To the Editor,
Adverse reactions to vaccines are usually insignificant, but there have been reports of myopericarditis after vaccination.1 Recently, several cases have been published of myopericarditis associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.2, 3
We describe the case of a 39-year-old male physician, with a past medical history of asthma, autoimmune hypothyroidism, chronic atrophic gastritis, an isolated episode of atrial fibrillation, and recurrent spontaneous pneumothorax with left apical lobectomy. In recent months, he had undergone several PCR and serology screening tests for SARS-CoV-2 infection, all of which were negative. The patient gave informed consent for the write-up and publication of this clinical case.
As per the COVID-19 vaccination program, he received the first dose of the BNT162b2 vaccine, with no significant adverse reactions. At 6 hours postvaccination with the second dose, 21 days after the first, he noted a persistent fever of above 38 °C, which was treated with antipyretics. He subsequently developed intermittent chest and interscapular pain, which lasted for several hours and was not relieved with conventional analgesia, so he decided to attend the emergency department.
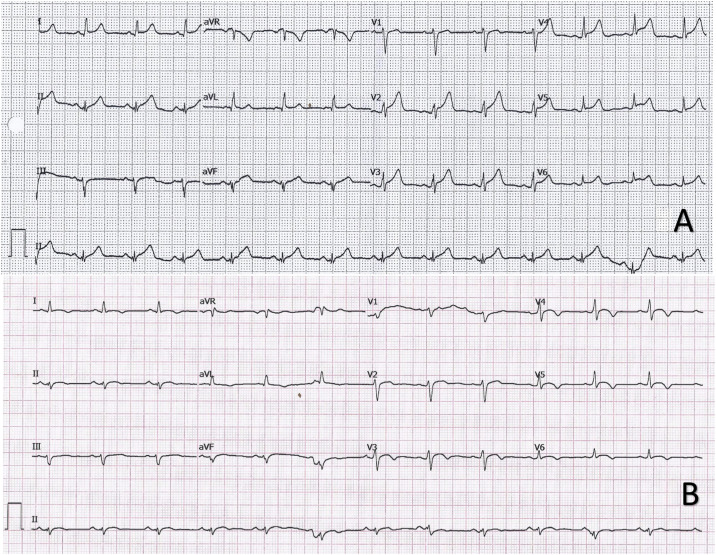
Electrocardiogram on arrival showed sinus tachycardia at 130 bpm with a narrow QRS complex and diffuse ST-segment elevation (figure 1A ). Chest X-ray showed no significant findings. Blood tests showed raised markers of myocardial damage, with a first high-sensitivity troponin T (hsTnT) of 139 ng/L. PCR test for SARS-CoV-2 was negative. In view of the symptoms, electrocardiographic changes, and blood test results, transthoracic echocardiography was performed, which showed good biventricular function, with no regional wall abnormalities, no significant valve disease, and no pericardial effusion. Acute aortic syndrome was excluded on computed tomography angiography (CT-angio) of the chest; a coronary study could not be carried out as the heart rate remained uncontrolled.
Figure 1.
A: electrocardiogram showing sinus rhythm with a reduced PR interval in I and V5-V6 and diffuse ST elevation with upward concavity. B: electrocardiogram in sinus rhythm with < 1 mm elevation and upward concavity in V3-V6 and negative T waves in I, II, aVL, and V3-V6.
Given diagnostic suspicion of acute myopericarditis, anti-inflammatory treatment was started, and the patient's symptoms resolved. However, it was decided to transfer him to a tertiary hospital for further work-up.
