Abstract
The aim of the current meta-analysis was to determine the effects of acute and chronic interval training (IT) on serum and plasma BDNF concentrations in healthy young adults. A literature search was performed using six databases until February 2020. The TESTEX scale was used to assess the quality of studies. Effect sizes (ES) were computed and two-tailed α values < 0.05 and non-overlapping 95% confidence intervals (95% CI) were considered statistically significant. Heterogeneity, inconsistency (I2), and small-study effects using the Luis Furuya–Kanamori (LFK) index were examined. Fifteen studies (n = 277 participants, age = 24 ± 3 years) were included. The overall effects of IT on circulating BDNF concentrations were moderate and significant (ES = 0.62, 95% CI 0.00, 1.24, heterogeneous (p < 0.001), highly inconsistent (I2 = 90%), and with major asymmetry (LFK index = 2.76). The acute effect of IT on peripheral BDNF levels was large and significant (ES = 1.10, 95% CI 0.07, 2.14), heterogeneous (p < 0.001), highly inconsistent (I2 = 92%), and with major asymmetry (LFK index = 3.34). The chronic effect of IT on circulating BDNF was large and significant (ES = 0.93, 95% CI 0.40, 1.46), heterogeneous (p < 0.001), with moderate inconsistency (I2 = 70%), and minor asymmetry (LFK index = 1.21). Acute and chronic IT elicited a moderate increase in serum and plasma BDNF concentrations in a healthy young population.
Subject terms: Neuroscience, Physiology
Introduction
Brain-derived neurotrophic factor (BDNF) was discovered in the early 1980s1 and belongs to the neurotrophin family of proteins2. Early studies in rodents showed an association between BDNF and synaptic plasticity, neuronal growth, neuronal survival, and cognitive processes3–6. BDNF binds to a specific tyrosine kinase receptor which induces TrkB tyrosine phosphorylation and activation in its cytoplasmic and kinase domains BDNF-brain-TrkB. The kinase domain recruits and activates specific proteins in the cytoplasm to activate signaling pathways that regulate cognition and synaptic plasticity7,8.
Although different cell types synthesize and release BDNF (e.g. adipocytes, skeletal muscle, immune cells, vascular endothelial cells, among others)9–11, the hippocampus of the brain is considered the main source of BDNF in mammals12–14. Interestingly, reports indicate that this neurotrophin can cross the blood–brain barrier15 and that peripheral circulating BDNF concentrations are associated with brain function16–18. In agreement, studies in humans demonstrate that peripheral BDNF concentrations are positively associated with hippocampus size and cognitive performance19,20, inversely associated with mood disorders21–23. Likewise, data suggest that BDNF has anti-inflammatory effects on brain in patients with Parkinson’s disease24.
Experimental studies, narrative reviews, and meta-analysis have indicated that aerobic exercise (moderate-intensity continuous training-MICT) increases circulating BDNF concentrations and improves brain function25–27. Thus, MICT is considered an effective strategy to induce neuroprotection28 and to improved brain function. Despite the many benefits of exercise, perceived or real “lack of time” is reported most frequently as the primary barrier that most individuals around developed or developing countries do not exercise regularly29,30.
Interval training (IT) modalities can be performed in a fraction of the time as MICT and have been shown to produce similar improvements in peripheral metabolism compared to MICT31–33. However, the latter effect is dependent on the population studied. Specifically, in untrained and patients with coronary artery disease, MICT may produce superior results than IT34–36. By its very nature, IT represents a potential solution for individuals that report “lack of time” as a barrier to exercise participation. IT consists of repeated short and long bouts of high-intensity exercise (near maximal, maximal, or supramaximal intensity -maximal heart rate, VO2max, peak power output, superior to maximal lactate state-state velocity). High-intensity bouts of exercise are interspersed with recovery periods (light-moderate exercise intensity or passive recovery), with a full-session of IT requiring ≤ 30 min to be completed37,38. Therefore, IT can be easily adapted to participants representing different ages, fitness levels, or health status. Although there is considerable variation and classifications in the literature, high-intensity interval training (HIIT) and sprint interval training (SIT) are the primary forms of IT reported. HIIT is characterized by near maximal bouts of exercise ranging from 2–4 min per interval With SIT, bouts are shorter in length (~ 30 s), but are maximal or supramaximal39,40 (See Table 1).
Table 1.
Studies included in the meta-analysis.
| Reference | Sample size (male/female) | Fitness | Age (yr.) M ± SD | BDNF collection | Exercise characteristics (duration/frequency/mode) | Protocol | HIIT classification (by Wen et al. 2019) | Main findings | TESTEX score |
|---|---|---|---|---|---|---|---|---|---|
| Cabral-Santos et al. 2016 | 10 (10/0) | Physically active | 25.2 ± 1.7 | Serum | Crossover acute high-intensity exercise/motorized treadmill | High (2.5 km) and low (1.25 km) session at 1:1 min VO2max velocity with passive recovery | Acute moderate-volume and moderate-interval | Both protocols increased BDNF concentrations. Nonetheless, the BDNF response was not dependent of exercise volume | 7 |
| DiBattista et al. 2018 | 11 (11/0) | Physically active | 28.8 ± 5.3 | Plasma | Acute and after 2 weeks/three times a week/cycle-ergometer | 3-min warm-up at 50 W, 8 × 60 s intervals in sessions 1–2, 10 × 60 s intervals in sessions 3 – 4, 12 × 60 s intervals in sessions 5 – 6 at Wpeak interspersed 75 s of active recovery and 3 min cool-down at 50 W | Short-term, moderate-volume and moderate-interval HIIT | Both first and last session increased BDNF concentrations immediately after the exercise session, ST-HIIT did not change BDNF levels at baseline | 6 |
| Figuereido et al. 2019 | 11 (11/0) | Physically active | 22.5 ± 5.4 | Serum | Acute and eight week intervention/motorized treadmill | 5-min warm-up at 50% VO2max speed, 60 s 100% sVO2max with 60 s passive recovery (no exercise) until completion of 5 km | Acute, high-volume, moderate-interval HIIE | BDNF levels increased after the session completion compared at baseline | 5 |
| Gmiat et al. 2017 | 14 (0/14) | Sedentary | 30.8 ± 18.6 | Serum | Acute high intensity exercise/whole body circuit | 3 × 30 s of AMRAP of 10 circuit whole body exercises (i.e., jumping jacks, push-ups, abdominal crunch, squat, plank, triceps dips, high knees/running, lunges, push-up with rotations and side plank) with 2-min recovery between sets | Acute, high-volume, short-interval HIIE | noted decrease of BDNF after 1 h HIIE was present in both young and middle age participants | 7 |
| Heibisz et al. 2018 | 26 (17/9) | Sportsmen | 19.2 ± 5.2 | Serum | 6 months/2 – 3 sessions a week/cycle-ergometer |
HIIT: 5 to 7 5-min bouts at 85 – 95% Pmax with 12-min of moderate activity efforts at 55 – 60% Pmax 2 × a week SIT: 3 × 3 to 4 30 s bouts at all-out effort with 90 s of active recovery at 50 W and 25-min of low effort (45 – 55% Pmax) between sets |
HIIT: Long-term, high-volume, Long-interval training SIT: Long-term, moderate-volume, SIT |
Decreases of BDNF concentrations 10 and 60 min after sprint test in the SIT group after 2 and 6 month intervention | 11 |
