Abstract
Coronavirus disease 2019 (COVID-19) has substantially challenged healthcare systems worldwide. By investigating population characteristics and prescribing profiles, it is possible to generate hypotheses about the associations between specific drug-utilisation profiles and susceptibility to COVID-19 infection. A retrospective drug-utilisation study was carried out using routinely collected information from a healthcare database in Campania (Southern Italy). We aimed to discover the prevalence of drug utilisation (monotherapy and polytherapy) in COVID-19 versus non-COVID-19 patients in Campania (~ 6 million inhabitants). The study cohort comprised 1532 individuals who tested positive for COVID-19. Drugs were grouped according to the Anatomical Therapeutic Chemical (ATC) classification system. We noted higher prevalence rates of the use of drugs in the ATC categories C01, B01 and M04, which was probably linked to related comorbidities (i.e., cardiovascular and metabolic). Nevertheless, the prevalence of the use of drugs acting on the renin-angiotensin system, such as antihypertensive drugs, was not higher in COVID-19 patients than in non-COVID-19 patients after adjustments for age and sex. These results highlight the need for further case–control studies to define the effects of medications and comorbidities on susceptibility to and associated mortality from COVID-19.
Subject terms: Epidemiology, Outcomes research, Health policy, Infectious diseases
Introduction
As of 24 April 2020, there has been ~ 3,000,000 coronavirus disease 2019 (COVID-19) cases and > 200,000 associated deaths worldwide1. COVID-19 is very contagious and has a wide spectrum of presentations. COVID-19 symptoms can range from no symptoms to severe illness, and the disease includes three phases (i.e., viral infection, pulmonary, hyperinflammation/systemic phases)2. Ageing and underlying diseases (e.g., heart disease, diabetes mellitus) have been reported to be risk factors for adverse outcomes, and male sex and a genetic predisposition to infection are under investigation as potential contributors3–7. Moreover, initial reports suggested a potential pro-infective effect of drugs. Two classes of drugs that have been implicated are angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs). These effects may be due to the interaction between the virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and ACE-2 receptors in the lungs, though this theory is controversial8–12.
There is a lack of data on drug use (monotherapy and polytherapy) in COVID-19 patients. The main aims of this study were to (1) discover the prevalence of drug utilisation (monotherapy and polytherapy) in COVID-19 versus non-COVID-19 patients in Campania, southern Italy and (2) ascertain the epidemiology and profiles of affected patients in relation to drug utilisation.
Methods
Study design
A retrospective drug-utilisation study was carried out using routinely collected information from healthcare databases in Campania. The Campania Region Database (CaReDB) includes information on patient demographics and the electronic records of outpatient pharmacy dispensing for ~ 6 million residents, comprising a well-defined population in Italy (~ 10% of the population of Italy). CaReDB is complete and includes data that has been validated in previous drug-utilisation studies13–20. The characteristics of CaReDB are described in Supplementary Table S1.
At the beginning of the COVID-19 epidemic, a surveillance system was implemented to collect the data of all cases identified by reverse transcription-polymerase chain reaction (RT-PCR) testing for SARS-CoV-2. These archives are linked together by a unique anonymous identifier that is encrypted to protect patient privacy. Our research protocol adhered to the tenets of the Declaration of Helsinki 1975 and its later amendments. Permission to use anonymised data for this study was granted to the researchers of the Centro di Ricerca in Farmacoeconomia e Farmacoutilizzazione (CIRFF) by the governance board of Unità del Farmaco della Regione Campania. The research did not involve a clinical study, and all patients’ data were fully anonymised and were analysed retrospectively. For this type of study, formal consent was not required according current national established by the Italian Medicines Agency, and according to the Italian Data Protection Authority, neither ethical committee approval nor informed consent was required for our study21.
Study population
People who had been dispensed medication according to CaReDB during 2019 were included in the study cohort. From regional surveillance system data, we obtained the information of patients with confirmed COVID-19 from the beginning of the epidemic (26 February 2020) to 30 March 2020 who were linked to the population identified in CaReDB. For the purposes of our investigation, the study population diagnosed with SARS-CoV-2 infection on or before the date of analysis was referred to as the ‘COVID-19 group’ (C19G). The remaining individuals were used as a comparator group in the analysis and were referred to as the ‘general population group’ (GPG).
Patient characteristics
The study population was categorised by sex and subdivided into four age groups: 0–39; 40–59; 60–79; and ≥ 80 years. The number of drug prescriptions, prevalence of drug use and polypharmacy regimens (classified as ‘no-polypharmacy’; ‘polypharmacy’; and ‘excessive polypharmacy’) were ascertained in 2019. Drugs were grouped according to the Anatomical Therapeutic Chemical (ATC) classification system. ATC II and ATC IV codes with a prevalence ≥ 3% in the C19G were included in the analysis.
Outcome
The drug-utilisation profile was evaluated as the prevalence of drug use. Drug use prevalence was estimated as the number of individuals dispensed ≥ 1 drug prescription per 100 inhabitants in 2019. The prevalence of drug use was evaluated in the C19G and GPG. Prevalence was stratified by age group and sex. Prevalence was probably influenced by the heterogeneous demographic distribution among the age groups, so we conducted direct standardisation.
