Abstract
Emotional experiences create durable memory traces in the brain, especially when these memories are consolidated in the presence of stress hormones such as cortisol. Although some research suggests cortisol elevation can increase long-term memory for emotional relative to neutral content, the impact of stress and cortisol on the consolidation of emotional and neutral aspects of memories when they are part of the same experience remains unknown. Here, after encoding complex scenes consisting of negative or neutral objects placed on neutral backgrounds, participants were exposed to a psychosocial stressor (or matched control condition) in order to examine the impact of stress and cortisol on early consolidation processes. The next day, once cortisol levels had returned to baseline, specific and gist recognition memory were tested separately for objects and backgrounds. Results indicate that while there was a numerical increase in memory for negative objects in the stress group, higher endogenous cortisol concentrations were specifically associated with decreased memory for the neutral backgrounds originally paired with negative objects. Moreover, across all participants, cortisol levels were positively correlated with the magnitude of the emotional memory trade-off effect. Specifically, while memory for negative objects was preserved, elevated cortisol during early consolidation was associated with decreased memory for neutral backgrounds that were initially paired with negative objects. These memory effects were observed in both the stricter specific measure of memory and the less conservative measure of gist memory. Together, these findings suggest that rather than influencing all aspects of an experience similarly, elevated cortisol during early consolidation selectively preserves what is most emotionally salient and adaptive to remember while allowing the loss of memory for less important neutral information over time.
Keywords: stress, cortisol, emotional memory, HPA axis, encoding, consolidation
INTRODUCTION
Emotional experiences leave durable traces in the brain and tend to be exceptionally well-remembered, especially when formed during times of stress. This bias to remember what is affectively salient likely serves an adaptive purpose, as remembering emotional experiences is critical to survival and can help us navigate a complex social world. A wealth of evidence accumulated over the past several decades suggests that stress hormones released during arousing experiences play a key role in emotional memory formation1-4 (but see 5 for review of some exceptions). The adrenergic system and the hypothalamic-pituitary-adrenal (HPA) axis work in concert to preferentially preserve emotional information in long-term memory,2 sometimes at the detriment of memory for neutral information.6-9 Such processes take time to fully develop and have their maximal influence during the consolidation phase of memory formation, which takes place in the minutes, hours, and days post-encoding.2,10
Following a stressful experience, concurrent glucocorticoid and norepinephrine activity in the basolateral amygdala (BLA) enhances interactions among the amygdala, hippocampus, and other memory-relevant regions of the brain, such as the ventromedial prefrontal cortex (vmPFC).2,11 Given the importance of this network for emotional memory, its potentiation by stress is thought to underlie behavioral evidence for the consolidation of emotionally salient memories over neutral ones that have less adaptive value.
In humans, elevated cortisol, resulting either from exposure to a stressful experience or exogenous cortisol administration, can facilitate long-term memory for emotionally arousing, relative to neutral, information,6,7,12,13 although there are some important exceptions.14-20 For example, Buchanan and Lovallo (2001) demonstrated that a 20mg dose of cortisol during learning enhanced the consolidation of emotionally arousing but not non-arousing pictures.12 Using a psychosocial stressor, Payne et al. (2007) demonstrated that stress exposure enhanced long-term memory for an emotionally arousing slideshow relative to a control condition, but impaired memory for a closely matched neutral slide show.7 Cahill et al. (2003) showed that participants who were exposed to cold pressor stress after watching a slideshow consisting of neutral and emotionally arousing slides remembered more emotional slides than non-stressed control participants, whereas memory for neutral slides was unaffected.13 Abercrombie, Speck, and Monticelli (2006) demonstrated that while cortisol elevations in humans are often correlated with enhanced memory consolidation for emotionally laden information, this is only the case when individuals are emotionally aroused.21 Similarly, in rats, stress hormones typically do not globally enhance memory consolidation, but tend to selectively modulate consolidation of emotionally arousing experiences.4,22-24
Underlying these effects at a neural level, work in animal models has clarified that while elevated stress often impairs hippocampal and PFC function, amygdala function and plasticity is enhanced.25,26 Likewise, human neuroimaging studies demonstrate that cortisol elevations at encoding can diminish hippocampal activity, while potentiating activity in the amygdala under certain conditions. For example, Pruessner et al. (2008) showed that acute stress provoked significant deactivation in regions of the limbic system, including the hippocampus, and the degree of hippocampal deactivation was significantly correlated with cortisol reactivity.27 Van Stegeren and colleagues (2007) found that endogenously elevated cortisol levels correlated with intensified amygdala activation at encoding and better future memory for emotional information, but only in the presence of sufficient noradrenaline in the amygdala.28
Each of these studies highlights the importance of stress- and arousal-related neuromodulators in the formation of emotional memories. An important next step is to examine precisely which aspects of memories of emotional events are influenced by the physiological stress response. This is an important question because memories of emotional events are not stored as precise replicas of the original experience, and thus stress may preferentially enhance some, but not all, aspects of our emotional experiences. For example, central, emotionally salient information is typically remembered at the expense of neutral background information,29-34 which is a type of emotional memory trade-off effect. Such trade-off effects in emotional memory are not restricted to the laboratory but can also be found in the real world. One ecologically relevant example is the weapon-focus effect, where victims vividly remember an assailant’s weapon but have poor memory for other aspects of the event even if it is useful information, such as the perpetrator’s face.35 This divergence in memory for central and peripheral aspects of emotional episodes depends in part on differential attention and encoding of these two aspects of the scene. However, we also know that these elements undergo qualitatively different processing after encoding, during the consolidation period, particularly if this period includes sleep.32,33,36-38
In prior studies,32,39-41 researchers presented participants with pictures of neutral (e.g., a harmless-looking chipmunk) or negatively arousing (e.g., a vicious-looking snake) objects placed on neutral backgrounds (e.g., a forest scene), and subsequently tested their memory for the objects and backgrounds separately. Even after a brief delay, there was evidence of an emotional trade-off effect;29 negative emotional objects were consistently better remembered than neutral objects, but the neutral backgrounds were more poorly remembered if they had been presented with negative objects than if they had been paired with neutral objects. Importantly for theories of consolidation, a period of sleep (which is considered to provide ideal conditions for memory consolidation processes to operate) magnified this trade-off effect compared to a period of wakefulness.32,33,42 This suggests that the two components of memory for scenes underwent differential processing during the consolidation phase. Thus, post-encoding processes play an important role in the fate of emotional memories.
Here, we examine whether post-encoding stress might affect the differential consolidation of these components of memory. This is important theoretically because we currently know little about how the different features of complex emotional experiences are processed and stored in memory, whether they change over time or remain the same, and whether stress affects their consolidation differently. For example, emotional scene memories could be stored as intact units, suffering some forgetting over time but retaining the same relative vividness for all components. Alternatively, the components could undergo differential memory processing, with a selective emphasis on what is most emotionally salient and important to remember.
Although numerous studies have demonstrated that increased cortisol around the time of learning benefit memory for emotionally arousing stimuli more than neutral stimuli,7,31,43,44 no study has yet examined the effect of elevated cortisol concentrations during the early consolidation phase on stimuli in which both emotional and neutral components are present. This is a particularly relevant question as a recent meta-analysis found that post-encoding stress was more likely to improve memory, and not all studies agree about how stress and cortisol affect neutral and emotional memory consolidation in general.5 Part of the reason for this lack of agreement may be because it remains unclear if HPA axis activity (i.e. including the release of cortisol) is required to produce these emotional memory benefits, or if non-specific aspects and/or the subjective experience of stress is sufficient to generate similar memory effects. In addition, benefits to emotional memory from post-encoding cortisol may be altered, lessened, or absent in some circumstances based on factors such as the method of cortisol induction, the degree of cortisol response, or the sex of the participant.14-16,18,45
Here, we predicted that HPA axis activity, measured as post-stressor cortisol concentration, would enhance the selective memory effect for emotional content. Specifically, we anticipated that cortisol increase would benefit consolidation of emotional aspects of scenes at the expense of their neutral backgrounds through the selective memory processing of information with greater salience. As such, we predicted that increased cortisol during the early consolidation period would magnify the emotional memory trade-off effect.
METHOD
Participants
Seventy-four participants (44 female, 30 male) from the University of Notre Dame (mean age 19.1 ± 1.1), who were part of a larger ongoing study examining sleep-stress interactions, completed a memory encoding session and retrieval session on two consecutive days. They participated for course credit or payment. Participants were native English speakers with normal or corrected-to-normal vision. Prior to enrollment in the study, participants were excluded if they reported a history of psychiatric illness (including anxiety or mood disorder), sleep disorder, or the use of medications that affect the central nervous system or endocrine system (e.g. antidepressants, ingested steroids, etc). All experimental protocols were approved by the University of Notre Dame IRB committee, and the methods were carried out in accordance with relevant guidelines and regulations. Informed consent was collected from all participants prior to participation. We prespecified sample sizes by calculating the sample size required to detect medium effects (Cohen’s d of 0.30, based on similar previous research32) with 85% power. We report all manipulations and measures. Any data excluded is discussed in detail below.
