Abstract
Objectives.
To quantify and model conversion of a normal to an abnormal coronary artery calcium (CAC) scan.
Background.
While absence of CAC is associated with excellent prognosis, progression to CAC>0 confers increased risk. The time interval for repeat scanning remains poorly defined.
Methods.
Our study included 3,116 participants from the Multi-Ethnic Study of Atherosclerosis (MESA) with baseline CAC=0 and follow-up scans over 10 years after baseline. Prevalence of incident CAC, defined by thresholds of either CAC>0, CAC>10, or CAC>100, was calculated and time-to-progression was derived from a Weibull parametric survival model. Warranty periods were modeled as a function of sex, race/ethnicity, cardiovascular risk, and desired yield of repeat CAC testing. We further analyzed the proportion of coronary events occurring in participants with baseline CAC=0 that precede and follow repeat CAC testing at different time intervals.
Results.
Mean age was 58 ± 9 years, with 63% women, and mean 10-year cardiovascular risk of 14%. Prevalence of CAC>0, CAC>10, and CAC>100 was 53%, 36%, and 8% respectively at 10 years. Using a 25% testing yield (Number Needed to Scan=4) the estimated warranty period of CAC>0 varied between 3 to 7 years depending on sex and race/ethnicity. Approximately 15% progressed to CAC>10 in 5 to 8 years, while 10-year progression to CAC>100 was rare. Presence of diabetes was associated with significantly shorter warranty period, while family history and smoking had small effects. 19% of all 10-year coronary events occurred in CAC=0 prior to performance of a subsequent scan at 3–5 years, while new detection of CAC>0 preceded 55% of future events and identified individuals at 3-fold higher risk of coronary events.
Conclusion.
In a large population of individuals with baseline CAC=0, we provide a robust estimation of the CAC=0 warranty period, considering progression to CAC>0, CAC>10, and CAC>100, and impact on missed vs. detectable 10-year year CHD events. Beyond age and sex, race/ethnicity and diabetes also have a significant impact on the warranty period. We suggest that evidence-based guidance would be to consider rescanning in 3 to 7 years depending on individual demographics and risk profile.
Keywords: risk assessment, coronary artery calcium, coronary artery disease
Introduction
Coronary artery calcium (CAC) detected using cardiac gated non-contrast computed tomography is now routinely used as a marker of coronary atherosclerosis burden. Absence of coronary calcification (i.e. CAC=0) is a powerful negative risk predictor, as occurrence of coronary heart disease (CHD) events without any coronary calcification is exceedingly rare. (1–4) Given the prognostic power of CAC=0 and the clinical significance of CAC progression, it is important to know when an initial CAC=0 scan should be repeated, defined here as the so-called “warranty period”, in order to guide appropriate timing of rescans. Our preliminary study of overall time to conversion from CAC=0 to CAC>0 suggested that the time frame in which development of CAC>0 after a normal CAC scan, i.e. the “warranty period” of CAC=0, lies between 3 to 5 years depending on sex, age, and atherosclerotic cardiovascular disease (ASCVD) risk category. (5) This general estimate of the warranty period of CAC=0 is a clinically important finding for appropriate timing of re-scans and timely detection and prevention of future CHD events. However, the impact of race/ethnicity and key individual risk factors identified in guidelines as modifying interpretation of CAC=0 – including diabetes, family history, and current smoking – has not yet been described.
While any detectable CAC is prognostically important, higher CAC scores of CAC>10 and CAC>100 have been more strongly associated with an increased risk of CHD as well as all-cause mortality. (6) In fact, most studies have found increased CAC burden to be a more robust predictor of coronary events in primary prevention than conventional risk scores such as the Pooled Cohort Equations (PCE). Detection of new CAC at higher thresholds, including CAC>10 and CAC>100, may therefore be most clinically important for guiding decision making in primary prevention. (7)
We therefore sought to provide a comprehensive analysis of the warranty period of CAC=0. In order to provide policymakers, guideline writers, and clinicians with the necessary data for a more precise and individualized warranty estimate, we considered the impact of race/ethnicity, cardiovascular risk factors, and varying thresholds for newly detectable CAC burden. The goal was to provide a precise yet easy to use look-up table for individualized risk estimation and appropriate timing of CAC rescans.
