Abstract
The development of selectively bred high and low alcohol-preferring mice (HAP and LAP, respectively) has allowed for an assessment of the polygenetic risk for pathological alcohol consumption and phenotypes associated with alcohol use disorder (AUD). Accumulating evidence indicates that the dorsal striatum (DS) is a central node in the neurocircuitry underlying addictive processes. Therefore, knowledge of differential gene, protein, and phosphorylated protein expression in the DS of HAP and LAP mice may foster new insights into how aberrant DS functioning may contribute to AUD-related phenotypes. To begin to elucidate these basal differences, a complementary and integrated analysis of DS tissue from alcohol-naïve male and female HAP and LAP mice was performed using RNA sequencing, quantitative proteomics, and phosphoproteomics. These datasets were subjected to a thorough analysis of gene ontology, pathway enrichment, and hub gene assessment. Analyses identified 2,108, 390, and 521 significant differentially expressed genes, proteins, and phosphopeptides, respectively between the two lines. Network analyses revealed an enrichment in the differential expression of genes, proteins, and phosphorylated proteins connected to cellular organization, cytoskeletal protein binding, and pathways involved in synaptic transmission and functioning. These findings suggest that the selective breeding to generate HAP and LAP mice may lead to a rearrangement of synaptic architecture which could alter DS neurotransmission and plasticity differentially between mouse lines. These rich data sets will serve as an excellent resource to inform future studies on how inherited differences in gene, protein, and phosphorylated protein expression contribute to AUD-related phenotypes.
Keywords: Alcohol, AUD, Dorsal Striatum, RNA-sequencing, Proteomics, Phosphoproteomics
Graphical Abstract

Introduction
Alcohol Use Disorder (AUD) is a significant public health burden that contributes to 88,000 deaths and costs approximately $250 billion per year in the United States, alone (Sacks et al. 2015; Stahre et al. 2014). AUD is heavily genetically-influenced, with increased individual risk of developing AUD corresponding with the extent of AUD diagnosis within an individual’s family (Kendler et al. 2018; Yoon et al. 2013). The extent to which the association between this genetic risk and aberrant neurobiology and function in humans can be explored is significantly limited by important ethical considerations. To this end, animal models of genetic AUD risk have been valuable tools to explore these types of questions in neuroscience.
One such model is the selectively bred high and low alcohol-preferring mice (HAP and LAP, respectively). To generate these selected lines, researchers phenotyped mice from a genetically heterogenous foundation stock of HS/Ibg mice (obtained from the Institute of Behavior Genetics at Boulder, CO) for preference of a 10% ethanol (alcohol; v/v) solution over water in a continuous access paradigm (see Oberlin et al., 2011 for a detailed description of these lines). Mice with the highest degree of alcohol preference were bred together and the same was done with the lowest preferers. The offspring of these animals were the first generation of HAP and LAP mice, respectively. As phenotyping and selective breeding continued over generations, the HAP and LAP lines increasingly diverged in alcohol preference and consumption (Matson & Grahame 2013; Oberlin et al. 2011). Eventually, the 10% alcohol solution accounted for nearly all of the daily fluid intake for HAP mice with consumption > 20 g/kg/day of alcohol and blood alcohol concentrations measured ~80–225 mg/dl, 80 mg/dl being equivalent to the legal driving limit in the United States (Matson & Grahame 2013). LAP mice on the other hand, became virtually complete abstainers and consumed < 5% of their daily fluid from the alcohol tube and had no measurable blood alcohol concentrations following access. The HAP and LAP lines thus model genetic propensities for excessive alcohol consumption (HAP) or avoidance (LAP).
An additional strength of this model is that HAP mice express other neurobehavioral phenotypes consistent with the clinical AUD description, thus increasing its translational validity. HAP mice exhibit a greater basal pharmacodynamic, but not pharmacokinetic, alcohol tolerance capacity relative to LAP mice and will drink alcohol to the point of both pharmacodynamic and pharmacokinetic tolerance development (Fritz & Boehm II 2014; Fritz et al. 2013). In addition, HAP mice are more impulsive than LAP mice (Oberlin & Grahame 2009), develop sensitization to alcohol’s psychomotor stimulating effects (Grahame et al. 2000), and demonstrate binge-like alcohol consumption (Linsenbardt & Boehm 2015). Collectively, these data strongly support the use of HAP and LAP mice as divergent genetic risk models for AUD development.
The dorsal striatum (DS) has received increased attention in the addiction field over recent years given its significant role in action selection and specific relevance to goal-directed and habitual behaviors, extremes of which may foster the development of AUD (Ma et al. 2017; Yin & Knowlton 2006; Corbit et al. 2012). Differential functioning of the DS as a result of differential gene and protein expression between the HAP and LAP mice may contribute to the observed divergent alcohol consumption in these lines. A recent study by our lab examined functional neurophysiological differences between alcohol-naïve HAP and LAP mice, specifically within the DS. We found significant differences in both excitatory and inhibitory DS neurotransmission between the HAP and LAP lines (Fritz et al. 2019). Although the HAP and LAP lines have distinct excitatory and inhibitory neurotransmission profiles in the DS, the mechanisms of these observations are not clear. To begin to elucidate explanations for these functional differences, DS tissue from both male and female, alcohol-naïve HAP and LAP mice was subjected to a thorough RNA-sequencing analysis and quantitative proteomic analyses of both global protein and phosphorylated peptide expression.
Materials and Methods
Animals
The animal experimental procedures in this study were approved by the Institutional Animal Care and Use Committee at the Indiana University School of Medicine (Protocol Number 19017). Guidelines set forth by the National Institutes of Health (Maryland, USA) for ethical treatment and care for experimental animals were followed. Adult (postnatal day 60–75) male and female HAP and LAP mice bred and maintained at Indiana University-Purdue University Indianapolis (IUPUI) facilities were transferred to our laboratory prior to tissue collection. All mice were alcohol-naïve and group-housed at the time of tissue collection. The vivarium space was maintained at 20°C and 50% relative humidity with ad libitum access to food and water. These lines were described in the introduction and information related to their development may be found elsewhere (Matson and Grahame, 2013; Oberlin et al., 2011). For the current work, mice from the 66th generation of the second replicate were used. Replicate lines are used to ensure that the alcohol preference selection phenotype could be produced in multiple lines. Our choice to only test replicate two here was based on the similar physiological measures between replicates two and three that we found previously (Fritz et al. 2019) and that the first LAP replicate is currently extinct. To minimize pain and distress of animals, animals were anesthetized under isoflurane by placement into a desiccator containing a gauze pad with approximately 200 uL of isoflurane. After approximately 30 seconds in the desiccator, animals were removed and rapid decapitation for tissue collection was performed after animals were assessed for the absence of a pedal withdrawal reflex. Isoflurane was chosen over other anesthetics due to the rapid onset of action and minimally invasive method (i.e. no injections) for deeply anesthetizing animals prior to decapitation (Gargiulo et al. 2012). Following longer periods of isoflurane exposure usually intended to model a surgical procedure, others have reported a significant impact of isoflurane on the brain phosphoproteome (Kohtala et al. 2016), protein kinases (Tao et al. 2016), and phosphatases (Cao et al. 2014). Given our very brief period of isoflurane exposure prior to tissue collection, the effect of isoflurane on differentially expressed phosphorylated proteins is likely minor; nonetheless, we cannot be confident that isoflurane did not interact with genetic background to alter the phosphorylation status of certain proteins.
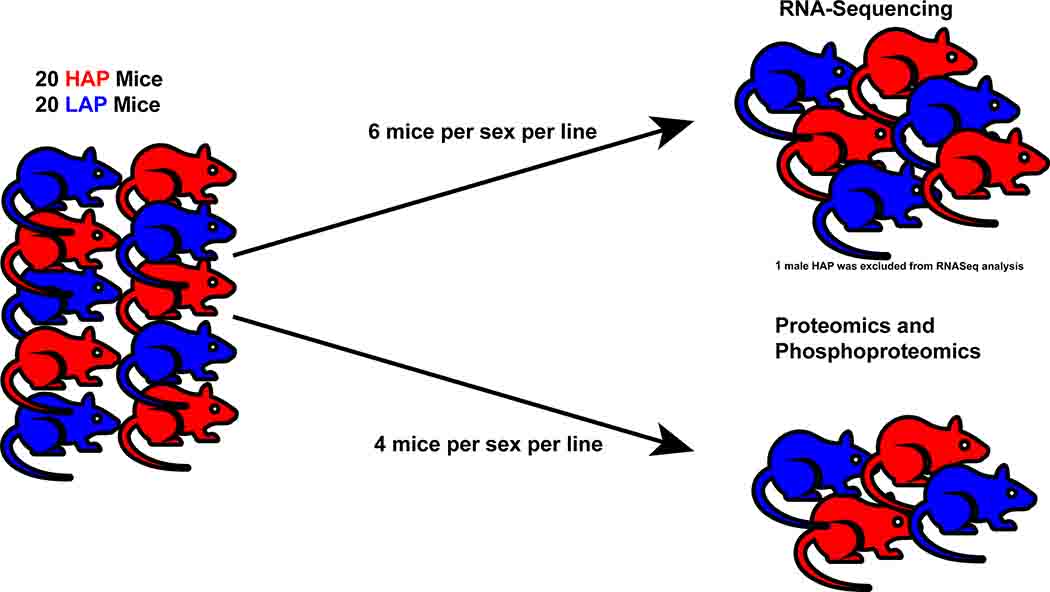
This study was not officially pre-registered nor were any a priori power calculations completed to determine sample size as this was an exploratory study. However, the number of biological replicates was determined from previously powered RNA-sequencing studies which used sample sizes of 2–6 per group (Boisvert et al. 2018; Li et al. 2017; Tanaka et al. 2013; Zhang et al. 2017) and proteomic/phosphoproteomic studies which used sample sizes of 3–4 per group (Boza-Serrano et al. 2018; Gonzalez-Lozano et al. 2016; Kohtala et al. 2016; Sharma et al. 2015). See Figure 1 for a brief workflow of the completed experiments.
Figure 1. Experimental Overview.
