Abstract
The present study is aimed at evaluating the effect of different processing techniques on astringency reduction, nutrient retention, and sensory attributes in cashew apple fruit and its juice. Astringency attribute was measured by tannin content, while nutrition profile by ascorbic acid, total sugars, and antioxidant activity. Hot water, steaming, and microwave were selected as the source of heat application for treating whole fruit, where the process variables were the temperature and exposure time. The non-thermal technique selected to treat juice was by using bio coagulants, i.e., dried okra pod and drumstick seed powder, where the independent parameters were concentration and settling time. The processes were optimized using a multivariate approach coupled with full factorial design. The obtained results indicated that samples, with 42.6% tannin removal, were rated as being the least astringent. The use of dried okra pod powder under optimal conditions (0.3% concentration, 0.5 h settling time) was found to be the best in reducing astringency while retaining the nutrient and desirable sensory attributes. Maximum tannin removal (48.9 ± 1.6%) with minimum loss of ascorbic acid (8.1 ± 0.9%), total sugar (4.8 ± 0.5%) and antioxidant activity (11.1 ± 1.0) with high sensory score (92.7 ± 1.6%) was achieved with composite desirability of 0.85.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04744-4) contains supplementary material, which is available to authorized users.
Keywords: Astringency, Bio coagulant, Cashew apple fruit, Cashew apple juice, Heat treatment, Tannins
Introduction
Cashew (Anacardium Occidentale L.), a tropical plantation crop, is being widely grown in several countries of Asia, Africa, and Central America as an economically important agricultural crop. The cashew nut and cashew apple are the two morphological parts of the cashew fruit. About 10–15 tons of cashew apples are obtained as a by-product for every ton of cashew nut produced. A fully developed cashew apple contains a significant amount of polyphenols, vitamins, minerals, and organic acids, making it a functional food (Das and Arora 2017). The primary reason for the unpopularity of the cashew apple, despite its high nutritional value, is its astringent taste, which is an undesirable sensory attribute. The polyphenols, especially tannins, are responsible for the astringency (Michodjehoun-Mestres et al. 2009).
Various methods of tannins removal have been reported for cashew apple juice (CAJ) and whole cashew fruit (CAF). The methods reported for CAJ are microfiltration, enzymatic treatment, blending with other fruit juice, use of coagulating agents, etc. (Talasila et al. 2012). Microfiltration is reported to be promising as the process occurs at mild operating conditions, thereby preserving heat-sensitive compounds present in the juice. However, given the high tannin content of the CAJ, the ultra-thin membranes of 0.1 and 0.2 μm pore size used for microfiltration get quickly fouled and rendered useless (Abreu et al. 2005). Pre-treating the juice with enzymes (tannase/cellulase) before microfiltration makes the process efficient as it reduces viscosity and fractionates the insoluble polysaccharides (Cormier 2008). Despite many advantages, the technology is not being adopted by cashew processors in India due to lack of resources to access this sophisticated technology. Several studies report the blending of fresh/untreated CAJ with other tropical fruit juice as a promising technique to reduce astringency (Marc et al. 2017). However, our lab study (unpublished data) indicated when beverages made from fresh CAJ blended with other tropical fruit juice, the organoleptic scores of mixes were significantly low (p < 0.05). Hence, it is desirable to reduce tannins from fresh CAJ before blending for better sensory acceptability. The use of coagulating agents is reported to be effective and efficient in removing polymerized tannins and phenols (Pena-Neira 2019). Polyvinyl pyrrolidone (PVP) and gelatine are widely used coagulants for reducing astringency from CAJ. However, the PVP is reported to be expensive and not readily available while the use of gelatine is of concern as it comes from animal sources (Talasila et al. 2012; Hameed et al. 2018). Currently, the growing concern with environmental issues has raised the interest in bio coagulants because they are the low-cost source, abundant in nature, and non-toxic. Jayalekshmy and John (2004) reported cassava starch as an efficient and economic coagulating agent for treating CAJ. The flip side of the process is long clarification time, i.e., more than 8 h (Cormier 2008). As reported by several researchers, dried okra pod powder (DOP) and dried drumstick seed powder (DDSP) showed promising results as bio coagulants (Okolo et al. 2015). However, there is no literature available on the use of DOP and DDSP to treat CAJ. There are studies with respect to astringency removal, from CAF, using hot water and steam (Mosha et al. 1995; Akinwale and Aladesua 1999).
To the best of our knowledge, none of the reported studies have investigated the nutrition profile and sensory attributes of treated CAJ and CAF in addition to astringency level. The effectiveness of astringency removal method must be quantified by maximum reduction in tannin content while simultaneously retaining its nutritional components as maximum as possible.
Hence, the present study aims at investigating the effectiveness of different techniques selected for CAF and CAJ, with respect to the final tannin content, nutrient profile (ascorbic acid, total sugar and antioxidant activity), and sensory evaluation (astringency, sweetness and overall preferences). The heat treatment methods, selected for CAF, were hot water (HWT), steaming (HT), and microwave (MW). The non-thermal technique, selected for treating CAJ, was the use of bio coagulants, DOP and DDSP. The objective also involves optimizing the process parameters using multivariate approach, to maintain an equilibrium between astringency level, nutritional quality, and sensory attributes.
Materials and methodology
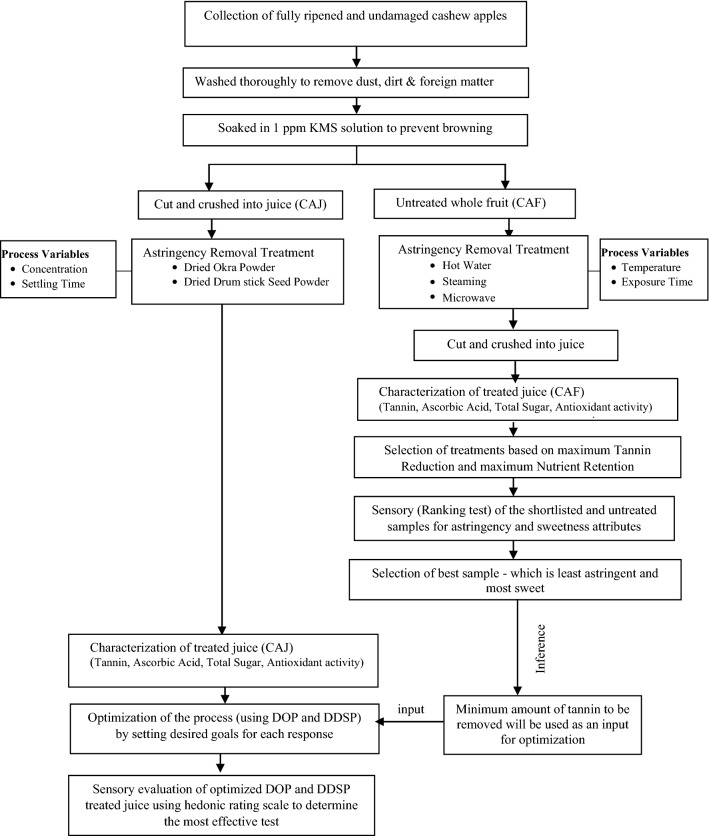
The experiment has been divided into two phases. During the 1st phase, CAF were treated with various thermal techniques. The effective treatments were shortlisted manually, on the basis of maximum tannin removal with the highest possible retention of the nutrient levels (ascorbic acid, total sugar, and antioxidant activity). The shortlisted samples were further subjected to sensory testing which assisted in inferring the minimum amount of tannin to be removed from the sample for it to be organoleptically acceptable. During the 2nd phase of the experiment, CAJ was treated with bio coagulants. The data obtained was subjected to optimization by setting the goals for each response variables (tannins and nutrient profile). The value for tannin removal obtained from the 1st phase was used as one of the inputs. The sensory test was conducted on the optimized samples to determine the most effective treatment. The detailed experimental methodology is described below, and also represented in Fig. 1.
