Abstract
Background
Treatment of recurrent, unresectable granulosa cell tumor (GCT) of the ovary can be challenging. Given the rarity of the tumor, alternative therapies have been difficult to evaluate in large prospective clinical trials. Currently, to our knowledge, there are no reports of the use of immune checkpoint inhibitors in GCT patients. Here, we present a case series of GCT patients treated with pembrolizumab who were enrolled in a phase II basket trial in advanced, rare solid tumors (Clinicaltrials.gov: NCT02721732).
Cases
We identified 5 patients with recurrent GCT (4 adult and 1 juvenile type); they had an extensive history of systemic therapy at study enrollment (range, 3–10), with most regimens resulting in less than 12 months of disease control. Pembrolizumab was administered in these patients, as per trial protocol. Although there were no objective responses according to the irRECIST guidelines, 2 patients with adult-type GCT experienced disease control for ≥ 12 months (565 and 453 days). In one, pembrolizumab represented the longest duration of disease control compared to prior lines of systemic therapy (565 days vs 13 months). In the other, pembrolizumab was the second longest systemic therapy associated with disease control (453 days vs 22 months) compared to prior lines of therapy. In this patient, pembrolizumab was discontinued following withdrawal of consent. PD-L1 expression was not observed in any baseline tumor samples. Pembrolizumab was well tolerated, with no grade 3 or 4 treatment-related adverse events.
Conclusions
Although our results do not support the routine use of pembrolizumab monotherapy in unselected GCT patients, some patients with adult-type GCT may derive a clinical benefit, with a low risk of toxicity. Future studies should investigate the role of immunotherapy and predictors of clinical benefit in this patient population.
Keywords: granulosa cell tumor of the ovary, pembrolizumab, immunotherapy, case report
Introduction
Granulosa cell tumors (GCTs) of the ovary represent 2%-5% of all ovarian neoplasms but comprise the majority (70%) of sex cord-stromal tumors[1, 2]. Given that most GCTs present at an early stage, intervention with tumor cytoreduction and surgical staging, with or without systemic adjuvant therapy, can be curative in many cases[3]. GCTs are classified as adult or juvenile type[3, 4]. Despite the favorable prognosis of adult-type GCT, recurrences are not uncommon, with relapses occurring even after 10 years[3–6]. In contrast, juvenile-type GCT tends to occur in younger patients and is typically more aggressive, with earlier recurrences[3, 5]. Regardless of the GCT type, cytoreductive surgery continues to be the mainstay of treatment for recurrent disease that is resectable with the addition of systemic chemotherapy, hormonal therapy, or radiation therapy[3, 7]. Unfortunately, patients with recurrent disease may require multiple surgical interventions and lines of systemic therapy, and responses to current systemic treatments are variable and limited[3, 6, 7]. Most patients with relapsed disease ultimately die of their disease, prompting a search for novel treatment modalities[3, 6, 7].
Immune checkpoint inhibitors have emerged as a treatment option for patients with malignancies such as metastatic melanoma and non-small cell lung carcinoma because they result in durable responses[8, 9]. Malignant cells may evade normal immune checkpoints through the programmed cell death protein-1 (PD-1)/programmed cell death ligand-1 (PD-L1) pathway that downregulates cytotoxic T-cell activity, thus favoring tolerance and tumor growth. As an anti-PD-1 monoclonal antibody, pembrolizumab can inhibit this immune escape pathway. In addition, pembrolizumab has demonstrated impressive clinical responses in microsatellite instability-high or mismatch repair-deficient tumors[10]. Given the clinical benefit, the US Food and Drug Administration (FDA) approved pembrolizumab for microsatellite instability-high/mismatch repair-deficient tumors that had not responded to prior treatment in May 2017; this represents the first tissue-agnostic indication for drug approval. This new indication highlights that pembrolizumab’s anti-tumor effect may also benefit patients with other uncommon tumors.
Given the rarity of GCT, alternative therapies have been difficult to evaluate in large prospective clinical trials, and to our knowledge, no reports exist of immune checkpoint inhibitor use in these patients. We report a case series of 5 heavily pre-treated patients with recurrent GCT who received single-agent pembrolizumab as part of a phase II basket trial for patients with advanced rare malignancies.
Cases
Clinical trial design
We evaluated 5 patients with recurrent GCT who were enrolled in cohort 10 (“other rare tumor histologies” category) of an open-label, phase II basket Clinicaltrials.gov: (NCT02721732) at The University of Texas MD Anderson Cancer Center (Houston, Texas). In brief, this phase II trial examined the clinical efficacy and safety of single-agent pembrolizumab (200 mg IV every 3 weeks) in 10 pre-specified cohorts of advanced, rare tumors, regardless of PD-L1 status. Details of the trial design have been reported elsewhere[11], All trial patients had PD-L1 (H-score ≥ 42.5 denoted positivity) and tumor-infiltrating lymphocyte (TIL) characterization of tumor tissue that was correlated with treatment response, as described previously[11].