Methods
Study population
The Multi-Ethnic Study of Atherosclerosis (MESA) study is a multi-ethnic observational cohort study of 6814 men and women aged 45–84 years without known cardiovascular disease at the time of enrollment. Participants were enrolled from July 2000 through September 2002 at six different centers in the USA (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Northern Manhattan and Bronx, New York; Los Angeles County, California; St. Paul, Minnesota). The institutional review boards at each site approved the study. For this study, a total of 3,116 MESA participants with baseline CAC=0 and at least one additional follow-up CAC scan were included. All participants gave written informed consent. Full details of the MESA study design have been published previously. (8–10)
CAC measurement
Baseline CAC was measured by electron beam CT at 3 sites (Chicago, Los Angeles, New York) and by multidetector CT at the other 3 centers (Baltimore, Forsyth County, St. Paul). Images were interpreted by a radiologist or cardiologist at the MESA CT reading center (Harbor-UCLA, Torrance, California). All participants were scanned twice at baseline, with mean CAC (Agatston) score used for all analyses. The details of the MESA CT scanning and interpretation methods are discussed elsewhere. (11)
Follow-up CAC scan
Participants in MESA had undergone a total of 5 study visits at the time of this analysis. Baseline CAC scans were conducted on all participants at visit 1. Following the initial baseline scan, subsets of participants underwent repeat CT scans (regardless of baseline CAC burden) during different follow-up visits, as per the pre-specified MESA protocol. MESA visits 2, 3, 4 and 5 included subjects who had follow-up CT scans at mean 1.7 ± 0.3, 3.2 ± 0.4, 4.9 ± 0.5, and 9.7 ± 0.6 years, respectively, following the initial baseline scan.
In MESA, not all participants had a follow-up scan at each of the visits. As part of the study design, approximately half of the participants with baseline CAC=0 received repeat scan at visit 2 and the other half at visit 3. Visit 4 prioritized participants without a scan at visit 3, and during visit 5, approximately one-half of the participants with CAC=0 on prior visits received a repeat scan preferentially including participants with visit 3 and 4 scans. Repeat CT scanning in MESA was unrelated to specific individual participant characteristics and risk factors. All participants included in this analysis with CAC=0 at baseline received at least 1 repeat scan (100%), while 47% had 2 total scans, 42% had 3 total scans, and 11% had 4 total scans over follow-up.
Risk factor assessment
During the baseline examination, study teams at each of the six centers collected information on cardiovascular risk factors. Demographics, medical conditions, smoking history, family history, and medication use were obtained by a questionnaire. Resting blood pressure was measured 3 times with the participant in a seated position; average of the last 2 measurements was used for analyses. Fasting glucose and lipid concentrations were measured by the central laboratory at the University of Minnesota (Minneapolis, MN, USA).
Definitions
Hypertension (HTN) status was classified according to JNC7.(12) Diabetes mellitus (DM) was classified according to American Diabetes Association (ADA) 2003 criteria. (13) Obesity was classified according to World Health Organization (WHO) classification. Smoking status was classified as never smoker, former smoker, and current smoker. Never smoker was defined as lifetime consumption of less than 100 cigarettes, and current smoker was defined as smoking within the last 30 days (14) Family history of CHD was defined as any immediate family member (parents, siblings, and children) with history of myocardial infarction, coronary angioplasty, or coronary artery bypass surgery.
Follow-up for CHD Events
The participants were followed over a total of 12.6 ± 2.3 years for incident total and hard CHD events. Total CHD endpoints included hard CHD events plus definite or probable angina followed by coronary revascularization. Hard CHD events included only CHD death, resuscitation cardiac arrest, and myocardial infarction. At intervals of 9–12 months, trained personnel called the participants to inquire about interim hospital admissions, outpatient diagnoses of cardiovascular disease, or deaths. Medical records were obtained, and events adjudicated by two or more members of the Morbidity and Mortality Committee. For reported out-of-hospital deaths, next of kin were interviewed and death certificates reviewed by the adjudication committee. A detailed description of the MESA follow-up methods is available at http://www.mesa-nhlbi.org.
Statistical analysis
Baseline characteristics of participants with baseline CAC=0 were calculated as mean and standard deviation or median and interquartile range for continuous variables, and as the total number and the percent of subjects for categorical variables.
Prevalence of new CAC was calculated as the total number of participants who developed CAC>0, CAC>10 and CAC>100 divided by the total number at risk. Since participants did not get yearly CAC scanning, it was not feasible to calculate a true annual incidence of conversion to CAC>0, CAC>10 and CAC>100. Therefore, the cumulative annual incidence at each study rescan interval was calculated as the total number of individuals with incident CAC>0, CAC>10 and CAC>100 at that time point divided by the total person-years at risk, with only rescanned individuals contributing person-time (see Supplementary figure 1 for further details). Prevalence and cumulative annual incidence were evaluated at 2, 3, 4, 5, 6, and 10 year follow-up based on MESA CAC re-scanning patterns.
Survival models were used to model time-to-conversion to CAC>0, CAC>10 and CAC>100, allowing calculation of the “warranty period” of CAC=0 in years. Due to the non-linear incidence of CAC>10 and CAC>100 as well as the lack of rescanning between years 6 and 8, the proportional hazards assumption was not satisfied after year 6. As a result, Cox proportional hazards models gave less reliable estimates. To achieve reliable estimates >6 years, a Weibull parametric survival model was used. The Weibull distribution was selected after the Akaike information criterion (AIC) and Bayesian information criterion (BIC) selection criterion identified this as the best fit.
Warranty calculations were based on different “yields of re-testing”, representing varying proportions developing new CAC>0. Test yields of 15%, 20% and 25% were used (corresponding to number needed to screen of 6, 5 and 4 respectively), allowing calculation of the mean estimated time (or warranty period) to reach new CAC>0 for each testing yield. Estimates were then repeated after stratification by race/ethnicity, family history of CHD, diabetes, and smoking.