Fig. 1.
Schematics of the experimental methodology followed
Procurement and processing of cashew apple
The fully ripened, undamaged, and fresh cashew apple (Variety: Yellow) collected from the local agricultural farms near Goa (India) (14° 53′ 54″ N, 74° 20′ 13″ E) were used for the experiments. The fruits were washed thoroughly to remove foreign materials and then soaked in preservative solution (1 ppm potassium metabisulphite (KMS)) until further treatment to prevent browning. The physico-chemical characteristics are shown in Table S1.
Techniques investigated for removal of astringency
Cashew apple fruit (CAF)
The different heat application methods selected to remove astringency from CAF were HWT, HT, and MW. The process variables were temperature (power level in the case of MW) and exposure time. The levels of independent variables were selected based on preliminary trials and published literature and details shown in Table 1a–c. Thermostatic water bath (Model-PB 28 L, Precision Water Bath) was used for the hot water treatment, in which CAF were placed at 100 ± 2 °C for different intervals (1, 5, 10, 15, and 25 min). Autoclave (Model-7407ST) was used for the steaming treatment, in which CAF were placed at 121 ± 1 °C (15.1 Psi) for different intervals (1, 5, 10, 15, and 25 min). A domestic microwave oven (2465 MHz; LG Make, Intellowave MC2886BRUM) was used for the MW treatment, in which CAF were subjected to various power levels (180 W, 360 W, 480 W) and exposure time (30 s, 60 s, 90 s). The juice extracted from treated CAF was used for further characterization.
Table 1.
Experimental details (process variables and their levels) to remove astringency from cashew apple fruit (CAF) and cashew apple juice (CAJ)
| (a) Hot water treatment (HWT)* | ||
|---|---|---|
| Independent variables | Dependent variables | |
| Temperature °C |
Exposure time min |
|
| 100 | 1 |
• Tannin • Ascorbic acid • Total sugar • Antioxidant activity |
| 100 | 5 | |
| 100 | 10 | |
| 100 | 15 | |
| 100 | 25 | |
| (b) Steaming (HT)* | ||
|---|---|---|
| Independent variables | Dependent variables | |
| Temperature °C |
Exposure time min |
|
| 121 | 1 |
• Tannin • Ascorbic acid • Total sugar • Antioxidant activity |
| 121 | 5 | |
| 121 | 10 | |
| 121 | 15 | |
| 121 | 25 | |
| (c) Microwave heating (MW)* | |||||
|---|---|---|---|---|---|
| Variables | Name (units) | Levels | Dependent variables | ||
| Lower limit | Centre point | Upper limit | |||
| Pw | Power level, W | 180 | 360 | 540 |
• Tannin • Ascorbic acid • Total sugar • Antioxidant activity |
| Et | Exposure time, s | 30 | 60 | 90 | |
| (d) Dried Okra pod powder (DOP) and dried drumstick seed powder (DDSP)** | |||||
|---|---|---|---|---|---|
| Variables | Name (units) | Levels | Dependent variables | ||
| Lower limit | Centre point | Upper limit | |||
| Cn | Concentration, g/100 ml | 0.1 | 0.3 | 0.5 |
• Tannin • Ascorbic acid • Total sugar • Antioxidant activity |
| St | Settling time,h | 0.5 | 1.0 | 1.5 | |
| Ps | Particle size, µm | < 150 | > 150 and ≤ 500 | > 500 and ≤ 1000 | |
*CAF, **CAJ
Cashew apple juice (CAJ)
The juice extracted from the fresh/untreated fruits was treated with the bio coagulants i.e., DOP and DDSP. They were prepared by drying sliced okra pods and drumstick seeds in a hot air oven, at 60 °C, until the coagulant reached constant weight. This was followed by grinding and sieving to obtain different particle sizes. The process variables considered were particle size (Ps), concentration (Cn), and settling time (St) varied between 150 and 1000 µm, 0.1–0.5%, and 0.5–1.5 h, respectively and details are shown in Table 1d. The measured amount of powdered coagulating agent was slowly poured into CAJ and constantly stirred at 120 RPM for 10 min (based on preliminary experiments) to achieve uniform dispersion of coagulant in CAJ. Subsequently, the mixture was allowed to rest for coagulation and flocculation. The cleared CAJ was then filtered and collected into a glass container for subsequent nutrient and sensory analysis.
Analysis of physcio-chemical parameters
The compositions i.e., tannins (Tn), total polyphenol content (TPC), ascorbic acid (AA), total sugars (Ts), and antioxidant activity (AOA) of both untreated and treated samples, were analysed using the methods outlined by AOAC (2012). TPC and Tn were determined spectrophotometrically using Folin-Ciocalteu and Folin-Danis reagent respectively and expressed as mg/100 ml of juice. Tannic acid and Gallic acid were used as the standard for Tn and TPC respectively with the absorbance read at 715 and 760 nm. The 2, 6-dichlorophenol indophenol titration method was used for the measuring AA content. Total sugars were determined using the phenol–sulphuric acid method and have been expressed as g/100 ml with absorbance read at 490 nm. The AOA was measured using 1, 1- diphenyl-2-picryl hydrazyl (DPPH) method. Percent inhibition of free radical DPPH was calculated using Eq. 1 and then expressed as a half-maximal effective concentration (IC50 value), i.e., the concentration of the sample required to inhibit 50% of DPPH radical scavenging activity.
| 1 |
where Acontrol Absorbance at 519 nm of DPPH without sample; Asample Absorbance at 519 nm of the reaction mixture containing DPPH and sample.
Statistical analysis
The experiments were based on 3-level, 3-factor FFD generating 27 experiments for CAJ (Table 1d). The responses measured were the percent reduction in Tn, AA, TS, and AOA. Response surface methodology (RSM) was used to develop a second-order polynomial equation for analysis of experimental data and to determine the relative contributions of variables on various responses under study. The quality of fit and statistical significance of the response function was expressed by the adjusted R2 and F-test respectively. One-way ANOVA was used to compare the percent reduction values (responses) using Tukey’s test at 95% confidence interval. The significance of all the terms (linear, quadratic, and interactions) in the response surface function was judged by computing the probability (p) at 0.05. Numerical optimization technique using desirability function was used for simultaneous optimization of the multiple responses (Giri and Prasad 2007). The objective was to maximize, minimize, or obtain the target value of the responses within the experimental range. In our study, optimization of independent variables was carried out based on maximizing the percent reduction of Tn and minimizing the percent reduction of AA, Ts & IC50 within the data obtained in experimental range. Depending on the objective, different desirability functions were employed. The overall desirability function D is calculated as the geometric average of the individual desirability functions (Myers and Montgomery 2002) using the equation given below. The value of ‘D’ ranges from zero (least desirable) to one (most desirable). The data were analysed using Minitab Statistical Software version 16 (Pennsylvania State University, USA).
| 2 |
where the function D(x) reflects the desirable ranges for each response (di), d1, d2,…,di are responses and n is the total number of responses.
Sensory evaluation
Sensory analysis was conducted using the ranking test and hedonics rating scale as described in ISO 8587: 2006 (E) (BS ISO 8587:2006) (https://www.iso.org/standard/36172.html).