Treatment response was evaluated using Immune-related Response Evaluation Criteria in Solid Tumors (irRECIST) guidelines on serial radiologic imaging at baseline, every 9 weeks for the first 6 months, and then every 12 weeks at the discretion of the investigator. Safety and tolerability were assessed by characterizing and grading adverse events using the National Cancer Institute Common Terminology Criteria for Adverse Events v403. This study received FDA and institutional review board approval, and all patients provided informed consent prior to enrollment.
The overall results of the phase II study and small cell neuroendocrine carcinoma of the female genital tract have been reported elsewhere[11, 12], In this case series, we report the outcomes of patients with relapsed GCT who had been treated with pembrolizumab monotherapy.
Patient characteristics
During the enrollment period of August 15, 2016–July 27, 2018, 5 patients with GCT of the ovary met the study inclusion criteria and provided informed consent to participate in the phase II trial. The baseline clinical and tumor characteristics of the 5 patients are shown in Table 1. Patients’ ages ranged from 23 to 73 years, and most (4 of 5) had adult-type GCT. At baseline prior to treatment initiation, all patients had recurrent disease; 4 had disease contained within the abdominal or pelvic cavity, and the remaining patient (patient 2) also had lung metastases. Patients’ treatment histories prior to pembrolizumab administration are shown in Table 2. They underwent a median of 2 (range, 2–5) and 7 (range, 3–10) prior cytoreductive surgeries and systemic therapies, respectively. The treatments with the longest duration of disease control after the first disease recurrence were carboplatin and paclitaxel, followed by anastrozole maintenance (9 months), single-agent anastrozole (13 months), carboplatin/docetaxel (52 months), carboplatin/taxane (8 months), and endoxifen (22 months) for patients 1–5, respectively. For patients 1, 3, and 4, these aforementioned systemic therapies were administered after optimal secondary tumor cytoreductive surgeries. Otherwise, the majority of these patients’ prior regimens resulted in less than 12 months of disease control.
Table 1.
Baseline clinical and tumor characteristics
| Patient | Age (years) | GCT type | Years since initial diagnosis | ECOG PS | FIGO stage1 | Metastatic sites2 | PD-L1 H-score | TIL infiltration |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | Adult | 16 | 1 | IC1 | A, P | 0 | 3 |
| 2 | 38 | Adult | 12 | 0 | I | A, L, P | 0 | 1 |
| 3 | 73 | Adult | 26 | 1 | IC | H, I, P, S | N/A3 | N/A3 |
| 4 | 23 | Juvenile | 2 | 1 | IIA | A, P | 0 | 1 |
| 5 | 70 | Adult | 8 | 1 | IIB | H, P | 0 | 2 |
GCT = granulosa cell tumor, ECOG PS = Eastern Cooperative Oncology Group Performance Status, FIGO = International Federation of Gynecology and Obstetrics, PD-L1 = programmed cell death ligand-1, TIL = tumor-infiltrating lymphocyte, I = intestinal metastases, A = adenopathy, P = peritoneal metastases, L = lung metastases, H = hepatic metastases, S = splenic metastases, N/A = not applicable.
Percentage and intensity of PD-L1 membrane staining on a scale from 0 to 300, with ≥ 42.5 defined as the threshold for positivity of PD-L1. TIL = intensity of TILs within tumor nests on a scale of 0 to 3; 0 = absence of TILs, 1 = low number of TILs, 2 = moderate number of TILs, 3 = high number of TILs.
FIGO 2015 staging.
Sites of metastatic disease prior to treatment.
Patient 3 had no tumor present in the tissue specimen for PD-L1 and TIL evaluation.
Table 2.