RNA-Sequencing
Separate brains from 12 HAP (6 male, 6 female) and 12 LAP (6 male, 6 female) mice were rapidly harvested by a blinded researcher and the DS, as a whole, was dissected bilaterally and homogenized. Tissue collection was completed in the morning to midday and samples were deidentified until tissue was fully processed and data was prepared for analysis. RNA was isolated from brain tissue using the RNeasy Plus Universal Mini Kit (Qiagen #73404) according to the manufacturer’s protocol. The paired-end library was prepared using the Dual Indexed KAPA mRNA Hyperprep Kit (KK8581; Roche). Bulk RNA-sequencing was performed on an Illumina HiSeq4000. Reads 75bp long were generated using a HiSeq3000/4000 PE cluster/SBS kits (Illumina #PE-410–1001, #FC-410–1002). Although single cell RNA-sequencing would have provided detailed data on differential gene expression across heterogenous cell populations in the DS of HAP and LAP mice, current technical capabilities limit the performance of single cell proteomics/phosphoproteomics. As our intent with the current study was to provide a complementary and integrative multi-omic analysis of the DS, we chose to employ bulk RNA-sequencing so that our findings could be integrated and compared to proteomic/phosphoproteomic findings. Quality control of the raw sequence data was performed using FastQC/MultiQC. The sequence reads were mapped to the UCSC mm10 reference genome using STAR (Spliced Transcripts Alignment to a Reference) for the B6 mouse (Dobin et al. 2013). Although the HAP/LAP mice are derived from a heterogenous stock, read mismatch parameters were not adjusted as we find the default STAR alignment settings regarding mismatches to be liberal. (STAR allows up to 10 mismatches across an alignment which equates to 10 mismatches in 150 (2×75 paired read) bases in this experiment. Uniquely mapped reads were retained by filtering for a MAPQ of 60 during alignment and 10 during counting. Therefore, we applied a balanced approach where mismatches were allowed but there was high confidence in the mapping location for a fragment to be counted.) To evaluate the quality of the RNA-sequencing data, the numbers of reads that map to different annotated regions (e.g. exonic, intronic, splicing junction, intergenic, promoter, UTR) were determined with bamUtils (Breese & Liu 2013). Low quality mapped reads (including reads mapped to multiple positions) were excluded. The tool ‘featureCounts’ was used to quantify gene level read counts (Liao et al. 2014). Differential gene expression analysis was performed with edgeR (Robinson et al. 2010). According to recommendations in the edgeR user’s guide, genes with read counts <0.5 CPM in ≥ 12 samples were filtered out and a negative binomial generalized linear model with likelihood ratio test was used. A multi-dimensional scaling (MDS) plot provided a visual representation of the similarities and differences among the experimental samples (Supplementary Figure 1A,B). Distances on the plot corresponded to the Euclidian distance (root-mean-square) of leading log2 fold change (FC) for the most variable genes between each pair of samples (Ritchie et al. 2015). Comparisons were adjusted for sex by implementing a batch factor according to the edgeR manual. One sample, a HAP male, was identified as an outlier and was found to have a much smaller library size with significantly more reads aligning to mitochondrial sequences when compared to other samples. Therefore, this sample was removed from the analyses. Full processed datasets and analyses may be found at https://github.com/dlhagger/AUD-Multi-Omics.
Quantitative Reverse Transcription PCR (RT-qPCR)
The remaining isolated RNA utilized for RNA-sequencing was used to confirm transcriptomics findings using RT-qPCR. RNA concentrations were determined by Nanodrop (ThermoFisher) and complementary DNA (cDNA) was generated from 1μg of total RNA using the iScript Reverse Transcription Supermix for RT-qPCR. (BioRad, Cat No: 1708840) The qPCR C5ar2 primer sequences were purchased from Integrated DNA Technologies (IDT) and designed as described previously (Miyabe et al. 2019) (Forward: 5′-ACCACCAGCGAGTATTATGACT-3′ and Reverse: 5′-GCAGGACTATCAGGTAGACATCA-3′) while Pla2g3 (Cat No: qMmuCIP0028420), Kcnj15 (Cat No: qMmuCEP0042967), and Ubc (Cat No: qMmuCIP0034811) were purchased from BioRad. qPCR was conducted by using the SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA; Cat No: 1725270) on an CFX Connect Real-Time PCR Detection System (Bio-Rad). The relative amount of each transcript was determined via normalization across all samples to the endogenous control, GAPDH. cDNA samples from each individual animal were run in duplicate. To quantify the relative expression levels of the genes for each mouse genotype, we calculated the difference (ΔCt) between the cycle threshold of each gene and the housekeeping gene, GAPDH. From these data, the ΔΔCt ([ΔCtGene(HAP)–ΔCtGene(LAP)]) was computed and converted to a relative quantitative (RQ) value using the formula 2−ΔΔCt. An ANOVA with line (HAP/LAP) and sex (M/F) as factors was used to compare RQ of C5ar2, Pla2g3, Kcnj15, and Ubc using GraphPad Prism (version 8.2). The level of significance was set at p<0.05.
Protein Preparation
Sample preparation, mass spectrometry analysis, bioinformatics, and data evaluation for quantitative proteomics and phosphoproteomics experiments were performed in collaboration with the Proteomics Core Facility at the Indiana University School of Medicine. Methods described below in brief were adaptations from literature reports (Levasseur et al. 2019) and vendor provided protocols.
Separate brains from 8 HAP (4 male, 4 female) and 8 LAP (4 male, 4 female) mice were rapidly harvested by a blinded researcher and the DS, as a whole, was dissected bilaterally and homogenized. Tissue collection was completed in the morning to midday and samples were deidentified until tissue was fully processed and data was prepared for analysis. Flash frozen brain lysates were prepared in 1 mL of 9 M urea (CHEBI: 16199) in 20 mM HEPES, pH 8.0 (CHEBI: 46756), 1x cOmpleteTM protease inhibitor cocktail (Roche Diagnostics cat. 11836153001). Tissues were homogenized using a BeadBugTM 6 (Benchmark scientific Cat No: D1036, 3mm zirconium beads Cat No: D1032–30, 10 rounds of 30 × 30 sec,4 °C). Samples were next sonicated in 1.5 mL Micro Tubes (TPX Plastic for Sonication from Diagende Inc.) using a Bioruptor® sonication system (Diagenode Inc. USA, North America cat number B01020001) with 30 sec/30 sec on/off cycles for 15 minutes in a water bath at 4 °C. After subsequent centrifugation at 12,000 rpm for 20 min, protein concentrations were determined by Bradford protein assay (BioRad Cat No: 5000006). 400 μg equivalent of protein from each brain were then treated with 5 mM tris(2-carboxyethyl)phosphine hydrochloride (Sigma-Aldrich Cat No: C4706) to reduce disulfide bonds and the resulting free cysteine thiols were alkylated with 10 mM chloroacetamide (Sigma Aldrich Cat No: C0267). Samples were next diluted with 100 mM Tris.HCl (Sigma-Aldrich Cat No: 10812846001; to a final urea concentration of 2 M to carry out Trypsin/Lys-C based overnight protein digestion to derive peptides (1:100 protease:substrate ratio, Mass Spectrometry grade, Promega Corporation, Cat No: V5072.) (Levasseur et al. 2019; Li et al. 2020)
Peptide Purification and Labeling
Peptides were desalted on Sep-Pak® Vac cartridges (WatersTM Cat No: WAT054955), dried down, and resuspended in 24 μL of 50 mM triethylammonium bicarbonate. Each sample was then labeled for two hours at room temperature, with 3 mg of Tandem Mass Tag Pro (TMTpro) reagent (16-plex kit, manufactures instructions Thermo Fisher Scientific,TMTpro™ Isobaric Label Reagent Set; Cat No: 44520, lot no. UL297970) (Li et al. 2020). Samples were randomized and labeled as indicated in the supplementary excel data files (https://github.com/dlhagger/AUD-Multi-Omics). Labelling reactions were quenched by adding 5% hydroxylamine to the reaction mixtures and holding at room temperature for 15 minutes. Labeled peptides were then mixed, desalted on 3 × 100 mg Sep-Pak® Vac (WatersTM Cat No: WAT036820) cartridges and dried by speed vacuum prior to phosphopeptide enrichment.
Phosphopeptide Enrichment
Phosphopeptides were enriched using High-Select™ TiO2 Phosphopeptide Enrichment Kits (Thermo Fisher Scientific, catalog A32993). After preparing spin tips as per manufacturer’s instructions, approximately 2 mg of labeled and mixed peptides were repeatedly applied to 3 separate TiO2 spin tips and eluted in 50 μL elution buffer. Elutions were combined for phosphoproteomics. The flow through from each tip was saved and combined for global proteomics.
High pH Basic Fractionation
Purified phosphopeptides and 300 μg of global peptides were reconstituted in 0.1% trifluoroacetic acid and fractionated on PierceTM High pH reversed-phase peptide fractionation spin columns using the vendor provided methodology (Thermo Fisher Cat No: A32993). Global TMT-peptides (flow through from TiO2 spin tips) were fractionated into 9 fractions, and TMT-labeled enriched phosphopeptides were fractionated into 6 fractions (including the 5% acetonitrile wash). Each fraction was dried by speed vacuum and reconstituted in 30 μL 0.1% formic acid (FA, CHEBI:30751).
Nano-LC-MS/MS Analysis
Nano-LC-MS/MS analyses were performed on an EASY-nLC™ HPLC system (SCR: 014993, Thermo Fisher Scientific) coupled to Orbitrap Fusion™ Lumos™ mass spectrometer (Thermo Fisher Scientific). One third of each fraction was loaded onto a reversed phase EasySprayTM C18 column (2 μm, 100 Å, 75 μm x 50 cm, Thermo Scientific Cat No: ES802A) at 400 nL/min. Peptides were eluted from 4–28% with mobile phase B (Mobile phases A: 0.1% FA, water; B: 0.1% FA, 80% Acetonitrile (Fisher Scientific Cat No: LS122500)) over 160 minutes; 28%−35% B over 5 mins; 35–50% B for 14 minutes; and dropping from 50–10% B over the final 1 min. The mass spectrometer method was operated in positive ion mode with a 4 sec cycle time data-dependent acquisition method with advanced peak determination and Easy-IC (internal calibrant). Precursor scans (m/z 400–1750) were done with an orbitrap resolution of 120000, RF lens% 30, maximum inject time 50 ms, standard AGC target, including charges of 2 to 6 for fragmentation with 60 sec dynamic exclusion. MS2 scans were performed with a fixed first mass of 100 m/z, 34% fixed CE, 50000 resolution, 20% normalized AGC target and dynamic maximum IT. The data were recorded using Thermo Fisher Scientific Xcalibur (4.3) software (Thermo Fisher Scientific Inc.).
SPS-MS3 Phosphopeptide Replicate
A replicate of the phosphopeptides was run using a SPS-MS3 method. LC conditions were the same as above. The mass spectrometer method was operated in positive ion mode with 3 sec cycle time data-dependent acquisition method with advanced peak determination and Easy-IC (internal calibrant). Precursor scans (m/z 400–1750) were done with an orbitrap resolution of 120000, RF lens% 30, maximum inject time 50 ms, standard AGC target, including charges of 2 to 7 for fragmentation with 60 sec dynamic exclusion. Data-Dependent MS2 scan properties included selection m/z 400–1200, exclusion mass width of 5 m/z, isobaric tag loss exclusion of TMTpro, an isolation window of 0.7 CID collision energy fixed at 35%, neutral loss mass 97.9673, detector: orbitrap, orbitrap resolution: 30000. MS3 data dependent scans in the scan range 100–500, based on 10 synchronous precursor selection (SPS) with 2 m/z isolation window, isolation offset off, HCD activation 65% collision energy, detector orbitrap, orbitrap resolution 50000. Data were recorded using Thermo Fisher Scientific Xcalibur (4.3) software (Thermo Fisher Scientific Inc.).
