Abstract
Background/Objective:
Optimizing blood pressure is an important target for intervention following pediatric traumatic brain injury (TBI). Existing literature has examined the association between systolic blood pressure (SBP) and outcomes. Mean arterial pressure (MAP) is a better measure of organ perfusion than SBP and is used to determine cerebral perfusion pressure but has not been previously examined in relation to outcomes after pediatric TBI. We aimed to evaluate the strength of association between MAP-based hypotension early after hospital admission and discharge outcome, and to contrast the relative strength of association of hypotension with outcome between MAP-based and SBP-based blood pressure percentiles.
Methods:
We examined the association between lowest age-specific MAP percentile within 12 hours after pediatric intensive care unit admission and poor discharge outcome (in-hospital death or transfer to a skilled nursing facility) in children with severe (Glasgow Coma Scale score <9) TBI who survived at least 12 hours. Poisson regression results were adjusted for maximum head Abbreviated Injury Scale (AIS) severity score, maximum non-head AIS, and vasoactive medication use. We also examined the ability of lowest MAP percentile during the first 12 hours to predict discharge outcomes using receiver operating curve characteristic analysis without adjustment for covariates. We contrasted the predictive ability and the relative strength of association of blood pressure with outcome between MAP and SBP percentiles.
Results:
Data from 166 children age <18 years were examined, of whom 20.4% had a poor discharge outcome. Poor discharge outcome was most common among patients with lowest MAP <5th percentile (42.9%; aRR 5.3 vs. 50th-94th percentile, 95% CI 1.2, 23.0) and MAP 5th-9th percentile (40%; aRR 8.5, 95% CI 1.9, 38.7). Without adjustment for injury severity or vasoactive medication use, lowest MAP percentile was moderately predictive of poor discharge outcome (AUC:0.75, 95% CI 0.66, 0.85). In contrast, lowest SBP was associated with poor discharge outcome only for the <5th percentile (50%; aRR 5.4, 95% CI 1.3, 22.2). Lowest SBP percentile was moderately predictive of poor discharge outcome (AUC: 0.82, 95% CI 0.74, 0.91).
Conclusions:
In children with severe TBI, a single MAP <10th percentile during the first 12 hours after Pediatric Intensive Care Unit admission was associated with poor discharge outcome. Lowest MAP percentile during the first 12 hours was moderately predictive of poor discharge outcome. Lowest MAP percentile was more strongly associated with outcome than lowest SBP percentile but had slightly lower predictive ability than SBP.
Keywords: Traumatic Brain Injury, Pediatric Intensive Care Unit, Blood Pressure/Physiology, Abbreviated Injury Scale, Patient Discharge, Hypotension, Humans, Retrospective Studies, Predictive Value of Tests
Introduction
Traumatic brain injury (TBI) is a leading cause of pediatric death and disability worldwide.1 While the severity of the primary injury may determine outcome, outcomes are also affected by second insults such as hypoxia, hypercarbia, and/or high intracranial pressure (ICP) that arise from injury cascades after initial TBI.2 Early systemic hypotension after severe TBI is a well-recognized cause of secondary brain injury and is associated with poor discharge outcomes3–7 Early detection and correction of hypotension after severe TBI is considered paramount to optimizing both cerebral and patient-level outcomes.
Several pediatric studies have examined the effect of blood pressure in severe TBI but there are currently no consistent or national-level age-specific recommendations for systemic blood pressure targets for children with TBI.2,8,9 Kokoska et al. reported that hypotension occurring during the first 24 hours of hospitalization post-injury was associated with worse Glasgow Outcome scale (GOS).5 Coates and Santama et al. demonstrated that systolic blood pressure (SBP) <5th percentile in the pre-hospital period and emergency department were more strongly associated with poor hospital discharge GOS than hypotension occurring later, suggesting a need for early intervention.7 We previously demonstrated that age-specific SBP percentiles were more strongly associated with discharge GOS <4 than the static measure of SBP <90mmHg.4 Suttipongkaeset et al. used a large dataset to demonstrate that SBP <75th percentile was associated with in-hospital mortality, suggesting that SBP percentiles higher than previously considered may be beneficial in pediatric TBI.3
While all pediatric TBI studies to date have defined hypotension using SBP percentile, mean arterial pressure (MAP) is a better measure of organ perfusion than SBP.10,11 In TBI care, MAP is essential in the estimation of cerebral perfusion pressure (CPP) by the formula CPP = MAP–ICP,12 and maintenance of CPP is recommended by the Brain Trauma Foundation to improve patient outcomes.9 However, ICP monitors are not always placed in severe TBI care, leaving clinicians with only MAP to estimate CPP. Furthermore, while invasive measurement of blood pressure is the gold-standard for detecting hypotension, invasive blood pressure monitors are not always placed routinely on pediatric patients with severe TBI We conducted this study to determine if and how non-invasively measured MAP can be used to understand outcomes in severe pediatric TBI relative to non-invasively measured SBP. Our primary objectives were to evaluate the strength of association between lowest MAP percentile in the first 12 hours after pediatric intensive care unit (PICU) admission and discharge outcome, to test the ability of lowest MAP percentile to predict discharge outcome, and to contrast the relative strength of association of blood pressure with outcome between MAP and SBP percentiles.