Materials
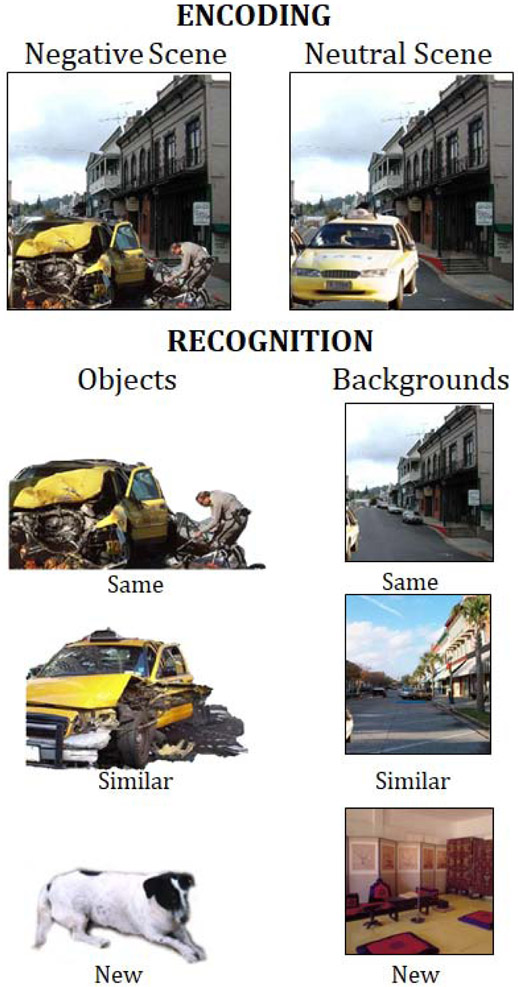
The scenes depicted negative arousing or neutral objects placed on plausible neutral backgrounds29,32,46. For each of 64 scenes (e.g., a car on a street), we created eight different versions by placing two similar neutral objects (e.g., two images of a car) and two related negative objects (e.g., two images of a car crash) on two neutral backgrounds (e.g., two images of a street; see Figure 1). An additional 32 scenes served as foils on the subsequent recognition memory test. The objects and backgrounds were normed on dimensions of valence and arousal using 7-point Likert scales in a prior experiment.47 All negative objects received arousal ratings of 5 to 7 (with higher scores representing an arousing image) and valence ratings lower than 3 (with lower scores representing a negative image). All neutral items (both objects and backgrounds) were rated as un-arousing (arousal values lower than 4) and neutral in valence (valence ratings between 3 and 5).
Figure 1.
Task. Demonstration of how the scenes were created for the emotional trade-off task. At encoding, full scenes were presented with a negative or neutral central object placed on a neutral background. At recognition, scene components were presented separately and one at a time and participants were asked to distinguish if the object or background was the same, similar, or completely new compared to what they saw during encoding.
Procedure
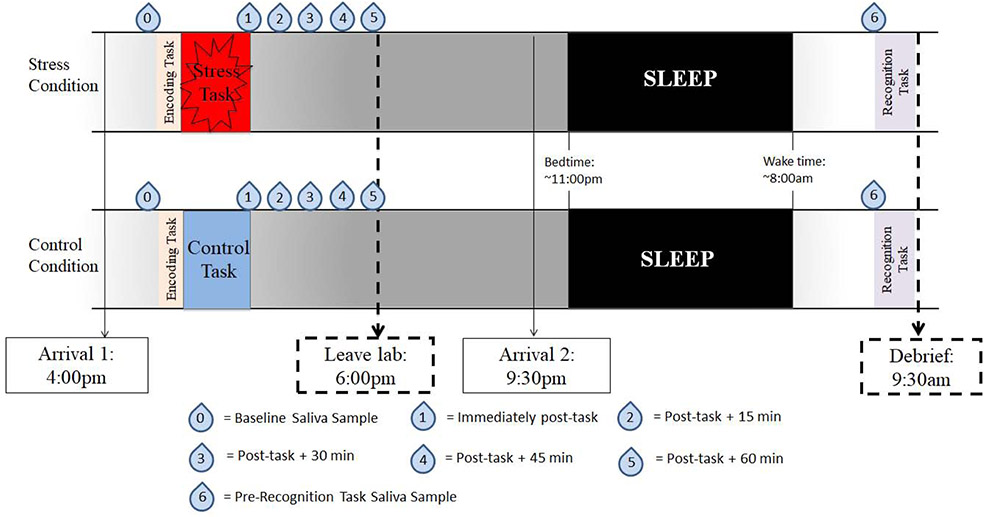
Figure 2 is a schematic of the study timeline. Participants were randomly assigned to a stress group (n = 39, female=22, mean age 19.0 ± 0.91) or a control group (n = 35, female=22 mean age 19.2 ± 1.2). Chi-square tests revealed no difference in the number of men and women in the stress and control conditions (χ2 = .32, p = .57). Participants encoded the stimuli in the late afternoon (between 4-5 pm), and everyone underwent the stress or control manipulation at approximately 5pm to control for circadian influences on cortisol secretion. During encoding, participants viewed 64 scenes (32 with a neutral object and 32 with a negative object, all placed on neutral backgrounds) for 5000ms each. For each scene, they indicated on a 7-point scale whether they would approach or back away from the scene if they encountered it in real life. This task was used to ensure that participants were paying attention to the scenes and to promote deeper encoding32,37,48. The studied version of each scene (of the eight possible versions) was counterbalanced across participants. Immediately following encoding of the scenes, participants were exposed to a validated psychosocial stressor, the Trier Social Stress Test (TSST; see Stress Manipulation below),49 or a matched control treatment, both of which lasted approximately 20 minutes. In addition to saliva sampling both prior and after the stress manipulation (see Cortisol Reactivity Assessment below), measures of state affect (Positive and Negative Affect Schedule; PANAS)50 and state anxiety (State-Trait Anxiety Inventory; STAI)51 were assessed at baseline, immediately after completion of the stress/control task, and again the next day prior to recognition testing.
Figure 2:
Schematic of study timeline.
The next morning at 9am, participants completed an unexpected, self-paced recognition task in which objects and backgrounds were presented separately and one at a time. Some of these objects and backgrounds were identical to the scene components that had been encoded (e.g., the same car accident), others were the alternate version of the object or background and thus shared the same verbal label but differed in specific visual details (a similar car accident), and others were objects or backgrounds that had not been seen at encoding (new). Participants either saw the same or the similar version of a particular item at test, never both. For each item, participants indicated whether it was an exact match to a previously viewed component (“same”), similar but not an exact match (“similar”), or not seen before (“new”).
The recognition task included 32 same objects (16 negative, 16 neutral), 32 similar objects (16 negative, 16 neutral), 32 new objects (16 negative, 16 neutral), 32 same backgrounds (16 previously presented with a negative object, 16 previously presented with a neutral object), 32 similar backgrounds (16 previously presented with a negative object, 16 previously presented with a neutral object), and 32 new backgrounds.
Stress Manipulation
The TSST is a well-established method of stress induction, reliably inducing cortisol elevations in laboratory settings.49 It combines social evaluative threat with stressor uncontrollability, which together typically produce a large HPA axis response in humans,52 although a number of individual difference factors can impact the response to this task.53-55 Participants undergoing the TSST are told that they will be judged on nonverbal and verbal performance while delivering a speech. They are then given a 10-min speech preparation period, followed by a 5-min speech on why they would be the best candidate for a job position, given without notes (notes are abruptly taken away from participants just before they begin their speech). The presentation could be on any job position, but they were required to use only truthful information and could not fabricate details about themselves.
Participants in the stress condition delivered their speeches standing in front of two judges wearing white lab coats and were given the impression that their performance was being audio- and video-recorded for later analysis. Immediately following the speech, participants performed a mental arithmetic task aloud (count backward from 1022 by 13’s as quickly and accurately as possible). Upon making a mistake, they were told to start over. After 5 minutes, the participants were told to stop and rejoin the experimenter to complete the first session of the study.
Participants in the control condition also prepared a speech on why they were the best candidate for a job, but prior to doing so were informed that they were in the control condition and would not be presenting it in front of anyone. Instead, after preparation, they read their speech aloud from their notes and completed the math task in an empty room with no audio or video equipment present. They were monitored (surreptitiously through a window) to ensure that they followed these directions and remained awake.