For development of CAC>10 and >100 test yields of 5%, 10%, or 15% were used (corresponding to number needed to screen of 20, 10, and 6 respectively), as the higher test yields used for CAC=0 would have resulted in time periods of well over 10 years. Estimates were then repeated after stratification by baseline 10-year ASCVD risk and age.
The incidence of CHD events before and after rescan were recorded for each pre-defined time interval, 0–2, 2–4, 4–6, and 6–10 years. Summary measures were calculated as follows:
Percentage of 10-year events that occurred before rescan:
# of individuals with an event before rescan
total number of events (before & after rescan)
Percentage of subsequent events after new CAC>0 detection:
# of individuals with incident CAC >0 on rescan & subsequently developed an event
total number of future events (after rescan)
Percentage of subsequent events occurring in participants with persistent CAC=0:
# of individuals with persistent CAC=0 on rescan & subsequently developed an event
total number of events (after rescan)
Results
Baseline characteristics
The baseline characteristics of the participants are shown in Table 1. Baseline characteristics of the participants scanned at various time intervals were not different see Supplementary table 1. Of the 6,814 participants enrolled in MESA, 3,116 had a CAC=0 at baseline. About 60% of these participants were younger than 60 years of age. All four ethnicities were well-represented (34% White, 31% Black, 23% Hispanic, 12% Chinese). About 9% of these participants had diabetes, 13% were active smokers and 37% had a family history of CHD. Using the ASCVD risk categories from the 2018 ACC/AHA/MS Cholesterol Guideline (15), 31%, 44% and 25% of these participants were classified as low, borderline/intermediate, and high ASCVD risk using the Pooled Cohort Equations, respectively.
Table 1.
Baseline demographic characteristics of the study population (participants with baseline CAC=0 and at least 1 repeat CAC scan)
| N=3116 | |
|---|---|
| Age (years) | 57.9 ± 9.1 |
| <60 years old | 60.5% |
| ≥60 years old | 39.5% |
| Female | 63% |
| Race/ethnicity | |
| White | 34.0% |
| Black | 31.0% |
| Chinese | 11.7% |
| Hispanic | 23.3% |
| At least high school education | 83.5% |
| BMI (kg/m2) | 28.3 ± 5.6 |
| Waist circumference | 96.5 ± 14.7 |
| Active smoking | 13.0% |
| Diabetes | 8.8% |
| Family history of CHD | 37.3% |
| Systolic blood pressure (mmHg) | 122.1 ± 20.2 |
| Diastolic blood pressure (mmHg) | 71.2 ± 10.2 |
| Total cholesterol (mg/dL) | 193.9 ± 34.8 |
| LDL cholesterol (mg/dL) | 116.3 ± 30.7 |
| HDL cholesterol (mg/dL) | 52.6 ± 15.0 |
| Triglycerides (mg/dL)* | 107 (75 – 154) |
| Anti-hypertensive medication | 28.2% |
| Lipid lowering medication | 10.6% |
| ASCVD risk score | 13.8 ± 13.3 |
| ASCVD risk categories | |
| Low risk (0–5%) | 31.3% |
| Intermediate risk (5–20%) | 44.2% |
| High risk (>20%) | 24.5% |
Descriptive statistics of participants with CAC=0 at baseline and with at least 1 repeat CAC scan at least 12 months after the baseline scan. ASCVD=Atherosclerotic cardiovascular disease; BMI=Body Mass Index; CAC=Coronary artery calcium, CHD=Coronary heart disease event; HDL=High-density lipoprotein; LDL=Low-density lipoprotein;
presented as median (interquartile range).
Cumulative Incidence and prevalence of CAC>0
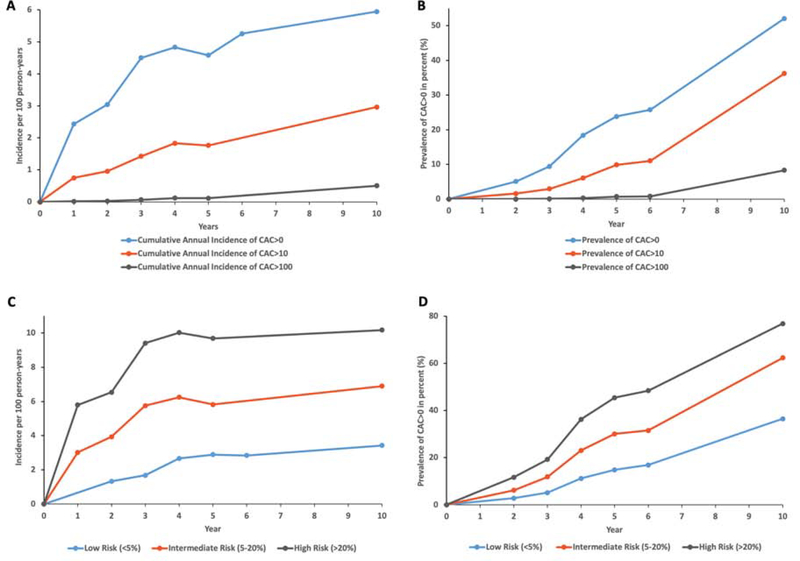
The proportion of participants with CAC>0 increased steadily over time from 11% in the 0–2 year, 20% in the 0–4 year, 28% in the 0–6 year, and 46 % in the 0–10 year rescan window (Table 2). Approximately half of participants with baseline CAC=0 developed incident CAC>0 at 10 years of follow-up (Figure 1B). The cumulative yearly incidence of CAC>0 increased over time, from between 2–3% per year over the first 2 years after baseline scan to about 4–5% per year in the 4 to 10 years after baseline scan (Figure 1A).
