Abstract
The cereal grains such as wheat, barley, sorghum, millets were evaluated before and after germination (24 h, 48 h and 72 h) and compared for their proximate composition, antioxidant activity, total phenolic content, total flavonoid content, pasting properties, in vitro starch digestibility and FTIR spectroscopy. Germination inversely affected the protein, fat, and ash content of different cereal grains. The germinated flours have less water content and higher oil absorption capacities along with reduced starch content. The contents of rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch (RS) in the ungerminated cereal flours ranged from 20.7 to 32.1%, 26.9 to 38.0% and 6.2 to 17.6% respectively but after germination of 72 h, the RDS content increased from 26.5 to 36.2% while SDS and RS content decreased from 26.1% (sorghum) to 16% (barley) and 14.7% (barley) to 4.6% (wheat) respectively. The drought–tolerant crops (sorghum, millets and barley) are potential sources of antioxidants and phenolic content and yielded lower hydrolysis index and estimated glycaemic index upon germination. The highest section of antiparallal β-sheet, α-helix and β-turns were found in wheat flour followed by sorghum flour and their proportion decreased with continuous germination. The continuous reduction of viscosity was evaluated with the progress in germination. Overall, germination is a way to get health-promoting compounds from less utilizing cereal such as millets, sorghum and barley and enhance their uses to nourish the huge population with the aim to fulfill their nutritional requirements.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04742-6) contains supplementary material, which is available to authorized users.
Keywords: Germination, ATR–FTIR, Starch digestion, Cereal grains
Introduction
Cereal grains belong to Monocotyledonous family, Gramineae or grass family. The main cereal crops are rice, wheat, maize, barley, millets, sorghum, oats and rye. India is one of the main grains producing country in the world (FAOSTAT 2017) and growing of cereal commodities is necessary for the nourishment of its large population. Barley, sorghum and millet are such grains which grow and develop in less time than wheat, adjust well in severe conditions of salinity stress, water shortage and are more appropriate for growing in drought susceptible regions that lead to sustainable agriculture (Manjunatha et al. 2006). Sorghum and millet are the important crops of semiarid regions of Africa and Asia. Barley is the most widely developed grain around the world and consumed as malted form in brewing and bakery industry. It constitutes the similar structure as wheat but varies in the proportion of bioactive phytochemicals such as β-glucan, antioxidants and phenolic compounds which are attaining a wide acceptance due to their health benefits (Madhujith and Shahidi 2007).
The germination or biological activation of grain is a natural, biological processing strategy that can be utilized to enhance the better nutritional, functional and sensory characteristics of cereal grains along with increased their micronutrients. The nutritive value of grains can be increased by germinating the grains from 12 to 48 h (Singh et al. 2017). The significant ecological conditions required for grain initiation are ideal moistness, accessibility of oxygen for aerobic respiration, a sufficient temperature and time for the diverse metabolic processes (Sangronis and Machado 2007). The germination technique is considered as environment friendly processing technique and such germinated grains and their flours are suitable for the development of novel foods (foods with low glycemic index) and value-added products (Malleshi and Klopfenstein 1998). The modifications in protein structure of cereal grains during germination have been described to be largely accountable for functional changes such as emulsification, oil absorption, and water absorption capacity (WAC). The amylograph viscosity and swelling power decreased during activation of grains and no variation or only minor ones in amylose content were noted during the germination process (Chinma et al. 2009). The phenolic compounds present in cereal grains contribute to the nutritional and functional properties of grains. The synthesis of enzymes and modification of grains took place during the germination process, which contributed to the enhancement of intrinsic phenolic compounds and antioxidant activity (Kaukovirta-Norja et al. 2004). The large volume of the prepared malts is dealt with in the brewing and distilling enterprises, while malts can likewise be used in different items, for example, bakery products, breakfast cereals, cereal bars, and non-alcoholic beverages (malt coffee or lactic acid matured refreshments). Therefore, this study was aimed to investigate the effect of germination time on physicochemical, nutritional and ATR–FTIR molecular interactions of flour prepared from germinated wheat, barley, sorghum and millet.
Materials and methods
Materials
Four cereal grains, wheat (Triticum aestivum) (HD-3086), barley (Hordeum vulgare) (PL-426), sorghum (Sorghum bicolor) (Sudex) and millet (Pennisetum glaucum) (FBC-16) from 2017 harvest, commonly grown in Punjab state, were procured from Punjab Agriculture University, Ludhiana, India. The climate of Punjab is semi-arid and subtropical with hot and dry winds during the summer months. The soil in Punjab is sandy loam to clayey. The grains were thoroughly cleaned to separate dirt, foreign material and any degraded seeds and then kept at 20◦C until use. The chemical and reagents utilized for this research were of analytical grade. The enzymes (porcine pancreatic α-amylase (Sigma A3176) and amyloglocosidase) used were obtained from Sigma-Aldrich (Taufkirchen, Germany) and GOD-POD (glucoseoxidase-peroxidase) kit was obtained from Megazyme, Ireland. The milli-Q water was employed for all observations.
Sample preparation
The cereal grains were first steeped in excess distilled water (25 °C) and then spread as a thin layer on two-layered damp muslin cloth and allowed to germinate for 24, 48 and 72 h at 25 °C in an incubator. The germinated grains were then subjected to dehydration in the cabinet drier at 45 °C (moisture content 10%) for 8–10 h. The dried germinated cereal grains were pulverized in supermill (Newport, Australia). The milled flour proceeded through 60 mm mesh size sieve and kept in plastic containers until further evaluation.
Germination percentage
Hundred seeds were taken in three replications from each cereal i.e. wheat, barley, sorghum and millet and then germinated according to the above-mentioned method. After the germination duration of 24, 48 and 72 h, the number of normal seedlings were counted and the radical length of 5 randomly selected seedlings was measured carefully and grouped according to the length. The germination percentage was determined from the ratio of germinated grains to the total grains.
Color characteristics
The color characteristics of native and germinated cereal flours were studied using a Hunter colorimeter (Hunter Associates Laboratory, U. S. A.). The colorimeter was equalized with standard tile. The flour was put in a sample cup and then it kept in the sample platform for its color identification as L*, à* and b* values. The L* values specified whiteness to darkness. The chromatic portion is examined by a* (+) redness and a* (−) greenness, b* (+) yellowness and b* (−) blueness.
Proximate composition of germinated grains
The proximate nutritional composition (protein, fat, ash) of the native and germinated cereal flour from different cereal grains was estimated by using the standard procedures of AACC (2000).
Water and oil absorption capacity
The water and oil absorption capacity of native and germinated cereal flours were analysed by the procedure of Elkhalifa and Bernhardt (2010). The flour sample of 3 g was weighed into a centrifuge tube and added 30 ml of distilled water/refined sunflower oil at room temperature. The samples were agitated and then centrifuged at 3000 rpm for 15 min. The supernatant was discharged and the tubes were permitted to decant for 5 min and the increase in weight was taken to calculate the % water and oil absorption capacity of flours.