Cortisol Reactivity Assessment
Upon arrival, participants were given 20 min to acclimate to the laboratory setting prior to collection of the baseline saliva sample (t0). Participants used the passive drool method using a straw to expectorate (i.e. no gum, cotton, or other saliva flow stimulants were used) and were instructed to fill the test tube to the 5mL line, which typically takes 5-10 minutes per sample. Following the stress or control task, participants gave 5 additional saliva samples in 15-minute increments, including immediately following the TSST (t1), 15-min (t2), 30-min (t3), 45-min (t4), and 60-min (t5) post-stressor. We also collected a saliva sample just prior to the recognition task in the morning to ensure no differences in cortisol levels between groups remained at retrieval. Several studies have reported sex differences in stress-induced cortisol response and have highlighted the importance of controlling for such factors when investigating differences in the effects of endogenous cortisol levels.54,56-58 Thus, for the purposes of this study investigating the general effects of cortisol levels on emotional memory, cortisol was log-transformed to approximate a normal distribution59-61 and then standardized within sex and assay kit (see Supplementary Materials for further details). High scores on the cortisol distribution indicate high levels relative to other individuals of the same sex and within the same assay kit. This data analysis strategy is in line with prior behavioral and cognitive research wherein sex differences in hormones are observed and raw hormone data need to be normalized for parametric testing.61-63 For all of our analyses, we employ these log-transformed, standardized scores as our measures of endogenous cortisol levels (e.g., basal cortisol, cortisol reactivity). For further details of the saliva collection and analysis process, see Supplementary Material.
Memory Analysis
To investigate varying specificity of memory following the stress manipulation, we calculated both specific and gist recognition memory scores. Consistent with prior studies, a less conservative general or gist recognition score was computed by summing the number of “same” and “similar” responses to same items, as this score reflects memory for at least some aspects of the studied item.29,32 That is, for same items identified as either “same” or “similar”, participants had to remember at least that a particular type of object or background had been studied (e.g., that they had seen a car accident or a street), because otherwise they would have instead indicated that the item was “new”. Thus, this more general recognition memory is a measure of a participant’s ability to remember at least the gist of the items (with or without specific detail). The more conservative specific recognition score (i.e., summing only “same” responses to same items), was computed to capture veridical memory for the precise visual details of a studied object or background. Specific and gist recognition scores were computed for each type of scene component (negative and neutral central objects and peripheral neutral backgrounds that had been studied with either a negative or neutral object). Both specific and gist recognition scores were corrected for response bias by subtracting the proportion of false alarms (“same” responses to new items) for both the central objects and the peripheral backgrounds of scenes. To assess the magnitude of the trade-off effect, we calculated a trade-off score by subtracting the corrected memory score for the paired (neutral) backgrounds from the corrected memory score for the objects (e.g., [negative object memory score] - [memory score for backgrounds originally paired with negative objects)]. The data were de-identified, processed, and analyzed in SPSS. All de-identified data, documentation, and SPSS output code are available at this link: https://osf.io/egjku/?view_only=1b88f97107d741e3b6b186f46d59867e.
RESULTS
Stressor Efficacy
Cortisol Reactivity
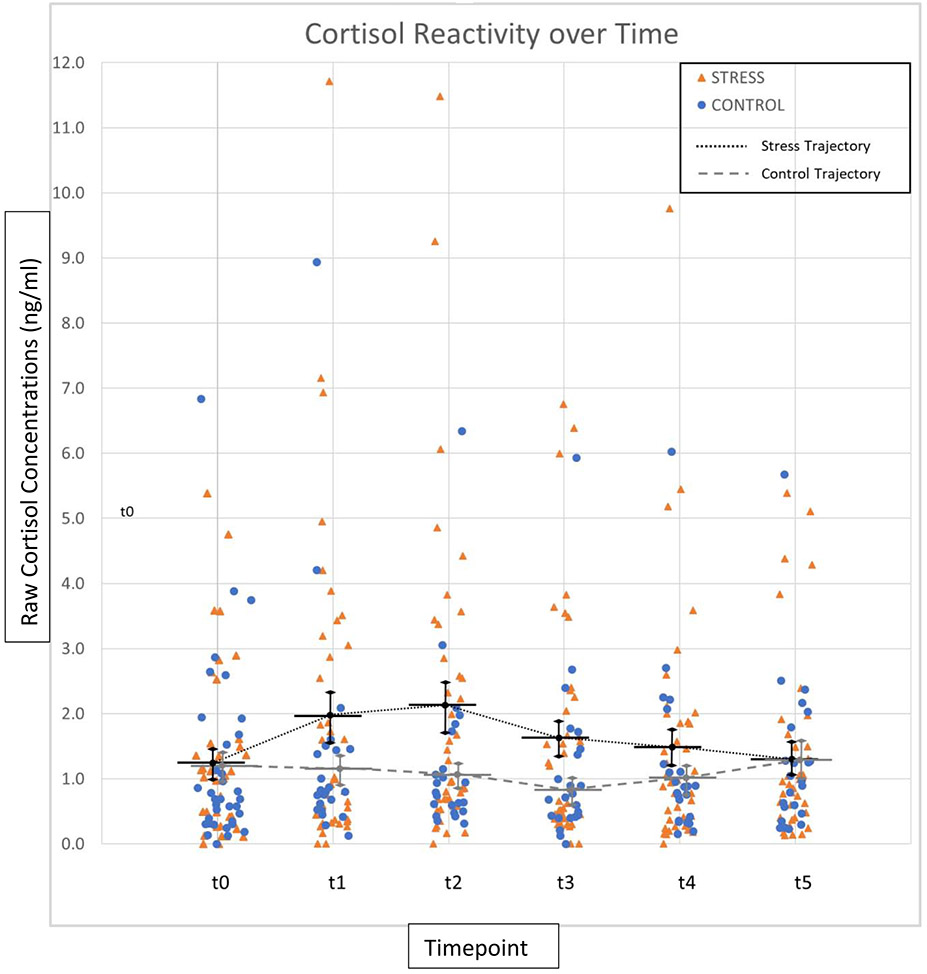
To assess the impact of the TSST vs. control manipulation on cortisol reactivity, measures of reactivity (baseline to post-TSST maximum cortisol value) were compared between groups. First, utilizing raw cortisol concentration levels, we found that while baseline cortisol concentration was nearly identical for the stress and control groups [t(72) = −.149, p = .88, d =0.03 , 95% CI (−.68, .59)], cortisol reactivity following the manipulation task measured as the difference in cortisol from pre-task baseline to post-task peak cortisol value revealed that the stress group demonstrated a significant increase in cortisol compared to the control group [t(72) = −2.34, p = .022, d = 0.55 , 95% CI (−1.78, −.14), see Figure 3]. Further, there was no difference in cortisol levels between groups in the morning prior to the recognition task [t(63) = −0.55, p = .59].t As mentioned above, the raw hormone data was then natural log transformed and then standardized to control for sex and assay kit (see 63 and Supplementary Material). Using these standardized scores, there was again no difference in baseline cortisol concentration between groups [t(72) = −.129, p = .90, d =0.03 , 95% CI (−.47, .42)], while the stress group showed a significant increase in cortisol over time compared to the control group [t(72) = −2.06, p = .042, d = 0.47 , 95% CI (−.90, −.01)], again measured as the difference in cortisol from baseline to peak cortisol value. These log-transformed and standardized data were used in all correlational analyses.
Figure 3.
Cortisol Response (ng/mol). Comparing raw salivary cortisol reactivity to stress and control conditions from baseline to 60 min after task. t0 = baseline sample, t1 = immediately post-stressor, t2 = 15 min, t3 = 30 min, t4 = 45 min, t5 = 60 min post-stressor. Orange Triangles = Stress Participants; Blue Circles = Control Participants; Black Dotted Line = Stress Group Cortisol Trajectory; Gray Dashed Line = Control Group Trajectory; Black Solid Bars = Stress Group Mean; Gray Solid Bars = Control Group Mean; Error bars = SEM
Subjective Measures of Stress and Negative Affect
To assess the efficacy of our stressor and to determine its impact on subjective affect, we conducted mixed ANOVAs, with time of assessment as the repeated measure, on ratings of state anxiety (STAI-state) and negative affect (PANAS- negative affect) in the stress and control groups (two control participants did not fill out the PANAS correctly and were excluded from analysis; see Table 1 for average scores). Analysis of state anxiety measures revealed a main effect of group [F1, 72 = 27.6, p < .001, η p2 = .28] as well as an interaction between group and time of assessment on anxiety symptoms [F1,72 = 16.5, p < .0001, η p2 = .187]. The interaction was driven by a significant increase in subjective anxiety following the speech task in the stress condition [t(38) = −5.14, p < .001, d = .95 , 95% CI (−10.07, −4.38)], while those in the control group reported a slight, nonsignificant decrease over time (see Table 1). ANOVA analysis of negative affect also revealed a main effect of group [F1, 70, = 13.0, p = .001, η p2= .15] and a significant interaction between group and time of assessment [F1,70 = 9.8, p = 0.003, η p2 = .12]. This interaction was similarly driven by a significant increase in negative affect following the speech task in the stress condition [t(38) = 3.0, p = .005, d = .48 , 95% CI (.80, 4.3)], while those in the control group again reported a nonsignificant decrease (see Table 1).
Table 1.