Proteome and Phosphoproteome Analysis
Resulting RAW files were analyzed in Proteome Discover™ 2.4 (Thermo Fisher Scientific, RRID: SCR_014477) with a mus musculus UniProt FASTA plus common contaminants. Quantification methods utilized isotopic impurity levels available from Thermo Fisher Scientific. SEQUEST HT searches were conducted with a maximum number of 3 missed cleavages; precursor mass tolerance of 10 ppm; and a fragment mass tolerance of 0.02 Da. Static modifications used for the search were, 1) carbamidomethylation on cysteine (C) residues; 2) TMTpro label on lysine (K) residues and the N-termini of peptides. Dynamic modifications used for the search were oxidation of methionines and acetylation of N-termini. Percolator False Discovery Rate was set to a strict setting of 0.01 and a relaxed setting of 0.05. IMP-ptm-RS node was used for all modification site localization scores. For SPS-MS3 quantification, SEQUEST HT searches were conducted with a maximum number of 3 missed cleavages; precursor mass tolerance of 10 ppm; and a fragment mass tolerance of 0.5 Da, and MS3 FTMS reporter ion tolerance of 20 ppm. Values from both unique and razor peptides were used for quantification. In the consensus workflows, peptides were normalized by total peptide amount with no scaling. Data shown is for HAP/LAP abundance value ratios (AR). Sex/Line ratios are provided at https://github.com/dlhagger/AUD-Multi-Omics. Resulting grouped abundance values for each sample type, AR values; and respective p-values (t-test) from Proteome Discover™ were exported to Microsoft Excel.
Using the exported sex, line, and AR values, principal component analyses were performed on the proteome and phosphoproteome. Analyses were conducted in python using pandas and NumPy to import and clean the data (McKinney 2010; Walt et al. 2011). AR values were preprocessed using the standard scaler function in scikit-learn (Pedregosa et al. 2011). Principal component analyses were done using the PCA function in scikit-learn with the total number of components for the analysis being chosen based on the cumulative sum of the components explaining 80% of the total variance. For the proteome, 8 components were chosen for analysis and for the phosphoproteome, 6 components were chosen for analysis. For both analyses, principal component 1 and principal component 2 were plotted with sex, line, or both labels to visualize the output data. Full processes datasets and analyses may be found at https://github.com/dlhagger/AUD-Multi-Omics and raw data files are available upon request.
Network Analyses
Differentially expressed genes were sorted and filtered with a cutoff of absolute log2 FC>±0.5, FDR<0.05 and differentially expressed total proteins and phosphopeptides were sorted and filtered for p values<0.05. The filtered differentially expressed genes, proteins, and phosphorylated proteins were submitted to String-db (https://string-db.org/) and were filtered with an edge confidence of 0.9 and for at least one interaction. Although several proteins had multiple phosphopeptide abundance changes, only the unique UniProt accession numbers were submitted to String-db from the filtered phosphopeptide list. Using String-db, gene ontology (GO), KEGG and Reactome pathway enrichment were determined for these filtered differentially expressed genes, proteins, and phosphorylated proteins. The filtered differentially expressed genes and global proteins were then analyzed in CytoScape software using the cytoHubba plugin to identify hub genes/proteins (nodes ≥ 7 degrees) (Chin et al. 2014). Hubs were categorized by number of degrees and maximal clique centrality (MCC) which is another validated predictor of how essential a particular node is within a network (Chin et al. 2014). Full network and enrichment results analyses may be found in the Supplementary Files.
Integrated Analysis
Differential gene (FDR<0.05), protein (p<0.05), and phosphorylated protein (p<0.05) expression profiles were classified and visualized using python and matplotlib-Venn (https://github.com/konstantint/matplotlib-Venn). Phosphorylated protein master accession numbers were converted to GeneIDs using UniProt’s mapping tool so the overlap of all three datasets could be computed and plotted in a Venn diagram. Similarly, the overlap in enriched GO terms and pathways from the network of significant differentially expressed genes (filtered using log2≥±0.5), proteins, and phosphorylated proteins were computed and used to create Venn diagrams. A linear regression was computed using GraphPad Prism (version 8.2) to model the relationship between log2FC of differentially expressed genes and the log2AR of differentially expressed proteins. We confirmed that residuals were normally distributed and the relationship between log2FC and log2AR was linear. An integrated pathway analysis was completed using the RNA-sequencing and proteomic data by the PaintOmics3 tool (Hernández-de-Diego et al. 2018). Significant differentially expressed genes and proteins log2FC and log2AR values, respectively, were used to generate overviews for pathways of interest by locating and displaying changed expression patterns. The PaintOmics3 job with the associated datasets of differentially expressed genes and proteins can be utilized for those who may have interest in other KEGG pathways (http://www.paintomics.org/?jobID=dCEK1fF2R4).
Results
Overview of Analyses
Multidimensional scaling of the RNA-sequencing data demonstrated clear data clustering distinguishing selected lines and sexes (Supplementary Figure 1A and B). As indicated in the Methods, comparisons between lines for RNA-sequencing datasets were adjusted for sex. Principal component analyses of the proteomic and phosphoproteomic data sets revealed limited clustering for lines, sex, and a line by sex interaction (Supplementary Figure 1C–H). Due to the lack of clustering for sexes, subsequent network analyses of proteomic and phosphoproteomic data did not include sex as a factor. However, specific differences in differentially expressed proteins and phosphopeptides between each sex and the HAP and LAP lines may be found at https://github.com/dlhagger/AUD-Multi-Omics. Although sex effects on alcohol drinking exist in HAP and LAP mice, these sex effects are present in rodents, in general, suggesting sex is not specifically related to the divergent alcohol consumption and related phenotypes associated with HAP and LAP mice (Oberlin et al. 2011). Furthermore, our prior work assessing DS neurotransmission demonstrated relatively consistent effects across sexes for each line (Fritz et al. 2019). Volcano plots revealing distributions for the RNA-sequencing, proteomic, and phosphoproteomic analysis can be found in Supplementary Figure 2.
RNA-Sequencing
A differential gene expression analysis was first performed to identify underlying differences that may account for the various endophenotypes of the selected lines and to initiate an exploration for potential molecular mechanisms that could be related to the physiological differences previously observed in the DS of HAP and LAP mice (Fritz et al. 2019). Of the 13,870 transcripts identified, 2,108 of these were found to be significantly differentially expressed between the lines (FDR<0.05). Table 1 lists the top 20 differentially expressed proteins in HAP relative to LAP mice. Although it is not feasible to validate all the differences identified by RNA-sequencing, we confirmed that C5ar2 (ANOVA: Line, F(1,20)=67.3, p<0.0001; Sex, F(1,20)=3.28, p=0.09; Interaction, F(1,20)=3.37, p=0.08) and Pla2g3 (ANOVA: Line, F(1,20=54.8, p<0.0001; Sex, F(1,20)=3.00, p=0.10; Interaction, F(1,20)=3.08, p=0.09) are increased in HAP mice relative to LAP mice and Kcnj15 (ANOVA: Line, F(1,19)=70.5, p<0.0001; Sex, F(1,19)=0.0156, p=0.90; Interaction, F(1,19)=0.0838, p=0.78) and Ubc (ANOVA: Line, F(1,20)=85.5, p<0.0001; Sex, F(1,20)=0.225, p=0.64; Interaction, F(1,20)=0.0145, p=0.91) are decreased in HAPs relative to LAPs using RT-qPCR (Supplementary Figure 3).
Table 1.
Top 20 Differentially Expressed Genes.
| Gene | Gene Name | Log2(FC HAP/LAP) |
|---|---|---|
| Increased in HAP vs LAP | ||
| Tuba1c | Tubulin, alpha 1C | 6.04 |
| C5ar2 | Complement component 5a receptor 2 | 3.64 |
| Vmn2r84 | Vomeronasal 2, receptor 84 | 3.63 |
| BC002163 | NADH dehydrogenase Fe-S protein 5 pseudogene | 2.94 |
| Gm1821 | Ubiquitin pseudogene | 2.52 |
| Gdpd3 | Glycerophosphodiester phosphodiesterase domain containing 3 | 2.25 |
| Pla2g3 | Phospholipase A2, group III | 1.94 |
| Itgal | Integrin alpha L | 1.88 |
| Cd84 | CD84 antigen | 1.85 |
| Mid1 | Midline 1 | 1.84 |
| Decreased in HAP vs LAP | ||
| Kcnj15 | Potassium inwardly-rectifying channel, subfamily J, member 15 | −3.61 |
| Oscar | Osteoclast associated receptor | −3.55 |
| Tnni1 | Troponin I, skeletal, slow 1 | −2.98 |
| Vmn2r29 | Vomeronasal 2, receptor 29 | −2.97 |
| Cd6 | CD6 antigen | −2.08 |
| Mxra8 | Matrix-remodeling associated 8 | −1.70 |
| Ubc | Ubiquitin C | −1.70 |
| Dcdc2b | Doublecortin domain containing 2b | −1.68 |
| Il12rb1 | Interleukin 12 receptor, beta 1 | −1.67 |
| Tmem40 | Transmembrane protein 40 | −1.60 |
FC, Fold Change.