Table 2.
Number of participants scanned at each follow-up range
| Follow-up | Number of participants rescanned with baseline CAC=0 | Number of participants with incident CAC (% of those re-scanned) |
|---|---|---|
| 0–2 years | 1294 | 147 (11.4%) |
| 2–4 years | 1601 | 320 (20.0%) |
| 4–6 years | 760 | 212 (27.9%) |
| 6–10 years | 1397 | 648 (46.4%) |
Number of participants re-scanned with baseline CAC=0 and with incident CAC (% of those re-scanned) at each follow-up range: 0–2 years: 2–4 years; 4–6 years and 6–10 years. CAC=Coronary artery calcium.
Figure 1. Cumulative annual incidence and prevalence of CAC, by incident CAC burden and ASCVD risk.
A Cumulative annual incidence and B prevalence of CAC>0, CAC>10, CAC>100 among CAC=0 MESA participants who were re-scanned during follow-up. C Cumulative annual incidence and D prevalence of CAC>0 by ASCVD risk categories in the study population. ASCVD=Atherosclerotic cardiovascular disease risk; CAC=Coronary artery calcium.
Similarly, the incidence of CAC>10 increased within the first 3 to 5 year period, although this was generally less than half that of CAC>0. The incidence of CAC>100 was very low and showed a modest increase only in the 6 to 10 year period (Figure 1A). The prevalence at 10 years was 52% for CAC>0, 36% for CAC>10 and 8% for CAC>100 (Supplementary table 2).
Cumulative incidence of CAC>0 after stratification by ASCVD risk categories is shown in Figure 1C. New CAC annual incidence rose to about 6%/year in the borderline/intermediate risk group and to about 3%/year in the low risk group. At 10 years, the prevalence of CAC>0 was 78% for the high risk group, 62% for the intermediate risk group, and 37% for the low risk group (Figure 1D).
Warranty period of CAC=0 overall and by key clinical characteristics
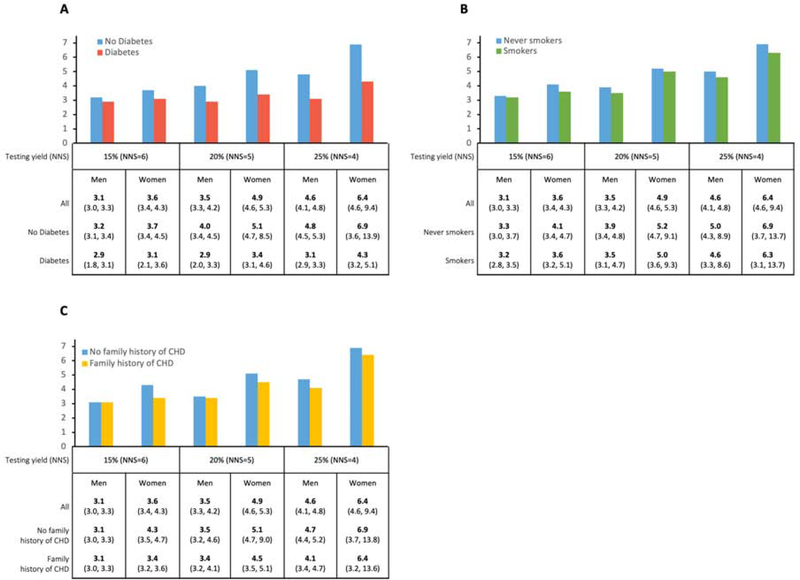
Figure 2 presents the warranty period of CAC=0 for men and women after stratification by race/ethnicity. The warranty period of CAC=0 for women showed less variance among different races/ethnicities compared to men. Using 25% conversion (NNS=4) to CAC>0, it would take an average of 7.4 years for Chinese women but only 6.0 years for White women to reach this threshold. Warranty periods for Black and Hispanic women lay in between Chinese and Whites. These racial/ethnic differences were more pronounced in men. Assuming the same testing yield of 25%, it would take 6.7 years for Chinese men but only 3.5 years for White men to reach CAC>0. In contrast to findings in women, Hispanic men had a slightly longer warranty period of 4.8 years than Black men with a warranty period of 4.4 years.
