Abstract
Pasteurella multocida causes fowl cholera, a highly contagious poultry disease of global concern, causing significant ecological and economic challenges to the poultry industry each year. This study evaluated the effects of novel multi-strain probiotics consisting of Lactobacillus plantarum, L. fermentum, Pediococcus acidilactici, Enterococcus faecium and Saccharomyces cerevisiae on growth performance, intestinal microbiota, haemato-biochemical parameters and anti-inflammatory properties on broilers experimentally challenged with P. multocida. A total of 120 birds were fed with a basal diet supplemented with probiotics (108 CFU/kg) and then orally challenged with 108 CFU/mL of P. multocida. Probiotics supplementation significantly (P < 0.05) improved growth performance and feed efficiency as well as reducing (P < 0.05) the population of intestinal P. multocida, enterobacteria, and mortality. Haemato-biochemical parameters including total cholesterol, white blood cells (WBC), proteins, glucose, packed cell volume (PCV) and lymphocytes improved (P < 0.05) among probiotic fed birds when compared with the controls. Transcriptional profiles of anti-inflammatory genes including hypoxia inducible factor 1 alpha (HIF1A), tumor necrosis factor- (TNF) stimulated gene-6 (TSG-6) and prostaglandin E receptor 2 (PTGER2) in the intestinal mucosa were upregulated (P < 0.05) in probiotics fed birds. The dietary inclusion of the novel multi-strain probiotics improves growth performance, feed efficiency and intestinal health while attenuating inflammatory reaction, clinical signs and mortality associated with P. multocida infection in broilers.
Subject terms: Microbiology, Applied microbiology
Introduction
Pasteurella multocida is a Gram-negative coccobacillus and readily transmitted bacterium which causes fowl or avian cholera, an acute and fatal septicemic infection affecting wide range of both wild and domestic bird species1,2. In poultry, it causes significant economic loss on back-yard and large-scale commercial poultry production globally3,4. Although not common, human cases of P. multocida infections are often associated with immunosuppressed individuals, older adults or rarely due to occupational exposure5,6. The route of infection and pathogenesis of P. multocida in poultry have not been clearly elucidated, however, accumulated evidence suggests the respiratory tract as the major entry point2. Furthermore, P. multocida is known to be normal microflora of the upper respiratory tract (URT) of most healthy animals including poultry, and it can inhabit the oropharynx of healthy hosts for elongated periods without causing disease2. However, virulent strains of P. multocida are able to colonize the mucosa of the upper respiratory tract and, subsequently, infect the air sacs and lungs of birds. Through an unknown mechanism, but possibly related to the migration in macrophages of the upper respiratory tract, the bacteria can access the blood circulation from the mucosa and multiply in different tissues, especially in the liver and spleen2,4.
In the poultry industry, antibiotics have been used over the years in the treatment and control of poultry infections and in some countries also as growth promoters. The control of P. multocida infections in poultry using antibiotics has been somewhat successful, nevertheless, there is often relapse after antibiotic withdrawal. More so, 80.5% of P. multocida infections have shown high degree of resistance to broad range of commonly used antibiotics7,8. Strains of P. multocida from ducks, chickens, geese, turkeys and quails have reportedly shown resistance to doxycycline-HCl, enrofloxacin, chloramphenicol, norfloxacin and lincomycin4,8,9.
With the phasing out of antibiotics in animal production due to immense public health concerns including the presence of drug residue in animal products, emergence and spread of resistance, dysbiosis of gut microflora and hypersensitivity among others, there is need for the application of naturally safe alternatives which will both improve animal growth performance as well as control infectious diseases. Probiotics, which are now widely accepted as alternatives to antibiotics, are viable microorganisms which confer wide range of nutritional and health benefits in animals when administered in sufficient amount. Probiotics ameliorate enteric infections through competitive exclusion of pathogens, and chronic inflammatory and allergic diseases, as well as immunomodulation and immune-stimulation, increased digestibility and nutrients assimilation in their host10–13.
The regular inclusion of probiotics in poultry diets may both minimize the risk of infections with pathogens such as P. multocida, E. coli, Campylobacter spp., Listeria monocytogenes and Salmonella as well as improving growth performance in birds14. This would significantly decrease the risks associated with poultry and poultry product contamination with animal pathogens of public health concern, hence reducing human spread as well as safeguarding the environment.
Recently, several successful reports have emerged on the treatment of poultry infections and multidrug resistant bacterial pathogens using probiotic strains13–18. Up to date, apart from few field studies on the effectiveness of antibiotic alternatives such as bacteriophages, vaccines, β-glucan, and certain proteins against P. multocida infections19–23, no study has evaluated the effectiveness of probiotics in ameliorating infections caused by P. multocida in poultry despite its devastating effects in the poultry industry. Being known as an ‘inscrutable’ pathogen, some strains of P. multocida have the potential of colonizing the nasopharynx, respiratory and the gastrointestinal tracts of animals including poultry thereby inducing severe disease. The use of probiotics in mitigating infection caused by P. multocida, which is rarely regarded as a gut-associated pathogen, would further aid in reducing the intestinal colonization and/or severity infection by other gut-associated and opportunistic pathogens.
In our previous studies, strains of LAB (lactic acid bacteria) were isolated, characterized and evaluated for antagonistic activity against poultry pathogens and in vitro probiotic properties17,18. We hypothesized that dietary multi-strains probiotic supplementation would improve the growth performance, modulate intestinal microflora and haemato-biochemical parameters as well as ameliorate P. multocida infection in broiler chickens. This study aimed to investigate the effect of dietary supplementation with novel multi-strain probiotic (consisting of Lactobacillus plantarum, L. fermentum, Pediococcus acidilactici, Enterococcus faecium and Saccharomyces cerevisiae) on the growth performance, intestinal microflora, haemato-biochemical parameters and anti-inflammatory properties on P. multocida infection in broilers.
Results
Growth performance
From the beginning of this experimental trial, i.e., days 1 to 14, no statistically significant (P > 0.05) differences were recorded in the body weight (BW), body weight gain (BWG), feed intake (FI) and feed conversion ratio (FCR) among all the treatments. The FCR differs significant (P < 0.05) between the probiotic supplemented groups (Pro− and Pro+) when compared with the with the NC− while other groups showed non-significantly higher values (Table 1). During the post—P. multocida challenge period, i.e., days 14 to 28, probiotics supplementation significantly (P < 0.05) affected growth performance and feed utilization (Table 1). There was severe depression in the growth, FI and FCR in the NC+ and PC+ treatments in the first- and second-week post infection compared with other treatments. In general, probiotics supplemented groups, with or without P. multocida challenged showed significantly (P < 0.05) higher BW and BWG with feed efficiency than other treatments at the end of the experiment. Birds in the challenged positive control (PC+) and the challenged negative control (NC+) treatments had the worse BW, BWG and FCR compared to others from day 21 onwards (Table 1).
Table 1.
Effects of probiotics supplementation on growth performance of Pasteurella multocida challenged broiler chickens.
| Growth performance | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| NC− | PC− | Pro− | Pro+ | PC+ | NC+ | SEM | P value | |
| BW 1 (g) | 47.00a | 46.86a | 46.55a | 46.95a | 47.05a | 46.75a | 0.145 | 0.071 |
| BW 7 (g) | 143.85a | 139.05a | 145.7a | 141.1a | 142.7a | 142.65a | 0.931 | 0.087 |
| BWG (g) | 96.85a | 92.45a | 99.15a | 94.15a | 95.25a | 96.1a | 0.941 | 0.999 |
| FI (g) | 231a | 239.2a | 234.45a | 238.15a | 253.7a | 230.1a | 3.520 | 0.105 |
| FCR | 2.385a | 2.587a | 2.365a | 2.529a | 2.664a | 2.394a | 0.051 | 0.083 |
| BW 14 (g) | 324.2b | 329.95b | 356.45ab | 352.1ab | 338.7b | 320.7b | 6.024 | 0.041 |
| BWG (g) | 277.2a | 283.67a | 309.9ab | 305.15ab | 291.25a | 274.15a | 6.005 | 0.050 |
| FI (g) | 435.5ab | 405ac | 410.5ab | 401.4ad | 400.6ae | 397.8aef | 5.694 | 0.012 |
| FCR | 1.571a | 1.428ab | 1.325b | 1.315b | 1.375b | 1.451ab | 0.039 | 0.031 |
| BW 21 (g) | 621.65c | 631.69c | 687.2a | 651.26b | 426.36d | 414.89d | 48.811 | 0.015 |
| BWG (g) | 574.65c | 585.11c | 640.65a | 604.21b | 379.81d | 368.11d | 48.792 | 0.032 |
| FI (g) | 957.00a | 903.58d | 804.07c | 701.11d | 641.73e | 662.44f | 53.643 | 0.029 |
| FCR | 1.665b | 1.544c | 1.255d | 1.160d | 1.690b | 1.799a | 0.105 | 0.044 |
| BW 28 (g) | 989.22c | 942.18d | 1201.72a | 1195.71b | 612.38e | 582.57f | 110.928 | 0.009 |
| BWG (g) | 942.22c | 895.58d | 1155.17a | 1148.76b | 564.93e | 536.02f | 110.969 | 0.010 |
| FI (g) | 1139.55a | 993.55b | 1096.53b | 999.05b | 865.17c | 699.46d | 65.883 | 0.011 |
| FCR | 1.209c | 1.109d | 0.949e | 0.870f | 1.531a | 1.305b | 0.099 | 0.025 |
Values are means of two replicates and standard errors of means.
Within each variable, values with the same superscript letter are not significantly different according to Duncan’s multiple range test (P > 0.05).
NC−: unchallenged negative control; PC−: unchallenged positive control; Pro−: unchallenged probiotics control; Pro+: challenged probiotic control; PC+: challenged positive control; NC+: challenged negative control, BW−: body weight; BWG−: body weight gain; F−: feed intake; FCR: feed conversion ration.
Clinical signs and mortality
In a pilot study to determine the infectivity, broilers were challenged with P. multocida and the clinical signs and death due to P. multocida were recorded (data not shown). Severe pasteurellosis due to the experimental inoculation of birds with P. multocida was initially induced in the NC+ group and later in the PC+ group about 12 and 24 h post-challenged (Fig. 1). Clinical signs manifested in challenged birds included diarrhoea, depression, severe weakness, nasal discharge, isolation, reduction in feed and water consumption, ruffled feathers, immobility and lameness which are the characteristics of P. multocida infection in poultry (Fig. 1). Although the most severe clinical manifestations recorded in this study were observed between 24 to 94 h post-infection, mild to moderate clinical signs persist in the challenged groups (PC+ and NC+) until the end of the experiment while no obvious clinical signs were observed in challenged probiotic (Prob+) group (Fig. 1). At the end of the trial, mortality rates attributed to P. multocida infection were 5.00, 60.00 and 65.00% for Pro+, PC+ and NC+ treatments respectively (Table 2). Mortality was significantly higher (P < 0.05) in birds in the PC+ and NC+ groups when compared with the Pro+ group. Similarly, the highest mortality recorded due to P. multocida occurred during the first 72 h post-infection, with 10 and 8 mortalities in PC+ and NC+ groups while the lone mortality recorded in Prob+ throughout the entire study occurred on 72 h post infection (Table 2).
