Abstract
Background
Eribulin is a microtubule-targeting agent approved for the treatment of advanced or metastatic breast cancer (BC) previously treated with anthracycline- and taxane-based regimens. PIK3CA mutation is associated with worse response to chemotherapy in oestrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−) metastatic BC. We aimed to evaluate the role of phosphoinositide 3-kinase (PI3K)/AKT pathway mutations in eribulin resistance.
Methods
Resistance to eribulin was evaluated in HER2− BC cell lines and patient-derived tumour xenografts, and correlated with a mutation in the PI3K/AKT pathway.
Results
Eleven out of 23 HER2− BC xenografts treated with eribulin exhibited disease progression. No correlation with ER status was detected. Among the resistant models, 64% carried mutations in PIK3CA, PIK3R1 or AKT1, but only 17% among the sensitive xenografts (P = 0.036). We observed that eribulin treatment induced AKT phosphorylation in vitro and in patient tumours. In agreement, the addition of PI3K inhibitors reversed primary and acquired resistance to eribulin in xenograft models, regardless of the genetic alterations in PI3K/AKT pathway or ER status. Mechanistically, PI3K blockade reduced p21 levels likely enabling apoptosis, thus sensitising to eribulin treatment.
Conclusions
PI3K pathway activation induces primary resistance or early adaptation to eribulin, supporting the combination of PI3K inhibitors and eribulin for the treatment of HER2− BC patients.
Subject terms: Breast cancer, Tumour biomarkers, Predictive markers, Cancer therapeutic resistance
Background
Metastatic breast cancer (mBC) is an incurable disease with a median 5-year overall survival (OS) of only 25%.1 The current clinical practice is defined based on the available clinical–pathological and immunohistochemistry (IHC)-based expression of human epidermal growth factor receptor 2 (HER2), oestrogen receptor (ER), progesterone receptor (PR) and the proliferation marker Ki-67. Hence, mBC is classified as HER2-positive (HER2+), luminal A (ER+ and PR+ and Ki-67 low), luminal B (ER+ or PR+ or Ki-67 index >14%) or triple negative (lack of HER2, ER and PR expression). The median OS of each subgroup is ~40% for HER2+ and luminal, and 30 and 10 for luminal B and triple-negative BCs (TNBCs), respectively.1 According to the fourth ESO-ESMO and St. Gallen’s Consensus Guidelines, anti-HER2 therapy and endocrine therapy are the preferred therapy for HER2+ and hormone receptor-positive (HR+) tumours, respectively.2,3 However, for ER/PR+ tumours resistant to endocrine therapy or with visceral crisis and TNBC, chemotherapy (mainly anthracyclines and taxanes) remains as recommended therapy. In this regard, microtubule-targeting agents (MTAs) are among the most active agents for the treatment of mBC. Particularly, eribulin has significantly improved OS in both ER+ and TN mBC patients, leading to the approval for the treatment of advanced or mBCs previously treated with an anthracycline and a taxane.4,5 However, tumours become resistant to eribulin therapy and patients succumb to the disease.
The phosphoinositide 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) signalling pathway is critical for regulation of cell growth, cell cycle, proliferation and cell survival.6 Among other target proteins, AKT phosphorylates the cyclin-dependent kinase inhibitor p21 (encoded in CDKN1A gene).7 When bound to CDK4/cyclin D1 complexes, p21 acts as both to positively and negatively regulate CDK4, depending on its phosphorylation status.8,9 p21 mediates nuclear translocation of cyclin D-dependent kinases,10,11 providing access to their nuclear substrates. However, unphosphorylated p21 can directly block CDK4/6 kinase active site.11,12 In addition, cytoplasmic p21 inhibits cell death by interacting with procaspase 3 and impede caspase 3 cleavage and Fas-mediated cell death induction.13
Activating mutations in PIK3CA or those inactivating PTEN are frequent in ER+ or in TNBC, respectively.14–17 Preclinical studies in different tumour types have shown that activation of the PI3K/AKT/mTOR pathway promote chemoresistance,18–20 providing further rationale for the development of PI3K pathway inhibitors in combination with chemotherapeutic agents.21,22 Interestingly, PI3K blockade increases the sensitivity of tumour cells to apoptosis-inducing agents,23–28 and recently it has been shown to synergise with eribulin in preclinical models of TNBC.29 Furthermore, recent studies also reported the benefit of combining eribulin and mTOR inhibitors in TNBC.30,31
An ongoing open-label, non-randomised, multicentre, phase 1/2b (dose escalation followed by expansion part) study (PIQHASSO, NCT02723877) is currently evaluating clinical safety and pharmacokinetics of PQR309 (a dual PI3K/mTORC1/2 inhibitor32) in combination with a standard dose of eribulin in patients with locally advanced or metastatic HER2− and efficacy in TNBC. Anticipating new biomarkers of response to eribulin will help to more accurately identify which patients are most likely to benefit from eribulin monotherapy and which from combination strategies.
In this study, we sought to investigate the predictive value of PI3K/AKT pathway alterations towards eribulin response, confirm the benefit of combining eribulin and PI3K inhibitors for the treatment of pre-treated metastatic HER2− BC (including ER+/HER2− and TNBC) in preclinical models, and uncover the molecular mechanisms of action for this combination.
Methods
Study design
This study was designed to evaluate the potential of PI3K inhibitors combined with eribulin. We assessed eribulin single agent and the combination with BKM120 sensitivity in a cohort of 23 and 6 murine xenografts from breast cancer patients, respectively. All animal procedures were approved by the Ethics Committee of Animal Research of the Vall d’Hebron Institute of Research and by the Catalan Government. For ethical issues, in vivo experiments were ended when the sum of the tumour volumes of a mouse surpassed 1500 mm3 or a decline in mouse welfare was observed. These experiments were not performed in a blinded fashion. Tumours were harvested and formaldehyde-fixed, paraffin-embedded (FFPE) and flash-frozen for subsequent proteomic and genomic analyses.
Cell lines and treatments
Cell lines were obtained from the American Type Culture Collection. HCC38, HCC70, HCC1143, HCC1395, T47D and ZR-75-1 cell lines were maintained in RPMI (Live Technologies Inc., Ltd). MDA-MB-468 cell line was kept in Dulbecco’s modified Eagle’s media (DMEM):F12 supplemented with 10% foetal bovine serum (FBS) and 2 mmol/l l-glutamine (Live Technologies Inc., Ltd). MDA-MB-231, KPL1, MCF7, CAL-51, CAL-120, BT20 and CAMA-1 were cultured in DMEM high glucose (PAA Laboratories GmbH) supplemented with 10% FBS, as well as BT-549, which was also supplemented with 10 μg/ml insulin. MDA-MB-175 cell line was maintained in L-15 (Live Technologies Inc., Ltd) supplemented with 10% FBS. MCF10A (both parental and PIK3CA H1047R/+) cell lines were purchased from Horizon Discovery Ltd and maintained in DMEM:F12 supplemented with 5% horse serum and 2.5 mM l-glutamine, 15 mM HEPES, 10 μg/ml insulin, 0.5 μg hydrocortisone (Live Technologies Inc., Ltd). The medium for MCF10A parental cells was supplemented with 5 ng/ml hEGF. Upon assay conditions, all MCF10A cells were cultured in DMEM-F12 supplemented with 2% horse serum and 2.5 mM l-glutamine, 15 mM HEPES, 10 μg/ml insulin, 0.5 μg/ml hydrocortisone (Live Technologies Inc., Ltd). All the cell lines were incubated at 37 °C in 5% CO2 in a humidified atmosphere, except for MDA-MB-175 that were kept at 37 °C in the absence of CO2. The pan-PI3K inhibitor BKM120 and the α-PI3K inhibitor BYL719 were kindly provided by Novartis. The pan-PI3K inhibitor GDC0941 was purchased at Haoyuan Chemexpress Co. Ltd. Paclitaxel, cisplatin and doxorubicin were purchased from Selleck Chemicals, Houston, Texas. Eribulin was purchased as eribulin mesylate (Halaven®, Eisai Europe Ltd).