Subjective State Measure Comparisons
| Subjective Measures | ||||||
|---|---|---|---|---|---|---|
| Stress Group | Control Group | |||||
| Baseline M (SEM) |
Post-task M (SEM) |
Difference M (SEM) |
Baseline M (SEM) |
Post-task M (SEM) |
Difference M (SEM) |
|
| STAI-State | 43.5 (1.4) | 50.7 (1.4) | 7.2 (1.4)* | 39.1 (1.8) | 37.3 (1.8) | − 1.4 (1.4) |
| PANAS-NA | 15.4 (0.8) | 18.0 (0.9) | 2.6 (0.9)* | 13.6 (1.0) | 12.6 (1.0) | − 1.0 (0.7) |
Subjective Measures: Mean scores and standard errors of subjective responses to STAI-State and the negative affect scale of the PANAS pre- and post-stress or control task.
= p <.05
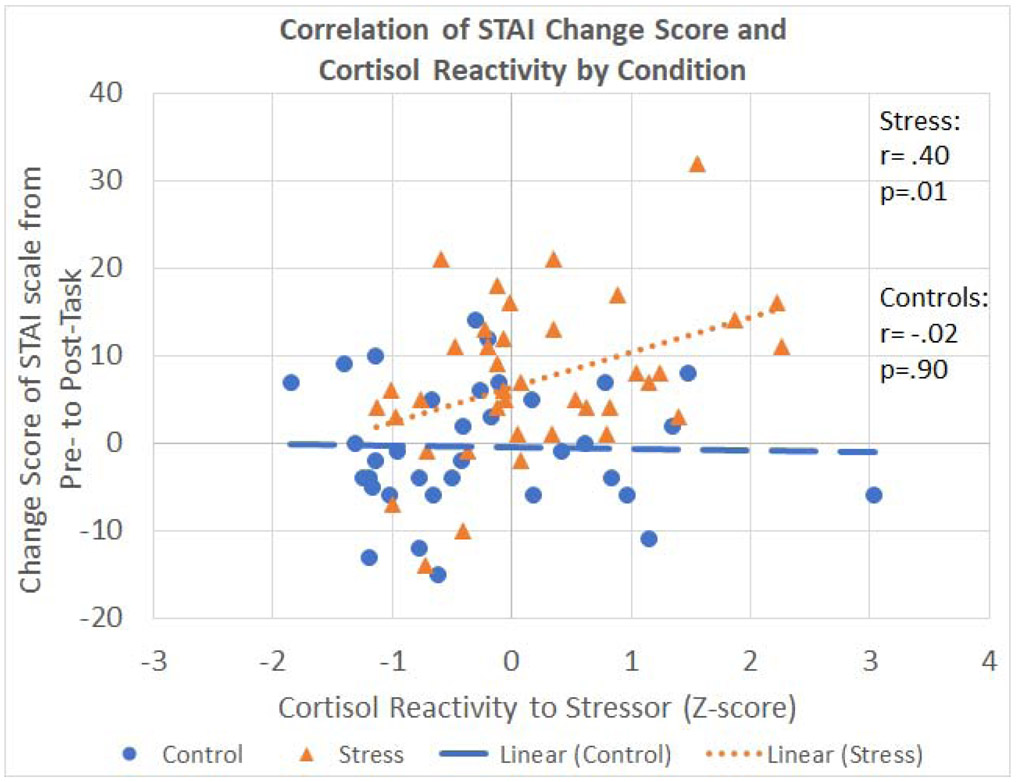
Importantly, the stress group reported significantly greater increases in state anxiety symptoms from baseline to post-TSST compared to the control group [t(72) = −4.1, p < .001, d = .95, 95% CI (−11.4, −3.9); see Table 1]. We then ran correlations between changes in reported anxiety and changes in cortisol levels. When all participants (stress and control) were included, the correlation between change in reported anxiety and change in cortisol concentration was significant [r(74) = 0.28, p = 0.015], indicating that changes in subjective reports of state anxiety and subsequent cortisol reactivity track each other (e.g.64,65). When we ran the analysis within each condition separately, the correlation between changes in reported anxiety and changes in cortisol reactivity was significant within the stress group [r(39) = 0.40, p = 0.011], but not within the control group [r(35) = −.023, p = 0.89; see Figure 4]. A Fisher-z comparison between stress and control group correlations failed to reach significance (p = .07).
Figure 4.
Stress Correlation. Within the stress group, there is a significant correlation between change in reported anxiety and change in cortisol concentrations, as measured through cortisol reactivity. This association was not present for participants in the control condition.
With regards to affect, the stress group similarly reported a significantly larger increase in negative affect from pre- to post-task compared to the control group [t(70) = −3.1, p = .003, d = .76, 95% CI (−5.8, −1.3); see Table 1]. Further, the association between change in negative affect and cortisol was trending in the stress group [r(39) = 0.29, p = 0.076] and across all participants [r(72) = 0.21, p = 0.076], but failed to reach significance. Change in negative affect did not correlate with cortisol reactivity within the control group [r(33) = −.04, p = 0.83; see Supplementary Figure 1 for plot of PANAS-NA and cortisol reactivity associations]. Again, a Fisher-z comparison between stress and control group correlations was not significant (p = .16).
The Impact of Stress on Selective Emotional Memory Consolidation
Specific Memory Group Comparison
We first compared the stress group to the control group on specific memory performance (i.e. responding “same” to same items). This was done using a 2 (condition: stress, control) x 2 (scene component: object, background) x 2 (valence: negative, neutral) mixed analysis of variance (ANOVA), with scene component and valence as repeated measures, on specific recognition memory. This analysis revealed a main effect of scene component [F1, 72 = 82.4, p < 0.0001, η p2 = .53] and a 2-way interaction between scene component and valence [F 1, 72 = 45.2, p < 0.0001, η p2 = .38], which again confirms the existence of the emotional memory trade-off effect.29 However, there was no main effect of condition [F1, 72 =.68, p =.68, η p2 = .002] and the three-way interaction was nonsignificant [F1, 72 = 1.32, p = 0.25, η p2 = .01]. While the stress group showed a numerical increase compared to controls in memory both for negative central objects [t(72) = 1.7, p = .09, d = .37, 95% CI (−0.01, 0.14)] and in the magnitude of the emotional trade-off score [t(72) = 1.8, p = .076, d = .44, 95% CI (−0.008, 0.16)], these differences did not reach significance. Memory for neutral scene information was nearly identical between groups (all p’s >.8; see Table 2 for group scores).
Table 2:
| Memory Performance | |||||||
|---|---|---|---|---|---|---|---|
| Stress Group | Control Group | ||||||
| Object M (SEM) |
Background M (SEM) |
Difference M (SEM) |
Object M (SEM) |
Background M (SEM) |
Difference M (SEM) |
||
| Specific Memory | Negative | 0.68 (.026) | 0.39 (.028) | 0.30 (.030) | 0.62 (.029) | 0.40 (.031) | 0.22 (.030) |
| Neutral | 0.56 (.028) | 0.48 (.027) | 0.08 (.031) | 0.55 (.043) | 0.49 (.035) | 0.07 (.039) | |
| Gist Memory | Negative | 0.85 (.021) | 0.57 (.024) | 0.28 (.023) | 0.83 (.020) | 0.56 (.029) | 0.28 (.031) |
| Neutral | 0.71 (.026) | 0.68 (.027) | 0.03 (.03) | 0.72 (.034) | 0.65 (.032) | 0.06 (.03) | |
Emotional Trade-off Memory Performance: Mean scores and standard errors for memory performance on scene components and magnitude of the emotional trade-off scores. Specific Memory = correctly identifying “old” stimuli as “old”; Gist Memory = identifying “old” stimuli as “old” or “similar”; Object = the memory for central object; Background = memory for neutral background paired with negative or neutral central object, Difference = difference score between object and background memory (magnitude of the object-background trade-off).
General (Gist) Memory Group Comparison
We also examined gist memory (i.e. responding “same” or “similar” to same items) by comparing performance between the stress and control groups. While the emotional memory trade-off effect for gist memory was apparent across groups [main effect of scene component: F1, 72 = 110.8, p < 0.0001, η p2 = .606; scene component x valence interaction: F 1, 72 = 70.02, p < 0.0001, η p2 = .49], there was no effect of stress on this less conservative form of memory with all ps ≥ .44 for all group comparisons, interactions, and main effect analyses.
The Impact of Cortisol on Selective Emotional Memory Consolidation
Specific Memory Correlation Analysis
To increase statistical power and variability in cortisol responses and subjectively reported anxiety, we next examined correlations between these continuous variables and emotional memory in all participants. For specific memory, we correlated cortisol level and change in subjective measures of anxiety (STAI-state) and negative affect (PANAS-Negative Affect) with object-background trade-off magnitude scores (see Memory Analysis). The log-transformed and standardized measures of cortisol were used for all correlations.
