Abstract
Autism spectrum disorders (ASD) are highly prevalent neurodevelopmental disorders; however, the neurobiological mechanisms underlying disordered behavior in ASD remain poorly understood. Notably, individuals with ASD have demonstrated difficulties generating implicitly derived behavioral predictions and adaptations. Although many brain regions are involved in these processes, the cerebellum contributes an outsized role to these behavioral functions. Consistent with this prominent role, cerebellar dysfunction has been increasingly implicated in ASD. In this review, we will utilize the foundational, theoretical contributions of the late neuroscientist Masao Ito to establish an internal model framework for the cerebellar contribution to ASD-relevant behavioral predictions and adaptations. Additionally, we will also explore and then apply his key experimental contributions towards an improved, mechanistic understanding of the contribution of cerebellar dysfunction to ASD.
Keywords: cerebellum, autism spectrum disorders, internal models, prediction, adaptation, implicit
INTRODUCTION
Autism spectrum disorders (ASD) are prevalent neurodevelopmental disorders characterized by abnormal social communication and interactions as well as restricted interests and repetitive behaviors. In addition to these core behavioral deficits, individuals with ASD also display a spectrum of comorbid symptoms which can include anxiety disorders, gastrointestinal problems, sleep disturbances, seizures, attentional disorders, and intellectual disability. Collectively, this varied symptomatology remains a significant challenge to treat. At the present time, targeted pharmacologic therapies for the core deficits in ASD are not available, with ASD treatment strategies focusing on treating associated comorbidities.
To improve therapeutic options for ASD, researchers have sought to understand the neurobiological underpinnings of the disorder. Studies have identified a strong genetic contribution to ASD, with now over a thousand genes linked to this disorder (Berg and Geschwind, 2012). As the functions of these genes have been delineated, various shared themes have emerged, including a critical role for many of these genes in the regulation of synapse and circuit function (Kenny et al., 2014; Bourgeron, 2015). Although initial studies focused on the contribution of circuit dysfunction in the neocortex, increasing studies have emerged that also support critical roles for sub-cortical structures in the pathogenesis of ASD – with the most consistently identified anatomic substrate implicated in ASD being the cerebellum.
CEREBELLAR CONTRIBUTIONS TO ASD
Many lines of evidence support the contribution of the cerebellum to ASD (for reviews see (D’Mello and Stoodley, 2015; Fatemi et al., 2012; Mosconi et al., 2015b)). The most consistently identified brain pathology reported in ASD is cerebellar, with postmortem studies in ASD reporting reduced numbers of cerebellar Purkinje cells (PCs) (Whitney et al., 2008). Cytoarchitecture within the rest of the cerebellum remains mostly intact, suggesting that PCs develop and then die instead of failing to develop altogether (Whitney et al., 2009). In addition to PC loss, human post-mortem studies also report cellular changes within the cerebellar output nuclei along with cerebellar projections to other brain regions (Bauman and Kemper, 1985; Fatemi et al., 2002; Palmen et al., 2004). Imaging studies in both individuals with ASD and rodent models of ASD, including Rett Syndrome, Fragile X Syndrome, and Tuberous Sclerosis Complex, also report cerebellar structural and functional differences when compared to control populations (Murakami et al., 1992; Ellegood et al., 2015; Stoodley et al., 2017; Kelly et al., 2020). Structural magnetic resonance imaging (MRI) studies in toddlers and young children report significantly larger cerebellar volumes in ASD subjects (Courchesne et al., 2011). Moreover, these volumetric differences are also observed in specific cerebellar lobules, many of which have been previously linked with cognitive functions (Murakami et al., 1992; Duerden et al., 2012; Stoodley, 2014). One consistent volumetric finding reported in human imaging studies is hypoplasia of the posterior cerebellar vermis in ASD, with the degree of gray matter loss within the vermis proportional to severity of repetitive behaviors and poorer social interaction scores (Courchesne et al., 1988; Scott et al., 2009; D’Mello et al., 2015). Human imaging studies also support altered vermis activation in ASD, with abnormal activation associated with processing facial expressions (Critchley et al., 2000; Wang et al., 2007). In addition to the posterior vermis, the degree of volumetric change in cerebellar lobule VII, specifically area right crus 1 (Rcrus1) of the cerebellar cortex, also correlates with the degree of ASD symptom severity, especially with social deficits (Riva et al., 2013; D’Mello et al., 2015). Additionally, regions of the cerebellar cortex including crus1, lobule VIII and lobule IX display consistently reduced gray matter volume in ASD (Duerden et al., 2012; Stoodley, 2014), and functional imaging studies suggest differential activation of these cerebellar regions during complex behavioral tasks in individuals with ASD (Pierce et al., 2004; Wang et al., 2007; Silani et al., 2008; Mostofsky et al., 2009; Solomon et al., 2009). Preclinical research studies manipulating cerebellar activity in mice with optogenetic and chemogenetic tools have also demonstrated the importance of area Rcrus1 and the posterior vermis in ASD-related behaviors (Stoodley et al., 2017; Badura et al., 2018; Kelly et al., 2020). This work demonstrates critical contributions of area Rcrus1 and the posterior vermis in the regulation of ASD-relevant behaviors and, importantly, suggests that stimulation of Rcrus1 and the posterior vermis ameliorates social preference / novelty phenotypes and repetitive behaviors (Stoodley et al., 2017; Kelly et al., 2020). Collectively, these studies point to the importance of the cerebellum in ASD and ASD-relevant behaviors; however, how the cerebellum modulates these complex processes and the precise regions of the cerebellum contributing to ASD-related phenotypes is still under investigation. To understand how the cerebellum might contribute to social behaviors and specifically how cerebellar dysfunction influences ASD-related behaviors, we must first examine the functions of the cerebellum.
During any discussion of cerebellar functions, one must highlight the immense contribution of the late, esteemed neuroscientist Masao Ito. Around the mid-20th century, the basic understanding of neuroscience was being transformed by the growing understanding of synaptic communication. This rapid growth in knowledge surrounding nervous system function also reached the cerebellum around this time, as several scientists produced pioneering work contributing to our current understanding of the cerebellum. Soon after John Eccles discovered the concept of inhibition in the spinal cord (Eccles et al., 1962), the young Japanese neuroscientist, Masao Ito joined his research group. Inspired by his early training with Eccles, Ito contributed decades of work demonstrating critical properties of the cerebellum, discoveries that ultimately pioneered advancements that are both practical and theoretical. Together with Eccles and János Szentágothai, Ito published ‘‘The Cerebellum as a Neuronal Machine,” a work highlighting the stereotyped anatomical connections within the cerebellum and postulating feedback/feed forward concepts that could be performed by cerebellar circuitry (Eccles et al., 1962). In this and subsequent studies, Ito made important theoretical contributions, postulating that the cerebellum provides the brain with a way to generate internal models, facilitating the smooth pursuit of motor actions (Ito, 2006). Additionally, Ito theorized that these internal models, which will be discussed in more detail below, are stored representations of dynamic behavioral actions, such as the movement of body parts (Ito and Ito, 1984). However, Ito’s foundational theories of internal models have grown to encompass much more beyond motor learning and sensorimotor behavior. As data has emerged supporting cerebellar involvement in non-motor behavior, Ito further expanded his theories of internal models to accommodate the cerebellar contribution to non-motor behaviors (Ito, 2008).
Ito’s work on the theoretical applications of cerebellar circuitry was complemented by his and other’s discoveries examining the cellular and physiological mechanisms underlying these internal models. One of Ito’s earliest experimental findings was that PCs are inhibitory neurons that utilize the neurotransmitter GABA (Ito and Yoshida, 1964; Ito et al., 1964). These findings secured the important role PCs play in regulating deep cerebellar nuclear output to the rest of the brain and showed for the first time that inhibition was a modality that could extend beyond local circuit networks.
Together, these findings of PC inhibitory function paired with local cerebellar circuitry and the theoretical framework of internal models creatively influenced other scientists also focused on studying cerebellar function. In fact, within five years of the publication of ‘‘The Cerebellum as a Neuronal Machine”, Marr and Albus both postulated that motor learning, and specifically motor memory, could be coded at the parallel fiber-PC synapse via changes in synaptic efficacy. In 1969, Marr suggested such a function would utilize long term potentiation (Marr, 1969), while Albus, in his conceptualization of the cerebellum as perceptron, predicted long term depression in 1971 (Albus, 1971). Despite many attempts to address these hypotheses, a conclusive answer remained elusive until Ito himself demonstrated the presence of LTD upon simultaneous climbing fiber and parallel fiber stimulation (Ito et al., 1982; Ito, 2001, 2002).
Collectively, these experimental findings made by Ito and his research group have changed our fundamental understanding of cerebellar structure and function and have provided a foundation for decades of further study. In this manuscript, we will first discuss Ito’s theoretical framework for the cerebellum and utilize this framework to discuss the growing literature pointing to the contribution of cerebellar dysfunction to ASD. We will then explore the mechanisms for cerebellar contributions to ASD through the lens of Ito’s discoveries detailing the inhibitory nature of cerebellar Purkinje cell output and the importance of LTD in cerebellar learning.
ITO’S CONCEPTUALIZATION OF INTERNAL MODELS
In his description of internal models, Ito provides numerous insights into the contributions of the cerebellum in ASD. An internal model can be defined as ‘‘any neural representation of the external world” (Ito, 2006). Ito proposed that the cerebellar contribution to internal models underlies an organism’s ability to learn motor skills and perform them with increasing ease and less conscious effort (Ito and Itō, 1984). For example, as we practice a movement pattern such as kicking a ball, with more repetitions we are able to perform the movement more precisely and to do so in an increasingly implicit and automatic manner. Therefore, practicing these motor patterns refines the internal model representing the motor action. With this refinement, the internal model can establish predictions about the outcome of the movement given the context and circumstances and update those predictions to improve future successful execution of the action (Requarth and Sawtell, 2014; Sokolov et al., 2017). Ito proposed that these internal models are dependent on cerebellar function. Since his original proposal, many studies have provided evidence to support the dependence of these internal models on cerebellar function. This cerebellar dependence has been demonstrated in both clinical studies of cerebellar injury or dysfunction (Fiez et al., 1992; Moberget et al., 2014; Cullen and Brooks, 2015; Bodranghien et al., 2016) and in multiple pre-clinical models where motor learning and performance is disrupted by cerebellar impairment (Piochon et al., 2014; Ha et al., 2016).