| Heisz et al. 2017 | 66 (24/42) | Sedentary | 20.7 ± 2.8 | Serum | 6 weeks/~ 3 times a week/cycle-ergometer | 3-min warm-up at 50 W, 10 × 60 s of high intensity bouts performed at ~ 80%Wmax/~ 85 – 95% HRpeak combined with 60 s active recovery at ~ 30%Wmax and 2-min cool-down at 50 W | Mid-term, moderate-volume, moderate-interval HIIT | BDNF did not significantly change from the intervention, however individual differences between low and high responders to exercise are directly related to the increase of BDNF concentrations | 11 |
| Kujach et al. 2019 | 36 (36/0) | Physically active | 21.3 ± 1.3 | Serum | Acute/cycle-ergometer | 5-min warm-up at 1.5 W/kg body mass followed by 6 × 30 s all-out bout with fly-wheel at 0.075 kg/kg with resting periods of 4.5-min | Acute, low-volume, SIT | Increase of peripheral BDNF in experimental group was correlated with the significant increase in blood Lactate | 11 |
| Murawska et al. 2015 | 12 (7/5) | Physically active | 25.6 ± 5.8 | Serum | 3 months/2 times a week/CrossFit, whole body workout combined with treadmill or cycling | 60-min of WOD: 15-min strength training (dumbbells and bars) followed with 10-min whole body aerobic exercise circuit and finishing with 15-min of cycling or treadmill | Long-term, high-volume, Long-interval HIIT | Baseline BDNF increased after intervention, then lowered after progressive Wingate test in males but no changes in women | 11 |
| Nicolini et al. 2019 | 18 (18/0) | Sedentary | 23.1 ± | Serum | 6 weeks/3 sessions a week/cycle-ergometer | 3-min warm up at 50% Wpeak, 5 × 60 s high bouts at 105–135% Wpeak with 90 s active recovery at 30% Wpeak | Mid-term, low-volume, moderate-interval HIIT | Improvement of cardiorespiratory fitness but no significant changes in BDNF concentrations after 18 sessions of HIIT in sedentary individuals | 8 |
| Rentería et al. 2019 | 17 (0/17) | Sedentary | 21.5 ± 1.6 | Serum | 4 weeks/3 times a week/cycle-ergometer | 15- to 25-min of 3 to 5 30 s bouts of high intensity at 80% of MAP with 4-min of active recovery at 40% MAP | Mid-term, low-volume, moderate-interval | BDNF concentrations increased after intervention and lowered after graded-exercise test post-HIIT | 7 |
| Reycraft et al. 2019 | 8 (8/0) | Physically active | 23.1 ± 3 | Plasma | Crossover acute training/self-propelled treadmill | 18-min, 4 × 30 s bouts of all-out running interspersed with 4 min of active recovery | Acute, low-volume, SIT | BDNF concentrations were increased immediately post SIT and recovered baseline concentrations 30 and 90 min after exercise, no incremental changes were observed in other modalities | 7 |
| Rodríguez et al. 2018 | 6 (6/0) | Physically active | 22.6 ± 0.7 | Serum | Acute high intensity exercise/motorized treadmill | 5-min warm-up at 50–60%VO2max followed by 4 4 min bouts at 85% VO2max combined with 3-min active recovery at 40%VO2max | Acute/high-volume, long-interval HIIT | Significant BDNF increase after HIIE but the changes were not correlated with the increase in lactate | 7 |
| Sadowska et al. 2019 | 8 (8/0) | Physically active | 23.1 ± 1.7 | Serum | 6 weeks/5 days a week/CrossFit combined with running track field | 50-min of WOD composed with aerobic whole body circuit, aerobic training and weightlifting of predetermined sets or AMRAP | Mid-term, high-volume, combination of repeated sprint and SIT | Slight increase of BDNF levels after CrossFit training, but no changes after aerobic testing were performed | 7 |
| Saucedo-Marquez et al. 2015 | 21 (21/0) | Physically active | 28 ± | Serum | Crossover acute training/cycle-ergometer | 20-min of 60 s at 90% VO2max with 60 s active rest period | Acute, moderate-volume, moderate-interval HIIT | Greater increase of peripheral BDNF in HIIT compared to CON, with a higher magnitude of change in contrast to baseline measures | 11 |
| Slusher et al. 2018 | 13 (13/0) | Sedentary | 23.6 ± 1.0 | Serum and Plasma | Acute high intensity exercise/cycle-ergometer | 5-min, 20 s 170% VO2peak at 5.5% bodyweight interspersed with 10 s active recovery | Acute, low-volume, SIT | Serum BDNF increased significantly after HIIE and remained higher following the completion of executive function test; otherwise plasma BDNF was not modified in either post-HIIE or after completion of executive function text | 7 |
Note: AMRAP: As many repetitions as possible, BDNF: Brain-Derived Neurotrophic Factor. CON: Control group, HIIE: High Intensity Intermittent Exercise, HIIT: High Intensity Interval Training, MAP: Maximal aerobic power, Pmax: Maximal power, SIT: Sprint Interval Training, ST-HIIT: Short-term High Intensity Interval Training, sVO2max: Maximal speed reached during VO2max, VO2max: Maximal oxygen consumption, VO2peak: Peak oxygen consumption, Wmax: Maximal Wattage, WOD: Workout of day, Wpeak: Peak Wattage.
Therefore, the purpose of this systematic review and meta-analysis was to examine the effects of acute and chronic IT on circulating BDNF concentrations in apparently healthy young adults. In regard that the exercise response of BDNF is influenced by gender41,42, fitness level43,44, and exercise intensity45–47 an analysis of moderator variables by subgroups were performed. Finally, we assess the differences of changes in circulating BDNF between serum and plasma after IT.
Methods
Overview
This study followed the methodologies to complete a systematic review and meta-analysis suggested by Moher et al. (2015)48 and the International Prospective Register of Systematic Reviews (PROSPERO). The protocol was registered at PROSPERO under the code CRD42019122687.
Eligibility criteria
Studies that met the following criteria were included: (1) randomized controlled trials (RCT) and controlled trials without randomization (pre-test), (2) healthy normal-weight participants (as determined by a body mass index (BMI) between 20 to 24 kg/m2 or a body fat mass < 20% for men and < 28% for women, (3) young adults (18 to 40 yr. old), (4) male and female of different ethnic groups, (5) interventional studies, (6) serum and plasma circulating BDNF, (7) studies including participants free of any pharmacological prescription medication or drug, or recreational smoking, (8) studies using the enzyme-linked immunosorbent assay (ELISA) method to determine circulating BDNF. Studies that met the following criteria were excluded: (1) studies involving overweight and obese participants (BMI > 25 kg/m2), (2) children and adolescents (< 18 years old), (3) middle age and elderly people (> 40 yr. old), (4) pregnant women, and (5) cross-sectional studies.
Information sources
Seven electronic databases (PubMed, Science Direct Collection, Scopus, SpringerLink, Taylor & Francis journals, Wiley Online Library, Web of Science) were searched for potentially eligible studies in English. In addition, cross-referencing from retrieved studies was conducted. The last searches were conducted on February 2020 by two researchers (PCG-S and AJ-M).
Search strategy
Search strategies were developed using text words as well as Medical Subject Headings associated with the effects of exercise on BDNF. The search strategy included the following key words in English language: interval training, BDNF, intermittent training, high intensity intermittent training, interval running, brain-derived neurotrophic factor, high-intensity interval training, HIIT, sprint interval training, SIT, CrossFit, Tabata. Boolean operators AND, OR, NOT OR Mesh option were used to concatenate the search terms (key words). A secondary search was performed by screening the reference list of the selected studies and relevant review articles. Finally, a forward citation tracking of the selected studies was conducted through Scopus. An example of the search strategy for one of the databases searched (PubMed) is shown in supplementary Fig S1 online.