Statistical methods
The baseline characteristics of the study population were analysed using descriptive statistics. Quantitative variables are described as means ± standard deviations. Categorical variables are described as counts and percentages. The chi-square test and t-test were performed to determine the difference between the C19G and GPG in terms of sex and age. A P-value of < 0.05 was considered significant. Crude and age-adjusted prevalence rates were calculated. Differences in the prevalence between the C19G and GPG are expressed as risk ratios (RRs) adjusted for sex and age with 95% confidence intervals (CIs). Standardisation was performed using a direct method whereby the Italian population up to 1 January 2019 was used as the standard population (available on the Demo Istat website22).
where (Ti = ni/n) = rate in stratum ‘i’ of the study population; ni = number of cases in stratum ‘i’ of the study population; N = size of the study population in stratum ‘i’; wi = size of stratum ‘i’ of the reference population; m = number of considered strata; k = multiplicative constant.
The age-adjusted RRs and 95% CIs were computed using standard methods. Data management was performed with SQL server v2018 (Microsoft, Redmond, WA, USA). Analyses were carried out with SPSS v17.1 (IBM, Armonk, NY, USA).
Results
C19G characteristics
A total of 1,532 individuals in Campania who tested positive for COVID-19 on 30 March 2020 were identified. Of these, 926 (60.4%) were males, and the median age of the entire sample was 55 ± 19 years. Among the C19G patients, 20.8% were aged 0–39 years, 36.1% were aged 40–59 years, 33.6% were aged 60–79 years, and 9.5% were aged > 80 years. The percentage of males was higher than that of females in all age groups except the > 80 years age group (43.8% males). Differences in age and the sex ratio between the C19G and GPG were statistically significant (p-value < 0.001).
The prevalence of drug use among the C19G was 74.5% and increased with age, reaching 93.8% in those aged > 80 years. The median number of prescriptions per patient (overall: 16 [interquartile range, IQR]: 5–42) ranged from 3 (IQR, 1–6) among people aged 0–39 years to 51 (IQR, 29–71) among individuals aged > 80 years.
Half of the COVID-19 patients aged 0–39 years had no exposure to any medication, whereas 45.5% of the COVID-19 patients were prescribed ≤ 4 medications, and 4.1% had polypharmacy regimens (5–9 drugs). The percentage of participants receiving polypharmacy increased with increasing age, at 18.3% in those aged 40–59 years and 34.8% in those aged 60–79 years; moreover, ~ 80% of participants aged > 80 years were prescribed polypharmacy or excessive polypharmacy (≥ 10 drugs) regimens. The C19G characteristics are shown in Table 1.
Table 1.
Characteristics of the COVID-19 population.
| Overall 1532 |
Age groups N (%) | ||||
|---|---|---|---|---|---|
| 0–39 years 319 (20.8) |
40–59 years 553 (36.1) |
60–79 years 514 (33.6) |
≥ 80 years 146 (9.5) |
||
| Sex N (%) | |||||
| Male | 926 (60.4) | 189 (59.2) | 335 (60.6) | 338 (65.8) | 64 (43.8) |
| Female | 606 (39.6) | 130 (40.8) | 218 (39.4) | 176 (34.2) | 82 (56.2) |
| Mean age ± SD | 55 (± 19) | 27 (± 9) | 51 (± 5) | 68 (± 6) | 85 (± 4) |
| Prevalence of drug use (%) | 74.54 | 49.53 | 69.98 | 89.49 | 93.84 |
| Median number of prescriptions (IQR) | 16 (5–42) | 3 (1–6) | 9 (3–20) | 28 (13–54) | 51 (29–71) |
| Polypharmacy group, N (%) | |||||
| 0 drugs | 387 (25.5) | 161 (50.5) | 163 (29.5) | 54 (10.5) | 9 (6.2) |
| No polypharmacy (1–4 drugs) | 600 (39.2) | 145 (45.5) | 264 (47.7) | 168 (32.7) | 23 (15.8) |
| Polypharmacy (5–9 drugs) | 351 (22.9) | 13 (4.1) | 101 (18.3) | 179 (34.8) | 58 (39.7) |
| Excessive polypharmacy (≥ 10 drugs) | 194 (12.7) | – | 25 (4.5) | 113 (22.0) | 56 (38.4) |
Drug-utilisation profiles of the C19G
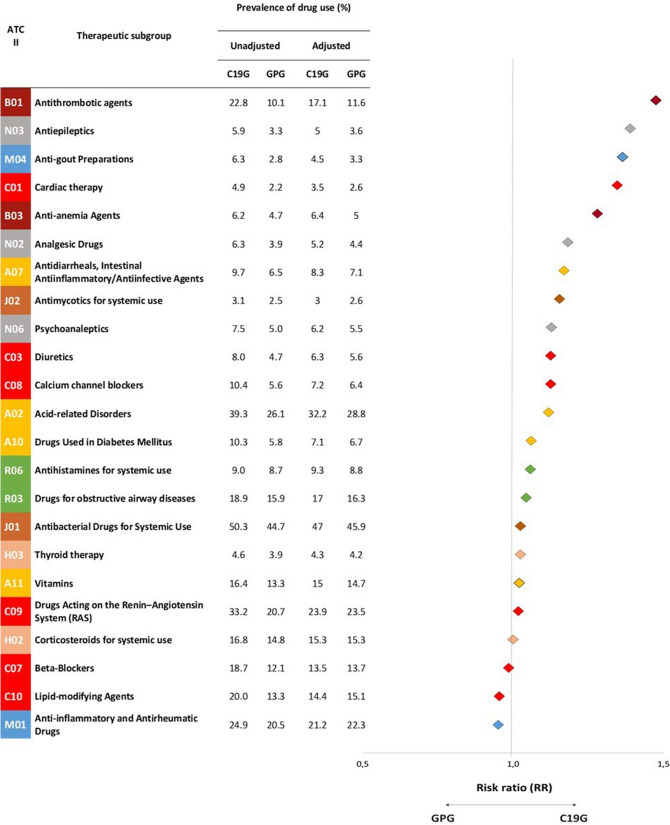
Twenty-three pharmacological ATC II groups and 39 ATC IV groups had a prevalence > 3% in the C19G. The highest unadjusted and adjusted prevalence rates of drug use in the ATC II groups were observed for drug categories J01, A02, C09, M01, B01 and R03 in the C19G and GPG (Fig. 1).