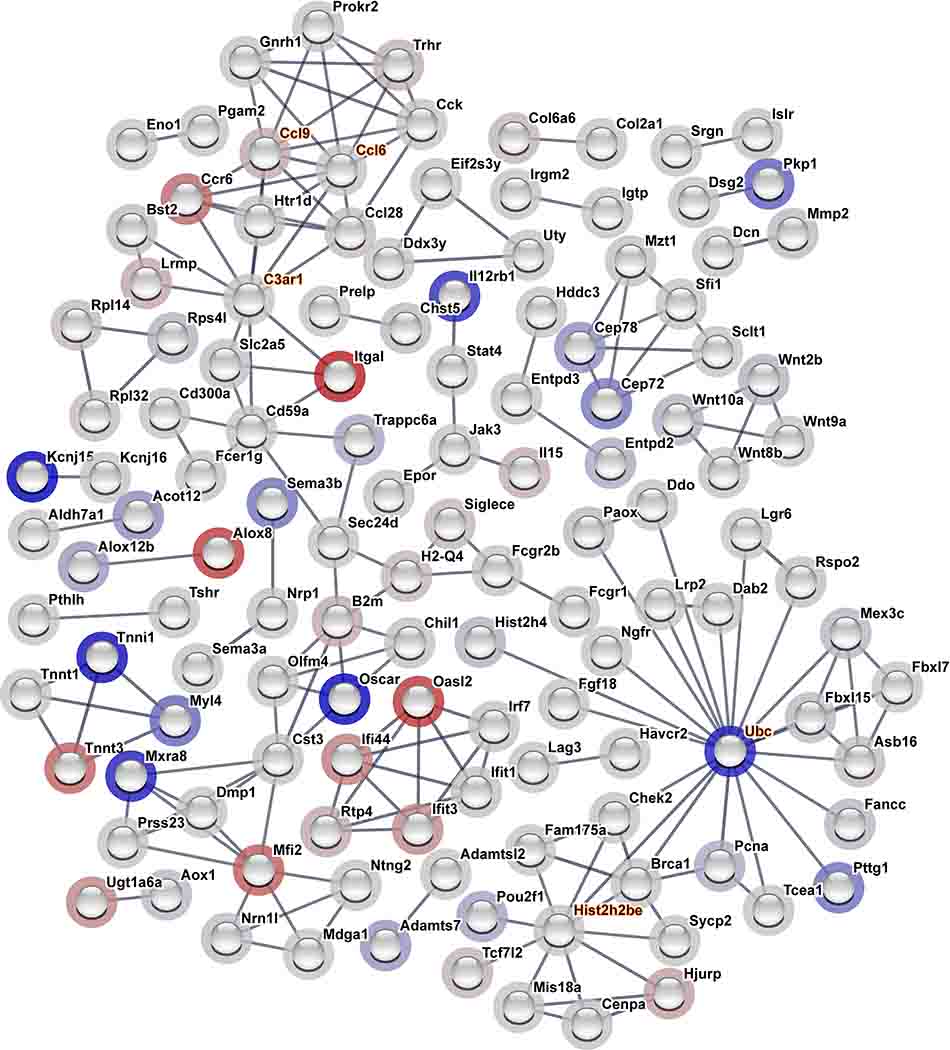
For network analysis, the gene list was filtered using a log2 FC HAP relative to LAP cut-off of ≥±0.5, which resulted in 485 significant differential expressed genes between lines. Of the 485 genes submitted for interaction analysis in String-db, 187 edges were discovered compared to an expected number of 158 edges (Figure 2; enrichment p=0.0126). Using Cytoscape’s CytoHubba, 8 hub genes were found in this differential gene expression network (See Supplementary File “Enrichment.Hubs_RNASeq” for full details on hub genes for network of differentially expressed genes) with the top five hub genes presented in Table 2 (also highlighted in orange text in Figure 2). Of these, Ubc displayed the greatest degrees, whereas Ccl6 and Ccl9 showed the greatest MCC. Although no KEGG or Reactome pathways were enriched in this network, the assessment of GO revealed 15 cellular components (CC), 137 biological processes (BP), and 3 molecular functions (MF) that were enriched in the network of differentially expressed genes submitted to String-db (See Supplementary Files for full details on significantly enriched GO terms for network of differentially expressed genes). In particular, immunity-related BPs and binding-related MFs were found to be enriched. We also noted an enrichment in extracellular-related CCs; however, it is worth noting that gene set functional enrichment analysis may be biased to long genes such as those found in the extracellular matrix (Mandelboum et al. 2019). Therefore, this finding must be viewed with caution.
Figure 2. Differentially Expressed Gene Interaction Network.
The 485 differentially expressed genes (log2 FC>±0.5, FDR<0.05) were submitted to String-db. Only high confidence interactions >0.9 with at least 1 connected gene are displayed. Node halo correlates with the log2 FC for HAP relative to LAP mice (red = increased expression and blue = decreased expression). Hub genes are highlighted in orange text. (n = 11 HAP and 12 LAP)
Table 2. Top Five Hub Genes from RNA-Sequencing Analysis.
Hub gene analysis was performed on the network of significant differentially expressed genes using CytoScape software with the cytoHubba plugin. Hub genes are highlighted in orange text in Figure 2.
| Gene | Gene Name | Log2(FC HAP/LAP) | Degree | MCC |
|---|---|---|---|---|
| Ubc | Ubiquitin C | −1.70 | 20 | 45 |
| Hist2h2be | Histone H2B type 2-E | 0.51 | 10 | 22 |
| C3ar1 | C3a anaphylatoxin chemotactic receptor | 0.65 | 10 | 128 |
| Ccl6 | C-C motif chemokine 6 | 0.79 | 9 | 246 |
| Ccl9 | C-C motif chemokine 9 | 1.02 | 9 | 246 |
FC, Fold change; MCC, maximal clique centrality.
Quantitative Proteomics
A quantitative LC/MS proteomic analysis of the DS in the selected lines was performed to further identify protein expression patterns which could account for the various behavioral and electrophysiological differences previously reported in these selected lines. A total of 4,682 protein groups and 29,554 peptide groups were identified with 390 unique proteins being differentially expressed between lines (p<0.05). Table 3 lists the top 20 differentially expressed proteins in HAP relative to LAP mice. Although the magnitude of differential expression for any single protein appears relatively small, ratio compression is a known effect of MS2 quantification with TMT-labels (Rauniyar & Yates 2014); therefore, actual differences in concentrations may be higher. Additionally, due to the use of 8 biological replicates per line and the precision of tandem mass tag labeling for quantification, any proteins with significant (p<0.05) differential expression between lines can be considered of interest biologically.
Table 3.
Top 20 Differentially Expressed Proteins.
| Gene | UniProt Accession | Protein | Log2(AR HAP/LAP) |
|---|---|---|---|
| Increased in HAP vs LAP | |||
| Serinc5 | Q8BHJ6 | Serine incorporator 5 | 0.49 |
| Gng5 | Q80SZ7 | Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-5 | 0.39 |
| Pvalb | P32848 | Parvalbumin alpha | 0.37 |
| Ttc34 | A0A140LHH00 | Tetratricopeptide repeat protein 34 | 0.36 |
| Mindy2 | Q6PDI6 | Ubiquitin carboxyl-terminal hydrolase MINDY-2 | 0.34 |
| Me3 | Q8BMF3 | NADP-dependent malic enzyme, mitochondrial | 0.31 |
| Mtmr6 | Q8VE11 | Myotubularin-related protein 6 | 0.30 |
| Itih3 | E9PVS1 | Inter-alpha-trypsin inhibitor heavy chain H3 | 0.26 |
| Sh3kbp1 | Q8R550 | SH3 domain-containing kinase-binding protein 1 | 0.26 |
| Phactr2 | B1AVP0 | Phosphatase and actin regulator | 0.26 |
| Decreased in HAP vs LAP | |||
| Entpd2 | O55026 | Ectonucleoside triphosphate diphosphohydrolase 2 | −0.72 |
| Pstpip1 | A0A0R4J0P5 | Proline-serine-threonine phosphatase-interacting protein | −0.64 |
| Fam177a1 | Q8BR63 | Protein FAM177A1 | −0.60 |
| Tmem33 | A0A0R4J1Z3 | Transmembrane protein 33 | −0.50 |
| Anln | Q8K298 | Anillin | −0.42 |
| Crocc | Q8CJ40 | Rootletin | −0.38 |
| Gdpgp1 | Q3TLS3 | GDP-D-glucose phosphorylase 1 | −0.38 |
| Pgam5 | Q8BX10 | Serine/threonine-protein phosphatase PGAM5, mitochondrial | −0.37 |
| Acad8 | D3YTT4 | Isobutyryl-CoA dehydrogenase, mitochondrial | −0.35 |
| Cst3 | A2APX3 | Cystatin | −0.34 |
AR, Abundance Ratio.
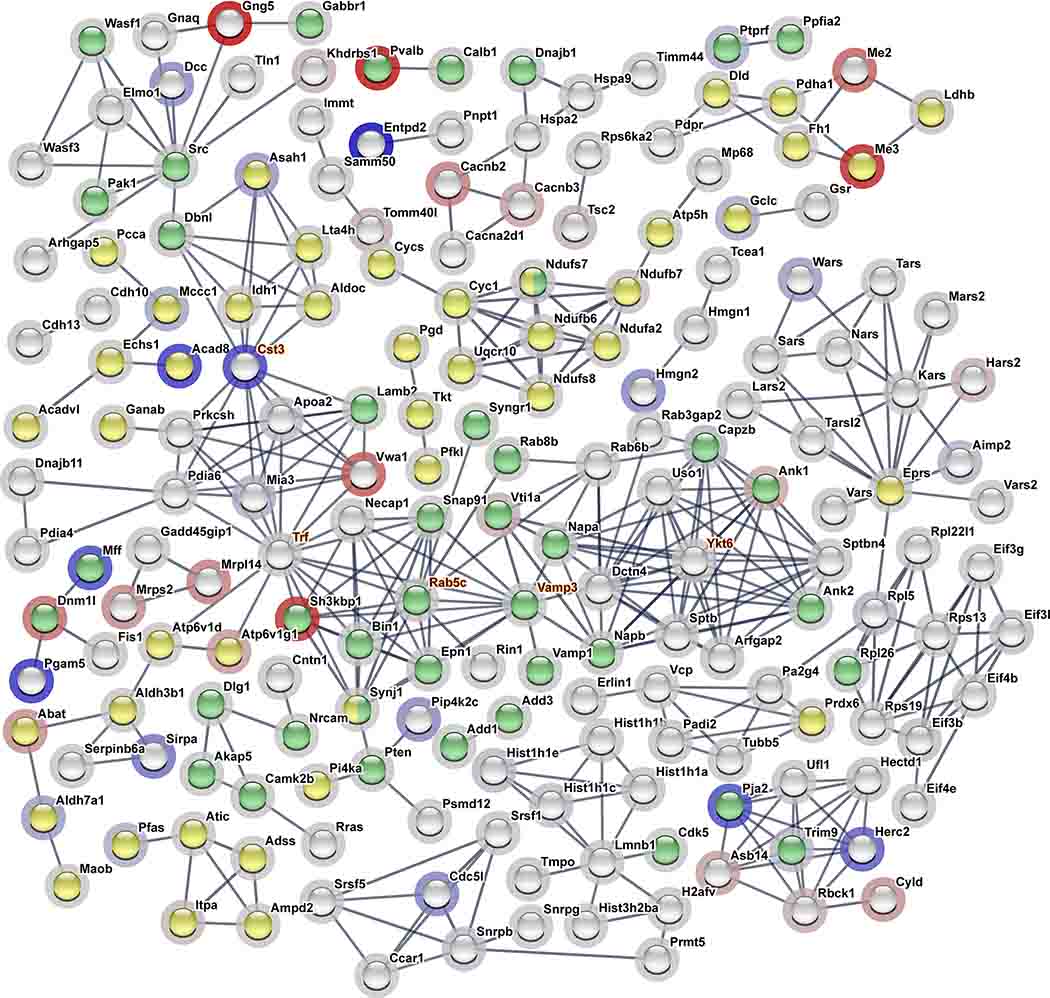
Many of the proteins that demonstrated the greatest differential expression were related to metabolic processes such as phosphoglycerate mutase 2, GDP-D-glucose phosphorylase, mitochondrial NAD- and NADP-dependent malic enzyme, and mitochondrial isobutyryl-CoA dehydrogenase. Of the 390 proteins submitted for interaction analysis in String-db, 376 edges versus an expected number of 239 edges were discovered (Figure 3; enrichment p=2.22×10−16). We identified 37 hub proteins in this highly enriched differential protein expression network (See Supplementary Files for full details on hub proteins for network of differentially expressed proteins). The top five are presented in Table 4 and are highlighted in orange text in Figure 3. Of these five hub proteins, serotransferrin exhibited the greatest degrees of interaction while synaptobrevin homolog Ykt6 displayed the greatest MCC. The assessment of enriched GO terms, KEGG pathways, and Reactome pathways revealed 485 BP, 103 MF, 148 CC, 31 KEGG pathways, and 37 Reactome pathways that were found to be enriched in the network of differentially expressed proteins (See Supplementary File “Enrichment.Hubs_Proteomic” for full details on significantly enriched GO terms network of differentially expressed proteins). The synapse CC (highlighted in green, Figure 3), mitochondrial CC, and metabolic-related BPs were particularly prominent. Additionally, the KEGG metabolic pathway (highlighted in yellow, Figure 3) and numerous other metabolic-related KEGG and Reactome pathways including the citric acid (TCA) cycle and respiratory electron transport, pyruvate metabolism, and citric acid (TCA) cycle, carbon metabolism, and valine, leucine and isoleucine degradation were among the top pathways found to be particularly enriched in this differential protein expression network (See Supplementary Dataset for full details on GO and pathways enrichment).