Ranking test (CAF)
The ranking test was conducted to differentiate the shortlisted treated CAF samples with untreated CAF as control sample based on astringency and sweetness attributes. The panel consisting of twenty untrained panellists with age between 24 and 35 years were asked to rank the treated samples using a quantitative scale with a score from one to four in order of intensity. The tasters were familiarized with the astringency and sweetness attributes. The evaluation was done in two sessions with 10 assessors in each session, and was completed in the same day. The samples were provided in 50 mL small cups randomly coded with three-digit numbers. Each juice sample was assessed in two replications. The least significant difference (LSD) for the sum of ranks was used for comparison between two individual products. Samples whose sum of ranks differed by more than LSD value (Eq. 3) were considered significantly different.
| 3 |
where J and K represent the number of samples ranked and panellists, respectively.
The hedonics rating scale
The hedonic test was carried out to evaluate the overall acceptability of the optimized CAJ sample according to the hedonic scale on 9 scale: extremely dislike = 1; very much dislike = 2; moderately dislike = 3; slightly dislike = 4; neither like nor dislike = 5; slightly like = 6; moderately like = 7; very much like = 8 and extremely like = 9. The acceptability index (AI) was calculated using Eq. 4, as shown below (Arruda et al. 2016). Higher the index value, the well-accepted the sample is.
| 4 |
Results and discussion
The results obtained in this study have been discussed in detail in the following sections, which include the effect of treatments on CAF and CAJ on the degree of astringency removal and the retention of nutrients. Percent reduction of Tn measured the astringency level while percent retention of AA, TS, and AOA decided the nutritional attributes of the treated juice.
Effect of applications of heat treatments on CAF
Tannins (Tn)
The different heat application methods (i.e., HWT, HT, and MW) were found to reduce the Tn content significantly (p < 0.05) in CAF (Table 2). The Tn of untreated CAF was determined to be 199.7 ± 49.6 mg/100 ml. The wide variation in value may be due to the inherent disparity of Tn among fruits or could be due to experiments being spread over a few months during the season. The variation has been normalized by converting the values to percent reduction to remove the confounding of individual fruit. The reduction of Tn was found to increase with the increase in exposure time, i.e., from 28.7–48.9%, 39.2–55.7%, and 15.7–30.1% for HWT, HT, and MW treated samples, respectively. The reduction was because of heat application and in agreement with previous studies, and the value was reported to be between 30-50% for steam treated lentils and 60% for hot water processed yam/sweet potato (Hefnawy 2011; Egbuonu and Nzew 2016). Emelike and Ebere (2016) reported the reduction of Tn was ~96% in CAF when subjected to HWT for 20 min. The reason attributed was the predominant tannins present in CAF are hydrolyzable, i.e., soluble in water. Quite contrary to this, other researchers reported that hydrolyzable and condensed tannins constitute around 20–40% and 30–55%, respectively, in cashew apple, implying the tannins reduction phenomenon is rather complex (Talasila et al. 2012; Prommajak et al. 2014). Other significant reasons could be the structural changes due to thermal degradation and the formation of insoluble complexes between Tn and other components such as protein and minerals present in CAF (Nithya et al. 2007). The percent reduction of Tn is the least for MW treated samples (15.7–30.1), and a similar result has been reported for lentil when subjected to MW heating (i.e., 35% reduction) (Hefnawy 2011). HWT and HT were found to be comparatively more effective in reducing the Tn, i.e., 28.7–48.9% and 39.2–55.7%, respectively than MW heating. The reason could be, the moisture conducts heat better than air. Hence the heat transfer rate is higher in the case of HWT and HT compared to MW, which is a dry heat. This partly explains the effect of different heat treatments on the percent reduction in Tn as observed in the study.
Table 2.
Effect of different treatments on total phenols (TPC), tannins (Tn), ascorbic acid (AA), total sugars (Ts), and antioxidant activity (AOA) in cashew apple fruit (CAF)
| Run | Temp (°C) | Time (min) | TPC | Tn | AA | Ts | AOA |
|---|---|---|---|---|---|---|---|
| Hot water treated cashew apple (HWT) | |||||||
| 1 | 100 | 1 | 31.4 ± 0.8b | 28.7 ± 1.0b | 26.7 ± 1.2c | 14.1 ± 0.6a | 50.7 ± 1.4c |
| 2* | 100 | 5 | 38.1 ± 0.6c | 42.6 ± 0.9c | 30.0 ± 2.0c | 14.3 ± 0.5a | 56.2 ± 1.4d |
| 3 | 100 | 10 | 48.8 ± 0.7d | 42.7 ± 1.3c | 30.9 ± 0.8c | 15.4 ± 0.6a | 63.1 ± 2.1e |
| 4 | 100 | 15 | 52.9 ± 2.1e | 47.4 ± 0.9d | 50.3 ± 0.9d | 16.3 ± 0.4a | 68.8 ± 3.1f |
| 5 | 100 | 25 | 54.2 ± 1.2e | 48.9 ± 1.1d | 55.6 ± 1.1d | 16.1 ± 0.8a | 73.5 ± 2.5f |
| Steam treated cashew apple (HT) | |||||||
| 1 | 121 | 1 | 50.2 ± 1.7d | 39.2 ± 1.6c | 61.7 ± 1.4e | 50.2 ± 0.6b | 71.1 ± 2.9f |
| 2 | 121 | 5 | 52.2 ± 0.7e | 44.9 ± 0.5c | 68.4 ± 0.6f | 52.1 ± 0.5b | 73.3 ± 2.7f |
| 3 | 121 | 10 | 66.8 ± 1.0f | 48.4 ± 1.3d | 68.9 ± 1.1f | 53.0 ± 0.4b | 78.8 ± 1.6 g |
| 4 | 121 | 15 | 68.2 ± 0.6f | 51.4 ± 1.1d | 72.9 ± 0.4f | 52.1 ± 0.8b | 81.7 ± 0.8 g |
| 5 | 121 | 25 | 70.1 ± 1.8f | 55.7 ± 1.3e | 73.2 ± 1.4f | 53.9 ± 0.9b | 88.3 ± 0.6 h |
| Microwave treated cashew apple (MW) | |||||||
| 1 | 180 | 30 | 17.3 ± 1.3a | 15.7 ± 1.9a | 7.1 ± 2.0a | 14.1 ± 0.4a | 32.7 ± 1.3a |
| 2 | 180 | 60 | 27.1 ± 1.4b | 27.3 ± 1.8b | 7.2 ± 2.7a | 14.6 ± 0.5a | 40.6 ± 2.1b |
| 3 | 180 | 90 | 30.1 ± 1.0b | 27.8 ± 1.9b | 9.4 ± 1.8b | 15.1 ± 0.5a | 41.2 ± 1.7b |
| 4 | 360 | 30 | 16.5 ± 1.6a | 15.0 ± 2.0a | 7.1 ± 0.3a | 14.7 ± 0.8a | 31.3 ± 1.8a |
| 5* | 360 | 60 | 27.7 ± 0.3b | 28.5 ± 1.8b | 7.5 ± 2.7a | 15.1 ± 0.8a | 43.4 ± 2.4b |
| 6 | 360 | 90 | 36.3 ± 1.9c | 29.0 ± 2.6b | 10.0 ± 1.1b | 15.3 ± 0.4a | 54.4 ± 2.8d |
| 7 | 540 | 30 | 19.2 ± 1.2a | 16.9 ± 1.5a | 7.1 ± 1.4a | 15.1 ± 0.6a | 40.6 ± 3.1b |
| 8 | 540 | 60 | 36.3 ± 1.8c | 28.5 ± 2.6b | 9.9 ± 1.7b | 16.3 ± 0.4a | 54.9 ± 3.7d |
| 9* | 540 | 90 | 40.3 ± 1.7c | 30.1 ± 1.3b | 11.1 ± 0.5b | 16.8 ± 0.5a | 60.9 ± 2.7e |
*Shortlisted treatments across methods
Means that do not share a common superscript letter in a column are significantly different (p < 0.05) from each other
Total phenol content (TPC) and antioxidant activity (AOA)
It was observed that the application of heat resulted in a significant loss of phenolic compounds. The value of TPC for untreated cashew apple was found to be 324.1 ± 40.8 mg/100 ml. Losses of phenols were the highest for HT treated samples (50.2–70.1) compared to HWT (31.4–54.2), and MW treated samples (17.3–40.3) (Table 2). The steaming process in the autoclave is characterized by high pressure (15.1 psi) and high temperature (121 °C), which increases the heat content of the samples and thereby diffusion rates, which promote loss of the phenols from the sample. The lower temperature of processing during HWT (100 °C) might have helped to preserve phenols a little better. Also, the degree of thermal processing is an essential factor in deciding the amount of reduction. It was observed, the percent reduction in TPC was found to increase with an increase in treatment time and temperature irrespective of the method of heat application. The results confirm with the previous studies where it has been reported that TPC of fruits and vegetables decreased significantly with the increase in temperature and time of thermal processing (Jafari et al. 2017). Several studies show a strong positive correlation between the amount of polyphenols and antioxidant activity (AOA), which implies that polyphenol contributes to radical scavenging activity (Mahattanatawee et al. 2006). It was observed that there is a decrease in phenol content (data in Table 2) when subjected to different heat treatments in turn it might have led to a reduction in antioxidant activity. The reduction in AOA ranged between 50.7% and 70.3%, 71.1% and 88.3%, and 32.7% and 60.9% for HWT, HT, and MW treatment, respectively (Table 2).