Pasting properties
Pasting properties of native and germinated flours were observed by using Rapid Visco-Analyzer (Model RVA-3, Newport Scientific Pvt. Ltd., Australia). The flour sample (3 g) was dispersed in 25 ml of distilled water and kept in the RVA sample canister to form a flour slurry of 28 g. The heating period of the sample ranged from 50 to 95 °C at 6 °C/min with 1 min of stability time at 50 °C and holding step of 5 min at 95 °C. After that, the cooling phase occurred by decreasing the temperature from 95 to 50 °C at 6 °C/min and holding step of 2 min at 50 °C. The analysed parameters were pasting temperature, holding viscosity, peak viscosity, final viscosity, breakdown viscosity (peak viscosity–trough viscosity) and setback viscosity (final viscosity–trough viscosity).
Antioxidant properties of germinated cereal grains
DPPH radical scavenging activity
DPPH radical scavenging activity of native and germinated cereal flour was measured using a modified version of the method explained by Sharma and Gujral (2010). The inhibition percentage of sample extract was expressed as Trolox equivalents (μmole/g).
Reducing power (RP)
The reducing power of the extracts obtained from native and germinated cereal flours was measured by following the method described by Zhao et al. (2008). The results were expressed as μg AAE/g dwb.
Total phenolic content (TPC)
The total phenolic content of native and germinated flours was determined by using the method explained by Sharma and Gujral (2010).
Total flavonoids content (TFC)
The total flavonoids content of different flours from germinated and ungerminated cereal grains was determined according to the method followed by Sharma and Gujral (2011). The flavonoids were extracted with 80% methanol. The absorbance was recorded at 510 nm using Catechin as the standard and the results were reported as µg CE/g of flour.
Fourier-transform infrared (FTIR) spectra analysis
FTIR spectra of germinated and ungerminated cereal flours were evaluated using FTIR spectrometer (Aligent Technologies, Cary 630 FTIR) with ATR method. The intensity evaluation was performed on either original or the deconvoluted spectra by determining the height of the absorbance bands from their baseline. The specific interest of areas that studied included the protein (Amide I, II and III groups), in addition to the secondary structure of protein i.e. α-helix and β-sheet in the IR regions of about 1800–800 cm−1. Furthermore, the starch crystalline regions approached from 1300 to 800 cm−1 and were computed to evaluate the changes in their relative intensities. The amide I region (1700–1600 cm−1) positioned in the spectral range of proteins was subjected to both Fourier self deconvolution (FSD) and second derivative analysis to acquire overlapping peaks in that region.
In vitro digestibility of germinated flours
In vitro digestibility of raw and cooked flour slurries before and after germination was determined according to the method described by Englyst et al. (1992). The starch fractions such as RDS (rapidly digestible starch), SDS (slowly digestible starch), and RS (resistant starch) were evaluated based on their digestion rates. The kinetics of hydrolysis starch and excepted glycaemic index were assessed by a non-linear model established by Goni et al. (1997).
Statistical analysis
The data reported in all the tables is averages of triplicate observations. The data was inspected by one-way analysis of variance (ANOVA) and means were compared by Fisher’s least significant difference (LSD) test at p < 0.05. The Pearson correlation coefficients (r) were computed. The principle component analysis was performed to compute the data. The data was subjected to statistical analysis using Minitab Statistical Software version 17 (Minitab Inc., USA).
Results and discussion
Germination percentage
The germination percentage of different cereal grains varied from 90.0 to 94.0% (Table S1). After 24 h of germination, the germination percentage of wheat (94%) was significantly (p < 0.05) higher followed by sorghum (92%), millets (91%) and barley (88%) grains. The germination rate significantly (p < 0.05) increased with the progression in germination duration. This may be attributed to the fact that after a longer duration of germination, the moisture diffuses up to the optimum points of grains and specific temperature become more available to grains that activates the enzymes required for germination. The radicals became visible after 24 h and at this time interval most were in the range of 0–2 mm, with the prolonged germination, most of the radicals from different germinated cereal grains were fallen in the category of 4–6 mm and 6–8 mm (Table S1).
Physico-chemical characteristics of flours from germinated cereal grains
Color characteristics
It was observed that the L* value i.e. brightness of germinated cereal flours decreased as the period of germination progressed (Table 1). However, both a*(redness) and b*(yellowness) values of cereal flours increased with proceeding germination time. During germination, there is an activation of some oxidative enzymes such as polyphenol oxidase and peroxidase and therefore browning occurred by the facilitation of enzymes, resulting in decreased lightness and increased redness and yellowness. This may also be attributed to the formation of more starch and protein hydrolysates with increasing germination time, and eventually, maillard reaction occurred between them during drying treatment. Similar results were evaluated by Tian et al. (2010) for germinated oats.
Table 1.
Color characteristics and physico-chemical properties of flours from different germinated cereals
| Cereal | Time (hrs) | Protein (%) | Fat (%) | Ash (%) | Total starch (%) | L* | a* | b* | WAC (g/g) | WSI (%) | OAC (g/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat | 0 | 13.7a ± 0.14 | 1.7g ± 0.05 | 1.32g ± 0.07 | 69.7ab ± 1.23 | 85.42c ± 0.30 | 1.84g ± 0.06 | 11.35fg ± 0.18 | 1.22ef ± 0.02 | 6.44a ± 0.08 | 2.23i ± 0.02 |
| 24 | 13.2b ± 0.10 | 1.4h ± 0.02 | 1.25h ± 0.08 | 62.4ef ± 1.15 | 84.71cd ± 0.24 | 1.86g ± 0.05 | 11.72e ± 0.05 | 1.18gh ± 0.01 | 6.22b ± 0.07 | 2.28gh ± 0.05 | |
| 48 | 12.4cd ± 0.08 | 1.1hi ± 0.03 | 1.22hi ± 0.05 | 55.1hi ± 1.20 | 83.88d ± 0.94 | 1.90fg ± 0.02 | 12.84b ± 0.08 | 1.11i ± 0.04 | 6.02c ± 0.05 | 2.33f ± 0.01 | |