Figure 2. Warranty period (in years) of CAC=0 in the total population and by race, sex, and desired yield of testing.
Warranty period (in years) of CAC=0 in the total population as a function of race, sex and desired yield of testing (NNS). CAC=Coronary artery calcium; NNS=Number needed to screen.
Figure 3 presents the warranty period of CAC=0 after simple stratification by diabetes, smoking, and family history of CHD. Of these three key risk factors, diabetes had the greatest impact on the warranty period, associated with a 38% shorter warranty in women (4.3 years for diabetes vs. 6.9 years for no-diabetes) and a 35% shorter period for men (3.1 years for diabetes vs 4.8 years for no-diabetes). Subgrouping by smoking and family history of CHD had only minor impact on the warranty period.
Figure 3. Warranty period (in years) of CAC=0 in key subgroups.
A Diabetes versus no diabetes B current smokers versus never-smokers and C family history of CHD versus no family history of CHD; warranty period shown as a function of sex and desired yield of testing. CAC=Coronary artery calcium; NNS=Number needed to screen.
Figure 4 presents the time period calculations for CAC conversions from CAC=0 to CAC>10, and from CAC=0 to CAC>100, respectively, stratified by ASCVD risk category and age. For 15% of the total population (NNS=6) to convert from CAC=0 to CAC>10 it would take between 4.5 to 8.1 years depending on ASCVD risk category and age. Given the scarcity of CAC>100 conversions, it would take over 10 years for 15% of the CAC=0 population (NNS=6) - irrespective of age or risk group - to convert to CAC>100, with the only exception being high ASCVD risk individuals of whom 15% would convert to CAC>100 after an average of 9.3 years.
Figure 4. Warranty period (in years) of CAC=0 in the total population using higher thresholds of detectable CAC (>10, >100), by estimated 10-year ASCVD risk, age. and desired yield of testing.
Warranty period (in years) of CAC=0 in the total population using higher thresholds of detectable CAC (>10, >100) as a function of estimated 10-year atherosclerotic cardiovascular disease risk A, age B and desired yield of testing (NNS). ASCVD=Atherosclerotic cardiovascular disease risk; CAC=Coronary artery calcium; NNS=Number needed to screen.
Interplay between CT rescan interval and 10-year CHD events
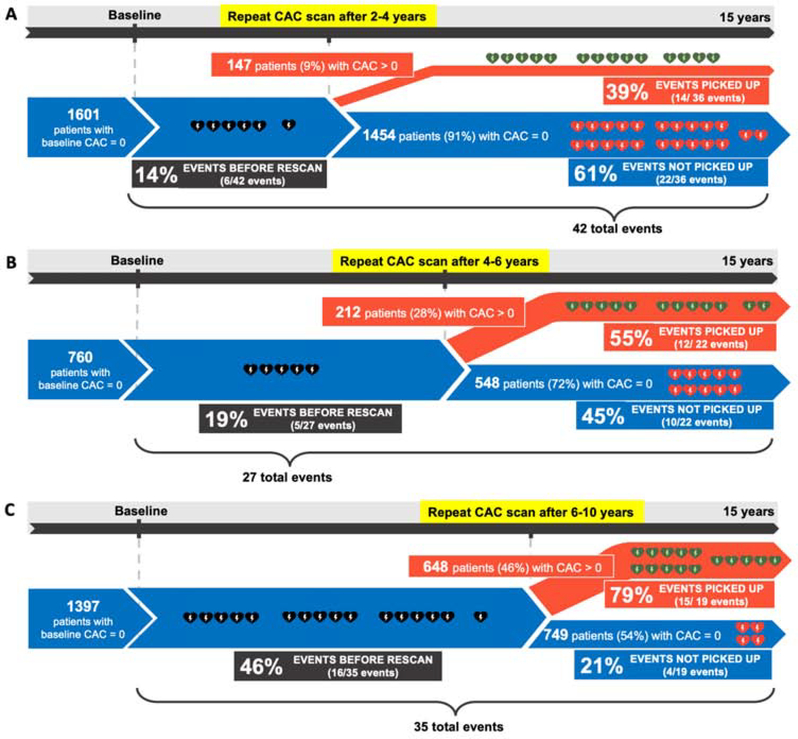
Figure 5 shows the implications of rescanning at different time windows for potential timely detection of participants at risk for CHD events. The number of “missed” CHD events (those occurring before the follow-up scan) in each respective group was low. Follow-up scans after 2–4 years (average 3.1 years) and 4–6 years (average 4.8 years), respectively, and would have picked up 39% (14 out of 36) and 55% (12 out of 22) of the rare participants developing CHD events in the following 8–10 years,, while missing just 14% (6 out of 42) and 19% (5 out of 27), respectively. Supplementary table 3 shows the data from Figure 5 in tabular form. Supplementary table 4 and Supplementary figure 2 present the same calculations for participants developing hard CHD events during study follow-up. Supplementary figure 3 present the event rates of those who converted within 5 years after baseline scan stratified by incident CAC score groups. Supplementary table 5 shows the mean follow-up time for events after each rescan interval.