Ascorbic acid (AA)
The AA content of untreated CAF was determined to be 196.9 ± 32.5 mg/100 ml. As explained in “Tannins (Tn)” section, the wide variation in value has been normalized by converting to percent reduction. Processing methods caused significant decreases in AA content (Table 2) and found to be highest for HT samples (60–70%) followed by HWT (30–50%). It is widely known that AA is thermolabile, i.e., thermal treatment can cause this to destroy and leach out in the medium during the processing (Lee and Kader 2000). The cause of destruction is ascorbic acid oxidation to dehydroascorbic acid, followed by hydrolysis, polymerization, and the formation of physiologically inactive substances; since heating accelerates oxidation, it results into the destruction of AA (Gregory 1996). The decrease was the least during MW heating, i.e., 7–11% and agrees with work reported by Kaur and Kapoor (2002) on the effect of MW (700 W for 3 min) and HWT blanching (100 °C for 5 min) on AA in green peas and carrot respectively. MW heating had the highest AA retention with 76%, while HWT had 34% retention. Short processing time (i.e., 30–90 s) in case of MW heating might have helped in preserving more AA than HWT and HT. In addition, moisture migration from CAF was not significant due to volumetric heating phenomena associated with MW heating. The combined effect of volumetric heating and short exposure time might not have disturbed the cell wall, which is supposed to be rich in ascorbic acid. Similar phenomena, i.e., distribution of AA in apple fruit studied by Li et al. (2008) showed that the skins of apple have the highest levels of AA than the flesh.
Total sugars (Ts)
Sugars are the important carbohydrates in juices as they not only give sweetness but also mask the astringency effects caused by tannins on its taste. Of the total sugars present in cashew apple, glucose, and fructose are predominant and contribute heavily to the sweet taste of cashew apple (de Silva et al. 2014). The untreated CAF had total sugar about 5.9 ± 1.08 g/100 ml. The reduction in total sugars was between 14 and 16% for both HWT and MW treated samples, while it is nearly 50% for HT treated samples (Table 2). The decrease is more with an increase in temperature and exposure time. Heating and roasting of food usually result in the loss of glucose, because of the interaction of glucose with amino acids at high temperatures, in a reaction called Maillard and caramelization (Woo et al. 2015).
Sensory evaluation
Sensory evaluation was carried out to rank the shortlisted treated samples (Table 1a–c) in terms of astringency and sweetness attribute. The effective treatments were shortlisted manually, on the basis of maximum tannin removal with the highest possible retention of the levels of AA, TS, and AOA. The treatments shortlisted were the “HWT (100 °C–5 min)” and “MW (360 W–60 s and 540 W–90 s)”. The sample with very intense astringency and sweetness got a rank four while the least intense sample got a rank one. The untreated sample was used as control. The intermediate samples were ranked two and three. Friedman’s non-parametric analysis of variance was performed to detect differences in the perception of attributes. The treatments were compared among themselves using Tukey’s test at 5% level of significance. The above three treatment samples, along with untreated sample were presented to panellist, and they were asked to rank according to the scale. Figure 2 shows that samples have been well differentiated in terms of astringency (Tn) and sweetness (Ts). For astringent attribute, T4 (control, i.e., untreated fruit) showed the highest sum of ranks signifying high astringency while the T1 (HWT:100 °C–5 min) had the lowest sum of ranks indicating the lowest astringency. Treatment T3 (MW: 540 W–90 s) and T2 (MW: 360 W–60 s) were intermittently ranked as two and three. Friedman’s analysis of variance showed that the astringency among the samples is significantly different. The highest sweetness was perceived for the T4, while the sample T3 showed the least. Sample T1 and T2 (360 W, 60 s) intermediately ranked with no significant difference among themselves. Based on the sweetness and astringency score, HWT sample (T1: 100 °C for 5 min) was organoleptically accepted, where Tn and Ts removals were about 42.6 ± 0.9% and 15.3 ± 0.5% respectively.
Fig. 2.
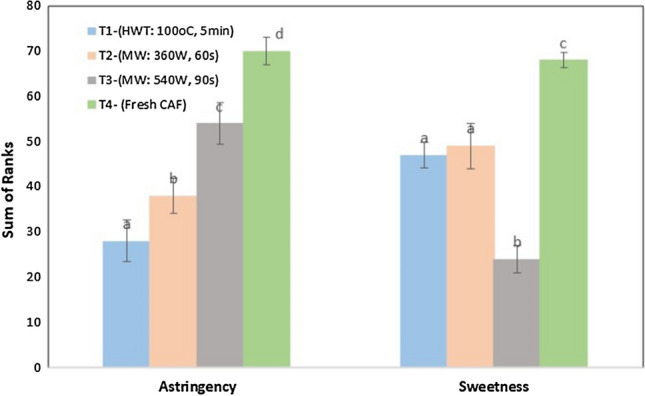
Results of ranking test for untreated and treated cashew apple fruit (CAF)
Effect of Coagulating Agent in Treating CAJ
Dried okra pod powder (DOP)
The effects of the process variables (Ps, Cn, and St), using DOP in CAJ, on responses have been discussed in detail below.
Effect of particle size (Ps)
Cashew apple juice treated with DOP at different experimental conditions were evaluated for percent reductions in Tn, AA, Ts and AOA and results are listed in Table 3a–c for three different Ps, i.e., “≤ 150 µm”, “> 150 and ≤ 500 µm”, “> 500 and ≤ 1000 µm”, respectively. The percent reduction in Tn was found to vary between 21.1 and 54.1 within the combinations of the variables studied. The interaction of tannin with the protein, starch and digestive enzymes results in the formation of complexes of higher molecular weight, which later precipitates, and leads to the reduction of tannin (Sieniawska and Baj 2017). The okra powder being rich in proteins and polysaccharides act as a promising flocculation agent. Analysis shows DOP contain 13–25% crude protein and about 35–45% of carbohydrates (Okolo et al. 2015). In their separate studies, De Jesus et al. (2013) have reported that using DOP as a coagulant helped in achieving up to 99% reduction in turbidity in wastewater within 10 min of sedimentation time. Several authors found that both hydrolysable and condensed tannins could also bind with starch and polysaccharides (Barros et al. 2012), and cashew apple has both the forms of tannins [“Tannins (Tn)” section].