Study records and selection
All studies to potentially be screened were imported into Mendeley software, version 1.19.3 (Elsevier Inc., New York, NY, USA). One author then removed duplicates both electronically and manually. A copy of the database was then provided to two authors for duplicate screening. The two authors selected all studies, independent of each other. The full report for each article was obtained for all titles and abstracts that appeared to meet the inclusion criteria or where there was any uncertainty. Reasons for exclusion were coded as one or more of the following: (1) duplicates (2) missing or incomplete descriptive statistics (3) inappropriate research design (4) language different to English (5) abstracts only and (6) animal model. Upon completion, the two authors met and reviewed their selections. Given the small number of studies selected, discrepancies were reached by consensus. Based on the final number of studies to be included, the overall precision of the searches was calculated by dividing the number of studies included by the total number of studies screened after removing duplicates. The number needed to read (NNR) was then calculated as the inverse of the precision49.
Data extraction
Titles and/or abstracts of studies retrieved using the search strategy and those from additional sources were screened independently by two review authors (PCGS and AJM) to identify studies that potentially met the inclusion criteria outlined above. The full text of these potentially eligible studies was retrieved and independently assessed for eligibility by two review team members. Any disagreement between them over the eligibility of particular studies was resolved through discussion with a third reviewer (IR).
The studies were retrieved in Mendeley software, version 1.19.3 (Elsevier Inc., New York, NY, USA) and exclusion reasons were recorded. Data were exported to a standardized, pre-piloted Excel spreadsheet used to extract data from the included studies for assessment of study quality and evidence synthesis. The extracted information included publication year, participant demographics and baseline characteristics (e.g., gender, age, cardiorespiratory fitness level), details of the intervention (e.g., exercise frequency, intensity, duration, session duration, total duration of the intervention, dropouts) and control conditions, outcomes (i.e., serum and plasma BDNF) (mean and standard deviation). Two review authors extracted data independently and discrepancies were identified and resolved through discussion with a third author. Missing data were requested from study authors.
Primary outcome
The primary outcome was the change in peripheral BDNF concentration between control and experimental conditions (i.e., repeated measures design) or groups (i.e., independent group design). It is worth noting the first post-exercise BDNF measure was considered for analysis.
Risk of bias assessment in individual studies
Two review authors independently assessed the risk of bias in included studies by using the Tool for the Assessment of Study Quality and Reporting in Exercise (TESTEX)50. The TESTEX is a 12-item (5 points for study quality and 7 points for reporting) and 15-point scale (5 points for study quality and 10 points for reporting) developed to facilitate a comprehensive review of exercise training trials. Disagreements between the review authors over the risk of bias in particular studies were resolved by discussion, with involvement of a third review author where necessary.
Data synthesis and calculation of effect sizes
The effect size (ES) was calculated as the difference between means according to the methodology proposed by Borenstein, Hedges, Higgins, and Rothstein (2009)51. For the calculation, the initial score (pre-test) of BDNF was compared with the final score (post-test) after an intervention (exercise). The ES was subsequently adjusted to take into account the bias introduced by small samples52. For the analysis, the random effects model was used, which assumes that ESs vary between studies51,53. In this study, ES was interpreted as trivial (0 to 0.19), small (0.20 to 0.49), moderate (0.50 to 0.79) and large (≥ 0.80)54. ANOVA and independent samples t-test were used to determine mean ES differences between categorical moderator variables.
Meta-biases
Small-study effects (publication bias, etc.) were assessed following current recommendations55,56. The degree of heterogeneity of the studies was analyzed through Cochran’s Q test57 and the degree of consistency between the studies was calculated through the I2 test58. The I2 statistic ranges from 0 to 100%, and is interpreted as low (≤ 25%), moderate (26–74%) and high (≥ 75%)58. The effect of the studies with small samples was determined by the Doi plot and LFK index55. LFK index values outside the interval between −1 and + 1 are considered consistent with asymmetry (i.e. publication bias)59. An α level ≤ 0.05% and 95% confidence intervals (95% CI) that did not include zero (0) were considered to represent statistically significant small-study effects.
Software used for data synthesis
All data were analyzed using IBM SPSS Statistics for Windows, Version 23.0 (Armonk, NY), Microsoft Excel V.2010 and the Meta XL V.5.3, 2016 add-in software for Excel (EpiGear Intl., Queensland, Australia).
Results
Study characteristics
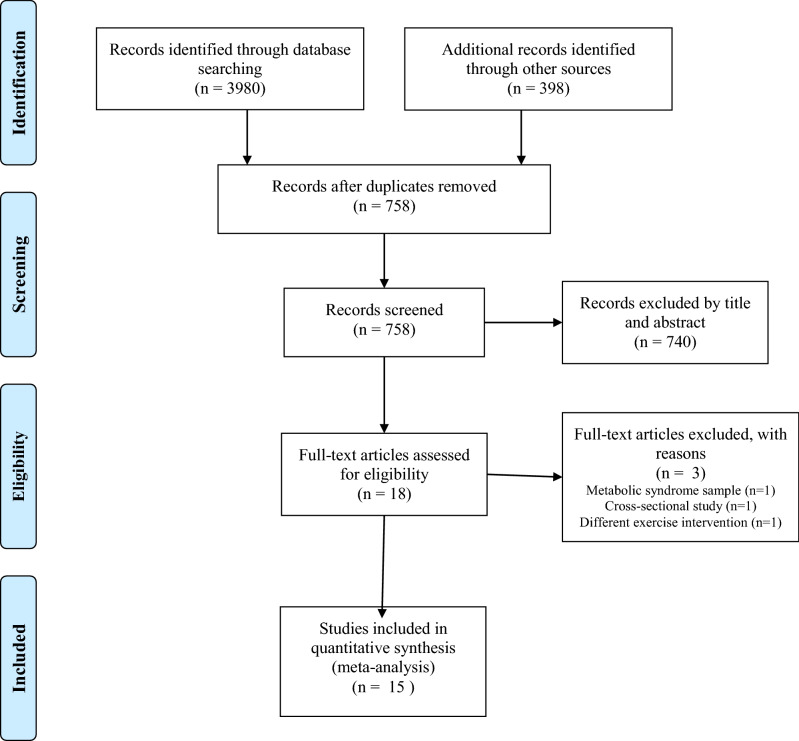
A flow diagram that depicts the search process for study selection is shown in Fig. 1. After initially identifying 4378 citations and removing 3620 duplicates both electronically and manually, 758 citations were screened. Of these, 18 studies met the criteria for inclusion, and three studies were excluded (one study used a sample with metabolic syndrome, one study used a cross-sectional design, and one study used a different exercise intervention). The major reasons for exclusion were: (1) duplicates (82.7%), (2) title and abstract did not meet the test subjects’ inclusion criteria (16.9%), (3) Cross-sectional study and other modalities different from IT (0.7%). The precision of the search, excluding duplicates, was 2% while the NNR was 51. Twenty-two ESs were computed from 15 studies representing 277 participants meeting the criteria for inclusion (Table 1).
Figure 1.
Flow diagram depicting the search process.
Participant and exercise characteristics
The mean age of the participants was 24.8 ± 4.4 yr., and the mean number of participants in the studies was 19 ± 15 participants, with most studies recruiting males (67%), mixed samples (20%), and a small number of studies recruiting females (13%). Participants in the studies were physically active (60%), sedentary (33%), and athletes (7%). Nine studies (60%) recorded acute exercise responses and six studies (40%) recorded chronic training effects. Specific types of activities included IT on a cycle-ergometer (54%), treadmill (27%), and combined (20%), including whole-body circuits, CrossFit, and running on a track field (Table 1). Overall, five studies assessed BDNF response on HIIT (34%) and ten studies in SIT (66%).