To generate this internal model, Ito predicted that models reliant on waiting for sensory feedback would operate on too slow a timescale to efficiently receive/predict motor outcomes to effectively compute motor commands (Ito and Itō 1984). Instead, he proposed that the cerebellum utilizes two types of predictive feedforward models: inverse and forward. In a motor context, the inverse model provides a motor command to fit a desired outcome, thereby replacing the need for another brain area (such as a cerebral-cortical area) to generate said command. In contrast, the forward model predicts the outcome of the performed action given the current context. Thus, an organism can generate an efference copy of the action and compare the activated command with the predicted outcome in real time while also comparing that predicted outcome with slower feedback from proprioceptive and other sensory information (Fig. 1) (Ito and Itō, 1984). This efference copy is proposed to be generated from a corollary discharge received by the cerebellum for motor action (Person, 2019). These two predictive feed forward programs are hypothesized to be coupled so that an inverse model learns to provide suitable commands for contexts in which the related forward model is trained to provide error predictions (Wolpert et al., 1998). In this way, the models work in tandem to generate proper sensorimotor predictions and intact motor control.
Fig. 1.
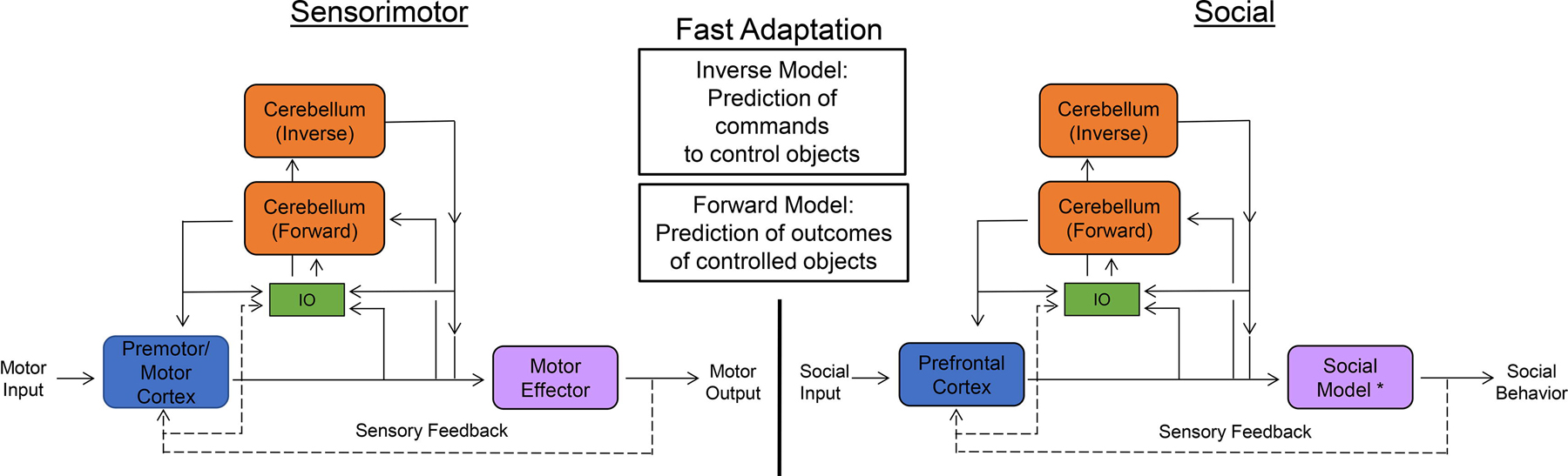
Block diagrams of internal model, including both cerebellar-mediated forward and inverse feedforward models. Diagram for sensorimotor (left) and for social behaviors (right) are shown. * ‘‘Social Model” represents the multiple effectors required for social behaviors. Sensory Feedback is represented by dashed lines. Error signals in the forward model are derived by comparison in the inferior olive (IO) of outputs from forward models and sensory feedback. Error is also derived by comparison of inverse model with all other input to the IO. These models are adapted from models presented by Masao Ito (See ‘‘Control of Mental Activities by Internal Models in the Cerebellum” Nat. Rev. Neuro. 2008).
In the context of a motor action, these postulated internal models are comprised of local cerebellar circuits that work in concert with connected neocortical areas which serve as a controller for the internal models of the cerebellum and are responsible for initiating the motor command (Fig. 1) (Ito, 2006). PC’s receive singular climbing fiber input from the inferior olive, tens to hundreds of thousands of granule cells via parallel fibers, and a variety of local interneurons. The integration of these many inputs is thought to represent the integration of proprioceptive, interoceptive, and exteroceptive sensory inputs. The cerebellar cortical circuit is stereotypically organized. As this circuit is described in great detail elsewhere in this special issue, it will not be discussed in detail here.
Although the initial models were based on a sensorimotor framework, Ito expanded his theoretical predictions to non-motor functions well before a cerebellar role in non-motor behaviors was widely accepted (Ito and Itō, 1984). He believed that the internal models of the cerebellum played a critical role in the processing and execution of more diverse functions including internal thoughts and language (Ito and Itō, 1984). As he conceptualized within the context of motor behaviors, Ito also theorized that an accurately predictive internal model would be essential for any complex behavior. Thus, we will next review data supporting the extension of Ito’s theories beyond motor function and, in specific, to autism-relevant behaviors.
INTERNAL MODELS – BEYOND MOTOR BEHAVIORS: PREDICTION
Performing social interactions depends on the ability to identify familiar contexts and individuals while recognizing sequences within a complex interplay of sensory information. From this sensory information, an organism would then need to be able to generate a predicted set of behaviors geared towards achieving the desired social outcome based on recognition of that sensory information. For example, factors such as another individual’s personality, mood, past experiences, cultural background, the underlying social context, and purposes of the social interaction are all critical variables to negotiate successful social interactions and would need to be included into a social predictive model. Likely outcomes can then be predicted and executed in consideration of these factors. Moreover, as with motor activity, the precise timing and temporal kinetics required for proper performance of a complex behavior such as social behavior require feedforward-based approaches, consistent with Ito’s theorized internal models.
Unsurprisingly, then, the ability to make accurate predictions has been shown to be disrupted in individuals with ASD in a number of behavioral spheres, including both social and non-social behaviors (Tseng et al., 2007; Sinha et al., 2014; Balsters et al., 2016). In sensorimotor domains, the majority of individuals with ASD have motor skill deficits (Lloyd et al., 2011), and individuals with ASD have been shown to make abnormal sensorimotor predictions (Ernst et al., 2019; Kinard et al., 2020). In addition, individuals with ASD who have difficulties generating accurate predictions demonstrate greater ASD symptom severity (Greene et al., 2019).
Difficulties with prediction also extend beyond sensorimotor domains. Individuals with ASD also struggle with interoceptive accuracy and generating predictions about their own internal state, and these challenges contribute significantly to processing and understanding internal emotions (DuBois et al., 2016; Garfinkel et al., 2016). Additionally, individuals with ASD demonstrate difficulties in making proper social predictions and inferences (Chambon et al., 2017). One way in which social prediction is examined in ASD is through Theory of Mind tasks. Theory of Mind refers to an individual’s ability to see the world from another’s point of view (Baron-Cohen, 1997). These tasks are by nature dependent on generating predictions (or intuiting previous events) based on current observations, a process in which individuals with ASD struggle (Baron-Cohen et al., 1985; Sinha et al., 2014). Dysfunctional social prediction and difficulties with Theory of Mind have been linked to cerebellar activity (Ernst et al., 2019; Kinard et al., 2020), and individuals with cerebellar dysfunction struggle with socio-emotional perception (Hoche et al., 2016). Functional (f)MRI imaging studies in individuals who listened to stories that either violate or meet social norms highlight an important role for the cerebellum in socially-relevant predictions. In these studies, cerebellar activation was noted (Berthoz et al., 2002), indicating that the cerebellum is involved in the calculation of social prediction.
In neuro-typical individuals, areas critical for prediction include not only the cerebellum but also the basal ganglia, anterior cingulate cortex, and striatum (Delgado et al., 2008; Schlerf et al., 2012; Ide et al., 2013; Tanaka et al., 2016). Interestingly, these are all ASD-implicated brain areas (Thakkar et al., 2008; Qiu et al., 2010; Fatemi et al., 2012; Araujo et al., 2015), and many of these areas which have been shown to be instrumental for social prediction/social prediction error also display aberrant function and connectivity to the cerebellum in individuals with ASD (Verly et al., 2014; Balsters et al., 2016).
INTERNAL MODELS – BEYOND MOTOR BEHAVIORS: ADAPTATION FROM ERROR-BASED LEARNING
Inherent in the generation of an internal model is a need to have a mechanism to manage inaccuracies of the model, or put another way, a prediction error. ‘‘Prediction error” can be defined as the ability to make predictions and utilize incoming sensory information to calculate error between the predicted and actual outcomes (Schlerf et al., 2012). This error calculation allows for the appropriate adaptation to minimize error in the future and thus refines the predicted action and internal model (Synofzik et al., 2008; Popa et al., 2016). In motor terms, whether it be a comparison of signals generated by either inverse versus forward models or comparing sensory feedback from the execution of the motor command to the efference feedforward signals, the existence of error can be examined (Requarth and Sawtell, 2014). If the predicted outcome matches the actual outcome, the signal can be voided (Brooks and Cullen, 2013); however, when the actual and predicted results are not alike, the difference constitutes a sensory prediction error. These error calculations allow for rapid modification of motor output and serve to reduce error upon prediction of subsequent movements (Bastian, 2006). The inferior olive derived climbing fiber signal is thought to play an important role in conveying information of both predicted and actual outcomes, as it receives cortical, subcortical, and cerebellar inputs, placing it in a prime position to perform sensory error-based functions (De Zeeuw et al., 1990, 1998). Interestingly, recent studies suggest that climbing fiber input may also provide additional information beyond an error signal and may also convey information about reward prediction (Heffley and Hull, 2019; Hull, 2020). The information carried via climbing fiber input is conveyed via complex spike activity onto PCs (Davie et al., 2008). Therefore, the features of the complex spike as well as the resulting pause in PC simple spike activity following initiation of the complex spike are likely to convey error, adaptation, and reward information. However, exactly how this information is encoded in these features remains to be fully elucidated (Hewitt et al., 2011; Popa et al., 2016).