Figure 1.
Differences in prevalence of drug use between the C19G and GPG according to Therapeutic Group (ATC II). C19G COVID-19 group; GPG general population group.
Crude differences (at least ± 20% in the overall prevalence of drug use between the C19G and GPG) were found in all 23 pharmacological ATC II groups and in 30 of 39 ATC IV groups included in the analysis (Fig. 1, Table 2). After adjustment, differences remained in six ATC II groups and eight ATC IV groups. With respect to drugs acting on the renin–angiotensin system (RAS) (C09), including beta-blockers (C07), antibacterial drugs for systemic use (J01) and anti-inflammatory and antirheumatic drugs (M01), the differences disappeared after adjustment. The large differences in antithrombotic agents (B01), cardiac therapy drugs (C01) and anti-epileptics (N03) diminished after adjustment, even though they were more common in the C19G than in the GPG after adjustment.
Table 2.
Differences in prevalence of drug use between the C19G and GPG according to Chemical Subgroup (ATC IV).
| ATC IV | Chemical subgroup | Prevalence of drug use (%) | Adjusted RR C19G/GPG (95%CI) | |||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | |||||
| C19G | GPG | C19G | GPG | |||
| A02AD | Aluminium, calcium and magnesium | 4.6 | 3.1 | 3.7 | 3.4 | 1.10 (1.099–1.109) |
| A02BC | Proton pump inhibitors | 36.8 | 23.4 | 29.6 | 26.0 | 1.14 (1.136–1.140) |
| A02BX | Other drugs for peptic ulcers | 6.9 | 5.1 | 6.1 | 5.5 | 1.10 (1.098–1.106) |
| A07AA | Antibiotics | 7.2 | 5.4 | 6.1 | 5.9 | 1.03 (1.026–1.033) |
| A10BA | Biguanides | 6.9 | 3.8 | 4.6 | 4.3 | 1.09 (1.083–1.092) |
| A11CC | Vitamin D and analogues | 16.4 | 13.3 | 15.0 | 14.7 | 1.02 (1.016–1.021) |
| B01AB | Heparin group | 5.2 | 2.2 | 4.7 | 2.5 | 1.88 (1.874–1.895) |
| B01AC | Platelet-aggregation inhibitors | 17.2 | 8.1 | 12.2 | 9.4 | 1.29 (1.286–1.294) |
| B03BB | Folic acid and derivatives | 4.0 | 2.8 | 3.9 | 3.0 | 1.31 (1.303–1.316) |
| C03CA | Sulfonamides | 5.9 | 3.6 | 4.7 | 4.4 | 1.07 (1.063–1.072) |
| C07AB | β-blocking agents, selective | 14.8 | 9.3 | 10.5 | 10.6 | 0.99 (0.988–0.994) |
| C08CA | Dihydropyridine derivatives | 9.6 | 5.2 | 6.7 | 6.0 | 1.11 (1.105–1.113) |
| C09AA | ACE inhibitors | 8.8 | 5.9 | 6.1 | 6.7 | 0.91 (0.902–0.909) |
| C09BA | ACE inhibitors and diuretics | 5.0 | 3.2 | 3.6 | 3.7 | 0.97 (0.962–0.971) |
| C09CA | Angiotensin-II receptor blockers | 10.2 | 5.7 | 7.4 | 6.5 | 1.13 (1.129–1.137) |
| C09DA | Angiotensin-II receptor blockers and diuretics | 8.6 | 5.2 | 6.5 | 5.9 | 1.10 (1.099–1.107) |
| C10AA | HMG CoA reductase inhibitors | 17.0 | 11.5 | 12.1 | 13.1 | 0.92 (0.922–0.926) |
| H02AB | Glucocorticoids | 16.8 | 14.8 | 15.3 | 15.3 | 1.00 (1.001–1.006) |
| H03AA | Thyroid hormones | 4.2 | 3.6 | 4.0 | 3.8 | 1.05 (1.044–1.053) |