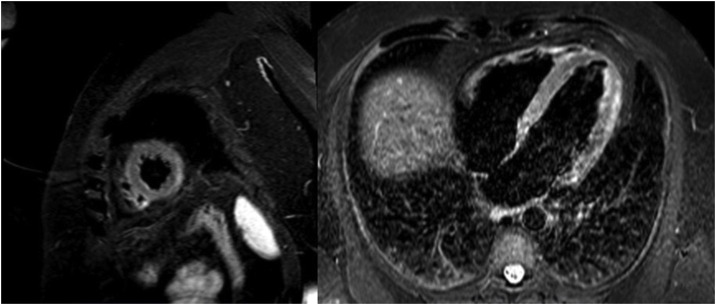
Upon arrival there, the patient was hemodynamically stable and asymptomatic, with occasional episodes of chest pain. Subsequent electrocardiograms showed partial resolution of the ST changes, with negative T-waves in the precordial leads (figure 1B). Peak hsTnT was 854 ng/L. Within the first 48 hours, a CT-coronary angiogram was performed, which ruled out coronary disease, and cardiac magnetic resonance imaging showed edema on T2-STIR sequences and subepicardial enhancement in the lateral mediastinal region compatible with acute myocarditis (figure 2 ). Investigations were completed with viral serology and plasma polymerase chain reaction (PCR) screening for the main cardiotropic viruses, as well as for SARS-CoV-2 on a new oropharyngeal sample, which was negative. Serological testing was positive for nonspecific IgM, positive for IgG (spike), and negative for IgG (nucleocapsid)—a pattern indicative of immunization after vaccination against SARS-CoV-2, as the spike protein is coded for by the mRNA contained in the vaccine.4 Due to the patient's low-risk profile and favorable progress, endomyocardial biopsy was not performed. The patient had a good clinical outcome, with symptom resolution, and he was discharged after 6 days in hospital.
Figure 2.
Magnetic resonance image with late enhancement in the lateral mediastinal region and edema on T2-enhanced STIR sequences.
Given the temporal association between vaccination and the onset of symptoms, and having excluded other acute cardiological disorders, we suggest that this acute myocarditis was an adverse reaction to the BNT162b2 vaccine.
Although causality has not been established, numerous cases have been described of myopericarditis associated with various vaccines, as collected in the United States vaccine adverse effect reporting system (VAERS)1; of 620 195 adverse effects reported, 708 (0.1%) are described as myopericarditis. However, this disease does not feature in the safety study of the BNT162b2 vaccine, but adverse effects of a cardiovascular nature are described: isolated cases of acute coronary syndrome, atrial fibrillation, ventricular extrasystole, and cardiac arrest (< 0.05%).5
Various authors have described a possible association between SARS-CoV-2 infection and the onset of autoimmune diseases via a mechanism of molecular mimicry and cross-reaction. It is suggested that these reactions may also be triggered following vaccination, particularly in genetically-predisposed individuals.4 In this case, of a patient with a past medical history of asthma, autoimmune hypothyroidism, and chronic atrophic gastritis, we hypothesize that the vaccine may have been the trigger of an autoimmune reaction manifesting as acute myocarditis.
Multiple cases of myocarditis secondary to COVID-19 have been published.2, 3 In the investigation of such cases, although endomyocardial biopsy did show evidence of myocardial inflammation, SARS-CoV-2 was not isolated in the human cardiomyocytes.2 In the case described, acute infection with this microorganism was excluded on 2 PCR tests, but a serological pattern compatible with postvaccination immunity was observed.4
In the case presented here, as often occurs in acute myocarditis, the definitive etiological diagnosis was difficult to determine.2 In light of the temporal association and the serological pattern compatible with postvaccination immunization, and having ruled out acute infection, it seems reasonable to attribute this patient's symptoms and signs to an adverse reaction to the BNT162b2 vaccine against COVID-19.
In conclusion, this is the first published case of acute myocarditis as an adverse reaction to the SARS-CoV-2 vaccine.
FUNDING
There were no funding sources for this article.
AUTHORS’ CONTRIBUTIONS
J. Bautista García, P. Peña Ortega and J.A. Bonilla Fernández wrote the manuscript. A. Cárdenes León was the main editor. L. Ramírez Burgos was the cardiologist responsible for the patient while in hospital. E. Caballero Dorta is head of department.
CONFLICTS OF INTEREST
All the authors declare no conflict of interest.
References
- 1.Su J.R., McNeil M.M., Welsh K.J., et al. Myopericarditis after vaccination, vaccine adverse event reporting system (VAERS), 1990-2018. Vaccine. 2021;39:839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 2.Mele D., Flamigni F., Rapezzi C., et al. Myocarditis in COVID-19 patients: current problems. Intern Emerg Med. 2021 doi: 10.1007/s11739-021-02635-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salamanca J., Díez-Villanueva P., Martínez P., Cecconi A., et al. COVID-19 “fulminant myocarditis” successfully treated with temporary mechanical circulatory support. JACC Cardiovasc Imaging. 2020;13:2457–2459. doi: 10.1016/j.jcmg.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases”. Clin Immunol. 2021;224:108665. doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]