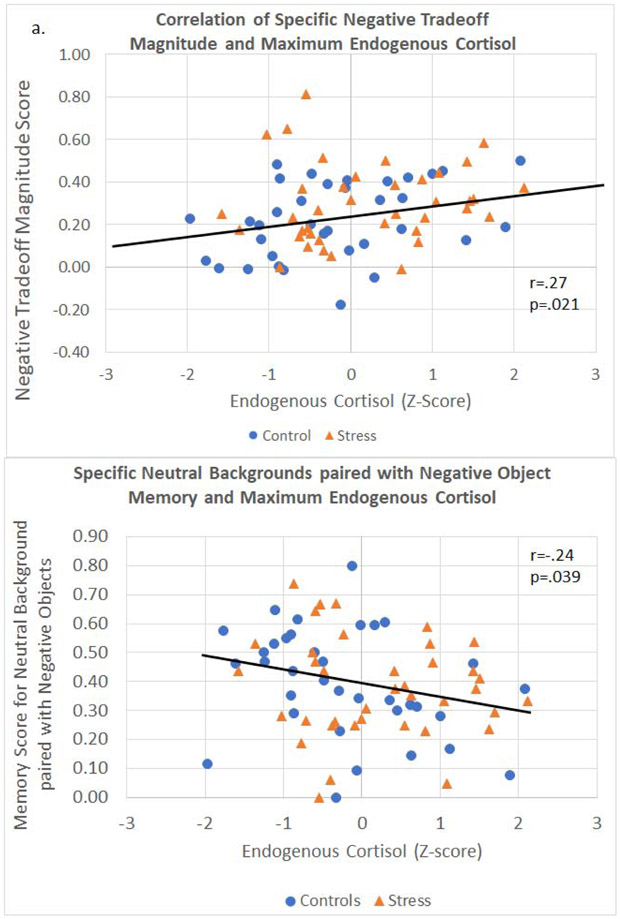
Correlational analyses across all participants revealed the predicted positive correlation between specific memory negative trade-off scores and peak post-task cortisol values [r(74) = .27, p = .021; see Figure 5a], indicating that higher endogenous cortisol concentrations during early consolidation were associated with a greater emotional memory trade-off magnitude score. Importantly, specific negative trade-off scores did not correlate with pre-encoding baseline cortisol levels [r(74) = −.007, p = .95] or the maximum change in cortisol from baseline to post-task [r(74) = .19, p = .11], indicating that the association between cortisol and the magnitude of the negative object-background trade-off was driven by peak levels of cortisol during the early consolidation phase and not pre-encoding cortisol levels or the magnitude of the change in cortisol across the testing session.
Figure 5.
Correlation of specific memory scores and maximum endogenous cortisol levels (as measured by the highest cortisol concentration taken from any time point after the stress or control manipulation) across all participants. Maximum endogenous cortisol during early consolidation has (a) a positive correlation with negative trade-off magnitude and (b) a negative correlation with neutral backgrounds paired with negative objects. This suggests that the primary effect of cortisol during the early consolidation period is a suppressive effect on neutral information paired with negative content.
As discussed, these trade-off scores are comprised of both object and background scores of different valences. Given this association between cortisol concentration and negative trade-off scores, we examined each scene component type to determine if cortisol acted upon a particular element of memory. These post-hoc analyses revealed that while there was not a significant association between cortisol and negative objects, cortisol was associated with poorer memory for the neutral backgrounds originally paired with negative objects at encoding [r(74) = −.24, p = .039; see Figure 5b]. The correlation values between all scene components and object-background trade-off scores can be found in Table 3, and plots of the non-significant correlations can be found in the Supplementary Section.
Table 3:
| Correlations between Memory Scores and Peak Post-Task Cortisol Levels | |||||||
|---|---|---|---|---|---|---|---|
| Negative Objects |
Backgrounds paired with Neg Objects |
Magnitude of Negative Object- Background trade-off |
Neutral Objects |
Backgrounds paired with Neu Objects |
Magnitude of Neutral Object- Background trade-off |
||
| Specific Memory | r | .04 | −.24 | .27 | −.02 | −.10 | .07 |
| P | .75 | .039* | .02* | .87 | .41 | .57 | |
| Gist Memory | r | .05 | −.26 | .29 | −.05 | −.16 | .10 |
| p | .68 | .028* | .01* | .70 | .19 | .39 | |
Correlations between Memory Score and Cortisol Levels: Pearson’s r and p-values for the association between peak post-task cortisol level and all memory scores (each scene component type and object-background trade-off value).
= p <.05
The relationship between subjective anxiety (difference in STAI score from baseline to post-task) and specific memory for negative objects across all participants did not achieve statistical significance [r(74) = .21, p = .065]. Similarly, no specific memory measures correlated with changes in reported negative affect within either condition or across all participants (all ps > .2).
Gist Memory Correlation Analysis
The same correlational analyses with cortisol and changes in subjective measures of anxiety and negative affect were then completed with measures of gist memory. Similar to specific memory, correlational analyses across all participants revealed a positive correlation between the emotional memory trade-off and peak post-task cortisol values [r(74) = .29, p = .012] and did not correlate with pre-encoding baseline cortisol levels [r(74) = −.04, p = .73] or the maximum change in cortisol from baseline to post-task [r(74) = .12, p = .30]. We again conducted follow-up analyses to decipher which elements of gist memory were influenced by cortisol. Again, while there was no relationship between cortisol level and negative objects, there was a significant negative correlation between cortisol and gist memory for the neutral backgrounds originally paired with negative objects at encoding [r(74) = −.27, p = .028; Table 3]. Plots for all gist memory correlations can be found in the Supplementary Section.
There were no associations between gist memory performance and measures of subjective stress and negative affect across all participants and when run within each condition separately (all ps ≥ .05).
DISCUSSION
Emotional episodic memories are often complex, with multiple components. The main goal of this study was to assess whether levels of endogenous cortisol during the initial stages of memory consolidation would impact subsequent memory for negative and neutral components of experience. Based on evidence from our lab and others (e.g. 5,7,12,66), we hypothesized that increased cortisol during the early consolidation period would selectively benefit memory for negative aspects of scenes through the preferential processing of information with greater salience, while memory for neutral elements would deteriorate.
Our results demonstrate that higher levels of endogenous cortisol during the early consolidation period of emotionally complex scenes is associated with the preservation of emotional components of scenes at the cost of peripheral neutral details (i.e. an increase in the emotional memory trade-off effect). Specifically, there was a clear preservation (and in fact, a numerical increase) of memory for negative objects in the stress group. Moreover, higher endogenous cortisol concentrations were significantly associated with a decrease in memory for the peripheral, neutral backgrounds of the scenes originally paired with negative objects at encoding. These memory effects were found in both the stricter specific measure of memory, and in the less conservative measure of gist memory in which participants get credit for identifying similar elements as “same” (suggesting that they at least have some memory trace of the original). From this, we can conclude that elevated cortisol during the early consolidation window leads to a greater trade-off in emotional memory, likely driven by a preservation of emotional objects and a simultaneous stripping away of less relevant, neutral scene backgrounds in which these emotional objects appear. This effect occurs in both specific, veridical memory and more general, gist-like memories.
Cortisol levels were strongly and positively associated with the magnitude of the trade-off effect. The higher the cortisol level during early consolidation, the greater the magnitude was in the difference between memory for negative objects and their associated neutral backgrounds. Higher cortisol levels were particularly associated with declines in memory for neutral backgrounds paired with negative objects. These findings suggest a potentially important role for the stress hormone cortisol in how emotional memories are consolidated, rather than merely being in a stress condition or subjectively reporting being anxious. It has been established that a variety of influences can alter a person’s response to a psychosocial stressor, and not everyone mounts a cortisol response to the TSST, with an estimated 20-30% of subjects typically failing to demonstrate a cortisol response to this challenge.53-55 Similarly, some participants likely mount a stressful response merely by participating in a research study about stress, which may have been further exacerbated by viewing emotional scenes. Others may be stressed for reasons unrelated to the experimental protocol (e.g., an upcoming exam, a recent argument with a friend, or running to the lab to be on time). As such, despite participating in a task designed to be stressful and having an overall larger increase in cortisol on average, there was still significant overlap in cortisol levels between the stress and control groups, as can be visualized in Figures 4 and 5. In fact, 28% of the control group had cortisol levels above the mean level found in the stress group. In cases like these, where participants assigned to stress and control conditions have high overlap in stress hormone levels or response, it may be useful to consider stress tasks like this as a means of increasing the variance of cortisol levels present during the consolidation period and use cortisol as a continuous variable across all participants, rather than dichotomizing the data for group comparisons. This has the additional benefit of increasing power.
