Abstract
Predictive models have played a critical role in local, national, and international response to the COVID-19 pandemic. In the United States, health care systems and governmental agencies have relied on several models, such as the Institute for Health Metrics and Evaluation, Youyang Gu (YYG), Massachusetts Institute of Technology, and Centers for Disease Control and Prevention ensemble, to predict short- and long-term trends in disease activity. The Mayo Clinic Bayesian SIR model, recently made publicly available, has informed Mayo Clinic practice leadership at all sites across the United States and has been shared with Minnesota governmental leadership to help inform critical decisions during the past year. One key to the accuracy of the Mayo Clinic model is its ability to adapt to the constantly changing dynamics of the pandemic and uncertainties of human behavior, such as changes in the rate of contact among the population over time and by geographic location and now new virus variants. The Mayo Clinic model can also be used to forecast COVID-19 trends in different hypothetical worlds in which no vaccine is available, vaccinations are no longer being accepted from this point forward, and 75% of the population is already vaccinated. Surveys indicate that half of American adults are hesitant to receive a COVID-19 vaccine, and lack of understanding of the benefits of vaccination is an important barrier to use. The focus of this paper is to illustrate the stark contrast between these 3 scenarios and to demonstrate, mathematically, the benefit of high vaccine uptake on the future course of the pandemic.
The rapid development and availability of vaccines have had a significant impact on the potential to control the COVID-19 pandemic. In the United States, 3 vaccines are currently approved by the Food and Drug Administration with emergency use authorization. A number of real-world studies have demonstrated high-level effectiveness in preventing symptomatic and asymptomatic infections with these available vaccines.1, 2, 3, 4 The current administration has suggested that everyone who would like to receive a vaccine will be able to receive one by May 1, 2021. However, surveys indicate that half of American adults are hesitant to receive a COVID-19 vaccine, and lack of understanding of the benefits of vaccination is an important barrier to use.5 , 6
With the potential concerns around vaccine hesitancy, it would be valuable to understand the impact of different levels of vaccination rates on controlling the pandemic. Various modeling approaches may be used to better understand the impact of vaccination on infection rates. Predictive models have played a critical role in local, national, and international response to the COVID-19 pandemic. In the United States, health care systems and governmental agencies have relied on several models, such as the Institute for Health Metrics and Evaluation,7 Youyang Gu (YYG),8 Massachusetts Institute of Technology,9 and Centers for Disease Control and Prevention (CDC) ensemble,10 to predict short- and long-term trends in disease activity. In this paper, we use the Mayo Clinic Bayesian SIR model to compare 3 vaccine uptake scenarios in the future course of the pandemic. The Mayo Clinic Bayesian SIR model was recently made available to the public.11 This model has been used by practice leadership at all Mayo Clinic hospitals across the country to safely manage patient volumes and has been shared with Minnesota state leadership to help inform critical decisions during the past year. One key to the accuracy of the Mayo Clinic model is its ability to adapt to the constantly changing dynamics of the pandemic and uncertainties of human behavior. Examples include changes in the rate of contact among the population over time and by geographic location and, more recently, the impact of new virus variants. The Mayo Clinic model can also be used to forecast COVID-19 trends in different hypothetical worlds in which no vaccine is available, vaccinations are no longer being accepted from this point forward, and 75% of the population is already vaccinated. The focus of this paper is to illustrate the stark contrast between these 3 scenarios and to demonstrate, mathematically, the impact of vaccine uptake on the future course of the pandemic.
Probabilistic Characterization of Future COVID-19 Cases and Hospitalizations
The Mayo Clinic Bayesian SIR model along with the corresponding vaccination model and all assumptions have been previously described by Storlie et al.11 However, we provide an overview in a supplement to this article for convenience because the results depend heavily on the vaccination model and assumptions being used.
