Abstract
Background:
Although head tremor (HT) and pain are prevalent in cervical dystonia (CD), their joint relationship to phenotypic features of focal dystonia remains unclear.
Objectives:
We examined how severity of HT and pain are associated with age of CD onset and duration, and whether HT subtypes (“jerky” or “regular”) exhibit distinct relationships between severity of HT and pain.
Methods:
The severity of HT and pain were assessed with the Toronto Western Spasmodic Torticollis Rating Scale in retrospective review of 188 CD patients recruited through the Dystonia Coalition.
Results:
HT severity was associated with longer CD duration (p < 0.0005), whereas pain severity was associated with younger age at onset (p = 0.043). HT severity and pain severity were not correlated for jerky HT (p = 0.996), but positively correlated for regular HT (p = 0.01).
Conclusions:
The distinct associations of HT and pain with age at onset, disease duration, and HT subtype further characterize the heterogeneity of CD’s clinical presentation and suggest similarly heterogeneous underlying mechanisms.
Keywords: head tremor, pain, cervical dystonia, dystonic tremor
Introduction
Two common characteristics of cervical dystonia (CD) are head tremor (HT) and pain, affecting up to 60% [1–5] and 90% [1, 6–9] of patients, respectively. HT [1, 7, 8] and pain [1, 6–15] have a substantial impact on overall CD severity, disability, and quality of life. However, the clinical course of HT and pain severity and the relationship between them remain unclear.
Age at onset is a key clinical characteristic in patients with isolated dystonias; generalized dystonias often have childhood or adolescent onset whereas focal dystonias usually have adult onset [16]. There is emerging evidence from natural history studies that some features, including HT and pain, vary with disease duration [1, 4, 17–20]. However, those studies have shown relationships between age at onset and disease duration and the presence of HT and pain, not their severity. Furthermore, they did not control for severity of both HT and pain. In addition, although previous research found that pain is more prevalent in CD patients with HT than without HT [2, 17], these prior studies did not take into account HT type. HT type can be characterized as either “jerky” or “regular” [21]. Jerky HT usually appears irregular, whereas regular HT appears sinusoidal [21]. Since the two HT types exhibit distinct kinematic properties [21], we hypothesized that they may differ in terms of their relationship with pain severity. More specifically, because of the biomechanical forces involved in the irregular muscle spasms associated with jerky HT, we hypothesized that pain severity would be positively correlated with HT severity for jerky but not regular HT patients.
The objectives of our study were twofold: first, to determine how the severity of HT and pain are related to age at onset and disease duration using analyses that account for both HT and pain; second, to determine the relationship between severity of pain and severity of HT subtypes.
Methods
We analyzed data on HT and pain collected from 208 patients with a clinical diagnosis of isolated cervical dystonia. Patients were enrolled across ten sites in a previous rating scale validation study of the Dystonia Coalition (https://clinicaltrials.gov/ct2/show/NCT01373424). The protocols for this study were approved by the Human Research Protection Offices at the Washington University School of Medicine (WUSM), Rush University Medical Center (RUMC), and the University of California, San Diego (UCSD; protocol 111255X). All patients provided informed consent prior to participation.
Clinical assessments and video recordings used for analyses were taken during a standard examination protocol between March 2011 and January 2013. All patients were assessed three or more months after their last BoNT injections, when much of the effect has dissipated. Age at onset was reported by patients. Movement disorder neurologists evaluated each patient for their predominant type and severity of HT. HT type was designated as “regular” or “jerky (irregular)” (referred to herein simply as “jerky”, as originally described in [22, 23]. This dichotomous descriptor was based on the complete examination. HT was deemed “regular” if it appeared sinusoidal and “jerky” if it appeared irregular. Severity of HT and pain were rated using the revised Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS-2, [12, 24]). HT severity, as with all of the other items on the TWSTRS-2 Motor Severity scale, was rated on a range of 0–4, with 0 = absent, 1 = slight, 2 = mild, 3 = moderate, and 4 = severe. Pain Severity was assessed using the TWSTRS-2 Pain scale. Pain severity was based on neck pain due to CD during the preceding week on a scale of 0–10 where 0 represents no pain and 10 represents the most excruciating pain imaginable. Patients were asked about the pain level at best, worst, and usual. Overall pain severity was computed from an average of these three values, with the “usual” pain severity being doubly weighted. Out of 208 patients, 20 patients were excluded due to missing data.