Risk of bias assessment
Results for risk of bias assessment using the TESTEX scale showed that overall, studies achieved 54.7% of the quality requirements. Therefore, 45.3% of the studies were at an unclear or high risk of bias concerning: (1) eligibility criteria specified (100%), (2) randomization specified (53%), (3) allocation concealment (53%), (4) groups similar at baseline (53%), (5) blinding of assessor (0%), (6) outcome measures assessed in 85% of patients (13%), (7) intention-to-treat analysis (73%), (8) between-groups statistical comparisons reported (50%), (9) point measures and measures of variability for all reported outcome measures (80%), (10) activity monitoring in control groups (73%), (11) relative exercise intensity remained constant (10%), and (12) exercise volume and energy expenditure (93%). Given the inability to truly blind participants in exercise intervention trials, all studies (100%) were considered to be at a high risk of bias for the categories “allocation concealment” and “blinding of assessor”. In addition, 87% of the studies did not report adverse effects and 13% of the studies reported adherence to exercise interventions.
Data synthesis
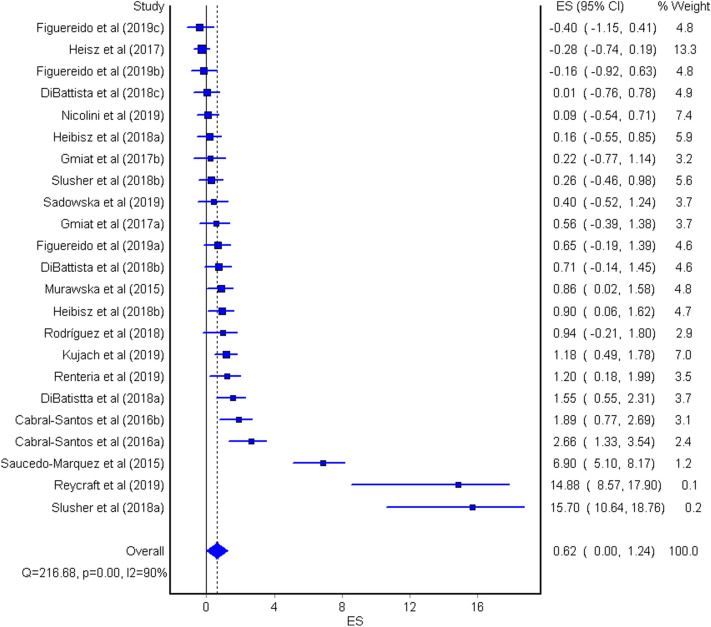
The overall effect of IT on peripheral circulating BDNF concentrations was moderate and significant (ES = 0.62, 95% CI = 0.00, 1.24, Fig. 2). The studies provided heterogeneous results (Q = 216.68, p < 0.001), showed high inconsistency (I2 = 90%), and major asymmetry (LFK index = 2.76, see Supplementary Fig. S2 online).
Figure 2.
The overall effect of interval training (IT) on peripheral BDNF concentration. The lines indicate 95% confidence intervals (CI), and the square reflects the standardized differences (SMD) for each study. The diamond in the forest plot indicates the overall effect size (ES).
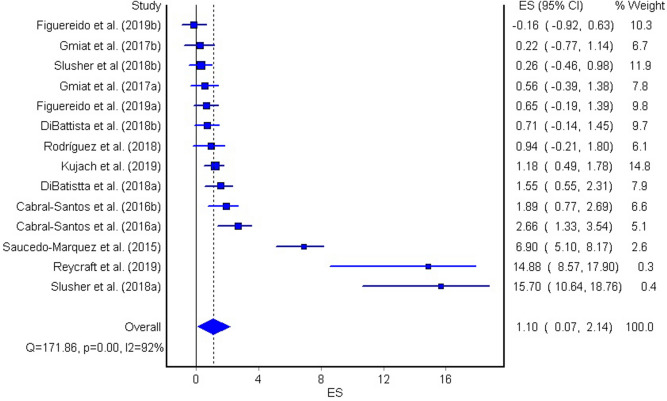
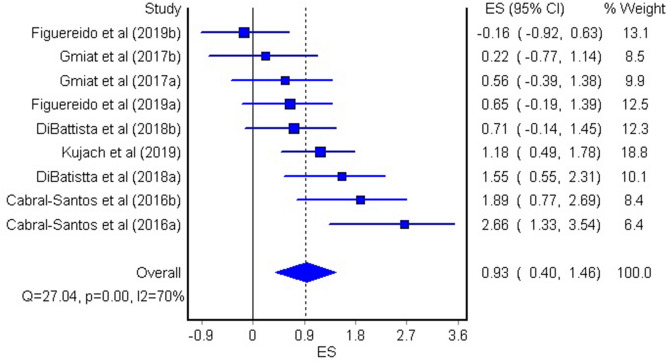
The acute effect of IT on circulating BDNF was large and significant (ES = 1.10, 95% CI = 0.07, 2.14, Fig. 3). However, similar to the overall effect, the studies provided heterogeneous results (Q = 171.86, p < 0.001), and showed high inconsistency (I2 = 92%), and major asymmetry (LFK index = 3.34, see Supplementary Fig. S3 online). The chronic effect of IT on BDNF was large and significant (ES = 0.93, 95% CI = 0.40, 1.46, Fig. 4). The studies provided heterogeneous results (Q = 27.04, p < 0.001), showed moderate inconsistency (I2 = 70%), and minor asymmetry (LFK index = 1.21, see Supplementary Fig. S4 online).
Figure 3.
The effect of acute interval training (IT) on circulating BDNF concentration. The lines indicate 95% confidence intervals (CI), and the square reflect the standardized differences (SMD) for each study. The diamond in the forest plot indicates the overall effect size (ES).
Figure 4.
The effect of chronic interval training (IT) on circulating BDNF concentration. The lines indicate 95% confidence intervals (CI), and the square reflects the standardized differences (SMD) for each study. The diamond in the forest plot indicates the overall effect size (ES).
Categorical moderator variable analysis on acute and chronic exercise interventions showed that there is no statistically significant subgroup effect for fitness level, type of training, and medium (serum vs. plasma) for acute and chronic IT (Table 2). There was a strong tendency (p = 0.052) for gender in chronic IT analysis; a higher ES was observed in females during chronic IT intervention compared with males (Table 2). For continuous moderators, no significant correlations were found between ES and age for acute (r = -0.18, p = 0.534) and chronic (r = -0.09, p = 0.805) exercise. No significant correlations were found between ES and sample size for acute (r = -0.20, p = 0.501) and chronic (r = -0.45, p = 0.192) exercise.
Table 2.
