Abstract
Objective
This systematic review, with meta-analysis and meta-regression aims to evaluate the effect of colchicine administration on mortality in patients with coronavirus disease 2019 (COVID-19) and factors affecting the association.
Methods
A systematic literature search using the PubMed, Scopus, and Embase databases were performed from inception of databases up until 3 March 2021. We included studies that fulfill all of the following criteria: 1) observational studies or randomized controlled trials (RCTs) that report COVID-19 patients, 2) reporting colchicine use, and 3) mortality within 30 days. There was no restriction on the age, inpatients or outpatients setting, and severity of diseases. The intervention was colchicine administration during treatment for COVID-19. The control was receiving placebo or standard of care. The outcome was mortality and the pooled effect estimate was reported as odds ratio (OR). Random-effects restricted maximum likelihood meta-regression was performed to evaluate factors affecting the pooled effect estimate.
Results
Eight studies comprising of 5530 patients were included in this systematic review and meta-analysis. There were three RCTs and five observational studies. Pooled analysis showed that colchicine was associated with lower mortality in patients with COVID-19 (OR 0.47 [0.31, 0.72], p = 0.001; I2: 30.9, p = 0.181). Meta-regression analysis showed that the association between colchicine and mortality was reduced by increasing age (OR 0.92 [0.85, 1.00], p = 0.05), but not gender (reference: male, p = 0.999), diabetes (p = 0.376), hypertension (p = 0.133), and CAD (p = 0.354).
Conclusion
This meta-analysis indicates that colchicine may reduce mortality in patients with COVID-19. Meta-regression analysis showed that the benefit was reduced as age increases.
PROSPERO: CRD42021240609.
Keywords: Colchicine, Coronavirus, COVID-19, Outcome, Treatment
Abbreviations: CAD, Coronary Artery Disease; COVID-19, Coronavirus Disease 2019; CRP, C-reactive Protein; IF, Interferon; IL-6, interleukin-6; TNF, Tumor Necrosis Factors; OR, Odds Ratio; PSM, Propensity-Score Matched; RCT, Randomized Controlled Trial; RoB, Risk of Bias; NOS, Newcastle-Ottawa Scale
1. Introduction
Patients with severe coronavirus disease (COVID-19) often experienced cytokine storm, characterized by an elevated level of inflammatory biomarkers such as tumor necrosis factor (TNF) -α, C-reactive protein (CRP), D-dimer, interferon (IF) -γ and interleukin-6 (IL-6).[1], [2] This pro-inflammatory state is thought to be responsible to the development of complications, ranging from acute respiratory distress syndrome ARDS, coagulopathy, sepsis, cardiopulmonary collapse, multi-organ dysfunction, and death.[3] Given there is no specific treatment for COVID-19 to date, various combinations of drugs are used to combat the novel coronavirus, including anti-viral medications, anti-inflammatory drugs, and immunomodulatory agents, as seen in pandemics caused by respiratory viruses (SARS, MERS, and H1N1).[4], [5], [6], [7] However, these medications are largely ineffective.[8]
Colchicine, an alkaloid derived from the plant family Colchicum autumnale or Gloriosa superba, contains anti-inflammatory properties and therefore may be useful in treating severe and critically-ill COVID-19 patients.[5], [9], [10] Colchicine may help in mitigating the cytokine release in patients with COVID-19 and thereby improve outcome in these patients. This systematic review, with meta-analysis and meta-regression aims to evaluate the effect of colchicine administration on mortality in patients with COVID-19 and factors affecting the association. We included both randomized controlled trials (RCTs) and observational studies that addresses colchicine administration compared to standard of care or placebo in patients with COVID-19 in terms of mortality.
2. Methods
This is a Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) compliant systematic review and meta-analysis. This study is registered in PROSPERO (CRD42021240609).
2.1. Search strategy and study selection
A systematic literature search using the PubMed, Scopus, and Embase databases were performed using the terms “(COVID-19 OR 2019-nCoV OR SARS-CoV-2) AND (colchicine)” from inception of databases up until 3 March 2021. The full search strategy can be accessed in the Supplementary Table 1. Two authors performed independent screening of title/abstracts. The full-text articles were then assessed for eligibility by evaluating inclusion and exclusion criteria. Discrepancies that arises were resolved by discussion.
2.2. Inclusion and exclusion criteria
We included studies that fulfill all of the following criteria: 1) observational studies or randomized controlled trials that report COVID-19 patients, 2) reporting colchicine use, and 3) mortality within 30 days. There was no restriction on the age, inpatients or outpatients setting, and severity of diseases.