| J01CA | Penicillins with extended spectrum | 3.7 | 4.0 | 3.4 | 4.1 | 0.83 (0.831–0.838) |
| J01CR | Combinations of penicillins | 22.8 | 21.3 | 21.2 | 21.8 | 0.97 (0.970–0.973) |
| J01DD | Third-generation cephalosporins | 16.8 | 13.4 | 15.5 | 14.1 | 1.10 (1.097–1.102) |
| J01FA | Macrolides | 14.2 | 12.7 | 13.8 | 12.9 | 1.07 (1.067–1.072) |
| J01MA | Fluoroquinolones | 14.6 | 10.1 | 12.0 | 11.0 | 1.09 (1.082–1.088) |
| J01XX | Other antibacterials | 5.6 | 4.5 | 5.4 | 4.9 | 1.11 (1.101–1.110) |
| J02AC | Antimycotic for systemic use | 3.1 | 2.5 | 3.0 | 2.6 | 1.17 (1.160–1.172) |
| M01AB | Acetic acid derivatives | 10.8 | 8.3 | 9.0 | 9.1 | 1.00 (0.994–1.000) |
| M01AE | Propionic acid derivatives | 12.3 | 10.8 | 10.7 | 11.7 | 0.92 (0.913–0.918) |
| M01AH | Coxibs | 4.1 | 3.2 | 3.3 | 3.5 | 0.94 (0.938–0.947) |
| M01AX | Other anti-inflammatory and antirheumatic agents, non-steroidal anti-inflammatory drugs | 4.0 | 4.3 | 3.0 | 4.7 | 0.63 (0.632–0.637) |
| M04AA | Preparations inhibiting uric acid | 5.9 | 2.7 | 4.2 | 3.2 | 1.29 (1.286–1.299) |
| N03AX | Other antiepileptics | 4.0 | 2.3 | 3.4 | 2.6 | 1.30 (1.294–1.308) |
| N06AB | Selective serotonin reuptake inhibitors | 3.9 | 3.4 | 3.3 | 3.8 | 0.86 (0.853–0.860) |
| N06AX | Other antidepressants | 3.8 | 1.7 | 3.0 | 2.0 | 1.54 (1.531–1.550) |
| R03AK | Adrenergics in combination with corticosteroids | 5.9 | 4.2 | 4.8 | 4.5 | 1.06 (1.058–1.066) |
| R03BA | Glucocorticoids | 11.2 | 10.4 | 10.7 | 10.3 | 1.03 (1.030–1.036) |
| R03BB | Anticholinergics | 4.0 | 1.9 | 2.8 | 2.2 | 1.25 (1.241–1.256) |
| R06AE | Piperazine derivatives | 4.8 | 4.5 | 5.0 | 4.6 | 1.10 (1.093–1.101) |
| R06AX | Other antihistamines for systemic use | 4.6 | 4.6 | 4.8 | 4.7 | 1.02 (1.016–1.023) |
C19G COVID-19 group; CI confidence interval; GPG general population group; RR, risk ratio.
ATC A: drugs targeting the alimentary tract and metabolism
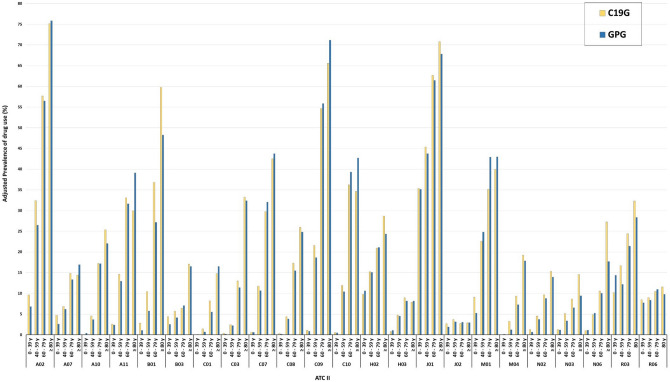
Drugs for acid-related disorders (ATC II: A02) had adjusted prevalence rates of 32.2% in the C19G and 28.8% in the GPG (RR, 1.12; 95% CI, 1.116–1.120) (Fig. 1). This difference increased mainly in those aged 40–59 years (32.4% vs. 26.5%; RR, 1.22) (Fig. 2). Regarding chemical subgroups, proton pump inhibitor (ATC IV: A02BC) use had a higher prevalence in the C19G than in the GPG, mainly in those aged 0–39 years (6.8% vs. 5.2%; RR, 1.36) and 40–59 years (30.1% vs. 22.8%; RR, 1.32) (Supplementary Tables S4, S5). The difference in the prevalence of drug use for diabetes mellitus (ATC II: A10) between the C19G and GPG after adjustment was very small. With regard to ATC IV, biguanide (A10BA) use had a higher prevalence in the C19G than in the GPG, mainly in those aged ≥ 80 years (14.6% vs. 10.7%; RR, 1.36) (Supplementary Tables S4, S5).