Figure 3. Differentially Expressed Protein Interaction Network.
The 390 differentially expressed proteins (p<0.05) were submitted to String-db. Only high confidence interactions >0.9 with at least 1 connected node are displayed. Node halo corresponds to the log2 AR HAP relative to LAP mice (red = increased expression and blue = decreased expression). Node color represents identified proteins associated with the significantly enriched metabolic pathways KEGG Pathway (yellow) and the Synapse Cellular Component (green). Hub proteins are highlighted in orange text (n = 8 per line)
Table 4. Top Five Hub Proteins from Proteomic Analysis.
Hub protein analysis was performed on the network of significant differentially expressed proteins using CytoScape software with the cytoHubba plugin. Hub proteins are highlighted in orange text in Figure 3.
| Gene | UniProt Accession | Protein | Log2(AR HAP/LAP) | Degree | MCC |
|---|---|---|---|---|---|
| Trf | Q921I1 | Serotransferrin | −0.07 | 17 | 45362 |
| Vamp3 | P63024 | Vesicle-associated membrane protein 3 | 0.09 | 13 | 40345 |
| Cst3 | A2APX3 | Cystatin-C | −0.34 | 12 | 5160 |
| Rab-5C | P35278 | Ras-related protein Rab-5C | 0.06 | 12 | 40324 |
| Ykt6 | Q9CQW1 | Synaptobrevin homolog YKT6 | 0.07 | 12 | 45384 |
MCC, maximal clique centrality; AR, Abundance Ratio.
Phosphopeptide Quantification
Phosphorylation is an important regulator of many molecular pathways and processes, which could potentially be an additional contribution to the divergent endophenotypes in HAP and LAP mice; therefore, phosphopeptide enrichment and quantitation across the DS was performed. A total of 7,289 phosphopeptide groups were quantified with 521 phosphopeptides (from 345 unique proteins) differentially expressed between lines (p<0.05). Table 5 identifies proteins of the top 20 phosphopeptides with the greatest differential expression in HAP relative to LAP mice.
Table 5. Proteins of the Top 20 Differentially Expressed Phosphorylated Peptides.
All peptides are also TMT modified.
| Gene | UniProt Accession | Protein | Peptide Position in Master Protein | Phosphorylations in Master Proteins (% localization probability if known) | Log2(AR HAP/LAP) |
|---|---|---|---|---|---|
| Increased in HAP vs LAP | |||||
| Ank2 | Q8C8R3 | Ankyrin-2 | 1815–1823 | 2xPhospho [S1816(100); S1819(100)] | 2.79 |
| Nefh | P19246 | Neurofilament heavy polypeptide | 565–582 | 2xPhospho [S571(100); S577(100)] | 1.75 |
| Ank2 | Q8C8R3 | Ankyrin-2 | 3217–3235 | 3xPhospho [S3226(100); S3229(100); S3230(100)] | 1.63 |
| Ank2 | Q8C8R3 | Ankyrin-2 | 1812–1823 | 2xPhospho [S1816(100); S1819(100)] | 1.14 |
| Nefh | P19246 | Neurofilament heavy polypeptide | 559–576 | 2xPhospho [S565(100); S571(100)] | 1.00 |
| Mapt | A0A0A0MQC7 | Microtubule-associated protein | 57–72 | 1xPhospho [T58(99.7)] | 0.98 |
| Bicc1 | G3X8S6 | Bicaudal C homolog 1 | 807–819 | 1xPhospho [S814(50); S819(50)} | 0.90 |
| Sgip1 | Q8VD37 | SH3-containing GRB2-like protein 3-interacting protein 1 | 76–99 | 1xPhospho [S95(100)] | 0.85 |
| Bsn | O88737 | Protein bassoon | 2858–2874 | 1xPhospho [S2860(100)] | 0.81 |
| Mical3 | Q8CJ19 | Protein-methionine sulfoxide oxidase MICAL3 | 515–520 | 1xPhospho [S517(100)] | 0.81 |
| Decreased in HAP vs LAP | |||||
| Ank2 | Q8C8R3 | Ankyrin-2 | 3763–3783 | 1xPhospho [S3781(91.5)] | −2.3 |
| Wdr37 | Q05BF4 | WD repeat-containing protein 37 | 20–26 | 2xPhospho [S22(100); S24(100)] | −1.39 |
| Dlg1 | D3Z3B8 | Disks large homolog 1 | 479–494 | 1xPhospho [S492(100)] | −1.39 |
| Amph | A0A0G2JEG8 | Amphiphysin | 495–509 | 1xPhospho [S504(100)] | −1.28 |
| Scg2 | Q03517 | Secretogranin-2 | 549–561 | 1xPhospho [S556(99.7)] | −1.21 |
| Naxe | Q8K4Z3 | NAD(P)H-hydrate epimerase | 41–52 | 1xPhospho [T45(89.4)] | −1.12 |
| Exoc7 | O35250 | Exocyst complex component 7 | 281–290 | 1xPhospho [S283(100)] | −1.1 |
| Scg2 | Q03517 | Secretogranin-2 | 549–561 | 1xPhospho [S556(99.7)] | −1.04 |
| Srsf10 | Q3TFP0 | Serine/arginine-rich-splicing factor 10 | 119–126 | 2xPhospho [S121(100); S123(100)] | −1.04 |
| Tgoln1 | Q62313 | Trans-Golgi network integral membrane protein 1 | 126–160 | 3xPhospho [S130(0.6); S131(32.8); T132(32.8); T135(32.8) | −0.99 |
AR, Abundance Ratio.
Of the 345 unique proteins that were submitted for interaction analysis in String-db, 221 edges were discovered versus an expected number of 96 edges (Figure 4; enrichment p < 1×10–16). The assessment of enriched GO and pathways revealed 532 BP, 91 MF, 190 CC, 20 KEGG pathways, and 26 Reactome pathways enriched in the network of proteins with differentially expressed phosphopeptides (See Supplementary File “Enrichment_Phospho” for full details on significantly enriched GO terms for network of differentially expressed phosphorylated proteins). In particular, the synapse CCs (highlighted in green, Figure 4) and the neuronal system, transmission across chemical synapses, trafficking of AMPA receptors, and clathrin-mediated endocytosis Reactome pathways were particularly prominent in this network of differential phosphorylated proteins. Additionally, cellular organization and cytoskeleton-related BPs and MFs such as cellular component organization and cytoskeletal protein binding were strongly enriched. Similarly, microtubule-associated protein 1B, ankyrin-2, neurofilament heavy polypeptide, and protein piccolo, which are critical structural and scaffolding proteins located in the synapse, demonstrated twelve or more unique phosphopeptides that were each found to be differentially expressed between lines (Table 6).
Figure 4. Differentially Expressed Phosphorylated Protein Interaction Network.
The 345 unique proteins with differential peptide phosphorylations (p<0.05) were submitted to String-db. Only high confidence interactions >0.9 with at least 1 connected node are displayed. Node color represents identified proteins associated with the significantly enriched Synapse Cellular Component (green). Node halos are not present in this network because many proteins displayed more than one significant differentially expressed phosphopeptide between lines (n = 8 per line)
Table 6.
Proteins with the Greatest Number of Unique Phosphorylated Peptides Differentially Expressed Between Lines
Integrated Analysis
Not surprisingly, many more differentially expressed genes than differentially expressed proteins or phosphopeptides were quantified. Of the 2,108 mRNA transcripts, 390 proteins, and 345 phosphorylated proteins differentially expressed (FDR or p<0.05) that could be matched to gene names or UniProt accession numbers, 87 transcripts and proteins, 34 proteins and phosphorylated proteins, and 45 transcripts and phosphorylated proteins overlapped between the respective datasets (Figure 5A). Eight transcripts, proteins, and phosphorylated proteins were quantified as significantly expressed differentially between lines in all three datasets (Figure 5A; Supplementary Table 1). Of the overlapping 87 transcripts/proteins found in the RNA-sequencing and quantitative proteomics analysis, 68 exhibited coordinated regulation (both mRNA and protein were increased or both mRNA and protein were decreased) and 19 demonstrated divergent regulation (increased in one analysis, decreased in the other). For these 87 overlapping genes and proteins, there was a moderately strong relationship between mRNA transcript log2 FC and protein log2 AR (R-square = 0.334, p<0.0001; Figure 5B). When comparing shared GO enrichment across the networks of differentially expressed genes, proteins, and phosphorylated proteins described above, 26 BP, 3 MF, and 3 CC were identified as enriched in all three networks (Figure 5C–E). Although no Reactome or KEGG pathways were enriched in the network of differentially expressed genes, 4 Reactome and 2 KEGG Pathways were found to be enriched in the network of both differentially expressed proteins and phosphorylated proteins (Figure 5F,G). The identity of the enriched GO terms and pathways shared across datasets can be found in Supplementary Table 2.
Figure 5. Integrated Analysis.