We excluded studies that fulfill one of the following criteria: 1) abstracts, 2) conference papers, 3) letters, and 4) commentaries. We did not impose any language restriction
2.3. Data extraction
Two authors independently extract data from the studies for the first author, study design, colchicine dosing, sample size, age, gender, diabetes, hypertension, coronary artery disease (CAD), the intervention, and the outcome. Discrepancies were resolved by discussion.
2.4. Risk of bias assessment
Two independent authors performed risk of bias assessment of the included studies using the Newcastle-Ottawa Scale (NOS)[11] and Cochrane Risk of Bias Assessment for randomized controlled trials [12]. NOS consists of three domains: 1) selection, 2) comparability, and 3) outcome. Cochrane Risk of Bias Assessment evaluates the possibilities of selection bias, performance bias, detection bias, attrition bias, selective reporting, and other biases. Discrepancies were resolved by discussion. The information derived the risk of bias assessment acts as a note for the included studies but did not modify data synthesis.
2.5. Intervention and outcome
The intervention was colchicine, defined as colchicine administration during treatment for COVID-19. The control was placebo or standard of care. Standard of care was defined as treatment regimen given by the respective study centers. The outcome was mortality, defined as clinically non-survivor/death. The pooled effect estimate was reported as odds ratio (OR).
2.6. Statistical analysis
STATA version 16.0 was used to perform meta-analysis. Der-Simonian Laird random-effects meta-analysis was used to calculate the pooled OR for the mortality from dichotomous values derived from colchicine and control group. Random-effects model was selected regardless of heterogeneity. Pooled effect estimate was considered as statistically significant if the p-value was below ≤0.05 and 95% CI did not cross 1.00. Cochran’s Q test and I2 statistic were used to evaluate heterogeneity, in which I2 values above 50% and p-value below 0.10 indicates substantial heterogeneity. Subgroup analyses were performed for the RCTs and non-RCTs. Sensitivity analysis was performed by removing the study with outpatients setting. Random-effects restricted maximum likelihood (REML) meta-regression analysis was performed to evaluate the effect of baseline characteristics (possible confounders) on the association between colchicine and mortality.
3. Results
3.1. Baseline characteristics
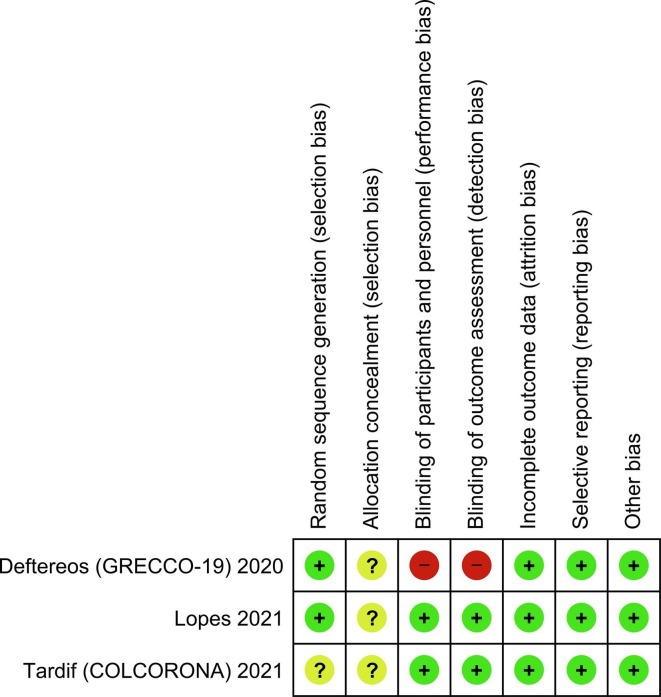
Eight studies comprising of 5530 patients were included in this systematic review and meta-analysis [Fig. 1 ].[4], [5], [9], [13], [14], [15], [16], [17] There were three randomized controlled trials and five observational studies. The baseline characteristics and risk of bias assessment based on NOS can be seen in Table 1 . Cochrane Risk of Bias assessment can be seen in Fig. 2 .
Fig. 1.
PRISMA Flowchart.
Table 1.