We used a multiple regression to evaluate the contribution of pain severity, age at onset, disease duration, and the interaction term between age at onset and disease duration to HT severity. We also used a multiple regression to evaluate the contribution of HT severity, age at onset, disease duration, and the interaction term between age at onset and disease duration to pain severity. We used a nonparametric Wilcoxon test to compare HT types (“jerky” and “regular”) in terms of their pain and HT severity. Lastly, we used linear regressions to evaluate the relationship between HT severity and pain severity, separately for each HT type. All statistical analyses were performed with John’s Macintosh Project (JMP [25]) with an alpha level of 0.05.
Results
The 188 patients included in this study had a median age of 60 (range 29–83) years at time of examination and consisted of 141 females (75%). The total TWSTRS-2 score, combining motor, disability, and pain, averaged 33 (range 5–61.75). Of this group, 118 (63%) had HT and 148 (79%) had pain.
HT severity was negatively correlated with age at onset (r(188) = −0.190, p = 0.009) (Fig. 1A), and positively correlated with disease duration (r(188) = 0.347, p < 0.0001) (Fig. 1B). Pain severity was negatively correlated with age at onset (r(188) = −0.188, p = 0.010) (Fig. 1C), but not correlated with disease duration (r(188) = 0.025, p = 0.735) (Fig. 1D).
Figure 1.
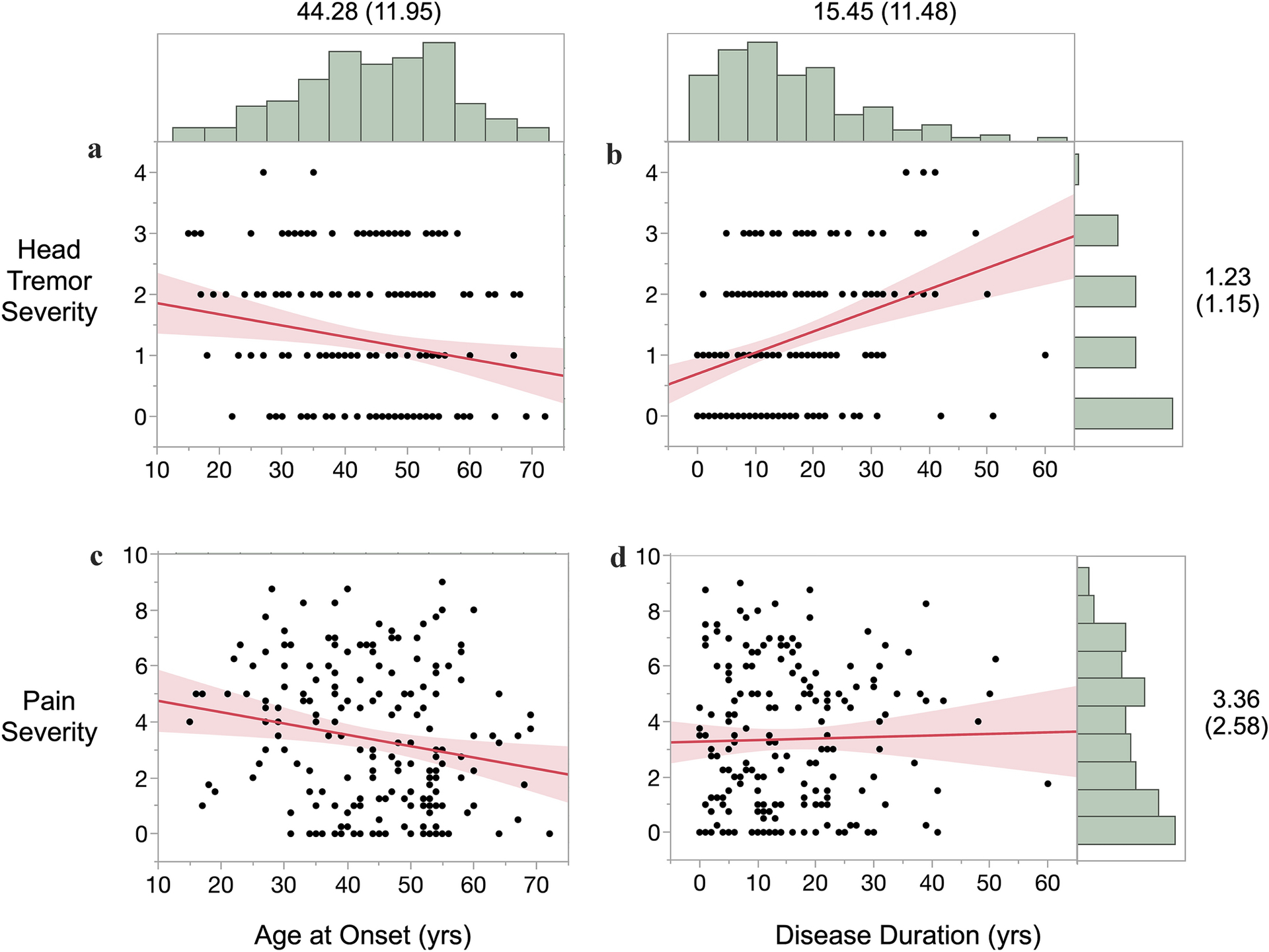