Cell proliferation
Cells were seeded and treated the following day with indicated single-drug treatments or combinations. Afterwards, cells were fixed with formaldehyde for 30 min, rinsed three times and stained for 1 h with crystal violet. Once dried, the staining was diluted in an aqueous solution containing acetic acid 10% for 10 min. The absorbance at 560 nm was then measured using a colorimeter.
Cell cycle and apoptosis
A total of 0.3 × 106 cells per well were seeded in 6-well plates and treated with the indicated single-drug treatments or combinations for 48 h for cell cycle analyses and apoptosis measures. Cell cycle and hypodiploid apoptotic cells were quantified by flow cytometry as described in refs. 33,34. Briefly, cells were trypsinised, washed with phosphate-buffered saline (PBS), fixed in cold 70% ethanol and then stained with propidium iodide while treated with RNaseA (Sigma-Aldrich). Quantitative analysis of cell cycle distribution and sub-G1 cells was carried out in a FACSCalibur cytometer using the FCS Express 4 Flow Research Edition.
Viral infections
HEK293T Phoenix-Ampho cells were transfected with FuGENE® HD Transfection Reagent (Promega Co.), following the manufacturer’s protocol. Specific knockdown of CDKN1A was conducted under the standard protocol of pGIPz retroviral vectors: pGIPz-Scramble-shRNA-puro and pGIPz-CDKN1A-shRNA-puro (RHS4430-200281172 clone V3LHS-322234 from Open Biosystems-Thermo Fisher Scientific). Lentiviral particles were collected twice a day for 3 days from transfected cells and supplemented to MCF10A’s medium. Afterwards, MCF10A wt/wt (wild type/wild type) and wt/H1047R cells were selected with puromycin (0.5 μg/ml).
Collection of tumour samples and establishment of PDXs
Fresh tumour samples from patients with BC were collected following an Institutional Research Board-approved protocol and the associated written informed consent. The study was compliant with the Declaration of Helsinki.
Experiments were conducted following the European Union’s animal care directive (2010/63/EU) and were approved by the Ethical Committee of Animal Experimentation of the Vall d’Hebron Research Institute, the Catalan Government or by the National Research Ethics Service, Cambridgeshire (ref. 35 and https://caldaslab.cruk.cam.ac.uk/bcape).
Six-week-old female athymic nude HsdCpb:NMRI-Foxn1nu and NSG (NOD.Cg-PrkdcSCIDIl2rgtm1Wjl/SzJ) mice were purchased from Janvier Laboratories or from Charles River Laboratories, respectively, housed in specific pathogen-free conditions, handled in air-filtered laminar flow cabinets with a 12-h light cycle and provided with food and water ad libitum. Biopsied or surgical tumours were subcutaneously implanted in anaesthetised mice. Established PDXs were expanded into new recepient mice for drug experiments (see below). The experiments were ended when the sum of the tumour volumes of a mouse surpassed 1500 mm3 or a decline in mouse welfare was observed, including mouse weight loss >20%.
Animals were supplemented with 1 μmol/l 17β-oestradiol (Sigma) in their drinking water.36 Upon xenograft growth, tumour tissue was re-implanted into recipient mice, and randomised when tumour volume was 150–300 mm3. Mice were treated with eribulin mesylated weekly on days 1, 3, 5 intravenously [0.1 mg/kg, in PBS] and with BKM120 daily per os [27.5 mg/kg in 5% N-methyl-2-pyrrolidone, 95% with polyethylene glycol] or with BYL719 daily per os [50 mg/kg in 0.5% carboximethylcellulose vehicle]. In combination treatments, eribulin and BKM120 or BYL719 were dosed simultaneously at the same dose and route of administration as in the single treatments. For the 4-arm experiments comparing tumour growth in vivo, four to six mice per group were determined as the minimal number of tumour-bearing according to Mead’s equation and taking into account the implantation rate of a tumour xenograft in these mouse strains.37 For tumour growth comparisons, tumours were used as the statistical unit and two-way analysis of variance (ANOVA) or mixed-effects models tests were used for the statistical analysis.
Tumour xenografts were measured with callipers and tumour volumes were determined using the ellipsoid formula: (length × width2) × (π/6). All the in vivo experiments contained an untreated control arm. The anti-tumour activity was determined by comparing tumour volume at 13–25 days to its baseline: % change in tumour volume = (V13–25 days − Vinitial)/Vinitial × 100. To classify the response of the subcutaneous implants, we modified the RECIST (Response Evaluation Criteria in Solid Tumours) criteria, to be based on the % change in tumour volume:38,39 CR (complete response), best response < −95%; PR (partial response), −95% < best response < −30%; SD (stable disease), −30% < best response < +20%; PD (progressive disease), best response > +20%. Those models that display a clinical benefit from each therapy compared to the untreated counterparts (SD, PR and CR with untreated tumours with a % change in tumour volume > +20%) were categorised as sensitive. For sensitive patient-derived xenograft (PDX), the best response was defined as the minimum value of % tumour volume change sustained for at least 10 days.
Treatment tolerability was analysed by weighing the mice. At the end of the experiment, animals were euthanised using CO2 inhalation. Tumour volumes are plotted as means and SEM.
Molecular subtype of the PDX
Immunohistochemical staining, performed on tissue sections from FFPE PDXs, against ER (Roche, Cat num. 790-4324), PR (Roche, Cat num. 790-2223) and HER2 (Roche, Cat num. 790-2991) were accomplished following the protocol provided by Ventana Medical Systems Inc. In short, the slides were heated in the instrument at 75 °C for 28 min and deparaffinised with EZ prep solution (Ventana Medical Systems). Then, antigen retrieval was performed at slightly basic pH at 95 °C for 56 and 40 min for ER and HER2 antibodies using Cell Conditioning 1 (Ventana Medical Systems), and 40 min with buffer CC2 for PR antibody. Finally, the slides were counterstained with Haematoxylin II and Bluing Reagent (Ventana Medical Systems) and mounted with Xylol-based mounting medium. An investigator blinded to identify the samples quantified the percentage of positively stained cells.
Targeted exome sequencing (MSK-IMPACTTM)
Flash-frozen pieces of tumour xenograft were used for DNA sequencing by the MSK-IMPACTTM (Integrated Mutation Profiling of Actionable Cancer Targets) gene test that involves hybridisation barcoded libraries to custom oligonucleotides (Nimblegen SeqCap) designed to capture all protein-coding exons and select introns of 410 commonly implicated oncogenes, tumour suppressor genes and members of pathways deemed actionable by targeted therapies.40 Barcoded sequence libraries were prepared using 100–250 ng genomic DNA (Kapa Biosystems) and combined into equimolar pools of 13–21 samples. The captured pools were subsequently sequenced on an Illumina HiSeq 2000 as paired-end 100 bp reads, producing a median of 588-fold coverage per tumour.