A) Venn diagram demonstrating the number of mRNA transcripts (red), proteins (green), and phosphorylated proteins (blue) differentially expressed between lines and the overlap between each dataset. B) The correlation between mRNA and protein log2 FC and AR, respectively for the overlapping 87 mRNA transcripts and proteins differentially expressed between lines. Venn diagrams representing the overlapping, enriched GO terms discovered across all three omics analyses including C) biological processes, D) molecular function, and E) cellular component. Enriched F) Reactome and G) KEGG pathways discovered from both the proteomics and phosphoproteomic analysis. See supplementary table 1 for the associated overlapping genes, proteins, and phosphorylated proteins, and supplementary table 2 for the overlapping GO terms and pathways enriched
The 2,108 mRNA transcripts and 390 proteins with differential expression between lines (FDR and p<0.05, respectively) were submitted to PaintOmics3 for an integrated KEGG pathway enrichment analysis (Hernández-de-Diego et al. 2018). The combined pathway analysis did not reveal any pathways significantly enriched for the combined datasets; however, images of genes/proteins that were differentially expressed in the present study were mapped onto the glutamatergic synapse and GABAergic synapse KEGG pathways as these pathways are of relevance to our previous work and may be of interest to the reader (Supplemental Figure 4).
Discussion
Here we present a complementary and integrated analysis of differential gene expression, protein regulation, and peptide phosphorylation in the DS of alcohol-naive mice genetically selected for high or low alcohol preference. Although prior genome wide analyses and microarrays have been performed in replicates of HAP and LAP mice (Bice et al. 2006; Bice et al. 2009; Bice et al. 2011; Mulligan et al. 2006), this study represents the first investigation of combined differential gene, protein, and phosphorylated protein expression and functional analyses of these differentially expressed networks in this mouse model of AUD. While considerable work has focused on the nucleus accumbens (a part of the ventral striatum) in alcohol research, the DS represents an additional region of interest where aberrant function is associated with pathological alcohol seeking and consumption. The DS receives dense glutamatergic inputs from the cortex, thalamus, and limbic regions, and these circuits play a critical role in alcohol drinking and alcohol-related neuroadaptations (Wang et al. 2010; DePoy et al. 2013; Fanelli et al. 2013; Corbit et al. 2012). Therefore, understanding differences in basal functioning between the DS of HAP and LAP mice is likely relevant to a number of the alcohol-related phenotypes observed in these animals. Our laboratory recently examined the electrophysiological properties of DS GABAergic medium spiny neurons (MSNs), which account for ~95% of the cells in the this region (Tepper et al. 2007), of alcohol-naïve HAP and LAP mice (Fritz et al. 2019). MSNs in the DS were significantly more excitable in HAP relative to LAP mice. In addition, our findings suggested that presynaptic glutamate and GABA release were significantly enhanced in HAP relative to LAP DS. Finally, the ratio of DS AMPA to NMDA receptor mediated transmission was significantly lower in HAP relative to LAP mice, indicating that lines differences in postsynaptic glutamate receptor characteristics exist (Fritz et al. 2019).
To probe potential mechanisms or gene/protein/phosphorylated protein networks related to the functional differences observed in our electrophysiology experiments, a multi-omic analysis of the DS in HAP and LAP mice was employed. Minimal overlap was observed in the transcriptome and proteome results which is commonly reported across numerous tissues and cell types (Chen et al. 2002; Pascal et al. 2008; Ghazalpour et al. 2011; Chen et al. 2019; Hausser et al. 2019). This discordance between mRNA and protein abundance is likely a result of post-transcriptional/post-translational processing and mRNA vs protein degradation rates. (Vogel & Marcotte 2012). Additionally, as mRNA transcripts are mostly located in cell bodies, the RNA-sequencing may not fully capture line differences present in the dense presynaptic inputs from other brain regions that project to the DS. However, proteomic and phosphoproteomic analyses possess a greater capacity to discover line differences in protein expression from both neurons of the DS and presynaptic inputs arriving from other brain regions. Alternatively, proteomics is less sensitive to lowly expressed proteins (as one cannot amplify proteins similar to amplifying mRNA), and additional biological replicates for the proteomics data may have increased the number of less abundantly expressed proteins identified as significantly differentially expressed between lines which could have altered the correlation between our transcriptome and proteome results. Minimal overlap was also observed for differentially expressed proteins and phosphorylated proteins (34 identified from 390 and 345 proteins and phosphorylated proteins, respectively) which suggests that the alterations in protein phosphorylation states reflect specific protein phosphorylation changes that are not simply due to changes in global protein abundance. This represents a strength of our complimentary multi-omics approach as RNA-sequencing and global proteomics cannot detect these dynamic and rapid peptide phosphorylations differentially occurring in the DS of HAP and LAP mice which our quantitative phosphoproteomics approach captured.
Despite the lack of apparent uniformity, a shared finding among the three separate analyses was the differential expression of structural genes, proteins, and phosphorylated proteins and an enrichment in BP related to cellular organization and structure. Many of these genes and proteins are concurrently associated with the synapse CC which was significantly enriched in both the proteomics and phosphoproteomics analyses. The hub proteins analysis of the proteome network discovered vesicle-associated membrane protein-3 and synaptobrevin homolog YKT6 which belong to the SNARE family proteins involved in the synaptic vesicle cycle in the presynaptic terminal (Schwarz 2013). In addition to these two hub proteins, numerous other genes, proteins, and phosphorylated proteins involved in presynaptic vesicle loading, docking, fusion, and neurotransmitter release emerged from our analyses as differentially expressed between lines. These included members of the RIMS family and associated binding proteins (Rims1, Rims2, Rimbp2), synaptotagmins (Syt3 and Syt12), syntaxins and related binding proteins, (Stx1b and Stxbp5l), synaptogyrin-1 (Syngr1), bassoon (Bsn), piccolo (Pcl), and amphiphysin (Amph). Differences in the expression or phosphorylation state of these proteins would be expected to alter presynaptic release of neurotransmitters which could contribute to the increased glutamate or GABA signaling previously observed in HAP mice (Fritz et al. 2019).
Differences in cytoskeletal-related genes, proteins, and phosphorylated proteins, and an enrichment in BP and MF related to the cytoskeleton was also particularly prominent across analyses between the lines. For instance, Tuba1c, a globular protein which polymerizes to form a necessary component of microtubules, displayed the greatest differential log2 FC in gene expression in the RNA-sequencing analysis. Similarly, Mapt, Map2, Map4, Map6, Map1a, and Map1b among others emerged from the analyses as other microtubule associated proteins that were differentially expressed genes, proteins, and/or phosphorylated proteins between lines. Microtubule-associated protein 1B (Map1b) contains numerous phosphorylation sites that impact microtubule stability and dynamics (Yang et al. 2012) and is involved in both presynaptic and postsynaptic regulation particularly at glutamatergic synapses (Palenzuela et al. 2017; Eriksson et al. 2010; Bodaleo et al. 2016). In vitro work also suggests Map1b may facilitate the transport of the Nav1.6 voltage-gated sodium channel to the neuronal membrane which could impact action potential firing rates (O’Brien et al. 2012). As the Map1b gene and protein was significantly upregulated and contained 16 differentially expressed phosphopeptides in the DS of HAP relative to LAP mice, these alterations in Map1b across lines could contribute to the enhanced excitability in HAP MSNs reported in our prior study (Fritz et al. 2019).
Ankyrin-2 (Ank2) and neurofilament heavy polypeptide (Nefh) are additional cytoskeletal proteins that play a role in proper formation, stability, and functioning of synapses (Yang et al. 2019; Koch et al. 2008; Galiano et al. 2012; Yuan et al. 2015). Our phosphoproteomics analysis revealed twelve significantly different ankyrin-2 phosphopeptides with many peptides in the top 20 differentially expressed phosphopeptides between lines. In drosophila, casein kinase 2 targets ankyrin-2 to maintain synapse stability suggesting certain phosphomodifications of ankyrin-2 may be important to preserve synaptic terminal integrity (Bulat et al. 2014). Elevated ankyrin-2 may also increase spontaneous excitatory postsynaptic current frequency (without affecting inhibitory currents) in mouse hippocampal neurons, creating an excitation/inhibition imbalance (Lippi et al. 2016). If ankyrin-2 functions similarly in the mouse DS, the differential phosphomodifications could contribute to increased excitation and glutamatergic activity demonstrated in HAP MSNs relative to LAP mice. Similarly, fifteen different neurofilament heavy polypeptide (NFH) phosphopeptides were found to be differentially expressed between groups. NFH is a known target of numerous protein kinases, and these phosphorylation events regulate the interaction between different neurofilament subunits and other cytoskeletal structures to provide stability to mature axons (Yuan et al. 2017). Although neurofilaments were previously thought to be axonal contaminants, accumulating evidence indicates neurofilaments are present in the synapse (Yuan & Nixon 2016), particularly the postsynaptic density where they have been shown to co-localize with the NR1 NMDA receptor subunit, the D1 dopamine receptor, and spinophilin (Baucum et al. 2010; Yuan et al. 2015; Ehlers et al. 1998; Hiday et al. 2017). Interestingly, chronic cocaine, morphine, nicotine, and alcohol administration reduces neurofilament concentrations in the ventral tegmental area suggesting changes to neurofilament integrity may be a shared drug-induced structural change in brain regions important for drug reward (Beitner-Johnson et al. 1992; Ortiz et al. 1995; Bunnemann et al. 2000).
These structural and cytoskeletal-related proteins are just a sampling of the differences detected as the synapse CC was highly enriched in the network of differential expressed proteins and phosphorylated proteins. Further work is necessary to fully characterize how the differential expression and phosphorylations of these structural and cytoskeletal-related proteins associated with the synaptic CC work in conjunction to produce the differential physiological properties of MSNs in the DS and potentially contribute to differential endophenotypes described in HAP and LAP mice. Unfortunately, we discovered no direct relationship between any single differentially expressed gene, protein, or phosphopeptide and the primary neurophysiological differences we previously observed between the selected lines. For example, higher voltage-gated Na+ channel expression in HAP mice could clearly associate with heightened excitability observed in HAP mice; although we did quantify a few peptides of some Na+ channels, the relative abundances were quite low and it is unclear if their differential expression or phosphorylation states directly relate to the differential excitability profiles of MSNs in the DS of HAP and LAP mice. It is quite probable that downstream signaling mechanisms or regulatory functions related to our findings will produce the differential neuronal excitability and neurotransmitter release phenotypes.
Given the large body of research examining the interaction between alcohol and the immune system, it is not surprising that over 60 of the 138 enriched BPs discovered in the differentially expressed gene network were related to immune signaling and immune system functioning. Similarly, C3ar1, Ccl6, Ccl9, and Cd59a, which are cell membrane receptors involved in innate immune signaling, were identified as hub genes in the differentially expressed gene network. Alcohol is known to activate Toll-like receptor 4 (TLR4) innate immune signaling pathways leading to NF-κB mediated transcription of pro-inflammatory cytokines and chemokines which may contribute to addictive processes such as escalation of intake, behavioral inflexibility, impulsivity, and negative affect (Crews et al. 2011; Crews et al. 2006; Montesinos et al. 2016; Zou & Crews 2010). Emerging evidence indicates cytokine and chemokine signaling also modulate synaptic transmission and plasticity. For instance, TNF-α, a cytokine produced upon TLR4 activation, drives AMPA receptor internalization in MSNs of the DS reducing AMPA/NMDA ratio driven currents (Lewitus et al. 2014). Our RNA-sequencing analysis provides further evidence that neuroimmune signaling is involved in the genetic risk for AUD, and follow up studies will be necessary to examine how differential expression of these numerous immune-related genes may contribute to the distinct DS excitability and neurotransmission we have previously described in HAP and LAP mice (Fritz et al. 2019).