Baseline Characteristics of the Included Studies.
| Authors | Design | Samples | Severity of COVID-19 (%) | Colchicine Dose (Initial) | Maintenance Dose | Control | Duration of Treatment | Age (years) | Male (%) | Diabetes (%) | Hypertension (%) | CAD (%) | Follow-up (days) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brunetti 2020 | PSM Cohort (1:1) | 66 (33 vs 33) | Severe: 100% | 1.2 mg (73%) | 0.6 mg twice daily | SOC | Unclear | 63 | 65 | 79 | 49 | 9 | 28 | 8 |
| Deftereos (GRECCO-19) 2020 | RCT | 105 (55 vs 50) | Unclear | 1.5 mg + 0.5 mg | 0.5 mg twice daily | SOC | Until discharge or 21 days | 58 | 58 | 20 | 45 | 13 | 21 | See RoB (Fig. 2) |
| Lopes 2021 | RCT | 75 (37 vs 38) | Percentage unknown, Enrol moderate-severe COVID-19 | 1.0 mg | 0.5 mg thrice daily | Placebo | 5 days | 55 | 46 | 39 | Unclear | Unclear | 26 | See RoB (Fig. 2) |
| Mahale 2020 | RC | 134 (39 vs 95) | Mild ARDS: 31.3* Moderate ARDS: 26.9* Severe ARDS: 13.4* Normal: 14.9* |
No loading | 0.5 mg per day | SOC | Unclear | 56 | 68 | 44 | 46 | 19 | Unclear | 7 |
| Pinzon 2021 | CS | 301 (145 vs 156) | Severe:100 | No loading | 0.5 mg twice daily | SOC | 7–14 days | 57 | 59 | 24 | 46 | 6 | Until Discharge | 7 |
| Sandhu 2020 | PC | 112 (34 vs 78) | Percentage unknown, Enrol moderate-severe COVID-19 | No loading | 0.6 mg twice daily | SOC | 12 days | 67 | 55 | 46 | 66 | 7 | Until Discharge | 7 |
| Scarsi 2020 | PC | 262 (122 vs 140) | Severe: 100 | No loading | 1.0 mg per day | SOC | Unclear | 64 | 12 | Unclear | Unclear | Unclear | 21 | 8 |
| Tardif (COLCORONA) 2021 | RCT | 4159 (2075 vs 2084) | Unclear, possibly there is no severe COVID-19 | No loading | 0.5 mg twice daily | Placebo | 30 days | 55 | 46 | 20 | 36 | 3 | 30 | See RoB (Fig. 2) |
CAD: Coronary Artery Disease, CS: Cross-sectional, PC: Prospective Cohort, PSM: Propensity-Score Matched; RC: Retrospective Cohort, RCT: Randomized Controlled Trial, RoB: Risk of Bias, NOS: Newcastle-Ottawa Scale.
Based on P/F ratio.
Fig. 2.
Summary of Cochrane Risk of Bias Assessment.
3.2. Colchicine and mortality
Pooled analysis showed that colchicine was associated with lower mortality in patients with COVID-19 (OR 0.47 [0.31, 0.72], p = 0.001; I2: 30.9%, p = 0.181) [Fig. 3 ]. Subgroup analysis for observational studies (cohorts) showed mortality reduction (OR 0.48 [0.28, 0.82], I2: 56.7%), however, pooled RCTs only showed a statistically non-significant trend towards mortality reduction (OR 0.43 [0.17, 1.08], I2: 0%). Sensitivity analysis by removing Tardif et al. (outpatients setting) showed that the mortality benefit is still statistically significant (OR 0.45 [0.28, 0.74], p = 0.002; I2: 40.5%, p = 0.121).
Fig. 3.
Colchicine and Mortality in COVID-19.
3.3. Meta-regression
Meta-regression analysis showed that the benefit of colchicine on mortality was reduced as age increases (OR 0.92 [0.85, 1.00], p = 0.05) [Fig. 4 A]. In contrast, gender (reference: male, OR 1.00 [0.97, 1.03], p = 0.999) [Fig. 4B], diabetes (OR 0.99 [0.95, 1.02], p = 0.376) [Fig. 4C], hypertension (OR 0.96 [0.91, 1.01], p = 0.133) [Fig. 4D], and CAD (OR 1.05 [0.94, 1.17], p = 0.354) [Fig. 4E] did not affect the benefit of colchicine on mortality.
Fig. 4.
Meta-regression Analysis for the Association between Colchicine and Mortality in COVID-19 using age (A), gender (B), diabetes (C), hypertension (D), and CAD (E) as covariates.