Sequence data were demultiplexed using CASAVA, and reads were aligned to the reference human genome (hg19) using BWA and post-processed using the Genome Analysis Toolkit (GATK) according to GATK best practices.
MuTect and GATK were used to call single-nucleotide variants and small indels, respectively. Candidate mutations were manually reviewed using the Integrative Genomics Viewer to eliminate likely false-positive calls. Because matched normal DNA was not available, tumours were compared to a pool of ten unmatched normal samples to eliminate common polymorphisms and systematic sequencing artefacts.
IHC and image analysis
Tumour xenografts pieces were fixed immediately after excision in 10% buffered formalin solution for a maximum of 24 h at room temperature before being dehydrated and paraffin-embedded under vacuum conditions (FFPE).
IHC was performed on FFPE PDX model-derived xenograft tissue sections (4 µm), using pAKT S473 (DAKO M3628, final concentration 14.1 µg/ml) or pHistone H3 S10 (pHH3, Cell Signalling #9701, diluted 1/100 in Envision Flex Antibody Diluent) primary antibodies. Sections were dewaxed, rehydrated and then antigen retrieved in pH9 (pAKT S473) or pH6 (pHH3) retrieval buffer (DAKO) using a Microwave Histoprocessor (Milestone). IHC staining was performed on Lab VisionTM Autostainer 720-2D (Thermo ScientificTM). Rabbit EnVisionTM + System-HRP (horseradish peroxidase) was used for pAKT S473 staining. Liquid DAB+ Substrate Chromogen System (DAKO) and Harris’ haematoxylin was used on all samples. Slides were scanned at ×20 using the Aperio AT2 scanner. For pAKT S473, the percentage of tumour cells with strong (3+), moderate (2+), weak (1+) or negative staining was scored by two independent blinded researchers and represented as H-score, which was calculated as: [(%1+ cells) + (%2+ cells × 2) + (%3+ cells × 3)]. For pHH3 S10, the percentage of positive cells was quantified by to independent researchers.
Immunohistochemical staining against Ki-67 was accomplished following the protocol provided by Ventana Medical Systems Inc. Briefly, the slides were heated in the instrument at 75 °C for 28 min and deparaffinised with EZ prep solution (Ventana Medical Systems). Then, antigen retrieval was performed at slightly basic pH at 95 °C for 64 min (Cell Conditioning 1 protocol from Ventana Medical Systems). Finally, the slides were counterstained with Haematoxylin II and Bluing Reagent (Ventana Medical Systems) and mounted with xylol-based mounting medium. An investigator blinded to identify the samples quantified the percentage of positively stained cells.
Western blot
Tumour samples were homogenised in ice-cold lysis buffer [Tris-HCl, pH 7.8, 20 mmol/l; NaCl, 137 mmol/l; EDTA, pH 8.0, 2 mmol/l; NP40 1%, glycerol 10%; supplemented with NaF, 10 mmol/l; leupeptin, 10 μg/ml; Na2VO4, 200 μmol/l; phenylmethylsulfonylfluoride, 5 mmol/l; and aprotinin (Sigma-Aldrich)]. Cell lines were also collected in ice-cold lysis buffer. Lysates were cleared by centrifugation at 13,000 r.p.m. for 10 min at 4 °C, and supernatants were removed and assayed for protein concentration using the Pierce BCA Protein Assay Kit (Thermo Scientific). Fifty micrograms of total lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose membranes. Membranes were hybridised with the following primary antibodies diluted 1/1000: pAKT T308 (#4056), pAKT S473 (#9271), AKT (#9272), pFOXO1/3A T24/T32 (#9464), pPRAS40 T246 (#2997), pS6 S235/6 (#2211), pS6 S240/4 (#2215), p4EBP1 T37/46 (#2855), p4EBP1 T70 (#9455), p21 (#2947) and pCDK1 Y15 (#4539) from CST; pCDK1 T161 (ab135775) and GAPDH (ab128915) from Abcam; CDK1 (33–1800) from Thermo Fisher Scientific; p27 (sc-528) from Santa Cruz Biotechnology. Rabbit HRP-conjugated secondary antibody (Amersham Biosciences) was used at 1:2000 in TBS-T/5% non-fat dry milk. Protein–antibody complexes were detected by chemiluminescence with the Immobilon Western HRP Substrate and images were captured with a Fujifilm LASS-3000 camera system or by X-ray radiography film (Fuji Medical X-ray film, Fujifilm Corporation).
Statistical analysis
GraphPad Prism 8.0 was used for statistical analysis. For the comparative experiments of biomarkers between eribulin single agent and combinations with PI3K inhibitors, we used one-way ANOVA with Bonferroni’s post-test in vitro. The tumour growth experiments were analysed with two-way ANOVA or mixed-effects model with Geisser-Greenhouse correction and Tukey’s multiple comparison for the evaluation of statistical difference among treatments and across time. The control group reached tumour volume endpoint before the experimental arms and therefore had to be excluded from the statistical analysis. For comparison of eribulin activity in PIK3CA wt/wt and H1047R/wt, we used unpaired t test with Welch’s correction.
Results
Activating mutations in PIK3CA or AKT1 are associated with eribulin resistance in vitro
Previous in vitro studies suggested that constitutive activation of the PI3K pathway limits the anti-cancer activity of chemotherapy.18–20,24,25 In line with this, we observed that the anti-proliferative activity of eribulin or paclitaxel was reduced in MCF10A PIK3CA-wt/p.H1047R cells compared to PIK3CA-wt/wt. In contrast, this effect was not observed when two DNA-damaging agents (doxorubicin and cisplatin) were tested (Fig. 1a, b). We also observed that MCF10A PIK3CA-wt/p.H1047R cells exhibited an attenuated accumulation of cells in the G2/M phase of the cell cycle upon eribulin treatment than MCF10A PIK3CA-wt/wt cells. No significant difference in apoptosis induction was observed in this isogenic, non-tumorigenic cell line model (Fig. 1c, d). We extended our analysis to a panel of 14 HER2− BC cell lines (Table S1). As expected, PIK3CA-mutant cells were more sensitive to the PI3K inhibitors BKM120 and BYL719 than PIK3CA-wt cells, and we also observed a trend towards eribulin resistance in PIK3CA-mutant cells in this panel (Fig. 1e).
Fig. 1. PIK3CA-activating mutations correlate with resistance to eribulin in vitro.
a Crystal violet staining of MCF10A cells harbouring PIK3CA wt or p.H1047R heterozygous mutation after 1 week of treatment with the indicated treatments. b Cell count of surviving MCF10A cells harbouring PIK3CA wt or p.H1047R heterozygous mutation after 1 week of treatment with eribulin 1 nM. Unpaired t test with Welch’s correction. Error bars represent SD. c Analysis of the cell cycle distribution of MCF10A cells harbouring PIK3CA wt or p.H1047R heterozygous mutation after 48 h of eribulin treatment by flow cytometry. Two-way ANOVA with Sidak’s multiple comparisons test. Error bars represent SD. d Analysis of the hypodiploid population of MCF10A cells harbouring PIK3CA wt or p.H1047R heterozygous mutation after 48 h of eribulin treatment by flow cytometry. Two-way ANOVA with Sidak’s multiple comparisons test. Error bars represent SD. e EC50 values of a panel of 13 established breast cancer cell lines regarding the PIK3CA status. Unpaired t test with Welch’s correction.