Figure 5. CHD events in sub-cohorts of participants defined by rescan interval.
CHD events in sub-cohorts of participants defined by rescan interval A 2–4 years; B 4–6 years; and C 6–10 years. CAC=Coronary artery calcium; CHD=Coronary heart disease.
Discussion
Based on data from over two decades of studies, the 2018 ACC/AHA/MS Cholesterol Guideline emphasized the clinical importance of CAC=0, stating that it is reasonable to withhold statin therapy and, in most patients, reassess risk status with CAC scoring in 5 to 10 years. (15) Given the clinical importance of CAC=0 and the clinical significance of CAC development, it is important to know when an initial CAC=0 scan should be updated to reassess risk in these patients. In this paper, we have defined this as the “warranty period” of CAC=0. We sought to extend our preliminary work (5), presenting a comprehensive, granular analysis of the warranty periods of CAC=0 considering conversion to CAC>0, CAC>10, and CAC>100 with additional accounting for race/ethnicity and individual risk factors. We provide flexible calculations to characterize the time period to incident CAC (“warranty period”) of a CAC=0 scan, thus defining the timeframe in which the probability of developing CAC>0 is reasonably low and in which repeating a CAC scan would likely result in a low testing yield based on a desired yield of testing of 15%, 20% and 25%.
The overarching goal was to provide policymakers, guideline writers, and health care providers with all the necessary data to make an informed decision on general re-scanning recommendations based on individual preferences, risk profiles, and varying approaches to primary prevention.
Two major studies have previously investigated the temporal conversion to CAC>0 after initial CAC=0. The first study was published in 2007 by Gopal et al and analyzed 710 patients with CAC=0 at baseline and a follow-up scan at least 12 months later. They found that 62% of patients did not show any progression to CAC>0 over the course of the study (about one third each was followed for 1–3 years, 3–5 years, and >5 years respectively). Based on their results Gopal et al suggested that a follow-up CAC scan should be conducted 5 years after baseline at the earliest. (16)
Min and colleagues conducted a prospective 5-year study in 2010 in which 422 patients with an inconspicuous baseline CAC scan and a comparison group of 621 subjects with baseline CAC>0. They showed that incidence of CAC>0 during the first 2 years was 0.5–1.2% and increased to 5.7%, 6.2% and 11.6% respectively in the following three years after that. Considering this non-linear increase of new CAC, Min et al recommended a time frame of about 4 years for repeating the CAC scan. (17)
Both of the aforementioned studies however had major limitations due to a relatively small study population, lack of ethnic diversity, and referral of patients by physicians which is likely to introduce significant selection bias. Also, neither of these two studies were able to distinguish between sex, age, race/ethnicity, and baseline cardiovascular risk profile in their recommendations for time between CAC scans. Also, neither of the two studies considered the desired yield of testing, an important element of shared decision making, when calculating respective warranty periods for CAC=0.
The question of the warranty period of CAC=0 is clinically important, as CAC=0 is now established as a powerful negative risk predictor that is strongly associated with CHD event-free and overall survival. (18–20) Moreover, studies have shown that CAC can serve as a valuable tool in the physician-patient interaction, thereby either motivating patients to emphasize a healthy lifestyle to prevent conversion from CAC=0 to CAC>0, or helping patients to decide about statin or other pharmacotherapy. (21) Therefore, the latest 2018 ACC/AHA/MS Cholesterol Guideline now endorses the CAC score as a decision-making aid in many clinical scenarios, and states that it is reasonable to withhold statin therapy in individuals with CAC=0 in the absence of diabetes, strong family history of premature CHD, and current smoking. (15) In contrast, commencement of statin therapy is favored in individuals with a conversion to CAC>0 and indicated if CAC>75th percentile or >100. Thus, progression from baseline CAC=0 to CAC>0 has considerable implications for patient management.
In order to further improve warranty estimates of CAC=0 we stratified our results by race/ethnicity as well as the three aforementioned risk factors in the prevention guidelines. We found that Whites had the shortest warranty period of CAC=0, consistent with known higher CAC scores in Whites as compared to Blacks and other race/ethnicities. Although the underlying biological reasons for these differences are incompletely described, they underscore the importance of race/ethnicity for estimation of the warranty period and the overall individual CHD risk profile. Diabetes had a major impact on the warranty period of CAC=0 in our study population, corresponding to a 40% shorter warranty period in both sexes. These results argue for close consideration of diabetes status in estimating the warranty period of CAC=0. Interestingly, both family history of CHD and smoking had only minor impact, with only about a 10% shorter warranty periods for both men and women. However, it should be noted that prevalence of smoking and family history of CHD was relatively low in the MESA population.