Table 3.
Effect of particle size (Ps) on percent reduction of tannins (Tn), ascorbic acid (AA), total sugars (Ts) and antioxidant activity (AOA) at different concentration (Cn) and settling time (St)
| Run | Independent variables | % Reduction (responses) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOP | DDSP | |||||||||
| Cn (%) | St (h) | Tn | AA | Ts | AOA | Tn | AA | Ts | AOA | |
| (a) Size: ≤ 150 µm | ||||||||||
| 1 | 0.1 | 0.5 | 44.3 ± 1.0 | 7.1 ± 1.0 | 3.7 ± 1.0 | 4.7 ± 0.4 | 20.0 ± 1.2 | 21.0 ± 0.3 | 1.0 ± 0.1 | 6.1 ± 0.8 |
| 2 | 0.1 | 1.0 | 47.1 ± 1.1 | 8.1 ± 0.9 | 10.1 ± 0.8 | 11.4 ± 0.5 | 36.9 ± 0.7 | 24.8 ± 0.6 | 3.3 ± 0.2 | 14.8 ± 0.9 |
| 3 | 0.1 | 1.5 | 48.9 ± 0.8 | 8.7 ± 0.3 | 12.9 ± 0.7 | 30.5 ± 0.3 | 52.0 ± 1.0 | 26.9 ± 0.7 | 26.8 ± 0.5 | 28.0 ± 0.9 |
| 4 | 0.3 | 0.5 | 45.1 ± 0.9 | 7.1 ± 1.5 | 4.1 ± 0.9 | 7.3 ± 0.3 | 22.8 ± 0.7 | 24.1 ± 0.9 | 2.5 ± 0.4 | 7.5 ± 1.0 |
| 5 | 0.3 | 1.0 | 46.9 ± 0.9 | 8.3 ± 1.1 | 11.5 ± 1.2 | 15.1 ± 0.6 | 40.2 ± 1.2 | 26.1 ± 0.9 | 11.6 ± 0.4 | 23.0 ± 1.0 |
| 6 | 0.3 | 1.5 | 53.1 ± 0.3 | 9.1 ± 1.0 | 14.2 ± 0.9 | 32.4 ± 0.3 | 56.9 ± 0.6 | 27.8 ± 1.3 | 31.6 ± 0.6 | 40.2 ± 0.6 |
| 7 | 0.5 | 0.5 | 48.8 ± 1.1 | 8.2 ± 1.2 | 6.4 ± 0.8 | 35.0 ± 0.6 | 27.1 ± 0.8 | 25.7 ± 1.6 | 6.1 ± 0.3 | 9.1 ± 0.8 |
| 8 | 0.5 | 1.0 | 51.1 ± 0.7 | 9.4 ± 0.8 | 14.8 ± 0.9 | 43.1 ± 0.3 | 46.4 ± 0.7 | 26.2 ± 0.7 | 11.9 ± 0.4 | 32.1 ± 0.8 |
| 9 | 0.5 | 1.5 | 53.9 ± 1.3 | 9.8 ± 1.0 | 17.1 ± 1.3 | 47.8 ± 0.3 | 61.1 ± 0.9 | 29.0 ± 0.8 | 32.0 ± 0.6 | 44.2 ± 0.7 |
| (b) Size: > 150 and ≤ 500 µm | ||||||||||
| 1 | 0.1 | 0.5 | 44.5 ± 0.4 | 6.9 ± 0.7 | 3.6 ± 0.1 | 4.4 ± 0.3 | 19.9 ± 0.5 | 20.9 ± 0.1 | 0.9 ± 0.1 | 5.9 ± 0.8 |
| 2 | 0.1 | 1.0 | 46.6 ± 0.4 | 8.1 ± 0.8 | 9.9 ± 0.1 | 11.4 ± 0.5 | 36.4 ± 0.7 | 24.5 ± 0.4 | 3.2 ± 0.1 | 14.6 ± 0.7 |
| 3 | 0.1 | 1.5 | 49.4 ± 1.0 | 8.9 ± 1.3 | 13.1 ± 0.6 | 30.2 ± 0.3 | 51.5 ± 0.3 | 26.6 ± 0.6 | 26.7 ± 0.2 | 27.8 ± 1.1 |
| 4 | 0.3 | 0.5 | 45.6 ± 0.4 | 7.1 ± 0.9 | 4.1 ± 0.3 | 6.9 ± 1.4 | 22.1 ± 0.2 | 23.9 ± 0.8 | 2.4 ± 0.1 | 7.30 ± 0.5 |
| 5 | 0.3 | 1.0 | 47.4 ± 0.7 | 8.3 ± 1.1 | 11.9 ± 0.7 | 15.6 ± 0.4 | 39.2 ± 0.4 | 25.8 ± 0.9 | 11.5 ± 0.1 | 23.3 ± 1.7 |
| 6 | 0.3 | 1.5 | 53.0 ± 0.1 | 9.1 ± 0.4 | 14.5 ± 0.2 | 32.5 ± 0.6 | 56.4 ± 0.6 | 27.4 ± 0.8 | 31.4 ± 0.2 | 40.0 ± 1.9 |
| 7 | 0.5 | 0.5 | 49.1 ± 0.8 | 8.2 ± 1.2 | 6.7 ± 0.2 | 34.7 ± 0.2 | 26.5 ± 0.9 | 25.4 ± 1.1 | 6.1 ± 0.2 | 9.9 ± 1.4 |
| 8 | 0.5 | 1.0 | 50.1 ± 0.7 | 9.4 ± 0.8 | 14.9 ± 0.1 | 42.9 ± 0.6 | 45.9 ± 0.1 | 25.9 ± 0.7 | 11.8 ± 0.5 | 31.8 ± 1.9 |
| 9 | 0.5 | 1.5 | 54.1 ± 0.7 | 9.7 ± 0.6 | 16.3 ± 0.4 | 47.8 ± 0.3 | 60.3 ± 0.4 | 28.2 ± 0.5 | 31.9 ± 0.2 | 45.3 ± 1.3 |
| (c) Size: > 500 and ≤ 1000 µm | ||||||||||
| 1 | 0.1 | 0.5 | 21.1 ± 1.8 | 5.1 ± 0.3 | 2.9 ± 0.3 | 3.1 ± 0.5 | 10.2 ± 1.0 | 15.9 ± 0.8 | 0.4 ± 0.1 | 4.1 ± 0.3 |
| 2 | 0.1 | 1.0 | 22.9 ± 1.0 | 6.0 ± 0.2 | 8.9 ± 0.8 | 7.7 ± 0.3 | 14.5 ± 0.8 | 17.2 ± 1.0 | 1.3 ± 0.1 | 11.7 ± 0.9 |
| 3 | 0.1 | 1.5 | 24.1 ± 1.5 | 6.7 ± 0.7 | 9.1 ± 0.9 | 13.2 ± 0.4 | 22.0 ± 1.2 | 19.4 ± 1.2 | 17.1 ± 0.8 | 21.2 ± 0.6 |
| 4 | 0.3 | 0.5 | 22.6 ± 0.8 | 5.2 ± 0.7 | 3.2 ± 0.5 | 4.5 ± 0.6 | 11.2 ± 0.9 | 16.0 ± 1.1 | 1.1 ± 0.2 | 5.6 ± 0.5 |
| 5 | 0.3 | 1.0 | 23.4 ± 0.8 | 6.6 ± 0.6 | 9.6 ± 1.1 | 10.1 ± 0.7 | 19.1 ± 1.1 | 18.6 ± 1.1 | 10.6 ± 0.4 | 20.2 ± 1.0 |
| 6 | 0.3 | 1.5 | 25.7 ± 0.9 | 7.7 ± 0.6 | 10.8 ± 0.4 | 19.2 ± 0.4 | 26.8 ± 0.8 | 20.3 ± 1.0 | 21.9 ± 0.5 | 33.6 ± 0.5 |
| 7 | 0.5 | 0.5 | 23.3 ± 0.9 | 6.1 ± 0.7 | 4.6 ± 0.4 | 19.8 ± 0.8 | 12.3 ± 0.8 | 18.1 ± 0.9 | 4.3 ± 0.2 | 6.8 ± 0.3 |
| 8 | 0.5 | 1.0 | 25.1 ± 1.0 | 7.4 ± 0.8 | 10.8 ± 0.3 | 23.6 ± 0.7 | 22.2 ± 1.2 | 19.0 ± 0.9 | 10.9 ± 0.9 | 27.7 ± 0.5 |
| 9 | 0.5 | 1.5 | 26.7 ± 1.1 | 8.1 ± 0.6 | 12.1 ± 0.8 | 33.3 ± 0.4 | 27.0 ± 1.1 | 22.2 ± 0.8 | 22.1 ± 0.3 | 38.8 ± 0.7 |
The percent reduction of Tn was observed to be a function of Ps and varied between 44.3–53.9 and 44.5–54.1 for Ps “≤ 150 µm” and “> 150 and ≤ 500 µm” respectively (Table 3a, b). However, when Ps increased to “> 500 and ≤ 1000 µm”, the decrease in reduction of Tn, is not significant i.e., between 21.1 and 26.7%. This indicated the smaller the Ps, the higher the surface area per unit weight of coagulant (Maina et al. 2016) this could have led to a higher percentage of removal of Tn.