We further assessed the potential of mutations in the PI3K/AKT pathway to predict eribulin resistance in 23 cell lines and PDXs. Of note, three of the PDXs were derived from patients who had received eribulin before the PDX was established (Table S2), and these responded consistently with the response achieved by the patient, consolidating the use of PDXs as preclinical models to evaluate drug responses (PDX244, PDX288 and PDX432).
Consistently with the in vitro results, most of the PIK3CA, PIK3R1 and AKT1 mutant models progressed to eribulin single agent, namely underwent PD as the best response (Fig. 2a, black bars and Tables S3 and S4). In this data set, the odds ratio for eribulin resistance when harbouring a PI3K/AKT pathway mutation was 8.75 (95% confidence interval (CI) 1.15–49.99, P = 0.036 Fisher’s exact test, Fig. 2a and Tables S3 and S4). In fact, 7 out of 11 eribulin-progressing models (64%) harboured mutations in PIK3CA/PIK3R1/AKT1, while only 2 out of 12 eribulin-sensitive models (16%) harboured mutations in PIK3CA/PIK3R1/AKT1. We did not observe an association between PTEN status and eribulin response, since PTEN-altered models were equally distributed among eribulin-resistant and -sensitive groups: 6 out of 11 eribulin-resistant models and 5 out of 12 eribulin-sensitive models exhibited alterations in PTEN (P = 0.684, Fisher’s exact test; Figs. 1e and 2a). Overall, these results suggest that genetic alterations in PIK3CA/PIK3R1/AKT1 could predict for eribulin resistance.
Fig. 2. PIK3CA/AKT1-activating mutations do not predict primary resistance to eribulin in vivo.
a Waterfall plot representing the tumour growth of 23 cell line or patient-derived xenografts (PDXs) treated with 0.1 mg/kg eribulin three times a week. The percentage change from the initial tumour volume is shown at the time-point of the best response. +20%, −30% and −95% are marked by dotted lines to indicate the range of progressive disease (PD), stable disease (SD), partial response (PR) and complete response (CR). Error bars indicate SD from at least two tumours. In the bottom panel, black and white boxes indicate ER+ and triple-negative breast cancer subtypes, respectively. All experiments were performed in athymic nude HsdCpb:NMRI-Foxn1nu mice except for the one indicated with an asterisk (*), which was performed in the NSG strain. b Kaplan–Meier curves of HER2-negative breast cancer patients treated with eribulin with tumours positive or negative for PIK3CA/AKT1 mutations.
In an attempt to validate these observations in patient samples, we analysed the predictive value of PIK3CA/AKT1 mutations, measured in the primary tumour sample, in a cohort of 44 HER2− mBC patients treated with eribulin in our Institution (30 with PIK3CA/AKT1 wt tumours, 11 PIK3CA mutant and 3 AKT1 mutant; baseline characteristics of the patients can be found in Table S5). Median progression-free survival (PFS) was 154 vs. 160 days for patients with mutant vs. wt tumours, respectively (log-rank (Mantel–Cox) test, P = 0.658, hazard ratio = 0.86, 95% CI 0.43–1.71) (Fig. 2b). These results contrast with our observations in preclinical models and warrant validation in larger cohorts using concurrent mutation status.
PI3K inhibition enhances eribulin anti-tumour activity by promoting apoptosis
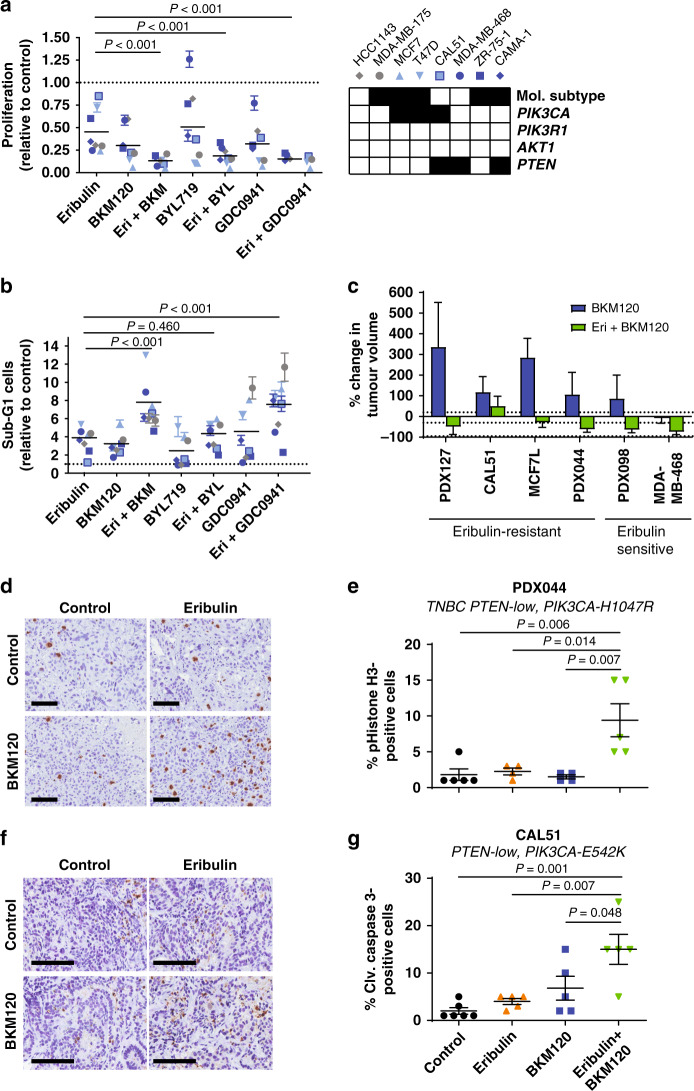
Preclinical studies suggested that PI3K or mTOR inhibition synergise with eribulin.29–31 We firstly aimed to assess if mutations in the PI3K/AKT pathway in tumour cells had an impact on the combination response. Therefore, we screened the anti-proliferative and proapoptotic activity of eribulin combined with either of three PI3K inhibitors (two pan-PI3K, and one α-specific class I PI3K inhibitors: BKM120 and GDC0941, and BYL719, respectively) in a panel of eight HER2− BC cell lines (five ER+ and three triple-negative) with or without genetic alterations in PIK3CA or PTEN (Fig. 3a, b). We observed that PI3K inhibition significantly enhanced the anti-proliferative activity of eribulin, regardless of the PI3K/AKT pathway mutational status of the cell line. Induction of apoptosis in the combination treatment was evidenced for pan-PI3K inhibitors but not for the PI3K-α inhibitor, likely due to its limited activity in PTEN-mutated cell lines.41,42
Fig. 3. PI3K inhibition increases the activity of eribulin in vitro and in vivo.
a Proliferation (top panel) and b apoptosis induction (bottom panel) of a cohort of established breast cancer cell lines upon treatment with eribulin (0.25 nM), PI3K inhibitors (BKM120 1 µM, BYL719 2.5 µM, GDC0941 1 µM) or their combination. Two-way ANOVA with Tukey’s multiple comparisons test. Relevant P values are shown. In the right panel, black and white boxes indicate ER+ and triple-negative breast cancer subtypes, respectively. White boxes indicate wild-type genes and black boxes indicate mutation and gene loss for PIK3CA and PTEN, respectively. c Waterfall plot representing the tumour growth of six cell lines or patient-derived xenografts (PDXs) treated with 27.5 mg/kg BKM120 daily or the combination with eribulin. The percentage change from the initial tumour volume is shown at the time point of the best response. +20%, −30% and −95% are marked by dotted lines to indicate the range of progressive disease (PD), stable disease (SD), partial response (PR) and complete response (CR). Error bars indicate SD from at least four tumours. d Immunohistochemistry scoring of pHistone H3 S10 in FFPE samples from PDX044 tumours from each arm after 9 days of treatment. Scale bar: 100 μm e Quantification of (d). Mean of the tumour staining in each group and SEM are indicated. P value, Mann–Whitney test. f Immunohistochemistry scoring of cleaved caspase 3 in FFPE samples from CAL51 tumours from each arm after 3 days of treatment. Scale bar: 100 μm g Quantification of (f). Mean of the tumour staining in each group and SEM are indicated. P value, Mann–Whitney test.