Figure 1.
Symptoms in Pasteurella multocida challenged groups supplemented with antibiotic (PC+) and control (NC+); (A) Yellowish-grey diarrhoea appeared on the cloaca, (B) Yellowish-grey diarrhoea appeared on the cloaca and littered, (C) Depression and isolation, (D) Moribund, lameness and ruffled feathers, (E) Healthy and active probiotics supplemented broilers challenged with Pasteurella multocida (Pro+), (F) Healthy and active probiotics supplemented broilers challenged with Pasteurella multocida (Pro+).
Table 2.
Mortality rate of broiler chickens supplemented with probiotics and challenged with Pasteurella multocida.
| Days | NC− | PC− | Pro− | Pro+ | PC+ | NC+ |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 2 | 3 |
| 3 | 0 | 0 | 0 | 0 | 4 | 2 |
| 4 | 0 | 0 | 0 | 1 | 4 | 3 |
| 5 | 0 | 0 | 0 | 0 | 0 | 3 |
| 6 | 0 | 0 | 0 | 0 | 1 | 1 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 | 0 | 1 | 0 |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | 0 | 0 | 0 | 0 | 0 | 1 |
| 14 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 0 | 0 | 0 | 1 | 12 | 13 |
| % mortality | 0c | 0c | 0c | 5b | 60a | 65a |
Within each variable, values with the same superscript letter are not significantly different according to Duncan’s multiple range test (P > 0.05). NC−: unchallenged negative control; PC−: unchallenged positive control; Prob−: unchallenged probiotics control; Pro+: challenged probiotic control; PC+: challenged positive control; NC+: challenged negative control.
Carcass and visceral organs weight
On day 21 of the experiment, the relative weight of the Bursa in the probiotics supplemented birds (Pro− and Pro+) were significantly (P < 0.05) higher when compares to birds in other treatments, with the NC+ having the least weight of 0.09 g as recorded. Similarly, the relative weight of ileum in Prob+ group differed significantly (P < 0.05) when compared with other P. multocida challenged groups (PC+ and NC+), and non challenged groups (PC− and NC−) (Table 3). The relative weights of the wing and dressing carcass recorded from birds in Pro− and Pro+ groups were significantly (P < 0.05) higher than NC+. Furthermore, whereas the relative weight of the liver from birds in Pro− group was significantly higher than birds from all the groups except Pro+ (which was numerically higher) on day 28, the relative weight of the spleen in the NC+ and PC− groups were significantly lower when compared with other groups. Also, although the dressing carcass of the PC− group was significantly (P < 0.05) higher than all other groups (except Pro+), the dressing carcass of birds in the Pro+ group similarly had significantly (P < 0.05) higher relative weight when compared with other challenged groups (PC+ and NC+) (Table 3). The relatively weights of carcass and visceral organs from the P. multocida challenged groups generally had the least values especially from birds in the NC+ group.
Table 3.
Relative weights (% BW) of organs from broilers supplemented with probiotics and challenged with Pasteurella multocida.
| Organ | Treatment | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| NC− | PC− | Pro − | Pro+ | PC+ | NC+ | SEM | ||
| Day 21 | ||||||||
| Heart | 0.74a | 0.54a | 0.74a | 0.72a | 0.69a | 0.53a | 0.040 | 0.298 |
| Liver | 2.083a | 2.36a | 2.99a | 3.29a | 2.77a | 2.53a | 0.179 | 0.107 |
| Spleen | 0.11a | 0.17a | 0.25a | 0.19a | 0.13a | 0.09a | 0.013 | 0.929 |
| Bursa | 0.12c | 0.13c | 0.30ab | 0.35a | 0.18bc | 0.09c | 0.043 | 0.021 |
| Gizzard | 3.46a | 3.11a | 3.54a | 3.82a | 3.50a | 3.61a | 0.094 | 0.183 |
| Ileum | 2.09b | 2.24b | 2.57b | 3.53a | 2.75b | 2.73b | 0.207 | 0.021 |
| Caecum | 0.57a | 0.46a | 0.58a | 0.71a | 0.63a | 0.38a | 0.048 | 0.092 |
| Thigh | 4.77a | 4.02a | 4.07a | 4.28a | 4.42a | 4.25a | 0.111 | 0.202 |
| Drumstick | 4.34a | 3.63a | 3.86a | 3.58a | 3.34a | 3.41a | 0.149 | 0.075 |
| Breast | 19.57ab | 20.15ab | 21.98a | 19.60ab | 17.30bc | 13.75c | 1.060 | 0.041 |
| Wing | 2.13ab | 1.81ab | 2.67a | 2.02ab | 1.90ab | 1.56b | 0.096 | 0.012 |
| Dressing | 55.07ab | 54.62ab | 61.47a | 57.53ab | 45.22bc | 41.37c | 1.720 | 0.023 |
| Day 28 | ||||||||
| Heart | 0.55a | 0.43a | 0.61a | 0.63a | 0.63a | 0.63a | 0.032 | 0.081 |
| Liver | 2.61b | 2.54b | 4.45a | 3.59ab | 2.60b | 2.63b | 0.108 | 0.016 |
| Spleen | 0.13ab | 0.02c | 0.17a | 0.18a | 0.15a | 0.07bc | 0.024 | 0.028 |
| Bursa | 0.46ab | 0.45ab | 0.94a | 0.75ab | 0.33b | 0.40ab | 0.036 | 0.011 |
| Gizzard | 2.92a | 2.69a | 2.43a | 2.83a | 3.61a | 3.33a | 0.175 | 0.083 |
| Ileum | 3.10a | 2.29bc | 3.44a | 2.89ab | 2.71ab | 1.88c | 0.195 | 0.001 |
| Caecum | 0.65ab | 0.46b | 0.77ab | 1.07a | 1.02ab | 0.52ab | 0.103 | 0.031 |
| Thigh | 4.92a | 4.56a | 4.90a | 4.28ab | 3.18c | 3.48bc | 0.279 | 0.012 |
| Drumstick | 4.35ab | 4.16ab | 4.93a | 4.24ab | 3.38b | 3.11b | 0.138 | 0.031 |
| Breast | 19.83ab | 18.05ab | 21.25a | 18.59ab | 16.72ab | 15.03b | 0.900 | 0.007 |
| Wing | 2.62ab | 2.56ab | 2.16a | 2.57ab | 2.75a | 2.46ab | 0.082 | 0.039 |
| Dressing | 56.27bc | 56.58bc | 66.30a | 64.64ab | 49.00c | 47.65c | 2.593 | 0.011 |
Values are means of two replicates and standard errors of means.
Within each variable, values with the same superscript letter are not significantly different according to Duncan’s multiple range test (P > 0.05).
NC−: unchallenged negative control; PC−: unchallenged positive control; Pro−: unchallenged probiotics control; Pro+: challenged probiotic control; PC+: challenged positive control; NC+: challenged negative control.
Enumeration of intestinal digesta intestinal bacteria, yeast and P. multocida
There was no significant (P > 0.05) differences in the numbers of total aerobes from the ileal and caecal contents on day 21 of the trial among all the treatments. However, the LAB counts in the gizzard contents of probiotics supplemented birds (groups Pro− and Pro+) was significant (P < 0.05) higher on day 28 when compared with other groups (Table 4). Also, the LAB counts in the ileum and caecum of birds from PC+ and NC+ were lower (P < 0.05) than probiotics supplemented birds (groups Pro− and Pro+). The numbers of enterobacteria recorded from the gizzard, ileum and caecum on day 21 of the trial were significantly (P < 0.05) higher in P. multocida challenged groups, PC+ and NC+ , when compared with other treatments, and this trend persist till the end of the trial. The numbers of yeast cells tended to be higher in the ileum and caecum of birds supplemented probiotics than other groups. However, the least yeast counts were recorded from the gizzards of all the birds across the treatments with birds in PC+ and NC+ groups having a significantly (P < 0.05) lowered counts on day 21.
Table 4.
Microbial counts (Log10 cfu/g) in the digesta of chickens supplemented with probiotics and challenged with Pasteurella multocida.