The Ps also affected the removal of other nutrients along with the tannins. The reductions were in the range of 5.1–9.8%, 2.9–17.1%, and 3.1–47.8% for AA, TS, and AOA (Table 3), respectively, for all the three particle sizes studied. Based on maximum Tn removal (set at ≥ 42%) and minimum nutrient loss, the particle size range selected was “≤ 150 µm” and “> 150 and ≤ 500 µm”. However, it was observed at Ps “≤ 150 µm” the CAJ was slimy, which posed filtration challenges. Also, the energy requirement is a function of the particle size reduction ratio. The smaller the particle size, the more energy required. Therefore, the most favourable range was Ps “> 150 and ≤ 500 µm”. A subsequent analysis was conducted using other two independent variables i.e. Cn and St, while keeping Ps (> 150 and ≤ 500 µm) as the constant variable.
Effect of concentration (Cn) and settling time (St)
The results of the percent reduction of Tn, AA, TS, and AOA for particle size “>150 and ≤ 500 µm” at different Cn and St are shown in Table 3b. The data were used to design a response function to study the combined effect of independent variables. ANOVA was conducted to confirm the adequacy of the response functions, and results are shown in Table S2. The adjusted R2 obtained for percent reduction in Tn, AA, TS, and AOA as a function of Cn and St were 0.92, 0.91, 0.98, and 0.97.
The data indicated that the Tn removal increased with an increase in Cn irrespective of St. It might be at higher Cn, the numbers of sites available for interaction with tannins are more, thereby resulting in higher removal of Tn (Van Buren and Robinson 1969). However, when Cn increased to ≥ 0.5%, it had minimal effect on Tn removal (results not shown). This could be due to particle overlapping and overcrowding, resulting in a reduction of the total adsorbent surface area. Similarly, there is a significant increase in percent reduction of Tn with an increase in St. However, beyond 1.5 h of St, there is a plateau, which might be due to most of the flocs were already settled by then. St has a more significant effect on the percent reduction of Tn (p < 0.05) than Cn (Table S2).
The percent reduction of AA and TS was also found to increase with the increase in Cn and St. This is attributed to the fact that there could be hydrogen bonding between coagulating agent and the other substances, leading to a formation of the high molecular weight compound, followed by precipitation. Results of this work were found to be consistent with the Naka et al., (2015) wherein they used cashew gum as a clarifying agent to reduce astringency in CAJ. AOA values were also observed to decrease with an increase in Cn and St between 4.5 and 47.8%, which were found to be strongly correlated with phenol content.
Dried drumstick seed powder (DDSP)
The following section describes the use of DDSP on response variables at different Ps, Cn and St.
Effect of particle size (Ps)
The percent reduction in Tn correspond to three different Ps, i.e., ≤ 150 µm, > 150 and ≤ 500 µm, and > 500 and ≤ 1000 µm is shown in Table 3a–c respectively. As explained earlier (“Effect of particle size (Ps)” section), the decrease in Tn could be due to the formation of insoluble tannin-protein complexes. Drumstick seeds contain active coagulating agents, i.e., dimeric cationic proteins with a molecular mass of 12–14 kDa, which act as flocculant inducing agents (Martín et al. 2012). Smaller Ps (i.e., “≤ 150 µm” and “> 150 and ≤ 500 µm”) resulted in higher removal of Tn, i.e., 20.0–61.1% and could be due to higher surface area per unit of coagulant.
The Ps also affected nutrient content along with the tannins. The reductions were in the range of 15.9–29.0, 0.4–32.0, and 4.1–45.3 for AA, TS, and AOA, respectively, for all the three different Ps studied (Table 3). It was observed that at Ps “> 500 and ≤ 1000 µm”, though there was maximum possible retention, which is desirable, but the Tn removed was not adequate, i.e., 10.2–27.0%. Based on the tests carried out on CAF (“Sensory evaluation” section), minimum Tn removal must be ≥ 42.6% to make the sample organoleptically acceptable. Hence, Ps “> 500 and ≤ 1000 µm” was rejected. Though the use of Ps “≤ 150 µm” could remove Tn up to 40–60%, it made the juice slimy, thereby posing difficulties during filtration. Therefore, the Ps “> 150 and ≤ 500 µm” was selected for subsequent analysis.
Effect of concentration (Cn) and settling time (St)
CAJ treated with DDSP at different combination of Cn and St (Ps: “> 150 and ≤ 500 µm”) was studied to determine the percent reduction in Tn, AA, TS, and AOA (Table 3b). The adjusted R2 obtained for response surface functions for percent reduction in Tn, AA, Ts, and AOA were 0.99, 0.87, 0.98, and 0.97, respectively. The removal of Tn increased with an increase in Cn from 19.9 to 60.3%. Both the independent variables significantly (p < 0.05) affected the reduction of Tn (Table S2). The increase in Cn and St increased the reduction in AA, and Ts i.e. from 20.9–28.2% and 1.0–31.9% respectively and were significantly higher compared to DOP treated CAJ (AA: 6.9–9.7% and Ts: 3.6–16.3%) (Table 3b). Highest removal of Tn was achieved at 1.5 h of St, irrespective of Cn, and a further increase in St did not show any changes. The percent reduction in AOA values ranged between 5.9 and 45.3 and found to be comparable with DOP treated CAJ (4.4–47.8).