| 72 | 11.5f ± 0.12 | 0.9i ± 0.01 | 1.20ij ± 0.09 | 51.2j ± 1.28 | 81.25e ± 0.15 | 1.97ef ± 0.07 | 13.45a ± 0.23 | 1.02j ± 0.01 | 5.84d ± 0.08 | 2.38e ± 0.04 | |
| Barley | 0 | 11.9ef ± 0.13 | 2.44f ± 0.04 | 1.37f ± 0.07 | 65.2c ± 1.45 | 88.85a ± 0.67 | 1.1j ± 0.12 | 8.42m ± 0.14 | 1.27cd ± 0.03 | 5.13e ± 0.06 | 2.56d ± 0.03 |
| 24 | 10.8g ± 0.07 | 2.40f ± 0.01 | 1.32g ± 0.06 | 63.1de ± 1.57 | 88.75ab ± 0.08 | 1.99e ± 0.02 | 8.62l ± 0.05 | 1.21efg ± 0.02 | 4.92f ± 0.05 | 2.61c ± 0.04 | |
| 48 | 10.2h ± 0.10 | 2.33f ± 0.03 | 1.24hi ± 0.03 | 61.4f ± 1.38 | 88.27ab ± 0.09 | 2.27d ± 0.05 | 8.83k ± 0.10 | 1.15h ± 0.05 | 4.75g ± 0.07 | 2.72b ± 0.05 | |
| 72 | 9.6i ± 0.08 | 2.28f ± 0.02 | 1.16j ± 0.02 | 57.2gh ± 1.45 | 87.47b ± 0.65 | 2.62c ± 0.10 | 9.06j ± 0.32 | 1.11i ± 0.02 | 4.57h ± 0.12 | 2.77a ± 0.02 | |
| Sorghum | 0 | 10.8g ± 0.12 | 3.81d ± 0.04 | 1.46e ± 0.06 | 71.7a ± 2.20 | 80.88ef ± 0.30 | 1.99e ± 0.03 | 11.2h ± 0.18 | 1.29c ± 0.01 | 2.65l ± 0.08 | 2.06k ± 0.01 |
| 24 | 9.7i ± 0.09 | 3.72d ± 0.07 | 1.40f ± 0.05 | 68.2b ± 2.31 | 79.72f ± 0.57 | 3.29b ± 0.04 | 11.8e ± 0.05 | 1.24de ± 0.07 | 2.46m ± 0.06 | 2.14j ± 0.07 | |
| 48 | 8.8j ± 0.13 | 3.55de ± 0.05 | 1.32g ± 0.10 | 65.3cd ± 2.64 | 77.28g ± 2.47 | 3.62a ± 0.06 | 12.36c ± 0.26 | 1.19fgh ± 0.02 | 2.21n ± 0.04 | 2.23hi ± 0.04 | |
| 72 | 8.1k ± 0.11 | 3.39e ± 0.02 | 1.25h ± 0.07 | 61.6ef ± 2.40 | 77.15g ± 2.10 | 3.65a ± 0.08 | 12.44c ± 0.61 | 1.16h ± 0.05 | 2.08o ± 0.07 | 2.29fg ± 0.02 | |
| Millets | 0 | 12.8bc ± 0.15 | 5.53a ± 0.06 | 2.24a ± 0.04 | 63.2de ± 1.87 | 75.99gh ± 0.74 | 1.48i ± 0.01 | 10.8i ± 0.17 | 1.49a ± 0.02 | 3.67i ± 0.10 | 2.33hi ± 0.05 |
| 24 | 12.1de ± 0.12 | 5.37ab ± 0.04 | 2.19b ± 0.08 | 60.8f ± 2.15 | 75.10hi ± 3.69 | 1.61h ± 0.02 | 11.29gh ± 0.63 | 1.38b ± 0.03 | 3.38j ± 0.05 | 2.40e ± 0.02 | |
| 48 | 11.7ef ± 0.13 | 5.13b ± 0.03 | 2.12c ± 0.05 | 58.5g ± 1.94 | 74.0i ± 1.07 | 2.26d ± 0.08 | 11.4f ± 0.05 | 1.31c ± 0.07 | 2.97k ± 0.04 | 2.52d ± 0.05 | |
| 72 | 10.6g ± 0.08 | 4.76c ± 0.05 | 2.02d ± 0.09 | 54.9i ± 2.08 | 74.11i ± 0.46 | 2.27d ± 0.04 | 12.08d ± 0.19 | 1.23def ± 0.03 | 2.08o ± 0.06 | 2.57cd ± 0.04 |
Mean ± SD with different superscripts in columns differ significantly (p < 0.05; n = 3)
WAC water absorption capacity, OAC oil absorption capacity
Proximate composition of germinated cereal flours
The cereal grains showed significant variation in their moisture content (Table 1) and it may be associated with their physical and molecular arrangement. The nutritional significance of cereal grains can be analysed from their proximate composition. The protein content of ungerminated grains varied from 10.6 to 13.7% (Table 1). The protein content was significantly higher in wheat flour while the sorghum flour had the lowest value. The protein content was significantly (p < 0.05) decreased after germination and it decreased continuously as the germination time progressed. It may be attributed to the enzymatic degradation of protein by protease enzymes during germination for the development of new seedlings (Afify et al. 2012). Germination duration was inversely proportional to the protein, ash and fat content of different cereal grains. The biochemical and physiological changes occurred during germination which provides energy for the emergence of new plant tissues. The fat content affected due to the increased activities of lipolytic enzymes during germination which converted the fat content into fatty acids and glycerol (Singh et al. 2017). The ash content decreased significantly (p < 0.05) as the minerals get depleted due to the rootlet and washing of grains to remove the foul smell that occurred owing to germination and degradation of the outer cell wall of cereal grains during vegetative growth. These results are agreed with Singh et al. (2017) and Afify et al. (2012) who also reported the decreased proximate composition in germinated sorghum grains.
Water absorption capacity and water solubility index
The water absorption capacity and water solubility index showed significant differences among the flours from different germinated cereal grains (Table 1). Water absorption capacity leads to the maximum portion of water that the flour can absorb and retain and it is the foremost functional property for many food applications (bakery goods). The millet flour was found to have the highest water absorption capacity (1.49%) as compared to other cereal flours. The water absorption capacity of cereal grains decreased with the progression in germination time. Similar results of decreasing water absorption capacity were reported by Cornejo and Rosell (2015) for germinated brown rice flour and Singh et al. (2017) for germinated sorghum flour. During germination, starch and fiber degrading enzymes get accelerated which facilitates the production of a greater proportion of dextrin and fermentable sugars by hydrolysing the starch. The release of these sugars initiate the formation of crosslinks in the amorphous region between the starch chains and consequence reduction in swelling of starch occurs that lowers its water hydration properties and therefore as a result, WAC was decreased (Cornejo and Rosell 2015).
Oil absorption capacity
The oil absorption capacity of cereal flours varied from 2.06 to 2.56% for different cereal grains (Table 1). Barley flour showed the highest oil absorption capacity while the sorghum flour had the lowest value. The oil absorption capacity was significantly (p < 0.05) increased with the prolonged germination time. As the germinated time increased from 24 to 72 h, the oil absorption capacity of different cereal grains was increased appreciably. The denaturation of protein molecule occurs during germination which makes more availability of lipophillic proteins and these hydrophobic amino acids attached with the hydrocarbon side chains of oil and therefore the oil absorption capacity of flours from germinated cereal grains get enhanced (Elkhalifa and Bernhardt 2010). The results reported by Elkhalifa and Bernhardt (2010) and Singh et al. (2017) in sorghum also revealed that the oil absorption capacity of flour from germinated cereal grains was higher as compared to control flour and it elevated progressively with the progression in germination time.
