Abstract
Clitoria ternatea or commonly known as ‘Butterfly pea’ has been used traditionally in Ayurvedic medicine in which various parts of the plants are used to treat health issues such as indigestion, constipation, arthritis, skin diseases, liver and intestinal problems. The flowers of C. ternatea are used worldwide as ornamental flowers and traditionally used as a food colorant. This paper reviews the recent advances in the extraction and biological activities of phytochemicals from C. ternatea flowers. The application of maceration or ultrasound assisted extraction greatly increased the yield (16–247% of increase) of phytochemicals from C. ternatea flowers. Various phytochemicals such as kaempferol, quercetin and myricetin glycosides as well as anthocyanins have been isolated from C. ternatea flowers. Clitoria ternatea flower extracts were found to possess antimicrobial, antioxidant, anti-inflammatory, cytotoxic and antidiabetic activities which are beneficial to human health. Clitoria ternatea flower is a promising candidate for functional food applications owing to its wide range of pharmacotherapeutic properties as well as its safety and effectiveness.
Keywords: Anthocyanin, Antibacterial, Anticancer, Anti-inflammatory, Antioxidant, Flavonol
Introduction
Aromatic and medicinal plants have been used for therapeutic, religious, cosmetic, nutritional, and beautification purposes since ancient times and humanity of all civilizations and culture are familiar with their usage (Senica et al. 2019; Senkal et al. 2019; Gecer et al. 2020). Clitoria ternatea plant is classified in the kingdom Plantae, phylum Tracheophyta, class of Magnoliopsida and a family of Fabaceae (Jamil et al. 2018). Clitoria ternatea is a perennial climber (2–3 m in height) and is known by its common name as butterfly pea or blue pea flower (Mukherjee et al. 2008). In other regions it is known as aparajita (Bengali), kajroti (India), cunha (Brazilian), cunhã, fula criqua (Portuguese), lan hu die (Chinese), bunga biru, tembang telang (Indonesian), bunga telang (Malaysian), clitoria azul (Spanish), dangchan (Thai), chi dậu biếc (Vietnamese) and mavi kelebek sarmaşığı (Turkish) (Kosai et al. 2015; Subramanian and Prathyusha 2011; Mukherjee et al. 2008). It is commonly grown as an ornamental plant and is also used as a revegetation species while in Southeast Asia, the blue flower pigment is traditionally used as food colorant (Havananda and Luengwilai 2019; Oguis et al. 2019) The plant is known to be suitable as a cover crop and green manure having the ability to not only suppress perennial weeds but able to enrich the soil by nitrogen fixation (Chauhan et al. 2012; Reid and Sinclair 1980). C. ternatea plant is widely distributed in India, Phillipines, in other tropical Asian countries, South and Central America, the Caribbean and Madagascar (Sivaranjan and Balachandran 1994; Ambasta 1988).
Clitoria ternatea is considered as a nootropic herb in Ayurvedic medicine (Chauhan et al. 2017). It grows well in full sunlight/partially shaded area in which seed germination takes about 1–2 weeks while 4 weeks is required for flowering to occur (Jamil et al. 2018; Nguyen et al. 2011). There are several lines of C. ternatea with different flower colours of light blue, dark blue, white and mauve which are 4–5 cm long (Fig. 1). Compounds reported to be found in the flowers are ternatin anthocyanins and various flavanol glycosides of kaempferol, quercetin and myricetin (Mukherjee et al. 2008; Kazuma et al. 2003). The leaves are pinnate with 5–7 leaflets, eliptic-oblong with a length and width range of 2.5–5.0 and 2.0–3.2 cm. They have flat linear beaked seed pods with a length range of 5–7 cm and is edible when tendered. The seed is oval in shape and has a blackish or yellowish brown colour with a length range of 4.5–7.0 mm and 3–4 mm wide. It has a taproot system with many slender lateral roots (Kosai et al. 2015; Mukherjee et al. 2008).
Fig. 1.

Clitoria ternatea flower
Nutritional analysis of C. ternatea flowers identified the percentage of protein, fibre, carbohydrate and fat to be 0.32, 2.1, 2.2 and 2.5% respectively while the moisture content was found to be 92.4%. The flower was also found to have high content of calcium (3.09 mg/g), magnesium (2.23 mg/g), potassium (1.25 mg/g), zinc (0.59 mg/g), sodium (0.14 mg/g) and iron (0.14 mg/g) (Neda et al. 2013). Several studies investigated, identified and isolated the bioactive compounds from C. ternatea flower. The anthocyanins known as ternatins are blue in colour and are acylated based on delphinidin (Fig. 2). Their structures were characterised as malonylated delphinidin 3,3′,5′-triglucosides having 3′,5′-side chains with alternative d-glucose and p-coumaric acid units at R and R1 with a total of 15 (poly) acylated delphinidin glucosides identified in all the blue petal lines namely ternatins A1-A3, B1-B4, C1-C4 and D1-D3 while some studies have identified several other delphinidin derivatives (Zakaria et al. 2018; Shen et al. 2016; Nair et al. 2015). Ternatins A1, A2, B1, B2, D1 and D2 (Fig. 3) are the six major anthocyanins present in the flowers (Mukherjee et al. 2008; Terahara et al. 1998). The flavonols (Fig. 4) identified in the petals are fourteen kaempferol, quercetin and myricetin glycosides which consist of H or OH at R1 and R2 and with H, rhamnosyl or malonyl at R3 and R4 (Mukherjee et al. 2008; Kazuma et al. 2003). Shen et al. (2016) identified various lipophilic compounds from C. ternatea being fatty acids (palmitic acid, stearic acids, petroselinic acids, linoleic acid, arachidic acid, behenic acid and phytanic acid), phytosterols (campesterol, stigmasterol, β-sitosterol and sitostanol) and tocols (α-tocopherol and γ-tocopherol). Several other components such as mome inositol, pentanal, cyclohexen, 1-methyl-4-(1-methylethylideme) and hirsutene were identified by Neda et al. (2013). In addition to the identification of various anthocyanins and flavonol glycosides, other components such as 6″‐malonylastragalin, phenylalanine, coumaroyl sucrose, tryptophan and coumaroyl glucose were determined (Zakaria et al. 2018).
Fig. 2.