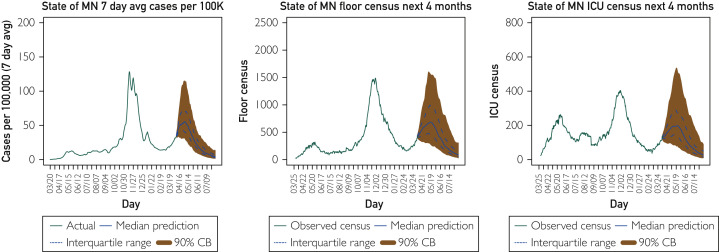
Figure 1 provides a forecast for cases and hospital census (general care and intensive care unit, respectively) in the state of Minnesota for the next 4 months as of April 6, 2021 (the time of this writing), under our best guess for vaccinations going forward. The vaccination model is described in the Supplemental Material (available online at http://www.mayoclinicproceedings.org) but essentially assumes that future vaccination trends will be similar to the past few weeks until we reach a point of dwindling demand (when 50% to 75% of the population has been vaccinated). These results suggest that the current rise in COVID cases in Minnesota is likely to continue for a few more weeks before decreasing into the summer. The interquartile range (dashed lines) and the 90% Bayesian credible interval indicate bounds that should contain the future trend with 50% and 90% certainty, respectively. Based on prospective validation, the Bayesian SIR model has been accurate with its probability statements representing this uncertainty, namely, close to 50% of future paths for cases and hospitalizations have fallen within the predictive interquartile range for time horizons up to 4 weeks and similar for the 90% bounds.11 These bounds indicate that a large rise approaching the magnitude of cases observed in November-December 2020 is possible, even if unlikely. It is also possible that cases and hospitalizations will stop increasing immediately and begin to fall off much sooner, although this is also unlikely.
Figure 1.
Mayo Clinic Bayesian SIR model 4-month forecast on April 6, 2021, for the state of Minnesota (MN) cases (7-day average per 100,000), hospital general care (floor) census, and intensive care unit (ICU) census.
Three Hypothetical Vaccination Scenarios
In this section, 3 hypothetical vaccination scenarios are considered. Whereas none of these scenarios are realistic, they serve to illustrate the critical impact of vaccination rates on COVID-19 cases and hospitalizations.
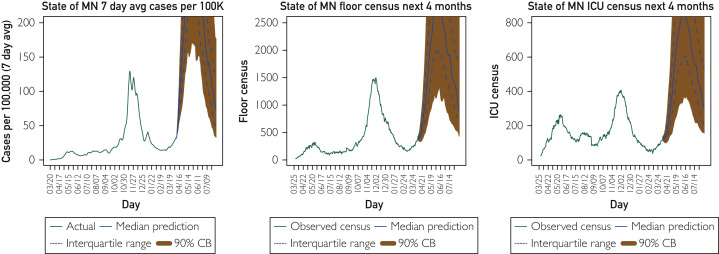
The first scenario depicted in Figure 2 shows a hypothetical future in which a COVID-19 vaccine did not exist under our current behavior patterns with current strains of the virus. For a given county and day, the model estimates a rate of spread for a given infected individual if all people were susceptible but then suppresses that rate by the proportion of the population that is not currently susceptible to infection. This model estimate for the unabated rate of spread can change as a result of new variants or changes in social behavior. To produce the “no vaccine” scenario, we simply placed the vaccinated individuals back into the susceptible pool and propagated the model forward with no impedance to the rate of spread due to vaccination. However, it is still assumed that there is impedance due to natural infection–acquired immunity.
Figure 2.
Hypothetical scenario: If no one were vaccinated, what would the future look like? Four-month forecast on April 6, 2021, for the state of Minnesota (MN) cases (7-day average per 100,000), hospital general care (floor) census, and intensive care unit (ICU) census assuming no vaccination.