DPPH radical scavenging activity
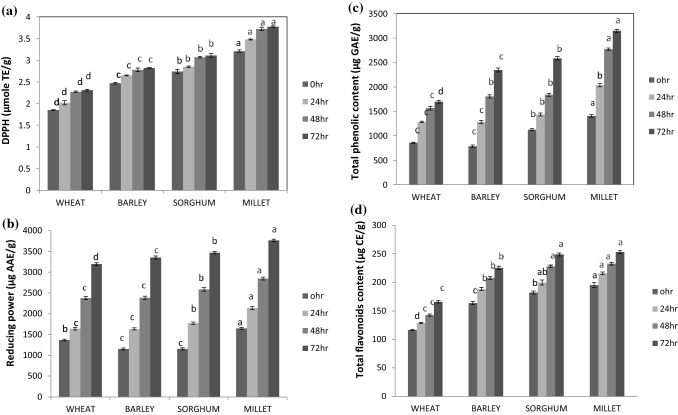
The DPPH is a stable free radical and commonly used to analyze the antioxidant potential or radical scavenging activity. The antioxidant activity significantly varied among different cereal flours (Fig. 1a) and ranged from 1.85 to 2.96 μmol TE/g. The millet flour exhibited the highest antioxidant activity while wheat flour had the lowest. There was a significant (p < 0.05) positive correlation (R = 0.956) between TPC and antioxidant activity. Sharma and Gujral (2010) and Lu et al. (2007) reported a positive correlation between the total phenolic content and antioxidant activity of germinated barley and wheat respectively. The germination of cereal grains increased the antioxidant activity of all the cereal flours ranged from 2.41 to 3.72 μmol TE/g after 72 h of germination, the highest increase was observed in millet flour and the lowest in wheat flour. Lu et al. (2007) concluded that the antioxidant activity of barley increased after germination. During germination, the bound phenolics get librated due to the modification of endosperm by hydrolytic enzymes i.e. activation of endogenous esterases that associated with the increase in antioxidant activity (Sharma and Gujral 2010).
Fig. 1. a.
DPPH scavenging activity of germinated cereal flour. Alphabets a–d show significant (p < 0.05) differences among germinated cereal grains (0–72 h). Error bars show standard deviation. b Reducing power of germinated cereal flours. Alphabets a–d show significant (p < 0.05) differences among germinated cereal grains (0–72 h). Error bars show standard deviation. c Total phenolic content of germinated cereal flours. Alphabets a–d show significant (p < 0.05) differences among germinated cereal grains (0–72 h). Error bars show standard deviation. d Total flavonoid content of germinated cereal flours. Alphabets a–d show significant (p < 0.05) differences among germinated cereal grains (0–72 h). Error bars show standard deviation
Reducing power
Reducing power is used as an indicator of antioxidant potential. During germination, hydrolytic enzymes take part in the modification of endosperm; therefore, some of the bound phenolics get liberated and play a role in antioxidant activity (Gujral et al. 2012). The reducing power may be primary or secondary antioxidants as they considered as electron donor compounds and can eliminate the oxidized intermediates of the lipid peroxidation reactions (Zhao et al. 2008). The reducing power activities of raw and germinated grains are presented in Fig. 1b. The germination process caused significant inclination in reducing power activity. The reducing power of flours from ungerminated cereals ranged from 1367 to 1648 µg AAE/g and increased significantly from 3192 to 3765 µg AAE/g after 72 h of germination. The millet flour showed the highest reducing power followed by sorghum and barley while wheat flour had the lowest value. The presence of reductones is usually concerned with the reducing attributes of antioxidants. According to Gordon (1990), the disintegration of the free radical chain by present a hydrogen atom is associated with the antioxidant action of reductones.
Total phenolic content (TPC)
The total phenolic content varied significantly among the different cereal grains (Fig. 1c). Germination carried out a noticeable increase in total phenolic content (TPC). The highest TPC was found in millet flour and it ranged from 1405 µg GAE/g to 3145 µg GAE/g after 72 h of germination. This might be contributed to the more appropriate extractability of phenolic components from the structures of grains after germination. Phenolic compounds are present either in a free state or bound to cell wall components in cereal grains. Free phenolic compounds are mainly located in the pericarp and can be extracted with an organic solvent. The TPC increased in brown rice (Donkor et al. 2012) and rough rice reported by Moongngarm and Saetung (2010) upon germination. The increase in total phenolic content might be contributed to the increased activity of enzymes such as polyphenol oxidases and peroxidases required for the oxidation of endogeneous phenolic compounds (Caceres et al. 2014). During germination, the endosperm gets modified by the hydrolytic enzymes and also extracts some of the bound components that play a role in antioxidant activity (Sharma and Gujral 2010). Tian et al. (2010) reported the increased content of total phenolic content in oat grains after germination.
Total flavonoid content
Flavonoids are located in the plant kingdom and exist as natural substances with different phenolic structures. They are predominately found in fruits, vegetables, grains, seeds and flowers (Fouad and Rehab 2015). Total flavonoids content of raw and germinated flours from different cereals are shown in Fig. 1d. Total flavonoids of flours from ungerminated or raw cereal grains varied from 116 to 195 µg CE/g. The flavonoids content in raw cereal grains was significantly (p ≤ 0.05) lower than germinated cereal grains. There was a significant increase in the total flavonoid content of cereal grains as a consequence of the germination process. The total flavonoid content was increased with the progression of germination duration. After 72 h of germination, the highest level of total flavonoids was observed ranged from 165 to 253 µg CE/g. During germination, the synthesized flavonoid pathway may be activated by the phenylpropanoid metabolic pathway, due to which the intermediates may facilitate the formation of acetyl coenzyme A esters that are converted to flavonoids (Ti et al. 2014).
Pasting properties
The effect of germination duration, type of cereal grain and their synergistic effect were significant on pasting properties of flours (Table 2). As the germination duration proceeded, the progressive reduction of viscosity was observed may be attributed to the degradation of starch molecules by enzymatic activity during the germination process. Peak viscosity represents the ability of starches to swell prior to their disintegration. Barley flour showed the highest PV and millet flour showed the lowest. Similar results were reported by Xu et al. (2012) for brown rice flour. The breakdown viscosity indicates the liability of the swollen granules to disrupt during consecutive heating and stirring of gelatinized starch slurry (Ingbian and Adegoke 2007). The germination activates the enzymes which hydrolyze the starch. Therefore, the viscosity of the starch suspension significantly decreased. Setback viscosity is the measure of solidification of cooled flour suspensions owing to the retrogradation of amylose, moreover, it also declined as the germination proceeds. Maximum final viscosity revealed the capability of flour to form a viscous paste. The germination induced a significant decrease of the final viscosity of flours. However, the pasting characteristics of starchy substance provide valuable guidance regarding the functionality, utilization and application of particular food products (Ghumman et al. 2016). The low viscosity of these germinated cereals flours is desirable for the preparation of weaning foods and also appropriate for the infants to consume the foods easily due to the added solids to the blend of weaning foods. However, the low viscosity of these cereal grains will help in elevating the nutrient profile of porridges and gruels which is beneficial to young ones (Tizazu et al. 2010).