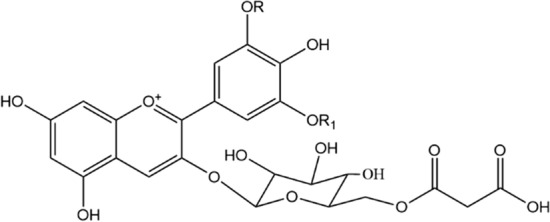
Delphinidin 3-malonyl glucoside
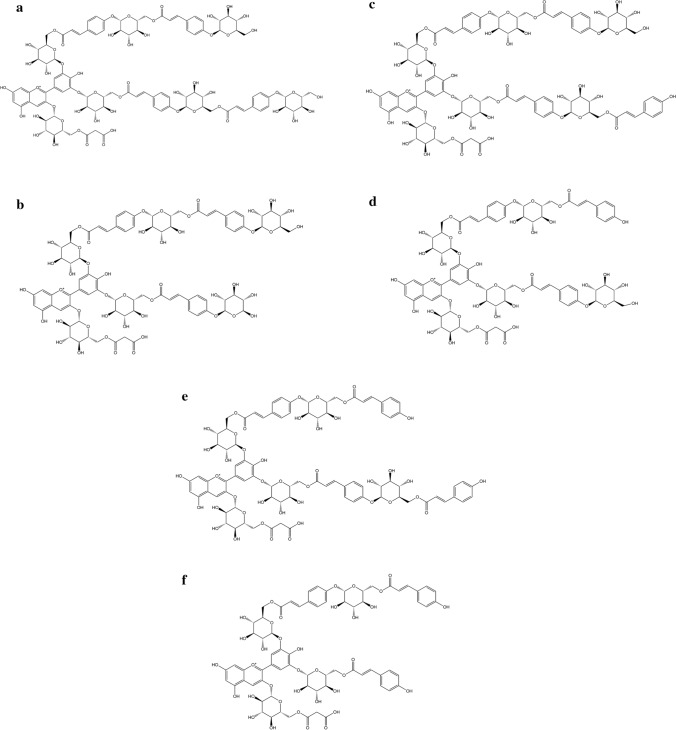
Fig. 3.
Major anthocyanins in C. ternatea flowers a Ternatin A1, b Ternatin A2, c Ternatin B1, d Ternatin B2, e Ternatin D1, and f Ternatin D2
Fig. 4.
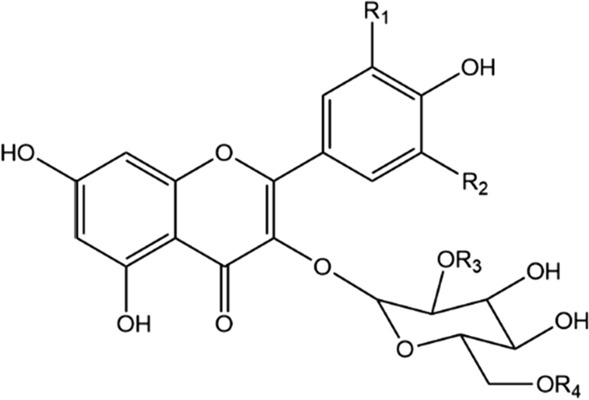
Flavonol glycosides
Clitoria ternatea has been used traditionally in ayurvedic medicine for various health issues. Its roots are used to treat indigestion, constipation, fever, arthritis, sore throat, skin diseases and eye ailments while its seeds are used as laxative, to treat colic and swollen joints. The traditional Cuban culture uses the decoction of the roots alone or combined with flowers to promote menstruation, induce uterine contractions as well as to treat liver and intestinal problems (Mukherjee et al. 2008; Fantz 1991). Various research studies have been done on the roots, seeds, flowers and leaves of C. ternatea. Clitoria ternatea flower is known for its potential health benefits in which several studies have shown the crude extract to have antidiabetic (Borikar et al. 2018), antioxidant (Chayaratanasin et al. 2015), antimicrobial (Leong et al. 2017) and antiproliferative/anticancer activities (Shen et al. 2016). Thus, C. ternatea flowers can be used as a natural source of antioxidants and/or a possible supplement in food or pharmaceutical industries. This paper reviews the current update on the extraction methods of C. ternatea flowers and its effect on the phytochemicals as well as the biological activities of these phytochemicals.
Extraction of phytochemicals
Extraction procedure of phytochemicals from plant materials is an important step. Various extraction procedures are available and the identification/selection of optimum parameters are important to ensure the enhancement of phytochemical yield (Azmir et al. 2013). Conventional and non-conventional extraction methods are available having respective advantages over each other thus careful selection of method should be evaluated depending on the suitability of samples and goals needed to be achieved. Prior to extraction, plant materials are usually reduced in size to increase the surface area for mixing with solvent and the samples used are either fresh, dried, grounded or powdered. Most studies on C. ternatea flowers utilized air/oven-dried, fresh flowers (Srichaikul 2018; Phrueksanan et al. 2014; Kamkaen and Wilkinson 2009) or grounded/powdered, dried flowers (Lakshan et al. 2019; López Prado et al. 2019; Mehmood et al. 2019; Pham et al. 2019; Adhikary et al. 2018; Rabeta and An Nabil 2013). Some studies utilized fresh flowers that were cut into smaller pieces, washed and stored in − 25 °C freezer and extracted within a month’s time (Chong and Gwee 2015) or freeze dried followed by grounding (Shen et al. 2016). Several conventional and non-conventional extraction methods have been used to obtain phytochemicals from C. ternatea flowers as discussed below.
Conventional extraction
Conventional extraction methods usually involve the use of different solvents with heat and/or mixing such as soxhlet extraction, maceration and hydrodistillation which though effective can be costly and require long extraction time (Wen et al. 2018; Azmir et al. 2013). Conventional extraction method is a classical method which has been widely used for the extraction of C. ternatea flower since the 1970s. Extraction studies on C. ternatea flower utilising aqueous solvent mixtures isolated and identified the structure of various phytochemicals mainly anthocyanins (Terahara et al. 1989, 1996, 1998) while other studies (Kazuma et al. 2003; Saito et al. 1985; Ranaganayaki and Singh 1979) focused on the flavonol constituents. Most studies employed extractions using aqueous solvent mixtures of ethanol or methanol rather than water alone with heating to investigate its potential bioactivities and phytochemical content while a number of studies investigated on the optimal solvent and/or extraction parameters (Table 1).
Table 1.