Without the ability of the cerebellum to perform precise prediction error calculations, the accurate and timely predictions of behavioral outputs would be difficult to achieve. In alliance with these concepts, individuals with ASD have impaired abilities to adapt predictions in a number of sensorimotor-related tasks such as oculomotor saccade and reaching tasks (Lawson et al., 2014; Van de Cruys et al., 2014). In these studies, individuals with ASD showed decreased adaptation, impaired accuracy, and dependence on slower, often explicit, feedback mechanisms (Mosconi et al., 2015a). These tasks have been previously demonstrated to rely on proper cerebellar function, further highlighting cerebellar dysfunction within ASD (Desmurget et al., 1998; Barash et al., 1999). These prediction errors also extend to social behaviors in individuals with ASD (Balsters et al., 2016; Greene et al., 2019; Kinard et al., 2020) who demonstrate difficulties with adaptation in social domains (Pellicano et al., 2007; Rutherford and Troje, 2012; d’Arc et al., 2020).
In addition, deficiencies in error-based adaptation are further implicated in ASD even beyond social interactions. Although prediction errors clearly apply to social functions as noted above, these difficulties also apply to the other core deficit category in ASD – behavioral rigidity, repetitive behaviors, and cognitive inflexibility. Individuals with ASD tend to hold tightly to specific behavior patterns, have difficulty changing plans, deviating from routines, and have trouble adapting to novel environments (de Vries and Geurts, 2012). The DSM-V describes these behaviors as, ‘‘insistence on sameness”. These restricted behaviors are often categorized as an expression of cognitive inflexibility. Cognitive flexibility, or the ability to switch quickly from one task to another, is a means traditionally ascribed to prefrontal cortex-regulated executive functions (Ragozzino et al., 1999; Monsell, 2003; Bissonette et al., 2008). However, cerebellar involvement in behavioral flexibility is logical given that the cerebellum and prefrontal cortex have been shown to be both structurally and functionally connected. Specifically, cerebellar domains implicated in ASD such as the cerebellar vermis have been shown to be connected to the mPFC, while these connections are disrupted in individuals with ASD (Buckner et al., 2011; Kelly et al., 2020). Moreover, studies in individuals with ASD demonstrate that the change in posterior vermis volume correlates with restricted, repetitive, and stereotyped behavioral scores on the Autism Diagnostic Interview-Revised (D’Mello et al., 2015). Moreover, recent studies in rodents demonstrate that proper function of the posterior vermis is necessary to prevent ASD-relevant behaviors (Badura et al., 2018; Kelly et al., 2020) and that modulation of function in this region is sufficient to improve repetitive and inflexible behaviors in an ASD-relevant mouse model (Kelly et al., 2020).
Accurate prediction error also provides the ability to discriminate between relevant and irrelevant sensory stimuli, including how to differentiate salient signals from noise. One example of this would be the ability to differentiate between background contexts that should be remembered for future behavioral relevance versus irrelevant sensory stimuli such as background noise (O’Shea et al., 2005; Baker et al., 2008; Brown and Dunn, 2010). This sensory processing and integration are known challenges for individuals with ASD, with many demonstrating hyper and/or hyposensitivity to various sensory stimuli, including auditory, visual, and tactile sensation (Leekam et al., 2007; Marco et al., 2011). These difficulties often manifest with individuals engaging in behaviors to manage this sensory information, sometimes by decreasing sensory input (plugging ears or covering eyes) or conversely by seeking out sensory feedback through actions such as rubbing their face or utilizing weighted blankets (Rosenhall et al., 2003; De Jonge et al., 2007; Güçlü et al., 2007). Interestingly, abnormal sensory responses have been shown to be correlated with the severity of social deficits in high functioning individuals with ASD (Hilton et al., 2010). Taken together, disruptions in both prediction and error-based adaptation as demonstrated in ASD are likely to contribute to both core and co-morbid behavioral disruption in ASD. Direct evidence linking cerebellar function with improved adaptation processes is currently under investigation.
INTERNAL MODELS – BEYOND MOTOR BEHAVIORS: IMPLICITY
Another key property of Ito’s proposed internal model is that the cerebellar capacity for error prediction and adaptation of learned tasks becomes ‘‘internal” and thus implicit. Implicit correction has been shown in multiple settings in motor paradigms and to be dependent on cerebellar function (Kim and Thompson, 1997; Boyden et al., 2004; Tseng et al., 2007). The cerebellum functions to hold programs required for executing behaviors and, once acquired, adapts them without conscious intervention (Wolpert et al., 1998). Implicit learning can be described as learning which occurs without conscious awareness or explicit understanding of how the learning has happened (Reber, 1993; Callenmark et al., 2014). For example, children often learn to follow grammatical rules, pronunciation, and syntax when learning to speak their native language without conscious knowledge of those rules and their acquisition (Vizcaino et al., 2006). Implicit learning also involves the ability to readily and unconsciously apply learned concepts to new contexts (Hoffmann and Koch, 1998).
In practice, activation of internal models during explicit learning promotes the generation of error-based signals as new movements and/or skills are acquired. Once acquired, internal models are refined and corrected through cerebellar generated error signals, and feedback loops supported via internal models promote the switching from explicit to implicit learning. Individuals with ASD and neurodevelopmental disorders in general struggle with implicitly learned paradigms and emotional perception (Klinger et al., 2007). Instead of utilizing implicit learning, many studies support that individuals with ASD rely instead on explicit strategies to navigate what would otherwise be implicit tasks (Callenmark et al., 2014). As with motor control, reliance on such strategies in social and cognitive domains, such as emotional perception, is predicted to result in significant impairment in functionality and likely contributes to social deficits and restricted behaviors seen in individuals with ASD (Paul and Cohen, 1985; Frith and Happé, 1999; Klinger et al., 2007; Senju, 2013; Callenmark et al., 2014). However, explicit learning strategies can assist learning in sensorimotor domains (Mazzoni and Krakauer, 2006; Taylor et al., 2014) and have been shown to impact social skill learning. Researchers have found that higher verbal age allows for more explicit tutelage and helps individuals with ASD successfully navigate what would be intuitive tasks for typically developing children (Happé, 1995; Klinger and Dawson, 1995; Bowler et al., 1997). This explicit learning approach also underlies one of the most common and effective ASD treatment options, applied behavioral analysis (ABA) (Ivy and Schreck, 2016). As previously mentioned, one type of task that ASD children struggle with is Theory of Mind, where one is expected to understand the mental/emotional state of others and make predictions based on that information. However, instructors have been able to help individuals with ASD successfully complete these tasks through the teaching of explicit rules that rely on effortful verbal strategies (Happé, 1995). At present, only a few studies have specifically evaluated the efficacy of explicit behavioral strategies on social skills (Begeer et al., 2011; Peters and Thompson, 2018). Direct evidence supporting these concepts and theories within ASD populations is currently under investigation. Thus, in ASD, altered cerebellar function and subsequent deficits in error-based learning impair prediction, adaptation, and implicit learning/processing resulting in the need to perform tasks explicitly and executing actions that depend on slower, feedback-based adaptation.
CEREBELLAR-CORTICAL CIRCUITRY AND INTERNAL MODELS
In order to generate an internal model within his theoretical framework, Ito proposed and showed evidence to support that the cerebellum communicated with cortical areas and specifically the prefrontal and parietal cortices for mental activities, language, and social interactions (Ito, 2008). Over many decades, studies have supported both structural and functional connectivity between the cerebellum and non-motor cortical areas, including the prefrontal and parietal cortices (Strick et al., 2009; Buckner et al., 2011). Recent work has shown that specific subtypes of cerebellar nuclear neurons may mediate these projections to differential cortical regions (Fujita et al., 2020). Cerebellar-cerebral cortical connections are exclusively polysynaptic and are classically thought of as organized in cerebellar-thalamo-cortical loops (Strick et al., 2009; Buckner et al., 2011; Voogd et al., 2012), although recent work also demonstrates connectivity through limbic regions such as the Ventral Tegmental Area (VTA) (Carta et al., 2019). It is through these reciprocal loops with the cortex that the cerebellum receives cortical information and in turn provides output back to relevant cortical regions.
Interestingly, communication between the cerebellum and cortical areas is also altered in ASD. Functional imaging studies have supported abnormal functional connectivity between the cerebellum and the cerebral cortex in ASD (Mostofsky et al., 2009; D’Mello and Stoodley, 2015; Stoodley et al., 2017). Resting state fMRI connectivity between the cerebellum and the cortex has been reported to be significantly diminished in ASD (Noonan et al., 2009; Khan et al., 2015). Specifically, areas implicated in ASD including cerebellar areas crus1 and crus2 have reduced connectivity to the dorsolateral and medial prefrontal cortex, and these connectivity alterations correlate with ASD symptom severity (Jung et al., 2014; Verly et al., 2014). Moreover, studies have similarly demonstrated disruptions in RcrusI connectivity to parietal areas in children with ASD and in ASD-relevant rodent models (Stoodley et al., 2017). Further, using structural connectivity approaches or an unbiased fMRI approach, disrupted connectivity can be identified between mPFC and Rcrus1 in both ASD-relevant rodent models and in individuals with ASD (Kelly et al., 2020).
Additional evidence supporting the importance of cerebellar circuitry in ASD comes from emerging work demonstrating roles for the cerebellum in processing reward. Studies have demonstrated outcome prediction and error calculation in cerebellar granule cells for reward behavior (Wagner et al., 2017). Additionally, climbing fiber activity has also been shown to convey reward related information (Hull, 2020), and connectivity from the cerebellum to VTA has been demonstrated to influence non-motor behaviors, including social behaviors (Carta et al., 2019). Human imaging studies have also found activation of cerebellar regions during reward-related tasks and differential cerebellar activation in drug users during reward learning and drug-related cravings and memories (Martin-Sölch et al., 2001; Ramnani et al., 2004; Anderson et al., 2006; Thoma et al., 2008). Studies in mice have further demonstrated atypical connectivity between the cerebellum and mPFC, and mPFC dopamine levels are altered in mouse models of PC loss and ASD (Rogers et al., 2011, 2013). Moreover, dopamine receptors in the cerebellar output nuclei have been shown to be critical for cognitive behaviors (Locke et al., 2018), while loss of tyrosine hydroxylase in PCs results in numerous non-motor deficits (Locke et al., 2020). Taken together, these interactions with reward pathways point to likely important roles for cerebellar-regulated predictions of reward and valence that may have critical impacts on non-motor behavior and cognition. However, the cerebellar networks and the precise interactions and neural circuits that underlie these cerebellar-regulated internal models remain to be fully established.