Methods
Study Design and Setting:
We performed a secondary analysis of the Pediatric Guidelines Adherence and Outcomes (PEGASUS) study cohort at Harborview Medical Center.13
Harborview Medical Center is a 450-bed mixed Level 1 adult and pediatric trauma hospital that serves the five-state Pacific Northwest region (Alaska, Idaho, Montana, Washington, and Wyoming). The 18-bed PICU admits approximately 120 children with TBI annually. Patients are discharged from Harborview Medical Center to their home, Seattle Children’s Hospital inpatient rehabilitation unit, skilled nursing facilities, or long-term care facilities.
The University of Washington Institutional Review Board approved this study with waiver of informed consent.
Study Participants
Patients were age <18 years with intracranial hemorrhage on head CT and GCS <9 at any point during their PICU stay who survived the first 12 hours after PICU admission.
Data Collection
Data on demographic characteristics, injury severity, and discharge outcomes were obtained from the Harborview Trauma Registry and electronic health record (EHR). Blood pressure values were extracted from the EHR.
SBP and Diastolic Blood Pressure (DBP) were made manually by an automated machine and entered by PICU nurses into the EHR at least hourly from the time of PICU admission. MAP was calculated by the formula: MAP = DBP + 1/3 * (SBP-DBP).14 MAP and SBP data were transformed to age-specific percentiles based on population distributions published by Haque et al (Supplementary Table 1).15 Each patient’s lowest MAP and SBP percentile during the first 12 hours from PICU admission was determined and categorized as <5th, 5th-9th, 10th-24th, 25th-49th, 50th-94th or ≥95th percentile. The first 12 hours was chosen to include the period of most active resuscitation for most patients while providing sufficient hypotension exposure time to be able to associate and predict outcomes. A CPP of >40 mmHg was targeted for all patients in the PEGASUS cohort.
Outcome Variables
Discharge outcome was categorized as either “favorable” (discharge home or to an inpatient or outpatient rehabilitation service) or “poor” (in-hospital death or discharge to a skilled nursing facility or long-term care facility). We performed sensitivity analyses using in-hospital mortality alone as the outcome.
Data Analysis
We used descriptive statistics to examine demographic and injury characteristics stratified by discharge outcome. To evaluate the concordance between MAP and SBP percentiles, we determined the median difference between each recorded MAP percentile and its associated SBP percentile. To examine the association between lowest MAP percentile over the first 12 hours from PICU admission and poor discharge outcome, we used unadjusted and adjusted Poisson regression with robust standard errors, modeling MAP percentile as a categorical variable: <5th, 5th-9th, 10th-24th, 25th-49th, 50th-94th or ≥95th percentile. The referent category was patients whose lowest MAP percentile over 12 hours was in the 50th-94th percentile.3,13 Covariates were selected by determining the minimum set of covariates that could account for the majority of the effect of any confounding. Analyses were adjusted for head Abbreviated Injury Scale (AIS) severity score, non-head AIS, and vasoactive medication use. To examine the extent to which there may have been confounding due to increased observation of more severe cases, we performed sensitivity analyses using only the first MAP percentile value per hour from PICU admit for each patient. We repeated each of these analyses using SBP percentile.
To examine the possible predictive utility of MAP percentile, we tested the ability of the lowest MAP percentile during the first 12 hours to predict poor discharge outcome or in-hospital mortality using receiver operating curve characteristic analysis. We performed a DeLong test to determine if the predictive ability of the lowest MAP percentile was significantly different than the predictive ability of the lowest SBP percentile. Youden indices were computed to evaluate thresholds that maximized sensitivity and specificity.
All statistical analyses were performed with R version 3.5.3.
Results
Participants
Of the PEGASUS patients admitted to the PICU with severe TBI, 166 (94.9%) survived the first 12 hours from PICU admission and constituted our analytical cohort. Thirty-four (20.4%) patients had poor discharge outcomes, including 29 (17.4%) patients who died in the hospital. Compared to patients with favorable discharge outcomes, patients with poor discharge outcome were younger (median [IQR] 8.9 [2.5, 16.3] vs. 11.8 [4.0, 15.9] years), more commonly had sustained abusive TBI (17.6% vs. 4.5%), had a higher maximum head AIS (median [IQR] 5 [5, 5] vs. 4 [4, 5] and non-head AIS (3 [2, 4] vs. 2 [1, 3]), and more frequently had vasoactive medication use (29.4% vs. 1.5%) (Table 1). Among a subset of individuals who experienced one or more MAP < 5th percentile, compared to patients with favorable discharge outcomes, patients with poor discharge outcomes had greater injury severity scores (Median [IQR] 40.0 [27.5, 58.5] vs. 27.0 [21.0, 34.3] (Supplementary Table 2).