The extraction of phytochemicals from C. ternatea flowers
| Extraction solvent | Phytochemical extracted | Optimal extraction condition | References |
|---|---|---|---|
| Ethanol, methanol, chloroform, acetonitrile, acetone, ethyl acetate, n-butyl, water, n-hexane and ethyl ether | Anthocyanins | Ethanol | Ludin et al. (2018) |
|
70% ethanol (40–80 °C) |
70 °C | ||
|
70% ethanol (pH 4–9) |
pH 4 | ||
|
Water (pH 2, 7 and 10) |
Anthocyanins | pH 2 | Mauludifia et al. (2019) |
| Methanol |
Anthocyanins (Ternatin A1, B2, B3, C2, D2, D3, delphinidin derivatives), kaempferol 3-neohesperidoside and quercetin 3-(2G-rhamnosylrutinoside), rutin, ellagic acid |
– | Shen et al. (2016) |
| Ethyl acetate and hexane (1:1) | Fatty acids (palmitic acid, stearic acids, petroselinic acids, linoleic acid, arachidic acid, behenic acid and phytanic acid), phytosterols (campesterol, stigmasterol, β-sitosterol and sitostanol) and tocols (α-tocopherol and γ-tocopherol) | – | |
|
50% ethanol (40–80 °C, 15–75 min, liquid–solid ratio of 10:1 to 30:1 mL/g) |
Anthocyanins | 60.6 °C, 6 min, liquid–solid ratio of 23:1 mg/L | Pham et al. (2019) |
|
Water (25–95 °C, 40–80 min liquid–solid ratio of 20:1 to 60:1) |
Extract yield | 54 °C, 74 min, liquid–solid ratio of 37:1 | Baskaran et al. (2019) |
|
Water (40–80 °C, 30–60 min liquid–solid ratio of 1 to 3 g/L) |
Phenolics | 59.6 °C, 37 min, liquid–solid ratio of 3 g/L | Lakshan et al. (2019) |
|
Water (11.7–68.3 °C, 8.78–51.21 min) |
Phenolics | 40 °C, 30 min | Escher et al. (2020) |
|
40% and 50% ethanol (with 30 min ultrasound or maceration alone for 1–7 days) |
Phenolics | 50% ethanol with 30 min ultrasound | Srichaikul (2018) |
| Flavonoids | Maceration in 40% ethanol for 7 days | ||
|
Water (30–50 °C, 30–150 min, 96–240 W, liquid–solid ratio of 2–15 mL/g) |
Anthocyanins | 50 °C, 150 min, liquid–solid ratio of 15 mL/g and 240 W | Chong and Gwee (2015) |
|
Water with ultrasound (50 °C, 150 min, liquid–solid ratio of 15 mL/g and 240 W) 95% ethanol without ultrasound (50 °C, 150 min, liquid–solid ratio of 15 mL/g) |
Water with ultrasound assistance | ||
|
Water with and without ultrasound (50 °C, 150 min, liquid–solid ratio of 15 mL/g and 240 W) |
Phenolics and flavonoids | Water with ultrasound assistance | Mehmood et al. (2019) |
|
Water with heating (3 h) Water with microwave (2 min) |
Dye (anthocyanins) | Water with microwave assistance | Sinha et al. (2012) |
Ludin et al. (2018) determined the extraction efficiency of anthocyanins using various solvents (high to low polarity) in which the ethanol extract was found to have the highest efficiency for anthocyanin extraction while ethyl ether extract had the lowest efficiency. The study found that solvent with higher polarity (ethanol) will have higher efficiency in extracting polar compounds such as anthocyanins.
Another study, compared and identified the differences in the phytochemical content of the flowers using hydrophilic (methanol) and hydrophobic (ethyl acetate and hexane; 1:1) extraction. The hydrophilic extract contained various anthocyanins, kaempferol and quercetin glycosides while the hydrophobic extract was composed of fatty acids, phytosterols and tocopherols showing the effect of compounds extracted depending on polarity of compounds (Shen et al. 2016).
Studies investigating the effect of pH for anthocyanin extraction found lower pH to be optimum (Mauludifia et al. 2019; Ludin et al. 2018) suggesting lower pH enhances the extraction of anthocyanins as increase in acidity changes anthocyanins to the flavium cation form (more stable) which may enhance vacuole cell wall breakage, increase solubility of pigments thus increasing the concentration of anthocyanins extracted. However, the possibility of inhibition of enzymatic oxidation of phenolics as well as the maintenance of the stability of extracted anthocyanins at low pH (Zhang et al. 2001) was suggested as a possible factor as well (Ruenroengklin et al. 2008).
Investigation of the extraction temperature for anthocyanin extraction was found to increase with an increase in temperature and 70 °C was found to be the optimum temperature (Ludin et al. 2018). The increase in extraction temperature facilitates higher extraction of anthocyanins as it increases the internal energy of the molecules which increases the diffusion and solubility of the pigments thus having a higher yield (Cacace and Mazza 2003). However, there was reduction in anthocyanin concentration at 80 °C which may be due to degradation of pigments (Ludin et al. 2018). A particular study compared the effect of extraction with water or aqueous methanol which found the latter to have a higher phenolic content. However, both extractions differed in time and temperature which may have influenced the outcome of the results thus suitable controls are required to have a fair justification and comparison of extraction variables (Rabeta and An Nabil 2013).
Several studies utilised the Response Surface Methodology (RSM) which adapts a multivariate system that fits experimental data in a statistical model by a response function to optimise the extraction of phytochemical of interest with variation of experiment parameters (temperature, extraction time, liquid–solid ratio). The utilisation of RSM enabled the identification of optimum parameters for the extraction of anthocyanins (114–132 mg/L), extract yield (23.7–53.4%) and phenolic content (594–692 mg GAE/100 g and 24–81 mg GAE/L) (Escher et al. 2020; Pham et al. 2019; Baskaran et al. 2019; Lakshan et al. 2019). Studies utilising RSM to enhance the extraction of phytochemical of interest is beneficial as it incorporates variables of interest (e.g. temperature, extraction time, liquid-solvent ratio) to be studied using a statistical approach which generates a mathematical model that optimizes extraction variables (Sreejith et al. 2014).
Non-conventional extraction methods
Non-conventional extraction methods are newer, highly efficient, safer to the environment having various advantages over conventional extraction method which include methods such ultrasound assisted extraction, microwave assisted extraction, enzyme assisted extraction, supercritical fluid extraction and pressurized liquid extraction (Wen et al. 2018; Azmir et al. 2013). To the author’s best knowledge, among the non-conventional extraction methods, only ultrasound and microwave assisted extraction have been employed to extract phytochemicals from C. ternatea flowers (Table 1).
Ultrasound assisted extraction works on the concept of acoustic waves production leading to molecular movement of solvent and sample which facilitates the leaching of organic and inorganic compounds (Herrera and De Castro 2005). Srichaikul (2018) compared the effect of short extraction time with ultrasound and long extraction time with maceration for 1–7 days for extraction efficiency of phenolic and flavonoid content using aqueous ethanol. Extraction of phenolic content was more efficient in the ultrasound assisted extraction. However, for flavonoid content, maceration in aqueous ethanol at day 7 was found to be higher than with ultrasound extraction (30 min). Although the flavonoid content was higher at day 7 in the aqueous ethanol extract without ultrasound assistance, a longer extraction time (7 days) was required to achieve this effect. A particular study utilised RSM to obtain the optimum extraction condition for anthocyanins using water by varying the extraction time, temperature, liquid–solid ratio and ultrasound frequency. The extraction using the optimized condition showed higher extraction yield of anthocyanins by 246.5% than 95% methanol without ultrasound assistance (Chong and Gwee 2015). Mehmood et al. (2019) further investigated this optimized condition by Chong and Gwee (2015) to determine the extraction efficiency of phenolic and flavonoid content from C. ternatea flowers by using water with and without ultrasound assistance. Similarly, ultrasound extraction showed a better extraction yield of phenolic and flavonoid content.