Table 2.
Pasting properties of flours from different germinated cereal grains
| Sample | Peak viscosity (Pa s) | Breakdown viscosity (Pa s) | Setback viscosity (Pa s) | Final viscosity (Pa s) | Holding viscosity (Pa s) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 0 h | 24 h | 48 h | 72 h | 0 h | 24 h | 48 h | 72 h | 0 h | 24 h | 48 h | 72 h | 0 h | 24 h | 48 h | 72 h | |
| Wheat | 1778 | 1582 | 1141 | 578 | 667 | 625 | 549 | 161 | 1125 | 1042 | 758 | 205 | 2115 | 1878 | 1352 | 362 | 1015 | 907 | 628 | 158 |
| Barley | 2752 | 2275 | 1631 | 592 | 1091 | 854 | 556 | 236 | 1400 | 1248 | 778 | 99 | 3061 | 2648 | 1334 | 257 | 1661 | 1421 | 1075 | 356 |
| Sorghum | 2681 | 2354 | 1563 | 667 | 905 | 782 | 416 | 189 | 2217 | 2009 | 1540 | 397 | 3993 | 3561 | 2687 | 875 | 1776 | 1572 | 1147 | 478 |
| Millets | 1665 | 1325 | 956 | 338 | 487 | 338 | 289 | 111 | 793 | 559 | 479 | 227 | 1971 | 1546 | 1146 | 482 | 1178 | 987 | 667 | 227 |
Total starch (%)
The total starch content showed significant differences in flours from germinated cereal grains (Table 1). Germination decreased the starch content from 71.7 to 61.6% in sorghum flour, 69.7 to 51.2% in wheat flour, 65.2 to 57.2% in barley flour and 63.2 to 54.9% in millet flour. The decrease in total starch content may be related to the enzymatic hydrolysis of starch by intrinsic amylases of seeds synthesized during germination (Yin et al. 2014). During germination, the various metabolic activities occurred and generate energy by utilizing the monosaccharides (Kataria and Chauhan 1988). The amylases and phosphorylase activated the amylolysis which may be devoted to the decreased total starch content of germinated cereal grains. The total starch was positively correlated with SDS (R = 0.889, p < 0.05), PV (R = 0.813), SV(R = 0.847, p < 0.05), BV (R = 0.713, p < 0.05), FV (R = 0.862, p < 0.05) and HV (R = 0.849, p < 0.05).
In vitro starch digestibility
The germination had a significant effect on their in vitro starch digestibility of different cereal grains evaluated in both raw and cooked forms (Fig. 2a, b). The raw wheat flour showed RDS content of 32.1% followed by sorghum (26.5%), millets (23.8%) and barley (20.7%). After germination, the RDS content increased while SDS and RS content get decreased. The germination process facilitates the metabolic and structural changes and therefore the residual components after germination exhibit higher susceptibility to enzymatic attack (Xu et al. 2012). During germination, starch hydrolysed by the amylolytic enzymes and became readily digestible as shown by the changes in RDS, SDS and RS contents (Fig. 2). Xu et al. (2012) suggested the increased digestibility by germination for rice. The variation in digestibility study may also be attributed to the structural changes of starch either in molecules or in crystallites (Kaur and Gill 2019).
Fig. 2. a.
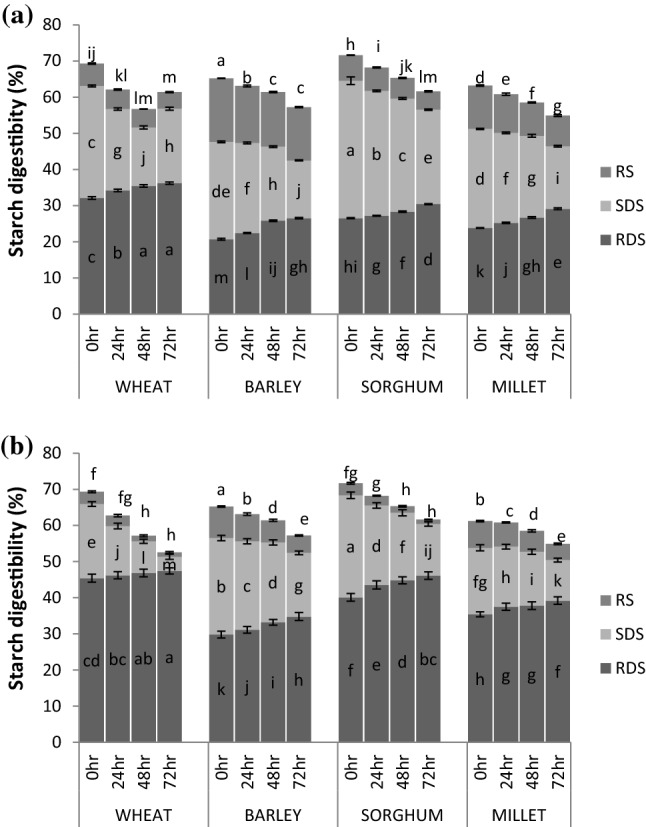
In vitro starch digestibility of germinated cereal flours; RDS rapidly digestable starch, SDS slowly digestible starch, RS resistant starch. Alphabets a-m show significant (p < 0.05) differences among germinated cereal grains (0–72 h). Error bars show standard deviation. b In vitro starch digestibility of cooked germinated cereal flours; RDS rapidly digestable starch, SDS slowly digestible starch, RS resistant starch. Alphabets a–m show significant (p < 0.05) differences among germinated cereal grains (0–72 h). Error bars show standard deviation
The cooking of flours from germinated grains greatly influence the digestibility of starch showed a considerable increase in RDS content followed by a significant decrease in SDS and RS content (Fig. 2b). SDS content of cooked cereal flours decreased significantly (p < 0.0 5) with increasing germination duration and also it was lower for cooked flours in contrast to their raw counterparts. As expected, the RS content decreased abruptly after the gelatinization of different cereal flours with increasing germination durations. Barley flour showed the highest RS content while sorghum ≈ wheat flour had the lowest RS content. The uncooked starch had intact starch granules assigned to their poor degradation by digestive enzymes and are liable for their higher RS content. The starch granules get gelatinized during cooking and solubilized to some degree and eventually became susceptible to digestive enzymes (Kaur and Gill 2019). The germination accompanied by cooking developed the optimum processing technique to accelerate starch gelatinization as germinated cooked flours had lower RS content as compared to the germinated uncooked flours (Fig. 2a, b). This technique (germinated and cooking) increased the RDS content of cereal flours. Therefore, the improved starch digestibility may assist the partial starch hydrolysis during germination that can actuate amylolysis due to the activation of amylases and phosphorylase. It has been also proposed that the cooked germinated grains may persuade metabolic and structural changes which may alter the nature of the association between starch and fiber/protein, exhibiting the starch more readily digestible (Cornejo et al. 2015).