Moderator variables for the effect of acute and chronic exercise on BDNF.
| Variable | n = studies | ES ± SD | 95%CI | p = | |
|---|---|---|---|---|---|
| Lower-limit | Upper-limit | ||||
| Acute exercise | |||||
| Gender | 0.405 | ||||
| Male | 12 | 3.93 ± 5.61 | 0.76 | 7.10 | |
| Female | 2 | 0.39 ± 0.24 | 0.06 | 0.72 | |
| Fitness | |||||
| Sedentary | 4 | 1.19 ± 7.68 | − 6.33 | 8.71 | 0.750 |
| Active | 10 | 3.12 ± 4.57 | 0.29 | 5.95 | |
| Type of training | 0.824 | ||||
| HIIT | 8 | 3.13 ± 5.57 | − 0.74 | 7.00 | |
| SIT | 6 | 3.81 ± 5.46 | − 0.56 | 8.18 | |
| Blood analysis | 0.698 | ||||
| Plasma | 4 | 4.35 ± 7.04 | − 2.55 | 11.3 | |
| Serum | 10 | 3.05 ± 4.88 | − 0.44 | 6.55 | |
| Chronic exercise | |||||
| Gender | |||||
| Male | 5 | 0.15 ± 0.40 | − 0.35 | 0.65 | 0.052 |
| Female | 2 | 1.25 ± 0.06 | 0.67 | 1.82 | |
| Mixed | 3 | 0.26 ± 0.60 | − 1.22 | 1.74 | |
| Fitness | 0.950 | ||||
| Sedentary | 3 | 0.34 ± 0.77 | − 1.58 | 2.25 | |
| Active | 5 | 0.39 ± 0.64 | − 0.41 | 1.19 | |
| Athlete | 2 | 0.53 ± 0.52 | − 4.17 | 5.23 | |
| Type of training | 0.208 | ||||
| HIIT | 4 | 0.48 ± 0.69 | − 0.62 | 1.57 | |
| SIT | 3 | − 0.08 ± 0.29 | − 0.80 | 0.64 | |
| CrossFit | 3 | 0.78 ± 0.46 | − 0.36 | 1.92 | |
Discussion
The present study was designed to systematically-review and meta-analyze the effects of acute and chronic IT on circulating BDNF concentration in young adults. Overall, acute and chronic IT increased peripheral BDNF concentration. In chronic IT, females showed greater increases in BDNF compared with males. Finally, the study showed that the fitness levels did not regulate the BDNF response after IT, at least in the studied population (apparently healthy young adults).
The data of the current study are in agreement with the previous report focused on the impact of aerobic exercise on peripheral BDNF60. In Dinoff’s study, the exercise protocols were longer than the interventions analyzed in the current meta-analysis (≤ 30 min/session). This finding suggests that IT is an effective treatment to improve brain health with more time efficiency than MICT. The latter condition is concordant with peripheral adaptations induced by IT (e.g., oxidative capacity in muscle, cardiometabolic markers)31,37,61.
IT is characterized by lactate accumulation in blood45,62–64. Studies in rodents have demonstrated that blood lactate (BLa) produced during exercise reaches the brain and enhances expression of genes associated with cognition (i.e. Bdnf)65,66. Although in humans this response has not been completely demonstrated, authors suggested a similar effect of BLa in brain45,63,67,68. Resulting in diverse improvements in executive function63. Unfortunately, in the current meta-analysis, there were not enough studies that reported blood lactate changes; consequently, it was not possible to run meta-regressions to identify the role of this metabolite in the BDNF response.
Non-statistical differences were found among BDNF changes in plasma and serum (Table 2). While some studies did not find statistical differences between BDNF changes in serum and plasma following physical exercise27, others reported significant changes in circulating BDNF in plasma compared with serum60. In previous studies, aerobic, strength, and concurrent training were analyzed, whereas, in the current meta-analysis, IT interventions were examined. Circulating BDNF changes are sensitive to training modality45,69; therefore, it was not possible to compare our data with other systematic and meta-analytic works27,60.
In serum, BDNF concentration is > 50 fold higher than plasma18,70,71. In the periphery, platelets store BDNF; therefore, these cells are considered the major reservoir of circulating BDNF71,72. Once activated, platelets release BDNF18,71. This process is considered the main mechanism to explain differences between serum and plasma concentrations73,74. The evidence suggests that chronic training improves the capacity of platelets to release BDNF18,75. Concerning this, we did not discard that the length of interventions examined in the current study was insufficient to modify the platelet’s capacity in the BDNF secretion; thus, further studies are necessary to elucidate this hypothesis. In addition, it is known that IT is an exercise modality that increases muscle damage76. We believe that this condition could be present in the participants and consequently will generate platelet activation71, releasing BDNF to repair muscle injuries77. This physiological response might explain the lack of differences among the BDNF changes in serum and plasma (Table 2). Finally, we did not discard that the small numbers of studies included in the current meta-analysys can explain the lack of differences among the blood mediums.
Furthermore, it is worth noting that plasma volume (PV) changes should be considered in studies that assess the impact of exercise on biomarkers such as neurotrophins. It is known that exercise modifies PV78, which can increase biomarker concentrations. Thus, the results of studies neglecting to measure PV changes should be viewed with caution78–80. In one study examined, there was no effect on circulating BDNF with IT when PV was not adjusted. In contrast, DiBatista et al. showed that IT increased BDNF levels following PV adjustment. Finally, in work conducted by Reycraft and colleagues,PV was not adjusted and the authors reported a significant effect of IT on BDNF. The results of these studies show that PV should be considered when evaluating the effects of IT on circulating BDNF levels. Moreover, studies where BDNF was assessed in plasma were fewer than studies where the biomarker was measured in serum. Thus, unequal distribution can be a confounding variable to find statistical differences.
Similarly, to the medium, fitness level did not significantly affect the BDNF response to IT. These findings are contrary to previous reports18,44,60,75,81. Despite the established negative correlation between fitness level and BDNF response during exhaustive or aerobic exercise44,60, biochemical and physiological mechanisms are not fully understood. One hypothesis suggests that well-trained participants have higher BDNF receptor levels in peripheral organs (e.g., skeletal muscle) which could attenuate circulating BDNF changes during exercise82. Once it activates the peripheral TrkB receptor, BDNF participates in the repair of skeletal muscle77. As indicated above, IT induces muscle damage in well-trained and untrained participants76; therefore, we did not discard that the low peripheral BDNF levels were induced by muscular damage after IT. That condition could partially explain the lack of significant differences in BDNF changes between athletes and untrained participants (Table 2). Another hypothesis suggests that trained participants show better cognitive performance than sedentary people44,83. Indeed, athletes and well-trained individuals have more efficient uptake and utilization of BDNF which has been shown to improve neural plasticity and improve performance in cognitive tasks compared to untrained participants44,83. The extensive utilization of BDNF in brain reflects a lower peripheral BDNF in athletes and well-trained participants with respect to untrained people44,83. Therefore, we do not discard the possibility that active participants show a high capacity to uptake BDNF in brain after IT compared with sedentary participants (Table 2). In contrast, sedentary participants have lower synthesis and release of BDNF. Both conditions combined resulted in a non-significant statistical effect among active and sedentary (Table 2).
The null findings observed in sedentary and active participants after acute IT can be explained by stress hormone activity. Specifically, IT is perceived as difficult and vigorous in well-trained and untrained population45,76,84–86. In agreement with this, IT increases systemic cortisol concentrations in athletes and untrained participants47,87–89. Cortisol is a hormone that decreases BDNF synthesis90. Therefore, higher cortisol levels could be present in the participant (sedentary, active, and athlete participants) after IT, reducing differences in BDNF changes (Table 2). Finally, we do not exclude the possibility that the small numbers of studies included in the current meta-analyses can explain the lack of differences among fitness levels.