| Content | Treatment | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| NC− | PC− | Pro− | Pro+ | PC+ | NC+ | SEM | ||
| Day 21 | ||||||||
| Gizzard | ||||||||
| Total aerobes | 8.45ab | 6.87c | 8.53ab | 9.39a | 7.91bc | 8.47ab | 0.345 | 0.008 |
| Enterobacteria | 4.80ab | 4.74ab | 3.54b | 3.54b | 5.19a | 5.30a | 0.329 | 0.017 |
| LAB | 7.55a | 7.37a | 8.11a | 8.48a | 7.39a | 7.91a | 0.181 | 0.205 |
| Yeast | 6.48a | 6.73a | 6.65a | 6.66a | 5.70b | 5.60b | 0.210 | 0.018 |
| P. multocida | 0.00b | 0.00b | 0.00b | 0.50b | 5.54a | 5.20a | 1.109 | 0.001 |
| Ileum | ||||||||
| Total aerobes | 8.78a | 7.77a | 9.06a | 8.95a | 8.35a | 8.46a | 0.194 | 0.402 |
| Enterobacteria | 5.64a | 5.73a | 3.99b | 3.48b | 5.40a | 5.86a | 0.379 | 0.020 |
| LAB | 8.25ab | 7.75b | 9.35a | 9.11ab | 7.87ab | 8.07ab | 0.273 | 0.015 |
| Yeast | 7.66a | 8.22a | 8.85a | 8.41a | 7.87a | 7.90a | 0.177 | 0.082 |
| P. multocida | 0.00c | 0.00c | 0.00c | 2.54b | 5.85a | 5.87a | 1.172 | 0.000 |
| Caecum | ||||||||
| Total aerobes | 9.55a | 9.00a | 9.67a | 9.08a | 8.69a | 9.09a | 0.149 | 0.087 |
| Enterobacteria | 5.84a | 6.29a | 3.06b | 3.33b | 6.22a | 6.51a | 0.438 | 0.017 |
| LAB | 8.54b | 8.99ab | 9.69a | 9.28ab | 8.65b | 8.59b | 0.187 | 0.014 |
| Yeast | 8.54c | 8.99bc | 9.68a | 9.26ab | 7.15d | 7.61d | 0.403 | 0.001 |
| P. multocida | 0.00c | 0.00c | 0.00c | 3.65b | 6.86a | 6.40a | 1.338 | 0.010 |
| Day 28 | ||||||||
| Gizzard | ||||||||
| Total aerobes | 8.34ab | 7.37b | 9.45a | 9.44a | 8.22ab | 9.20a | 0.341 | 0.041 |
| Enterobacteria | 4.80b | 4.89b | 3.03c | 2.98c | 5.45ab | 5.92a | 0.415 | 0.025 |
| LAB | 8.45bc | 7.83c | 8.93b | 9.96a | 7.77c | 8.36bc | 0.331 | 0.012 |
| Yeast | 7.26a | 7.13a | 7.29a | 7.00a | 6.00a | 7.20a | 0.201 | 0.218 |
| P. multocida | 0.00c | 0.00c | 0.00c | 1.50b | 4.65a | 4.74a | 0.941 | 0.013 |
| Ileum | ||||||||
| Total aerobes | 8.26ab | 7.74b | 9.31a | 9.55a | 8.23ab | 8.75ab | 0.283 | 0.041 |
| Enterobacteria | 6.22b | 6.34ab | 2.41c | 3.06c | 6.60ab | 7.04a | 0.448 | 0.000 |
| LAB | 7.75c | 8.25abc | 9.35a | 9.14ab | 8.18bc | 8.20bc | 0.253 | 0.002 |
| Yeast | 8.22b | 7.69b | 9.47a | 8.23b | 7.15b | 7.69b | 0.324 | 0.040 |
| P. multocida | 0.00c | 0.00c | 0.00C | 2.65b | 6.72a | 7.01a | 1.126 | 0.001 |
| Caecum | ||||||||
| Total aerobes | 9.35b | 9.00c | 9.89a | 9.67a | 9.10c | 8.62d | 0.193 | 0.042 |
| Enterobacteria | 6.81ab | 6.17b | 3.13c | 3.52c | 7.13ab | 7.81a | 0.514 | 0.007 |
| LAB | 8.54b | 8.48b | 9.84a | 9.69a | 8.92b | 8.47b | 0.254 | 0.039 |
| Yeast | 7.75b | 8.33b | 9.93a | 8.70ab | 7.61a | 7.75a | 0.359 | 0.043 |
| P. multocida | 0.00c | 0.00c | 0.00c | 3.28b | 6.53a | 6.62a | 1.332 | 0.006 |
Values are means of two replicates and standard errors of means. Within each variable, values with the same superscript letter are not significantly different according to Duncan’s multiple range test (P > 0.05).
NC−: unchallenged negative control; PC−: unchallenged positive control; Pro−: unchallenged probiotics control; Pro+: challenged probiotic control; PC+: challenged positive control; NC+: challenged negative control.
Throughout this experimental trial, birds in the probiotic control, Pro+ treatment had significantly (P < 0.05) lowered counts of P. multocida in their gizzards, ilea and caeca when compared with those of birds in the other challenged treatments, PC+ and NC+ respectively (Table 4). Whereas the gizzards of birds in the probiotic control treatment, Prob+ had the least counts of P. multocida, 0.50 log10CFU/g on day 21, significantly higher counts of P. multocida ranging between 6.62 and 7.01 log10CFU/g were recorded from the ilea and caeca of birds in the PC+ and NC+ treatments on day 28 of the trial. The numbers of P. multocida in the ileal and caecal contents were significantly (P < 0.05) reduced in Pro+ group when compared with other challenged groups, PC+ and NC+ on days 21 and 28 (i.e., 7 and 14 days post-challenged) respectively. Birds from all the treatments were confirmed to be culture-negative for Pasteurella before inoculation with P. multocida on day 14 of the experiment. Also, the non-P. multocida challenged treatments (i.e., NC−, PC− and Pro−) were Pasteurella negative throughout the experimental period.
Intestinal digesta pH
Generally, this study recorded an increasing pH concentration from acidity to neutrality with the gizzard digesta of birds having the lowest pH levels followed by the ileal and then the caecal contents respectively (Table 5). The pH of the ileal contents from birds in PC+ groups were significantly (P < 0.05) lowered when compared with other challenged groups, PC+ and NC+ on days 21 and 28 respectively.
Table 5.
Effects of supplementation with probiotics and challenged with Pasteurella multocida on pH of GIT of broilers.
| pH | NC − | PC − | Pro − | Pro+ | PC+ | NC+ | SEM | P-value |
|---|---|---|---|---|---|---|---|---|
| Day 21 | ||||||||
| Gizzard | 3.53ab | 3.84ab | 3.18b | 3.38b | 3.75ab | 4.1b | 0.137 | 0.057 |
| Ileum | 5.62a | 5.83a | 4.71bc | 3.93c | 5.7a | 5.61ba | 0.298 | 0.021 |
| Caecum | 6.47a | 6.14a | 6.17a | 5.89a | 6.04a | 6.27a | 0.139 | 0.287 |
| Day28 | ||||||||
| Gizzard | 3.63a | 3.80a | 3.01a | 2.95a | 3.76a | 3.65a | 0.155 | 0.093 |
| Ileum | 5.73ab | 5.97a | 5.36ab | 5.09b | 5.71ab | 6.05a | 0.119 | 0.026 |
| Caecum | 7.15a | 6.72a | 6.43a | 6.31a | 6.64a | 6.65a | 0.115 | 0.105 |
Values are means of two replicates and standard errors of means.
Within each variable, values with the same superscript letter are not significantly different according to Duncan’s multiple range test (P > 0.05).
NC−: unchallenged negative control; PC−: unchallenged positive control; Pro−: unchallenged probiotics control; Pro+: challenged probiotic control; PC: challenged positive control; NC+: challenged negative control.
Haemato-biochemical parameters
The total cholesterol concentration in birds from the probiotics supplemented groups, Pro− and Pro+ were significantly (P < 0.05) lower than P. multocida challenged groups, PC+ and NC+ on days 21 and 28 of the experiment. Nevertheless, there was no significant (P > 0.05) difference in the HDL cholesterol and LDL cholesterol concentrations across the treatment all through the experiment. On day 28, while triglyceride levels were reduced (P > 0.05) in probiotics supplemented groups but higher (P < 0.05) in NC−, glucose levels were significantly(P < 0.05) reduced in the same groups. Similarly, protein levels were significantly (P < 0.05) higher in probiotics supplemented groups when compared with other groups on day 28 (Table 6).
Table 6.
Effects of probiotics supplementation on biochemical parameters of Pasteurella multocida challenged broiler chickens.
| Parameter | Treatment | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| NC− | PC− | Pro− | Pro+ | PC+ | NC+ | SEM | ||
| Day 21 | ||||||||
| Total cholesterol (mg/dL) | 92ab | 94.04ab | 74.00b | 81.5b | 111a | 108a | 4.333 | 0.024 |
| HDL cholesterol (mg/dL) | 76a | 74a | 72.5a | 82.5a | 91a | 93a | 4.197 | 0.095 |
| LDL cholesterol (mg/dL) | 26a | 20.5a | 24.5a | 22a | 24a | 25.5a | 0.864 | 0.217 |
| Triglyceride (mg/dL) | 56.5a | 60a | 54.5a | 52a | 70a | 60a | 3.829 | 0.089 |
| Total cholesterol-HDL ratio | 1.18a | 1.25a | 1.23a | 1.23a | 1.22a | 1.16a | 0.014 | 0.101 |
| Total protein (g/dl) | 2.53bc | 2.8abc | 3.26a | 2.95ab | 2.52bc | 2.25c | 0.114 | 0.021 |
| Glucose (mmol/L) | 12.34a | 13.65a | 11.12a | 11.38a | 14.18a | 13.64a | 0.386 | 0.082 |
| Day 28 | ||||||||
| Total cholesterol (mg/dL) | 100.5a | 110.5a | 76.5b | 79b | 105.5a | 107a | 5.581 | 0.031 |
| HDL cholesterol (mg/dL) | 66a | 91a | 68a | 73.5a | 73a | 86a | 4.098 | 0.097 |
| LDL cholesterol (mg/dL) | 28.6a | 30.5a | 20.7a | 27a | 37.5a | 31.5a | 2.260 | 0.310 |
| Triglyceride (mg/dL) | 113.5a | 111.5ab | 71.5b | 91ab | 103ab | 105ab | 9.681 | 0.021 |
| Total cholesterol-HDL ratio | 1.27a | 1.21a | 1.09a | 1.20a | 1.27a | 1.26a | 0.027 | 0.472 |
| Total protein (g/dL) | 2.81bc | 2.89bc | 3.64a | 3.51ab | 2.25c | 2.38c | 0.232 | 0.009 |
| Glucose (mmol/L) | 12.21a | 15.05a | 9.04b | 8.17b | 13.87a | 12.22a | 0.434 | 0.026 |
Values are means of two replicates and standard errors of means.
Within each variable, values with the same superscript letter are not significantly different according to Duncan’s multiple range test (P > 0.05).
NC−: unchallenged negative control; PC−: unchallenged positive control; Pro−: unchallenged probiotics control; Pro+: challenged probiotic control; PC+: challenged positive control; NC+: challenged negative control.
HDL high density lipid, LDL low density lipid, RISK total cholesterol-HDL ratio.
The results of the haematogical parameters analyzed are shown in Table 7. Whereas no difference (P > 0.05) was recorded in total RBC, haemoglobin, ESR, PCV, basophiles, monocytes, total platelet count and MPV across the treatments on day 21, statistical difference were recorded for total WBC and neutrophils between the probiotics supplemented groups when compared with other treatments. Nevertheless, on day 28 of the experiment MCV and RDW were significantly (P > 0.05) higher in probiotics supplemented groups Pro– and Pro+ when compared with non-P. multocida challenged negative control group, NC−. Also, probiotics supplemented birds with or without P. multocida challenge significantly (P < 0.05) increased total WBC counts at the end of the trial. Also, the concentrations of lymphocytes and monocytes were higher in probiotics supplemented groups while PC− and Prob− had higher (P > 0.05) total platelet counts on day 28 of the experiment.
Table 7.