HT severity and pain severity as a function of CD age at onset and duration (marginal distributions labeled with mean (SD); shaded areas are 95% confidence intervals).
In a multiple regression, pain severity, age at onset, disease duration, and the interaction term of age at onset and disease duration were used to predict the severity of HT (F(4,187) = 6.64, p < 0.0001, R2adjusted = 0.11). Of those predictors, only disease duration was a significant predictor of HT severity (B = 0.044, t = 4.12, p < 0.0001). Similarly, HT severity, age at onset, disease duration, and the interaction term of age at onset and disease duration were used in a multiple regression to predict pain severity (F(4,187) = 2.52, p = 0.043, R2adjusted = 0.03). Only age at onset was a significant predictor of pain severity (B = −0.06, t = −3.11, p = 0.002).
Of 118 CD patients, the HT type was characterized as “regular” for 33 (28%) and as “jerky” for 85 (72%). The regular and jerky HT patients did not differ in terms of HT severity (means of 2.09 and 1.88, respectively, Z = 1.236, p = 0.215) or in terms of pain severity (means of 3.31 and 3.26, respectively; Z = 0.102, p = 0.919).
For patients with regular HT, pain severity was positively correlated with tremor severity (r(33) = 0.441, p = 0.010) (Fig. 2A). For patients with jerky HT, pain severity was not correlated with tremor severity (r(85) = −0.001, p = 0.996) (Fig. 2B). The correlations were significantly different between the two groups (2-sided Fisher’s z = 2.224, p = 0.026; Zou’s confidence interval = 0.0526 – 0.7631) [26].
Figure 2.