In microwave assisted extraction, electromagnetic energy is converted to heat following ionic conduction and dipole rotation mechanisms which promotes the release of solutes from the sample matrix to solvent (Alupului et al. 2012; Jain 2009). Sinha et al. (2012) compared the extraction efficiency of C. ternatea dye (anthocyanins) using conventional extraction with water and heating to microwave assisted extraction. Microwave assisted extraction was more efficient than conventional extraction having a higher dye yield. Microwave assisted extraction was also more time efficient requiring 2 min as opposed to conventional extraction method which required 3 h.
Although conventional extraction has widely been used for the extraction of these flowers, the use of non-conventional extraction method (ultrasound assistance) has shown to be superior and beneficial for the extraction of phytochemicals. Thus exploration on the use of other non-conventional extraction methods which are considered as “green techniques” would be beneficial in determining extraction efficiency of various phytochemicals. Other studies have employed the use of ultrasound, pulsed-electric field, pressurized liquid and microwave assisted extraction which were more effective than the conventional extraction method for extraction of phenolics and anthocyanins which required shorter extraction time and were also useful in preventing oxidation of compounds (Caldas et al. 2018; Corrales et al. 2008, 2009).
Biological activities
Clitoria ternatea flower contains a significant amount of phytochemicals which exhibits great antioxidant, antimicrobial, antidiabetic, anti-inflammatory and antiproliferative/anticancer properties (López Prado et al. 2019; Mahmad et al. 2018; Nair et al. 2015; Rajamanickam et al. 2015; Neda et al. 2013). Acute toxicity study using albino Wistar rats treated orally with aqueous ethanol extract (2000 mg/kg bodyweight) of the flower showed no signs of mortality or abnormality and there was no significant difference in the haematological values The extract did not display acute toxicity effects and are safe for consumption (Srichaikul 2018). Clitoria ternatea flowers can potentially be utilised as a functional food incorporated into various food products or even as a pharmaceutical supplement/drug combined with commercial drugs to improve treatment efficacy of patients.
Antioxidant activity
Oxidative stress plays a part in the development of chronic and degenerative illness such as cancer, autoimmune disorders, cardiovascular and neurodegenerative diseases. The discovery of antioxidants from natural sources is beneficial to human health (Admassu et al. 2018; Pham-Huy et al. 2008). Various studies investigated the antioxidant activity of C. ternatea flowers using antioxidant assays such as 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) radical scavenging, ferric reducing antioxidant power (FRAP), hydroxyl radical scavenging activity (HRSA), hydrogen peroxide scavenging, oxygen radical absorbance capacity (ORAC), superoxide radical scavenging activity (SRSA), ferrous ion chelating power, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radical scavenging and Cu2+ reducing power assays. Clitoria ternatea flower has been shown to have potent antioxidant activity (Table 2).
Table 2.
The antioxidant activity of C. ternatea flowers from various research studies
| Extract | Antioxidant assay | Results | References |
|---|---|---|---|
| Water extract and 100% ethanol extract | DPPH radical scavenging |
Water extract IC50 = 1 mg/mL Ethanol extract IC50 = 4 mg/mL Water extract in gel formulation for inhibition of DPPH reduction = 28% at 0.5 mg/mL |
Kamkaen and Wilkinson (2009) |
| 100% Methanol extract | DPPH radical scavenging | IC50 = 327 µg/mL | Nithianantham et al. (2013) |
| Citrate buffer extract | DPPH radical scavenging | EC50 = 0.49 mg/mL | Siti Azima et al. (2014) |
| FRAP | 13.3 mM/g based on trolox equivalent antioxidant capacity (TEAC) | ||
| Water extract | DPPH radical scavenging | IC50 = 84.15 µg/mL | Iamsaard et al. (2014) |
| FRAP | 0.33 mmol/mg ascorbic equivalent | ||
| Water extract | DPPH radical scavenging | IC50 = 470 µg/mL | Phrueksanan et al. (2014) |
| Oxygen radical absorbance capacity (ORAC) | 17.54 μg trolox equivalents/mg extract | ||
| Reduction of free radical-induced erythrocyte hemolysis (4 h) | 96.3% at 400 µg/mL | ||
| Inhibition of lipid peroxidation (4 h) | 72.7% at 400 µg/mL | ||
| Water extract | DPPH radical scavenging | IC50 = 0.47 mg/mL | Chayaratanasin et al. (2015) |
| Trolox equivalent antioxidant capacity (TEAC) | 0.17 mg trolox/mg dried extract | ||
| Ferric reducing antioxidant power (FRAP) | 0.38 mmol FeSO4/mg dried extract | ||
| HRSA | IC50 = 19.2 mg/mL | ||
| SRSA | IC50 = 26.3 mg/mL | ||
| Ferrous ion chelating power | > 103 mg EDTA/mg dried extract | ||
|
Methanol/acetone/water (5:4:1) extract |
ORAC | 490.7 μmol trolox equivalent/g extract | Nair et al. (2015) |
| 95% methanol extract | DPPH radical scavenging | IC50 = 95.3 µg/mL | Rajamanickam et al. (2015) |
| Water extract | ABTS radical scavenging | 4.2 µM trolox equivalent/g extract | Azima et al. (2017) |
| DPPH radical scavenging | EC50 = 0.76 mg/mL | ||
| FRAP | 10.9 mM trolox equivalent/g extract | ||
| ORAC | 15.8 µmol trolox equivalent/g extract | ||
| Water extract | ABTS radical scavenging | IC50 = 42.9 µg/mL | Zakaria et al. (2018) |
| DPPH radical scavenging | IC50 = 195.5 µg/mL | ||
| Water extract with ultrasound assistance (US) and water extract with heat assistance at 50 °C (AGE) | DPPH radical scavenging |
US = 931.5 µg trolox equivalent/g extract AGE = 764.3 µg trolox equivalent/g extract |
Mehmood et al. (2019) |
| ABTS radical scavenging |
US = 13,488 µg trolox equivalent/g extract AGE = 11,720.3 µg trolox equivalent/g extract |
||
| FRAP |
US = 5834.6 µg trolox equivalent/g extract AGE = 4195.3 µg trolox equivalent/g extract |
||
| Reducing power |
US = 4539.0 µg trolox equivalent/g extract AGE = 6154.1 µg trolox equivalent/g extract |
||
| Cu2+ reducing power |
US = 12,696 µg trolox equivalent/g extract AGE = 9549 µg trolox equivalent/g extract |
||
| Xanthine oxidase inhibition |
US = 1.01 mg/mL (IC50) AGE = 1.22 mg/mL (IC50) |
||
| Water extract, 100% and 50% methanol extract at 6 h (best condition) | DPPH radical scavenging |
Water extract = 11.7 mM trolox equivalent/g extract 100% methanol extract = 6.99 mM trolox equivalent/g extract 50% methanol extract = 12.2 mM trolox equivalent/g extract |
López Prado et al. (2019) |
| Inhibition of cholesterol oxidation |
Water extract = 79.8% 100% methanol extract = 49.7% 50% methanol extract = 89.8% |
In the DPPH assay, 100% methanol extract of C. ternatea flower extract was found to be more potent than vitamin E (Nithianantham et al. 2013), whereas the water extract was found to be lower than ascorbic acid (vitamin C) (Chayaratanasin et al. 2015; Phrueksanan et al. 2014; Iamsaard et al. 2014) Some studies investigated and compared the antioxidant activity (DPPH assay) of the extracts using different solvents in which the water extract was found to be more potent than 100% ethanol extract at 15 min extraction time (Kamkaen and Wilkinson 2009). However, in another study the best extraction time was determined (6 h) for the water extract, 100% and 50% methanol extract in which the water extract and 50% methanol were found to be equally potent and had a higher activity than 100% methanol extract (López Prado et al. 2019). The optimum condition was investigated using water extract in another study with and without ultrasound at a fixed temperature and liquid-solvent ratio in which the extraction with ultrasound was found to have higher antioxidant activity (Mehmood et al. 2019). The in vitro chemical assays to measure antioxidant activity (Table 2) need to be carefully interpreted as they bear no similarity to biological systems including the absorption of antioxidants by the human body (Gengatharan et al. 2015). In a cell based study, the water extract was found to potently inhibit 2,2′-azobis-2-methyl-propanimidamide dihydrochloride (AAPH)-induced hemolysis and oxidative damage of canine erythrocytes (Phrueksanan et al. 2014). In another study, the pre-treatment of human HaCaT keratinocytes with the water extract reduced UV-induced mitochondrial DNA damage (Zakaria et al. 2018). In a randomised crossover study, acute ingestion of C. ternatea flower extract/beverage was found to have increased plasma antioxidant capacity and the effect was further enhanced when consumed together with sucrose in healthy men (Chusak et al. 2018). These studies attribute the flavonols and anthocyanins for the antioxidant activity.