Effects of probiotics supplementation on haematological parameters of Pasteurella multocida challenged broiler chickens.
| Parameter | Treatment | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| NC− | PC− | Pro− | Pro+ | PC+ | NC+ | SEM | ||
| Day 21 | ||||||||
| RBC | ||||||||
| Total RBC (mil/Cmm) | 1.68a | 2.59a | 2.71a | 2.48a | 2.62a | 2.17a | 0.158 | 0.310 |
| Haemoglobin (g/dL) | 6.65a | 7.58a | 7.45a | 7.62a | 7.3a | 6.75a | 0.318 | 0.092 |
| ESR (mm/1 h) | 3a | 2a | 3a | 4a | 2a | 3.5a | 0.327 | 0.420 |
| PCV (%) | 22.15a | 33.4a | 36.7a | 34.3a | 35.4a | 29a | 2.212 | 0.198 |
| MCV (fl) | 130.4bc | 129.2c | 142.1ab | 144.25a | 135.45abc | 134.05abc | 2.677 | 0.032 |
| MCH (pg) | 34.15a | 30.2bc | 32.4ab | 33ab | 28.05c | 31.1abc | 0.891 | 0.024 |
| MCHC (g/dL) | 26.25a | 23.35ab | 22.45b | 22.9a | 20.65b | 23.2ab | 0.741 | 0.040 |
| RDW (%) | 9.35c | 9.35c | 11.85b | 10.15c | 12.95a | 9.75c | 0.609 | 0.022 |
| WBC | ||||||||
| Total WBC (C/mm) | 63845c | 171760abc | 233860ab | 266850a | 123320bc | 110985bc | 19,785.52 | 0.001 |
| Neutrophils (%) | 40.5a | 36.5a | 34ab | 32.00ab | 23.5b | 36a | 5.567 | 0.013 |
| Lymphocytes (%) | 57b | 59.5b | 80ab | 91.8a | 73ab | 58.4b | 6.167 | 0.036 |
| Monocytes (%) | 1a | 0.5a | 2a | 2.5a | 1a | 1a | 0.307 | 0.415 |
| Basophiles (%) | 0.5a | 0.5a | 0.67a | 1.27a | 0.75a | 0.78a | 0.196 | 0.172 |
| Platelets | ||||||||
| Total platelet count (C/mm) | 3500a | 2500a | 2350a | 2200a | 2000a | 2500a | 202.76 | 0.113 |
| MPV (fl) | 8.9a | 7.9a | 10.8a | 11.45a | 11.65a | 10.55a | 0.609 | 0.081 |
| Day 28 | ||||||||
| RBC | ||||||||
| Total RBC (mil/Cmm) | 2.08a | 3.07a | 3.08a | 2.78a | 2.615a | 2.66a | 0.150 | 0.107 |
| Haemoglobin (g/dL) | 6.51ab | 6.65ab | 8.78a | 7.77ab | 6.58ab | 6.22b | 0.230 | 0.035 |
| ESR (mm/1 h) | 3a | 2a | 3a | 4a | 2a | 3.5a | 0.327 | 0.195 |
| PCV (%) | 28.15c | 30.4bc | 36.7c | 34.05ab | 31.25abc | 25.63c | 1.383 | 0.004 |
| MCV (fl) | 129.8c | 135.15bc | 148.20a | 141.80ab | 135.59bc | 126.9c | 3.312 | 0.026 |
| MCH (pg) | 32.75a | 29.3a | 31.26a | 33.2a | 27.04a | 30.16a | 0.938 | 0.387 |
| MCHC (g/dL) | 23.45a | 23.19a | 22.75a | 24.1a | 24a | 23.26a | 0.210 | 0.410 |
| RDW (%) | 8.97b | 10.61ab | 10.98ab | 11.38a | 11.70a | 9.54ab | 0.437 | 0.031 |
| WBC | ||||||||
| Total WBC (C/mm) | 172070c | 202866c | 302440ab | 346652a | 235780bc | 223933bc | 26,314.05 | 0.001 |
| Neutrophils (%) | 39a | 39.5a | 35a | 37a | 31.5a | 38.5a | 1.243 | 0.201 |
| Lymphocytes (%) | 52.5b | 57.7b | 88.6a | 93.5a | 67ab | 55.75b | 7.566 | 0.021 |
| Monocytes (%) | 0.75b | 1b | 2.5a | 3.5a | 1.25b | 0.75b | 0.338 | 0.023 |
| Basophiles (%) | 1a | 0.5a | 1.25a | 1.02a | 1a | 0.75a | 0.106 | 0.719 |
| Platelets | ||||||||
| Total platelet count (C/mm) | 4000ab | 5500ab | 7500a | 5250ab | 3750b | 2750b | 681.35 | 0.019 |
| MPV (fl) | 11.25a | 9.1a | 9.95a | 10.85a | 11.15a | 11.95a | 0.417 | 0.231 |
Values are means of two replicates and standard errors of means.
Within each variable, values with the same superscript letter are not significantly different according to Duncan’s multiple range test (P > 0.05).
NC−: unchallenged negative control; PC−: unchallenged positive control; Pro−: unchallenged probiotics control; Pro+: challenged probiotic control; PC+: challenged positive control; NC+: challenged negative control.
MPV mean platelets volume, MVC mean corpuscular volume, MCH mean corpuscular haemoglobin, MCHC mean corpuscular haemoglobin concentration, RDW RBC distribution width, ESR erythrocyte sedimentation rate.
Anti-inflammatory gene expression
On 14-day post P. multocida challenged, dietary supplementation of birds with probiotics significantly (P < 0.05) upregulated the mRNA profiles of anti-inflammatory genes including HIF1A (hypoxia inducible factor 1 alpha) and TSG-6 (Tumor necrosis factor- (TNF) stimulated gene-6) on the caecal mucosa when compared to the birds in the control group (Table 8). However, when both anti-inflammatory genes are compared, probiotic effect in the upregulating the expression of HIF1A was higher than for PTGER2. There was no difference in the expression of both anti-inflammatory genes in birds supplemented with antibiotic and the negative control except for TSG-6 (Table 8).
Table 8.
Effect of probiotics supplementation on anti-inflammatory gene expression in caecal mucosa of Pasteurella multocida challenged broilers.
| Anti-inflammatory gene | Pasteurella multocida infection | P value | |||
|---|---|---|---|---|---|
| Treatment | |||||
| Prob+ | PC+ | NC+ | SEM | ||
| 14 days post challenged (2−ΔΔCt) | |||||
| PTGER2 | 1.13a | 0.75a | 1.02a | 0.113 | 0.884 |
| HIF1A | 134.58a | 36.82b | 13.23b | 37.148 | 0.011 |
| TSG-6 | 13.71a | 9.86b | 1.05c | 3.747 | 0.003 |
Values are means of two replicates and standard errors of means.
Within each variable, values with the same superscript letter are not significantly different according to Duncan’s multiple range test (P > 0.05). Pro+: challenged probiotic control; PC+: challenged positive control; NC+: challenged negative control.
HIF1A hypoxia inducible factor 1 alpha, PTGER2 prostaglandin E receptor 2, TSG-6 tumor necrosis factor- (TNF) stimulated gene-6, Pro+ challenged probiotic, PC+ challenged antibiotic; NC+ challenged control.
Discussion
Avian cholera caused by P. multocida is a highly contagious poultry disease of global concern, causing significant ecological and economic challenges to the poultry industry each year3,24,25. The ability of P. multocida to survive asymptomatically in carrier birds for a longer period of time even after the disappearance of clinical signs have often led to frequent recurrence of P. multocida outbreaks with high mortality4,25. Also, P. multocida has been reported to persist for several months in the environment, water supplies, and insects24,26,27. In this study, we proposed that the supplementation of poultry with multistrain probiotics containing L. plantarum, L. fermentum, P. acidilactici, E. faecium and S. cerevisiae can control P. multocida infection in broiler chickens through mitigating the manifestation of clinical signs and the reduction of mortality associated with P. multocida while improving the overall performance.
Although some studies have previously tried the effectiveness of vaccines as antibiotic alternatives in the control of P. multocida in poultry, studies evaluating the possible role of probiotics in the control of P. multocida colonization and infections in poultry production are lacking. With the successful reports of probiotics effectiveness in the control and mitigation of the colonization and infection by poultry pathogens including Salmonella14,28,29, Campylobacter15,30,31, E. coli32,33, Eimeria spp.34,35, L. monocytogenes36–38 and Clostridium perfringens39,40, the trial of probiotics in the control of P. multocida would further unravel probiotics effectiveness against this devastating poultry pathogen.
Generally, the dietary supplementation of probiotics has been reported to positively influence animal health and productivity. The results obtained from this study shows that dietary inclusion of probiotics significantly improved the performance and feed efficiency in broiler chickens with beneficial impact on the intestinal microbiota composition and health, hence decreasing the severity of the FC in birds. The significant improvement in BW, BWG and FCR recorded among broilers supplemented with probiotics in comparison with the controls from this study confirmed the positive impact of probiotics supplementation on the performance of broilers. This finding is significant not only to confirm the improvement of intestinal health after probiotic supplementation, but also to mitigate the economic losses due to P. multocida infections in poultry production. Our results agreed with the findings of Smialek et al., Massacci et al., Mountzouris et al., Olnood et al. who reported significantly improved growth performance and feed efficiency of probiotics supplemented broilers challenged with either Campylobacter and Salmonella respectively14,15,28,30. Furthermore, apart from the adoption of biosecurity measures and vaccination (which are most potent preventive measures) in endemic regions of the world, the routine use of probiotics in the control and prevention of enteric infections in poultry production could further help in reducing the severity of cases of fowl cholera hence, diminishing the spread of the pathogen.
Changes in the relative weight of visceral organs and carcass in broilers is one principal effect mostly attributed to probiotic supplementation in poultry. The symptoms of pasteurellosis in the P. multocida challenged positive control (PC+) and negative control (NC+) were accompanied by decrease in the live BW of birds, visceral organs and dressing carcass in these treatments with higher increase in gizzard weights on day 28 of the experiment. These trends were similarly reported previously by Olnood et al., and Park and Kim, after experimentally infecting broiler chickens with Salmonella spp.41,42. The improved growth performance of birds due to probiotic supplementation as observed in the current study was also reported by other researchers after challenging broilers with different pathogens. The dietary supplementation of broilers with B. subtilis as probiotic significantly increased the relative weight of spleen by 3.8% without significantly affecting the relative weights of liver and bursa of Fabricius43. In agreement with our study, Pedroso et al. reported that the dietary inclusion of Lactobacillus reuteri and L. johnsonii as probiotics significantly increased intestinal weight in 21-day old broilers44. Probiotics effect on the weight of visceral organs and intestines of animals is inexplicit, and can also be determined by the nature and amount of microbial strains used as probiotics. It has been reported that probiotics consistently influence the intestinal morphology and micro-structure which often increases the absorptive function of the ileum14,45. Also, Pelicano et al. reported significant improvement in the leg yield and breast of birds fed with probiotics46.