We tested the anti-tumour activity of eribulin, BKM120 or the combination of both drugs in six HER2− BC xenograft models in mice, three derived from cell lines (CAL51, MCF7L and MDA-MB-468) and three from patient tumour samples (PDX127, PDX044 and PDX098), and a similar phenotype was observed in vivo. All models except MDA-MB-468 were resistant to BKM120. Importantly, the combination of eribulin plus BKM120 resulted in tumour regression in vivo in five out of six xenografts (Fig. 3c and Table S3). Mechanistically, we observed that eribulin plus BKM120 increased the percentage of phosphorylated Histone H3-positive cells and of cleaved caspase 3 in PDX044 and CAL51, respectively (Fig. 3d–g), indicating that the combination of drugs can induce mitotic arrest or apoptosis in a tumour-dependent manner in vivo. This combination was well tolerated in vivo, as no significant body weight loss was measured throughout the treatment duration (Fig. S1).
Early adaptation via PI3K pathway activation is a putative mechanism of resistance to eribulin
The anti-tumour activity of the combination of eribulin plus a PI3K inhibitor observed both in PI3K-activated and wt models was unexpected. We posited that eribulin induced AKT activation, such as described for other chemotherapies, and this was counteracted by PI3K inhibition, thereby enhancing its anti-tumour response.19 In this sense, we observed that eribulin treatment induced the phosphorylation of AKT S473 and its downstream targets, including FOXO1/3A T24/32 and PRAS40 T243, in MCF10A PIK3CA-wt and H1047R in vitro (Fig. 4a). Long-term treatment with eribulin also resulted in supra-activation of pAKT (Fig. S2). Similarly, 5 out of 17 (29%) primary HER2−, stage I–II, breast tumours treated with eribulin showed higher pAKT S473-positive cells in post-treatment samples compared to pre-treatment levels (Fig. 4b, c).
Fig. 4. PI3K inhibition reverses secondary resistance to eribulin single agent and restores therapy response.
a Biochemical dose–response analysis of the PI3K/AKT pathway to eribulin treatment by Western blot in MCF10A cells harbouring PIK3CA wt or p.H1047R heterozygous mutation. b Analysis of pAKT S473 levels in tumour specimens by immunohistochemistry from patients treated with neoadjuvant eribulin in the NeoEribulin trial (NCT01669252). Tumour biopsies were harvested at screening (before therapy initiation), at day 28 of treatment (day 1 of cycle 2, 1 cycle: dosing at day 1 and day 8 every 21 days). A ×100 magnification was used to capture the pictures. c Quantification of (b). d Tumour growth of CAL51 xenografts treated with eribulin and BKM120 as single agents, or in combination upfront or after progression to eribulin single agent. e Tumour growth of PDX044 treated with eribulin and BYL719 as single agents, or in combination upfront or after progression to eribulin single agent. f Tumour growth of PDX127 treated with eribulin and BKM120 as single agents, or in combination upfront or after progression to eribulin single agent. Dashed lines indicated the threshold between PD and SD, no change from initial tumour volume and the threshold between SD and PR. The symbols * and # indicate statistically significant differences between the combination-treated arm (eribulin plus BKM120 or BYL719) and eribulin single agent or BKM120/BYL719 respectively. */#P < 0.05; **/##P < 0.01; ***P < 0.001; ****P < 0.0001.
In view of this adaptive response of the PI3K/AKT pathway upon treatment with eribulin, we presumed that addition of PI3K inhibitors to eribulin at disease progression would result in tumour growth control. Our results using three independent tumour models in vivo showed that addition of BKM120 or BYL719 to eribulin resulted in tumour regression or disease stabilisation (Fig. 4d–f). Overall, these results provide a rationale for combining eribulin with PI3K inhibitors in the clinical setting, either upfront or after progression to eribulin monotherapy.
PI3K inhibition sensitises to eribulin via downmodulation of p21
The primary mode of action of eribulin is the impairment of microtubule growth that results in mitotic arrest,43 but the mechanism whereby PI3K inhibition sensitises to eribulin in BC is unknown. We observed that treatment with eribulin resulted in an accumulation of the CDK inhibitor p21, at 0.5 nM (half-maximal effective doses, EC50, Fig. 5a).
Fig. 5. Downmodulation of p21 re-sensitises to eribulin treatment.
a Analysis of the PI3K/AKT pathway and p21 by Western blot in MCF10A harbouring PIK3CA wt or p.H1047R heterozygous mutation treated with eribulin, BKM120 and BYL719 treatment as a single agent or in combination. b Crystal violet staining of MCF10A cells harbouring PIK3CA wt or p.H1047R heterozygous mutation after 1 week of treatment with eribulin 1 nM in the presence or absence of short-hairpin RNA against CDKN1A, of one representative experiment.
Mechanistically, the addition of a PI3K inhibitor (BKM120 or BYL719) to eribulin-treated MCF10A cells resulted in reduction of pAKT T308 and S473, downmodulation of the AKT-downstream targets, including the eribulin-induced p21 upregulation, regardless of the PIK3CA status (Fig. 5a). We further unravelled if p21 modified eribulin response and we knocked down CDKN1A, the gene encoding for p21, by means of shRNA (Fig. S3B). As posited, reduction of p21 sensitised MCF10A cells to eribulin (Fig. 5b and Fig. S3B). Altogether, these results suggest that eribulin induces a pAKT/p21-dependent response and that the addition of a PI3K inhibitor may unleash the proapoptotic response.
Discussion
Eribulin has demonstrated clinical efficacy in late-line HER2− mBC.4 This study aimed to investigate if PI3K pathway activation is associated with an attenuated response to eribulin and if there is any benefit of combining it with PI3K inhibitors. Using cell line models and PDX models, we provide evidence that PI3K pathway activation is associated with eribulin resistance. In addition, we show that PI3K inhibition reverses primary resistance to eribulin by preventing eribulin-induced activation of the AKT/p21 axis, resulting in reduced proliferation as well as increased apoptosis.