When combining analyses across all participants, our findings with regard to the effect of cortisol on memory fall in line with previous research. In another study investigating the impact of cortisol on the emotional memory trade-off effect, higher cortisol levels at the time of encoding (in the absence of an explicit stressor) were found to positively correlate with selective memory for negative arousing objects in the trade-off task, but only after a period of sleep. Bennion et al. concluded that elevations in cortisol helped “tag” the emotional content within scenes at the time of encoding as important, and that such tags enabled sleep-dependent processes to preferentially consolidate this information. We have also previously shown that arousal as measured by psychophysiological reactivity to the trade-off task images at encoding predict memory for negative information at recognition, but again, only following a period of sleep.33 These findings may be particularly relevant to the current study, in which all subjects obtained a night of sleep during the consolidation interval in order to ensure that the physiological effects of the previous day’s stressor had completely washed out prior to the retrieval stage, further suggesting that stress and sleep may work together to promote a greater object-background trade-off in emotional memory, above and beyond what is seen simply with a night of sleep.66 Interestingly, here we did not find an association between baseline cortisol and subsequent performance on the emotional trade-off task (all ps >.16). In addition to several notable protocol differences between our study and Bennion et al. (e.g. time of cortisol assessment and encoding, length of time between encoding and bed, etc.), this could suggest that cortisol levels during the early consolidation phase may be most critical to subsequent emotional memory processing. In our study, cortisol levels were manipulated using a stress task and as such changed substantially from baseline to early consolidation. By contrast, in the studies by Bennion et al. and Cunningham et al, stress was not manipulated and as such baseline levels were likely highly related to cortisol concentrations during the consolidation window as well.33,66 Future research is needed to further distinguish the effects of cortisol on these different phases of memory processing.
The results reported here have important implications for emotional memory formation following stress exposure, and thus have real-world relevance. In participants with elevated cortisol, there was a divergence of memory for the components of negative scenes (i.e. objects and backgrounds). Rather than conferring a general benefit on memory for negative scenes in their entirety, cortisol appears to preserve only memory for central emotional objects while allowing memory for the neutral details in the periphery to dissipate over time. This result may suggest that memory for the individual components of the scene become “unbound” during consolidation and cortisol impacts the unbound elements differently, allowing the brain to selectively preserve only what is calculated to be most salient and thus important to remember – an outcome with clear adaptive relevance.31,36
While a number of studies have demonstrated that stress at encoding benefits long-term emotional memory in humans,7,12 fewer studies have explored the effects of stress administered post-encoding, during the early consolidation phase of emotional memory processing, and, to our knowledge, this is the first to examine the impact of stress on differential components of emotional and neutral memory within stimuli. In one important study, Cahill and colleagues (2003) utilized a cold-pressor task to elicit stress during early consolidation of emotionally arousing and neutral pictures.13 After a week-delay, they found that those that underwent the stressor had increased performance in long-term emotional memory, while memory for neutral information was similar between groups. Smeets, Otgaar, Candel, and Wolf (2008) applied a cold-pressor stressor during encoding, early consolidation, and retrieval of neutral and emotional word lists.67 Similar to the Cahill et al. study, when the stressor was applied during the early consolidation window there was a benefit in memory for emotional words, but not neutral words. Critically, however, these studies tested memory for word lists or full scenes and were not designed to distinguish the memory consolidation effects between emotional and neutral information within the same scenes. Thus, while our results build upon these previous findings, they are novel in that they demonstrate that cortisol concentration during early consolidation impacts memory for emotional experience by selectively enhancing memory only for the central emotional aspects of scenes, while reducing memory for the neutral background details. Additionally, we found that cortisol levels have no effect on neutral scene memory - for either the neutral objects or their associated backgrounds. Thus, cortisol appears to selectively benefit the consolidation of emotionally arousing and negative aspects of experience, and reduces neutral elements of memories with competing emotional content.
Importantly, prior research has shown that in order for elevated cortisol to effectively enhance subsequent memory for emotionally salient information, the observer must perceive and experience the information as emotionally arousing.21,23 The negative stimuli used in our study have been verified both to elicit higher arousal ratings,29 and to induce greater visceral responses, as measured by heart rate deceleration and skin conductance response, than the neutral images.33 Emotional arousal such as this typically results in the release of norepinephrine (NE) and increases in amygdalar activity. This release of NE at encoding alone has been shown to enhance emotional memory formation through the modulation of key neural networks (i.e. hippocampus, amygdala, frontal cortex; see 11 for review). When the occurrence of a stressor is in close temporal proximity with emotional arousal, either as a single emotionally stressful experience or concurrently as in our design (emotional encoding task followed by TSST), one possibility is that norepinephrine and cortisol work together to further enhance amygdalar, hippocampal, and prefrontal cortex connectivity, leading to an additional boost of emotional memory during the consolidation interval.68 Another possibility is that during stress, amygdala activation (involved in encoding emotional items along with the perirhinal cortex) is bolstered, while the hippocampus (involved in encoding contextual information) is inhibited, thus leading to a preservation of emotional items while the contextual information (i.e., neutral backgrounds) deteriorate.69-72 Critically, the subjective anxiogenic and mood effects of experiencing a stressor alone during the consolidation period was not sufficient to generate changes in emotional memory traces beyond typical emotional content enhancements. Rather, HPA axis activity overall, regardless of group, was necessary to further influence memory during the consolidation window, when memories are particularly labile and open to external influence. This HPA activation and subsequent cortisol concentration during early consolidation leads to increased cortisol in the memory networks of the brain, increasing amygdalar and hippocampal connectivity68 and boosting emotional memory.73 Here, we demonstrate that cortisol elevation during the early consolidation period preserves the emotional components of scenes while reducing memory for neutral, peripheral details, provoking an increase in the magnitude of the emotional memory trade-off effect.
Critically, the impact of stress during the consolidation of neutral and emotional information is not consistent throughout the literature.5 Factors such as the cortisol induction method (psychosocial stressor14, physiological stressor15-19), the amount of cortisol or cortisol change achieved,17,19,74 the sex of the participant (see Supplementary Materials for exploratory analysis of sex within this dataset),14,18 the initial value of cortisol,17,66 the type of memory examined (e.g. recollection vs familiarity18,19) or when interacting with other neurotransmitters or physiological systems, such as norepinephrine,15-17 hormonal contraceptives,15 or phases in the menstrual cycle16 have all been shown to influence the impact of stress on different valences of memory. In fact in certain circumstances, stress has been shown to benefit long-term memory for neutral information15,16 or lead to an inverted-U effect on memory.19 Notably, across many of these studies the type of memory tested differed and there were other important methodological differences (timing of stressor, number of tests, etc.), and the present study is the first to employ the emotional trade-off task using the post-learning stress protocol. As such, continued research will be paramount in sorting out these conflicting reports.
Our study was focused on measuring stress reactivity via elevations in cortisol concentration. One limitation of this study is that a physiological stress response is associated with a whole host of psychological, hormonal, neurochemical, and brain activation changes15,16,18,49 not investigated within our narrow focus. Together with the fact that the cortisol response builds slowly (even in the case of non-genomic effects), taking at least several minutes before having an effect on the brain,75-77 this finding suggests that it is not only possible, but likely that cortisol is not acting alone on the brain to generate this memory effect. Rather it is likely working collaboratively with other neurohormones released during emotion and stress responses (such as norepinephrine) to “tag” certain information as being particularly critical for additional processing.78,79 Further research will be critical in determining the individual impact of cortisol and other features of the stress response in affecting memory processing. Additionally, in light of the results reported here offering support for the theory that memory elements become “unbound” during processing and consolidation and that this effect may be enhanced by cortisol, it will be important to further explore the effects of stress and cortisol in both different types of memory assessments and more ecologically-relevant scenarios. For instance, future research could incorporate associative recognition tests into similar designs and explore if information is able to be more flexibly applied to new situations after being unbound. A final important consideration is that to ensure the memories would still be labile at the time of the stressor, we kept the stress task as temporally close to encoding as possible. Because the passive drool technique takes time, as mentioned above, we were unable to collect saliva samples both during the encoding task and immediately after encoding, prior to the start of the stressor. Although studies such as Abercrombie, Speck, & Monticelli, 2006 suggest that the viewing of emotional scenes alone does not generate a significant increase in cortisol,21 we cannot rule it out in the current study. This may in part explain some of the overlap between conditions. There is potential for individual differences and this is an open area for further investigation, particularly when attempting to distinguish the impact of stress and cortisol on different phases of memory.
To our knowledge, this study is the first to demonstrate that the manipulation of endogenous cortisol following the encoding of negatively arousing stimuli selectively preserves the consolidation of individual emotional aspects of memory while allowing peripheral, neutral details to be forgotten. Thus, the interaction of adrenal activity from stress, likely in concert with emotional arousal from viewing the pictures33 influences the magnitude of the emotional memory trade-off effect. Based on recent neuroimaging work,25,80 this likely occurs by increasing connectivity between emotional memory centers of the brain, such as the hippocampus and amygdala. This finding helps shed light on the influence that stress and cortisol can have on our memories -- in this case by selectively benefitting only the emotional components of experience. In particular, our results show that an experience stressful enough to elicit HPA axis activation during the early consolidation window may prime our memory systems to preferentially remember negatively arousing information, which we argue is adaptive,36 at least up to a point where excessive negative remembering becomes pathological in clinical conditions such as depression and anxiety.30,31,81 This finding elicits several fruitful questions for future research, such as how hyperactive adrenal networks in clinical conditions (e.g. depression) may further potentiate negative memory, or how learning to cope with stress to reduce physiological responses may influence long-term memory consolidation for our emotional experiences.
Supplementary Material
Highlights.