We found a high ES (strong tendency) for females compared with males; a difference shown principally in chronic IT (Table 2). This may be explained by the role of steroid hormones since it is known the positive effect of 17β estradiol on BDNF synthesis in the brain91–95. The estrogen hormone concentrations change during the menstrual cycle96; particularly, high levels of estrogen are found during the late follicular phase97. In the studies analyzed in the current meta-analysis, the menstrual cycle was not coded; therefore, we do not discard the possibility that some of the blood collection made in females was performed during the follicular phase, resulting in an enhancement effect of estrogen to IT impact on BDNF changes compared to males. Additionally, as discussed previously, platelets store and release BDNF71,72. In this sense, classic and emerging studies show that women have higher platelet content than men98–100. In light of this, we do not discard that platelet count could contribute to a higher BDNF response in women compared with men (Table 2). Additionally, authors have previously suggested that skeletal muscle uptake BDNF; once captured the neurotrophin regulates metabolic and neuromuscular responses101–103. In females, muscle mass is lower than males104–106. Therefore, it is possible that differences skeletal muscle mass among sex, can explain the larger ES in women compared with men (Table 2).
The current meta-analysis highlights that IT is an effective strategy to increase peripheral BDNF concentrations in young healthy adults. Our findings are in agreement with prior meta-analysis focused on assessing the impact of physical exercise (e.g., aerobic and strength exercise) on circulating BDNF in young adult and healthy population27,60,107 (Fig. 5). This finding adds relevant information to previous studies reporting a positive impact of IT on fitness levels108–110, and hemodynamic variablesy111. Therefore, the state of the art, based on quantitative analysis suggests that IT may be considered an adequate physical exercise modality to strengthen the health (brain and peripheral physiological functions) in an apparently healthy young adult population.
Figure 5.
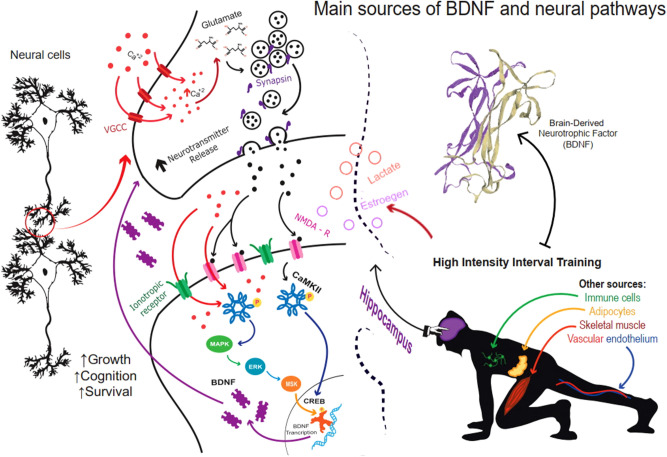
Interval training increases circulating BDNF levels in healthy adults (upper right). During this response, the brain (hippocampal region) seems be the main BDNF source; nevertheless, other tissues function as BDNF synthesizers. The mechanism of activation during IT has not elucidated yet (above right). In brain, BDNF synthesis is activated by an increase of calcium (Ca2+) concentrations in the cytosol. Inside neurons, Ca2+ activates calmodulin dependent kinase II (CaMKII), triggering activation of the MAPK/ERK/MSK cascade resulting in an increase in the expression and phosphorylation of cAMP response element-binding protein (CREB). CREB initiates BDNF transcription resulting in increased BDNF synthesis and release (left). Once secreted, the neurotrophin regulates molecular mechanisms associated with neuronal growth, cognition, and neuron survival (above left). Finally, scientific evidence suggests that other circulating molecules such as lactate and estrogen enhance BDNF synthesis in brain (center). The putative mechanism indicate that lactate increases calcium current in the neurons, and estrogens activates nuclear estrogen receptors and membrane estrogen receptors that enhance the BDNF synthesis. Figure made with adobe illustrator cs6. https://www.adobe.com/products/illustrator/free-trial-download.html. Figure conceived and designed for PCGS.
Supplementary Information
Acknowledgements
The authors are deeply thankful to Fabio Santos-Lira, Eugenia Murawska-Cialowicz, Aaron L. Slusher, Chun-Jung Huang and Alex Di Battista by providing raw BDNF data for the completion of this meta-analysis.
Author contributions
A.J.-M. conceived the review focus. A.J.-M. and P.C.G.S. reviewed the literature, and analyzed the data. I.R. and J.M.J. contributed with the data analysis and interpretation, E.P.P. contributed to the critical revision of the manuscript. A.J.M. drafted the manuscript. P.C.G.S. and E.P.P. finalized the manuscript. P.C.G.S. conceived and designed the Fig. 5. All authors approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88496-x.
References
- 1.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binder DK, Scharfman HE. Brain derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall J, Thomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat. Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 4.Kang HJ, Schuman EM. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J. Physiol. - Paris. 1995;89:11–22. doi: 10.1016/0928-4257(96)80547-X. [DOI] [PubMed] [Google Scholar]
- 5.Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. U. S. A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acheson, A. et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Lett. to Nat.374, (1995). [DOI] [PubMed]
- 7.Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: Mechanisms and functions. Physiology. 2005;20:70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- 8.Finkbeiner S, et al. CREB: A major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/S0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- 9.Matthews VB, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 10.Chaldakov GN, Tonchev AB, Aloe L. NGF and BDNF: from nerves to adipose tissue, from neurokines to metabokines. Riv. Psichiatr. 2009;44:79–87. [PubMed] [Google Scholar]
- 11.Nakahashi T, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/S0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen P, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 13.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of Brain-Derived Neurotrophic Factor. BDNF) Protein and mRNA in the Normal Adult Rat CNS; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/S0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 16.Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci. Lett. 2008;431:62–65. doi: 10.1016/j.neulet.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Suliman S, Hemmings SM, Seedat S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: Systematic review and meta-regression analysis. Front. Integr. Neurosci. 2013;7:1–11. doi: 10.3389/fnint.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gejl AK, et al. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-45976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue DS, et al. Acute increases in brain-derived neurotrophic factor following high or moderate-intensity exercise is accompanied with better cognition performance in obese adults. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-019-56847-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karege F, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/S0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 22.Impact on BDNF levels and psychopathology Kauer-Sant’Anna, M. et al. Traumatic life events in bipolar disorder. Bipolar Disord. Suppl. 2007;9:128–135. doi: 10.1111/j.1399-5618.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 23.Molendijk ML, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: State-trait issues, clinical features and pharmacological treatment. Mol. Psychiatry. 2011;16:1088–1095. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zoladz JA, et al. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in parkinson’s disease patients. J. Physiol. Pharmacol. 2014;65:441–448. [PubMed] [Google Scholar]
- 25.Zoladz JA, et al. Endurance Training Increases Plasma Brain-Derived Neurotrophic Factor Concentration in Young Healthy Men. J. Physiol. Pharmacol. 2008;59:119–132. [PubMed] [Google Scholar]
- 26.Huang T, Larsen KT, Ried-Larsen M, Møller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sport. 2014;24:1–10. doi: 10.1111/sms.12069. [DOI] [PubMed] [Google Scholar]
- 27.Dinoff, A., Herrmann, N., Swardfager, W., one, C. L.-P. & 2016, undefined. The effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): a meta-analysis. ncbi.nlm.nih.gov. [DOI] [PMC free article] [PubMed]