Table 1:
Clinical characteristics of patients admitted with severe TBI who survived the first 12 hours from PICU admission (n=166)
| Poor discharge outcome n=34 (20.5%) | Favorable discharge outcome n=132 (79.5%) | |
|---|---|---|
| Age (years), n (%) | ||
| < 1 | 5 (14.8) | 9 (6.8) |
| 1 – 5 | 11 (32.4) | 37 (28.0) |
| 6 – 12 | 2 (5.9) | 23 (17.4) |
| 13 – 17 | 16 (47.7) | 63 (47.7) |
| Males, n (%) | 22 (64.7) | 93 (70.5) |
| Mechanism of TBI, n (%) | ||
| Motor vehicle crash | 10 (29.4) | 45 (34.1) |
| Fall | 6 (17.6) | 34 (25.8) |
| Struck by vehicle | 6 (17.6) | 30 (22.7) |
| Abusive TBI | 6 (17.6) | 6 (4.5) |
| Other | 6 (17.6) | 17 (13.9) |
| GCS score on admission to PICU, median [IQR] | 3.0 [3.0, 4.0] | 8.0 [6.0, 10.3] |
| Highest AIS head score, median [IQR] | 5.0 [5.0, 5.0] | 4.0 [4.0, 5.0] |
| Highest AIS non-head score, median [IQR] | 3.0 [2.0, 4.0] | 2.0 [1.0, 3.0] |
| Injury Severity Score, median [IQR] | 40.0 [27.5, 58.5] | 27.0 [21.0, 34.3] |
| Polytrauma, n (%) | 20 (58.8) | 52 (39.4) |
| Vasoactive medication use, n (%) | 10 (29.4) | 2 (1.5) |
| Any surgery, n (%) | 17 (50.0) | 87 (65.9) |
| Craniotomy, n (%) | 10 (29.4) | 37 (28.0) |
| Intracranial pressure monitoring, n (%) | 11 (32.4) | 52 (39.4) |
| Experienced brain herniation | 23 (67.6) | 37 (28.0) |
| Avoidance of unwanted hypocarbia, n (%) * | 5 (50.0) | 61 (75.3) |
| Maintenance of all CPP > 40mmHg, n (%) | 13 (38.2) | 93 (70.5) |
| Early initiation of nutrition, n (%) | 15 (44.1) | 124 (93.9) |
AIS=Abbreviated Injury Scale. CPP=cerebral perfusion pressure. ED=emergency department. EMS=emergency medical services. GCS=Glasgow Coma Scale. IQR=interquartile range. PICU=pediatric intensive care unit. TBI=traumatic brain injury.
In patients without brain herniation
Exposure Data
Overall, 3,321 MAP data points were recorded for the cohort during the first 12 hours after PICU admission. Patients had a median of 19 MAP values each (IQR 13, 24). MAP percentiles were an average of 10% (SD 17.1) lower than their associated SBP percentiles (Supplemental Figure 1). Arterial blood pressure was available for 87 (52.4%) of patients.
Association between MAP and Discharge Outcome
Poor discharge outcome most commonly occurred in children with lowest MAP in the <5th percentile (18/42, 42.9%) and 5-9th percentile (4/10, 40%), while the lowest proportion of poor discharge outcome occurred in children with lowest MAP in the 50th-94th percentile (2/42, 4.8%) (Table 2). Relative to patients with lowest MAP in the 50th-94th percentile, patients with lowest MAP <5th percentile had an adjusted relative risk (aRR) of 5.3 (95% CI 1.2, 23.0) for poor discharge outcome after adjustment for head AIS, non-head AIS, and vasoactive medication use. Patients with lowest MAP in the 5th-9th percentile had an aRR of 8.5 (95% 1.9, 38.7) for poor discharge outcome. No other MAP percentile categories were associated with discharge outcome. There was no statistically different risk of poor discharge outcome between patients with lowest MAP <5th percentile and those with lowest MAP 5th – 9th percentile.
Table 2:
Association between lowest mean arterial pressure percentile and lowest systolic blood pressure percentile over the first 12 hours from PICU admit and poor discharge disposition among patients admitted to the PICU with severe TBI who survived 12 hours from PICU admission (n=166).