A significantly higher mortality was recorded in P. multocida challenged birds supplemented with antibiotic and challenged negative control when compared with the P. multocida challenged birds supplemented with probiotic. The change in behavior and the manifestation of clinical signs in the control groups were consistent with the reported signs characterizing fowl cholera in poultry such as diarrhoea, depression, listlessness, severe weakness, nasal discharge, recumbency and moribund status, isolation, anorexia combined with reduction in feed and water consumption, ruffled feathers, immobility and lameness4,22,23. Within 24 h post-P. multocida infection, birds in the control groups showed mild to moderate signs with mortality which increased in severity till about 92 h post infection. A similar trend was reported in experimental birds challenged with P. multocida25,47,48. Depending on the strain, the incubation period of P. multocida usually varied between 12 and 48 h with 100% mortality majorly between 24 and 72 h post infection49. There is generally limited information about the clinical pathology of pasteurellosis in poultry. Also, Wilkie et al. reported that broiler chickens experimentally challenged with P. multocida died within 22–72 h post infection2. Furthermore, the rapid death of the host animal including broilers due to acute form of fowl cholera is a characteristic of septicaemia induced by P. multocida47. The persistence of P. multocida strains at the site of infection as well as their migration to other host tissues and organs, and the eventual time of the host death depend primarily on the host immune response and the characteristics of P. multocida strain causing the infection which may also influence the shedding and isolation of the pathogen2,47,50. The inhibitory effect of each of the probiotic strain i.e. L. plantarum, L. fermentum, P. acidilactici, E. faecium and S. cerevisiae (used in this study) against P. multocida and other poultry pathogens have been evaluated previously and their probiotic potentials elucidated17,18.
Information regarding in vivo antimicrobial activity of probiotics strains including LAB and Saccharomyces against P. multocida and P. multocida infections are lacking. However, the inhibitory activity of probiotics consisting of strains of LAB and S. cerevisiae in broilers as recorded in this study indicates that these probiotic strains can be successfully used as alternatives for growth-promoting antibiotics in poultry production. The numbers of enterobacteria in the P. multocida challenged control groups were higher in the ileum and ceacum than the unchallenged groups both on days 21 and 28 of the experiment. Probiotic supplemented groups showed higher number of LAB and yeast in the gizzard, ileum and caecum which significantly decreased the numbers of P. multocida on both sampling days during the experiment. The reduction of enterobacteria by beneficial gut microflora may be attributed to the bacteriostatic effect of volatile fatty acids (VFA) secreted in the GIT of birds14. In vitro evaluation has demonstrated that VFA inhibited enterobacterial growth at the pH of 651. Therefore, probiotics supplementation may have increased the concentration of VFA in the gut of the birds examined. This finding is in agreement with the results of Lan et al.52 who reported significant decrease in the number of enterobacteria after broiler chickens were supplemented with multi-strain probiotics containing a mixture of L. agilis, L. acidophilus/gallinarum and L. salivarius. The inclusion of strains of Bacillus, Clostridium and Lactobacillus as multi-strain probiotics at the level of 106 to 109 CFU/kg of diet reportedly suppressed the growth of enterobacteria53–55. Also, probiotics’ antimicrobial effects come from their secretion of antimicrobial compounds including bacteriocins, organic acids (acetic, lactic, propionic, succinic acid, etc.), short-chain fatty acids, hydrogen peroxide and other low molecular weight substances56. The combination of the strains of LAB and yeast used as multi-strain probiotic in this study possibly synergized to form a robust antimicrobial activity against P. multocida and enterobacteria in the gut of the birds supplemented with the study probiotics.
Furthermore, these probiotic strains are able to competitively exclude pathogens, hence preventing their attachment to intestinal walls thereby improving intestinal microbial balance. In agreement with Olnood et al. there was a gradual increase in pH concentration from the proximal to the distal GIT regions with probiotic supplemented birds having a more lowered pH level especially in the gizzards14,41. The reduced pH among probiotic supplemented broiler chickens also contributed in the reduction of the numbers of P. multocida and enterobacteria.
Dietary conditions and pathological stress commonly determine the haematological changes and health status of birds57. The reduction in major haematological parameters including Hb, PCV, ESR and total RBC in P. multocida challenged control groups clearly depicts the onset of anaemia. The occurrence of anaemia in avian cholera infection in poultry has been properly reported48. The cause of anaemia in P. multocida challenged birds as recorded in this study may be attributed to bacterial septicaemia. The concentration of total WBC and lymphocytes were also higher in probiotics supplemented birds. Probiotics are known to modulate host immune system response primarily through balance between anti-inflammatory and proinflammatory cytokines36. Similarly, after dietary supplementation of B. subtilis-based probiotics, Park and Kim and Lee et al. showed the reduction of coccidiosis clinical signs and improved immune response in broiler chickens challenged with Eimeria maxima42,58. The improvement of gut health through the modulation of gut microflora and the modulation of intestinal inflammatory and immune response may significantly inhibit the P. multocida colonization and proliferation within the gut hence influencing haptoglobin concentration. The dietary inclusion of probiotics positively influenced haematopoiesis which among others increase the WBC counts, hence enhancing immune cells synthesis which further protects the host against invading pathogens59,60. The presence of congested blood vessels and haemorrhages observed in the lungs, livers, hearts and intestines of P. multocida infected birds as a result of fowl cholera is similar to the findings of Shivachandra et al. and Sonone et al.9,48. The supplementation of probiotics as revealed in this study significantly reduced the severity of P. multocida infection throughout the experiment, hence, reflecting in the improved haematological parameters as clearly shown.
The reduction in the concentration of total cholesterol, triglycerides, glucose and LDL cholesterol which are major biochemical parameters as reported in our study due to probiotic supplementation agrees with the report of Arun et al. and Al-Kassie et al. who separately reported a significant reduction in total cholesterol, triglycerides and glucose by dietary inclusion of 100 mg/kg diet of L. sporogene probiotic and the combination of probiotic (Aspergillus niger) and prebiotic (Taraxacum officinale) in broilers61,62. Total cholesterol reduction in probiotic supplemented birds could be as a result of direct assimilation of cholesterol by bacterial cells (which causes reduction in the cholesterol absorption and synthesis in the GIT), 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibition and bile salt hydrolysis63,64. Furthermore, triglyceride reduction in probiotic treated birds may be as a result of increased hydrolysis of bile salt which causes inadequate lipid absorption in the small intestine10. Strains of Lactobacillus are known to show high hydrolytic activity on bile salt which consequently leads to bile salts deconjugation within the GIT65. Also, the concentration of total protein was significantly higher in probiotics fed birds. This corroborated with the findings of Dimcho et al. and Alkhalf et al. who reported probiotic effects on total protein concentration in chickens10,66.
Maintaining intestinal health and the integrity of intestinal barrier function is essential for the growth and wellbeing of animals. Several pathogenic factors such as stress and pathogenic bacteria challenges can cause inflammation and damage to the intestinal barrier67. On day 14 post P. multocida challenge, greater effects were found for probiotic supplementation on HIF1A, and TSG-6 (P < 0.05). The data obtained from this study implies that probiotics supplementation can attenuate inflammatory reactions through the upregulation of the secretion of anti-inflammatory factors.
In the gut of animals, pathogens including P. multocida readily cause different degree of inflammatory damage of intestinal epithelial cells by establishing hypoxic microenvironments68. One of the major transcriptional factors that dampen hypoxia-induced inflammation in the gut is HIF1A. HIF1A enhances the synthesis and signaling effects of anti-inflammatory signaling molecules69. TSG-6 is multifunctional protein that has been implicated as having important anti-inflammatory and tissue protective properties70. From this study, HIF1A and TSG-6 in the control treatment with P. multocida infection expressed the lowest mRNA profiles, whereas they were upregulated with probiotics supplementation, depicting that these 2 genes are collaboratively associated with either P. multocida or probiotics. This is the first work that assessed the expression of these 2 genes on probiotics supplemented chickens infected with P. multocida. Unfortunately, no information exists about the expression of these genes in the presence of probiotics and P. multocida. However, in a recent work Deng et al. reported the upregulation of HIF1A in probiotic supplemented chickens infected with Listeria monocytogenes36. Therefore, further study on the mechanisms responsible for the dampening of inflammation and the upregulation of anti-inflammatory factors, HIF1A and TSG-6 in probiotics supplemented birds is needed. The higher population of Enterobacteria and P. multocida in the control group could be associated with the invasion of these bacteria and the pathogenesis of P. multocida resulting in the clinical manifestation of P. multocida infection and high mortality. A pool of P. multocida strains could be evaluated to determine their persistence, spread and multiplication in host tissues and shedding in future research.
Materials and methods
Ethical approval of the study
The field trial was approved by the Animal Care and Use Committee of the Faculty of Biological Science and Technology, Jashore University of Science and Technology, Jashore, Bangladesh (certification number: ERC/FBST/JUST/2019-32). The health status of birds in the field were routinely monitored by a veterinarian. The birds were kept under controlled environmental conditions in the animal house of Jashore University of Science and Technology, Jashore, Bangladesh, throughout the experimental period. Authors confirmed that all experiments were performed in accordance with relevant guidelines and regulations and in compliance with the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines.
Strains used for the study and diets
Five potential probiotic strains previously isolated from broiler chicken and raw milk, and identified using 16S rRNA sequencing as Lactobacillus plantarum, L. fermentum, Pediococcus acidilactici, Enterococcus faecium and Saccharomyces cerevisiae with suitable probiotic properties including antagonistic activity against broad range of poultry pathogens including P. multocida, survivability in simulated gastric juice, bile salts and phenol tolerance, adhesion to ileum epithelial cells, aggregation and hydrophobicity abilities, α-glucosidase inhibitory activity, and competitive exclusion of pathogens were selected for this field trial17,18. Basal diets (starter and grower/finisher) formulated in our laboratory were provided as pellets all through the trial and were based on wheat, soybean meal and corn as shown in Table 9. The five probiotic strains were mixed at equal ratio and added into respective experimental treatments at dose of 108 CFU/kg of diet.
Table 9.