Prior treatments before pembrolizumab treatment
| Patient | Prior RT | No. of prior cytoreductive surgeries | No. of prior systemic therapies | Prior systemic therapies1 |
|---|---|---|---|---|
| 1 | Yes | 5 | 7 | 1 – Letrozole2 2 – Carboplatin/paclitaxel, followed by anastrozole maintenance* 3 – Bevacizumab 4 – Leuprolide 5 – Tamoxifen 6 – Carboplatin/paclitaxel 7 – Lurbinectedin |
| 2 | No | 2 | 7 | 1 – Carboplatin/paclitaxel2 2 – Carboplatin/paclitaxel/bevacizumab 3 – Bleomycin/etoposide/cisplatin 4 – Anastrozole* 5 – Leuprolide 6 – Megestrol 7 –Megestrol/tamoxifen |
| 3 | No | 4 | 4 | 1 – Carboplatin/docetaxel* 2 – Carboplatin/paclitaxel 3 – Anastrozole 4 – Lurbinectedin |
| 4 | No | 2 | 3 | 1 – Bleomycin/etoposide/cisplatin with exemestane2 2 – Carboplatin/paclitaxel followed by carboplatin/docetaxel* 3 – Lurbinectedin |
| 5 | No | 2 | 10 | 1 – Carboplatin/paclitaxel2 2 – Carboplatin/paclitaxel 3 – Letrozole 4 – Leuprolide 5 – Bevacizumab 6 – Endoxifen* 7 – Temozolomide/methoxyamine 8 – Ixazomib/vorinostat 9 – Tamoxifen 10 – Lurbinectedin |
RT = radiation therapy.
Lines of systemic therapy are ordered chronologically.
Postoperative adjuvant therapy following surgical staging.
Longest duration of disease control following first disease recurrence.
Treatment response and treatment-related adverse events
All patients were evaluable using irRECIST criteria; Figs. 1 and 2 demonstrate the greatest percentage changes in target tumor lesions and dynamic tumor volume changes over time, respectively. Patients 2 and 5 had stable disease as their best objective response, while patients 1, 3, and 4 had progressive disease, resulting in a clinical benefit rate of 40% (2 of 5). Patient 2 had stable disease after 3 cycles of treatment and derived clinical benefit for a duration of 565 days, until disease progression after cycle 25. Patient 5 had stable disease after 3 cycles of treatment and derived clinical benefit from pembrolizumab for a duration of 453 days, until withdrawal of consent after cycle 22. Patients 1, 3, and 4 all had progressive disease that was identified at the first on-treatment imaging scan after 3 cycles of pembrolizumab.
Fig 1:
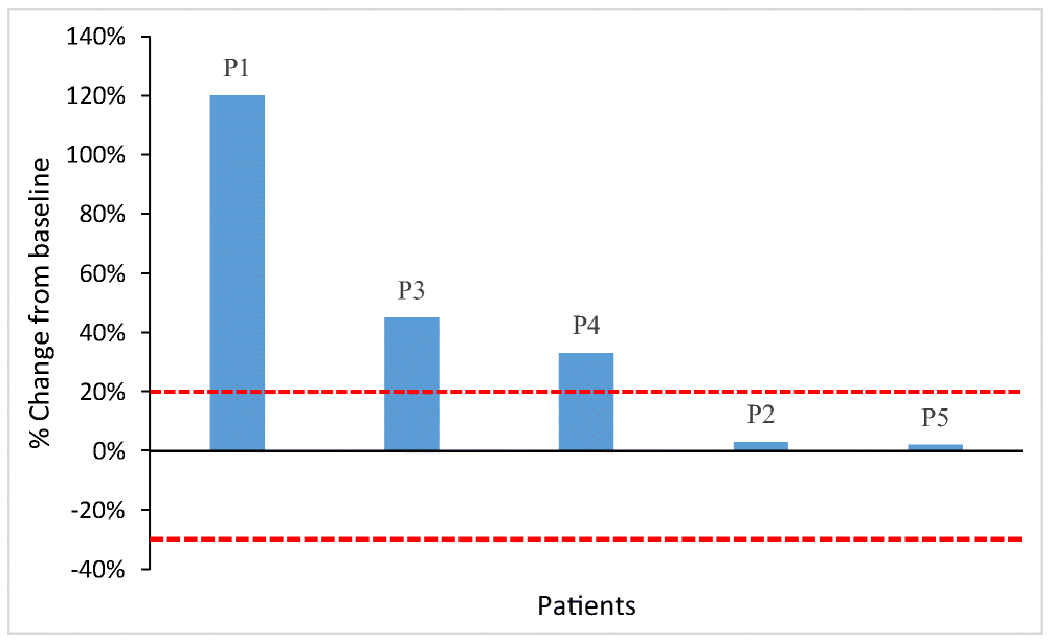
Radiologic response to pembrolizumab in patients with granulosa cell tumor of the ovary. Waterfall plot illustrating the best objective response to pembrolizumab in the 5 patients (P1—5) using irRECIST criteria. Each bar represents a patient and shows the maximum percentage change from the baseline in the sum of the longest diameters of all target lesions and any new lesions while on pembrolizumab. The area above the upper red dotted line represents progressive disease (≥ 20% increase in the sum of the diameters of the target lesions compared with the baseline). The area between the upper and lower red dotted lines represents stable disease.