| Mean Arterial Pressure | Systolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| Lowest blood pressure percentile | Poor discharge outcome N (%) | Relative Risk |
Poor discharge outcome N (%) | Relative Risk |
||
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |||
| < 5th | 18/42 (42.9) | 9.0 (2.2, 36.4) | 5.3 (1.2, 23.0) | 28/56 (50.0) | 6.8 (1.7, 26.3) | 5.4 (1.3, 22.2) |
| 5th - 9th | 4/10 (40.0) | 8.4 (1.8, 39.6) | 8.5 (1.9, 38.7) | 3/22 (13.6) | 1.8 (0.3, 10.1) | 1.8 (0.3, 10.0) |
| 10th – 24th | 5/35 (14.3) | 3.0 (0.6, 14.5) | 2.7 (0.6, 12.3) | 0/28 (0.0) | n/a | n/a |
| 25th - 49th | 4/30 (13.3) | 2.8 (0.5, 14.3) | 2.7 (0.5, 13.6) | 1/31 (3.2) | 0.4 (0, 4.5) | 0.4 (0, 4.7) |
| 50th-95th | 2/42 (4.8) | Referent | Referent | 2/27 (7.4) | Referent | Referent |
| ≥ 95th | 1/7 (14.3) | 3.0 (0.3, 28.8) | 2.7 (0.3, 24.8) | 0/2 (0.0) | n/a | n/a |
AIS=Abbreviated Injury Scale severity score. PICU=Pediatric Intensive Care Unit. TBI=traumatic brain injury.
Analyses adjusted for severe head injury (head AIS > 3), severe non-head injury (maximum non-head AIS > 3), and vasoactive use within the first twelve hours of PICU admission (Y/N)
Association between SBP and Discharge Outcome
SBP was associated with poor discharge outcome in only the lowest percentile category, with 50% of patients with a lowest SBP <5th percentile experiencing poor discharge outcome (aRR 5.4 vs. 50th-94th percentile, 95% CI 1.3, 22.2) More than 80% of poor outcomes occurred in the <5th percentile category (Table 2).
Sensitivity Analyses
In-hospital death was highest among those with lowest MAP <5th percentile (17/42, 40.5%) and 5th-9th percentile (3/10, 30%) and the lowest mortality occurred among patients with a lowest MAP in the 50th-94th percentile (2/42, 4.8%) (Table 3). Relative to patients with lowest MAP in the 50th-94th percentile, patients with lowest MAP <5th percentile had an aRR of 5.3 (95% CI 1.2, 23.5) for death and patients with lowest MAP in the 5th-9th percentile had an aRR of 7.2 (95% 1.4, 36.3). No other MAP percentile categories were significantly associated with risk of death. There was no statistically significant different in risk of death between patients with lowest MAP <5th percentile and 5-9th percentile (p= 0.43). For SBP, 42.9% of patients with SBP <5th percentile died (aRR 4.6, 95% CI 1.1, 19.9) and no other SBP percentile categories were significantly associated with risk of death.
Table 3:
Association between lowest mean arterial pressure percentile and lowest systolic blood pressure percentile over the first 12 hours from PICU admit and in-hospital mortality among patients admitted to the PICU with severe TBI who survived 12 hours from PICU admission (n=166).
| Mean Arterial Pressure | Systolic Blood Pressure | |||||
|---|---|---|---|---|---|---|
| Lowest blood pressure percentile | In-hospital mortality N (%) | Relative Risk |
In-hospital mortality N (%) | Relative Risk |
||
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | |||
| < 5th | 17/42 (40.5) | 8.5 (2.1, 34.5) | 5.3 (1.2, 23.5) | 24/56 (42.9) | 5.8 (1.5, 22.7) | 4.6 (1.1, 19.9) |
| 5th - 9th | 3/10 (30.0) | 6.3 (1.2, 32.8) | 7.2 (1.4, 36.3) | 2/22 (9.1) | 1.2 (0.2, 8.0) | 1.3 (0.2, 8.8) |
| 10th – 24th | 4/35 (11.4) | 2.4 (0.5, 12.3) | 2.3 (0.5, 11.4) | 0/28 (0) | n/a | n/a |
| 25th - 49th | 2/30 (6.7) | 1.4 (0.2, 9.4) | 1.4 (0.2, 9.5) | 1/31 (3.2) | 0.4 (0, 4.5) | 0.5 (0, 4.7) |
| 50th-95th | 2/40 (4.8) | Referent | Referent | 2/27 (7.4) | Referent | Referent |
| ≥ 95th | 1/7 (14.3) | 3.0 (0.3, 28.8) | 3.1 (0.3, 28.7) | 0/2 (0) | n/a | n/a |
AIS=Abbreviated Injury Scale severity score. PICU=Pediatric Intensive Care Unit. TBI=traumatic brain injury.
Analyses adjusted for severe head injury (head AIS > 3), severe non-head injury (maximum non-head AIS > 3), and vasoactive use within the first twelve hours of PICU admission (Y/N)
When blood pressure measurements were restricted to a single recording per hour, lowest MAP <5th percentile was similarly associated with poor discharge outcome (aRR 3.6, 95% CI 1.3, 9.8) and in-hospital mortality (aRR 3.4, 95% CI 1.1, 9.9) relative to lowest MAP in the 50th-94th percentile, though associations were reduced in lowest MAP in the 5th-9th percentile (aRR 2.5 95% CI 0.8, 8.0) (Supplemental Table 3). Similar to the primary analysis, SBP <5th percentile was associated with both poor discharge outcome (aRR 6.6, 95% CI 1.6, 26.8) and in-hospital mortality (aRR 5.0, 95% CI 1.2, 21.2) (Supplemental Table 4).