THE IMPACT OF LOST PURKINJE CELL INHIBITION ON CORTICAL DYSFUNCTION IN ASD
One prominent theory in ASD is that individuals with ASD have disruptions in cortical excitatory/inhibitory (E/I) balance (Rubenstein and Merzenich, 2003; Nelson and Valakh, 2015). In this formulation, the ratio of cortical excitation to inhibition is altered, with the balance shifted toward an increased excitatory-inhibitory ratio (Rubenstein and Merzenich, 2003; Nelson and Valakh, 2015). In its formulation, function of the cortex is altered when the balance between excitatory and inhibitory circuits is disrupted with too little inhibition, excess excitation, or a combination of both factors. Appropriate amounts of inhibition allow for excitatory output neurons to fire with high signal to noise ratio, precise timing, and to have the gain necessary to encode relevant tasks (Rubenstein and Merzenich, 2003; Vogels and Abbott, 2009). Without proper balance, the timing, quantity, and synchronization necessary for the appropriate computation are lost (Vogels and Abbott, 2009; Nelson and Valakh, 2015; Peterson and Voytek, 2017). Some of the strongest support for altered excitatory-inhibitory balance comes from the high rates of epilepsy in ASD in addition to electroencephalography studies pointing to excessive circuit excitation. Approximately 30% of ASD patients have epilepsy (Tuchman et al., 2009), and even in the absence of epilepsy, epileptiform EEG patterns and cortical hyperactivation are observed in significant proportions of individuals with ASD (Chez et al., 2006; Spence and Schneider, 2009).
Similar findings for disruptions in E/I balance have also been demonstrated in a number of mouse models of ASD-linked genes (Gogolla et al., 2009; Peñagarikano et al., 2011; Rothwell et al., 2014). Moreover, mouse models with loss of many ASD-related genes demonstrate increased cortical excitability, often with accompanying seizures (Meikle et al., 2007; Gibson et al., 2008; Peñagarikano et al., 2011; Selimbeyoglu et al., 2017). Furthermore, exogenous alterations in excitation and inhibition with a biasing toward increased excitation or decreased inhibition affects both social and repetitive behaviors in these models (Gogolla et al., 2009; Peñagarikano et al., 2011; Rothwell et al., 2014). Elevating E/I imbalance in the mPFC by exciting pyramidal neurons or by inhibiting parvalbumin expressing, inhibitory interneurons in wild-type mice resulted in disrupted social interactions (Gogolla et al., 2009; Mehta et al., 2011; Yizhar et al., 2011). Additionally, optogenetic inhibition of somatostatin expressing interneurons abolishes affective state discrimination, which can also be elicited by synchronous somatostatin neuron activation (Scheggia et al., 2019). Importantly, reducing excitatory tone in the mPFC has also been shown to rescue social deficits in a mouse mutant of the ASD-linked genes Cnt-nap2 and Tsc1 (Selimbeyoglu et al., 2017; Kelly et al., 2020). Reduction of mediodorsal or ventromedial thalamic activity (thalamic nuclei which project to the mPFC) also impairs ASD-related behaviors, including cognitive flexibility and social interaction, while activation of parvalbumin neurons in the mPFC can mitigate these impairments (Parnaudeau et al., 2013; Bolkan et al., 2017; Ferguson and Gao, 2018; Kelly et al., 2020). Together these findings support that proper excitatory-inhibitory circuit function is essential for social behaviors.
One of Ito’s most fundamental discoveries was that the sole output from the cerebellar cortex, the PC, is inhibitory and utilizes the inhibitory neurotransmitter GABA (Ito et al., 1982). Thus, these cells play critical roles in providing efferent inhibitory influence on downstream cerebellar nuclear activity (Ito et al., 1982). As noted above, the most consistently identified pathology in ASD is loss of PCs, while cerebellar output dysfunction is observed in ASD, consistent with impaired PC function (Ryu et al., 1999; Whitney et al., 2008). Further support for a critical role for PCs in ASD has emerged from pre-clinical studies. Mouse mutant models with targeted loss of ASD-related genes in PCs have consistently demonstrated PC dysfunction. Moreover, these models overwhelmingly demonstrate social impairment and repetitive and inflexible behavioral phenotypes, demonstrating that PC dysfunction is sufficient to generate these behaviors. These models include PC knockout of Tsc1, Tsc2, and Pten (Tsai et al., 2012; Reith et al., 2013; Cupolillo et al., 2016). Additionally, other ASD-related mouse models such as Shank2 knockout mice demonstrate PC dysfunction and similar ASD-related behavioral phenotypes (Peter et al., 2016). In addition, targeted disruption of the cerebellum using chemogenetic approaches has also shown that cerebellar dysfunction is sufficient to generate ASD-relevant phenotypes including social impairment and inflexible behaviors (Stoodley et al., 2017; Badura et al., 2018; Kelly et al., 2020).
Although the precise relationship between PC and cerebellar nuclear neuronal activity continues to be actively studied (Person and Raman, 2012), activation of PC activity results in inhibition of cerebellar nuclear neuronal activity (Chabrol et al 2019) and the loss of this important inhibitory output from PCs is consistent with increased output from the cerebellar nuclei, as is observed in individuals with ASD (Asano et al., 2001). Thus, alterations in cerebellar nuclear output would be predicted to impact neocortical activity via previously discussed connections to the cerebral cortex and would potentially impact the excitatory inhibitory imbalance observed in ASD. Recent studies support an impact of PC activity on neocortical activity consistent with this prediction (Stoodley et al., 2017; Chabrol et al., 2019; Kelly et al., 2020). As discussed, Ito proposed that cerebellar-mPFC pathways likely contribute to generation of internal models of behaviors. In alliance with this proposal, mouse models of PC loss such as the lurcher model display increased mPFC activity (Rogers et al., 2011, 2013). PC hypoactivity and PC loss is also seen across many mouse models of ASD with reported increases in cortical excitation. For example, individuals with Fragile X Syndrome, a neurodevelopmental disorder which is the leading mono-genetic contributor to ASD, as well as Fmr1 (gene disrupted in Fragile X) mouse mutants have been shown to have reduced PC activity as well as cortical hyperexcitability and sensory hypersensitivity (Koekkoek et al., 2005; Gibson et al., 2008; Hays et al., 2011).
Recent investigations have also demonstrated that decreased cerebellar activity results in increased activity in downstream mPFC and parietal cortical areas, thereby contributing to the elevated E/I balance in these cortical regions (Stoodley et al., 2017; Kelly et al., 2020). These studies in mice demonstrated anatomic connections between the ASD-implicated cerebellar regions, Rcrus1 and the posterior vermis, and the mPFC and also confirmed that output from these cerebellar regions to the mPFC is essential for normal behaviors (Badura et al., 2018; Kelly et al., 2020). Moreover, studies further identified disruptions in connectivity between Rcrus1 and the mPFC in a cohort of over 100 mouse models of ASD-linked genes, suggesting a potentially shared circuit disruption across many genetic ASD models (Kelly et al., 2020). Lastly, these studies highlight the potential translational applicability of these findings, as modulation at the cerebellum and along the cerebellar-mPFC circuit results in improved social behaviors in an ASD-relevant genetic mouse model (Stoodley et al., 2017; Kelly et al., 2020).
Ito’s discovery of the inhibitory nature of the PC thus may inform one of the leading theories in ASD dysfunction, E/I imbalance (Fig. 2). As discussed above, excitatory-inhibitory imbalance has been shown to alter cortical function and behaviors relevant to ASD. Although there has been significant research conducted in this area, additional examination beyond the present studies (Stoodley et al., 2017; Kelly et al., 2020) of the cerebellar contribution to cortical function in ASD will continue to be an important avenue of future study.
Fig. 2.