The role of mutations activating the PI3K/AKT pathway on the response to chemotherapy in BC patients is controversial. Some studies indicated that PIK3CA mutation was associated with improved patients’ outcome, possibly due to its correlation with other prognostic factors such as ER+ status or patient’s older age at diagnosis.44,45 In this sense, results from the SAFIR02 trial (NCT02299999) suggest that the prognostic value of PIK3CA mutations depends on the BC subtype: while PIK3CA mutation correlates with resistance to chemotherapy and worse OS in ER+ HER2− BC, it correlates with improved OS in TNBC.46 Our data using a panel HER2− BC preclinical models show that mutations in PIK3CA, AKT1 and PIK3R1 loss are associated with eribulin resistance. Our results are also consistent with larger clinical studies reporting that patients with PIK3CA mutations had lower pathologic complete response rate to either anthracycline- or taxane-based therapies than those with wt (P = 0.035);47 and the marked increase of a hotspot PIK3CA mutation in a tumour after relapse to paclitaxel (14–34%).48 Regarding our retrospective analysis of the predictive value of PI3K/AKT pathway mutations in patients treated with eribulin, we highlight some limitations. First, the small sample size in both wt and mutant groups included. Second, we could not screen for other molecular alterations of the PI3K/AKT/mTOR pathway, such as PIK3R1 loss, encoding for the regulatory subunit of PI3K, TSC1/2 loss and MTOR mutations, which also activate the PI3K/AKT/MTOR pathway and may lead to eribulin resistance. Third, other prognostic factors, such as the line of eribulin treatment as well as previous therapies or the number of metastatic sites among others, could not be taken into account also due to the small sample size and may have an impact on the survival data of the cohort. Therefore, the predictive value of mutations in the PI3K/AKT pathway towards eribulin still remains a question of interest and warrants prospective evaluation.
PI3K inhibition reverses eribulin resistance and efficiently impairs tumour growth to promote tumour regression in xenograft models. Remarkably, we used a dose of BKM120 without off-target microtubule-destabilising activity.49 This is consistent with recent publications showing that PI3K and mTOR inhibitors synergise with eribulin in TNBC in the preclinical setting.29–31 Accordingly, a phase 1 trial testing the safety and preliminary activity of the combination of eribulin with the mTOR allosteric inhibitor everolimus has shown promising results with 18 out of 25 patients achieving either a partial response or a disease stabilisation as best response (NCT02120469).50 In this work, we have shown that the combination of eribulin and PI3K inhibition is also effective in ER+/HER2− tumours, hence expanding the applicability of this therapeutic strategy. However, the clinical response to other combinatorial therapies, namely using MTAs and anti-PI3K/AKT inhibitors, have shown discordant results. Two trials assessing the activity of adding buparlisib (BKM120) or capivasertib (an AKT1-3 inhibitor) to paclitaxel in HER2− BC patients did not show evidence of prolongation of PFS (BELLE-4 (NCT01572727) and BEECH (NCT01625286) trials, respectively22,51). On the other hand, two trials demonstrated that the addition of capivasertib or ipatasertib (another AKT inhibitor) to paclitaxel extended PFS in TNBCs (PAKT (NCT02423603) and LOTUS (NCT02162719)). Additionally, another trial (FAIRLANE (NCT02301988) trials) showed a trend for increased rates of pathological complete response rate to neoadjuvant treatment with the combination of paclitaxel and ipatasertib in patients with early TNBC.52–54 Because the first group of clinical trials recruited both TNBC and ER+ BC patients, it seems likely that ER-positivity may undermine the response to such combinations. In fact, inhibition of the PI3K/AKT pathway releases ER function and its transcriptional programme.55,56 Although the mechanisms of action of eribulin and paclitaxel to block microtubule dynamics slightly differ,57,58 we envision similar clinical outcomes for the combination of PI3K inhibitors and eribulin. Therefore, the promising combination of MTAs, such as paclitaxel or eribulin, with PI3K/AKT inhibitors may need to be complemented with endocrine therapy in ER+ tumours to prevent ER-driven resistances. In contrast with this observation, our preclinical study indicates that PI3K inhibition sensitises eribulin-resistant models and promotes tumour regressions, regardless of ER status or mutational profile of the PI3K/AKT pathway, suggesting that some endocrine-resistant tumours or PIK3CA wt tumours may also benefit from this drug combination.
Here, we have also shown that eribulin induces the activation of the PI3K/AKT survival pathway in BC tumour samples as previously described in breast, ovarian and soft tissue sarcoma cell lines.19,59,60 Consistently with Wallin et al., AKT is phosphorylated and translocated to the nucleus upon treatment with doxorubicin (Fig. 4b and ref. 19). We have shown that eribulin promotes pAKT S473, which has been reported to be a target of phosphorylation by DNA-dependent protein kinase in the nucleus in response to DNA stress.61 In our cell line models, the phosphorylation of AKT S473 coincides with p21 upregulation, as described after DNA damage and microtubule targeting.62,63
In conclusion, our study supports the clinical testing of eribulin and PI3K inhibitor combination upfront or after progression to eribulin monotherapy in HER2− metastatic BC patients, as well as the analysis of the predictive value of activating alterations in the PI3K/AKT/mTOR pathway.
Supplementary information
Acknowledgements
We are grateful to all the patients who kindly consented to the use of their tumours to develop this study and to all the personnel involved in sample collection from the Breast Surgical Unit, Breast Cancer Centre, and Department of Radiology Vall d’Hebron University Hospital and Molecular Oncology Group at Vall d’Hebron Institute of Oncology (VHIO). We also thank the Cellex Foundation for providing research facilities and equipment, as well as Ana Vivancos and the Cancer Genomics Group at VHIO and Joanne Soong and the Centre for Molecular Oncology at the MSKCC for providing technical and analytical support with the patient and PDX sequencing.
Author contributions
A.G.-O., J.C. and V.S. designed the experiments and wrote the manuscript. A.G.-O., Y.H.I, M.A.R., C.G.-G., M.S.-G., F.R.-P., C.V., J.M.P.-G., A.L.-C., J.G., M.P., M.G., O.R., P.A., P.C., M.T.C., A.B., J.A., C.C., R.D., P.N., M.O., J.C. and V.S. helped in the acquisition of data and/or provided technical support (provided animals, acquired and managed patients, provided facilities, etc.). A.G.-O., Y.H.I., M.A.R., C.G.-G., M.S.-G., F.R.-P., C.V., R.D., M.O., J.C. and V.S. contributed in the analysis of data (e.g. statistical analysis, biostatistics, computational analysis and construction of databases).
Ethics approval and consent to participate
Fresh tumour samples from patients with breast cancer were collected following an Institutional Research Board-approved protocol and the associated written informed consent. The study was compliant with the Declaration of Helsinki.
Experiments were conducted following the European Union’s animal care directive (2010/63/EU) and were approved by the Ethical Committee of Animal Experimentation of the Vall d’Hebron Research Institute, the Catalan Government or by the National Research Ethics Service, Cambridgeshire (ref. 35 and https://caldaslab.cruk.cam.ac.uk/bcape/).
Data availability
All data generated or analysed during this study are included in this published article [and its Supplementary information files].