We explore how elevated cortisol affects the consolidation of memories.
Using the emotional trade-off task, we explore effects on negative and neutral stimuli
Increased cortisol correlates with greater emotional memory trade-off
This was due to impaired memory for neutral backgrounds paired with negative objects
These effects were found for both specific and gist calculations of memory
Acknowledgements:
This work was supported by funding from National Science Foundation (BCS-2001025). The author TJC would like to thank his NIH T32 funding source for supporting his work and ongoing training. TJC is currently funded by the Research Training Program in Sleep, Circadian and Respiratory Neurobiology (NIH T32 HL007901) through the Division of Sleep Medicine at Harvard Medical School and Brigham & Women’s Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interest: The authors declare no competing interests
Disclaimer: Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
Analysis of morning cortisol was in 64 of the 74 total participants. We did not initiate this part of the protocol for the first 9 participants (controls = 5, stress = 4), and the morning sample for 1 control participant was not usable. As such this analysis was run with an n = 29 for control participants, and an n = 35 for stress participants.
REFERENCES
- 1.Gold PE, McGaugh JL. A single-trace, two-process view of memory strage processes. Short-Term Mem. Published online 1975:355–378. [Google Scholar]
- 2.McGaugh JL. THE AMYGDALA MODULATES THE CONSOLIDATION OF MEMORIES OF EMOTIONALLY AROUSING EXPERIENCES. Annu Rev Neurosci. 2004;27(1):1–28. doi: 10.1146/annurev.neuro.27.070203.144157 [DOI] [PubMed] [Google Scholar]
- 3.McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev. 2012;36(7):1750–1762. doi: 10.1016/j.neubiorev.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roozendaal B, Hermans EJ. Norepinephrine effects on the encoding and consolidation of emotional memory: improving synergy between animal and human studies. Curr Opin Behav Sci. 2017;14:115–122. doi: 10.1016/j.cobeha.2017.02.001 [DOI] [Google Scholar]
- 5.Shields GS, Sazma MA, McCullough AM, Yonelinas AP. The Effects of Acute Stress on Episodic Memory: A Meta-Analysis and Integrative Review. Psychol Bull. 2017;143(6):636–675. doi: 10.1037/bul0000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payne J, Jackson E, Ryan L, Hoscheidt S, Jacobs J, Nadel L. The impact of stress on neutral and emotional aspects of episodic memory. Memory. 2006; 14(1):1–16. doi: 10.1080/09658210500139176 [DOI] [PubMed] [Google Scholar]
- 7.Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn Mem. 2007;14(12):861–868. doi: 10.1101/lm.743507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoladz PR, Peters DM, Cadle CE, et al. Post-learning stress enhances long-term memory and differentially influences memory in females depending on menstrual stage. Acta Psychol (Amst). 2015;160:127–133. doi: 10.1016/j.actpsy.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 9.Nadel L, Payne JD, Jacobs WJ. The Relationship Between Episodic Memory and Context: Clues from Memory Errors Made While Under Stress. 2002;51:10. [PubMed] [Google Scholar]
- 10.McGaugh JL. Memory--a Century of Consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248 [DOI] [PubMed] [Google Scholar]
- 11.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651 [DOI] [PubMed] [Google Scholar]
- 12.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26(3):307–317. doi: 10.1016/S0306-4530(00)00058-5 [DOI] [PubMed] [Google Scholar]
- 13.Cahill L, Gorski L, Le K. Enhanced Human Memory Consolidation With Post-Learning Stress: Interaction With the Degree of Arousal at Encoding. Learn Mem. 2003;10(4):270–274. doi: 10.1101/lm.62403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preuß D, Wolf OT. Post-learning psychosocial stress enhances consolidation of neutral stimuli. Neurobiol Learn Mem. 2009;92(3):318–326. doi: 10.1016/j.nlm.2009.03.009 [DOI] [PubMed] [Google Scholar]
- 15.Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biol Psychol. 2013;92(2):257–266. doi: 10.1016/j.biopsycho.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. NeuroImage. 2010;53(4):1286–1293. doi: 10.1016/j.neuroimage.2010.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larra MF, Schulz A, Schilling TM, et al. Heart rate response to post-learning stress predicts memory consolidation. Neurobiol Learn Mem 2014;109:74–81. doi: 10.1016/j.nlm.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 18.McCullough AM, Yonelinas AP. Cold-pressor stress after learning enhances familiarity-based recognition memory in men. Neurobiol Learn Mem. 2013;106:11–17. doi: 10.1016/j.nlm.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullough AM, Ritchey M, Ranganath C, Yonelinas A. Differential effects of stress-induced cortisol responses on recollection and familiarity-based recognition memory. Neurobiol Learn Mem. 2015;123:1–10. doi: 10.1016/j.nlm.2015.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preuß D, Schoofs D, Wolf OT. Associations between endogenous cortisol levels and emotional memory in young women: Influence of encoding instructions. Stress. 2009;12(5):379–387. doi: 10.1080/10253890802524592 [DOI] [PubMed] [Google Scholar]
- 21.Abercrombie HC, Speck NS, Monticelli RM. Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. :10. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y-L, Chao P-K, Lu K-T. Systemic and Intra-Amygdala Administration of Glucocorticoid Agonist and Antagonist Modulate Extinction of Conditioned Fear. Neuropsychopharmacology. 2006;31(5):912–924. doi: 10.1038/sj.npp.1300899 [DOI] [PubMed] [Google Scholar]
- 23.Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci. 2006;103(17):6741–6746. doi: 10.1073/pnas.0601874103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roozendaal B, Barsegyan A, Lee S. Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. In: Progress in Brain Research Vol 167. Elsevier; 2007:79–97. doi: 10.1016/S0079-6123(07)67006-X [DOI] [PubMed] [Google Scholar]
- 25.Ghosh S, Laxmi TR, Chattarji S. Functional Connectivity from the Amygdala to the Hippocampus Grows Stronger after Stress. J Neurosci. 2013;33(17):7234–7244. doi: 10.1523/JNEUROSCI.0638-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic Stress Induces Contrasting Patterns of Dendritic Remodeling in Hippocampal and Amygdaloid Neurons. J Neurosci. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pruessner JC, Dedovic K, Khalili-Mahani N, et al. Deactivation of the Limbic System During Acute Psychosocial Stress: Evidence from Positron Emission Tomography and Functional Magnetic Resonance Imaging Studies. Biol Psychiatry. 2008;63(2):234–240. doi: 10.1016/j.biopsych.2007.04.041 [DOI] [PubMed] [Google Scholar]
- 28.van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof F, Rombouts SARB. Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem. 2007;87(1):57–66. doi: 10.1016/j.nlm.2006.05.008 [DOI] [PubMed] [Google Scholar]
- 29.Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. J Mem Lang. 2007;56(4):575–591. doi: 10.1016/j.jml.2006.05.004 [DOI] [Google Scholar]
- 30.Payne JD. Learning, Memory, and Sleep in Humans. Sleep Med Clin. 2011;6(1):15–30. doi: 10.1016/j.jsmc.2010.12.005 [DOI] [Google Scholar]
- 31.Payne JD, Nadel L, Britton WB, Jacobs WJ. The Biopsychology of Trauma and Memory. In: Reisberg D, Hertel P, eds. Memory and Emotion. Oxford University Press; 2004:76–128. doi: 10.1093/acprof:oso/9780195158564.003.0003 [DOI] [Google Scholar]
- 32.Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep Preferentially Enhances Memory for Emotional Components of Scenes. Psychol Sci. 2008;19(8):781–788. doi: 10.1111/j.1467-9280.2008.02157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham TJ, Crowell CR, Alger SE, et al. Psychophysiological arousal at encoding leads to reduced reactivity but enhanced emotional memory following sleep. Neurobiol Learn Mem. 2014;114:155–164. doi: 10.1016/j.nlm.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 34.Reisberg D, Heuer F. Memory for Emotional Events. In: Reisberg D, Hertel P, eds. Memory and Emotion. Oxford University Press; 2004:1–41. doi: 10.1093/acprof:oso/9780195158564.003.0001 [DOI] [Google Scholar]
- 35.Stanny CJ, Johnson TC. Effects of stress induced by a simulated shooting on recall by police and citizen witnesses. Am J Psychol. 2000;113(3):359–386. doi: 10.2307/1423364 [DOI] [PubMed] [Google Scholar]
- 36.Payne JD, Kensinger EA. Sleep’s Role in the Consolidation of Emotional Episodic Memories. Curr Dir Psychol Sci. 2010;19(5):290–295. doi: 10.1177/0963721410383978 [DOI] [Google Scholar]
- 37.Bennion KA, Payne JD, Kensinger EA. Selective effects of sleep on emotional memory: What mechanisms are responsible? Transl Issues Psychol Sci. 2015;1(1):79–88. doi: 10.1037/tps0000019 [DOI] [Google Scholar]