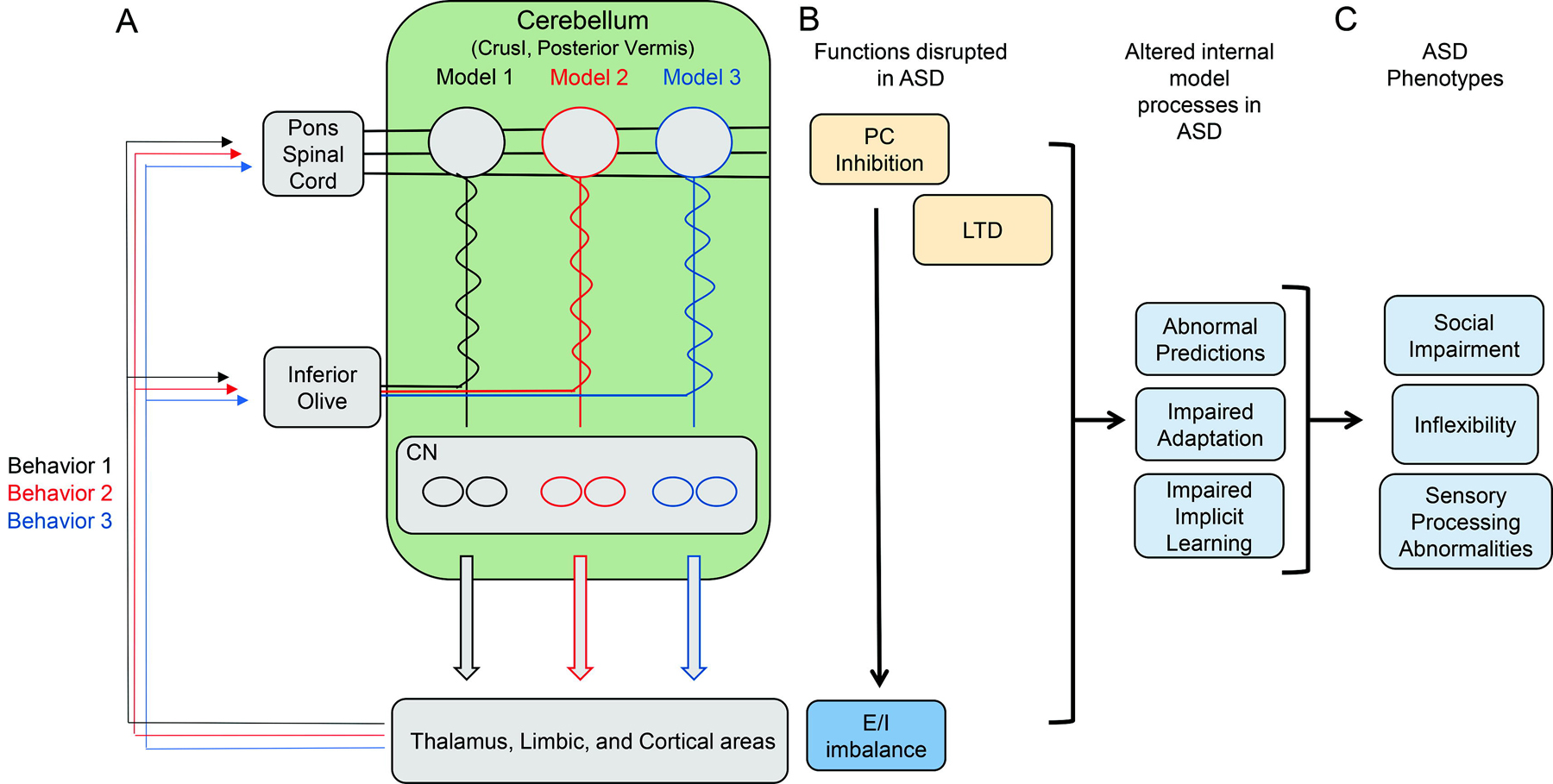
Model of cerebellar and cerebral-cortical circuits composing internal models for discrete behaviors (A) Dysfunction present in autism spectrum disorders and the sequelae of dysfunction on internal model function (B) and resulting autism-related behavioral phenotypes (C). ASD: autism spectrum disorder; PC: Purkinje cell; DCN: deep cerebellar nuclei, LTD: long term depression, E/I: Excitatory/Inhibitory.
LTD AND CEREBELLAR LEARNING IN ASD
The cerebellum makes important contributions to Ito’s theoretical generation of internal models; however, the exact mechanisms by which the cerebellum performs those functions remain largely undefined. Although PCs have a critical role in cerebellar function and have been implicated in ASD, whether disruptions in cerebellar-regulated learning are involved in ASD remains unknown.
As noted previously, for decades, competing theories were proposed regarding the neural mechanisms underlying cerebellar learning. In 1979, Ito showed experimental evidence that concurrent parallel fiber and climbing fiber activation resulted in LTD (Ito et al., 1979) and that this mechanism to generate plasticity could underlie cerebellar prediction learning (Ito and Kano, 1982; Ito et al., 1982). Consistent with the critical role for the inferior olive-generated climbing fiber in the representation of error, this mechanism does not occur in the absence of error or in the absence of climbing fiber input (Ito, 2001; Popa et al., 2016). While much evidence supports that the error signal is carried predominantly by climbing fiber inputs (Kawato and Gomi, 1992; Wolpert et al., 1998; Ito, 2002, 2013), recent studies suggest that input from climbing fibers may provide a richer source of information (Hull, 2020). Although studies continue to build on these original observations of cerebellar learning through LTD, we can apply our present understanding of these insights to better understand cerebellar dysfunction in ASD (Fig. 2).
One process that is dependent upon LTD-dependent cerebellar learning is eye-blink conditioning. Eye-blink conditioning is a classical conditioning task which requires the pairing of the unconditioned stimulus (air puff to the eye) with a conditioned stimulus (usually a tone) (Kim and Thompson, 1997). Through numerous lesion studies, electrophysiological recordings, and imaging studies, it has been shown that this task is dependent upon the cerebellum and more specifically is dependent on cerebellar LTD in rodents, rabbits, and humans (Woodruff-Pak et al., 1985; Molchan et al., 1994; Shibuki et al., 1996; Schreurs et al., 1997). Individuals with ASD have been found to have deficits in delayed eye-blink conditioning, further supporting disruptions in LTD-dependent behaviors in ASD (Sears et al., 1994; Oristaglio et al., 2013). Moreover, many mouse models for ASD-linked genes have also been shown to have deficits in eye-blink conditioning. ASD models of loss of Cnt-nap2, Mecp2, Shank3, Fmr1, Tsc2 and PC knockout of Tsc1 all have reported perturbations in associative sensory learning in the form of delayed eye-blink conditioning (Auerbach et al., 2011; Kloth et al., 2015). Moreover, mice with 15q11–13 duplication, one of the most prevalent genetic abnormalities in ASD, display aberrant cerebellar LTD and eye-blink conditioning deficits in addition to social deficits (Piochon et al., 2014). Alongside this mouse model, a mouse model of the non-syndromic ASD-linked gene Neuroligin-3 also displays dysregulated LTD in the cerebellum, further implicating the prevalence of LTD deficits in the setting of autism-related gene mutations (Baudouin et al., 2012a). In addition to changes in cerebellar plasticity, these models also are accompanied by impairments in PC function, which is postulated to contribute to the timing of parallel fiber and climbing fiber activation, thereby potentially contributing to knock on effects on LTD seen in these models (Tsai et al., 2012; Cupolillo et al., 2015; Chao et al., 2020). Alterations in LTD likely contribute to the learning, motor, and neuropsychiatric relevant phenotypes exhibited in these models (Koekkoek et al., 2005; Baudouin et al., 2012b; Piochon et al., 2014). Although LTD as the mechanism behind cerebellar learning is strongly supported, there is still a need to further elucidate the specific roles that LTD might play in ASD-relevant behaviors. Additionally, the role of lesser studied cell types in the cerebellum such as Bergmann glia may hold deeper insights not only into Ito’s theories of LTD and cerebellar learning but also in ASD. Studies have shown that Bergmann glia, which are present at synapses on PC dendrites play a role in the clearing of glutamate and the process of LTD (Shibuki et al., 1996; Grosche et al., 2002). Bergmann glia have also been indirectly associated with ASD and may provide another mechanistic contribution to the pathogenesis of ASD (Koirala and Corfas, 2010; Chrobak and Soltys, 2017).
A CONTINUED LEGACY
Masao Ito theorized that the cerebellum provides a critical contribution to internal models of both motor and non-motor behavior. He proposed and demonstrated that cerebellar cortical structure and learning capabilities allowed for generation of implicit predictions and adaptation of those predictions to a changing sensory environment. Ito theorized that these cerebellar functions would thus apply not only to motor actions but to any complex behavior, including an individual’s internal thoughts, social behaviors, and behavioral flexibility, the hallmark behaviors that are disordered in ASD. Consistent with these predictions, cerebellar-regulated functions of implicitly performed prediction and adaptation are disrupted in ASD, while increasing evidence implicates cerebellar dysfunction in ASD. Included in this dysfunction, disruptions in two of Ito’s experimental contributions – PC function and PC-mediated plasticity – have been consistently identified in ASD and ASD models, which together would be predicted to result in the disruptions in cerebellar-governed prediction and behavioral adaptation observed in ASD (Fig. 2). In addition, a specific anatomical topography for the cerebellar contribution to ASD is beginning to emerge with a regional organization to behaviors relevant to ASD. However, the cerebellum, in addition to gross anatomical organization, is also organized into functional modules or microzones (Apps et al., 2018). These microzones can be further defined by specific PC molecular expression (including aldolase C, PLCβ3 and 4, EAAT4, Pcdh10) while emerging single cell expression studies are further defining molecularly-characterized cerebellar subsets (Apps et al., 2018; Fujita et al., 2020). A more detailed examination of the contribution of cerebellar organization to specific behavioral phenotypes will be a critical avenue of future study.
In addition, Ito also believed that connectivity between the cerebellum and cortex is critical for proper execution and performance of behavior. As previously noted, cerebellar-cerebral cortical circuits are organized in cerebello-cortical loops (Strick et al., 2009; Buckner et al., 2011; Voogd et al., 2012) through which the cerebellum receives cortical information and provides critical computations to cortical regions. The application of these internal models onto different behavioral phenotypes will likely depend on the regions of the cerebellum and cerbral cortex which are connected in these loops. In this way there is a functional topography of the cerebellum, representing the associated cortical regions and functions (Buckner et al., 2011; D’Mello and Stoodley, 2015). Recent work has demonstrated the importance of these circuit loops in ASD, especially those including cerebellar cortical areas Rcrus1 and the posterior vermis to the mPFC (Kelly et al., 2020). However, altered function of these circuit loops is unlikely limited to one cerebellar or cortical region. In fact, studies have demonstrated decreased connectivity between the cerebellum and numerous cortical areas and abnormal activation of cerebellar and cerebral-cortical areas in individuals with ASD (Mueller et al., 2013; von dem Hagen et al., 2013). Each of these connected brain areas may play unique roles in ASD-related behaviors, which may contribute to the vast heterogeneity in behavioral symptomatology observed in ASD.
Ito further hypothesized that cortical areas, such as the mPFC, send out commands to the cerebellar-generated internal models, accounting for the cortical-cerebellar projection leg of the closed loop. However, both he and others also postulated important reciprocal functions of the return signaling from the cerebellum back to the cortex, suggesting a more complicated model than the traditionally held view of a top down relationship from the cortex to cerebellum. Recent work has suggested that cerebellar function and PC activity dictates mPFC activity, dopamine release, mPFC function, and mPFC behavioral regulation (Rogers et al., 2013; Parker et al., 2014; Badura et al., 2018; Kelly et al., 2020). These studies are just beginning to scratch the surface of the precise cerebellar contribution to the pathogenesis of ASD and the precise role for the cerebellum in the regulation of ASD-relevant behaviors.