Figure 2.
Prevalence of drug use between the C19G and GPG stratified by age group. C19G COVID-19 group; GPG general population group
ATC B: drugs targeting blood and blood-forming organs
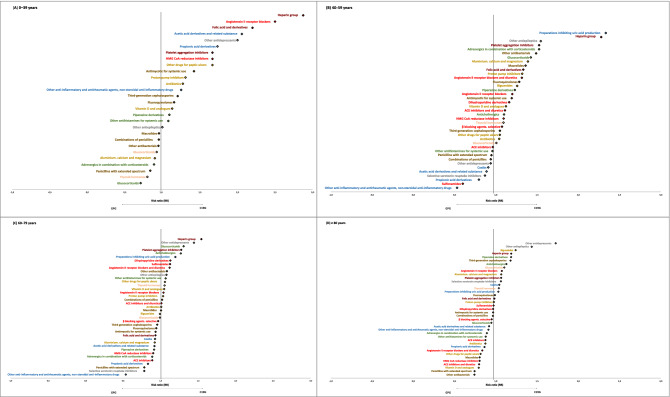
Antithrombotic agents (ATC II: B01) was the therapeutic group with the highest adjusted prevalence difference between the C19G and GPG (17.1% vs. 11.6%; RR: 1.47; 95% CI: 1.467–1.475) (Fig. 1). All age groups showed differences in adjusted prevalence rates between the C19G and GPG, with higher RRs observed in the younger age groups (Supplementary Tables S2, S3). An identical trend was observed for ATC IV. Heparin (B01AB) and platelet aggregation inhibitor (B01AC) use had higher adjusted prevalence rates in the C19G than in the GPG, with higher RRs in participants < 60 years of age (heparin: RR, 3.19 for 0–39 years and RR, 2.27 for 40–59 years; platelet aggregation inhibitors: RR, 1.94 for 0–39 years and RR, 1.52 for 40–59 years) (Fig. 3). Folic acid and derivative (B03BB) use had a higher prevalence in the C19G than in the GPG, mainly in those aged 0–39 years (3.3% vs. 1.5%; RR, 2.22) (Supplementary Tables S4, S5).
Figure 3.
Chemical Subgroup of the C19G with the highest adjusted relative differences in prevalence stratified by age group. (A) Patients aged 0–39 years. (B) Patients aged 40–59 years. (C) Patients aged 60–79 years. (D) Patients aged > 80.
ATC C: drugs targeting the cardiovascular system
Among drugs targeting the cardiovascular system, cardiac therapy (ATC II: C01) use had the largest differences in adjusted prevalence rates between the C19G and GPG overall and in each age group; the difference decreased with age (0–39 years: RR, 4.63; 40–59 years: RR, 2.09; 60–79 years: RR, 1.50) (Supplementary Table S3).
The other ATC II therapeutic group that pertained to the cardiovascular system did not show a relevant difference in the overall adjusted prevalence between the C19G and GPG (Fig. 1). Nevertheless, after stratification by age group, a higher RR (C19G/GPG) in people aged < 60 years was noted. In those older than 80 years, the differences disappeared or reversed, specifically for agents acting on the RAS (ATC II: C09) and lipid-modifying agents (ATC II: C10) (65.6% vs. 71.2% and 34.6% vs. 42.7% in the C19G vs. GPG, respectively) (Fig. 2).
ATC J: anti-infectives for systemic use
Relevant differences were not observed in the overall adjusted prevalence between the C19G and GPG for the therapeutic group (ATC II) included in this drug category (Fig. 1). Nevertheless, focusing on chemical subgroups (ATC IV), among people less than 40 years of age, third-generation cephalosporin (J01DD) use had a higher prevalence in the C19G than in the GPG (11.8% vs. 9.8%; RR, 1.20). In the 40–59 years group, macrolide (J01FA) and fluoroquinolone (J01MA) use had higher prevalence rates in the C19G group than in the GPG group (16.2% vs. 11.9%, RR, 1.37; 13.1% vs. 10.2%, RR, 1.29, respectively). Among those aged > 80 years, third-generation cephalosporin (J01DD) use had a higher prevalence in the C19G than in the GPG (37.3% vs. 29.1%, RR, 1.28) (Fig. 3 and Supplementary Tables S4, S5).
With regard to anti-mycotics for systemic use (ATC IV: J02AC), a large sex difference in the overall adjusted prevalence in the C19G was noted (male RR: 1.41) (Supplementary Tables S5).
ATC M: drugs targeting the musculoskeletal system
Regarding anti-inflammatory and antirheumatic drug (ATC II: M01) use, no significant differences were observed in the overall adjusted prevalence rates between the C10G and CPG (Fig. 1). Focusing on chemical subgroups (ATC IV), acetic acid derivative and related substance (M01AB; RR, 2.07) use and propionic acid derivative (M01AE; RR, 1.75) use had a higher prevalence rates in those aged > 40 years (Fig. 3).