3.4. Discussion
This meta-analysis indicates that colchicine may reduce mortality in patients with COVID-19. The largest study (COLCORONA Trial) was done in patients that did not require hospitalization which has low mortality rate. The trial study did not indicate mortality benefit, however, it reduces the rate of hospitalization due to COVID-19 (OR 0.75 [0.57, 0.99]. The rate of pneumonia was lower in the colchicine group (2.9% vs. 4.1%, p = 0.02). Thus the benefit of colchicine use is more apparent in hospitalized patients, especially in those with moderate-severe disease, as shown by Brunetti et al., Sandhu et al., and Scarsi et al. studies.[9], [14], [15] Meta-regression analysis showed that the benefit was reduced as age increases.
We included both placebo or standard of care as a control group because of the lack of studies, while this might pose as a source of bias due to different methodological quality in double-blinded studies compared to open label studies, it is unlikely that administration of placebo on top of standard of care will cause considerable difference in terms of mortality. Nevertheless, varying COVID-19 treatment protocol among institutions and countries may cause heterogeneity. Subgroup analysis showed that the effect is more pronounced in the observational studies. The RCTs subgroup has 0% heterogeneity and trend towards lower mortality but was not statistically significant. The RCTs comprised of two inpatients and one outpatients study, the two inpatients study have small sample size and event rates that may not provide adequate power to detect significance. This is in contrast with the observational studies, in which the incidence of mortality was higher, and the sample size was larger. However, observational studies were prone to biases, although NOS indicates that the studies have low-moderate risk of bias, it is not as robust as the RCTs. The RCTs included in the pooled analysis pose no serious risk of bias except for one study which was open-label. There was serious risk of imprecision in the RCTs but not observational studies. Further RCTs in the hospitalized patients with larger samples are required.
Colchicine, an alkaloid derived from the plant family Colchicum autumnale or Gloriosa superba, contains both anti-viral and anti-inflammatory properties and therefore may be useful in treating severe and critically-ill COVID-19 patients.[5], [9], [10] Colchicine binds with tubulin to form complexes which disrupts neutrophil chemotaxis, adhesion, and mobilization, interferes superoxide generation, inflammasome activation, destabilization, and degradation, as well as TNF inhibition.[9], [13], [14], [18] One of the main mechanism is the inhibition of Nod-like receptor protein 3 (NLRP-3), an inflammasome associated with the severe COVID-19 infection and poor clinical outcome, which consequently leads to decreased in IL-1β and IL-18 concentrations.[5], [15] Initially, colchicine prevents purinergic type 2 (P2X7) receptor activation and ASC polymerization, thereby hindering interaction between pyrin-like domains, and then suppressing mitochondrial transport and subsequent approximation of apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) to NLRP3.[17]
Within the host cell, intracellular transport of viral particles assisted by microtubules and associated proteins is also hindered by colchicine. Viral load reduction results in a decrease in the secretion of pro-inflammatory cytokines, which contributes to soothe uncontrolled cytokine storm.[9] Furthermore, colchicine has a cardioprotective effect because it improves endothelial function and enhances the concentration of peripheral white blood cells.[13], [18] This can be useful considering that cardiovascular complications is a possibility for patients with COVID-19.[19], [20]
Patients with advanced age, obesity, frailty, and pre-existing non-communicable conditions, are associated with an increased risk of experiencing cytokine storm and developing severe COVID-19.[21], [22] These comorbidities generally cause a low-grade, chronic, systemic pro-inflammatory state.[23], [24], [25] Colchicine has been used in many hyperinflammatory conditions, including gout arthropathy, acute pericarditis, and periodic febrile illnesses.[14], [18] In COVID-19 cases, the use of colchicine was associated with a reduction in the concentration of inflammatory parameters, including CRP, D-dimer, ferritin and IL-6.[5], [9], [13], [14], [15] Therefore, colchicine can be a promising option for battling hyperinflammation of COVID-19, given that this medication is widely available at low cost and has a well-known safety profile with only mild side effects.[14], [15], [18] The most commonly observed adverse events were gastro-intestinal symptoms, such as diarrhea, vomiting, and abdominal pain.[13], [14], [16]
There are several limitations of this systematic review and meta-analysis. First is the small number of studies and the variety of dose regimens of colchicine, ranging from 0.5 to 1.5 mg per day. Secondly, there might be small-study effects because large studies are weighted in close approximation with the small study. Thirdly, there are studies with small number of events which might be underpowered.