Antibacterial activity
The emergence of antibiotic resistance microbes limits the effectiveness of current drugs significantly causing treatment failure of infections (Scheffler et al. 2013). In regard to this challenge, there is a need to develop alternative approaches in addition to searching for new antibacterial compounds. In vitro activity of an antimicrobial (antibacterial or antifungal) agent can be tested through various methods such as broth or agar dilution, and disc diffusion methods (Balouiri et al. 2016). Several studies investigated on the antibacterial potential of C. ternatea flowers. The methanol extract of C. ternatea flower was tested against 12 bacterial species (Bacillus cereus, Bacillus subtilis, Bacillus thuringiensis, Staphylococcus aureus, Streptococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, Enterobacter aerogens, Proteus mirabilis and Herbaspirillum spp.) and was found to have the most potent activity against Bacillus thuringiensis with a minimum inhibitory concentration (MIC) of 12.5 mg/mL and minimum bactericidal concentration (MBC) of 25 mg/mL with an inhibition zone of 15.7 mm using agar disc diffusion technique (Kamilla et al. 2009). In another study, the water, methanol, petroleum ether, hexane and chloroform extract of C. ternatea flower (4 mg) were tested against E. coli, K. pneumoniae, S. enteritidis, S. typhimurium and P. aeruginosa to determine its antibacterial activity. The methanol extract was found to have the highest activity when tested using agar disc diffusion technique with an inhibition zone range of 16–26 mm in E. coli, K. pneumonia and P. aeruginosa but had no activity against S. typhi and S. enteritidis. The highest zone of inhibition 26 mm was observed against K. pneumonia and P. aeruginosa (Uma et al. 2009). Leong et al. (2017) determined the antibacterial activity of anthocyanins of C. ternatea flower ethanol extract paste against B. cereus, B. subtilis, S. aureus, B. subtilis subsp. spizizenii, Proteus mirabilis, K. pneumoniae, Yersinia enterocolitica and E. coli. The extract was found to have good antibacterial activity against B. cereus, B. subtilis, S. aureus, P. mirabilis and K. pneumonia with the most potent activity against K. pneumonia with a MIC of 1.6 mg/mL and minimum lethal concentration (MLC) of 25 mg/mL while in another study, the anthocyanin fraction obtained from the ethanol extract of C. ternatea flower had the best effect against B. subtilis with a disc diffusion inhibition zone of 10 mm (Mahmad et al. 2018). The findings from these studies suggest the potential of the anthocyanins for its antibacterial activity.
Antifungal activity
A rise in resistance towards most antifungal agents in diverse pathogens which calls for the need to identify new therapeutic agents (Perfect 2016). The methanol extract of C. ternatea flower (100 mg/mL) tested against Candida albicans, Rhizopus and Penicillium spp. had the highest activity against Candida albicans with an inhibition zone of 19 mm in agar disc diffusion. However, in broth dilution method, it only had activity against Penicillium spp. and Rhizopus with similar MIC value of 0.8 mg/mL and MFC value of 1.6 mg/ml (Kamilla et al. 2009). The anthocyanin fraction obtained from the ethanol extract of C. ternatea flower tested against Fusarium sp., A. niger and Trichoderma sp. had the highest activity against Fusarium sp. with an inhibition zone of 10 mm in agar disc diffusion technique (Mahmad et al. 2018). The anthocyanins of C. ternatea flower ethanol extract paste (50 mg/mL) tested against A. niger, P. expansum and R. stolonifera only exhibited activity against P. expansum with an inhibition zone of 15.5 mm in agar disc diffusion while it had an MIC value of 12.5 mg/mL and MLC value of 25 mg/mL. The mode of action for the antifungal activity against P. expansum was investigated and found to be mediated by the alteration of morphology of P. expansum fungal hyphae which had flattened empty hyphae resulting from cell wall disruption and damage of conidiophore. The germination of P. expansum conidia was completely inhibited with suppressed conidial development (Leong et al. 2017).
Anti-inflammatory activity
The current available non-steroidal anti-inflammatory drugs (NSAIDs), including acetaminophen and aspirin are associated with side effects, particularly gastrointestinal and cardiovascular effects as they are known to affect both COX-1 and COX-2. The discovery of new or alternate strategies is needed to reduce the risks associated with NSAIDs while achieving sufficient pain relief (Brune and Patrignani 2015).