Predictive Analyses
Using separate, unadjusted models where MAP/SBP was the sole predictor, the receiver operating curve analyses for lowest MAP and SBP percentiles to predict poor discharge outcome and in-hospital mortality are shown in Figure 1. When predicting poor discharge outcomes, lowest MAP percentile had an AUC=0.75 (95% CI 0.66, 0.85), and lowest SBP percentile had an AUC=0.82 (95% CI 0.72, 0.91). The difference in AUC was found to be statistically significant (p = 0.003). When predicting in-hospital mortality, lowest MAP percentile had an AUC=0.76 (95% CI 0.66, 0.87) and lowest MAP percentile had an AUC=0.82 (95% CI 0.74, 0.91), which was also a statistically significant difference (p=0.02).Peak Youden index (YI, sensitivity + sensitivity − 1) to detect poor discharge outcome occurred at a lowest MAP percentile of 10.2% (YI=0.45) and lowest SBP percentile of 6.7% (YI=0.62). The peak YI to determine likelihood of in-hospital mortality occurred at a lowest MAP percentile of 10.2% (YI=0.49) and lowest SBP percentile of 4.6% (YI=0.60) (Figure 2).
Figure 1. Receiver operating characteristic curve depicting accuracy of mean arterial pressure (MAP) percentile and systolic blood pressure (SBP) percentile.
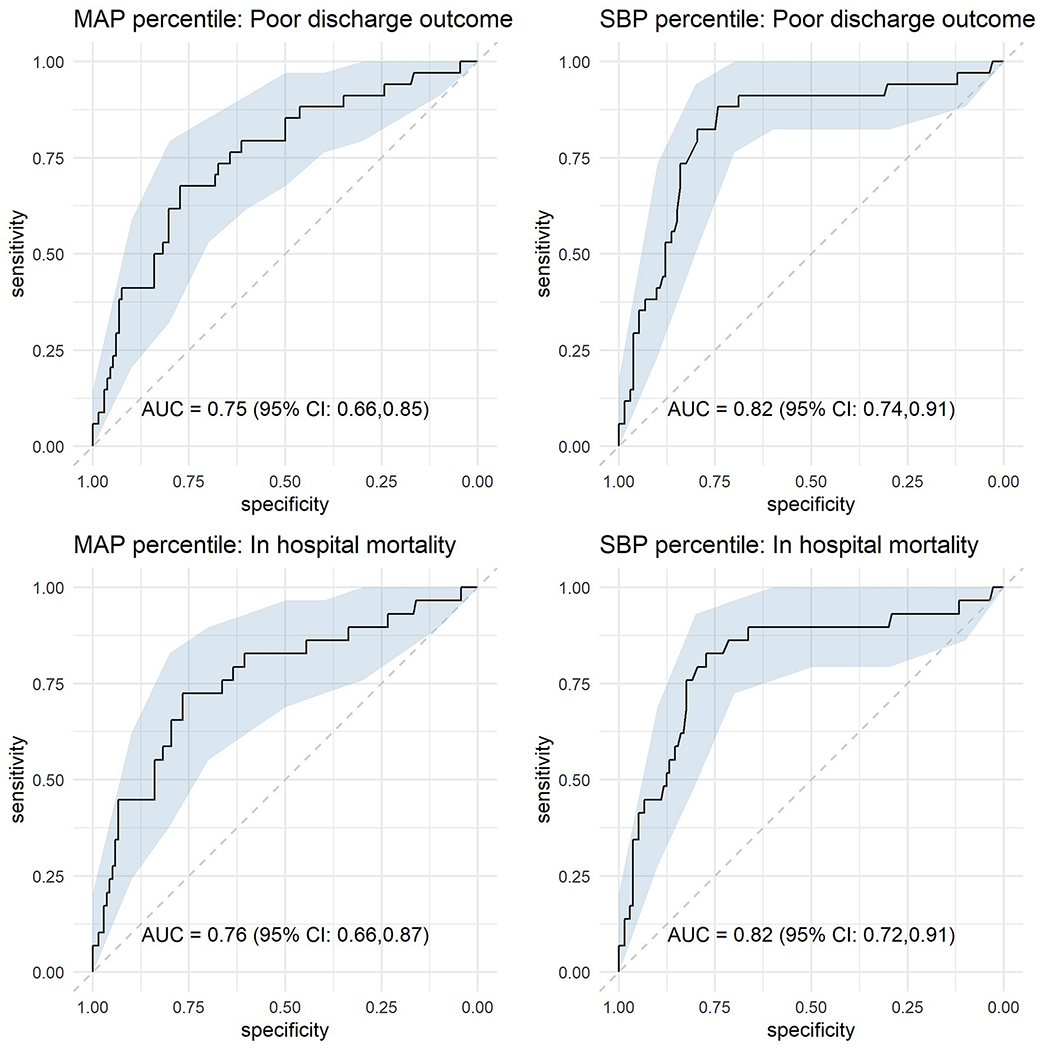
Area under curves (AUC) for MAP and SBP percentile to detect favorable discharge outcome and likelihood of survival are shown.