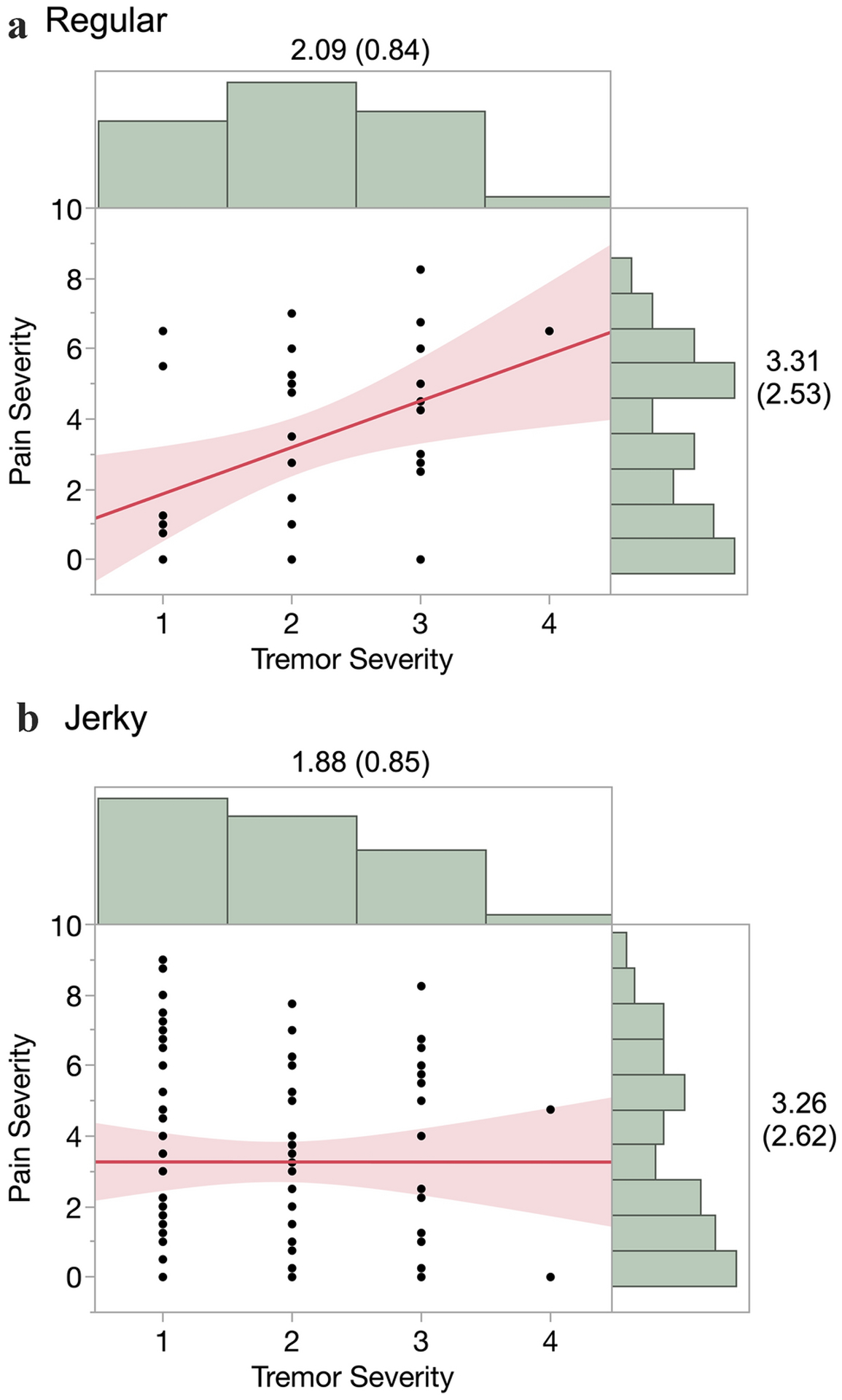
Pain severity as a function of HT severity for regular (A) and jerky (B) tremor types (marginal distributions labeled with mean (SD), N = 33 for Regular and N = 85 for Jerky, shaded areas are 95% confidence intervals).