The petroleum ether extract of C. ternatea flowers was evaluated for anti-inflammatory activity using carrageenan paw edema method with healthy albino rats of either sex. The extract (200 and 400 mg/kg) significantly inhibited paw edema compared to control untreated group while in Eddy’s hot plate method, the treatment group (400 mg/kg) had significant increased reaction time (time recorded when animals licked their fore or hind paws or jump response, whichever appear first) compared to control untreated group. The study suggests the possibility of the extracts to have a protective effect against the release of prostaglandins, kinnins and other substances in carrageenan induced edema (Shyamkumar and Ishwar 2012). In another study, the anthocyanin and flavonol fraction obtained from C. ternatea flower extract (extracted in a mixture of MeOH/acetone/H2O at ratio of 5:4:1) were investigated for its anti-inflammatory potential. In the lipopolysaccharides (LPS)-induced inflammation in RAW-264.7 macrophage cells, the flavonols had mild suppression of ROS while the anthocyanins had no effect on ROS production. The anthocyanins were also found to have higher inhibition of nitric oxide production compared to the flavonols. In western blot studies, only the anthocyanins inhibited nuclear factor-κB translocation and iNOS protein expression whereas the flavonols significantly inhibited COX-2 expression but not the anthocyanins (Nair et al. 2015).
Cytotoxic and anti-proliferative/anticancer activities
Chemotherapy, radiation therapy and targeted therapy are among the approaches used for the treatment and management of cancer but they are not able to provide a permanent cure and have been associated with various side effects and toxicities (Curigliano et al. 2012). Thus, new agents that are safe, available and effective are urgently needed. Several studies investigated the anticancer potential of C. ternatea flower extracted using different solvents. The 100% petroleum ether extract (IC50 = 36 µg/mL) was found to be more potent than the 100% ethanol extract (IC50 value of 57 µg/mL) in the in vitro cytotoxic assay against Dalton’s lymphoma ascites (DLA) cells at 3 h which could be due to different phytochemical composition in both extracts. The petroleum ether extract was found to have presence of saponins, tannins, steroids and triterpernoids while the ethanol extract had flavonols only (Kumar and Bhat 2011). In another study, the water extract was more potent than the methanol extract having much lower IC50 values with activity against hormone dependent breast cancer cell line (MCF-7), non-hormone-dependent breast cancer cell line (MDA-MB-231), human ovary cancer cell line (Caov-3), and human liver cancer cell line (HepG2) at 72 h. However, the methanol extract had activity against MCF-7 and MDA-MB-231 cells only with higher IC50 values. The extracts were not toxic against the normal cell line (Hs27). The study suggests the aqueous extract to have more significant anti-proliferative activity than the methanol extract as it may have more active compounds (flavonoids) present (Neda et al. 2013). Shen et al. (2016) found the anticancer effect of the hydrophilic (100% methanol) extract to be more potent than the lipophilic (hexane:ethyl acetate, 1:1) extract on human epithelial laryngeal carcinoma (Hep-2) cell line. The potent active compounds identified in the hydrophilic extract were mainly ternatins, kaempferol and quercetin responsible for the antiproliferative effect as opposed to the lipophilic extract which constitutes of fatty acids, phytosterols and tocopherols.
Antidiabetic activity
Oral antidiabetic medications such as biguanides, meglitinide, thiazolidinedione, sulfonylureas and dipeptidyl peptidase 4 are known to be associated with various side effects (Chaudhury et al. 2017). Herbal based medications are worth exploring for potential use in the management of diabetes as they are considered to be safer and may have reduced side effects (Borikar et al. 2018). Several studies investigated the in vitro and in vivo potential of C. ternatea flower extract for antidiabetic activity. The water extract reduced the formation of fluorescent advanced glycation end products having the highest activity at day 28 (49.4% at 1 mg/mL) as well as significant reduction in fructosamine level (14.47–36.66%) in glycated bovine serum albumin. The study suggests the potential of the extract in the prevention of the formation of advanced glycation end products to be mediated through its free radical scavenging ability mainly attributed to the active compounds present being the ternatin anthocyanins, delphinidin derivatives and kaempferol (Chayaratanasin et al. 2015). In vivo study for antidiabetic activity in alloxan-induced diabetic rats (wistar albino) by Rajamanickam et al. (2015) utilising 95% methanol, ethyl acetate and chloroform extract were found to have significantly reduced blood glucose level, increased serum protein levels and restored serum albumin to normal levels. The extracts also significantly decreased serum urea, creatinine, cholesterol and triglyceride levels compared to control untreated diabetic rats. A similar trend was also observed in the in vivo studies by Borikar et al. (2018) utilising 100% methanol extract and water extract in the study by Daisy and Rajathi (2009). In a randomised crossover study, acute ingestion of C. ternatea flower extract/beverage was found to have suppressed postprandial plasma glucose and insulin levels when consumed with sucrose in healthy men (Chusak et al. 2018). Overall, these studies suggested the hypoglycemic activity may be exerted by the flavonoid principles (flavonol glycosides and anthocyanins) and alkaloids present in the extract which may involve the potentiation of insulin secretion from the β-cell or by enhancement of the transport of blood glucose from plasma to peripheral tissues.
Other biological activities of phytochemicals studied in C. ternatea flowers
Clitoria ternatea flower has shown to have potential antioxidant, antimicrobial, anticancer and antidiabetic activity. However, there are also various studies which have looked into its potential for other beneficial activities. Adhikary et al. (2018) found the 100% methanol extract of C. ternatea flower and its purified compound quercetin-3β-d-glucoside for its anti-arthritic potential in a mice model. Quercetin-3β-d-glucoside was found to be more potent than the extract to significantly reduce myeloperoxidase activity, decrease in release of pro-inflammatory cytokines, chemokines, reactive oxygen species (ROS)/reactive nitrogen species production. It also significantly reduced tumor necrosis factor α-receptor 1, toll-like receptor 2, inducible isoform of nitric oxide synthase, COX-2 and matrix metalloproteinase-2 expression.
The anti-allergy effects of C. ternatea flower extract was also found in a study by Singh et al. (2018). The 98% ethanol extract was able to attenuate histamine-induced contraction in both goat tracheal chain and isolated guinea pig ileum preparations. The extract was also found to attenuate histamine-induced dyspnoea and ovalbumin-induced changes of various inflammatory cytokines in animal models. The extract also displayed antitussive activity in sulfur dioxide- and citric acid-induced cough in experimental animals and attenuated inflammation in carrageenan and acetic acid challenged rodents. Clitoria ternatea flower extract was also found to have other beneficial effects in various other studies such as anthelmintic (Nirmal et al. 2008), larvicidal (Mathew et al. 2009), anti-aging (Zakaria et al. 2018), hepatoprotective (Nithianantham et al. 2013), testicular damage protection (Iamsaard et al. 2014), anti-adipogenesis (Chayaratanasin et al. 2019) and starch digestion (Chusak et al. 2019) activity.