Figure 2A and 2B.
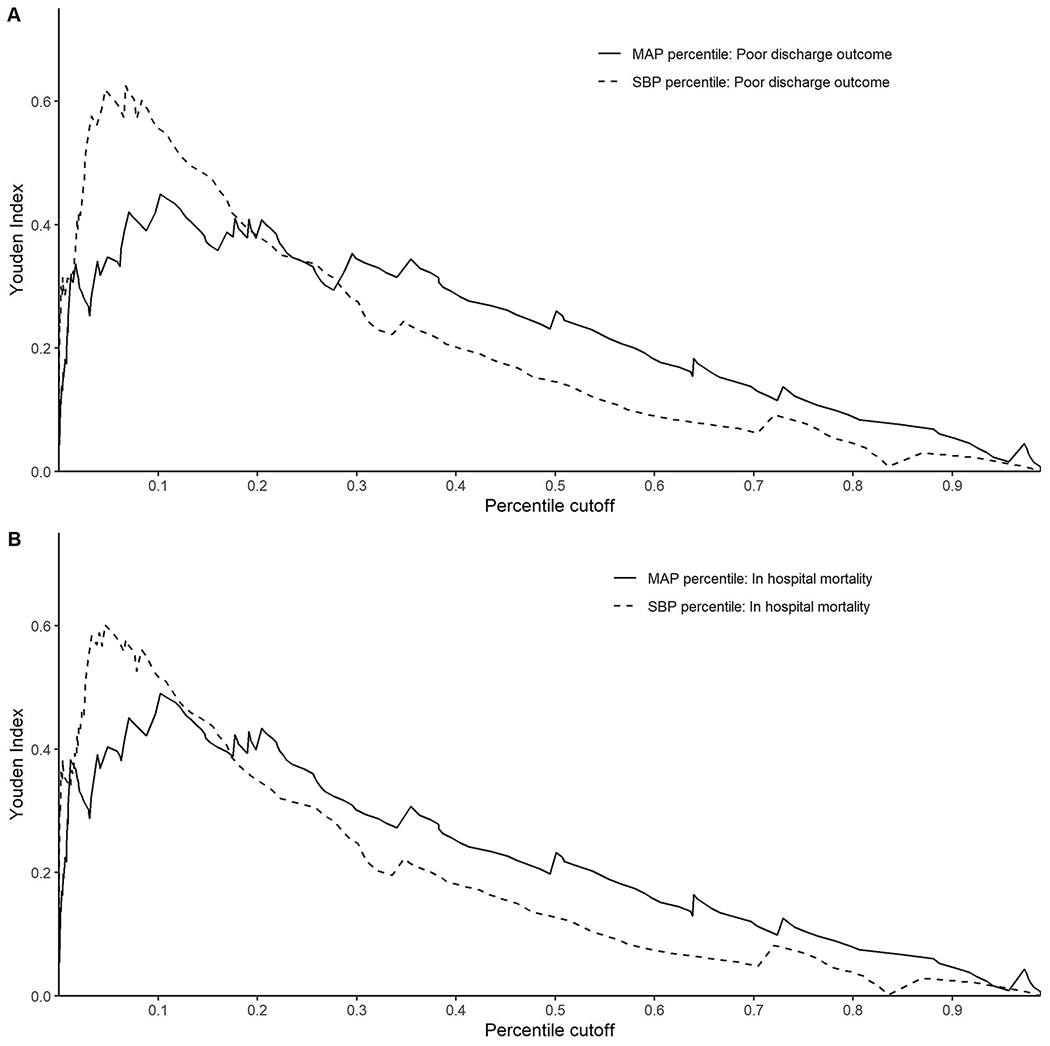
Youden index corresponding to cutoffs established at corresponding lowest mean arterial pressure (MAP) percentiles and systolic blood pressure (SBP) percentiles at 12 hours from PICU admission to detect likelihood of poor discharge outcome (2A) and in-hospital mortality (2B).
Discussion
This study examining MAP and outcomes following severe pediatric TBI demonstrated that a single MAP <10th percentile in the first 12 hours after PICU admission was strongly associated with poor discharge outcome, and that the lowest MAP percentile in the first 12 hours had moderate ability to predict discharge outcomes. When compared to the lowest SBP percentile in the first 12 hours, MAP percentile was more strongly associated with outcome but its ability to predict outcome without adjustment for injury severity or vasoactive medication use was slightly lower than for SBP percentile. Thresholds of <10.2 percentile for MAP and <6.7 percentile for SBP provided the greatest overall sensitivity and specificity for poor outcome. To the best of our knowledge, this is the first study to evaluate MAP as a measure of hypotension in severe pediatric TBI. This study demonstrates the potentially important role of MAP percentile as a therapeutic target early in PICU care.