In addition to the warranty period of CAC=0, time to progression to CAC>10 and CAC>100 is important as these higher CAC scores may be more clinically actionable than extremely low CAC burdens <10. Indeed, CAC scores of CAC>100 are associated with markedly elevated risk. (7) Individuals from MESA with higher incident CAC scores showed higher event rates; this likely indicates that such individuals converted to CAC>0 further in the past. In our analysis we found that conversion from CAC=0 to a CAC score of >10 would take on average 5 to 8 years depending on ASCVD risk category and age. Conversion to CAC>100 would take at least 9 years for high risk individuals and was exceedingly rare in a 10-year timeframe in all other risk and age groups.
In this study we provide flexible calculations for the time period to CAC>10 and CAC>100 progression based on a desired yield of testing of 5%, 10% and 15%, with the goal of enabling patients, physicians, as well as policy makers to make informed decisions about CAC rescanning. The time periods proposed in this discussion are based on a desired yield of identifying 15% of adults developing CAC>10 and >100 progression; however, some patients and providers might perceive testing yields of 5% and 10% as more acceptable. Balance between missed events before rescan and detecting CAC>0 prior to events is another important factor to take into account for determining the best rescan interval. However, acceptable limits for miss and pick-up rates of CHD events indicated by CAC>0 have not been established, again emphasizing the value of our data in balancing sensitivity and specificity, and guiding shared decision-making including patient preferences.
To our knowledge, MESA is the only large-scale U.S. study population that allows for these types of calculations as it provides a prospective dataset that contains assessment of baseline ASCVD-risk and CAC, follow-up CAC scans at different intervals, and long-term follow-up for CHD events. Despite the importance of timely detection of CAC>0, existing guidelines are not based on strong supporting data guiding CAC rescanning, providing only general recommendation of repeat CAC scans in 5 to 10 years. (15)
Our study has some limitations. In MESA, not all participants were scanned at every visit. This made the calculation of a true annual incident CAC unfeasible, and therefore we used cumulative yearly incidence as a measure of incident CAC over time. Although MESA is a relatively large study population with 6,814 individuals of which 3,116 were included in this study, the total number of CHD events in each respective scan group was still relatively low, and we cannot confirm that all events were atherosclerotic in nature. While this does limit the statistical power of the findings presented in this study, low CHD event rates are to be expected in a healthy study population with baseline CAC=0. In addition, a CAC scan is unable to diagnose early non-calcified plaques, and therefore our study is not suited to inform the warranty of maintaining “no plaque”. Finally, while we could not observe a statistically significant effect of smoking and positive family history of CHD on CAC conversion, this may be a result of the relatively low prevalence of smoking (13%) and positive family history of CHD (37%) in the study population. Larger studies or a patient population with a higher prevalence of these risk factors are needed confirm this finding.
In summary, this study extends our preliminary work suggesting 3–7 years as a good estimate for the warranty period of baseline CAC=0 (Table 3). In addition to sex, age, baseline ASCVD risk and desired yield of testing we show that race/ethnicity as well as diabetes are major risk factors which influence the warranty period of CAC=0. Moreover, this is the first study that provides time periods for progression to CAC>10 or CAC>100. We believe our data will inform policy and guidelines, as well as help guide appropriate, early initiation of preventive therapy in selecting patients with new CAC>0 in clinical practice.
Table 3.
Summary look-up table for individualized risk estimation and appropriate timing of CAC rescans
| Risk Group | Recommended rescan interval |
|---|---|
| Low-risk (<5% 10-year risk) | 6–7 years |
| Borderline to Intermediate risk (5–20% 10-year risk) | 3–5 years |
| High risk (>20% 10-year risk) | 3 years |
| Diabetes | 3 years |
Look-up table for individualized risk estimation and appropriate timing of CAC rescans. CAC=Coronary artery calcium.
Supplementary Material
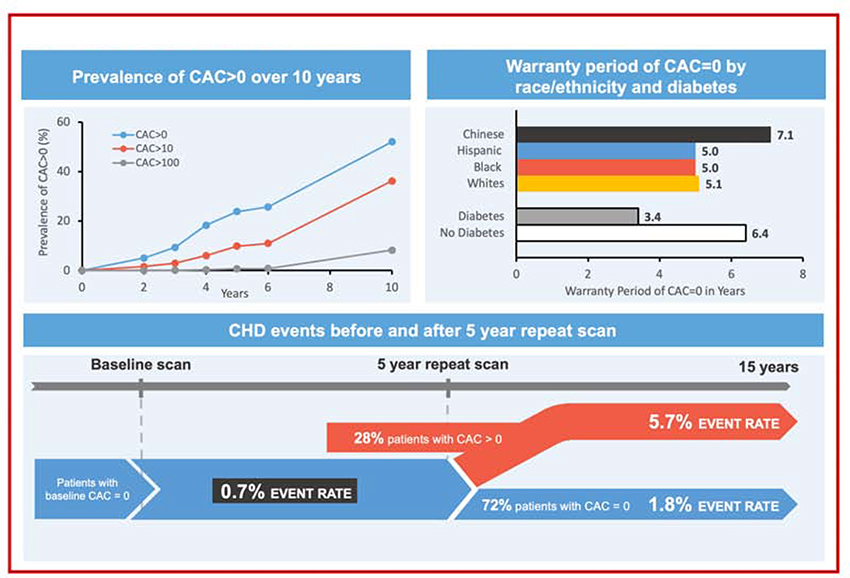
Central Illustration. Warranty period of zero coronary artery calcium.