Conclusion
Clitoria ternatea is a versatile plant known for its traditional application in ayurvedic medicine, food colourant and cover crop among others. Various beneficial studies have been done on this plant along the years which have found it to have many health benefits thus giving a greater insight on its potential uses. Extraction studies have been a very important step in identifying variables which influences the extraction of phytochemicals. Conventional extraction such as the use of solvent for maceration of samples has been the most common approach and shown to be efficient. However, several disadvantages have been associated with this method due to high cost, requiring long extraction time and is not environmentally friendly with the use of solvent. Thus newer studies have explored and compared extraction methods such as ultrasound/microwave assisted extraction (non-conventional method) which are not only environmentally friendly but are much more time efficient than conventional extraction methods. Thus, future studies could be geared towards the use of non-conventional extraction for C. ternatea flowers as well as exploring other environmentally safe methods such as pulsed-electric field, pressurized liquid, enzyme-assisted and pressurised liquid extraction. Findings from acute toxicity studies for C. ternatea flowers are rather encouraging as it has shown to be safe for consumption in vivo studies. Thus it is recommended for further studies to evaluate it for subchronic/chronic toxicity, histopathology as well as clinical studies to determine its effect and safety for long term consumption. Most studies have shown the phytochemicals in C. ternatea flowers, mainly anthocyanins, quercetin and kaempferol glycosides to probably be responsible for the beneficial biological effects. There are variants of anthocyanins, quercetin and kaempferol present in the flowers. Thus it would be beneficial for future studies to explore and compare the effect between anthocyanin/flavonol fractions which would help guide the potential fraction to further isolate the compound most probably responsible for the studied effect. Cytotoxicity studies have shown the extract to be more selective towards cancer cell lines than normal cells which may have less side effects in the human body. Some studies have determined the mode of action of these bioactive constituents which provides a lead in understanding the causative biological effect observed. Exploring the mechanism of action of the extract/active compounds in eliciting the biological effect are crucial to understand how it may affect/modulate certain pathways/molecular targets in the human body and are thus warranted in further studies. Numerous studies have shown C. ternatea flower for its potential antioxidant activity not only in chemical based assays but in cell based assays and in vivo studies. The consumption of C. ternatea flower extract/beverage was shown to have potential antioxidant and antihyperglycemic effects in human subjects. However, the effect was only studied in healthy subjects which may not be generalised to all population. Future studies looking on the effect on subjects with particular health conditions compared to healthy subjects are recommended to better understand its effect and determine its potential. Owing to the many benefits displayed by this flower, it is worthwhile to carry out suggested further studies to better understand the biological effects which have been reported so far as well as exploring other bioactivities. The bioactive compounds of C. ternatea flower are promising candidates for development as novel and effective pharmaceutical agents as well as applications as functional foods to promote human health and wellbeing.
Acknowledgement
This work was funded by the School of Science, Monash University Malaysia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adhikary R, Sultana S, Bishayi B. Clitoria ternatea flower petals: effect on TNFR1 neutralization via downregulation of synovial matrix metalloproteases. J Ethnopharmacol. 2018;210:209–222. doi: 10.1016/j.jep.2017.08.017. [DOI] [PubMed] [Google Scholar]
- Admassu H, Gasmalla MA, Yang R, et al. Bioactive peptides derived from seaweed protein and their health benefits: antihypertensive, antioxidant, and antidiabetic properties. J Food Sci. 2018;83:6–16. doi: 10.1111/1750-3841.14011. [DOI] [PubMed] [Google Scholar]
- Alupului A, Calinescu I, Lavric V. Microwave extraction of active principles from medicinal plants. UPB Sci Bull Ser B. 2012;74:129–142. [Google Scholar]
- Ambasta SP (1988) The Wealth of India: A Dictionary of India Raw Materials and Industrial Products, vol. II. Publication and Information Directorate, CSIR, New Delhi, India, p. 233. ISBN: 8185038902
- Azima AS, Noriham A, Manshoor N. Phenolics, antioxidants and color properties of aqueous pigmented plant extracts: Ardisia colorata var. elliptica, Clitoria ternatea, Garcinia mangostana and Syzygium cumini. J Funct Foods. 2017;38:232–241. doi: 10.1016/j.jff.2017.09.018. [DOI] [Google Scholar]
- Azmir J, Zaidul ISM, Rahman MM, et al. Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng. 2013;117:426–436. doi: 10.1016/j.jfoodeng.2013.01.014. [DOI] [Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran A, Mudalib SKA, Izirwan I. Optimization of aqueous extraction of blue dye from butterfly pea flower. J Phys Conf Ser. 2019;1358:012001. doi: 10.1088/1742-6596/1358/1/012001. [DOI] [Google Scholar]
- Borikar SP, Kallewar NG, Mahapatra DK, et al. Dried flower powder combination of Clitoria ternatea and Punica granatum demonstrated analogous anti-hyperglycemic potential as compared with standard drug metformin: in vivo study in Sprague Dawley rats. J Appl Pharm Sci. 2018;8:75–79. doi: 10.7324/japs.2018.81111. [DOI] [Google Scholar]
- Brune K, Patrignani P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J Pain Res. 2015;8:105. doi: 10.2147/jpr.s75160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacace JE, Mazza G. Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng. 2003;59:379–389. doi: 10.1016/s0260-8774(02)00497-1. [DOI] [Google Scholar]
- Caldas TW, Mazza KE, Teles AS, et al. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind Crop Prod. 2018;111:86–91. doi: 10.1016/j.indcrop.2017.10.012. [DOI] [Google Scholar]
- Chauhan NS, Singh NK, Gupta JK et al (2017) A Review on Clitoria ternatea (Linn.): Chemistry and Pharmacology. Medicinal Plants and its Therapeutic Uses. OMICS Group eBooks, CA, USA. ISBN: 1632780747
- Chaudhury A, Duvoor C, Dendi R, et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol. 2017;8:6. doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan N, Rajvaidhya S, Dubey BK. Pharmacognostical, phytochemical and pharmacological review on Clitoria ternatea for antiasthmatic activity. Int J Pharm Sci Res. 2012;3:398. [Google Scholar]