Given the known physiological relationship between MAP and organ perfusion, we hypothesized that low MAP may be strongly associated with poor discharge outcome.11 Previous research has identified an association between SBP and poor discharge outcomes.3–5,7 However, many factors that are important to brain perfusion may mediate, confound, or modify the relationship between hypotension and outcomes but are not considered when using SBP definitions.3,16 An example is cerebral autoregulation, a homeostatic process in which decreases in MAP (and by extension CPP) result in vasodilation in the presence of hypotension to preserve cerebral perfusion. When this protective homeostatic mechanism is disrupted, decreases in MAP result in decreases in CPP, causing ischemia and poor outcomes. Impaired autoregulation is common following TBI and contributes to poor outcome.17 A key consequence of this compromise is a decrease in CPP and cerebral blood flow as blood pressure decreases.
Our results suggest that interventions to maintain MAP above the 10th percentile for age may merit examination to mitigate the neurologic injury resulting from impaired cerebral autoregulation. Identifying appropriate MAP percentile thresholds may, additionally, allow clinicians to preemptively “account” for autoregulatory impairment in determining blood pressure targets.18 Our study lays the foundation for this future work to identify whether the associations we have identified are potential treatment targets. Finally, use of MAP percentile may be more desirable than SBP percentile in contexts where ICP monitoring is not common.19,20 Despite historical use of SPB goals and targets, estimates of brain perfusion based on MAP rather than SBP are more physiologic. We cannot assume a given ICP without direct monitoring but further development of MAP thresholds may allow for empirical bedside adjustments of MAP goals while either pending ICP monitor placement or as empirical targets. Given the worldwide TBI burden, a MAP-based hypotension measure may have widespread application.
While our study lacked the sample size to definitively address whether MAP-based percentile thresholds were significantly more associated with poor discharge outcome than SBP-based percentile thresholds, we did identify that the relative strength of association of blood pressure with outcome was stronger for MAP than for SBP. Additionally, the MAP threshold associated with poor discharge outcome was higher than the SBP threshold associated with poor outcome, demonstrating that age-based percentiles of MAP and SBP are not equivalent. Future work is needed to evaluate the various definitions of hypotension in relation to organ perfusion and outcomes in pediatric TBI and pediatric trauma, especially when comparing MAP percentiles to SBP percentiles.
We intentionally examined prognostication to complement our explanatory analysis, which allowed us to test the value of unadjusted MAP and SBP percentiles over the first 12 hours as predictors of discharge outcome. Our results demonstrate that lowest MAP and SBP percentiles within 12 hours of PICU admission are moderate predictors of future poor discharge outcome. The Youden index is a summary statistic of the ROC curve which determines the cut-point that optimizes a threshold’s differentiating ability when equal weight is given to sensitivity and specificity; a value of zero corresponds to agreement only by chance and a value of one corresponds to complete agreement between metrics. The Youden index can also be interpreted as the reciprocal of the number needed to diagnose (NND), or the number of patients who need to be examined to correctly detect one person with the disease of interest in a study population of persons with and without the known disease.21 We observed a peak Youden index accuracy for MAP to be 0.62 at the 10.2 percentile, which corresponds to a NND of 1.61. This means that approximately 3 patients will be diagnosed correctly for discharge outcome for every 5 patients evaluated for whether the patient’s minimum MAP percentile over the first twelve hours from PICU admission was greater than or less than 10.2.
Notably, the AUC for SBP was higher than the AUC for MAP, suggesting that the single lowest SBP value may be more strongly predictive of discharge outcome than the single lowest MAP value when not adjusted for injury severity or vasoactive medication use. While predictive analysis is explicitly not causal, it can be used as a basis for future exploratory work. Our analyses suggest a potentially new value for the role of MAP percentiles in not only identifying patients at risk of poor outcomes but also highlighting thresholds for future research to examine potential interventions to improve outcomes. Future work should confirm MAP thresholds identified in our study to examine their validity and further utility in relation to SBP thresholds.
There were several limitations to this study. First, this was a secondary analysis of data from an observational study and was likely subject to residual confounding. Second, our analysis excluded patients who did not survive the first 12 hours which limits the generalizability of our results, but this timeframe was important to ensure all patients had an equal opportunity for exposure. Third, this was a single center study and our findings must be externally validated. Fourth, we noted a decreased association between poor discharge outcome and lowest MAP percentile when a single hourly value was used, suggesting potential bias arising from more frequent monitoring of the most severely injured patients. However, this limitation is also informative in that future work should consider use of more datapoints during external validation. Fifth, we were unable to control for several factors in the collection of blood pressure. As data was collected approximately hourly, we were unable to capture beat to beat variability which may have resulted in a loss of study accuracy. Additionally, we were unable to isolate the location and method by which blood pressure was measured. As blood pressure measurements differ by measurement location this may have introduced confounding in our study. The use of automated cuff measurements of SBP may have overestimated the association between MAP and outcomes. Sixth, although we had a rationale for examining MAP at the 12-hour mark, this may or may not be the optimal time point for prediction. Seventh, our covariate choice was conservative to minimize collinearity, however this may have resulted in uncontrolled confounding. Finally, due to the low availability of invasive blood pressure measurements we were unable to assess the relative accuracy of non-invasively measured BP.