Competing interests
V.S. declares non-commercial research agreements with Genentech and Novartis. J.C. reports consulting for Roche, Celgene, Cellestia, AstraZeneca, Biothera Pharmaceutical, Merus, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Servier, Merck Sharp&Dohme, GSK, Leuko, Bioasis, Clovis Oncology and Boehringer Ingelheim; honoraria for Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, Merck Sharp&Dohme and Daiichi Sankyo; research funding to the Institution: Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardanth Health, Merck Sharp&Dohme, Pfizer, Piqur Therapeutics, Puma C and Queen Mary University of London; stock, patents and intellectual property of MedSIR; and travel, accommodation and other expenses for Roche, Novartis, Eisai, Pfizer and Daiichi Sankyo. M.O. declares research support from AstraZeneca, Genentech and Philips Healthcare; consultant role for Roche, GSK, PUMA Biotechnology and AstraZeneca; and has received honoraria from Roche. M.S.-G. is on the scientific advisory board of Menarini Ricerche and the Bioscience Institute, has received research funds from Puma Biotechnology, Daiichi-Sankio, Targimmune, Immunomedics and Menarini Ricerche, and is a cofounder of Medendi.org. C.C. is a member of AstraZeneca’s External Science Panel, of Illumina’s Scientific Advisory Board and is a recipient of research grants (administered by the University of Cambridge) from AstraZeneca, Genentech, Roche and Servier. R.D. is on advisory role of AstraZeneca, Roche and Boehringer-Ingelheim and has received speaker’s fees from Roche, Symphogen, IPSEN, Amgen, Servier, Sanofi and MSD; and research support from Merck. J.M.P.-G. reports an advisory role with Roche and Lilly. A.L.-C. has been a consultant for Roche, GlaxoSmithKline, Novartis, Celgene, Eisai and AstraZeneca in the previous 12 months and has stock options for Medica Scientia Innovation Research SL (MedSIR). All other authors declare no competing interests.
Funding information
We acknowledge the GHD-Pink programme, the FERO Foundation and the Orozco Family for supporting this study [to V.S.], as well as Fundación Mútua Madrileña [to J.C.]. This study has also been supported by the Catalan Agency AGAUR [2017 SGR 540 to V.S.]. V.S. is supported by the Miguel Servet Programme (ISCIII) [CPII19/00033]. A.G.-O. was awarded with a fellowship from the Agència de Gestió d’Ajuts Universitaris i de Recerca (FI-AGAUR, 2015 FI_B 01075), and M.S.-G. with a Marie Slodowska-Curie Innovative Training Networks (MSCA-ITN) Ph.D. fellowship (H2020-MSCA-ITN-2015_675392). The xenograft programme in the Caldas Laboratory was supported by Cancer Research UK and also received funding from an EU H2020 Network of Excellence (EuroCAN).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Javier Cortés, Violeta Serra
Contributor Information
Javier Cortés, Email: jacortes@vhio.net.
Violeta Serra, Email: vserra@vhio.net.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01293-1.
References
- 1.Cardoso F, Spence D, Mertz S, Corneliussen-James D, Sabelko K, Gralow J, et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005–2015) Breast. 2018;39:131–138. doi: 10.1016/j.breast.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann. Oncol. 2018;29:1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balic M, Thomssen C, Würstlein R, Gnant M, Harbeck N. St. Gallen/Vienna 2019: a brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care. 2019;14:103–110. doi: 10.1159/000499931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): A phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre K, O’Shaughnessy J, Schwartzberg L, Glück S, Berrak E, Song JX, et al. Phase 2 study of eribulin mesylate as first-line therapy for locally recurrent or metastatic human epidermal growth factor receptor 2-negative breast cancer. Breast Cancer Res Treat. 2014;146:321–328. doi: 10.1007/s10549-014-2923-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong K-K, Engelman JA, Cantley LC. Targeting the PI3K pathway in cancer. Curr. Opin. Genet Dev. 2009;8:627–644. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: A function for each cell compartment? Trends Cell Biol. 2003;13:65–70. doi: 10.1016/s0962-8924(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 8.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 9.Blain SW. Switching cyclin D-Cdk4 kinase activity on and off. Cell Cycle. 2008;7:892–898. doi: 10.4161/cc.7.7.5637. [DOI] [PubMed] [Google Scholar]
- 10.Labaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 11.Reynisdóttir I, Massagué J. The subcellular locations of pl5(Ink4b) and p27(Kip1) coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 12.Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, et al. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell. 1995;6:387–p400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki A, Kawano H, Hayashida M, Hayasaki Y, Tsutomi Y, Akahane K. Procaspase 3/p21 complex formation to resist Fas-mediated cell death is initiated as a result of the phosphorylation of p21 by protein kinase A. Cell Death Differ. 2000;7:721–728. doi: 10.1038/sj.cdd.4400706. [DOI] [PubMed] [Google Scholar]
- 14.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.López-Knowles E, O’Toole SA, McNeil CM, Millar EKA, Qiu MR, Crea P, et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int. J. Cancer. 2010;126:1121–1131. doi: 10.1002/ijc.24831. [DOI] [PubMed] [Google Scholar]
- 16.Bareche Y, Venet D, Ignatiadis M, Aftimos P, Piccart M, Rothe F, et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann. Oncol. 2018;29:895–902. doi: 10.1093/annonc/mdy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang WC, Hung MC. Induction of Akt activity by chemotherapy confers acquired resistance. J. Formos. Med. Assoc. 2009;108:180–194. doi: 10.1016/S0929-6646(09)60051-6. [DOI] [PubMed] [Google Scholar]
- 19.Wallin JJ, Guan J, Prior WW, Edgar KA, Kassees R, Sampath D, et al. Nuclear phospho-Akt increase predicts synergy of PI3K inhibition and doxorubicin in breast and ovarian cancer. Sci. Transl. Med. 2010;2:1–9. doi: 10.1126/scitranslmed.3000630. [DOI] [PubMed] [Google Scholar]
- 20.Clark AS, West K, Streicher S, Dennis PA, Brueggemeier RW, Shapiro CL, et al. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol. Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- 21.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 22.Martín M, Chan A, Dirix L, O’Shaughnessy J, Hegg R, Manikhas A, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4) Ann. Oncol. 2017;28:313–320. doi: 10.1093/annonc/mdw562. [DOI] [PubMed] [Google Scholar]
- 23.Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 24.VanderWeele DJ, Zhou R, Rudin CM. Akt up-regulation increases resistance to microtubule-directed chemotherapeutic agents through mammalian target of rapamycin. Mol. Cancer Ther. 2004;3:1605–1613. [PubMed] [Google Scholar]
- 25.Fujiwara Y, Hosokawa Y, Watanabe K, Tanimura S, Ozaki K-I, Kohno M. Blockade of the phosphatidylinositol-3-kinase-Akt signaling pathway enhances the induction of apoptosis by microtubule-destabilizing agents in tumor cells in which the pathway is constitutively activated. Mol. Cancer Ther. 2007;6:1133–1142. doi: 10.1158/1535-7163.MCT-06-0639. [DOI] [PubMed] [Google Scholar]
- 26.Wallin JJ, Guan J, Prior WW, Lee LB, Berry L, Belmont LD, et al. GDC-0941, a novel class I selective PI3K inhibitor, enhances the efficacy of docetaxel in human breast cancer models by increasing cell death in vitro and in vivo. Clin. Cancer Res. 2012;18:3901–3911. doi: 10.1158/1078-0432.CCR-11-2088. [DOI] [PubMed] [Google Scholar]