Discussion
We found that HT severity was positively associated with CD duration but not with age at onset, whereas pain severity was inversely associated with age at onset but not disease duration. Thus, in models that account for both HT and pain, our analyses revealed a double dissociation between HT and pain in terms of how they vary with age at onset and disease duration. This dissociation suggests that the two relationships are independent of each other. Although in most cross-sectional studies, including ours, a younger age at onset is associated with a longer disease duration, our models implicitly account for this relationship by explicitly incorporating a term for the interaction of age at onset and disease duration. The effect sizes are modest, explaining a small percentage of the overall variance, especially for pain. Nevertheless, our sample size was sufficiently large to detect these statistically significant effects. Other factors likely contribute to the effects, such as variable intervals since last injection, comorbid anxiety and depression, and pain medication status. While it is commonly assumed that neck pain in patients with CD is secondary to motor features, it could also arise from sensory dysfunction, which is increasingly recognized in dystonia [27]. CD patients in particular exhibit altered descending pain control [28]. In addition, CD patients reporting pain are more likely to have an effective sensory trick than those who do not [19, 20]. To at least some degree independent of pain sensitivity, patients who are depressed or anxious are known to score higher on pain ratings [29]. Although numerous motor and non-motor aspects of CD outside the purview of this study likely contribute to patient reports of pain, our results suggest HT severity should be included in future studies.
Counter to our hypothesis, we found that pain severity was positively correlated with HT severity only for patients with regular HT, whereas no such association was present for patients with jerky HT. This difference in associations is present despite distributions of HT severity and pain severity that are nearly identical between the two HT types. Our results, at least for the “regular” HT patients, are consistent with Chan et al. [1], who found that pain in CD is strongly associated with the presence of spasms (“jerky movements or forced transient spasms of the head”) and the persistence and severity of head turning. However, their descriptions do not clearly map to current concepts of “jerky” vs. “regular” HT. Our study also builds upon a more recent report using the Italian Dystonia Registry [19] that found no relationship between the presence of HT and the reported presence of pain but did not investigate the relationship between the two phenomena in terms of severity and did not distinguish between HT subtypes. The reasons for the marked difference between the two groups are unclear. Perhaps pain severity depends on the proportion of time patients exert effort to compensate for and control HT. Is the tremor present all day long or only for brief periods? How does it depend on moment-to-moment position? How frequently do patients assume these positions in their daily living? Our results suggest that patients with regular HT exhibit a broader spectrum of proportion of time HT is present than patients with jerky HT. On the other hand, perhaps the two subtypes of HT reflect differential involvement of the cerebellum. CD patients with HT (but not patients without HT) exhibit pathological eyeblink classical conditioning [30], proprioceptive acuity [31], and motor perception [32], all consistent with a cerebellar role in HT [33]. To address these questions, it would be valuable to have more objective measures of HT. Ultimately digital technologies including video-based head pose estimation will enable HT type and severity measures that are not only truly objective but also convenient for more frequent or even continuous use in patients’ daily lives.
This study has limitations. First, age at onset and therefore calculated disease duration are based on self-report and are therefore susceptible to recall bias. CD patients may be more likely to report age at onset earlier if HT is their initially presenting symptom, because HT may be more visible than mild postural abnormalities. Conversely, age at onset may be reported later if it is associated with a delayed diagnosis, which is more common in CD with HT as an initial symptom. Second, quantifying HT severity is difficult. Traditional clinical rating scales such as the TWSTRS-2 are based on human judgement and are therefore inherently subjective. This always raises concerns about the validity of the measure. The HT item in the TWSTRS-2 does not correlate with total motor severity [12]. However it does exhibit excellent inter- and intra-rater reliability (ICCs = 0.77 and 0.89, respectively; [34]). Thus although HT may be a phenomenon that is relatively distinct from other aspects of CD, the assessment of its severity with the TWSTRS-2 is reliable. Third, inter-rater agreement for distinguishing “regular” and “jerky” types of HT has not yet been assessed and may be poor. Characterization of HT remains a matter of debate among movement disorders neurologists. It may be an over-simplification to have the type of HT dichotomized into “regular” and “jerky”, as it is certainly possible that individual patients may have features of both, e.g. some patients might have jerks superimposed on top of more regular tremor or transient jerkiness might appear when the patients attempt to actively counter pulling muscles setting tonic abnormal posture. This is but one reason why HT subtyping is an active area of research [21]. In our study, the raters making these assessments were not given detailed operational guidelines by which to make this dichotomous determination. Instead, they were asked to simply provide a gross assessment based on their judgement as movement disorders neurologists with extensive experience in dystonia. Fourth, pain is difficult to quantify from patient report and likely reflects a multi-factorial symptom. Although we found statistically significant effects for the demographic and HT contributions to pain severity, our findings should be viewed as having limited clinical significance because other factors outside the scope of this study must play a substantial role. Finally, our cohort of CD patients may be more severe and have longer disease duration than the broader population of CD because all recruiting was through tertiary academic care centers.