Conclusions
MAP <10th percentile during the first 12 hours after PICU admission was associated with a higher risk of poor discharge outcome in children with severe TBI when compared to children whose lowest MAP percentile was between the 50th and 94th percentile. MAP percentile predicted poor outcomes and the highest accuracy for this prediction was a MAP threshold of the 10.2 percentile during the first 12 hours of PICU admission. Notably our data does not yet support targeting MAP as a hemodynamic target. Future studies should examine the benefit of using MAP percentile over SBP percentile in children with severe TBI.
Supplementary Material
Acknowledgments
This study was funded by National Institutes of Health 5R01NS072308-06 (Vavilala). Funder had no influence on study design or reporting of the results.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
The authors have no conflicts of interest relevant to this article to disclose. The authors have no financial relationships relevant to the article to disclose.
References
- 1.Rubiano AM, Carney N, Chesnut R, Puyana JC. Global neurotrauma research challenges and opportunities. Nature 2015;527(7578):S193–S197. [DOI] [PubMed] [Google Scholar]
- 2.Mayer T, Walker M. Pediatric head injury: The critical role of the emergency physician. Annals of Emergency Medicine 1985;14(12): 1178–84. [DOI] [PubMed] [Google Scholar]
- 3.Suttipongkaset P, Chaikittisilpa N, Vavilala MS, et al. Blood Pressure Thresholds and Mortality in Pediatric Traumatic Brain Injury. Pediatrics 2018;142(2):e20180594. [DOI] [PubMed] [Google Scholar]
- 4.Vavilala MS, Bowen A, Lam AM, et al. Blood Pressure and Outcome after Severe Pediatric Traumatic Brain Injury: The Journal of Trauma: Injury, Infection, and Critical Care 2003;55(6): 1039–44. [DOI] [PubMed] [Google Scholar]
- 5.Kokoska ER, Smith GS, Pittman T, Weber TR. Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma. Journal of Pediatric Surgery 1998;33(2):333–8. [DOI] [PubMed] [Google Scholar]
- 6.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. Journal of Pediatric Surgery 1993;28(3):310–6. [DOI] [PubMed] [Google Scholar]
- 7.Coates BM, Vavilala MS, Mack CD, et al. The Influence of Definition and Location of Hypotension on Outcome Following Severe Pediatric Traumatic Brain Injury. 2006;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannan N, Wang J, Mink RB, et al. Timely Hemodynamic Resuscitation and Outcomes in Severe Pediatric Traumatic Brain Injury: Preliminary Findings. Pediatric Emergency Care 2018;34(5):325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines. Pediatric Critical Care Medicine 2019;20(3):S1–S82. [DOI] [PubMed] [Google Scholar]
- 10.Pinsky MR, Payen D. Functional hemodynamic monitoring. Crit Care 2005;9(6):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehman LH, Saeed M, Talmor D, Mark R, Malhotra A. Methods of Blood Pressure Measurement in the ICU*: Critical Care Medicine 2013;41(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson CS. Management of Cerebral Perfusion Pressure after Traumatic Brain Injury. 2001;95(6):5. [DOI] [PubMed] [Google Scholar]
- 13.Vavilala MS, King MA, Yang J, et al. The Pediatric Guideline Adherence and Outcomes ( PEGASUS ) programme in severe traumatic brain injury: a single-centre hybrid implementation and effectiveness study. The Lancet child and Adolescent Health 2019;3(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazinsky M, Zaritsky A, Nadkarni V. Pediatric Advanced Life Support Provider Manual. Dallas, TX: American Heart Association; 2002. [Google Scholar]
- 15.Haque IU, Zaritsky AL. Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children: Pediatric Critical Care Medicine 2007;8(2): 138–44. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamoorthy V, Chaikittisilpa N, Kiatchai T, Vavilala M. Hypertension After Severe Traumatic Brain Injury: Friend or Foe? Journal of Neurosurgical Anesthesiology 2017;29(4): 382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaiwat O, Sharma D, Udomphorn Y, Armstead WM, Vavilala MS. Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J Neurotrauma 2009;26(5):657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vavilala MS, Lee LA, Boddu K, et al. Cerebral autoregulation in pediatric traumatic brain injury*: Pediatric Critical Care Medicine 2004;5(3):257–63. [DOI] [PubMed] [Google Scholar]
- 19.Van Cleve W, Kernic MA, Ellenbogen RG, et al. National Variability in Intracranial Pressure Monitoring and Craniotomy for Children With Moderate to Severe Traumatic Brain Injury. Neurosurgery 2013;73(5):746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesnut RM, Temkin N, Carney N, et al. A Trial of Intracranial-Pressure Monitoring in Traumatic Brain Injury. N Engl J Med 2012;367(26):2471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youden W Index for rating diagnostic tests. Cancer 1950;3(1):32–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