Altogether, Masao Ito has propelled the field of cerebellar research forward with his innumerable contributions, which have not only enlightened us to the molecular and microcircuit structure of the cerebellum but have also challenged the conceptual framework of the cerebellar computation. His foundational discoveries continue to guide work furthering our understanding of the internal models of the cerebellum, how they operate to perform, predict, and adapt complex behaviors, and how these processes are disrupted in neuropsychiatric disorders such as ASD. Additionally, while this review focuses on cerebellar contributions to ASD, similar cerebellar-regulated mechanisms of disrupted prediction and adaptation may underlie and contribute to neuropsychiatric dysfunction observed across other disorders.
Abbreviations:
- ASD
autism spectrum disorders
- E/I
excitatory/inhibitory
- MRI
magnetic resonance imaging
- PCs
Purkinje cells
- VTA
Ventral Tegmental Area
REFERENCES
- Albus JS (1971) A theory of cerebellar function. Math Biosci 10:25–61. [Google Scholar]
- Anderson CM, Maas LC, Frederick BD, Bendor JT, Spencer TJ, Livni E, Lukas SE, Fischman AJ, Madras BK, Renshaw PF (2006) Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology 31:1318–1326. [DOI] [PubMed] [Google Scholar]
- Apps R, Hawkes R, Aoki S, Bengtsson F, Brown AM, Chen G, Ebner TJ, Isope P, Jorntell H, Lackey EP, et al. (2018) Cerebellar modules and their role as operational cerebellar processing units: a consensus paper [corrected]. Cerebellum 17:654–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo DJ, Anderson AG, Berto S, Runnels W, Harper M, Ammanuel S, Rieger MA, Huang H-C, Rajkovich K, Loerwald KW (2015) FoxP1 orchestration of ASD-relevant signaling pathways in the striatum. Genes Dev 29:2081–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Chugani D, Muzik O, Behen M, Janisse J, Rothermel R, Mangner T, Chakraborty P, Chugani H (2001) Autism in tuberous sclerosis complex is related to both cortical and subcortical dysfunction. Neurology 57:1269–1277. [DOI] [PubMed] [Google Scholar]
- Auerbach BD, Osterweil EK, Bear MF (2011) Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature 480:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badura A, Verpeut JL, Metzger JW, Pereira TD, Pisano TJ, Deverett B, Bakshinskaya DE, Wang SS (2018) Normal cognitive and social development require posterior cerebellar activity. Elife 7 e36401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AE, Lane A, Angley MT, Young RL (2008) The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: a pilot study. J Autism Dev Disord 38:867–875. [DOI] [PubMed] [Google Scholar]
- Balsters JH, Apps MAJ, Bolis D, Lehner R, Gallagher L, Wenderoth N (2016) Disrupted prediction errors index social deficits in autism spectrum disorder. Brain 140:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P (1999) Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci 19:10931–10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S (1997). Mindblindness: an essay on autism and theory of mind (MIT press; ). [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U (1985) Does the autistic child have a ‘‘theory of mind”. Cognition 21:37–46. [DOI] [PubMed] [Google Scholar]
- Bastian AJ (2006) Learning to predict the future: the cerebellum adapts feedforward movement control. Curr Opin Neurobiol 16:645–649. [DOI] [PubMed] [Google Scholar]
- Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P, Tanaka KF, Spooren W, Hen R, De Zeeuw CI (2012) Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science 338:128–132. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL (1985) Histoanatomic observations of the brain in early infantile autism. Neurology 35:866. [DOI] [PubMed] [Google Scholar]
- Begeer S, Gevers C, Clifford P, Verhoeve M, Kat K, Hoddenbach E, Boer F (2011) Theory of Mind training in children with autism: a randomized controlled trial. J Autism Dev Disord 41:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JM, Geschwind DH (2012) Autism genetics: searching for specificity and convergence. Genome Biol 13:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoz S, Armony JL, Blair RJR, Dolan RJ (2002) An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain 125:1696–1708. [DOI] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM (2008) Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci 28:11124–11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodranghien F, Bastian A, Casali C, Hallett M, Louis ED, Manto M, Mariën P, Nowak DA, Schmahmann JD, Serrao M, et al. (2016) Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. Cerebellum (London, England) 15:369–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan SS, Stujenske JM, Parnaudeau S, Spellman TJ, Rauffenbart C, Abbas AI, Harris AZ, Gordon JA, Kellendonk C (2017) Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci 20:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T (2015) From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci 16:551–563. [DOI] [PubMed] [Google Scholar]
- Bowler DM, Matthews NJ, Gardiner JM (1997) Asperger’s syndrome and memory: Similarity to autism but not amnesia. Neuropsychologia 35:65–70. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Raymond JL (2004) Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Annu Rev Neurosci 27:581–609. [DOI] [PubMed] [Google Scholar]
- Brooks JX, Cullen KE (2013) The primate cerebellum selectively encodes unexpected self-motion. Curr Biol 23:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NB, Dunn W (2010) Relationship between context and sensory processing in children with autism. Am J Occup Ther 64:474–483. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callenmark B, Kjellin L, Rönnqvist L, Bölte S (2014) Explicit versus implicit social cognition testing in autism spectrum disorder. Autism 18:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K (2019) Cerebellar modulation of the reward circuitry and social behavior. Science 363:eaav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrol FP, Blot A, Mrsic-Flogel TD (2019) Cerebellar contribution to preparatory activity in motor neocortex. Neuron 103. 506–519 e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon V, Farrer C, Pacherie E, Jacquet PO, Leboyer M, Zalla T (2017) Reduced sensitivity to social priors during action prediction in adults with autism spectrum disorders. Cognition 160:17–26. [DOI] [PubMed] [Google Scholar]
- Chao OY, Marron Fernandez de Velasco E, Pathak SS, Maitra S, Zhang H, Duvick L, Wickman K, Orr HT, Hirai H, Yang Y-M (2020) Targeting inhibitory cerebellar circuitry to alleviate behavioral deficits in a mouse model for studying idiopathic autism. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed]
- Chez MG, Chang M, Krasne V, Coughlan C, Kominsky M, Schwartz A (2006) Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy Behav 8:267–271. [DOI] [PubMed] [Google Scholar]
- Chrobak AA, Soltys Z (2017) Bergmann glia, long-term depression, and autism spectrum disorder. Mol Neurobiol 54:1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Webb S, Schumann C (2011) From toddlers to adults: the changing landscape of the brain in autism. Autism Spectrum Disorders:611–631.
- Courchesne E, Yeung-Courchesne R, Hesselink J, Jernigan T (1988) Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med 318:1349–1354. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P (2000) The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain 123:2203–2212. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Brooks JX (2015) Neural correlates of sensory prediction errors in monkeys: evidence for internal models of voluntary self-motion in the cerebellum. Cerebellum 14:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupolillo D, Hoxha E, Faralli A, De Luca A, Rossi F, Tempia F, Carulli D (2015) Autistic-like traits and cerebellar dysfunction in Purkinje cell PTEN knock-out mice. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed]
- Cupolillo D, Hoxha E, Faralli A, De Luca A, Rossi F, Tempia F, Carulli D (2016) Autistic-like traits and cerebellar dysfunction in Purkinje cell PTEN knock-out mice. Neuropsychopharmacology 41:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Arc BF, Devaine M, Daunizeau J (2020) Social behavioural adaptation in autism. PLoS Comput Biol 16 e1007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, Crocetti D, Mostofsky SH, Stoodley CJ (2015) Cerebellar gray matter and lobular volumes correlate with core autism symptoms. NeuroImage: Clinical 7:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello AM, Stoodley CJ (2015) Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie JT, Clark BA, Häusser M (2008) The origin of the complex spike in cerebellar Purkinje cells. J Neurosci 28:7599–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonge M, Kemner C, De Haan E, Coppens J, Van den Berg T, Van Engeland H (2007) Visual information processing in high-functioning individuals with autism spectrum disorders and their parents. Neuropsychology 21:65. [DOI] [PubMed] [Google Scholar]
- de Vries M, Geurts HM (2012) Cognitive flexibility in ASD; task switching with emotional faces. J Autism Dev Disord 42:2558–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw C, Holstege J, Ruigrok T, Voogd J (1990) Mesodiencephalic and cerebellar terminals terminate upon the same dendritic spines in the glomeruli of the cat and rat inferior olive: an ultrastructural study using a combination of [3H]-leucine and wheat germ agglutinin coupled horseradish peroxidase anterograde tracing. Neuroscience 34:645–655. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoogenraad CC, Koekkoek S, Ruigrok TJ, Galjart N, Simpson JI (1998) Microcircuitry and function of the inferior olive. Trends Neurosci 21:391–400. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Li J, Schiller D, Phelps EA (2008) The role of the striatum in aversive learning and aversive prediction errors. Philos Trans Royal Soc B 363:3787–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Pelisson D, Urquizar C, Prablanc C, Alexander G, Grafton S (1998) Functional anatomy of saccadic adaptation in humans. Nat Neurosci 1:524–528. [DOI] [PubMed] [Google Scholar]
- DuBois D, Ameis SH, Lai M-C, Casanova MF, Desarkar P (2016) Interoception in autism spectrum disorder: a review. Int J Dev Neurosci 52:104–111. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Mak-Fan KM, Taylor MJ, Roberts SW (2012) Regional differences in grey and white matter in children and adults with autism spectrum disorders: an activation likelihood estimate (ALE) meta-analysis. Autism Res 5:49–66. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD (1962) Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol 161:282–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegood J, Anagnostou E, Babineau B, Crawley J, Lin L, Genestine M, Dicicco-Bloom E, Lai J, Foster J, Penagarikano O (2015) Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Mol Psychiatry 20:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst TM, Brol AE, Gratz M, Ritter C, Bingel U, Schlamann M, Maderwald S, Quick HH, Merz CJ, Timmann D (2019) The cerebellum is involved in processing of predictions and prediction errors in a fear conditioning paradigm. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE (2012) Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11:777–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, Thuras P, Merz A (2002) Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol 22:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BR, Gao W-J (2018) Thalamic control of cognition and social behavior via regulation of gamma-aminobutyric acidergic signaling and excitation/inhibition balance in the medial prefrontal cortex. Biol Psychiatry 83:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE, Cheney MK, Raichle ME (1992) Impaired non-motor learning and error detection associated with cerebellar damage: a single case study. Brain 115:155–178. [DOI] [PubMed] [Google Scholar]
- Frith U, Happé F (1999) Theory of mind and self-consciousness: What is it like to be autistic? Mind Language 14:82–89. [Google Scholar]
- Fujita H, Kodama T, du Lac S (2020) Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Tiley C, O’Keeffe S, Harrison NA, Seth AK, Critchley HD (2016) Discrepancies between dimensions of interoception in autism: implications for emotion and anxiety. Biol Psychol 114:117–126. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM (2008) Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of Fragile X Syndrome. J Neurophysiol 100:2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, LeBlanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK (2009) Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord 1:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene RK, Zheng S, Kinard JL, Mosner MG, Wiesen CA, Kennedy DP, Dichter GS (2019) Social and nonsocial visual prediction errors in autism spectrum disorder. Autism Res 12:878–883. [DOI] [PubMed] [Google Scholar]
- Grosche J, Kettenmann H, Reichenbach A (2002) Bergmann glial cells form distinct morphological structures to interact with cerebellar neurons. J Neurosci Res 68:138–149. [DOI] [PubMed] [Google Scholar]
- Güçlü B, Tanidir C, Mukaddes NM, Ünal F (2007) Tactile sensitivity of normal and autistic children. Somatosens Mot Res 24:21–33. [DOI] [PubMed] [Google Scholar]
- Ha S, Lee D, Cho YS, Chung C, Yoo Y-E, Kim J, Lee J, Kim W, Kim H, Bae YC (2016) Cerebellar Shank2 regulates excitatory synapse density, motor coordination, and specific repetitive and anxiety-like behaviors. J Neurosci 36:12129–12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé FG (1995) The role of age and verbal ability in the theory of mind task performance of subjects with autism. Child Dev 66:843–855. [PubMed] [Google Scholar]
- Hays SA, Huber KM, Gibson JR (2011) Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J Neurosci 31:14223–14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffley W, Hull C (2019) Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt AL, Popa LS, Pasalar S, Hendrix CM, Ebner TJ (2011) Representation of limb kinematics in Purkinje cell simple spike discharge is conserved across multiple tasks. J Neurophysiol 106:2232–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton CL, Harper JD, Kueker RH, Lang AR, Abbacchi AM, Todorov A, LaVesser PD (2010) Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. J Autism Dev Disord 40:937–945. [DOI] [PubMed] [Google Scholar]
- Hoche F, Guell X, Sherman JC, Vangel MG, Schmahmann JD (2016) Cerebellar contribution to social cognition. Cerebellum 15:732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J, Koch I. (1998). Implicit learning of loosely defined structures.