- 27.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 28.McDonald GT, Sullivan R, Paré GC, Graham CH. Inhibition of phosphatidylinositol 3-kinase promotes tumor cell resistance to chemotherapeutic agents via a mechanism involving delay in cell cycle progression. Exp. Cell Res. 2010;316:3197–3206. doi: 10.1016/j.yexcr.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Rajput S, Guo Z, Li S, Ma CX. PI3K inhibition enhances the anti-tumor effect of eribulin in triple negative breast cancer. Oncotarget. 2019;10:3667–3680. [PMC free article] [PubMed] [Google Scholar]
- 30.Owusu-Brackett N, Kenerson HL, Riggle KM, Turnham R, Sullivan K, Bauer R, et al. TAK228 enhances antitumor activity of eribulin in triple negative breast cancer. Oncotarget. 2019;10:5011–5019. doi: 10.18632/oncotarget.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen W, Marcinkowski E, Luyimbazi D, Luu T, Xing Q, Yan J, et al. Eribulin synergistically increases anti-tumor activity of an mTOR inhibitor by inhibiting pAKT/pS6K/pS6 in triple negative breast. Cancer Cells. 2019;8:1–15. doi: 10.3390/cells8091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beaufils F, Cmiljanovic N, Cmiljanovic V, Bohnacker T, Melone A, Marone R, et al. 5-(4,6-Dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine (PQR309), a potent, brain-penetrant, orally bioavailable, pan-class i PI3K/mTOR inhibitor as clinical candidate in oncology. J. Med. Chem. 2017;60:7524–7538. doi: 10.1021/acs.jmedchem.7b00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong JP, Traganos F, Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal. Biochem. 1994;218:314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- 34.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J. Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruna A, Rueda OM, Greenwood W, Batra AS, Callari M, Batra RN, et al. A Biobank of breast cancer explants with preserved intra-tumor heterogeneity to screen anticancer compounds. Cell. 2016;167:260–274.e22. doi: 10.1016/j.cell.2016.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tentler JJ, Tan AC, Weekes CD, Jimeno A, Leong S, Pitts TM, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Festing MFW. Design and statistical methods in studies using animal models of development. ILAR J. 2006;47:5–14. doi: 10.1093/ilar.47.1.5. [DOI] [PubMed] [Google Scholar]
- 38.Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015;21:1318–1325. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 39.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 40.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial Sloan Kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature. 2015;518:240–244. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wee S, Wiederschain D, Maira S-M, Loo A, Miller C, DeBeaumont R, et al. PTEN-deficient cancers depend on PIK3CB. Proc. Natl Acad. Sci. USA. 2008;105:13057–13062. doi: 10.1073/pnas.0802655105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jordan MA. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 2005;4:1086–1095. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- 44.Kalinsky K, Jacks LM, Heguy A, Patil S, Drobnjak M, Bhanot UK, et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin. Cancer Res. 2009;15:5049–5059. doi: 10.1158/1078-0432.CCR-09-0632. [DOI] [PubMed] [Google Scholar]
- 45.Zardavas D, Te Marvelde L, Milne RL, Fumagalli D, Fountzilas G, Kotoula V, et al. Tumor PIK3CA genotype and prognosis in early-stage breast cancer: a pooled analysis of individual patient data. J. Clin. Oncol. 2018;36:981–990. doi: 10.1200/JCO.2017.74.8301. [DOI] [PubMed] [Google Scholar]
- 46.Mosele F, Stefanovska B, Lusque A, Tran Dien A, Garberis I, Droin N, et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann. Oncol. 2020;31:377–386. doi: 10.1016/j.annonc.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Yuan H, Chen J, Liu Y, Ouyang T, Li J, Wang T, et al. Association of PIK3CA mutation status before and after neoadjuvant chemotherapy with response to chemotherapy in women with breast cancer. Clin. Cancer Res. 2015;21:4365–4372. doi: 10.1158/1078-0432.CCR-14-3354. [DOI] [PubMed] [Google Scholar]
- 48.Murtaza M, Dawson SJ, Tsui DWY, Gale D, Forshew T, Piskorz AM, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 49.Brachmann SM, Kleylein-Sohn J, Gaulis S, Kauffmann A, Blommers MJJ, Kazic-Legueux M, et al. Characterization of the mechanism of action of the pan class I PI3K inhibitor NVP-BKM120 across a broad range of concentrations. Mol. Cancer Ther. 2012;11:1747–1757. doi: 10.1158/1535-7163.MCT-11-1021. [DOI] [PubMed] [Google Scholar]
- 50.Lee JS, Yost SE, Blanchard S, Schmolze D, Yin HH, Pillai R, et al. Phase I clinical trial of the combination of eribulin and everolimus in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2019;21:1–13. doi: 10.1186/s13058-019-1202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner NC, Alarcón E, Armstrong AC, Philco M, López Chuken YA, Sablin M-P, et al. BEECH: a dose-finding run-in followed by a randomised phase II study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with estrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA. Ann. Oncol. 2019;30:774–780. doi: 10.1093/annonc/mdz086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmid P, Abraham J, Chan S, Wheatley D, Brunt M, Nemsadze G, et al. AZD5363 plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (PAKT): a randomised, double-blind, placebo-controlled, phase II trial. J. Clin. Oncol. 2018;36(Suppl.):1007–1007. [Google Scholar]
- 53.Kim S-B, Dent R, Im S-A, Espié M, Blau S, Tan AR, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18:1360–1372. doi: 10.1016/S1470-2045(17)30450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliveira M, Saura C, Nuciforo P, Calvo I, Andersen J, Gil M. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann. Oncol. 2019;30:1289–1297. doi: 10.1093/annonc/mdz177. [DOI] [PubMed] [Google Scholar]
- 55.Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci. Transl. Med. 2015;7:283ra51. doi: 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toska E, Osmanbeyoglu HU, Castel P, Chan C, Hendrickson RC, Elkabets M, et al. PI3K pathway regulates ER-dependent transcription in breast cancer through the epigenetic regulator KMT2D. Science. 2017;335:1324–1330. doi: 10.1126/science.aah6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain S, Vahdat LT. Eribulin mesylate. Clin. Cancer Res. 2011;17:6615–6622. doi: 10.1158/1078-0432.CCR-11-1807. [DOI] [PubMed] [Google Scholar]
- 58.Agoulnik S, Kuznetsov G, Tendyke K, Parent LA, Marsh JP, Twine N, et al. Sensitivity to halichondrin analog E7389 and hemiasterlin analog E7974 correlates with βIII tubulin isotype expression in human breast cancer cell lines. J. Clin. Oncol. 2017;23(Suppl.):2012–2012. [Google Scholar]
- 59.Mabuchi S, Ohmichi M, Kimura A, Hisamoto K, Hayakawa J, Nishio Y, et al. Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J. Biol. Chem. 2002;277:33490–33500. doi: 10.1074/jbc.M204042200. [DOI] [PubMed] [Google Scholar]
- 60.Hayasaka N, Takada K, Nakamura H, Arihara Y, Kawano Y, Osuga T, et al. Combination of eribulin plus AKT inhibitor evokes synergistic cytotoxicity in soft tissue sarcoma cells. Sci. Rep. 2019;9:1–8. doi: 10.1038/s41598-019-42300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBα/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol. Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 62.Mitsuuchi Y, Johnson SW, Selvakumaran M, Williams SJ, Hamilton TC, Testa JR. The phosphatidylinositol 3-kinase/AKT signal transduction pathway plays a critical role in the expression of p21(WAF1/CIP1/SDI1) induced by cisplatin and paclitaxel. Cancer Res. 2000;60:5390–5394. [PubMed] [Google Scholar]
- 63.Charrier-Savournin FB, Château MT, Gire V, Sedivy J, Piette J, Dulić V. p21-mediated nuclear retention of cyclin B1-Cdk1 in response to genotoxic stress. Mol. Biol. Cell. 2004;15:3965–3976. doi: 10.1091/mbc.E03-12-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its Supplementary information files].