Optimization
An optimization approach using desirability functions of multiple responses was performed using Minitab statistics software. The goals for optimization were set at obtaining tannin removal at ≥ 42.6% (based on input from 1st phase of the experiment on CAF), and minimizing percent reduction in AA, Ts, and AOA values within the data obtained in the experimental range (shown in Fig. S1 a and b for DOP and DDSP, respectively). The optimum conditions were determined to be 0.3% of Cn and 0.5 h of St for DOP-treated CAJ and 0.1% of Cn, and 1.2 h of St for DDSP-treated CAJ with composite desirability of 0.85 and 0.69 respectively (Fig. 3a, b). The percent reduction in Tn, AA, Ts, and AOA values were also experimentally determined under optimum conditions. The predicted reduction values for DOP treated CAJ were 46.4%, 7.2%, 4.5%, 10.1% and DDSP treated CAJ were 44.9%, 25.5%, 14.5%, 22.5% for Tn, AA, Ts, and AOA respectively. The experimental values were relatively close to the software-generated values (Table S3) hence confirming the validity of the optimized results. The contour plots of all response variables (Tn, AA, Ts, and AOA values), as a function of Cn, and St, were superimposed together, and the optimum regions (white shaded areas in the overlay plots) are shown in Fig. 3a, b for DOP-treated and DDSP-treated CAJ respectively.
Fig. 3.
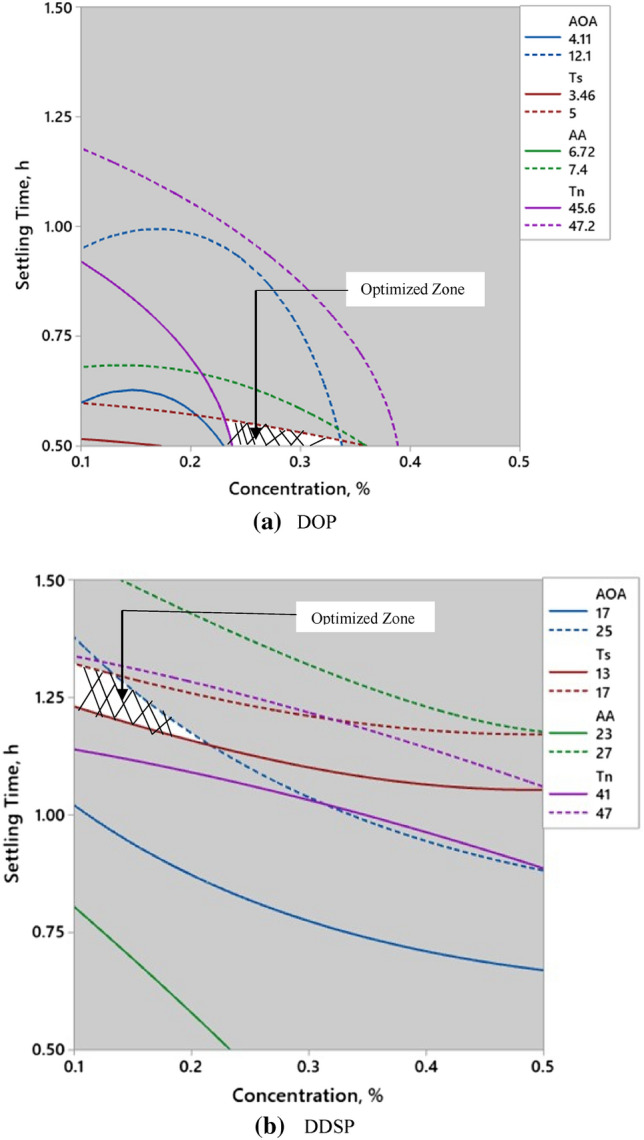
Graphical optimization (shaded area) of concentration and settling time for percent reductions in tannins (Tn), ascorbic acid (AA), total sugars (Ts) and antioxidant activity (AOA) of the treated CAJ using a Dried okra pod powder (DOP) and b Dried drumstick seed powder (DDSP)
Sensory evaluation using hedonics rating-test
Among the optimized DOP and DDSP treated samples, the DOP treated CAJ had a significant higher sensory acceptance index (92.7 ± 1.6%) than DDSP treated CAJ (79.1 ± 2.1%). The overall acceptability of DOP treated CAJ ranged between 8 (Like very much) and 9 (Like extremely) while for DDSP; it varied between 6 (Like Slightly) and 7 (Like moderately). Overall liking may be strongly correlated to astringency and sweetness attributes. Hence, the ranking test was further carried out to determine the astringency and sweetness score for optimized DOP and DDSP treated CAJ. HWT treated samples (100 °C for 5 min, the best one among the CAF) was used as the reference sample (“Sensory evaluation” section). The astringency score was found to be in the range of 34–37 for DOP, DDSP, and HWT treated CAJ with no significant difference among each other. While the sweetness score was the highest for DOP treated CAJ (i.e., 55) followed by HWT (i.e., 39) with least score obtained for DDSP treated CAJ (i.e., 20). Based on the astringency removal, nutrition retention, and sensory score, the optimized DOP treated CAJ (0.3% of Cn and 0.5 h of St) was found to be the best.
Conclusion
The study aimed to compare the different techniques to reduce the Tn while retaining its nutrient content, i.e., AA, Ts, and AOA as maximum as possible from both CAF and CAJ. The obtained results indicated that treated samples, with 42.6% tannin removal, were rated as the least astringent among all the samples. The numerical and graphical optimization indicated that the use of DOP at Cn of 0.3% and St of 0.5 h was optimal, where maximum Tn removal (46.4%) with minimum reduction in AA (7.2%), Ts (4.5%) and AOA (10.1%) was predicted with high acceptability index (92.7%). Since corresponding experimental values (48.9 ± 1.6, 8.1 ± 0.9, 4.8 ± 0.5, 11.1 ± 1.0 for Tn, AA, Ts, and AOA respectively) were close to the predicted values, the optimum conditions obtained in this study could be used as a standard or baseline information for industrial processing of this particular cashew apple variety. Retention of high concentrations of AA, Ts, and AOA makes it an excellent option for the beverages market in the form of juice blends.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the University Grant Commission (UGC), India (F.15-1/2015-17/PDFWM-2015-17-ORI-35369) for granting research fellowship and Tata Centre for Technology and Design, IIT Bombay (DGDON 422) for providing financial support for this study.
Abbreviations
- CAF
Cashew apple fruit
- CAJ
Cashew apple juice
- DOP
Dried okra pod powder
- DDSP
Dried drumstick seed powder
- HWT
Hot water treated
- HT
Steam treated
- MW
Microwave treated
- AA
Ascorbic acid (g/100 ml)
- AOA
Antioxidant activity
- Cn
Concentration (%, g/100 ml)
- PS
Particle size (µm)
- St
Settling time (h)
- TPC
Total phenol (g/100 ml)
- Tn
Tannin content (g/100 ml)
- TS
Total sugar (g/100 ml)
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ipsita Das, Email: ipsita.das@iitb.ac.in.
Sonia Sasmal, Email: sst2901@gmail.com.
Amit Arora, Email: aarora@iitb.ac.in.