Fig 2:
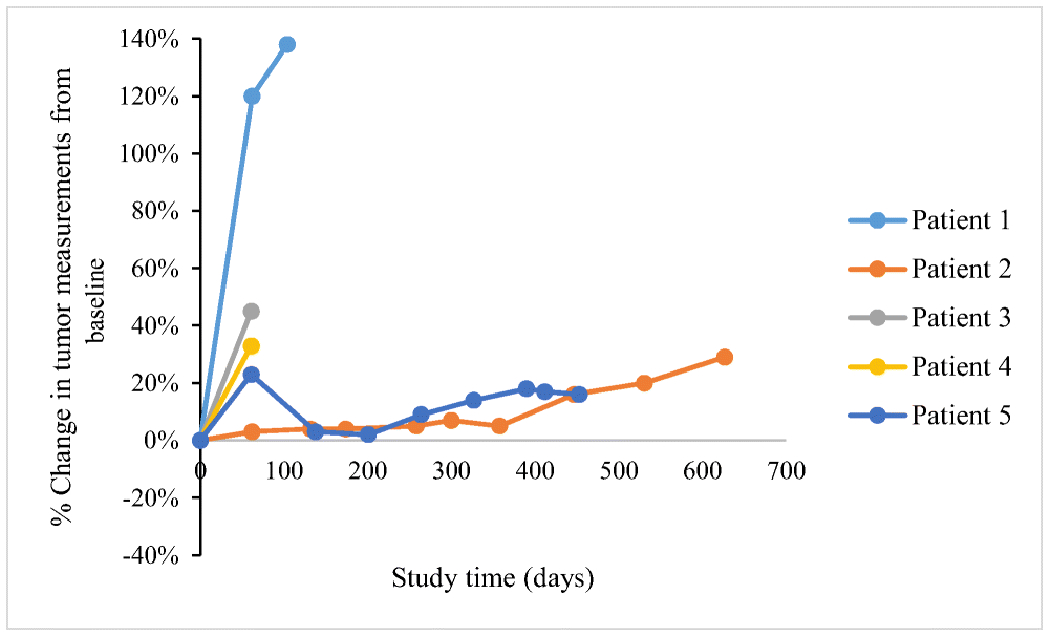
Tumor response according to irRECIST criteria over time. This spider plot demonstrates the tumor measurements from the baseline using irRECIST criteria during the course of treatment with pembrolizumab in the 5 patients. Patients 1, 3, and 4 experienced disease progression as their best objective response, while patients 2 and 5 had stable disease. All patients experienced disease progression prior to stopping treatment except for patient 5, who withdrew consent.
Three of the 5 patients experienced 1 or more treatment-related adverse events of any grade; none were grade 3 or 4. Two experienced immune-related adverse events: patient 2 had a grade 1 maculopapular rash that resolved after 47 days, and patient 5 had grade 2 hypothyroidism and required daily thyroid supplementation. Other non-immune treatment-related adverse events included fatigue (n = 1; patient 2), hyperthyroidism (n = 1; patient 2), nausea (n = 1; patient 4), and pruritus (n = 1; patient 5). Although unrelated to pembrolizumab therapy, a grade 3 hematoma from a tumor developed in patient 5 and resulted in a subsequent withdrawal of consent for further study participation.
PD-L1, TIL scoring, and somatic tumor mutations
Table 1 lists the PD-L1 H-scores and TIL scores of the 5 patients. One patient (patient 3) was not evaluable for PD-L1 H-scoring or TIL infiltration because of the absence of identifiable tumor cells on the specimen. For the remaining 4 patients, PD-L1 staining was absent (H-score of 0) and TIL infiltration was present in all baseline specimens. Patient 1 had a high number of TILs, patient 5 had a moderate number, and patients 2 and 4 had a low number.
Somatic tumor mutation gene panel results were available for 4 patients. Patients 1, 3, and 5 had FoxL2 (c.402C>G p.C134W) mutations. In addition, patient 1 had an ATM (c.1010G>A p.R337H) mutation, while patient 5 had an NFI mutation (c.2158C>T p.R720W) and patient 2 had TP53 mutations (c.733G>C p.G245R and c.490A>G p.K164E). Patient 4 had no detectable somatic mutations, and patient 3 did not undergo somatic tumor mutation gene panel testing.