Ingredient composition and calculated nutrient of basal diet used for the study.
| Item | Starter | Finisher |
|---|---|---|
| Ingredient, g/kg | ||
| Wheat (26.00%) | 262.00 | 214.00 |
| Sorghum (15.5%) | 350.25 | 400.00 |
| Mung beans | 100.00 | 100.00 |
| Soybean meal (46%) | 157.00 | 81.50 |
| Limestone | 15.50 | 16.00 |
| Salt | 1.75 | 1.50 |
| Soybean oil | 2.00 | 2.50 |
| Moisture | 8.00 | 6.70 |
| Ash | 7.60 | 6.00 |
| Dicalcium phosphate | 1.50 | 1.50 |
| Lysine | 2.10 | 0.40 |
| Methionine | 2.10 | 1.30 |
| Threonine | 0.20 | 0.15 |
| Vitamin and mineral premix* | 2.50 | 2.50 |
| Crude protein | 200.00 | 190.00 |
| Crude fibre | 35.17 | 43.14 |
| Crude fat | 52.16 | 54.47 |
*Nutrition value per Kg of vitamin and mineral premix contains 140,000 IU of vitamin A, 70 mg of vitamin E, 3000 IU of vitamin D3, 4 mg of vitamin K, 3 mg of thiamine, 10 mg of vitaminB2, 8 mg of vitamin B6, 0.04 mg of vitamin B12, 48 mg of niacin, 20 mg of calcium d-pantothenate, 500 mg of choline chloride, 0.20 mg of biotin, 1.8 mg of folic acid, 80 mg of manganese, 70 mg of zinc, 50 mg of iron, 10 mg of copper, 3 mg of iodine, 0.4 mg of selenium, and 0.2 mg of cobalt.
Experimental design and treatments
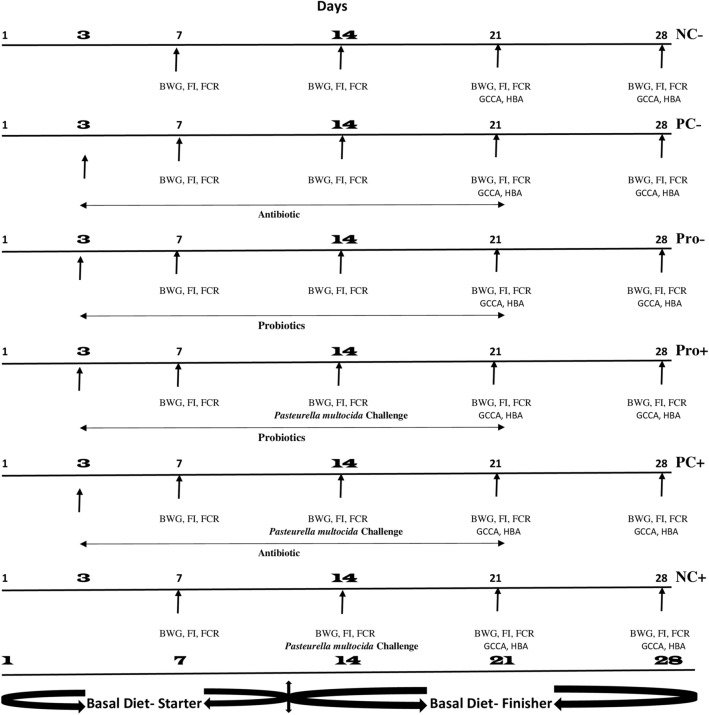
A total of 120 one-day-old Cobb 500 broiler mixed-sex chicks were purchased from a commercial hatchery (NOURISH FARMS, DHAKA, BANGLADESH), weighed individually and randomly assigned to 6 experimental treatments with 2 replicate groups containing 10 chicks each after they were allowed to acclimatize for 2 days. Birds in each treatment were housed in a floor pen containing sawdust litter. Twenty-three hours of light was provided during the first week and then reduced to 18 h throughout the 28 days of the experiment. The 6 experimental treatments adopted in this trial included: (1) negative control (NC−), non-probiotic and unchallenged with P. multocida; (2) positive control (PC−), supplemented with doxycycline HCL (0.5 g/mL), non-probiotic and unchallenged with P. multocida; (3) probiotic control (Pro−), probiotics supplemented and unchallenged with P. multocida; (4) probiotic challenged (Pro+), as probiotics supplemented and challenged with P. multocida; (5) positive challenged (PC+), as doxycycline HCl, supplemented and P. multocida challenged; and (6) negative challenge (NC+), as non-antibiotic, non-probiotic and challenged with P. multocida. In all the treatments, feed and water were provided ad libitum according to the experimental design. Birds in the antibiotic treatments were administered 1 g/L of the doxycycline HCL following the manufacturer’s instructions. Both probiotics and antibiotic were administered between days 3–21 of the experiment (Fig. 2).
Figure 2.
Schematic illustration of the experimental design. Birds were fed basal diet all through the experiment. Treatment groups included: NC−: unchallenged negative control; PC−: unchallenged positive control; Pro−: unchallenged probiotics control; Pro+: challenged probiotic control; PC+: challenged positive control; NC+: challenged negative control. BWG body weight gain, FCR feed conversion ratio, FI feed intake, GCCA gut content and carcass analysis, HBA haemato-biochemical analysis.
Pathogen challenge
For the pathogen challenge experiment, strain P-931of P. multocida subsp. Multocida ATCC 12945 (capsular Type A) isolated from fowl and known to cause fowl cholera was obtained and used for the experimental infection of birds. Stock culture of P. multocida stored at − 80 °C was revived and grown overnight at 37 °C in brain heart infusion broth (LIOFICHEM, ABRUZZI, ITALY) with shaking at 150 rpm. Cells were harvested by washing (8000×g, 10 min) three times with sterile PBS and finally reconstituted in PBS to 108 CFU/mL4. At day 14 of the trial, each bird in experimental challenged treatments was orally inoculated with 1 mL of 108 CFU of P. multocida as previously described2. Birds were examined at 12 h intervals for typical clinical manifestations of fowl cholera until the end of the experiment. Clinical signs and mortality were recorded accordingly. Major clinical signs and symptoms characterizing fowl cholera that were examined included nasal discharge, diarrhoea, lameness, weakness, moribund state, reduced activity, reduced water and feed intake, ruffled feathers and rapid breathing4,49.
Sample collection and processing
The individual weight of all the chickens were measured before grouping them into respective treatment pens. Individual bird and leftover feed from each treatment were weighed weekly and the feed intake (FI) and body weight gain (BWG) recorded. Also, feed conversion ratio (FCR; feed intake/weight gain) and mortality (when it occurred) for each treatment were also calculated41,71.
On days 21 and 28 of the experiment, four birds from each pen were selected at random and sacrificed by cervical dislocation after exposing them to overdose of isoflurane anesthesia. All efforts were made to minimize suffering. Visceral organs of each of the sacrificed bird were carefully removed and weighed after opening the abdominal cavity. After emptying the contents into sterile plastic containers, the weight of gizzard, ileum and caecum were recorded. Also, the weight of heart, liver, bursa, spleen, thigh, drumstick, breast, wing and dressing were recorded and expressed as the percentage of the body weight43.
Enumeration of intestinal bacteria, yeast and detection of P. multocida
For each bird sacrificed, fresh gizzard, ileum and caecum digesta were immediately collected within 1 h for microbial enumeration. Using 0.85% normal saline solution, approximately 1 g of the fresh digesta samples were serially diluted for the enumeration of total aerobes, enterobacteria (coliforms and lactose negative enterobacteria), lactic acid bacteria, total yeast and P. multocida by conventional microbiological techniques using selective media including nutrient agar (LIOFILCHEM, ITALY), MacConkey agar (LIOFILCHEM, ITALY), De Man, Rogosa and Sharpe (MRS) agar (LIOFILCHEM, ITALY), Sabouraud dextrose agar (LIOFILCHEM, ITALY) and 5% defibrinated sheep blood agar supplemented with amikacin (2 mg/L), vancomycin (4 mg/L), and amphotericin B (4 mg/L) respectively72,73. Microbiota enumerations were conducted in duplicate and the average determined. Results of the bacterial counts obtained were expressed as log10CFU/g (base-10 logarithm colony-forming units per gram) of gizzard, ileal and caecal digesta.
Determination of digesta pH
Exactly 1 g of fresh digesta samples from gizzard, ileum and caecum of each sacrificed bird on days 21 and 28 were transferred into 9 mL of distilled water in 15 mL tubes and vigorously mixed. After thorough stomaching, the suspension was mixed with a stirrer after which the pH was measured using glass electrode (HANNA INSTRUMENTS, INC., WOONSOCKET, RI, USA)74.
Haemato-biochemical parameters
Complete blood counts and lipid profile determining the haemato-biochemical parameters were carried out. Approximately 4 mL of blood samples from each bird sacrificed were collected from the jugular vein into plane tubes (for biochemical analyses) and anticoagulant tubes (for haematological analysis) on days 21 and 28 of the trial. Haematological assays were conducted using automatic SYSAM-XN-1000, XN-550 AL Random Access Haematology Machine (SYSMEX CORPORATION, JAPAN) and checked manually while the biochemical analyses were carried out by Siemens Dimension RxL/Max/Vitros350 Random Access Chemistry Analyzer (SIEMENS HEALTHCARE DIAGNOSTICS INC, USA) after obtaining the serum through centrifugation. The average of results obtained from the haemato-biochemical analyses per treatment were determined.
Total RNA extraction and mRNA quantification
Approximately 1 g of caecal mucosa was aseptically collected from each of the sacrificed bird for gene quantification. Total RNA was isolated from the caecal tissues samples using the DIRECT-ZOL RNA extraction Kits (ZYMO RESEARCH, IRVINE, USA), following the manufacturer’s protocol, and finally using a Nanodrop 2000 spectrophotometer (THERMO SCIENTIFIC, WILMINGTON, DE), the concentration and purity of RNA were quantified. The purified RNA was converted into complementary DNA (cDNA) using ProtoScript II First Strand cDNA Synthesis Kit (NEW ENGLAND BIOLABS, INC., MASSACHUSETTS, USA) following procedure described by the manufacturer. Real-time qPCR was performed with the BIO-RAD CFX96 Real-time PCR system (BIO-RAD LABORATORIES, CA, USA). The target genes and primers sequences36 are shown in Table 10. The PCR reaction was conducted using POWERUP SYBR Green Master Mix (THERMO FISHER SCIENTIFIC INC., MASSACHUSETTS, USA) consisting a total of 20 μL PCR reaction-mix containing 10 μL 2× SYBR Green PCR Master Mix, 2 μL primers (0.5 μM of 1 μL forward and 1 μL reverse primer) 6 μL template cDNA (~ 10 ng/μL) and 2 μL nuclease-free water. The qPCR cycling condition consisting of initial heat activation at 95 °C for 10 min, following by 40 cycles of denaturation at 95 °C for 15 s, annealing at 58 °C for 30 s and finally 30 s of extension at 72 °C. The optimum annealing temperature of target and reference genes were determined by the gradient protocol of BIO-RAD CFX96 Real-time PCR System (BIO-RAD LABORATORIES, CA, USA). The procedure by Livak and Schmittgen75 was used to determine the transcriptional profiles of specific gene of interest and were expressed as the relative expression to the housekeeping gene used (2−ΔΔCt).