References
- Abreu F, Perez AM, Dornier M, Reynes M. Potentialités de la microfiltration tangentielle sur membranes minérales pour la clarification du jus de pomme de cajou. Fruits. 2005;60(1):33–40. doi: 10.1051/fruits:2005010. [DOI] [Google Scholar]
- Akinwale TO, Aladesua OO. Comparative study of the physico chemical properties and the effect of different techniques on the quality of cashew juice from Brazilian and local varieties. Nig J Tree Crop Res. 1999;3:60–66. [Google Scholar]
- AOAC . Official methods of analysis of AOAC International. 19. Arlington: Association of Official Analytical Chemist; 2012. [Google Scholar]
- Arruda HS, Botrel DA, de Barros Fernandes RV, de Almeida MEF. Development and sensory evaluation of products containing the Brazilian Savannah fruits araticum and cagaita. Braz J Food Technol. 2016;19:e2015105. doi: 10.1590/1981-6723.10515. [DOI] [Google Scholar]
- Barros F, Awika JM, Rooney LW. Interaction of tannins and other sorghum phenolic compounds with starch and effects on in vitro starch digestibility. J Agric Food Chem. 2012;60(46):11609–11617. doi: 10.1021/jf3034539. [DOI] [PubMed] [Google Scholar]
- Cormier R. Clarification of cashew apple juice and commercial applications. Benin: Oxfarm Quebec; 2008. pp. 1–9. [Google Scholar]
- Das I, Arora A. Post-harvest processing technology for cashew apple: a review. J Food Eng. 2017;194:87–98. doi: 10.1016/j.jfoodeng.2016.09.011. [DOI] [Google Scholar]
- de Jesus E, Cruz PV, Pacifico JA, Silva AS. Removal of turbidity, suspended solids and ions of Fe from aqueous solution using okra powder by coagulation-flocculation process. Am J Water Res. 2013;1(3):20–24. doi: 10.12691/ajwr-1-3-1. [DOI] [Google Scholar]
- de Silva LMR, de Figueiredo EAT, Nagila Ricardo MPS, et al. Quantification of bioactive compounds in pulps and by-products of tropical fruits from Brazil. Food Chem. 2014;15:398–404. doi: 10.1016/j.foodchem.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Egbuonu ACC, Nzew DC. Influence of blanching on some nutrient and anti-nutrient compositions of bitter yam. Br J Appl Sci Technol. 2016;16(2):1–8. doi: 10.9734/BJAST/2016/25650. [DOI] [Google Scholar]
- Emelike NJT, Ebere CO. Effect of treatments on the tannin content and quality assessment of cashew apple juice and the kernel. Eur J Food Sci Technol. 2016;4(3):25–36. [Google Scholar]
- Giri S, Prasad S. Optimization of microwave vacuum drying of button mushrooms using response surface methodology. Dry Technol. 2007;25:901–911. doi: 10.1080/07373930701370407. [DOI] [Google Scholar]
- Gregory J. Vitamins. In: Fennema O, editor. Food chem. New York: Marcel Dekker; 1996. pp. 531–616. [Google Scholar]
- Hameed AM, Asiyanbi-H T, Idris M, Fadzillah N, Mirghani MES. A Review of gelatin source authentication methods. Trop Life Sci Res. 2018;29(2):213–227. doi: 10.21315/tlsr2018.29.2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefnawy TH. Effect of processing methods on nutritional composition and anti-nutritional factors in lentils. Ann Agric Sci. 2011;56(2):57–61. doi: 10.1016/j.aoas.2011.07.001. [DOI] [Google Scholar]
- Jafari SM, Sarem Nejad SF, Dehnad D, Rashidi AM. Effects of thermal processing by nanofluids on vitamin C, total phenolics and total soluble solids of tomato juice. J Food Sci Technol. 2017;54:679–686. doi: 10.1007/s13197-017-2505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayalekshmy VG, John PS. Sago-A natural product for cashew apple juice clarification. J Trop Agric. 2004;42:67–68. [Google Scholar]
- Kaur C, Kapoor HC. Anti-oxidant activity and total phe-nolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37(2):153–161. doi: 10.1046/j.1365-2621.2002.00552.x. [DOI] [Google Scholar]
- Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20:207–220. doi: 10.1016/S0925-5214(00)00133-2. [DOI] [Google Scholar]
- Li MJ, Ma FW, Zhang M, Pu F. Distribution and metabolism of ascorbic acid in apple fruits (Malus domestica Borkh cv. Gala) Plant Sci. 2008;174:606–612. doi: 10.1016/j.plantsci.2008.03.008. [DOI] [Google Scholar]
- Mahattanatawee K, Manthey JA, Luzio G, Talcott ST, Goodner K, Baldwin EA. Total antioxidant activity and fiber content of select florida-grown tropical fruits. J Agric Food Chem. 2006;54:7355–7363. doi: 10.1021/jf060566s. [DOI] [PubMed] [Google Scholar]
- Maina IW, Obuseng V, Nareetsile F. Use of Moringa oleifera (Moringa) seed pods and Sclerocarya birrea (morula) nut shells for removal of heavy metals from wastewater and borehole water. J Chem. 2016 doi: 10.1155/2016/9312952. [DOI] [Google Scholar]
- Marc A, Olivier KK, Claver KD. Technical sheet of valorisation of cashew apple juice (Anacardium occidentale L.) by association with passion fruit juice (Paciflora edulis) J Pharmacy. 2017;7(1):61–64. [Google Scholar]
- Martín JS, Heredia JB, Peres JA. Improvement of the flocculation process in water treatment by using Moringa oleifera seeds extract. Braz J Chem Eng. 2012;29(3):495–501. doi: 10.1590/S0104-66322012000300006. [DOI] [Google Scholar]
- Michodjehoun-Mestres L, Souquet JM, Fulcrand H, Meudec E, Reynes M, Brillouet JM. Characterization of highly polymerized from skin and flesh of four cashew apple (Anacardium occidentale L.) genotypes. Food Chem. 2009;114(3):989–999. doi: 10.1016/j.foodchem.2008.10.052. [DOI] [Google Scholar]
- Mosha TC, Gaga HE, Pace RD, Laswai HS, Mtebe K. Effect of blanching on the content of antinutritional factors in selected vegetables. Plant Foods Nutr. 1995;47(4):361–367. doi: 10.1007/BF01088275. [DOI] [PubMed] [Google Scholar]
- Myers RH, Montgomery DC. Response surface methodology: product and process optimization using designed experiments. 4. New York: Wiley; 2002. [Google Scholar]
- Naka T, Kouakou MD, Soumaila D, Patrice KL. Assessment of some bio chemical parameters of apple juices from two cashew varieties as affected by three regions of Côte d’Ivoire. J Adv Agric. 2015;5(2):621–633. [Google Scholar]
- Nithya S, Ramachandramurty B, Krishnamoorthy VV. Assessment of anti-nutritional factors, minerals and enzyme activities of the traditional and hybrid pearl millet (Pennisetum glaucum) as Influenced by different processing methods. J Appl Sci Res. 2007;2:1164–1168. [Google Scholar]
- Okolo B, Nnaji P, Menkiti M, Onukwuli O. A kinetic investigation of the pulverized okra pod induced coag-flocculation in treatment of paint wastewater. Am J Anal Chem. 2015;6:610–622. doi: 10.4236/ajac.2015.67059. [DOI] [Google Scholar]
- Pena-Neira A (2019) Management of astringency in wine. In: Morata A (ed), Red wine technology, Academic Press, pp 257–272. ISBN -978-0-12-814399-5
- Prommajak T, Leksawasdi N, Rattanapanone N. Biotechnological vaporization of cashew apple: a review. CMU J Nat Sci. 2014;13(2):159–182. [Google Scholar]
- Sieniawska E, Baj T. Tannins. Plant metabolites: their chemistry. In: Badal S, Delgoda R, editors. Pharmacognosy: fundamentals, applications and strategies. Oxford: Academic Press; 2017. pp. 199–232. [Google Scholar]
- Talasila U, Vechalapu RR, Shaik KB. Clarification, preservation, and shelf life evaluation of cashew apple juice. Food Sci Biol Technol. 2012;21(3):1–6. [Google Scholar]
- Van Buren JP, Robinson WB. Formation of complexes between protein and tannic acid. J Agric Food Chem. 1969;17:772–777. doi: 10.1021/jf60164a003. [DOI] [Google Scholar]
- Woo KS, Kim HY, Hwang IG, Lee SH, Jeong HS. Characteristics of the thermal degradation of glucose and maltose solutions. Prev Nutr Food Sci. 2015;20(2):102–109. doi: 10.3746/pnf.2015.20.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.