. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America guidelines on the diagnosis of coronavirus disease 2019 (COVID-19): serologic testing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1343. published online Sept 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cabello A, Zamarro B, Nistal S, et al. COVID-19 in people living with HIV: a multicenter case-series study. Int J Infect Dis. 2021;102:310–315. doi: 10.1016/j.ijid.2020.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Härter G, Spinner CD, Roider J, et al. COVID-19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020;48:681–686. doi: 10.1007/s15010-020-01438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gervasoni C, Meraviglia P, Riva A, et al. Clinical features and outcomes of patients with human immunodeficiency virus with COVID-19. Clin Infect Dis. 2020;71:2276–2278. doi: 10.1093/cid/ciaa579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shalev N, Scherer M, LaSota ED, et al. Clinical characteristics and outcomes in people living with human immunodeficiency virus hospitalized for coronavirus disease 2019. Clin Infect Dis. 2020;16:2294–2297. doi: 10.1093/cid/ciaa635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vizcarra P, Pérez-Elías MJ, Quereda C, et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. Lancet HIV. 2020;7:e554–e564. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inciarte A, Gonzalez-Cordon A, Rojas J, et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: a single-center, prospective observational study. AIDS. 2020;34:1775–1780. doi: 10.1097/QAD.0000000000002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Etienne N, Karmochkine M, Slama L, et al. HIV infection and COVID-19: risk factors for severe disease. AIDS. 2020;34:1771–1774. doi: 10.1097/QAD.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyashita H, Kuno T. Prognosis of coronavirus disease 2019 (COVID-19) in patients with HIV infection in New York City. HIV Med. 2021;22:e1–e2. doi: 10.1111/hiv.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020;173:536–541. doi: 10.7326/M20-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shapiro AE, Bender Ignacio RA, Whitney BM, et al. COVID-19 cases & hospitalizations in a US multisite cohort of people with HIV. Virtual Conference on Retroviruses and Opportunistic Infections; San Francisco, CA; March 6–10, 2021 (abstr 543).
- 67.Barbera LK, Kamis KF, Abdo MH, et al. Risk factors for hospitalization in people with HIV and COVID-19. Virtual Conference on Retroviruses and Opportunistic Infections; San Francisco, CA; March 6–10, 2021 (abstr 546).
- 68.Moran CA, Oliver N, Szabo BV, et al. Comorbidity burden is associated with hospitalization for COVID-19 among PWH. Virtual Conference on Retroviruses and Opportunistic Infections; San Francisco, CA; March 6–10, 2021 (abstr 547).
- 69.Mang S, Kaddu-Mulindwa D, Metz C, et al. Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome coronavirus 2 coinfection in a patient with newly diagnosed HIV-1 infection. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa906. published online July 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bouaré F, Laghmari M, Etouche FN, et al. Unusual association of COVID-19, pulmonary tuberculosis and human immunodeficiency virus, having progressed favorably under treatment with chloroquine and rifampin. Pan Afr Med J. 2020;35(suppl 2):110. doi: 10.11604/pamj.supp.2020.35.2.24952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choy CY, Wong CS. It's not all about COVID-19: pneumocystis pneumonia in the era of a respiratory outbreak. J Int AIDS Soc. 2020;23 doi: 10.1002/jia2.25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoffmann C, Casado JL, Härter G, et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2020 doi: 10.1111/hiv.13037. published online Dec 27. [DOI] [PubMed] [Google Scholar]
- 73.Blanco JL, Casado JL, Blanco J-R, et al. A prospective case-cohort study of COVID-19 in persons with HIV: CoVIH-19 study. Virtual Conference on Retroviruses and Opportunistic Infections San Francisco, CA; March 6–10, 2021 (abstr 641).
- 74.Lee MJ, Smith C, Fidler S, et al. HIV and COVID-19 inpatient outcomes: a matched retrospective multicentre analysis. Virtual Conference on Retroviruses and Opportunistic Infections; San Francisco, CA; March 6–10, 2021 (abstr 142).
- 75.Tang ME, Gaufin T, Anson R, Cachay ER. HIV is associated with a higher rate of COVID-19 diagnosis but no adverse outcomes. Virtual Conference on Retroviruses and Opportunistic Infections; San Francisco, CA; March 6–10, 2021 (abstr 542).