In summary, we found that patients with longer disease duration are more likely to have more severe HT and that patients with earlier age at onset are more likely to have more severe pain. This suggests that they may arise from at least partially independent mechanisms. Our findings of relationships between HT severity and pain severity that differ depending on the type of HT also contribute to the growing body of evidence for distinct CD subtypes based on their HT phenomenology. Future studies trying to clarify the complex relationship between CD and tremor should go beyond somatotopic distribution of symptoms [35] to incorporate the clinical course and subtype of HT.
Acknowledgments
We gratefully acknowledge Laura Wright and Matt Hicks (WUSM) for assistance with providing data access.
This research was conducted via the Dystonia Coalition, which is part of the Rare Diseases Clinical Research Network, an initiative funded by the Office of Rare Diseases Research at the National Center for Advancing Translational Sciences (U54 TR001456) in collaboration with the National Institute of Neurological Disorders and Stroke (U54 NS065701 and U54 NS116025) at the National Institute of Health (NIH). This work was also supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Peer Reviewed Medical Research Program under Award No. W81XWH-17-1-0393. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest/Competing interests: Not applicable
Original data available from the Dystonia Coalition upon reasonable request.
Contributor Information
Elizabeth Cisneros, Institute for Neural Computation, University of California, San Diego, La Jolla, CA, USA.
Richard L Barbano, Department of Neurology, University of Rochester, Rochester, NY, USA.
Christopher G Goetz, Department of Neurological Sciences, Rush University Medical Center, Chicago, IL, USA.
Joseph Jankovic, Parkinson’s Disease Center and Movement Disorders Clinic, Department of Neurology, Baylor College of Medicine, Houston, TX.
Hyder A Jinnah, Departments of Neurology and Human Genetics, Emory University, Atlanta, GA.
Joel S Perlmutter, Department of Neurology, Washington University School of Medicine, St. Louis, MO; Departments of Radiology, Neuroscience, Physical Therapy, and Occupational Therapy, Washington University School of Medicine, St. Louis, MO.
Brian D Berman, Department of Neurology, Virginia Commonwealth University, Richmond, VA, USA.
Mark I Appelbaum, Department of Psychology, University of California, San Diego, La Jolla, CA, USA.
Cynthia L Comella, Department of Neurological Sciences, Rush University Medical Center, Chicago, IL, USA.
David A Peterson, Institute for Neural Computation, University of California, San Diego, La Jolla, CA, USA; Computational Neurobiology Laboratory, Salk Institute for Biological Studies, La Jolla, CA.