Table 10.
Primer sequences used for RT-qPCR.
| Gene | GenBank | Sequence 5′ → 3′ | Length (bp) |
|---|---|---|---|
| HIF1A | NM_204297.1 | F-CCAGCAGTTCCTCATGCAAT | 215 |
| R-AAATGCTGCTAGCCCTTCCC | |||
| PTGER2 | NM_001083365.1 | F-TTGCACGTCACCTTCTCGTT | 235 |
| R-TGATGGTCATGATGGCGAGG | |||
| TSG-6 | DQ275160.1 | F-ATGGACAGCGGATTCACCTC | 219 |
| R-TCTGAAACCCACCAGCAGTC | |||
| ACTB | NM_205518.1 | F-TTACTCGCCTCTGTGAAGGC | 228 |
| R-TCCTAGACTGTGGGGGACTG |
ACTB beta-actin, HIF1A hypoxia inducible factor 1 alpha, PTGER2 prostaglandin E receptor 2, TSG-6 tumor necrosis factor- (TNF) stimulated gene-6, F forward, R reverse.
Statistical analysis
Data were collected and analyzed by analysis of variance as a completely randomized design using the GLM procedure as described by GRAPHPAD PRISM version 5.0 for Windows (GRAPHPAD SOFTWARE, SAN DIEGO, CA, USA) and SAS software (version 9.4, SAS Institute Inc., Cary, NC). Viable counts of the gizzard, ileum and caecum contents were subjected to logarithmic conversion (Log10) before statistical analysis. All the results were presented as means of two independent experiments, and differences between treatment groups were determined using the Duncan’s multiple range test. Probability values less than 0.05 (P < 0.05) was considered as significant.
Acknowledgements
This research was supported by the Bangladesh Academy of Science under BAS-USDA program with Grant code number LSc-33.
Author contributions
R.C.R. performed all experiments and drafted the manuscript. S.L.S. designed the study and edited the manuscript. H.I. and A.A.S. performed the molecular analysis and results interpretation. P.C.R. was collected and interpreted data, and revised manuscript. I.K.J. designed the study, coordinated the research and revised the manuscript. All the participating authors read and approved the submitted manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilson BA, Ho M. Pasteurella multocida: From zoonosis to cellular microbiology. Clin. Microbiol. Rev. 2013;26:631–655. doi: 10.1128/CMR.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkie IW, Harper M, Boyce JD, Adler B. Pasteurella multocida diseases and pathogenesis. Curr. Top. Microbiol. Immunol. 2012;361:1–22. doi: 10.1007/82_2012_216. [DOI] [PubMed] [Google Scholar]
- 3.Christensen JP, Bojesen AM, Bisgaard M. Chapter 10—Fowl cholera. Poult. Dis. 2008 doi: 10.1016/B978-0-7020-2862-5.50015-5. [DOI] [Google Scholar]
- 4.Petruzzi B, et al. Biofilm formation and avian immune response following experimental acute and chronic avian cholera due to Pasteurella multocida. Vet. Microbiol. 2018;222:114–123. doi: 10.1016/j.vetmic.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Baillot R, Voisine P, Côté L, Longtin Y. Deep sternal wound infection due to Pasteurella multocida: the first case report and review of literature. Infection. 2011;39:575–578. doi: 10.1007/s15010-011-0141-5. [DOI] [PubMed] [Google Scholar]
- 6.Abreu F, et al. Human Pasteurella multocida infection with likely zoonotic transmission from a pet dog, Spain. Emerg. Infect. Dis. 2018;24:1145–1146. doi: 10.3201/eid2406.171998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kehrenberg C, Schulze-Tanzil G, Martel JL, Chaslus-Dancla E, Schwarz S. Antimicrobial resistance in Pasteurella and Mannheimia: epidemiology and genetic basis. Vet. Res. 2001;32:323–339. doi: 10.1051/vetres:2001128. [DOI] [PubMed] [Google Scholar]
- 8.Khamesipour F, Momtaz H, Azhdary Mamoreh M. Occurrence of virulence factors and antimicrobial resistance in Pasteurella multocida strains isolated from slaughter cattle in Iran. Front. Microbiol. 2014;5:536. doi: 10.3389/fmicb.2014.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shivachandra SB, et al. Detection of multiple strains of Pasteurella multocida in fowl cholera outbreaks by polymerase chain reaction-based typing. Avian Pathol. 2005;34:456–462. doi: 10.1080/03079450500367963. [DOI] [PubMed] [Google Scholar]
- 10.Alkhalf A, Alhaj M, Al-homidan I. Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi J. Biol. Sci. 2010;17:219–225. doi: 10.1016/j.sjbs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhama K, et al. Applications of probiotics in poultry: Enhancing immunity and beneficial effects on production performances and health—A review. J. Immunol. Immunopathol. 2011;13:1–19. [Google Scholar]
- 12.Fellah JS, Jaffredo T, Nagy N, Dunon D. Development of avian immune system. In: Schat KA, Kaspers B, Kaiser P, editors. Avian Immunology. 2. Berlin: Academic Press; 2014. pp. 45–65. [Google Scholar]
- 13.Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: A summary of the evidence. Am. Fam. Phys. 2017;96:170–178. [PubMed] [Google Scholar]
- 14.Olnood CG, Beski SSM, Choct M, Iji PA. Use of Lactobacillus johnsonii in broilers challenged with Salmonellasofia. Anim. Nutr. 2015;1:203–212. doi: 10.1016/j.aninu.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smialek M, Burchardt S, Koncicki A. The influence of probiotic supplementation in broiler chickens on population and carcass contamination with Campylobacter spp. Field study. Res. Vet. Sci. 2018;118:12–316. doi: 10.1016/j.rvsc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Bortoluzzi C, et al. Bacillus subtilis DSM 32315 supplementation attenuates the effects of Clostridium perfringens challenge on the growth performance and intestinal microbiota of broiler chickens. Microorganism. 2019;7:71. doi: 10.3390/microorganisms7030071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuben RC, Roy PC, Sarkar SL, Rubayet-Ul-Alam ASM, Jahid IK. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019;53:1–20. doi: 10.1186/s12866-019-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reuben RC, Roy PC, Sarkar SL, Rubayet-Ul-Alam ASM, Jahid IK. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020;103:1223–1237. doi: 10.3168/jds.2019-17092. [DOI] [PubMed] [Google Scholar]
- 19.Herath C, et al. Experimental iron-inactivated Pasteurella multocida A: 1 vaccine adjuvanted with bacterial DNA is safe and protects chickens from fowl cholera. Vaccine. 2010;28:2284–2289. doi: 10.1016/j.vaccine.2009.12.068. [DOI] [PubMed] [Google Scholar]
- 20.Palócz O, et al. Alternative treatment of serious and mild Pasteurella multocida infection in New ZealandWhite rabbits. BMC Vet. Res. 2014;10:276. doi: 10.1186/s12917-014-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varinrak T, Poolperm P, Sawada T, Sthitmatee N. Cross-protection conferred by immunization with an rOmpH-based intranasal fowl cholera vaccine. Avian Pathol. 2017;46:515–525. doi: 10.1080/03079457.2017.1321105. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Sun E, Yang L, Song J, Wu B. Therapeutic application of bacteriophage PHB02 and its putative depolymerase against Pasteurella multocida capsular type A in mice. Front. Microbiol. 2018;9:1678. doi: 10.3389/fmicb.2018.01678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Q, et al. Protection of chickens against fowl cholera by supernatant proteins of Pasteurella multocidacultured in an iron- restricted medium. Avian Pathol. 2019;48:221–229. doi: 10.1080/03079457.2019.1568390. [DOI] [PubMed] [Google Scholar]
- 24.Glisson JR, Hofacre CL, Christensen JP. Fowl cholera. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DA, editors. Diseases of Poultry. 11. Ames: Iowa State University Press; 2003. pp. 658–676. [Google Scholar]
- 25.Xiao K, Liu Q, Liu X, Hu Y, Zhao X, Kong Q. Identification of the Avian Pasteurella multocida phoP gene and evaluation of the effects of phoP deletion on virulence and immunogenicity. Int. J. Mol. Sci. 2016;17:12. doi: 10.3390/ijms17010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bredy JP, Botzler RG. The effects of six environmental variables on Pasteurella multocida populations in water. J. Wildl. Dis. 1989;25:232–239. doi: 10.7589/0090-3558-25.2.232. [DOI] [PubMed] [Google Scholar]
- 27.Wubet W, et al. Evaluation of inactivated vaccine against fowl cholera developed from local isolates of Pasteurella multocida in Ethiopia. Afr. J. Microbiol. 2019;13:500–509. doi: 10.5897/AJMR2019.9096. [DOI] [Google Scholar]
- 28.Mountzouris KC, et al. Evaluation of yeast dietary supplementation in broilers challenged or not with Salmonella on growth performance, cecal microbiota composition and Salmonella in ceca, cloacae and carcass skin. Poult. Sci. 2015;94:2445–2455. doi: 10.3382/ps/pev243. [DOI] [PubMed] [Google Scholar]
- 29.Micciche AC, Foley SL, Pavlidis HO, McIntyre DR, Ricke SC. A review of prebiotics against Salmonella in poultry: Current and future potential for microbiome research applications. Front. Vet. Sci. 2018;5:1–1. doi: 10.3389/fvets.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massacci FR, et al. Dietary Saccharomyces cerevisiae boulardii CNCM I-1079 positively affects performance and intestinal ecosystem in broilers during a Campylobacter jejuni infection. Microorganism. 2019;7:596. doi: 10.3390/microorganisms7120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahimi S, Kathariou S, Fletcher O, Grimes JL. Effect of a direct-fed microbial and prebiotic on performance and intestinal histomorophology of turkey poults challenged with Salmonellaand Campylobacter. Poult. Sci. 2019;98:6572–6578. doi: 10.3382/ps/pez436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao GT, et al. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 2013;92:2949–2955. doi: 10.3382/ps.2013-03366. [DOI] [PubMed] [Google Scholar]
- 33.Huang L, et al. Effects of the dietary probiotic, Enterococcus faecium NCIMB11181, on the intestinal barrier and system immune status in Escherichia coli O78-challenged Broiler chickens. Prob. Antimicrob. Prot. 2019;11:946–956. doi: 10.1007/s12602-018-9434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giannenas I, et al. Assessment of probiotics supplementation via feed or water on the growth performance, intestinal morphology and microflora of chickens after experimental infection with Eimeria acervulina, Eimeria maxima and Eimeria tenella. Avian Pathol. 2014;43:209–216. doi: 10.1080/03079457.2014.899430. [DOI] [PubMed] [Google Scholar]