- 76.Yendewa GA, Perez JA, Schlick KA, et al. Characterizing COVID-19 presentation and clinical outcomes in HIV patients in the US. Virtual Conference on Retroviruses and Opportunistic Infections; San Francisco, CA; March 6–10, 2021 (abstr 548).
- 77.Berenguer J, Díez C, Martín-Vicente M, et al. Prevalence and factors associated with SARS-CoV-2 antibodies in a Spanish HIV cohort. Virtual Conference on Retroviruses and Opportunistic Infections San Francisco, CA; March 6–10, 2021 (abstr 549).
- 78.Ayerdi O, Puerta T, Clavo P, et al. Preventive efficacy of tenofovir/emtricitabine against severe acute respiratory syndrome coronavirus 2 among pre-exposure prophylaxis users. Open Forum Infect Dis. 2020;7:a455. doi: 10.1093/ofid/ofaa455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.RECOVERY Collaborative Group Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2020;396:1345–1352. doi: 10.1016/S0140-6736(20)32013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.WHO Solidarity Trial Consortium Repurposed antiviral drugs for COVID-19—interim WHO Solidarity Trial Results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324:1048–1057. doi: 10.1001/jama.2020.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19–final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2020;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Libster R, Pérez Marc G, Wappner D, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with COVID-19. N Engl J Med. 2020;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV. 2020;7:e629–e640. doi: 10.1016/S2352-3018(20)30211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simões D, Stengaard AR, Combs L, Raben D. Impact of the COVID-19 pandemic on testing services for HIV, viral hepatitis and sexually transmitted infections in the WHO European Region, March to August 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.47.2001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panel de expertos del Grupo de Estudio de SIDA . GESIDA; Madrid: 2020. Consensus document on teleconsultation (TC) for people living with HIV infection (PLHIV) (in Spanish) [Google Scholar]
- 98.Stowe MJ, Calvey T, Scheibein F, et al. Access to healthcare and harm reduction services during the COVID-19 pandemic for people who use drugs. J Addict Med. 2020;14:e287–e289. doi: 10.1097/ADM.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 99.Lapeyre-Mestre M, Boucher A, Daveluy A, et al. Addictovigilance contribution during COVID-19 epidemic and lockdown in France. Therapie. 2020;75:343–354. doi: 10.1016/j.therap.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mallet J, Dubertret C, Le Strat Y. Addictions in the COVID-19 era: current evidence, future perspectives a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2021;106 doi: 10.1016/j.pnpbp.2020.110070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trujols J, Larrabeiti A, Sànchez O, Madrid M, De Andrés S, Duran-Sindreu S. Increased flexibility in methadone take-home scheduling during the COVID-19 pandemic: should this practice be incorporated into routine clinical care? J Subst Abuse Treat. 2020;119 doi: 10.1016/j.jsat.2020.108154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531–542. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santos G-M, Ackerman B, Rao A, et al. Economic, mental health, HIV prevention and HIV treatment impacts of COVID-19 and the COVID-19 response on a global sample of cisgender gay men and other men who have sex with men. AIDS Behav; 25: 311–32. [DOI] [PMC free article] [PubMed]
- 104.Ballivian J, Alcaide ML, Cecchini D, Jones DL, Abbamonte JM, Cassetti I. Impact of COVID-19-related stress and lockdown on mental health among people living with HIV in Argentina. J Acquir Immune Defic Syndr. 2020;85:475–482. doi: 10.1097/QAI.0000000000002493. [DOI] [PubMed] [Google Scholar]
- 105.Joska JA, Andersen L, Rabie S, et al. COVID-19: increased risk to the mental health and safety of women living with HIV in South Africa. AIDS Behav. 2020;24:2751–2753. doi: 10.1007/s10461-020-02897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Reyniers T, Rotsaert A, Thunissen E, et al. Reduced sexual contacts with non-steady partners and less PrEP use among MSM in Belgium during the first weeks of the COVID-19 lockdown: results of an online survey. Sex Transm Infect. 2020 doi: 10.1136/sextrans-2020-054756. published online Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hammoud MA, Maher L, Holt M, et al. Physical distancing due to COVID-19 disrupts sexual behaviors among gay and bisexual men in Australia: implications for trends in HIV and other sexually transmissible infections. J Acquir Immune Defic Syndr. 2020;85:309–315. doi: 10.1097/QAI.0000000000002462. [DOI] [PubMed] [Google Scholar]