The hydrolysis index (HI) as well as the estimated glycemic index (eGI) of flours from different cereals were significantly reduced with germination (Table S2), therefore flours varied from medium to low eGI. Our results agreed with the study revealing low HI and eGI of bread prepared from germinated brown rice (Cornejo et al. 2015). The wheat flour showed the highest eGI followed by sorghum, millets and barley. Barley had the least eGI because it contains a higher amount of dietary fiber and unabsorbed carbohydrates. The significant declined of glycemic index prompted by germination might be corresponding to the internal modification in the starch granules during germination. After cooking, the increase in eGI of cereal flours is attributed to the starch gelatinization. Ovando-Martínez et al. (2011) concluded that the surface of the bean starch granules enhanced rough and flattened appearance after cooking. This structural modification was followed by disorganization and distortion of the cell during cooking. The starch gelatinized by heating was easily decomposed by enzymes, consequently releasing glucose and enhancing the eGI value. The estimation of low glycemic index values are regarded as beneficial for well-being, particularly as a tool to prevent health issues where controlled glycemic plays a significant role, such as obesity, diabetes and hyperlipidemia.
Fourier transform infrared spectroscopy (FTIR)
The ATR–FTIR was used to analyse the molecular differentiation in the carbohydrates and proteins section of flours obtained from germinated cereal grains in the absorption spectra from 1000 to 1200 and 1500 to 1700 cm−1 (Fig. 3). The proportion of each secondary structure was calculated to observe their relative proportion and is described in Table S3. The bands located at 1650 cm−1 equivalent to α-helical conformed proteins. The antiparallel β-sheets, β-sheets and β-turns bands are promulgated at frequencies varying between 1612–1640 cm−1, 1621–1640 and 1650–1658 respectively (Carbonaro and Nucara 2010). The differences among the germinated flours in amide 1 zone as well as carbohydrate zone assigned to the partial depolymerization of starch and protein molecules during germination. The occurrence of amide | bands due to C = O straightening of the peptide group. The presence of α-helix and β-sheets were at higher proportion followed by β-turns and antiparallel β-sheets (Table S3). The prominent change occurred in germinated cereal flours that had decreased ratio of β-sheets, β-turns, antiparallel β-sheets and α-helix with progress in the germination of different cereal grains; however, this decrease was not cumulative in all trends. As the proportion of these secondary structures of proteins declined during germination, it indicates the change in surface structure and interaction due to the germination process (Fig. 3c). The deconvolution interpretation of the spectra provides valuable instruction concerning to the secondary structures of protein as well as the existence of crystalline and amorphous starch structures. The index of crystallinity related to starch granular integrity can be estimated from the absorbance ratio 1047/1022 cm−1 as it quantified the proportion of ordered crystalline structures relative to amorphous material in starches. The starch zone displayed evident changes during germination, in this respect, there was a decreasing trend on 1047/1022 cm−1 ratio in germinated cereal flours (Table S3). Therefore, the germinated flours encouraged the shifting of crystal ratio from a highly ordered state to disorganized amorphous molecules. This phenomenon could be explained the molecular changes in starch after germination. In this study, antiparallel β-sheets, β-sheets and α-helix presented a positive correlation with HI (R = 0.852, p < 0.05; R = − 0.822, p < 0.05; R = 0.832, p < 0.05) and RDS (R = 0.711, p < 0.05; R = 0.677, p < 0.05; R = 0.742, p < 0.05) respectively showing the higher proportion of ordered structures promoted by germination technique. RS was found to be negatively correlated with antiparallel β-sheets, β-sheets and α-helix (R = − 0.755, p < 0.05; R = − 0.762, p < 0.05; R = − 0.665, p < 0.05), β-turns (R = 0.658, p < 0.05) and α-helix (R = 0.644, p < 0.05) depicting the evident changes in protein zone after digestion.
Fig. 3. a.
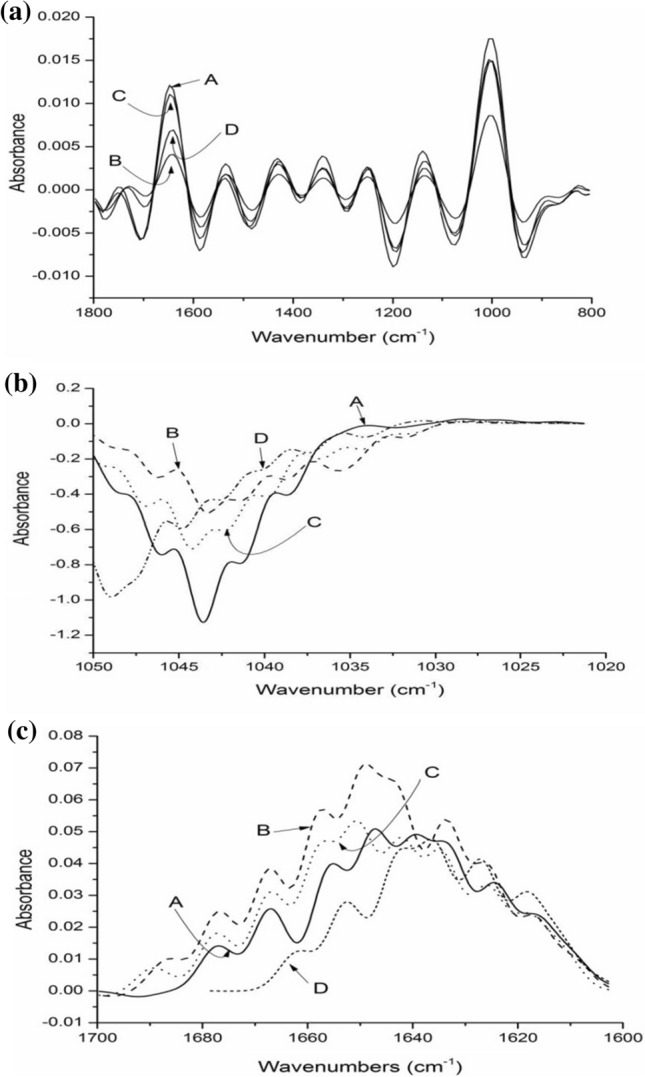
FTIR spectra of native cereal flours from wave numbers 800 to 1800 cm−1: (A) wheat flour, (B) barley flour, (C) sorghum flour (D) millet. b Deconvoluted spectra of native and germinated sorghum flours (A) sorghum flour (B) sorghum flour germinated for 24 h (C) sorghum flour germinated for 48 h (D) sorghum flour germinated for 72 h (c) Deconvoluted spectra of native and germinated millet flours (A) millet flour (B) millet flour germinated for 24 h (C) millet flour germinated for 48 h (D) millet flour germinated for 72 h
Principal component analysis
In this study, we use PCA to summarize the interrelationships between the physicochemical, nutritional and structural properties of cereal flours due to different duration of germination treatment (Fig. 4). The first and second components (PC1 and PC2) accounted for an accumulative variability of 64.6%. The loading plot (Fig. 4a) of PCA contains the correlations among some physicochemical and structural properties and the starch digestion fractions. PC1 represented 37.0% of the accumulated variability, in which the main contributor factors were the antiparallel β-sheets, β-sheets, β-turns, HI, eGI, RDS and SDS. The first component has a negative association with OAC, RS, AOA and TPC. PC2 has a large positive association with AOA, TPC, a*, b*, RDS and negative coalition with SDS, RS, PV, BV, FV, and HV. RDS was positively correlated to GI and HI while negatively correlated with RS. The loading plot (Fig. 4b) constitutes the comparability among flours from different germinated cereals. PCA revealed that barley flour (72 h of germination) was located at centre left core of the score plot carried a positive score in PC2 and this showed that barley flour (72 h of germination) was significantly different from all other germinated cereal flours in terms of their measured properties. The wheat flour (germinated for 72 h) with a positive score in PC1 and sorghum flour (72 h) with a negative score in PC2 was located at top of the score plot.