There are a number of deviations from the protocol. First, subgroup analyses were performed for RCTs and observational studies, since the authors did not know whether subgroup analyses were possible considering the number of studies, these were not pre-specified. Secondly, we did not perform funnel-plot analysis or Egger’s test due to because there were less than ten studies. Thirdly, to provide a more specific and clear definition, we defined the control group into placebo or standard of care. Finally, we one of three RCTs was outpatient setting, at first the authors only wanted to evaluate hospitalized patients; however, COLCORONA (Tardif et al.) is an important trial with a large sample size. Thus, the authors decided to include the study and added a sensitivity analysis instead, both pooled effect estimates with or without COLCORONA inclusion demonstrate statistical significance.
In conclusion, this meta-analysis indicates that colchicine may reduce mortality in patients with COVID-19. Meta-regression analysis showed that the benefit was reduced as age increases.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
None.
Funding
None.
Ethical Approval
Not Applicable.
Informed Consent
Not Applicable.
Data Availability
Data are available on reasonable request
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2021.107723.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Huang I., Pranata R., Lim M.A., Oehadian A., Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yonas E., Alwi I., Pranata R., Huang I., Lim M.A., Yamin M., Nasution S.A., Setiati S., Virani S.S. Elevated interleukin levels are associated with higher severity and mortality in COVID 19 – A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. Clin. Res. Rev. 2020;14:2219–2230. doi: 10.1016/j.dsx.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim M.A., Pranata R., Huang I., Yonas E., Soeroto A.Y., Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can. J. Kidney Heal. Dis. 2020;7 doi: 10.1177/2054358120938573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Purandare B., Rajhans P., Jog S., Dalvi P., Prayag P., Marudwar P., Godavarthy P., Dhundi U., Pawar H.S., Pawar B., Mahale N., Narasimhan V.L., Oak G., Marreddy S., Bedekar A., Akole P., Bhurke B., Chavan S., Telbhare V., Diwane D., Shahane M., Prayag A., Gugale S., Bhor S. A retrospective observational study of hypoxic COVID-19 patients treated with immunomodulatory drugs in a tertiary care hospital. Indian J. Crit. Care Med. 2020;24:1020–1027. doi: 10.5005/jp-journals-10071-23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.M. Alejandro Pinzón, C. Medellin Doris Cardona Arango, J. Felipe Betancur, C. Arias Arias, B. Javier Muñoz, J. Felipe Llano Clínica Medellín Pablo Montoya, Clinical outcome of patients with COVID-19 pneumonia treated with corticosteroids and colchicine in colombia, Res. Sq. (2020) 1–12. 10.21203/rs.3.rs-94922/v1. [DOI] [PMC free article] [PubMed]
- 6.Hui D.S., Lee N., Chan P.K., Beigel J.H. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res. 2018;150:202–216. doi: 10.1016/j.antiviral.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siemieniuk R.A.C., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Pardo-Hernandez H., Rochwerg B., Lamontagne F., Han M.A., Kum E., Liu Q., Agarwal A., Agoritsas T., Alexander P., Chu D.K., Couban R., Darzi A., Devji T., Fang B., Fang C., Flottorp S.A., Foroutan F., Heels-Ansdell D., Honarmand K., Hou L., Hou X., Ibrahim Q., Loeb M., Marcucci M., McLeod S.L., Motaghi S., Murthy S., Mustafa R.A., Neary J.D., Qasim A., Rada G., Bin Riaz I., Sadeghirad B., Sekercioglu N., Sheng L., Switzer C., Tendal B., Thabane L., Tomlinson G., Turner T., Vandvik P.O., Vernooij R.W.M., Viteri-García A., Wang Y., Yao L., Ye Z., Guyatt G.H., Brignardello-Petersen R. Drug treatments for covid-19: Living systematic review and network meta-Analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunetti L., Diawara O., Tsai A., Firestein B.L., Nahass R.G., Poiani G., Schlesinger N. Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19. J. Clin. Med. 2020;9:2961. doi: 10.3390/jcm9092961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della-Torre E., Della-Torre F., Kusanovic M., Scotti R., Ramirez G.A., Dagna L., Tresoldi M. Treating COVID-19 with colchicine in community healthcare setting. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells G., Shea B., O’Connell D., Peterson J. Ottawa Hosp. Res; Inst: 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, Ottawa. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 12.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopes M.I., Bonjorno L.P., Giannini M.C., Amaral N.B., Menezes P.I., Dib S.M., Gigante S.L., Benatti M.N., Rezek U.C., Emrich-Filho L.L., Sousa B.A.A., Almeida S.C.L., Luppino Assad R., Veras F.P., Schneider A., Rodrigues T.S., Leiria L.O.S., Cunha L.D., Alves-Filho J.C., Cunha T.M., Arruda E., Miranda C.H., Pazin-Filho A., Auxiliadora-Martins M., Borges M.C., Fonseca B.A.L., Bollela V.R., Del-Ben C.M., Cunha F.Q., Zamboni D.S., Santana R.C., Vilar F.C., Louzada-Junior P., Oliveira R.D.R. Beneficial effects of colchicine for moderate to severe COVID-19: A randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7 doi: 10.1136/rmdopen-2020-001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandhu T., Tieng A., Chilimuri S., Franchin G. A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe covid-19 infection. Can. J. Infect. Dis. Med. Microbiol. 2020;2020:1–9. doi: 10.1155/2020/8865954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarsi M., Piantoni S., Colombo E., Airó P., Richini D., Miclini M., Bertasi V., Bianchi M., Bottone D., Civelli P., Cotelli M.S., Damiolini E., Galbassini G., Gatta D., Ghirardelli M.L., Magri R., Malamani P., Mendeni M., Molinari S., Morotti A., Salada L., Turla M., Vender A., Tincani A., Brucato A., Franceschini F., Furloni R., Andreoli L. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann. Rheum. Dis. 2020;79:1286–1289. doi: 10.1136/annrheumdis-2020-217712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tardif J., Bouabdallaoui N., Allier P.L.L. Efficacy of Colchicine in Non-Hospitalized Patients with COVID-19. MedRxiv. 2021 [Google Scholar]
- 17.Deftereos S.G., Giannopoulos G., Vrachatis D.A., Siasos G.D., Giotaki S.G., Gargalianos P., Metallidis S., Sianos G., Baltagiannis S., Panagopoulos P., Dolianitis K., Randou E., Syrigos K., Kotanidou A., Koulouris N.G., Milionis H., Sipsas N., Gogos C., Tsoukalas G., Olympios C.D., Tsagalou E., Migdalis I., Gerakari S., Angelidis C., Alexopoulos D., Davlouros P., Hahalis G., Kanonidis I., Katritsis D., Kolettis T., Manolis A.S., Michalis L., Naka K.K., Pyrgakis V.N., Toutouzas K.P., Triposkiadis F., Tsioufis K., Vavouranakis E., Martinèz-Dolz L., Reimers B., Stefanini G.G., Cleman M., Goudevenos J., Tsiodras S., Tousoulis D., Iliodromitis E., Mehran R., Dangas G., Stefanadis C. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes A.Z., Hu K.A., Teperman J., Wampler Muskardin T.L., Tardif J.C., Shah B., Pillinger M.H. Anti-inflammatory therapy for COVID-19 infection: The case for colchicine. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-219174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 20.R.M. Inciardi, L. Lupi, G. Zaccone, L. Italia, M. Raffo, D. Tomasoni, D.S. Cani, M. Cerini, D. Farina, E. Gavazzi, R. Maroldi, M. Adamo, E. Ammirati, G. Sinagra, C.M. Lombardi, M. Metra, Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19), JAMA Cardiol. 5 (2020) 819–824. 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed]
- 21.J. July, R. Pranata, Prevalence of dementia and its impact on mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis, Geriatr. Gerontol. Int. 21 (2021) 172–177. 10.1111/ggi.14107. [DOI] [PubMed]
- 22.Pranata R., Supriyadi R., Huang I., Permana H., Lim M.A., Yonas E., Soetedjo N.N.M., Lukito A.A. The Association between chronic kidney disease and new onset renal replacement therapy on the outcome of COVID-19 patients: a meta-analysis. Clin. Med. Insights Circ. Respir. Pulm. Med. 2020;14 doi: 10.1177/1179548420959165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B., Meyer M. Body mass index and outcome in patients with COVID-19: a dose–response meta-analysis. Diabetes Metab. 2021;47 doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pranata R., Henrina J., Lim M.A., Lawrensia S., Yonas E., Vania R., Huang I., Lukito A.A., Suastika K., Kuswardhani R.A.T., Setiati S. Clinical frailty scale and mortality in COVID-19: A systematic review and dose-response meta-analysis: Clinical Frailty Scale in COVID-19. Arch. Gerontol. Geriatr. 2021;93 doi: 10.1016/j.archger.2020.104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuty Kuswardhani R.A., Henrina J., Pranata R., Anthonius Lim M., Lawrensia S., Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin Res. Rev. 2020;14:2103–2109. doi: 10.1016/j.dsx.2020.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request