Discussion
We present the first reported findings of the use of pembrolizumab monotherapy in recurrent GCT. Although no objective responses were seen among the 4 adult-type GCTs and 1 juvenile-type GCT, we observed stable disease, according to irRECIST criteria, in 2 patients that lasted for more than 12 months (patient 2: 565 days and patient 5: 453 days). Although the clinical benefit of pembrolizumab in these 2 patients may be overestimated because of the indolent nature of adult-type GCT, an examination of these patients’ prior systemic therapies in the recurrence setting may provide context. Most prior lines of systemic therapy received in the recurrent setting in adult-type GCT patients resulted in a duration of disease control of less than 12 months. Furthermore, in patient 2, pembrolizumab provided a greater duration of disease control than did the best prior line of systemic therapy: single-agent anastrozole (565 days vs. 13 months, respectively). For patient 5, although the longest duration of disease control was 22 months with endoxifen, the other lines of therapy were not as beneficial, with the second longest duration of disease control being leuprolide (9 months). In patient 5’s case, it is possible that pembrolizumab would have had an even longer duration of disease control, but treatment ended prematurely because of an unrelated grade 3 hematoma from a tumor bleed.
Interestingly, the 2 patients who had durable disease control also experienced immune-related adverse events that were attributable to pembrolizumab (maculopapular rash and hypothyroidism). This association between immune-related adverse events and relatively improved treatment response is suggestive of an active immune system, and this observation has been similarly reported in multiple studies[13–15]. Furthermore, pembrolizumab had low toxicity in patients with no grade 3 or 4 treatment-related adverse events.
The overall lack of objective anti-tumor response to pembrolizumab may be attributable to the absence of PD-L1 expression in the tumor microenvironment, as evidenced by the PD-L1 H-score of 0 in 4 of 5 patients’ tumor samples (the remaining tumor sample being non-evaluable). With multiple definitions of PD-L1 positivity in the literature, PD-L1 status has been described as a predictive biomarker of response to PD-1 inhibitor monotherapy[16–18]. Although the presence of TILs has also been associated with improved response to PD-1 inhibitors, the absence of PD-L1 expression may explain the lack of anti-tumor response to pembrolizumab in patient 1, who had the highest TIL infiltration[17, 18], In addition, Mills et al. recently demonstrated that a minority of GCT patients had PD-L1 expression or TIL infiltration; the authors suggested that immune checkpoint inhibitors have a minimal role in the disease[19]. It should be noted, however, that the absence of PD-L1 expression does not preclude anti-PD-1 therapy, as the combination of CTLA4 and PD-1 inhibitors demonstrated a synergistic therapeutic response, independent of PD-L1 status[20, 21].
Somatic FOXL2 mutations were present in 3 of the 4 adult-type GCT patients (the remaining patient had no available somatic mutation gene panel information). As a member of the forkhead transcription factor, FOXL2 plays an integral role in normal granulosa cell development [22], The detection of the FOXL2 (c.402C>G p.C134W) mutation in these patients is to be expected, as it is present in nearly all adult-type GCTs; this mutation is thought to be a driver of malignancy development and recurrence[23, 24], Patient 5 had 2 TP53 mutations: 1 pathogenic (c.733G>C p.G245R) and the other a variant of unknown significance (c.490A>G p.K164E). Along with TERT and inactivated KMT2D/MLL2 mutations, pathogenic TP53 mutations have been implicated in relapsed adult-type GCT[25–27], Roze et al. performed whole-genomic sequencing on tumor samples from 33 patients and found pathogenic TP53 mutations in tumors with the highest tumor mutational burden[26, 27]. Higher tumor mutational burden has been associated with a greater response to immune checkpoint inhibitors and may have been a contributing factor to disease control in patient 5[28]. Unfortunately, these patients did not undergo tumor mutational burden or MSI testing. Other somatic mutations detected in patients 2 (ATM) and 5 (NF1) were variants of unknown significance and are unlikely to be implicated in the response to pembrolizumab.
Relapsed GCT that is unresectable or metastatic continues to pose therapeutic challenges and has prompted a search for alternative treatments[3, 7]. The field of immunotherapy has revolutionized cancer therapeutics and has brought clinically durable treatment responses in multiple tumor types that have been refractory to conventional therapy[8, 9]. In gynecologic cancers, immune checkpoint inhibitors have led to improved treatment responses, resulting in FDA approval for the use of pembrolizumab for MSI-H endometrial cancer and PD-L1-positive cervical cancers. Furthermore, a recent trial of ipilimumab (a cytotoxic T-lymphocyte-associated protein 4 [CTLA4] inhibitor) and nivolumab (a PD-1 inhibitor) has demonstrated promising objective responses in patients with recurrent epithelial ovarian cancer (especially clear cell histologic type), irrespective of PD-L1 status[20]. Although the scope of the benefit of immune checkpoint inhibitors has expanded to include larger numbers of gynecologic malignancies, immunotherapy efficacy has been difficult to evaluate in GCT because of the rarity of the tumor.