- 38.Alger SE, Chambers AM, Cunningham T, Payne JD. The Role of Sleep in Human Declarative Memory Consolidation. In: Meerlo P, Benca RM, Abel T, eds. Sleep, Neuronal Plasticity and Brain Function. Vol 25. Current Topics in Behavioral Neurosciences. Springer; Berlin Heidelberg; 2014:269–306. doi: 10.1007/7854_2014_341 [DOI] [PubMed] [Google Scholar]
- 39.Payne J, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Front Integr Neurosci. 2012;6. doi: 10.3389/fnint.2012.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Payne JD, Kensinger EA. Sleep Leads to Changes in the Emotional Memory Trace: Evidence from fMRI. J Cogn Neurosci. 2011;23(6):1285–1297. doi: 10.1162/jocn.2010.21526 [DOI] [PubMed] [Google Scholar]
- 41.Waring JD, Payne JD, Schacter DL, Kensinger EA. Impact of individual differences upon emotion-induced memory trade-offs. Cogn Emot. 2010;24(1):150–167. doi: 10.1080/02699930802618918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payne JD, Kensinger EA, Wamsley EJ, et al. Napping and the selective consolidation of negative aspects of scenes. Emotion. 2015;15(2):176–186. doi: 10.1037/a0038683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.JELICI M, GERAERTS E, MERCKELBACH H, GUERRIERI R. Acute Stress Enhances Memory for Emotional Words, but Impairs Memory for Neutral Words. Int J Neurosci 2004;114(10):1343–1351. doi: 10.1080/00207450490476101 [DOI] [PubMed] [Google Scholar]
- 44.Wolf OT. Stress and memory in humans: Twelve years of progress? Brain Res. 2009;1293:142–154. doi: 10.1016/j.brainres.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 45.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of Gender, Menstrual Cycle Phase, and Oral Contraceptives on the Activity of the Hypothalamus-Pituitary-Adrenal Axis. Psychosom Med. 1999;61(2):154–162. [DOI] [PubMed] [Google Scholar]
- 46.Cunningham TJ, Chambers AM, Payne JD. Prospection and emotional memory: how expectation affects emotional memory formation following sleep and wake. Front Psychol. 2014;5. doi: 10.3389/fpsyg.2014.00862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. J Mem Lang 2006;54(1):99–112. doi: 10.1016/j.jml.2005.05.005 [DOI] [Google Scholar]
- 48.Waring JD, Kensinger EA. Effects of emotional valence and arousal upon memory trade-offs with aging. Psychol Aging. 2009;24(2):412–422. doi: 10.1037/a0015526 [DOI] [PubMed] [Google Scholar]
- 49.Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test’ – A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology 1993;28(1-2):76–81. doi: 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- 50.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 51.Spielberger CD. State-Trait Anxiety Inventory. In: Weiner IB, Craighead WE, eds. The Corsini Encyclopedia of Psychology John Wiley & Sons, Inc.; 2010:corpsy0943. doi: 10.1002/9780470479216.corpsy0943 [DOI] [Google Scholar]
- 52.Dickerson SS, Kemeny ME. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- 53.Kajantie E, Phillips D. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 54.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 55.Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology 2005;30(1):80–91. doi: 10.1016/j.psyneuen.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 56.Juster R-P, Raymond C, Desrochers AB, et al. Sex hormones adjust “sex-specific” reactive and diurnal cortisol profiles. Psychoneuroendocrinology. 2016;63:282–290. doi: 10.1016/j.psyneuen.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 57.Kudielka BM, Hellhammer DH, Wüst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34(1):2–18. doi: 10.1016/j.psyneuen.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 58.Liu JJW, Ein N, Peck K, Huang V, Pruessner JC, Vickers K. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology. 2017;82:26–37. doi: 10.1016/j.psyneuen.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 59.Wirth MM, Welsh KM, Schultheiss OC. Salivary cortisol changes in humans after winning or losing a dominance contest depend on implicit power motivation. Horm Behav. 2006;49(3):346–352. doi: 10.1016/j.yhbeh.2005.08.013 [DOI] [PubMed] [Google Scholar]
- 60.Mehta PH, Welker KM, Zilioli S, Carré JM. Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinology. 2015;56:88–99. doi: 10.1016/j.psyneuen.2015.02.023 [DOI] [PubMed] [Google Scholar]
- 61.Mehta PH, Josephs RA. Testosterone and cortisol jointly regulate dominance: Evidence for a dual-hormone hypothesis. Horm Behav. 2010;58(5):898–906. doi: 10.1016/j.yhbeh.2010.08.020 [DOI] [PubMed] [Google Scholar]
- 62.Tackett JL, Herzhoff K, Harden KP, Page-Gould E, Josephs RA. Personality × hormone interactions in adolescent externalizing psychopathology. Personal Disord Theory Res Treat. 2014;5(3):235–246. doi: 10.1037/per0000075 [DOI] [PubMed] [Google Scholar]
- 63.Panizzon MS, Hauger RL, Xian H, et al. Interactive effects of testosterone and cortisol on hippocampal volume and episodic memory in middle-aged men. Psychoneuroendocrinology. 2018;91:115–122. doi: 10.1016/j.psyneuen.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wüst S. Covariance Between Psychological and Endocrine Responses to Pharmacological Challenge and Psychosocial Stress: A Question of Timing: Psychosom Med. 2008;70(7):787–796. doi: 10.1097/PSY.0b013e3181810658 [DOI] [PubMed] [Google Scholar]
- 65.Brand HS. Anxiety and Cortisol excretion correlate prior to dental treatment. Int Dent J. 1999;49(6):330–336. doi: 10.1111/j.1875-595X.1999.tb00533.x [DOI] [PubMed] [Google Scholar]
- 66.Bennion KA, Mickley Steinmetz KR, Kensinger EA, Payne JD. Sleep and Cortisol Interact to Support Memory Consolidation. Cereb Cortex 2015;25(3):646–657. doi: 10.1093/cercor/bht255 [DOI] [PubMed] [Google Scholar]
- 67.Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33(10):1378–1386. doi: 10.1016/j.psyneuen.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 68.Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn Sci. 2011;15(6):280–288. doi: 10.1016/j.tics.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 69.Ritchey M, Wang S-F, Yonelinas AP, Ranganath C. Dissociable medial temporal pathways for encoding emotional item and context information. Neuropsychologia. 2019;124:66–78. doi: 10.1016/j.neuropsychologia.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bisby JA, Horner AJ, Hørlyck LD, Burgess N. Opposing effects of negative emotion on amygdalar and hippocampal memory for items and associations. Soc Cogn Affect Neurosci. 2016;11(6):981–990. doi: 10.1093/scan/nsw028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madan CR, Fujiwara E, Caplan JB, Sommer T. Emotional arousal impairs association-memory: Roles of amygdala and hippocampus. NeuroImage. 2017;156:14–28. doi: 10.1016/j.neuroimage.2017.04.065 [DOI] [PubMed] [Google Scholar]
- 72.Dimsdale-Zucker HR, Ritchey M, Ekstrom AD, Yonelinas AP, Ranganath C. CA1 and CA3 differentially support spontaneous retrieval of episodic contexts within human hippocampal subfields. Nat Commun. 2018;9(1):294. doi: 10.1038/s41467-017-02752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Quervain DJ-F, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30(3):358–370. doi: 10.1016/j.yfrne.2009.03.002 [DOI] [PubMed] [Google Scholar]
- 74.Cunningham TJ, Leal SL, Yassa MA, Payne JD. Post-encoding stress enhances mnemonic discrimination of negative stimuli. Learn Mem. 2018;25(12):611–619. doi: 10.1101/lm.047498.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dolcos F The fast and the slow sides of cortisol’s effects on emotional interference and sustained attention. Front Neurosci. 2014;8. doi: 10.3389/fnins.2014.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sapolsky RM. Stressed-Out Memories. Sci Am Mind. 2004;14(5):28–33. [Google Scholar]
- 77.Strelzyk F, Hermes M, Naumann E, et al. Tune It Down to Live It Up? Rapid, Nongenomic Effects of Cortisol on the Human Brain. J Neurosci 2012;32(2):616–625. doi: 10.1523/JNEUROSCI.2384-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Payne JD, Kensinger EA. Stress, sleep, and the selective consolidation of emotional memories. Curr Opin Behav Sci. 2018;19:36–43. doi: 10.1016/j.cobeha.2017.09.006 [DOI] [Google Scholar]
- 79.Kim SY, Payne JD. Neural correlates of sleep, stress, and selective memory consolidation. Curr Opin Behav Sci. 2020;33:57–64. doi: 10.1016/j.cobeha.2019.12.009 [DOI] [Google Scholar]
- 80.Vaisvaser S, Lin T, Admon R, et al. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front Hum Neurosci. 2013;7. doi: 10.3389/fnhum.2013.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cunningham TJ, Payne JD. Emotional Memory Consolidation During Sleep. In: Axmacher N, Rasch B, eds. Cognitive Neuroscience of Memory Consolidation. Studies in Neuroscience, Psychology and Behavioral Economics. Springer International Publishing; 2017:133–159. doi: 10.1007/978-3-319-45066-7_9 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.