- 108.Sanchez TH, Zlotorzynska M, Rai M, Baral SD. Characterizing the impact of COVID-19 on men who have sex with men across the United States in April, 2020. AIDS Behav. 2020;24:2024–2032. doi: 10.1007/s10461-020-02894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stephenson R, Chavanduka TMD, Rosso MT, et al. Sex in the time of COVID-19: results of an online survey of gay, bisexual and other men who have sex with men's experience of sex and HIV prevention during the US COVID-19 epidemic. AIDS Behav. 2020 doi: 10.1007/s10461-020-03024-8. published online Sept 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sociedad Española de Farmacia Hospitalaria . Sociedad Española de Farmacia Hospitalaria; Madrid: 2020. Documento de posicionamiento de la Sociedad Española de Farmacia Hospitalaria sobre la telefarmacia. [Google Scholar]
- 111.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodrigues CMC, Pinto MV, Sadarangani M, Plotkin SA. Whither vaccines? J Infect. 2017;74(suppl 1):S2–S9. doi: 10.1016/S0163-4453(17)30184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 114.Kwong PD, Mascola JR, Nabel GJ. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol. 2013;13:693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 115.Kwong PD, Mascola JR. HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity. 2018;48:855–871. doi: 10.1016/j.immuni.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 116.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Geretti AM, Doyle T. Immunization for HIV-positive individuals. Curr Opin Infect Dis. 2010;23:32–38. doi: 10.1097/QCO.0b013e328334fec4. [DOI] [PubMed] [Google Scholar]
- 118.Lee K-Y, Tsai M-S, Kuo K-C, et al. Pneumococcal vaccination among HIV-infected adult patients in the era of combination antiretroviral therapy. Hum Vaccin Immunother. 2014;10:3700–3710. doi: 10.4161/hv.32247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ceravolo A, Orsi A, Parodi V, Ansaldi F. Influenza vaccination in HIV-positive subjects: latest evidence and future perspective. J Prev Med Hyg. 2013;54:1–10. [PMC free article] [PubMed] [Google Scholar]
- 120.European AIDS Clinical Society Statement on risk of COVID-19 for people living with HIV (PLWH) and SARS-CoV-2 vaccine advice for adults living with HIV. Jan 15, 2021. https://www.eacsociety.org/home/bhiva-daig-eacs-gesida-polish-scientific-aids-society-and-portuguese-association-for-the-clinical-study-of-aids-apecs-statement-on-risk-of-covid-19-for-people-living-with-hiv-plwh-and-sars-cov-2-vaccine-advice-for-adults-living-with-hiv.html
- 121.US Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting: FDA Briefing Document—Moderna COVID-19 Vaccine. Dec 17, 2020.
- 122.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 VACCINE. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.NOVAVAX Press releases. https://ir.novavax.com/press-releases
- 124.Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS. 2020;34:F3–F8. doi: 10.1097/QAD.0000000000002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.WHO Global COVID-19 Clinical Data Platform for clinical characterization and management of hospitalized patients with suspected or confirmed COVID-19. https://www.who.int/teams/health-care-readiness-clinical-unit/covid-19/data-platform
- 126.WHO HIV/HEP/STI COVID-19 Questionnaire. November 2020. https://www.who.int/docs/default-source/hq-hiv-hepatitis-and-stis-library/hhs-service-disruption-slides-dec-2020.pdf?sfvrsn=be10f39d_12
- 127.WHO Coronavirus disease (COVID-19) technical guidance: maintaining essential health services and systems. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/maintaining-essential-health-services-and-systems
- 128.Buchbinder SP, McElrath MJ, Dieffenbach C, Corey L. Use of adenovirus type-5 vectored vaccines: a cautionary tale. Lancet. 2020;396:e68–e69. doi: 10.1016/S0140-6736(20)32156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]