The rise under this scenario is stark and almost unbelievable. This is because the actual rise that is currently happening in Minnesota is in the face of an approximately 35% vaccinated population (according to CDC data), which means the unabated spread rate in the model right now is very high. That is, if no one were vaccinated, we would likely be seeing a much higher reproduction number right now than we have seen since very early in the pandemic. It is difficult to untangle how much of this elevated rate of spread right now is due to new variants as opposed to changes in social behavior. Regardless of the reason, the absence of vaccinations in the current environment would have been likely to result in by far the largest surge to date.
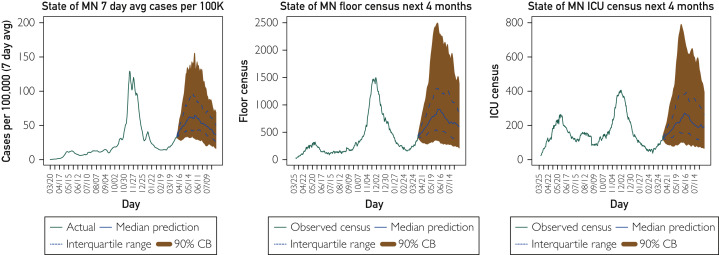
Thankfully, we do have several effective vaccines that people are receiving. As of this writing, according to CDC data, 33% of the US population has received at least 1 dose of vaccine.12 The second hypothetical scenario examines resulting cases and hospitalizations in the event that no further vaccinations are given (Figure 3 ). Our model demonstrates that existing vaccinations are substantially blunting the rise predicted in Figure 2. However, without further vaccinations, the risk of a substantial increase is much greater and would last much longer than what is expected under our best guess scenario presented in Figure 1.
Figure 3.
Hypothetical scenario: If no more people were vaccinated, what would the future look like? Four-month forecast on April 6, 2021, for the state of Minnesota (MN) cases (7-day average per 100,000), hospital general care (floor) census, and intensive care unit (ICU) census assuming no further vaccination from this point forward.
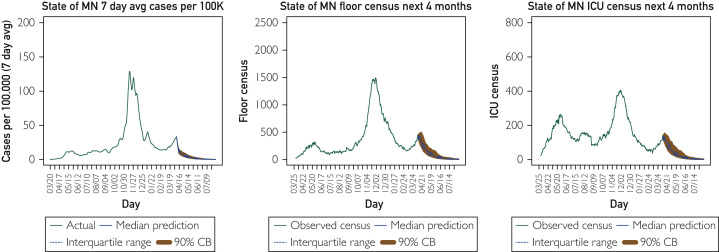
Finally, the last scenario in Figure 4 explores what would happen if the population were 75% vaccinated right now. This forecast is as stark as the no vaccination scenario. According to the model, this level of vaccination would completely suppress the growth (even in the face of the recent elevated spread rates) and immediately drive cases and hospitalizations down to very low levels.
Figure 4.
Hypothetical scenario: If 75% of the population were vaccinated right now, what would the future look like? Four-month forecast on April 6, 2021, for the state of Minnesota (MN) cases (7-day average per 100,000), hospital general care (floor) census, and intensive care unit (ICU) census assuming 75% already vaccinated.
Discussion
The Mayo Clinic Bayesian SIR model was developed during the early weeks of the COVID-19 pandemic by a predictive modeling task force. The goal was to provide COVID-19 census forecasting to help clinical leaders understand the potential short- and long-term impacts of the pandemic on hospital operations. Since inception, the model has been continuously revised to account for more data sources and the evolving dynamics of the pandemic, most recently the addition of vaccination. Several vaccination scenarios show how vaccination rates will shape the future of the COVID-19 pandemic.