- 35.Awais MM, et al. Immunomodulatory and ameliorative effects of Lactobacillus and Saccharomyces based probiotics on pathological effects of eimeriasis in broilers. Microb. Path. 2019;126:101–108. doi: 10.1016/j.micpath.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 36.Deng Q, Hanyi S, Yiran L, Heping Z, Ning L. Effect of dietary Lactobacilli mixture on Listeria monocytogenes infection and virulence property in broilers. Poult Sci. 2020;99:3655–3662. doi: 10.1016/j.psj.2020.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drolia R, Tenguria S, Durkes AC, Turner JR, Bhunia AK. Listeria adhesion protein induces intestinal epithelial barrier dysfunction for bacterial translocation. Cell Host Microbe. 2018;23:470–484. doi: 10.1016/j.chom.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajabi S, Darban D, Tabatabaei RR, Hosseini F. Antimicrobial effect of spore-forming probiotics Bacillus laterosporus and Bacillus megaterium against Listeria monocytogenes. Arch. Microbiol. 2020;202:2791–2797. doi: 10.1007/s00203-020-02004-9. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, et al. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018;9:25. doi: 10.1186/s40104-018-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rathnapraba S, Kanagaraju P, Vijayarani K. Effect of dietary supplementation of probiotic and BMD on the growth performance of broiler chickens challenged with Clostridium perfringens induced necrotic enteritis. Int. J. Chem. Stud. 2018;6:13–15. [Google Scholar]
- 41.Olnood CG, Beski SSM, Choct M, Iji PA. Novel probiotics: Their effects on growth performance, gut development, microbial community and activity of broiler chickens. Anim. Nutr. 2015;1:184–191. doi: 10.1016/j.aninu.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JH, Kim IH. The effects of the supplementation of Bacillus subtilis RX7 and B2A strains on the performance, blood profiles, intestinal Salmonella concentration, noxious gas emission, organ weight and breast meat quality of broiler challenged with Salmonella typhimurium. J. Anim. Physiol. Anim. Nutr. 2015;99:326–334. doi: 10.1111/jpn.12248. [DOI] [PubMed] [Google Scholar]
- 43.Zhang ZF, Cho JH, Kim IH. Effects of Bacillus subtilisUBT-MO2 on growth performance, relative immune organ weight, gas concentration in excreta, and intestinal microbial. Shedding in broiler chickens. Livestock Sci. 2013;155:343–347. doi: 10.1016/j.livsci.2013.05.021. [DOI] [Google Scholar]
- 44.Pedroso AA, et al. Performance and organ morphology of broilers fed microbial or antimicrobial additives and raised in batteries of floor pens. Rev. Bras. Cienc. Avic. 2003;5:2–10. [Google Scholar]
- 45.van Dijk JE, Huisman J, Koninkx JF. Structure and functional aspects of a healthy gastrointestinal tract. In: Blook MC, Vahl HA, DeLange L, Vande Braak AE, Hemke G, editors. Nutrition and Health of the Gastrointestinal Tract. Academic Publishers; 2002. pp. 71–92. [Google Scholar]
- 46.Pelicano ERL, et al. Effect of Different Probiotics on Broiler Carcass and Meat Quality. Braz. J. Poult. Sci. 2003;5:207–214. doi: 10.1590/S1516-635X2003000300009. [DOI] [Google Scholar]
- 47.Boyce JD, Harper M, Wilkie IW, Adler B. Pasteurella. In: Gyles CL, Prescott JF, Songer G, Thoen CO, editors. Pathogenesis of Bacterial Infections in Animals. 4. Blackwell Publishing; 2010. pp. 325–346. [Google Scholar]
- 48.Sonone S, Swamy M, Verma Y. Ameliorative effect of Mannan Oligosaccharide on pathology of fowl cholera in broiler birds. Asian J. Poult. Sci. 2011;5:86–93. doi: 10.3923/ajpsaj.2011.86.93. [DOI] [Google Scholar]
- 49.Sarkozy G, Semjen G, Laczay P, Horvath E. Treatment of experimentally induced Pasteurella multocida infections in broilers and turkeys: Comparative studies of different oral treatment regimens. J. Vet. Med. B. 2002;49:130–134. doi: 10.1046/j.1439-0450.2002.00518.x. [DOI] [PubMed] [Google Scholar]
- 50.Pilatti RM, et al. Establishment of a pathogenicity index for one-dayold broilers to Pasteurella multocida strains isolated from clinical cases in poultry and swine. Braz. J. Poult. Sci. 2016;18:255–260. doi: 10.1590/1806-9061-2015-0089. [DOI] [Google Scholar]
- 51.Van Immerseel F, et al. Invasion of Salmonella enteritidis in avian intestinal epithelial cells in vitro is influenced by short-chain fatty acids. Int. J. Food Microbiol. 2003;85:237–248. doi: 10.1016/S0168-1605(02)00542-1. [DOI] [PubMed] [Google Scholar]
- 52.Lan PT, Binh LT, Benno Y. Impact of two probiotic Lactobacillus strains feeding on faecal lactobacilli and weight gains in chicken. J. Gen. Appl. Microbiol. 2003;49:29–36. doi: 10.2323/jgam.49.29. [DOI] [PubMed] [Google Scholar]
- 53.Van der Wielen PW, Lipman LJA, van Knapen F, Biesterveld S. Competitive exclusion of Salmonella enterica serovar enteritidis by Lactobacillus crispatus and Clostridium lactatifermentans in a sequencing fed-batch culture. J. Appl. Environ. Microbiol. 2002;68:555–559. doi: 10.1128/AEM.68.2.555-559.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teo AY, Tan HM. Evaluation of the performance and intestinal gut microflora of broilers fed on corn-soy diets supplemented with Bacillus subtilis PB6 (CloSTAT) J. Appl. Poult. Res. 2007;16:296–303. doi: 10.1093/japr/16.3.296. [DOI] [Google Scholar]
- 55.Zhang Z, Kim I. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult. Sci. 2014;93:364–370. doi: 10.3382/ps.2013-03314. [DOI] [PubMed] [Google Scholar]
- 56.Grosu-Tudor SS, Stancu MM, Pelinescu D, Zamfir M. Characterization of some bacteriocins produced by lactic acid bacteria isolated from fermented foods. World J. Miocrobiol. Biotechnol. 2014;30:2459–2469. doi: 10.1007/s11274-014-1671-7. [DOI] [PubMed] [Google Scholar]
- 57.Elagib HAA, Ahmed ADA. Comparative study on haematological values of blood of indigenous chickens in Sudan. Asian J. Poult. Sci. 2011;5:41–45. doi: 10.3923/ajpsaj.2011.41.45. [DOI] [Google Scholar]
- 58.Lee KW, et al. Effect of Bacillus-based directed microbials on Eimeria maxima infection in broiler chickens. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:E105–E110. doi: 10.1016/j.cimid.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 59.LaFleur BM, LaFleur BD. Exploring Medical Language: A Student-Directed Approach. 7. Elsevier; 2008. p. p398. [Google Scholar]
- 60.Gaggıa F, Mattarelli P, Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010;141:15–28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 61.Arun KP, Rama R, Savaram V, Mantena VLN, Raju SRS. Dietary supplementation of Lactobacillus Sporogenes on performance and serum biochemico-lipid profile of broiler chickens. J. Poult. Sci. 2006;43:235–240. doi: 10.2141/jpsa.43.235. [DOI] [Google Scholar]
- 62.Al-Kassie G, Al-Jumaa Y, Jameel Y. Effect of probiotic (Aspergillus niger) and prebiotic (Taraxacum officinale) on blood picture and biochemical properties of broiler chicks. Int. J. Poult. Sci. 2008;7:1182–1184. doi: 10.3923/ijps.2008.1182.1184. [DOI] [Google Scholar]
- 63.Fukushima M, Nakano M. The effect of probiotic on faecal and liver lipid classes in rats. Br. J. Nutr. 1995;73:701–710. doi: 10.1079/BJN19950074. [DOI] [PubMed] [Google Scholar]
- 64.Mohan B, Kadirvel R, Natarajan M, Bhaskaran M. Effect of probiotic supplementation on growth, nitrogen utilization and serum cholesterol in broilers. Brit. Poult. Sci. 1996;37:395–401. doi: 10.1080/00071669608417870. [DOI] [PubMed] [Google Scholar]
- 65.Surono IS. In vitro probiotic properties of indigenous Dadih lactic acid bacteria. Asian-Austral. J. Anim. Sci. 2003;16:726–731. doi: 10.5713/ajas.2003.726. [DOI] [Google Scholar]
- 66.Dimcho D, Svetlana B, Tsvetomira S, Tatiana V. Effect of feeding Lactina probiotic on performance, some blood parameters and caecal microflora of mule ducklings. Trakia J. Sci. 2005;3:22–28. [Google Scholar]
- 67.Jiang J, et al. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants. 2019;8:575. doi: 10.3390/antiox8120575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian T, et al. Regulation of CD11b by HIF-1a and the STAT3 signaling pathway contributes to the immunosuppressive function of B cells in inflammatory bowel disease. Mol. Immunol. 2019;111:162–171. doi: 10.1016/j.molimm.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 69.Fujii M, et al. HIF1a inhibits LPS-mediated induction of IL-6 synthesis via SOCS3-dependent CEBPb suppression in human dental pulp cells. Biochem. Biophys. Res. Commun. 2020;522:308–314. doi: 10.1016/j.bbrc.2019.11.032. [DOI] [PubMed] [Google Scholar]
- 70.Day AJ, Milner CM. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019;78–79:60–83. doi: 10.1016/j.matbio.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 71.Gao J, Zhang HJ, Yu SH. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008;87:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- 72.Engberg RM, Steenfeldt S, Hedemann MS, Jensen BB. The influence of whole wheat and xylanase on broiler performance and microbial composition and activity in the digestive tract. Poult. Sci. 2004;83:925–938. doi: 10.1093/ps/83.6.925. [DOI] [PubMed] [Google Scholar]
- 73.Hatfaludi T, et al. Screening of 71 P. multocida proteins for protective efficacy in a fowl cholera infection model and characterization of the protective antigen PlpE. PLoS ONE. 2012;7:e39973. doi: 10.1371/journal.pone.0039973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar S, et al. Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS ONE. 2018;13:e0192450. doi: 10.1371/journal.pone.0192450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Metals. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]