References
- 1.Chan J, Brin MF, Fahn S (1991) Idiopathic cervical dystonia: Clinical characteristics. Mov Disord 6:119–126. 10.1002/mds.870060206 [DOI] [PubMed] [Google Scholar]
- 2.Godeiro C, Felicio AC, Aguiar PC, et al. (2008) Head tremor in patients with cervical dystonia: Different outcome? Arq Neuropsiquiatr 66:805–808. 10.1590/S0004-282X2008000600005 [DOI] [PubMed] [Google Scholar]
- 3.Chandran V, Pal PK, Reddy JYC, et al. (2012) Non-motor features in essential tremor. Acta Neurol Scand 125:332–337. 10.1111/j.1600-0404.2011.01573.x [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Vu JP, Cisneros E, et al. (2020) Postural Directionality and Head Tremor in Cervical Dystonia. Tremor Other Hyperkinet Mov (N Y) 10:. 10.7916/tohm.v0.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris SA, Jinnah HA, Espay AJ, et al. (2016) Clinical and demographic characteristics related to onset site and spread of cervical dystonia. Mov Disord 31:1874–1882. 10.1002/mds.26817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skogseid IM, Malt UF, Røislien J, Kerty E (2007) Determinants and status of quality of life after long-term botulinum toxin therapy for cervical dystonia. Eur J Neurol 14:1129–1137. 10.1111/j.1468-1331.2007.01922.x [DOI] [PubMed] [Google Scholar]
- 7.Molho ES, Agarwal N, Regan K, et al. (2009) Effect of cervical dystonia on employment: A retrospective analysis of the ability of treatment to restore premorbid employment status. Mov Disord 24:1384–1387. 10.1002/mds.22622 [DOI] [PubMed] [Google Scholar]
- 8.Pekmezovic T, Svetel M, Ivanovic N, et al. (2009) Quality of life in patients with focal dystonia. Clin Neurol Neurosurg 111:161–164. 10.1016/j.clineuro.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 9.Avenali M, De Icco R, Tinazzi M, et al. (2018) Pain in focal dystonias – A focused review to address an important component of the disease. Park Relat Disord 54:17–24. 10.1016/j.parkreldis.2018.04.030 [DOI] [PubMed] [Google Scholar]
- 10.Molho ES, Stacy M, Gillard P, et al. (2016) Impact of Cervical Dystonia on Work Productivity: An Analysis From a Patient Registry. Mov Disord Clin Pract 3:130–138. 10.1002/mdc3.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jahanshahi M, Torkamani M, Beigi M, et al. (2014) Pallidal stimulation for primary generalised dystonia: Effect on cognition, mood and quality of life. J Neurol 261:164–173. 10.1007/s00415-013-7161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comella CL, Perlmutter JS, Jinnah HA, et al. (2016) Clinimetric testing of the comprehensive cervical dystonia rating scale. Mov Disord 31:563–569. 10.1002/mds.26534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jankovic J, Adler CH, Charles D, et al. (2015) Primary results from the Cervical Dystonia Patient Registry for Observation of OnabotulinumtoxinA Efficacy (CD PROBE). J Neurol Sci 349:84–93. 10.1016/j.jns.2014.12.030 [DOI] [PubMed] [Google Scholar]
- 14.van den Dool J, Tijssen MAJ, Koelman JHTM, et al. (2016) Determinants of disability in cervical dystonia. Park Relat Disord 32:48–53. 10.1016/j.parkreldis.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 15.Skogseid IM, Røislein J, Claussen B, Kerty E (2005) Long-term botulinum toxin treatment increases employment rate in patients with cervical dystonia. Mov Disord 20:1604–1609. 10.1002/mds.20670 [DOI] [PubMed] [Google Scholar]
- 16.Albanese A, Sorbo F Del, Comella C, et al. (2013) Dystonia rating scales: Critique and recommendations. Mov Disord 28:874–883. 10.1002/mds.25579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal PK, Samii A, Schulzer M, et al. (2000) Head Tremor in Cervical Dystonia. Can J Neurol Sci / J Can des Sci Neurol 27:137–142. 10.1017/s0317167100052240 [DOI] [PubMed] [Google Scholar]