Current treatment strategies for GCT are heterogeneous, including chemotherapeutic regimens (carboplatin/paclitaxel, BEP [bleomycin, etoposide, and cisplatin], CAP [cyclophosphamide, doxorubicin, and cisplatin], VBP [vinblastine, bleomycin, and cisplatin]), hormonal therapy (progestins, aromatase inhibitors, and gonadotropin-releasing hormone agonists), and radiation therapy with variable results, especially when combined with cytoreductive surgery[3, 7]. Vascular endothelial growth factor has been shown to be highly expressed on GCT tumors and has prompted the use of angiogenesis inhibitors (e.g., bevacizumab)[29–31]. In a phase II Gynecologic Oncology Group cooperative trial of bevacizumab in 36 patients with sex cord-stromal tumors (mostly GCTs), the authors demonstrated partial response and stable disease rates of 16.7% and 77.8%, respectively[31]. The median progression-free survival duration was 9.3 months[31]. In the recently published ALIENOR/ENGOT-ov7 multicenter randomized control trial, the authors found that the addition of bevacizumab to weekly paclitaxel improved objective response rates (from 25% to 44%) in a cohort of sex-cord stromal tumors (most of which were adult-type GCT)[32]. However, it did not improve progression-free survival[32]. As treatment options are limited, other agents, such as lurbinectedin, a selective RNA polymerase II inhibitor, were used in 4 of the 5 patients in our study (3 adult-and 1 juvenile-type GCT) prior to study enrollment. However, the treatment duration was short (2-6 cycles) because of disease progression or withdrawal of consent. Overall, the effectiveness of these systemic therapies vary; thus, cytoreductive surgery (when feasible) to no residual disease continues to be the most effective treatment option for patients with recurrent GCT.
Despite the lack of objective responses observed in this trial, some adult-type GCT patients derived clinical benefit, with a disease control duration of greater than 12 months. Thus, a further investigation to determine more accurate predictive markers of response to immunotherapy is warranted. Currently, there is an ongoing, open-label phase II basket trial (SWOG 1609; NCT02834013) of ipilimumab and nivolumab in rare solid tumors (including GCT). The SWOG 1609 trial also includes a planned biomarker evaluation (including whole exome sequencing, RNA sequencing, and multiplex immune profiling); we eagerly await the trial results.
The strengths of our study include the evaluation of the use of pembrolizumab in a rare cohort of GCT patients, correlated with translational PD-L1 and TIL data. Furthermore, tumor responses were objectively measured in an independent review by experienced radiologists using irRECIST criteria.
The small sample size, although expected because of GCT’s rarity, is a limitation of this study. Furthermore, given the differing tumor biologic characteristics of adult-type and juvenile-type GCTs, the efficacy of pembrolizumab may not be generalizable. Juvenile-type GCT represents a minority of GCT cases (approximately 5%) and thus presents an even greater challenge when evaluating new therapies in prospective clinical trials.
In conclusion, we demonstrated that single-agent pembrolizumab is generally safe to use for GCT of the ovary. Although durable disease control may be observed, the routine use of pembrolizumab is unlikely to be effective at decreasing tumor burden in unselected patients with relapsed GCT, and more accurate biomarkers are needed to predict clinical benefit. Given the rarity of the tumor, genomic profiling of GCTs should be undertaken to identify pathways that can be targeted with novel investigational therapeutics.
Highlights.
Pembrolizumab can provide durable disease control in patients with recurrent adult granulosa cell tumors.
Pembrolizumab had an acceptable safety profile in patients with granulosa cell tumors.
In this cohort, granulosa cell tumors of the ovary had low expression of PD-L1.
Acknowledgements:
Sunita Patterson and Ann Sutton from the Editing Services, Research Medical Library at The University of Texas MD Anderson Cancer Center who provided editorial assistance. We thank the patients and their families and caregivers for participating in the study.
Funding: This work was supported by Merck MDS and the NIH/NCI under award numbers P30 CA016672 (supporting the MD Anderson Clinical Trials Office), P50 CA217685 (ovarian SPORE), and T32 CA101642 JH (T32 training grant). This work was also supported by the Dr. Henry R. Shibata Fellowship Award/Cedars Cancer Foundation (JH), and, in part, by grant CA217685, the Frank McGraw Memorial Chair in Cancer Research (AKS), grant 1P50 CA217685-01, and a Gynecologic Oncology Group Foundation Scholar Investigator Award (SNW).