Fig. 4.
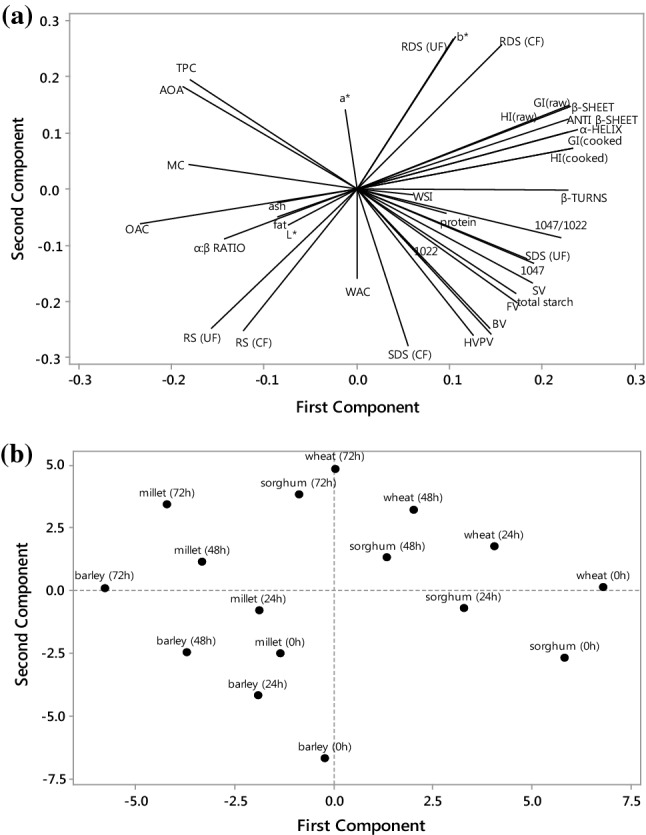
Principal component analysis of germinated cereal flours. a Loading plots RDS(UF) rapidly digestible starch of uncooked flour, RDS(CF) rapidly digestible starch of cooked flour, SDS(UF) slowly digestible starch of uncooked flour, SDS(CF) slowly digestible starch of cooked flour, RS(UF) resistant starch of uncooked flour, RS(CF) resistant starch of cooked flour, AOA antioxidant activity, TPC total phenolic content, BV breakdown viscosity, PV peak viscosity, SV setback viscosity, FV final viscosity, HV holding viscosity, WAC water absorption capacity, WSI water solubility index, OAC oil absorption capacity, HI hydrolysis index, GI glycemic index. b score plot of germinated cereal flours
Conclusion
The use of germination treatment for different durations on less utilized cereals grains could be an appropriate technique to obtain functional products and foods with a low glycemic index. The germinated flours presented molecular interactions between starch and proteins, which consequently decreased the starch hydrolysis. This study showed the improved antioxidant properties of germinated flours that aimed towards to impart unique functional and nutraceutical characteristics. The increase in germination time from 24 to 72 h resulted in a significant reduction in SDS, RS, water absorption capacity and pasting properties of flours from different cereal grains. The prolonged germination improved the RDS and oil absorption capacity. Although a large amount of the produced malts is utilized in the brewing and distilling industries, malts can also be found applications in various other products ranged from bakery goods to breakfast cereals, cereal bars and non-alcoholic beverages.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The author Harpreet Kaur acknowledges UGC for award of BSR Fellowship.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC . Approved methods of american association of cereal chemists. 10. St. Paul: The Association; 2000. [Google Scholar]
- Afify MR, El-Moneim ABD, El-Beltagi HS, Abd El-Salam SM, Omran AA. Effect of soaking, cooking, germination and fermentation processing on proximate analysis and mineral content of three white sorghum varieties (Sorghum bicolor L. moench) Not Bot Horti Agrobot Cluj Nap. 2012;40:92–98. doi: 10.15835/nbha4027930. [DOI] [Google Scholar]
- Caceres PJ, Martınez-Villaluenga C, Amigo L, Frias J. Maximising the phytochemical content and antioxidant activity of Ecuadorian brown rice sprouts through optimal germination conditions. Food Chem. 2014;152:407–414. doi: 10.1016/j.foodchem.2013.11.156. [DOI] [PubMed] [Google Scholar]
- Carbonaro M, Nucara A. Secondary structure of food proteins by fourier transform spectroscopy in the mid-infrared region. Amino Acid. 2010;38:679–690. doi: 10.1007/s00726-009-0274-3. [DOI] [PubMed] [Google Scholar]
- Chinma CE, Adewuyi O, Abu JO. Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus) Food Res Int. 2009;42:1004–1009. doi: 10.1016/j.foodres.2009.04.024. [DOI] [Google Scholar]
- Cornejo F, Rosell CM. Influence of germination time of brown rice in relation to flour and gluten free bread quality. J Food Sci Technol. 2015;52:6591–6598. doi: 10.1007/s13197-015-1720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo F, Caceres PJ, Martínez-Villaluenga C, Rosell CM, Frias J. Effects of germination on the nutritive value and bioactive compounds of brown rice breads. Food Chem. 2015;173:298–304. doi: 10.1016/j.foodchem.2014.10.037. [DOI] [PubMed] [Google Scholar]
- Donkor ON, Stojanovska L, Ginn P, Ashton J, Vasiljevic T. Germinated grains-Sources of bioactive compounds. Food Chem. 2012;135:950–959. doi: 10.1016/j.foodchem.2012.05.058. [DOI] [PubMed] [Google Scholar]
- Elkhalifa AEO, Bernhardt R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010;121:387–392. doi: 10.1016/j.foodchem.2009.12.041. [DOI] [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Euro J Clini Nutri. 1992;46:S33–S50. [PubMed] [Google Scholar]
- FAOSTAT (2017) Database of Food and Agricultural Organization. http://www.faostat3.fao.org/browse/rankings/commodities_by_regions/E. Accessed 18 Mar 2019
- Fouad AA, Rehab FMA. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris medik.) sprouts. Acta Sci Pol Technol Aliment. 2015;14:233–246. doi: 10.17306/J.AFS.2015.3.25. [DOI] [PubMed] [Google Scholar]
- Ghumman A, Kaur A, Singh N. Impact of germination on flour, protein and starch characteristics of lentil (Lens culinari) and horsegram (Macrotyloma uniflorum L.) lines. LWT Food Sci Technol. 2016;65:137–144. doi: 10.1016/j.lwt.2015.07.075. [DOI] [Google Scholar]
- Goni I, Garcia-Alonso A, Saura-Calixto FA. Starch hydrolysis procedure to estimate glycemic index. Nutri Res. 1997;17:427–437. doi: 10.1016/S0271-5317(97)00010-9. [DOI] [Google Scholar]
- Gordon MH. The mechanism of the antioxidant action in vitro. In: Hudson BJF, editor. Food antioxidants. Elsevier: London; 1990. pp. 1–18. [Google Scholar]