Anti-gout preparation (ATC II: M04) use had adjusted prevalence rates of 4.5% in the C19G and 3.3% in the GPG (RR, 1.37; 95% CI, 1.36–1.37) (Fig. 1). A large sex difference in the overall adjusted prevalence in the C19G was observed (female RR, 1.55) (Supplementary Table S3.
Focusing on chemical subgroups (ATC IV), use of preparations inhibiting uric acid production (M04AA) had a higher prevalence in the C19G in those aged 40–59 years (2.8% vs. 1.2%; RR, 2.36) and 60–79 years (8.5% vs. 7.1%; RR, 1.21) (Supplementary Tables S4, S5).
ATC N: drugs targeting the nervous system
Among drugs targeting the nervous system, anti-epileptic (ATC II: N03) use had the largest prevalence difference between the C19G and GPG (5.0% vs. 3.6%; RR, 1.39) (Fig. 1). For the pertaining chemical subgroup of other anti-epileptics (ATC VI: N03AX), the RR in COVID-19 patients increased with age, reaching its highest value in those aged > 80 years (11.7% vs. 7.2%; RR, 1.62) (Supplementary Tables S4, S5). Psychoanaleptic (ATC II: N06) use had adjusted prevalence rates of 6.2% in the C19G and 5.5% in the GPG (RR, 1.12; 95% CI, 1.114–1.122) (Fig. 1).
Focusing on chemical subgroups, other antidepressant (ATC IV: N06AX) use had a high prevalence in COVID-19 patients in all age groups except for those aged 40–59 years (Fig. 3).
Sex differences were observed for analgesic drug (N02) use (male RR, 1.41), other anti-epileptic (N03AX) use (female RR, 1.55) and selective serotonin reuptake inhibitor (N06AB) use (male RR, 0.67) (Supplementary Tables S3, S5).
ATC R: drugs targeting the respiratory system
Marked differences in the prevalence of therapeutic group (ATC II) use were not observed between the C19G and GPG (Fig. 1).
However, focusing on chemical subgroups (ATC IV), inhaled anticholinergic agent (R03BB) use had a larger sex difference in the overall adjusted prevalence in the C19G (male RR, 1.44) (Supplementary Table S5). Adrenergic agent combined with corticosteroid (R03AK) use had the highest prevalence in the C19G (6.1% vs. 4.0%; RR, 1.53) among those aged 40–59 years (Supplementary Table S5). Glucocorticoid (R03BA) use had the highest prevalence in the C19G among those aged 40–59 years (10.4% vs. 7.3%; RR, 1.42) (Supplementary Table S5) and those aged 60–79 years (14.9% vs. 11.4%; RR, 1.31) (Supplementary Table S5). Higher prevalence rates of anticholinergic (R03BB) use (11.9% vs. 9.8%; RR, 1.23) and piperazine derivative (R06AE) use (7.1% vs. 5.5%; RR, 1.30) were observed in the C19G among those aged > 80 years (Supplementary Table S5).
Discussion
The COVID-19 pandemic has imposed great challenges on healthcare systems worldwide. Some literature has been published on the clinical aspects of, possible treatments for and risk factors in patients with COVID-1923–26. Nevertheless, apart from a few studies, the epidemiology of and drug use profiles in patients with COVID-19 has not been studied. To our knowledge, this is the first study addressing this topic.
Most of our COVID-19 population comprised middle-aged men (55 ± 19 years; 80% were > 40 years of age) and those receiving ≥ 1 drug (74.5% of patients, including 35% with a polypharmacy regimen).
In general, our results revealed four profiles. The first comprises an age range of 0–39 (median age, 27 ± 9) years, male sex, no exposure to any drug in approximately half of the patients and a very low prevalence of polytherapy. The second comprises an age range of 40–59 (median age, 51 ± 5) years, male sex, use of 1–4 drugs in nearly half of the patients and a low prevalence of polytherapy (< 25%). The third comprises an age range of 60–79 (median age, 68 ± 6) years, male sex, use of ≥ 1 drug in 90% of the patients and polytherapy in more than half of the patients. The final profile comprises an age > 80 (median age 85 ± 4) years, female sex, use of ≥ 1 drug in 94% of the patients and polytherapy in 78% of the patients.
The analyses of drug-utilisation profiles highlighted differences between the C19G and GPG in terms of the prevalence of drug exposure. The drug categories with a difference ≥ 30% were antithrombotic agents (B01), antiepileptics (N03), anti-hyperuricaemics/anti-gout (M04) and cardiac therapy agents (C01). The higher prevalence rates associated with drugs in categories C01, B01 and M04 indicate a frequent pattern of cardiovascular and metabolic comorbidities in COVID-19 populations, as reported in other studies4,5,8. It is of some relevance that B01 drug use had the largest difference between the COVID-19 and general populations. This therapeutic profile indicates the presence of cardiovascular complications (including venous thromboembolism), supporting the hypothesis of an increased risk associated with COVID-19 infection in these patients8.