Disclaimer: The views expressed in this manuscript are the authors’ own views and are not the official position of the institution or supporting funding sources.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethics approval and consent to participate: The protocol was approved by the US Food and Drug Administration (FDA) and the Institutional Review Board at The University of Texas MD Anderson Cancer Center. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. All of the study participants provided written informed consent before enrollment.
Consent for participation and publication: All of the study participants provided written informed consent prior to clinical trial enrollment.
Availability of data and material: Data are available upon reasonable request. The datasets used or analyzed during the current study are available from the corresponding author on reasonable request and approval from the study sponsor according to the guidelines that were available at the time of the request.
Competing interests:
Jeffrey How reports grants from the NIH (T32 CA101642) and the Cedars Cancer Foundation during the conduct of the study.
Amir Jazaeri reports personal fees from Gerson and Lehrman Group, Guidepoint, Iovance Advisory Board Meeting, Nuprobe, Simcere, and Pact Pharma; grants from AstraZeneca, BMS, Iovance, Aravive, Pfizer, Immatics USA, and Eli Lilly; and other funding from AstraZeneca outside of the submitted work.
Shannon N. Westin reports grants from the NIH and the GOG Foundation during the conduct of the study; grants and personal fees from AstraZeneca, Clovis Oncology, GSK/Tesaro, Roche/Genentech, and Novartis; personal fees from Merck, Pfizer, Eisai, CIrculogene, and Zentalis; and grants from Cotinga Pharmaceuticals, Bayer, and ArQule outside of the submitted work.
Anil K. Sood reports grants from the NIH (CA109298 and CA209904) during the conduct of the study and other funding from Merck, Biopath, Kiyatec, and M-Trap outside of the submitted work.
Vivek Subbiah reports grants and other funding from LOXO Oncology/Eli Lilly, Novartis, Pharmamar, and Medimmune; grants from Roche/Genentech, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwest Biotherapeutics, Berghealth, Incyte, Fujifilm, D3, Pfizer, Multivir, Amgen, Abbvie, Alfa-sigma, Agensys, Boston Biomedical, Idera Pharma, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, the National Comprehensive Cancer Network, NCI-CTEP, The University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, and Boston Pharmaceuticals; and other funding from Helsinn, R-Pharma US, Incyte, QED Pharma, ASCO, ESMO, and Medscape during the conduct of the study.
Jordi A. Rodon reports personal fees from Eli Lilly, Orion Pharmaceuticals, Peptomyc, Roche Pharmaceuticals, Ellipses Pharma, Certera, and Ionctura SA; personal fees and other funding from Kelun Pharmaceuticals/Klus Pharma, Novartis, Spectrum Pharmaceuticals, Inc., Pfizer, and Bayer; and other funding from European Journal of Cancer, VHIO/Ministero De Empleo Y Seguridad Social, Chinese University of Hong Kong, SOLTI, Elsevier, GlaxoSmithKline, ESMO, Department of Defense, Merck Sharp & Dohme, Louisiana State University, Huntsman Cancer Institute, Cancer Core Europe, Karolinska Cancer Institute, King Abdullah International Medical Research Center, WIN Consortium, Janssen, Tocagen, Symphogen, BioAlta, GenMab, CytomX, Kelun-Biotech, Takea-Millenium, and Ipsen outside of the submitted work.
Aung Naing reports grants from NCI, EMD Serono, MedImmune, Healios Onc. Nutrition, Atterocor, Amplimmune, ARMO BioSciences, Eli Lilly, Karyopharm Therapeutics, Incyte, Novartis, Regeneron, Merck, BMS, Pfizer, CytomX Therapeutics, Neon Therapeutics, Calithera Biosciences, TopAlliance Biosciences, Kymab, PsiOxus, and Immune Deficiency Foundation (spouse) and other funding from CytomX Therapeutics, Novartis, Kymab, Genome, and ARMO BioSciences outside of the submitted work.
Lois M. Ramondetta, Mingxuan Xu, Abdulrahman Abonofal, Daniel D. Karp, Bettzy Stephen, and Fei Yang have no conflicts of interest to disclose.