- Gujral HS, Sharma P, Kumar A, Singh B. Total phenolic content and antioxidant activity of extruded brown rice. Int J Food Prop. 2012;15:301–311. doi: 10.1080/10942912.2010.483617. [DOI] [Google Scholar]
- Ingbian EK, Adegoke GO. Proximate compositions, pasting and rheological properties of mumu: a roasted maize meal. Int J Food Sci Technol. 2007;42:762–767. doi: 10.1111/j.1365-2621.2007.01563.x. [DOI] [Google Scholar]
- Kataria A, Chauhan BM. Contents and digestibility of carbohydrates of mung beans (Vigna radiata L.) as affected by domestic processing and cooking. Plant Food Hum Nutri. 1988;38:51–59. doi: 10.1007/BF01092310. [DOI] [PubMed] [Google Scholar]
- Kaukovirta-Norja A, Wilhemson A, Poutanen K. Germination: a means to improve the functionality of oat. Agr Food Sci. 2004;13:100–112. doi: 10.2137/1239099041838049. [DOI] [Google Scholar]
- Kaur H, Gill BS. Effect of high-intensity ultrasound treatment on nutritional, rheological and structural properties of starches obtained from different cereals. Int J Bio Macromol. 2019;126:367–375. doi: 10.1016/j.ijbiomac.2018.12.149. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhao H, Chen J, Fan W, Dong J, Kong W, Sun Y, Cao Y, Cai G. Evolution of phenolic compounds and antioxidant activity during malting. J Agri Food Chem. 2007;55:10994–11001. doi: 10.1021/jf0722710. [DOI] [PubMed] [Google Scholar]
- Madhujith T, Shahidi F. Antioxidative and antiproliferative properties of selected barley (Hordeum vulgare L.) cultivars and their potential for inhibition of low density lipoprotein (LDL) cholesterol oxidation. J Agri Food Chem. 2007;55:5018–5024. doi: 10.1021/jf070072a. [DOI] [PubMed] [Google Scholar]
- Malleshi NG, Klopfenstein CF. Nutrient composition, amino acid and vitamin contents of malted sorghum, pearl millet, finger millet and their rootlets. Int J Food Sci Nutri. 1998;49:415–422. doi: 10.3109/09637489809086420. [DOI] [Google Scholar]
- Manjunatha T, Bisht IS, Bhat KV, Singh BP. Genetic diversity in barley (Hordeum vulgare L.) landraces from Uttaranchal. Gen Res Crop Evol. 2006;54:55–65. doi: 10.1007/s10722-005-1884-6. [DOI] [Google Scholar]
- Moongngarm A, Saetung N. Comparison of chemical compositions and bioactive compounds of germinated rough rice and brown rice. Food Chem. 2010;122:782–788. doi: 10.1016/j.foodchem.2010.03.053. [DOI] [Google Scholar]
- Ovando-Martínez M, Osorio-Diaz P, Whitney K, Bello-Perez LA, Simsek S. Effect of the cooking on physicochemial and starch digestibility properties of two varieties of common bean (Phaseolus vulgaris L.) grown under different water regimes. Food Chem. 2011;129:358–365. doi: 10.1016/j.foodchem.2011.04.084. [DOI] [PubMed] [Google Scholar]
- Sangronis E, Machado CJ. Influence of germination on the nutritional quality of Phaseolus vulgaris and Cajanus cajan. LWT Food Sci Technol. 2007;40:116–120. doi: 10.1016/j.lwt.2005.08.003. [DOI] [Google Scholar]
- Sharma P, Gujral HS. Antioxidant and polyphenols oxidase activity of germinated barley and its milling fractions. Food Chem. 2010;120:673–678. doi: 10.1016/j.foodchem.2009.10.059. [DOI] [Google Scholar]
- Sharma P, Gujral HS. Effect of sand roasting and microwave cooking on antioxidant activity of barley. Food Res Int. 2011;44:235–240. doi: 10.1016/j.foodres.2010.10.030. [DOI] [Google Scholar]
- Singh A, Sharma S, Singh B. Effect of germination time and temperature on the functionality and protein solubility of sorghum flour. J Cereal Sci. 2017;76:131–139. doi: 10.1016/j.jcs.2017.06.003. [DOI] [Google Scholar]
- Ti H, Zhang R, Zhang M, Li Q, Wei Z, Zhang Y, Tang X, Deng Y, Liu L, Ma Y. Dynamic changes in the free and bound phenolic compounds and antioxidant activity of brown rice at different germination stages. Food Chem. 2014;161:337–344. doi: 10.1016/j.foodchem.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Tian B, Xie B, Shi J, Wu J, Cai Y, Xu T, Xue S, Deng Q. Physicochemical changes of oat seeds during germination. Food Chem. 2010;119:1195–1200. doi: 10.1016/j.foodchem.2009.08.035. [DOI] [Google Scholar]
- Tizazu S, Urga K, Abuye C, Retta N. Improvement of energy and nutrient density of sorghum based complementary foods using germination. Africa J Food Agri Nutr Dev. 2010;10:2927–2942. [Google Scholar]
- Xu J, Zhang H, Guo X, Qian H. The impact of germination on the characteristics of brown rice flour and starch. J Sci Food Agri. 2012;92:380–387. doi: 10.1002/jsfa.4588. [DOI] [PubMed] [Google Scholar]
- Yin Y, Yang R, Gu Z. Organ-specific proteomic analysis of NaCl-stressed germinating soybeans. J Agri Food Chem. 2014;62:7233–7244. doi: 10.1021/jf500851r. [DOI] [PubMed] [Google Scholar]
- Zhao H, Fan W, Dong J, Lu J, Chen J, Shan L, Lin Y, Kong W. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem. 2008;1007:296–304. doi: 10.1016/j.foodchem.2007.08.018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.