- Hull C (2020) Prediction signals in the cerebellum: beyond supervised motor learning. eLife 9 e54073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Shenoy P, Angela JY, Chiang-Shan RL (2013) Bayesian prediction and evaluation in the anterior cingulate cortex. J Neurosci 33:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M (2001) Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev 81:1143–1195. [DOI] [PubMed] [Google Scholar]
- Ito M (2002) The molecular organization of cerebellar long-term depression. Nat Rev Neurosci 3:896–902. [DOI] [PubMed] [Google Scholar]
- Ito M (2006) Cerebellar circuitry as a neuronal machine. Prog Neurobiol 78:272–303. [DOI] [PubMed] [Google Scholar]
- Ito M (2008) Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci 9:304–313. [DOI] [PubMed] [Google Scholar]
- Ito M (2013) Error detection and representation in the olivo-cerebellar system. Front Neural Circuits 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Itō M. (1984). The cerebellum and neural control (Raven press; ). [Google Scholar]
- Ito M, Kano M (1982) Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett 33:253–258. [DOI] [PubMed] [Google Scholar]
- Ito M, Nisimaru N, Shibuki K (1979) Destruction of inferior olive induces rapid depression in synaptic action of cerebellar Purkinje cells. Nature 277:568–569. [DOI] [PubMed] [Google Scholar]
- Ito M, Sakurai M, Tongroach P (1982) Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol 324:113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yoshida M (1964) The cerebellar-evoked monosynaptic inhibition of Deiters’ neurones. Experientia 20:515–516. [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshida M, Obata K (1964) Monosynaptic inhibition of the intracerebellar nuclei induced from the cerebellar cortex. Experientia 20:575–576. [DOI] [PubMed] [Google Scholar]
- Ivy JW, Schreck KA (2016) The efficacy of ABA for individuals with autism across the lifespan. Curr Dev Disord Rep 3:57–66. [Google Scholar]
- Jung M, Kosaka H, Saito DN, Ishitobi M, Morita T, Inohara K, Asano M, Arai S, Munesue T, Tomoda A (2014) Default mode network in young male adults with autism spectrum disorder: relationship with autism spectrum traits. Mol Autism 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M, Gomi H (1992) A computational model of four regions of the cerebellum based on feedback-error learning. Biol Cybern 68:95–103. [DOI] [PubMed] [Google Scholar]
- Kelly E, Meng F, Fujita H, Morgado F, Kazemi Y, Rice LC, Ren C, Escamilla CO, Gibson JM, Sajadi S, et al. (2020) Regulation of autism-relevant behaviors by cerebellar-prefrontal cortical circuits. Nat Neurosci 23:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny EM, Cormican P, Furlong S, Heron E, Kenny G, Fahey C, Kelleher E, Ennis S, Tropea D, Anney R, et al. (2014) Excess of rare novel loss-of-function variants in synaptic genes in schizophrenia and autism spectrum disorders. Mol Psychiatry 19:872–879. [DOI] [PubMed] [Google Scholar]
- Khan AJ, Nair A, Keown CL, Datko MC, Lincoln AJ, Müller R-A (2015) Cerebro-cerebellar resting-state functional connectivity in children and adolescents with autism spectrum disorder. Biol Psychiatry 78:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Thompson RE (1997) Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci 20:177–181. [DOI] [PubMed] [Google Scholar]
- Kinard JL, Mosner MG, Greene RK, Addicott M, Bizzell J, Petty C, Cernasov P, Walsh E, Eisenlohr-Moul T, Carter RM (2020) Neural mechanisms of social and nonsocial reward prediction errors in adolescents with autism spectrum disorder. Autism Research 13:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger LG, Dawson G (1995) A fresh look at categorization abilities in persons with autism. Learn Cognition Autism (Springer; ):119–136. [Google Scholar]
- Klinger LG, Klinger MR, Pohlig RL (2007) Implicit learning impairments in autism spectrum disorders. New developments in autism. Future Today:76–103.
- Kloth AD, Badura A, Li A, Cherskov A, Connolly SG, Giovannucci A, Bangash MA, Grasselli G, Peñagarikano O, Piochon C (2015) Cerebellar associative sensory learning defects in five mouse autism models. Elife 4 e06085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, et al. (2005) Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron 47:339–352. [DOI] [PubMed] [Google Scholar]
- Koirala S, Corfas G (2010) Identification of novel glial genes by single-cell transcriptional profiling of Bergmann glial cells from mouse cerebellum. PLoS ONE 5 e9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RP, Rees G, Friston KJ (2014) An aberrant precision account of autism. Front Hum Neurosci 8:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Nieto C, Libby SJ, Wing L, Gould J (2007) Describing the sensory abnormalities of children and adults with autism. J Autism Dev Disord 37:894–910. [DOI] [PubMed] [Google Scholar]
- Lloyd M, MacDonald M, Lord C (2011) Motor skills of toddlers with autism spectrum disorders. Autism 17:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke TM, Fujita H, Hunker A, Johanson SS, Darvas M, du Lac S, Zweifel LS, Carlson ES (2020) Purkinje cell-specific knockout of tyrosine hydroxylase impairs cognitive behaviors. Front Cell Neurosci 14:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke TM, Soden ME, Miller SM, Hunker A, Knakal C, Licholai JA, Dhillon KS, Keene CD, Zweifel LS, Carlson ES (2018) Dopamine D1 receptor–positive neurons in the lateral nucleus of the cerebellum contribute to cognitive behavior. Biol Psychiatry 84:401–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LBN, Hill SS, Nagarajan SS (2011) Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res 69:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D (1969) A theory of cerebellar cortex. J Physiol 202:437–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Sölch C, Magyar S, Künig G, Missimer J, Schultz W, Leenders K (2001) Changes in brain activation associated with reward processing in smokers and nonsmokers. Exp Brain Res 139:278–286. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW (2006) An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26:3642–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MV, Gandal MJ, Siegel SJ (2011) mGluR5-antagonist mediated reversal of elevated stereotyped, repetitive behaviors in the VPA model of autism. PLoS ONE 6:e26077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ (2007) A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci 27:5546–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget T, Gullesen EH, Andersson S, Ivry RB, Endestad T (2014) Generalized role for the cerebellum in encoding internal models: evidence from semantic processing. J Neurosci 34:2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan SE, Sunderland T, McIntosh A, Herscovitch P, Schreurs BG (1994) A functional anatomical study of associative learning in humans. Proc Natl Acad Sci 91:8122–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S (2003) Task switching. Trends Cognitive Sci 7:134–140. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Mohanty S, Greene RK, Cook EH, Vaillancourt DE, Sweeney JA (2015a) Feedforward and feedback motor control abnormalities implicate cerebellar dysfunctions in autism spectrum disorder. J Neurosci 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi MW, Wang Z, Schmitt LM, Tsai P, Sweeney JA (2015b) The role of cerebellar circuitry alterations in the pathophysiology of autism spectrum disorders. Front Neurosci 9:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ (2009) Decreased connectivity and cerebellar activity in autism during motor task performance. Brain 132:2413–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Keeser D, Samson AC, Kirsch V, Blautzik J, Grothe M, Erat O, Hegenloh M, Coates U, Reiser MF (2013) Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: a multimodal MRI study. PLoS ONE 8 e67329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami JW, Courchesne E, Haas R, Press G, Yeung-Courchesne R (1992) Cerebellar and cerebral abnormalities in Rett syndrome: a quantitative MR analysis. AJR Am J Roentgenol 159:177–183. [DOI] [PubMed] [Google Scholar]
- Nelson Sacha B, Valakh V (2015) Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Müller R-A (2009) Aberrant functional connectivity in autism: evidence from low-frequency BOLD signal fluctuations. Brain Res 1262:48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea AG, Fein DA, Cillessen AH, Klin A, Schultz RT (2005) Source memory in children with autism spectrum disorders. Dev Neuropsychol 27:337–360. [DOI] [PubMed] [Google Scholar]
- Oristaglio J, West SH, Ghaffari M, Lech MS, Verma BR, Harvey JA, Welsh JP, Malone RP (2013) Children with autism spectrum disorders show abnormal conditioned response timing on delay, but not trace, eyeblink conditioning. Neuroscience 248:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C (2004) Neuropathological findings in autism. Brain 127:2572–2583. [DOI] [PubMed] [Google Scholar]
- Parker KL, Narayanan NS, Andreasen NC (2014) The therapeutic potential of the cerebellum in schizophrenia. Front Syst Neurosci 8:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaudeau S, O’Neill P-K, Bolkan SS, Ward RD, Abbas AI, Roth BL, Balsam PD, Gordon JA, Kellendonk C (2013) Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 77:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Cohen DJ (1985) Comprehension of indirect requests in adults with autistic disorders and mental retardation. J Speech Language Hear Res 28:475–479. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Jeffery L, Burr D, Rhodes G (2007) Abnormal adaptive face-coding mechanisms in children with autism spectrum disorder. Curr Biol 17:1508–1512. [DOI] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A (2011) Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL (2019) Corollary discharge signals in the cerebellum. Biol Psychiatry: Cognitive Neurosci Neuroimaging. [DOI] [PMC free article] [PubMed]