- 28.Dishman RK, et al. Neurobiology of exercise. Obesity. 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- 29.Hoare E, Stavreski B, Jennings G, Kingwell B. Exploring Motivation and Barriers to Physical Activity among Active and Inactive Australian Adults. Sports. 2017;5:47. doi: 10.3390/sports5030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costello E, Kafchinski M, Vrazel J, Sullivan P. Motivators, barriers, and beliefs regarding physical activity in an older adult population. J. Geriatr. Phys. Ther. 2011;34:138–147. doi: 10.1519/JPT.0b013e31820e0e71. [DOI] [PubMed] [Google Scholar]
- 31.Gibala MJ, et al. Short-term sprint interval versus traditional endurance training: Similar initial adaptations in human skeletal muscle and exercise performance. J. Physiol. 2006;575:901–911. doi: 10.1113/jphysiol.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little JP, Jung ME, Wright AE, Wright W, Manders RJF. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Appl. Physiol. Nutr. Metab. 2014;39:835–841. doi: 10.1139/apnm-2013-0512. [DOI] [PubMed] [Google Scholar]
- 33.Gillen JB, et al. Twelve Weeks of Sprint Interval Training Improves Indices of Cardiometabolic Health Similar to Traditional Endurance Training despite a Five-Fold Lower Exercise Volume and Time Commitment. PLoS ONE. 2016;11:e0154075. doi: 10.1371/journal.pone.0154075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster C, et al. The effects of high intensity interval training vs steady state training on aerobic and anaerobic capacity. J. Sport. Sci. Med. 2015;14:747–755. [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang X, et al. Endurance training but not high-intensity interval training reduces liver carcinogenesis in mice with hepatocellular carcinogen diethylnitrosamine. Exp. Gerontol. 2020;133:110853. doi: 10.1016/j.exger.2020.110853. [DOI] [PubMed] [Google Scholar]
- 36.Tschentscher, M. et al. High-intensity interval training is not superior to other forms of endurance training during cardiac rehabilitation. Eur. J. Prev. Cardiol.0, 1–7 (2014). [DOI] [PubMed]
- 37.Gillen JB, Gibala MJ. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. 2018;39:409–412. doi: 10.1139/apnm-2013-0187. [DOI] [PubMed] [Google Scholar]
- 38.Billat LV. Interval Training for Performance: A Scientific and Empirical Practice. Sport. Med. 2001;31:13–31. doi: 10.2165/00007256-200131010-00002. [DOI] [PubMed] [Google Scholar]
- 39.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J. Physiol. 2017;595:2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle: Part II: Anaerobic energy, neuromuscular load and practical applications. Sports Med. 2013;43:927–954. doi: 10.1007/s40279-013-0066-5. [DOI] [PubMed] [Google Scholar]
- 41.Forti LN, et al. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp. Gerontol. 2015;70:144–149. doi: 10.1016/j.exger.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Chan CB, Ye K. Sex Differences in Brain-Derived Neurotrophic Factor Signaling and Functions. J. Neurosci. Res. 2017;95:328–335. doi: 10.1002/jnr.23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antunes BM, Rossi FE, Teixeira AM, Lira FS. Short-time high-intensity exercise increases peripheral BDNF in a physical fitness-dependent way in healthy men. Eur. J. Sport Sci. 2019;20:1–8. doi: 10.1080/17461391.2019.1611929. [DOI] [PubMed] [Google Scholar]
- 44.Babaei P, Damirchi A, Mehdipoor M, Tehrani BS. Long term habitual exercise is associated with lower resting level of serum BDNF. Neurosci. Lett. 2014;566:304–308. doi: 10.1016/j.neulet.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Saucedo Marquez, C. M., Vanaudenaerde, B., Troosters, T. & Wenderoth, N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J. Appl. Physiol.119, 1363–1373 (2015). [DOI] [PubMed]
- 46.Jeon, Y. K. & Ha, C. H. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ. Health Prev. Med.22, (2017). [DOI] [PMC free article] [PubMed]
- 47.Rodriguez AL, et al. Acute high-intensity interval exercise induces greater levels of serum brain-derived neurotrophic factor in obese individuals. Exp. Biol. Med. 2018;243:1153–1160. doi: 10.1177/1535370218812191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:148–160. doi: 10.1186/s13643-015-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee E, et al. An optimal search filter for retrieving systematic reviews and meta-analyses. BMC Med. Res. Methodol. 2012;12:1–11. doi: 10.1186/1471-2288-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smart NA, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies. Int. J. Evid. Based. Healthc. 2015;13:9–18. doi: 10.1097/XEB.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 51.Borenstein, M., Hedges, L. V., Higgins, J. P. T. & Rothstein, H. R. Introduction to Meta-Analysis. Introduction to Meta-Analysis (John Wiley & Sons, Ltd, 2009). doi:10.1002/9780470743386.
- 52.Morris SB. Estimating Effect Sizes From Pretest-Posttest-Control Group Designs. Organ. Res. Methods. 2008;11:364–386. doi: 10.1177/1094428106291059. [DOI] [Google Scholar]
- 53.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 54.Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 55.Furuya-Kanamori L, Barendregt JJ, Doi SAR. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based. Healthc. 2018;16:195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 56.Sterne, J. A. C. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ343, (2011). [DOI] [PubMed]
- 57.Cochran WG. The Combination of Estimates from Different Experiments. Biometrics. 1954;10:101. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 58.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furuya-Kanamori, L., Doi, S. A., Furuya-Kanamori, L. & Doi, S. A. L. F. K. Stata module to compute LFK index and and Doi plot for detection of publication bias in meta-analysis (2020).
- 60.Dinoff A, Herrmann N, Swardfager W, Lanctôt KL. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur. J. Neurosci. 2017;46:1635–1646. doi: 10.1111/ejn.13603. [DOI] [PubMed] [Google Scholar]
- 61.Burgomaster KA, et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008;586:151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cabral-Santos C, et al. Inflammatory Cytokines and BDNF Response to High-Intensity Intermittent Exercise: Effect the Exercise Volume. Front. Physiol. 2016;7:1–8. doi: 10.3389/fphys.2016.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto T, et al. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. 2018;32:1417–1427. doi: 10.1096/fj.201700381RR. [DOI] [PubMed] [Google Scholar]
- 64.Kujach S, et al. Acute Sprint Interval Exercise Increases Both Cognitive Functions and Peripheral Neurotrophic Factors in Humans: The Possible Involvement of Lactate. Front. Neurosci. 2020;13:1–14. doi: 10.3389/fnins.2019.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Hayek L, et al. Lactate mediates the effects of exercise on learning and memory through sirt1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF) J. Neurosci. 2019;39:2369–2382. doi: 10.1523/JNEUROSCI.1661-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. 2014;111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schiffer T, et al. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci. Lett. 2011;488:234–237. doi: 10.1016/j.neulet.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 68.Brooks GA. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 69.Domínguez-sanchéz MA, Bustos-cruz RH, Velasco-orjuela GP. Acute Effects of High Intensity, Resistance, or Combined Protocol on the Increase of Level of Neurotrophic Factors in Physically Inactive Overweight Adults : The BrainFit Study. Front. Physiol. 2018;9:1–12. doi: 10.3389/fphys.2018.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radka SF, Holst PA, Fritsche M, Altar CA. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996;709:122–130. doi: 10.1016/0006-8993(95)01321-0. [DOI] [PubMed] [Google Scholar]
- 71.Fujimura H, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002;87:728–734. doi: 10.1055/s-0037-1613072. [DOI] [PubMed] [Google Scholar]
- 72.Karege F, et al. Low Brain-Derived Neurotrophic Factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Polyakova M, et al. Stability of bdnf in human samples stored up to 6 months and correlations of serum and edta-plasma concentrations. Int. J. Mol. Sci. 2017;18:1–11. doi: 10.3390/ijms18061189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maffioletti E, Zanardini R, Gennarelli M, Bocchio-Chiavetto L. Influence of clotting duration on brain-derived neurotrophic factor (BDNF) dosage in serum. Biotechniques. 2014;57:111–114. doi: 10.2144/000114204. [DOI] [PubMed] [Google Scholar]