References
- 1.Schumer ST and Cannistra SA, Granulosa cell tumor of the ovary. J Clin Oncol, 2003. 21(6): p. 1180–9. [DOI] [PubMed] [Google Scholar]
- 2.Malmström H, et al. , Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecol Oncol, 1994. 52(1): p. 50–5. [DOI] [PubMed] [Google Scholar]
- 3.Levin G, et al. , Granulosa cell tumor of ovary: A systematic review of recent evidence. Eur J Obstet Gynecol Reprod Biol, 2018. 225: p. 57–61. [DOI] [PubMed] [Google Scholar]
- 4.Chen VW, et al. , Pathology and classification of ovarian tumors. Cancer, 2003. 97(10 Suppl): p. 2631–42. [DOI] [PubMed] [Google Scholar]
- 5.Young RH, Dickersin GR, and Scully RE, Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol, 1984. 8(8): p. 575–96. [DOI] [PubMed] [Google Scholar]
- 6.Mangili G, et al. , Long-term follow-up is crucial after treatment for granulosa cell tumours of the ovary. Br J Cancer, 2013. 109(1): p. 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurumurthy M, Bryant A, and Shanbhag S, Effectiveness of different treatment modalities for the management of adult-onset granulosa cell tumours of the ovary (primary and recurrent). Cochrane Database Syst Rev, 2014. 2014(4): p. Cd006912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topalian SL, et al. , Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med, 2012. 366(26): p. 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, et al. , Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med, 2010. 363(8): p. 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le DT, et al. , Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (New York, N.Y.), 2017. 357(6349): p. 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naing A, et al. , Phase 2 study of pembrolizumab in patients with advanced rare cancers. J Immunother Cancer, 2020. 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frumovitz M, et al. , Phase II study of pembrolizumab efficacy and safety in women with recurrent small cell neuroendocrine carcinoma of the lower genital tract. Gynecol Oncol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujii T, et al. , Incidence of immune-related adverse events and its association with treatment outcomes: the MD Anderson Cancer Center experience. Invest New Drugs, 2018. 36(4): p. 638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua C, et al. , Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol, 2016. 152(1): p. 45–51. [DOI] [PubMed] [Google Scholar]
- 15.Hosoya K, et al. , Association Between Early Immune-related Adverse Events and Clinical Outcomes in Patients With Non-Small Cell Lung Cancer Treated With Immune Checkpoint Inhibitors. Clin Lung Cancer, 2020. [DOI] [PubMed] [Google Scholar]
- 16.Taube JM, et al. , Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res, 2014. 20(19): p. 5064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardoll DM, The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer, 2012. 12(4): p. 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii T, et al. , Biomarkers of response to immune checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol, 2018. 130: p. 108–120. [DOI] [PubMed] [Google Scholar]
- 19.Mills AM, et al. , Emerging biomarkers in ovarian granulosa cell tumors. Int J Gynecol Cancer, 2019. 29(3): p. 560–565. [DOI] [PubMed] [Google Scholar]
- 20.Zamarin D, et al. , Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J Clin Oncol, 2020: p. Jco1902059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curran MA, et al. , PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A, 2010. 107(9): p. 4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocquet J, et al. , Evolution and expression of FOXL2. J Med Genet, 2002. 39(12): p. 916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SP, et al. , Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med, 2009. 360(26): p. 2719–29. [DOI] [PubMed] [Google Scholar]
- 24.Yanagida S, et al. , Clinical and genetic analysis of recurrent adult-type granulosa cell tumor of the ovary: Persistent preservation of heterozygous c.402C>G FOXL2 mutation. PLoS One, 2017. 12(6): p. e0178989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillman RT, et al. , KMT2D/MLL2 inactivation is associated with recurrence in adult-type granulosa cell tumors of the ovary. Nat Commun, 2018. 9(1): p. 2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Da Cruz Paula A, et al. , Genomic profiling of primary and recurrent adult granulosa cell tumors of the ovary. Mod Pathol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roze J, et al. , Whole Genome Analysis of Ovarian Granulosa Cell Tumors Reveals Tumor Heterogeneity and a High-Grade TP53-Specific Subgroup. Cancers (Basel), 2020. 12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman AM, et al. , Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther, 2017. 16(11): p. 2598–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao X, et al. , Anti-angiogenesis therapy with bevacizumab for patients with ovarian granulosa cell tumors. Gynecol Oncol, 2009. 114(3): p. 431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Färkkilä A, et al. , Vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 are highly expressed in ovarian granulosa cell tumors. Eur J Endocrinol, 2011. 164(1): p. 115–22. [DOI] [PubMed] [Google Scholar]
- 31.Brown J, et al. , Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: results of a phase 2 trial of the Gynecologic Oncology Group. Cancer, 2014. 120(3): p. 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray-Coquard I, et al. , Effect of Weekly Paclitaxel With or Without Bevacizumab on Progression-Free Rate Among Patients With Relapsed Ovarian Sex Cord-Stromal Tumors: The ALIENOR/ENGOT-ov7 Randomized Clinical Trial. JAMA Oncol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