- Person AL, Raman IM (2012) Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature 481:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter S, ten Brinke MM, Stedehouder J, Reinelt CM, Wu B, Zhou H, Zhou K, Boele H-J, Kushner SA, Lee MG, et al. (2016) Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat Commun 7:12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters LC, Thompson RH (2018) How teaching perspective taking to individuals with autism spectrum disorders affects social skills: findings from research and suggestions for practitioners. Behav Anal Pract 11:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EJ, Voytek B (2017) Alpha oscillations control cortical gain by modulating excitatory-inhibitory background activity. bioRxiv 185074. [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E (2004) The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain 127:2703–2716. [DOI] [PubMed] [Google Scholar]
- Piochon C, Kloth AD, Grasselli G, Titley HK, Nakayama H, Hashimoto K, Wan V, Simmons DH, Eissa T, Nakatani J, et al. (2014) Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat Commun 5:5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa LS, Streng ML, Hewitt AL, Ebner TJ (2016) The errors of our ways: understanding error representations in cerebellar-dependent motor learning. Cerebellum 15:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Adler M, Crocetti D, Miller MI, Mostofsky SH (2010) Basal ganglia shapes predict social, communication, and motor dysfunctions in boys with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 49. 539–551. e534. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP (1999) Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci 19:4585–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Elliott R, Athwal B, Passingham R (2004) Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage 23:777–786. [DOI] [PubMed] [Google Scholar]
- Reber AS (1993) Implicit learning and tacit knowledge: an essay on the cognitive unconscious. New York: Oxford University Press. [Google Scholar]
- Reith RM, McKenna J, Wu H, Hashmi SS, Cho S-H, Dash PK, Gambello MJ (2013) Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol Dis 51:93–103. [DOI] [PubMed] [Google Scholar]
- Requarth T, Sawtell Nathaniel B (2014) Plastic corollary discharge predicts sensory consequences of movements in a cerebellum-like circuit. Neuron 82:896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Annunziata S, Contarino V, Erbetta A, Aquino D, Bulgheroni S (2013) Gray matter reduction in the vermis and CRUS-II is associated with social and interaction deficits in low-functioning children with autistic spectrum disorders: a VBM-DARTEL Study. Cerebellum 12:676–685. [DOI] [PubMed] [Google Scholar]
- Rogers TD, Dickson PE, Heck DH, Goldowitz D, Mittleman G, Blaha CD (2011) Connecting the dots of the cerebro-cerebellar role in cognitive function: neuronal pathways for cerebellar modulation of dopamine release in the prefrontal cortex. Synapse 65:1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TD, Dickson PE, McKimm E, Heck DH, Goldowitz D, Blaha CD, Mittleman G (2013) Reorganization of circuits underlying cerebellar modulation of prefrontal cortical dopamine in mouse models of autism spectrum disorder. Cerebellum 12:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenhall U, Nordin V, Brantberg K, Gillberg C (2003) Autism and auditory brain stem responses. Ear Hear 24:206–214. [DOI] [PubMed] [Google Scholar]
- Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK, Fowler SC, Malenka RC, Südhof TC (2014) Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell 158:198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J, Merzenich MM (2003) Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav 2:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford M, Troje NF (2012) IQ predicts biological motion perception in autism spectrum disorders. J Autism Dev Disord 42:557–565. [DOI] [PubMed] [Google Scholar]
- Ryu YH, Lee JD, Yoon PH, Kim DI, Lee HB, Shin YJ (1999) Perfusion impairments in infantile autism on technetium-99m ethyl cysteinate dimer brain single-photon emission tomography: comparison with findings on magnetic resonance imaging. Eur J Nucl Med 26:253–259. [DOI] [PubMed] [Google Scholar]
- Scheggia D, Managò F, Maltese F, Bruni S, Nigro M, Dautan D, Latuske P, Contarini G, Gomez-Gonzalo M, Requie LM (2019) Somatostatin interneurons in the prefrontal cortex control affective state discrimination in mice. Nat Neurosci:1–14. [DOI] [PubMed]
- Schlerf J, Ivry RB, Diedrichsen J (2012) Encoding of sensory prediction errors in the human cerebellum. J Neurosci 32:4913–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Mcintosh AR, Bahro M, Herscovitch P, Sunderland T, Molchan SE (1997) Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response. J Neurophysiol 77:2153–2163. [DOI] [PubMed] [Google Scholar]
- Scott JA, Schumann CM, Goodlin-Jones BL, Amaral DG (2009) A comprehensive volumetric analysis of the cerebellum in children and adolescents with autism spectrum disorder. Autism Res 2:246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Finn PR, Steinmetz JE (1994) Abnormal classical eye-blink conditioning in autism. J Autism Dev Disord 24:737–751. [DOI] [PubMed] [Google Scholar]
- Selimbeyoglu A, Kim CK, Inoue M, Lee SY, Hong ASO, Kauvar I, Ramakrishnan C, Fenno LE, Davidson TJ, Wright M, et al. (2017) Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci Transl Med 9:eaah6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A (2013) Atypical development of spontaneous social cognition in autism spectrum disorders. Brain Dev 35:96–101. [DOI] [PubMed] [Google Scholar]
- Shibuki K, Gomi H, Chen L, Bao S, Kim JJ, Wakatsuki H, Fujisaki T, Fujimoto K, Katoh A, Ikeda T (1996) Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron 16:587–599. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U (2008) Levels of emotional awareness and autism: an fMRI study. Soc Neurosci 3:97–112. [DOI] [PubMed] [Google Scholar]
- Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, Diamond SP, Held RM (2014) Autism as a disorder of prediction. Proc Natl Acad Sci 111:15220–15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov AA, Miall RC, Ivry RB (2017) The cerebellum: adaptive prediction for movement and cognition. Trends Cognitive Sci 21:313–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS (2009) The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia 47:2515–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SJ, Schneider MT (2009) The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatr Res 65:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ (2014) Distinct regions of the cerebellum show gray matter decreases in autism, ADHD, and developmental dyslexia. Front Syst Neurosci 8:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, Gibson JM, Kelly E, Meng F, Cano CA, et al. (2017) Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci 20:1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA (2009) Cerebellum and nonmotor function. Annu Rev Neurosci 32:413–434. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Lindner A, Thier P (2008) The cerebellum updates predictions about the visual consequences of one’s behavior. Curr Biol 18:814–818. [DOI] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S (2016). Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. In Behavioral economics of preferences, choices, and happiness (Springer; ), pp. 593–616. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB (2014) Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34:3023–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Polli FE, Joseph RM, Tuch DS, Hadjikhani N, Barton JJ, Manoach DS (2008) Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD). Brain 131:2464–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma P, Bellebaum C, Koch B, Schwarz M, Daum I (2008) The cerebellum is involved in reward-based reversal learning. Cerebellum 7:433. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M (2012) Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488:647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng Y-W, Diedrichsen J.r., Krakauer JW, Shadmehr R, and Bastian AJ (2007). Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98, 54–62. [DOI] [PubMed] [Google Scholar]
- Tuchman R, Moshé SL, Rapin I (2009) Convulsing toward the pathophysiology of autism. Brain Dev 31:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Cruys S, Evers K, Van der Hallen R, Van Eylen L, Boets B, de-Wit L, Wagemans J (2014). Precise minds in uncertain worlds: Predictive coding in autism. Psychol Rev 121, 649. [DOI] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, Emsell L, Boets B, Noens I, Steyaert J (2014) Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. NeuroImage: Clinical 4:374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino CN, Perez JM, Comi ML, and Gonzalez PM (2006). New developments in autism: The Future is Today (Jessica Kingsley Publishers; ). [Google Scholar]
- Vogels TP, Abbott L (2009) Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat Neurosci 12:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen EA, Stoyanova RS, Baron-Cohen S, Calder AJ (2013) Reduced functional connectivity within and between ‘social’resting state networks in autism spectrum conditions. Social Cognitive Affect Neurosci 8:694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Schraa-Tam CK, van der Geest JN, De Zeeuw CI (2012) Visuomotor cerebellum in human and nonhuman primates. Cerebellum 11:392–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L (2017) Cerebellar granule cells encode the expectation of reward. Nature 544:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M (2007) Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch Gen Psychiatry 64:698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ (2008) Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum 7:406–416. [DOI] [PubMed] [Google Scholar]
- Whitney ER, Kemper TL, Rosene DL, Bauman ML, Blatt GJ (2009) Density of cerebellar basket and stellate cells in autism: evidence for a late developmental loss of Purkinje cells. J Neurosci Res 87:2245–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M (1998) Internal models in the cerebellum. Trends Cognitive Sci 2:338–347. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF (1985) Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res 348:249–260. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. (2011) Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]