- 75.Cho HC, et al. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO 2max performance in healthy college men. Neurosci. Lett. 2012;519:78–83. doi: 10.1016/j.neulet.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 76.Cipryan L. The effect of fitness level on cardiac autonomic regulation, IL-6, total antioxidant capacity, and muscle damage responses to a single bout of high-intensity interval training. J. Sport Heal. Sci. 2016;7:363–371. doi: 10.1016/j.jshs.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao Yu, Yun Chang, Xiao Lin Gao, Han Li, P. Z. et al. Dynamic Expression and the Role of BDNF in Exercise-induced Skeletal Muscle Regeneration. Physiol. Biochem.38, 959–966 (2017). [DOI] [PubMed]
- 78.Alis R, Ibañez-Sania S, Basterra J, Sanchis-Gomar F, Romagnoli M. Effects of an acute high-intensity interval training protocol on plasma viscosity. J. Sports Med. Phys. Fitness. 2014;55:647–653. [PubMed] [Google Scholar]
- 79.Matomäki P, Kainulainen H, Kyröläinen H. Corrected whole blood biomarkers – the equation of Dill and Costill revisited. Physiol. Rep. 2018;6:1–3. doi: 10.14814/phy2.13749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alis R, et al. Hemoconcentration induced by exercise: Revisiting the Dill and Costill equation. Scand. J. Med. Sci. Sport. 2015;25:e630–e637. doi: 10.1111/sms.12393. [DOI] [PubMed] [Google Scholar]
- 81.Correia PR, et al. Increased basal plasma brain-derived neurotrophic factor levels in sprint runners. Neurosci. Bull. 2011;27:325–329. doi: 10.1007/s12264-011-1531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nofuji Y, et al. Different Circulating BDNF Responses to Acute Exercise Between Physically Active and Sedentary Subjects. J. Sports Sci. Med. 2012;11:83–88. [PMC free article] [PubMed] [Google Scholar]
- 83.Currie J, Ramsbottom R, Ludlow H, Nevill A, Gilder M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci. Lett. 2009;451:152–155. doi: 10.1016/j.neulet.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 84.Saanijoki T, et al. Affective Adaptation to Repeated SIT and MICT Protocols in Insulin-Resistant Subjects. Med. Sci. Sports Exerc. 2018;50:18–27. doi: 10.1249/MSS.0000000000001415. [DOI] [PubMed] [Google Scholar]
- 85.Heisz JJ, Tejada MGM, Paolucci EM, Muir C. Enjoyment for high-intensity interval exercise increases during the first six weeks of training: Implications for promoting exercise adherence in sedentary adults. PLoS ONE. 2016;11:1–10. doi: 10.1371/journal.pone.0168534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Slusher AL, Patterson VT, Schwartz CS, Acevedo EO. Impact of high intensity interval exercise on executive function and brain derived neurotrophic factor in healthy college aged males. Physiol. Behav. 2018;191:116–122. doi: 10.1016/j.physbeh.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 87.García-Pinillos F, Soto-Hermoso VM, Latorre-Román PA. How does high-intensity intermittent training affect recreational endurance runners? Acute and chronic adaptations: A systematic review. J. Sport Heal. Sci. 2017;6:54–67. doi: 10.1016/j.jshs.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Monje C, et al. Effects of a high intensity interval session on mucosal immune function and salivary hormones in male and female endurance athletes. J. Sport. Sci. Med. 2020;19:436–443. [PMC free article] [PubMed] [Google Scholar]
- 89.Tanner AV, Nielsen BV, Allgrove J. Salivary and plasma cortisol and testosterone responses to interval and tempo runs and a bodyweight-only circuit session in endurance-trained men. J. Sports Sci. 2014;32:680–689. doi: 10.1080/02640414.2013.850594. [DOI] [PubMed] [Google Scholar]
- 90.Issa G, Wilson C, Terry AVB, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: Data from human postmortem and animal studies. Neurobiol. Dis. 2010;39:327–333. doi: 10.1016/j.nbd.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 91.Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2013;239:34–45. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Front. Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Langhnoja JM, Buch LK, Pillai PP. 17β-estradiol modulates NGF and BDNF expression through ERβ mediated ERK signaling in cortical astrocytes. Biologia (Bratisl). 2018;73:907–915. doi: 10.2478/s11756-018-0099-1. [DOI] [Google Scholar]
- 94.Kight KE, McCarthy MM. Sex differences and estrogen regulation of BDNF gene expression, but not propeptide content, in the developing hippocampus. J. Neurosci. Res. 2017;95:345–354. doi: 10.1002/jnr.23920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu YWC, Du X, Van Den Buuse M, Hill RA. Analyzing the influence of BDNF heterozygosity on spatial memory response to 17β-estradiol. Transl. Psychiatry. 2015;5:e498–e498. doi: 10.1038/tp.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farage MA, Neill S, MacLean AB. Physiological changes associated with the menstrual cycle a review. Obstet. Gynecol. Surv. 2009;64:58–72. doi: 10.1097/OGX.0b013e3181932a37. [DOI] [PubMed] [Google Scholar]
- 97.Draper CF, et al. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-32647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bain BJ. Platelet count and platelet size in males and females. Scand. J. Haematol. 1985;35:77–79. doi: 10.1111/j.1600-0609.1985.tb00804.x. [DOI] [PubMed] [Google Scholar]
- 99.Butkiewicz AM, et al. Platelet count, mean platelet volume and thrombocytopoietic indices in healthy women and men. Thromb. Res. 2006;118:199–204. doi: 10.1016/j.thromres.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 100.Ranucci M, et al. Gender-based differences in platelet function and platelet reactivity to P2Y12 inhibitors. PLoS ONE. 2019;14:1–11. doi: 10.1371/journal.pone.0225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology. 2013;28:330–358. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 102.Sakuma, K. & Yamaguchi, A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J. Biomed. Biotechnol.2011, (2011). [DOI] [PMC free article] [PubMed]
- 103.Delezie J, et al. BDNF is a mediator of glycolytic fiber-type specification in mouse skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 2019;116:16111–16120. doi: 10.1073/pnas.1900544116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schorr M, et al. Sex differences in body composition and association with cardiometabolic risk. Biol. Sex Differ. 2018;9:1–10. doi: 10.1186/s13293-018-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cichy I, et al. Sex differences in body composition changes after preseason training in elite handball players. Int. J. Environ. Res. Public Health. 2020;17:1–8. doi: 10.3390/ijerph17113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He X, et al. Age- and sex-related differences in body composition in healthy subjects aged 18 to 82 years. Med. (United States) 2018;97:12–17. doi: 10.1097/MD.0000000000011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu, J. xin, Zhu, L., Li, P. jun, Li, N. & Xu, Y. bing. Effectiveness of high-intensity interval training on glycemic control and cardiorespiratory fitness in patients with type 2 diabetes: a systematic review and meta-analysis. Aging Clin. Exp. Res.31, 575–593 (2019). [DOI] [PMC free article] [PubMed]
- 109.Milanović Z, Sporiš G, Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sport. Med. 2015;45:1469–1481. doi: 10.1007/s40279-015-0365-0. [DOI] [PubMed] [Google Scholar]
- 110.Sultana RN, Sabag A, Keating SE, Johnson NA. The Effect of low-volume high-intensity interval training on body composition and cardiorespiratory fitness: A systematic review and meta-analysis. Sports Med. 2019;49:1687–1721. doi: 10.1007/s40279-019-01167-w. [DOI] [PubMed] [Google Scholar]
- 111.Caldas-Costa E, et al. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in adults with pre- to established hypertension: A systematic review and meta-analysis of randomized trials. Sport. Med. 2018;48:2127–2142. doi: 10.1007/s40279-018-0944-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on request.