(Top left) Cumulative annual prevalence of CAC>0, CAC>10, CAC>100 among CAC=0 MESA participants who were re-scanned during follow-up. (Top right) Warranty period (in years) of CAC=0 in the total population by race and diabetes. (Bottom) CHD event rate in sub-cohort of participants defined by 4–6 years rescan interval. CAC=Coronary artery calcium; CHD=Coronary heart disease.
Clinical Perspectives.
Competency in medical knowledge:
In a large population of individuals with baseline CAC=0, we provide a robust estimation of the CAC=0 warranty period, considering progression to CAC>0, CAC>10, and CAC>100 as well as impact on distribution of CHD events before and after rescan. We suggest that evidence-based guidance should consider rescanning in 3 to 7 years depending on individual demographics and risk profile.
Translational outlook:
Additional clinical studies are required to confirm the optimal timeframe for reporting the CAC=0 warranty period as well as to validate its prognostic implications.
Acknowledgment:
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This publication was developed under the Science to Achieve Results (STAR) research assistance agreements, No. RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
Abbreviations:
- ACC/AHA/MS
American College of Cardiology /American Heart Association / Multisociety
- AIC
Akaike information criterion
- ASCVD
Atherosclerotic cardiovascular disease
- BIC
Bayesian information criterion
- CAC
Coronary artery calcium
- CHD
Coronary heart disease event
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- MESA
Multi-Ethnic study of atherosclerosis
- NNS
Number needed to scan
- PCE
Pooled cohort equation
Footnotes
Disclosures: The authors declare that they have no conflicts of interest relevant to the content of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mieres JH, Shaw LJ, Arai A et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 2005;111:682–96. [DOI] [PubMed] [Google Scholar]
- 2.Blaha MJ, Cainzos-Achirica M, Greenland P et al. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaha MJ, Blankstein R, Nasir K. Coronary Artery Calcium Scores of Zero and Establishing the Concept of Negative Risk Factors. J Am Coll Cardiol 2019;74:12–14. [DOI] [PubMed] [Google Scholar]
- 4.Valenti V, B OH, Heo R et al. A 15-Year Warranty Period for Asymptomatic Individuals Without Coronary Artery Calcium: A Prospective Follow-Up of 9,715 Individuals. JACC Cardiovasc Imaging 2015;8:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dzaye O, Dardari ZA, Cainzos-Achirica M et al. Incidence of New Coronary Calcification: Time to Conversion From CAC = 0. J Am Coll Cardiol 2020;75:1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol 2018;72:434–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahabadi AA, Mohlenkamp S, Lehmann N et al. CAC Score Improves Coronary and CV Risk Assessment Above Statin Indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging 2017;10:143–153. [DOI] [PubMed] [Google Scholar]
- 8.Detrano R, Guerci AD, Carr JJ et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Detrano R, Peterson D et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2005;111:1313–20. [DOI] [PubMed] [Google Scholar]
- 11.Carr JJ, Nelson JC, Wong ND et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 2005;234:35–43. [DOI] [PubMed] [Google Scholar]
- 12.Chobanian AV, Bakris GL, Black HR et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 13.Genuth S, Alberti KG, Bennett P et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–7. [DOI] [PubMed] [Google Scholar]
- 14.Berlin I, Lin S, Lima JA, Bertoni AG. Smoking Status and Metabolic Syndrome in the Multi-Ethnic Study of Atherosclerosis. A cross-sectional study. Tob Induc Dis 2012;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM, Stone NJ, Bailey AL et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019;73:3168–3209. [DOI] [PubMed] [Google Scholar]
- 16.Gopal A, Nasir K, Liu ST, Flores FR, Chen L, Budoff MJ. Coronary calcium progression rates with a zero initial score by electron beam tomography. Int J Cardiol 2007;117:227–31. [DOI] [PubMed] [Google Scholar]
- 17.Min JK, Lin FY, Gidseg DS et al. Determinants of coronary calcium conversion among patients with a normal coronary calcium scan: what is the “warranty period” for remaining normal? J Am Coll Cardiol 2010;55:1110–7. [DOI] [PubMed] [Google Scholar]
- 18.Blaha M, Budoff MJ, Shaw LJ et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging 2009;2:692–700. [DOI] [PubMed] [Google Scholar]
- 19.Sarwar A, Shaw LJ, Shapiro MD et al. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging 2009;2:675–88. [DOI] [PubMed] [Google Scholar]
- 20.Budoff MJ, Young R, Lopez VA et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61:1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Lau E, Varshney R et al. The Identification of Calcified Coronary Plaque Is Associated With Initiation and Continuation of Pharmacological and Lifestyle Preventive Therapies: A Systematic Review and Meta-Analysis. JACC Cardiovasc Imaging 2017;10:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.