An unexpected yet intriguing result was the enrichment in metabolic-related BP and pathways in the proteomic network of differentially expressed proteins. The enrichment was observed for multiple metabolic pathways including those involving amino acids, nucleic acids, fatty acids, and sugars. Alcohol is primarily metabolized by the liver to acetaldehyde and eventually acetate, generating reduced NADH (nicotinamide adenine dinucleotide) in the process. This increase in the NADH/NAD+ ratio directly and indirectly modulates lipid, carbohydrate, protein, lactate, and uric acid metabolism (Zakhari 2013). Furthermore, alcohol-derived acetate produced by peripheral sources can regulate neuronal gene transcription producing long lasting changes in behavior (Mews et al. 2019). A recent proteomic analysis of human prefrontal and motor cortex tissue from individuals with AUD revealed diverse changes in many energetic metabolic enzymes associated with increased alcohol-derived acetate utilization in the brain of AUD subjects (Enculescu et al. 2019). Although the animals used in this study were alcohol-naïve, it is quite possible that mice selected over generations for high vs low alcohol preference could have basal differences in metabolic pathways that alcohol and its metabolites interfere with in the brain. While HAP mice do not demonstrate more efficient basal metabolic clearance of alcohol relative to LAP mice, HAP mice exhibit reduced weights, increased locomotion, and greater preference for saccharin solution than LAP counterparts, possibly indicating basal differences in energetic metabolism may be present (Grahame et al. 2000; Chester et al. 2003; Grahame et al. 1999; Can et al. 2012). Exactly how these metabolic differences in neuronal tissues such as the DS may relate to HAP and LAP AUD-related phenotypes, however, remains unclear at this time.
There are some limitations to this work that must be considered. It is important to bear in mind that this analysis used tissue from the entire DS while our previous study examined the two major subregions of the DS (the dorsolateral and dorsomedial striatum). It is therefore possible that subregion-specific effects may have been obscured. Although, subregions could have been used for RNA-sequencing, the lack of total tissue available from these subregions in each animal prevented us from completing subregion specific proteomics and phosphoproteomics which require more tissue to accurately quantify protein abundance. Additionally, we cannot be confident that the results from our analyses are unique to the DS or if similar enrichments in GO or pathways are observed in other brain regions relevant to AUD. For the present study, HAP and LAPs from replicate 2 were used. Although behavioral phenotypes (Oberlin et al. 2011) and electrophysiological measures in the DS between replicates two and three are quite similar (Fritz et al. 2019), it remains possible that different replicates may not contain identical gene-sets which may limit the generalizability of our current findings. Lastly, the present study does not specifically probe how changes in the expression of these genes and phosphorylation patterns of peptides directly produces changes in neuronal function nor the maladaptive AUD-related behavior associated with HAP mice. Further studies are necessary to examine the hypotheses and speculations proposed herein. Nevertheless, this integrated mutli-omic analysis yielded bountiful intriguing differences between the HAP and LAP lines in the DS, and we encourage readers to carefully examine our full datasets for questions that may be of interest to them. These complementary and integrative -omics analyses may be a valuable resource that can inform future studies of basal gene and protein expression and phosphopeptide patterns in animal models of AUD.
Supplementary Material
Funding Sources
This work was supported by NIH grants R01AA027214 (BKA), F32 AA026488 (BMF), T32AA007462 (Czachowski, Training Grant on Genetic Aspects of Alcoholism), UL1TR002529 (Indiana Clinical and Translational Sciences Institute), and institutional funds from the Indiana University Health and Stark Neurosciences Research Institute. Work in the IUSM Proteomics Core was supported, in part, with support from the Indiana Clinical and Translational Sciences Institute which is funded by Award Number UL1TR002529 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Acquisition of the IUSM Proteomics core instrumentation used for this project, the Orbitrap Fusion Lumos, was provided by the Indiana University Precision Health Initiative.
Abbreviations:
- AR
abundance ratio
- AUD
Alcohol Use Disorder
- DS
dorsal striatum
- FC
fold change
- GO
Gene Ontology
- HAP
high alcohol preference
- LAP
low alcohol preference
- MCC
maximal clique centrality
- RRID
Research Resource Identifier (see scicrunch.org)
Footnotes
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article
--Human subjects --
Involves human subjects:
If yes: Informed consent & ethics approval achieved:
=> if yes, please ensure that the info “Informed consent was achieved for all subjects, and the experiments were approved by the local ethics committee.” is included in the Methods.
ARRIVE guidelines have been followed:
Yes
=> if it is a Review or Editorial, skip complete sentence => if No, include a statement in the “Conflict of interest disclosure” section: “ARRIVE guidelines were not followed for the following reason:
“
(edit phrasing to form a complete sentence as necessary).
=> if Yes, insert in the “Conflict of interest disclosure” section:
“All experiments were conducted in compliance with the ARRIVE guidelines.” unless it is a Review or Editorial
None of the authors have conflicts of interest to disclose.
REFERENCES
- Baucum AJ 2nd, Jalan-Sakrikar N, Jiao Y, Gustin RM, Carmody LC, Tabb DL, Ham AJ and Colbran RJ (2010) Identification and validation of novel spinophilin-associated proteins in rodent striatum using an enhanced ex vivo shotgun proteomics approach. Molecular & cellular proteomics : MCP 9, 1243–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitner-Johnson D, Guitart X and Nestler EJ (1992) Neurofilament proteins and the mesolimbic dopamine system: common regulation by chronic morphine and chronic cocaine in the rat ventral tegmental area. The Journal of neuroscience : the official journal of the Society for Neuroscience 12, 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bice P, Valdar W, Zhang L et al. (2009) Genomewide SNP screen to detect quantitative trait loci for alcohol preference in the high alcohol preferring and low alcohol preferring mice. Alcoholism, clinical and experimental research 33, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bice PJ, Foroud T, Carr LG, Zhang L, Liu L, Grahame NJ, Lumeng L, Li T-K and Belknap JK (2006) Identification of QTLs Influencing Alcohol Preference in the High Alcohol Preferring (HAP) and Low Alcohol Preferring (LAP) Mouse Lines. Behavior Genetics 36, 248. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Lai D, Zhang L and Foroud T (2011) Fine Mapping Quantitative Trait Loci that Influence Alcohol Preference Behavior in the High and Low Alcohol Preferring (HAP and LAP) Mice. Behavior Genetics 41, 565–570. [DOI] [PubMed] [Google Scholar]
- Bodaleo FJ, Montenegro-Venegas C, Henríquez DR, Court FA and Gonzalez-Billault C (2016) Microtubule-associated protein 1B (MAP1B)-deficient neurons show structural presynaptic deficiencies in vitro and altered presynaptic physiology. Sci Rep 6, 30069–30069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert MM, Erikson GA, Shokhirev MN and Allen NJ (2018) The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Reports 22, 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boza-Serrano A, Yang Y, Paulus A and Deierborg T (2018) Innate immune alterations are elicited in microglial cells before plaque deposition in the Alzheimer’s disease mouse model 5xFAD. Sci Rep 8, 1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese MR and Liu Y (2013) NGSUtils: a software suite for analyzing and manipulating next-generation sequencing datasets. Bioinformatics 29, 494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulat V, Rast M and Pielage J (2014) Presynaptic CK2 promotes synapse organization and stability by targeting Ankyrin2. Journal of Cell Biology 204, 77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunnemann B, Terron A, Zantedeschi V, Merlo Pich E and Chiamulera C (2000) Chronic nicotine treatment decreases neurofilament immunoreactivity in the rat ventral tegmental area. European journal of pharmacology 393, 249–253. [DOI] [PubMed] [Google Scholar]
- Can A, Grahame NJ and Gould TD (2012) Affect-related behaviors in mice selectively bred for high and low voluntary alcohol consumption. Behav Genet 42, 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Wang Z, Mi W and Zuo Z (2014) Isoflurane unveils a critical role of glutamate transporter type 3 in regulating hippocampal GluR1 trafficking and context-related learning and memory in mice. Neuroscience 272, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Chandrashekar DS, Varambally S and Creighton CJ (2019) Pan-cancer molecular subtypes revealed by mass-spectrometry-based proteomic characterization of more than 500 human cancers. Nature Communications 10, 5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Gharib TG, Huang CC et al. (2002) Discordant protein and mRNA expression in lung adenocarcinomas. Molecular & cellular proteomics : MCP 1, 304–313. [DOI] [PubMed] [Google Scholar]
- Chester JA, Lumeng L, Li TK and Grahame NJ (2003) High- and low-alcohol-preferring mice show differences in conditioned taste aversion to alcohol. Alcoholism, clinical and experimental research 27, 12–18. [DOI] [PubMed] [Google Scholar]
- Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and Lin CY (2014) cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol 8 Suppl 4, S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H and Janak PH (2012) Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biological psychiatry 72, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L and Zou J (2006) BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcoholism, clinical and experimental research 30, 1938–1949. [DOI] [PubMed] [Google Scholar]
- Crews FT, Zou J and Qin L (2011) Induction of innate immune genes in brain create the neurobiology of addiction. Brain, behavior, and immunity 25 Suppl 1, S4–s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePoy L, Daut R, Brigman JL et al. (2013) Chronic alcohol produces neuroadaptations to prime dorsal striatal learning. Proceedings of the National Academy of Sciences of the United States of America 110, 14783–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Fung ET, O’Brien RJ and Huganir RL (1998) Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. The Journal of neuroscience : the official journal of the Society for Neuroscience 18, 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enculescu C, Kerr ED, Yeo KYB, Schenk G, Fortes MRS and Schulz BL (2019) Proteomics Reveals Profound Metabolic Changes in the Alcohol Use Disorder Brain. ACS chemical neuroscience 10, 2364–2373. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Samuelsson H, Bjorklund S, Tortosa E, Avila J, Samuelsson EB, Benedikz E and Sundstrom E (2010) MAP1B binds to the NMDA receptor subunit NR3A and affects NR3A protein concentrations. Neuroscience letters 475, 33–37. [DOI] [PubMed] [Google Scholar]