There are several important caveats to these results. These results are dependent on the uncertainties of our model, although it is a model that has made accurate predictions for COVID surges thus far.11 Given our current knowledge,13, 14, 15 it is likely that vaccination-acquired (and natural infection–acquired) immunity will wane and the population will once again become vulnerable to SARS-CoV-2 infection. Our best understanding based on experience with other coronaviruses is that duration of immunity may be on the order of 1 to 2 years (on average),16 , 17 with some becoming susceptible again to infection sooner than others. This time frame also depends greatly on the impact of new virus variants on vaccine efficacy. It is also understood that the fewer cases there are, the less opportunity that SARS-CoV-2 has to mutate and to generate new strains that can escape vaccine-induced immunity. The vaccine is not 100% effective, even in the next few months after it has been received, so it will not prevent all infections, and this is considered in the modeling results. However, vaccination has also been shown to decrease the severity of infections and potential need for hospitalizations.
Conclusion
Comparing the range of predictions between scenarios offers compelling evidence for what many have assumed to be true, namely, without vaccinations, a large surge would be imminent. Conversely, vaccinations will suppress an otherwise inevitable surge of cases, but only if enough individuals take advantage of what modern science has provided. With continued education, perseverance, and exemplary leadership at the local, state, and national levels, attaining a 75% vaccination rate is a realistic goal. This is the shortest path to return to life as we knew it before the pandemic.
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Tande A.J., Pollock B.D., Shah N.D. Impact of the COVID-19 vaccine on asymptomatic infection among patients undergoing pre-procedural COVID-19 molecular screening. Clin Infect Dis. 2021 Mar 10:ciab229. doi: 10.1093/cid/ciab229. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M., Clemens S.A., Madhi S.A. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK [erratum appears in Lancet. 2021;397(10269):98] Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szilagyi P.G., Thomas K., Shah M.D. National trends in the US public's likelihood of getting a COVID-19 vaccine—April 1 to December 8, 2020. JAMA. 2020;325(4):396–398. doi: 10.1001/jama.2020.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidry J.P.D., Laestadius L.I., Vraga E.K. Willingness to get the COVID-19 vaccine with and without emergency use authorization. Am J Infect Control. 2021;49(2):137–142. [Google Scholar]
- 7.COVID I.H.M.E., Murray C.J. Forecasting the impact of the first wave of the COVID-19 pandemic on hospital demand and deaths for the USA and European Economic Area countries. medRxiv. https://doi.org/10.1101/2020.04.21.20074732 Preprint posted online April 26, 2020.
- 8.Gu Y. Covid-19 projections using machine learning. https://covid19-projections.com Accessed ∗∗∗.
- 9.Kissler S.M., Tedijanto C., Goldstein E., Grad Y.H., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray E.L., Wattanachit N., Niemi J. Ensemble forecasts of coronavirus disease 2019 (COVID-19) in the US. medRxiv. https://doi.org/10.1101/2020.08.19.20177493 Preprint posted online August 22, 2020.
- 11.Storlie C.B., Rojas R.L., Demuth G.O. A hierarchical Bayesian model for stochastic spatiotemporal SIR modeling and prediction of COVID-19 cases and hospitalizations. arXiv. https://arxiv.org/abs/2104.04033 Preprint posted online April 8, 2021.
- 12.Centers for Disease Control and Prevention COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccinations Accessed ∗∗∗.
- 13.Lavine J.S., Bjornstad O.N., Antia R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science. 2021;371(6530):741–745. doi: 10.1126/science.abe6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabino E.C., Buss L.F., Carvalho M.P. Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence. Lancet. 2021;397(10273):452–455. doi: 10.1016/S0140-6736(21)00183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altmann D.M., Boyton R.J. SARS-CoV-2 T cell immunity: specificity, function, durability, and role in protection. Sci Immunol. 2020;5(49):eabd6160. doi: 10.1126/sciimmunol.abd6160. [DOI] [PubMed] [Google Scholar]
- 16.Stokel-Walker C. What we know about covid-19 reinfection so far. BMJ. 2021;372:n99. doi: 10.1136/bmj.n99. [DOI] [PubMed] [Google Scholar]
- 17.Edridge A.W., Kaczorowska J., Hoste A.C. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26(11):1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.