With regard to the higher prevalence of use of drugs in the M04 category, a retrospective cohort study including 131,565 patients and 252,763 controls, using data from the UK Clinical Practice Research Datalink, reported an increased risk of pneumonia (hazard ratio, 1.27; 95% CI 1.18–1.36) in patients with gout27.
There is no clear association between epilepsy and the risk of developing COVID-19. Nevertheless, epilepsy may be associated with other comorbidities or a component of congenital/inherited syndromes that may affect the immune system. Additionally, anti-epileptic agents can be used in association with other medications that can influence the immune system (e.g., adrenocorticotropic hormones, corticosteroids, everolimus, immunotherapy agents), and this may increase the infection risk28. Moreover, these patients may require frequent clinical evaluation, which may explain (at least in part) their increased risk of healthcare-associated infections.
Notably, the adjusted prevalence of the use of drugs acting on the RAS (C09) was not different between the C19G and GPG (RR, 1.02; 95% CI, 1.01–1.02). This result is in accordance with evidence from a retrospective study involving a COVID-19 cohort in Italy29 and supports the position of the European Society of Cardiology30. Furthermore, no major differences were noted for any category of antihypertensive drugs. Corroborating our results, a recent study carried out in the United States revealed no association between ACEI or ARB use and COVID-19, supporting the recommendation of continuing ACEI and ARB use in the setting of the COVID-19 pandemic31. This was further explored in a recent Brazilian study that confirmed that among patients hospitalised with mild to moderate COVID-19 who were taking ACEIs or ARBs before hospital admission, there were no significant differences in the mean number of days alive and out of the hospital between those who discontinued and continued these medications32.
Stratification by age showed a higher prevalence of use of drugs in categories B01, B03, C09 and C10 in people aged < 40 years. This evidence should be interpreted with caution because the number of such patients was very small. Nevertheless, a morbidity pattern similar to that in older patients was observed in these patients. Conversely, in COVID-19 patients aged > 60 years, there was no significant difference in the prevalence of drug use for cardiometabolic diseases compared with that in the GPG, but the prevalence rates of drug use for respiratory diseases and neurological diseases were increased in the C19G.
A large number of males took analgesics (N02) and drugs for cardiac therapy (C01). A high number of females took anti-anaemia agents (B03) and anti-epileptic agents (N03). Early descriptions of COVID-19 suggested a male preponderance23,24,33. Sex-based immunological, genetic, and lifestyle differences (e.g., tobacco smoking) have been postulated to explain the male preponderance of COVID-1934. In a population of 507 patients with COVID-19 between 13 and 31 January 2020 (including 364 from mainland China), 281 patients were male (55%), and the median age was 46 (IQR, 35–60) years35. Zhou and colleagues described 191 COVID-19 patients from Wuhan (Hubei Province, China) during the first month of the outbreak. That cohort had a median age of 56 (IQR, 46.0–67.0) years, with 62% being male and 48% with comorbidities23. Additionally, data from Italy revealed a higher prevalence of COVID-19 in males than in females36,37. However, sex- and age-disaggregated data revealed the opposite to be true for women aged > 80 years in Campania. National data from Italy revealed that in those aged 20–29 years, 56.5% of the diagnosed patients were female, and only after the age of 50 years does the male preponderance of COVID-19 increase. Thus, the male preponderance of COVID-19 should be interpreted with caution because sex-disaggregated data are incomplete, and more robust evidence is needed.
Our study was not designed to define the association between drug use, comorbidities, risk of adverse outcomes and outcomes in COVID-19 patients. The associations between the use of certain drugs and susceptibility to SARS-CoV-2 infection (e.g., predictive factors for poor outcome) must be studied in a large cohort with a control group and robust clinical data. This was a retrospective study of health records. Additional detailed patient information (mainly regarding clinical outcomes) was not available at the time of the analysis. Despite these limitations, we delineated the drug use profiles and epidemiological and demographic characteristics of 1532 Italian patients with COVID-19. This information provides the first evidence of the association between drug utilisation and COVID-19 risk, giving us a solid background for further analyses and interpretations using new data.
Conclusions
In conclusion, the current data provide baseline information about the complexity of patients affected by COVID-19, showing frequencies and differences in drug utilisation profiles in COVID-19 patients compared with the general population. The higher prevalence rates of C01, B01 and M04 use were probably linked to related comorbidities (i.e., cardiovascular, metabolic). Nevertheless, the prevalence of the use of drugs acting on RAS, such as other anti-hypertensive drugs, did not show a higher prevalence among COVID-19 patients than among the general population. Since these pilot data were derived from the first month of documented COVID-19 cases in the Campania region (southern Italy), our results highlight the need for further case–control studies to define the effects of medications and comorbidities on susceptibility to and associated mortality from COVID-19 infection. Finally, to better understand the global epidemiology of COVID-19, reproducible and comparable results from cohorts from multiple countries and regions are needed for further investigation and meta-analysis.