- Fanelli RR, Klein JT, Reese RM and Robinson DL (2013) Dorsomedial and dorsolateral striatum exhibit distinct phasic neuronal activity during alcohol self-administration in rats. The European journal of neuroscience 38, 2637–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BM and Boehm II SL (2014) The effect of prior alcohol consumption on the ataxic response to alcohol in high-alcohol preferring mice. Alcohol 48, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BM, Grahame NJ and Boehm SL (2013) Selection for high alcohol preference drinking in mice results in heightened sensitivity and rapid development of acute functional tolerance to alcohol’s ataxic effects. Genes, Brain and Behavior 12, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz BM, Muñoz B and Atwood BK (2019) Genetic Selection for Alcohol Preference in Mice Alters Dorsal Striatum Neurotransmission. Alcoholism: Clinical and Experimental Research 43, 2312–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiano Mauricio R., Jha S, Ho Tammy S.-Y., Zhang C, Ogawa Y, Chang K-J, Stankewich Michael C., Mohler Peter J. and Rasband Matthew N. (2012) A Distal Axonal Cytoskeleton Forms an Intra-Axonal Boundary that Controls Axon Initial Segment Assembly. Cell 149, 1125–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A and Vesce G (2012) Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR journal 53, E55–69. [DOI] [PubMed] [Google Scholar]
- Ghazalpour A, Bennett B, Petyuk VA et al. (2011) Comparative analysis of proteome and transcriptome variation in mouse. PLoS genetics 7, e1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lozano MA, Klemmer P, Gebuis T, Hassan C, van Nierop P, van Kesteren RE, Smit AB and Li KW (2016) Dynamics of the mouse brain cortical synaptic proteome during postnatal brain development. Sci Rep 6, 35456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Li TK and Lumeng L (1999) Selective breeding for high and low alcohol preference in mice. Behav Genet 29, 47–57. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Rodd-Henricks K, Li TK and Lumeng L (2000) Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology 151, 252–260. [DOI] [PubMed] [Google Scholar]
- Hausser J, Mayo A, Keren L and Alon U (2019) Central dogma rates and the trade-off between precision and economy in gene expression. Nature Communications 10, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-de-Diego R, Tarazona S, Martínez-Mira C, Balzano-Nogueira L, Furió-Tarí P, Pappas GJ Jr. and Conesa A (2018) PaintOmics 3: a web resource for the pathway analysis and visualization of multi-omics data. Nucleic Acids Res 46, W503–w509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiday AC, Edler MC, Salek AB, Morris CW, Thang M, Rentz TJ, Rose KL, Jones LM and Baucum AJ 2nd (2017) Mechanisms and Consequences of Dopamine Depletion-Induced Attenuation of the Spinophilin/Neurofilament Medium Interaction. Neural Plast 2017, 4153076–4153076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Ohlsson H, Bacanu S, Sundquist J and Sundquist K (2018) The risk for drug abuse, alcohol use disorder, and psychosocial dysfunction in offspring from high-density pedigrees: its moderation by personal, family, and community factors. Molecular psychiatry, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch I, Schwarz H, Beuchle D, Goellner B, Langegger M and Aberle H (2008) Drosophila Ankyrin 2 Is Required for Synaptic Stability. Neuron 58, 210–222. [DOI] [PubMed] [Google Scholar]
- Kohtala S, Theilmann W, Suomi T, Wigren H-K, Porkka-Heiskanen T, Elo LL, Rokka A and Rantamäki T (2016) Brief Isoflurane Anesthesia Produces Prominent Phosphoproteomic Changes in the Adult Mouse Hippocampus. ACS chemical neuroscience 7, 749–756. [DOI] [PubMed] [Google Scholar]
- Levasseur EM, Yamada K, Pineros AR et al. (2019) Hypusine biosynthesis in beta cells links polyamine metabolism to facultative cellular proliferation to maintain glucose homeostasis. Science signaling 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Pribiag H, Duseja R, St-Hilaire M and Stellwagen D (2014) An adaptive role of TNFα in the regulation of striatal synapses. The Journal of neuroscience : the official journal of the Society for Neuroscience 34, 6146–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Qing T, Zhu J, Wen Z, Yu Y, Fukumura R, Zheng Y, Gondo Y and Shi L (2017) A Comprehensive Mouse Transcriptomic BodyMap across 17 Tissues by RNA-seq. Sci Rep 7, 4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Van Vranken JG, Pontano Vaites L et al. (2020) TMTpro reagents: a set of isobaric labeling mass tags enables simultaneous proteome-wide measurements across 16 samples. Nature methods 17, 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Smyth GK and Shi W (2014) featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN and Boehm SL (2015) Relative fluid novelty differentially alters the time course of limited‐access ethanol and water intake in selectively bred high‐alcohol‐preferring mice. Alcoholism: Clinical and Experimental Research 39, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Fernandes CC, Ewell LA et al. (2016) MicroRNA-101 Regulates Multiple Developmental Programs to Constrain Excitation in Adult Neural Networks. Neuron 92, 1337–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Barbee B, Wang X and Wang J (2017) Alcohol induces input-specific aberrant synaptic plasticity in the rat dorsomedial striatum. Neuropharmacology 123, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelboum S, Manber Z, Elroy-Stein O and Elkon R (2019) Recurrent functional misinterpretation of RNA-seq data caused by sample-specific gene length bias. PLoS biology 17, e3000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson LM and Grahame NJ (2013) Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addiction Biology 18, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney W (2010) Data structures for statistical computing in python. In: Proceedings of the 9th Python in Science Conference, Vol. 445, pp. 51–56. Austin, TX. [Google Scholar]
- Mews P, Egervari G, Nativio R et al. (2019) Alcohol metabolism contributes to brain histone acetylation. Nature 574, 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabe Y, Miyabe C, Mani V, Mempel TR and Luster AD (2019) Atypical complement receptor C5aR2 transports C5a to initiate neutrophil adhesion and inflammation. Science Immunology 4, eaav5951. [DOI] [PubMed] [Google Scholar]
- Montesinos J, Alfonso-Loeches S and Guerri C (2016) Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcoholism, clinical and experimental research 40, 2260–2270. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ et al. (2006) Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proceedings of the National Academy of Sciences 103, 6368–6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JE, Sharkey LM, Vallianatos CN, Han C, Blossom JC, Yu T, Waxman SG, Dib-Hajj SD and Meisler MH (2012) Interaction of voltage-gated sodium channel Nav1.6 (SCN8A) with microtubule-associated protein Map1b. The Journal of biological chemistry 287, 18459–18466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin B, Best C, Matson L, Henderson A and Grahame N (2011) Derivation and characterization of replicate high-and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behavior genetics 41, 288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG and Grahame NJ (2009) High‐alcohol preferring mice are more impulsive than low‐alcohol preferring mice as measured in the delay discounting task. Alcoholism: Clinical and Experimental Research 33, 1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS and Nestler EJ (1995) Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse (New York, N.Y.) 21, 289–298. [DOI] [PubMed] [Google Scholar]
- Palenzuela R, Gutierrez Y, Draffin JE, Lario A, Benoist M and Esteban JA (2017) MAP1B Light Chain Modulates Synaptic Transmission via AMPA Receptor Intracellular Trapping. The Journal of neuroscience : the official journal of the Society for Neuroscience 37, 9945–9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal LE, True LD, Campbell DS, Deutsch EW, Risk M, Coleman IM, Eichner LJ, Nelson PS and Liu AY (2008) Correlation of mRNA and protein levels: cell type-specific gene expression of cluster designation antigens in the prostate. BMC genomics 9, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa F, Varoquaux G, Gramfort A et al. (2011) Scikit-learn: Machine learning in Python. the Journal of machine Learning research 12, 2825–2830. [Google Scholar]
- Rauniyar N and Yates JR (2014) Isobaric labeling-based relative quantification in shotgun proteomics. J Proteome Res 13, 5293–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W and Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ and Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE and Brewer RD (2015) 2010 national and state costs of excessive alcohol consumption. American journal of preventive medicine 49, e73–e79. [DOI] [PubMed] [Google Scholar]
- Schwarz TL (2013) Chapter 7 - Release of Neurotransmitters. In: Fundamental Neuroscience (Fourth Edition), (Squire LR, Berg D, Bloom FE, du Lac S, Ghosh A and Spitzer NC eds.), pp. 139–161. Academic Press, San Diego. [Google Scholar]
- Sharma K, Schmitt S, Bergner CG et al. (2015) Cell type– and brain region–resolved mouse brain proteome. Nature Neuroscience 18, 1819–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahre M, Roeber J, Kanny D, Brewer RD and Zhang X (2014) Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis 11, E109–E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Nishimura M, Ihara F, Yamagishi J, Suzuki Y and Nishikawa Y (2013) Transcriptome Analysis of Mouse Brain Infected with <span class=“named-content genus-species” id=“named-content-1”>Toxoplasma gondii. Infection and Immunity 81, 3609–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao G, Xue Q, Luo Y, Li G, Xia Y and Yu B (2016) Isoflurane Is More Deleterious to Developing Brain Than Desflurane: The Role of the Akt/GSK3B Signaling Pathway. BioMed Research International 2016, 7919640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Abercrombie ED and Bolam JP (2007) Basal ganglia macrocircuits. Progress in brain research 160, 3–7. [DOI] [PubMed] [Google Scholar]
- Vogel C and Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nature Reviews Genetics 13, 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walt S. v. d., Colbert SC and Varoquaux G (2011) The NumPy Array: A Structure for Efficient Numerical Computation. Computing in Science & Engineering 13, 22–30. [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S and Ron D (2010) Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 10187–10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Wu M, Xia P et al. (2012) The role of microtubule-associated protein 1B in axonal growth and neuronal migration in the central nervous system. Neural Regen Res 7, 842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Walder-Christensen KK, Kim N et al. (2019) ANK2 autism mutation targeting giant ankyrin-B promotes axon branching and ectopic connectivity. Proceedings of the National Academy of Sciences 116, 15262–15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH and Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nature Reviews Neuroscience 7, 464–476. [DOI] [PubMed] [Google Scholar]
- Yoon G, Westermeyer J, Kuskowski MA and Nesheim L (2013) Impact of the number of parents with alcohol use disorder on alcohol use disorder in offspring: a population-based study. The Journal of clinical psychiatry 74, 795–801. [DOI] [PubMed] [Google Scholar]
- Yuan A and Nixon RA (2016) Specialized roles of neurofilament proteins in synapses: Relevance to neuropsychiatric disorders. Brain Res Bull 126, 334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Rao MV, Veeranna and Nixon RA (2017) Neurofilaments and Neurofilament Proteins in Health and Disease. Cold Spring Harb Perspect Biol 9, a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Sershen H, Veeranna et al. (2015) Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol Psychiatry 20, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhari S (2013) Alcohol metabolism and epigenetics changes. Alcohol research : current reviews 35, 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang Y, Levran O, Randesi M, Yuferov V, Zhao C and Kreek MJ (2017) Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: a RNA sequencing study. Psychopharmacology 234, 2259–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J and Crews F (2010) Induction of innate immune gene expression cascades in brain slice cultures by ethanol: key role of NF-κB and proinflammatory cytokines. Alcoholism, clinical and experimental research 34, 777–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.