- 18.Merola A, Dwivedi AK, Shaikh AG, et al. (2019) Head tremor at disease onset: an ataxic phenotype of cervical dystonia. J Neurol 266:1844–1851. 10.1007/s00415-019-09341-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tinazzi M, Erro R, Mascia MM, et al. (2020) Demographic and clinical determinants of neck pain in idiopathic cervical dystonia. J Neural Transm. 10.1007/s00702-020-02245-4 [DOI] [PubMed] [Google Scholar]
- 20.Di Biasio F, Marchese R, Abbruzzese G, et al. (2020) Motor and Sensory Features of Cervical Dystonia Subtypes: Data From the Italian Dystonia Registry. Front Neurol 10.3389/fneur.2020.00906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaikh AG, Zee DS, Jinnah HA (2015) Oscillatory head movements in cervical dystonia: Dystonia, tremor, or both? Mov Disord 30:834–842. 10.1002/mds.26231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahn S (1984) The Varied Clinical Expressions of Dystonia. Neurol Clin 2:541–554. 10.1016/S0733-8619(18)31090-9 [DOI] [PubMed] [Google Scholar]
- 23.Beylergil SB, Singh AP, Zee DS, et al. (2019) Relationship between jerky and sinusoidal oscillations in cervical dystonia. Park Relat Disord 66:130–137. 10.1016/j.parkreldis.2019.07.024 [DOI] [PubMed] [Google Scholar]
- 24.Comella CL, Fox SH, Bhatia KP, et al. (2015) Development of the Comprehensive Cervical Dystonia Rating Scale: Methodology. Mov Disord Clin Pract 2:135–141. 10.1002/mdc3.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(2019) JMP®, Version 14.0
- 26.Diedenhofen B, Musch J (2015) Cocor: A comprehensive solution for the statistical comparison of correlations. PLoS One. 10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel N, Jankovic J, Hallett M (2014) Sensory aspects of movement disorders. Lancet Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tinazzi M, Squintani GM, Bhatia KP, et al. (2019) Pain in cervical dystonia: Evidence of abnormal inhibitory control. Park Relat Disord. 10.1016/j.parkreldis.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 29.Lautenbacher S, Spernal J, Schreiber W, Krieg JC (1999) Relationship between clinical pain complaints and pain sensitivity in patients with depression and panic disorder. Psychosom Med. 10.1097/00006842-199911000-00015 [DOI] [PubMed] [Google Scholar]
- 30.Antelmi E, Di Stasio F, Rocchi L, et al. (2016) Impaired eye blink classical conditioning distinguishes dystonic patients with and without tremor. Park Relat Disord. 10.1016/j.parkreldis.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 31.Avanzino L, Cherif A, Crisafulli O, et al. (2020) Tactile and proprioceptive dysfunction differentiates cervical dystonia with and without tremor. Neurology 10.1212/WNL.0000000000008916 [DOI] [PubMed] [Google Scholar]
- 32.Martino D, Bonassi G, Lagravinese G, et al. (2020) Defective Human Motion Perception in Cervical Dystonia Correlates With Coexisting Tremor. Mov Disord. 10.1002/mds.28017 [DOI] [PubMed] [Google Scholar]
- 33.Batla A, Sánchez MC, Erro R, et al. (2015) The role of cerebellum in patients with late onset cervical/segmental dystonia?-Evidence from the clinic. Park Relat Disord. 10.1016/j.parkreldis.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 34.Comella C, Perlmutter J, Jinnah H, et al. (2015) Reliability of the Severity subscale of the revised Toronto Spasmodic Torticollis Rating Scale (TWSTRS-2) (S15.001). Neurology [Google Scholar]
- 35.Hvizdošová L, Nevrlý M, Otruba P, et al. (2020) The Prevalence of Dystonic Tremor and Tremor Associated with Dystonia in Patients with Cervical Dystonia. Sci Rep. 10.1038/s41598-020-58363-2 [DOI] [PMC free article] [PubMed] [Google Scholar]