Supplementary Information
Author contributions
V.O. and E.M. conceived the study. I.G. and S.M. conducted the study. V.O., E.M. and G.L. analysed the results and wrote the original draft. E.C., A.P. and U.T. reviewed the manuscript. All authors agreed with the final version of the manuscript.
Funding
This study was supported by grants from the Italian Medicine Agency (AIFA), funding on Pharmacovigilance of the Campania Region (Progetto per la valutazione e l’analisi della prescrizione farmaceutica in Regione Campania; Osservatorio sull’uso appropriato dei farmaci).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Giuseppe Limongelli and Enrica Menditto.
Contributor Information
Valentina Orlando, Email: valentina.orlando@unina.it.
Enrica Menditto, Email: enrica.menditto@unina.it.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88398-y.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19), Situation Report—95. www.who.int/docs/default-source/coronaviruse/situation-reports/20200424-sitrep-95-covid-19.pdf?sfvrsn=e8065831_4. Accessed on 4 April 2020.
- 2.Yuen KS, Ye ZW, Fung SY, Chan CP, Jin DY. SARS-CoV-2 and COVID-19: the most important research questions. Cell Biosci. 2020;10(1):1–5. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenham C, Smith J, Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10227):846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li B, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tignanelli CJ, et al. Antihypertensive drugs and risk of COVID-19? Lancet Respir. Med. 2020;8:e30–e31. doi: 10.1016/S2213-2600(20)30153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi RT, Lynch JB, del Rio C. Mild or moderate Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 8.Guo T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi GP, Sanga V, Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9:e57278. doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.South AM, Diz D, Chappell MC. COVID-19, ACE2 and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronson JK, Ferner RE. Drugs and the renin-angiotensin system in covid-19. BMJ. 2020;369:m1313. doi: 10.1136/bmj.m1313. [DOI] [PubMed] [Google Scholar]
- 12.Sommerstein R, Kochen MM, Messerli FH, Gräni C. Coronavirus disease 2019 (COVID-19): do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J. Am. Heart Assoc. 2020;9(7):e016509. doi: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Juste A, et al. Treatment patterns of diabetes in Italy: a population-based study. Front. Pharmacol. 2019;10:870. doi: 10.3389/fphar.2019.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerriero F, et al. Biological therapy utilization, switching, and cost among patients with psoriasis: retrospective analysis of administrative databases in Southern Italy. Clinicoecon. Outcomes Res. 2017;9:741. doi: 10.2147/CEOR.S147558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Putignano D, et al. Differences in drug use between men and women: an Italian cross sectional study. BMC Women’s Health. 2017;17(1):73. doi: 10.1186/s12905-017-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iolascon G, et al. Osteoporosis drugs in real-world clinical practice: an analysis of persistence. Aging Clin. Exp. Res. 2013;25(1):137–141. doi: 10.1007/s40520-013-0127-5. [DOI] [PubMed] [Google Scholar]
- 17.Orlando V, et al. Prescription patterns of antidiabetic treatment in the elderly. Results from Southern Italy. Curr. Diabetes Rev. 2016;12(2):100–106. doi: 10.2174/1573399811666150701120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menditto E, et al. Adherence to chronic medication in older populations: application of a common protocol among three European cohorts. Patient Prefer. Adherence. 2018;12:1975. doi: 10.2147/PPA.S164819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casula M, et al. Assessment and potential determinants of compliance and persistence to antiosteoporosis therapy in Italy. Am J Manag. Care. 2014;20(5):e138–e145. [PubMed] [Google Scholar]
- 20.Orlando V, et al. Drug utilization pattern of antibiotics: the role of age, sex and municipalities in determining variation. Risk Manag. Healthc. Policy. 2020;13:63. doi: 10.2147/RMHP.S223042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Italian Data Protection Authority. General authorisation to process personal data for scientific research purposes—1 March 2012 [1884019]. 10.1094/PDIS-11-11-0999-PDN.
- 22.Demo-Geodemo. Mappe, Popolazione, Statistiche Demografiche dell’ISTAT. http://demo.istat.it/. Accessed on 1 March 2020.
- 23.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vellingiri B, et al. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020;725:138277. doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spaetgens B, et al. Risk of infections in patients with gout: a population-based cohort study. Sci. Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-01588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beghi E, Shorvon S. Antiepileptic drugs and the immune system. Epilepsia. 2011;52:40–44. doi: 10.1111/j.1528-1167.2011.03035.x. [DOI] [PubMed] [Google Scholar]
- 29.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The European Society of Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic (2020). www.escardio.org/static_file/Escardio/Education-General/Topic%20pages/Covid-19/ESC%20Guidance%20Document/ESC-Guidance-COVID-19-Pandemic.pdf. Accessed on 21 Apr 2020.
- 31.Mehta N, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes RD, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325(3):254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir. Med. 2020;8(4):e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun K, Chen J, Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digit. Health. 2020;2(4):e201–e208. doi: 10.1016/S2589-7500(20)30026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riccardo F, et al. Epidemiological characteristics of COVID-19 cases in Italy and estimates of the reproductive numbers one month into the epidemic. medRxiv. 2020 doi: 10.1101/2020.04.08.20056861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.