- Chayaratanasin P, Barbieri MA, Suanpairintr N, et al. Inhibitory effect of Clitoria ternatea flower petal extract on fructose-induced protein glycation and oxidation-dependent damages to albumin in vitro. BMC Complement Altern Med. 2015;15:27. doi: 10.1186/s12906-015-0546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayaratanasin P, Caobi A, Suparpprom C, et al. Clitoria ternatea flower petal extract inhibits adipogenesis and lipid accumulation in 3T3-L1 preadipocytes by downregulating adipogenic gene expression. Molecules. 2019;24:1894. doi: 10.3390/molecules24101894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong FC, Gwee XF. Ultrasonic extraction of anthocyanin from Clitoria ternatea flowers using response surface methodology. Nat Prod Res. 2015;29:1485–1487. doi: 10.1080/14786419.2015.1027892. [DOI] [PubMed] [Google Scholar]
- Chusak C, Thilavech T, Henry CJ, et al. Acute effect of Clitoria ternatea flower beverage on glycemic response and antioxidant capacity in healthy subjects: a randomized crossover trial. BMC Complement Altern Med. 2018;18:1–11. doi: 10.1186/s12906-017-2075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chusak C, Ying JAY, Zhien JL, et al. Impact of Clitoria ternatea (butterfly pea) flower on in vitro starch digestibility, texture and sensory attributes of cooked rice using domestic cooking methods. Food Chem. 2019;295:646–652. doi: 10.1016/j.foodchem. [DOI] [PubMed] [Google Scholar]
- Corrales M, Toepfl S, Butz P, et al. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innov Food Sci Emerg Technol. 2008;9:85–91. doi: 10.1016/j.ifset.2007.06.002. [DOI] [Google Scholar]
- Corrales M, García AF, Butz P, et al. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J Food Eng. 2009;90:415–421. doi: 10.1016/j.jfoodeng.2008.07.003. [DOI] [Google Scholar]
- Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23:55–66. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- Daisy P, Rajathi M. Hypoglycemic effects of Clitoria ternatea Linn. (Fabaceae) in alloxan-induced diabetes in rats. Trop J Pharm Res. 2009;8:393–398. doi: 10.4314/tjpr.v8i5.48082. [DOI] [Google Scholar]
- Escher GB, Marques MB, do Carmo MAV, et al. Clitoria ternatea L. petal bioactive compounds display antioxidant, antihemolytic and antihypertensive effects, inhibit α-amylase and α-glucosidase activities and reduce human LDL cholesterol and DNA induced oxidation. Food Res Int. 2020;128:108763. doi: 10.1016/j.foodres.2019.108763. [DOI] [PubMed] [Google Scholar]
- Fantz PR. Ethnobotany of Clitoria (Leguminosae) Econ Bot. 1991;45:511–520. doi: 10.1007/BF02930715. [DOI] [Google Scholar]
- Gecer MK, Kan T, Gundogdu M, et al. Physicochemical characteristics of wild and cultivated apricots (Prunus armeniaca L.) from Aras valley in Turkey. Genet Resour Crop Eviron. 2020;67:935–945. doi: 10.1007/s10722-020-00893-9. [DOI] [Google Scholar]
- Gengatharan A, Dykes GA, Choo WS. Betalains: natural plant pigments with potential application in functional foods. LWT-Food Sci Technol. 2015;64:645–649. doi: 10.1016/j.lwt.2015.06.052. [DOI] [Google Scholar]
- Havananda T, Luengwilai K. Variation in floral antioxidant activities and phytochemical properties among butterfly pea (Clitoria ternatea L.) germplasm. Genet Resour Crop Eviron. 2019;66:645–658. doi: 10.1007/s10722-018-00738-6. [DOI] [Google Scholar]
- Herrera MC, De Castro ML. Ultrasound-assisted extraction of phenolic compounds from strawberries prior to liquid chromatographic separation and photodiode array ultraviolet detection. J Chromatogr A. 2005;1100:1–7. doi: 10.1016/j.chroma.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Iamsaard S, Burawat J, Kanla P, et al. Antioxidant activity and protective effect of Clitoria ternatea flower extract on testicular damage induced by ketoconazole in rats. J Zhejiang Univ Sci B. 2014;15:548–555. doi: 10.1631/jzus.b1300299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain T. Microwave assisted extraction for phytoconstituents: an overview. Asian J Chem. 2009;2:19–25. [Google Scholar]
- Jamil N, Zairi MNM, Nasim NAIM, et al. Influences of environmental conditions to phytoconstituents in Clitoria ternatea (butterfly pea flower): a review. J Sci Technol. 2018;10:208–228. doi: 10.30880/jst.2018.10.02.029. [DOI] [Google Scholar]
- Kamilla L, Mnsor SM, Ramanathan S, et al. Antimicrobial activity of Clitoria ternatea (L.) extracts. Pharmacologyonline. 2009;1:731–738. [Google Scholar]
- Kamkaen N, Wilkinson JM. The antioxidant activity of Clitoria ternatea flower petal extracts and eye gel. Phytother Res. 2009;23:1624–1625. doi: 10.1002/ptr.2832. [DOI] [PubMed] [Google Scholar]
- Kazuma K, Noda N, Suzuki M. Flavonoid composition related to petal color in different lines of Clitoria ternatea. Phytochemistry. 2003;64:1133–1139. doi: 10.1016/s0031-9422(03)00504-1. [DOI] [PubMed] [Google Scholar]
- Kosai P, Sirisidthi K, Jiraungkoorskul K, et al. Review on ethnomedicinal uses of memory boosting herb, butterfly pea, Clitoria ternatea. J Nat Remedies. 2015;15:71–76. doi: 10.18311/jnr/2015/480. [DOI] [Google Scholar]
- Kumar BS, Bhat KI. In-vitro cytotoxic activity studies of Clitoria ternatea linn flower extracts. Int J Pharma Sci Rev Res. 2011;6:120–121. [Google Scholar]
- Lakshan SAT, Jayanath NY, Abeysekera WPKM, et al. A commercial potential blue pea (Clitoria ternatea L.) flower extract incorporated beverage having functional properties. Evid Based Complement Altern Med. 2019;2019:1–13. doi: 10.1155/2019/2916914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong CR, Azizi K, Afif M, et al. Anthocyanins from Clitoria ternatea attenuate food-borne Penicillium expansum and its potential application as food biopreservative. Nat Prod Sci. 2017;23:125–131. doi: 10.20307/nps.2017.23.2.125. [DOI] [Google Scholar]
- López Prado AS, Shen Y, Ardoin R, et al. Effects of different solvents on total phenolic and total anthocyanin contents of Clitoria ternatea L. petal and their anti-cholesterol oxidation capabilities. Int J Food Sci Technol. 2019;54:424–431. doi: 10.1111/ijfs.13953. [DOI] [Google Scholar]
- Ludin AA, Al-Alwani MA, Mohamad AB, et al. Utilization of natural dyes from Zingiber officinale leaves and Clitoria ternatea flowers to prepare new photosensitisers for dye-sensitised solar cells. Int J Electrochem Sci. 2018;13(8):7451–7465. doi: 10.20964/2018.08.04. [DOI] [Google Scholar]
- Mahmad N, Taha RM, Othman R, et al. Anthocyanin as potential source for antimicrobial activity in Clitoria ternatea L. and Dioscorea alata L. Pigm Resin Technol. 2018;47:490–495. doi: 10.1108/prt-11-2016-0109. [DOI] [Google Scholar]
- Mathew N, Anitha MG, Bala TSL, et al. Larvicidal activity of Saraca indica, Nyctanthes arbor-tristis, and Clitoria ternatea extracts against three mosquito vector species. Parasitol Res. 2009;104:1017–1025. doi: 10.1007/s00436-008-1284-x. [DOI] [PubMed] [Google Scholar]
- Mauludifia F, Astrinia SD, Meiranti KA, et al. Production of natural colorant powder from Clitoria ternatea L. using tray dryer which is dehumidified by zeolite. J Phys Conf Ser. 2019;1295:012018. doi: 10.1088/1742-6596/1295/1/012018. [DOI] [Google Scholar]
- Mehmood A, Ishaq M, Zhao L, et al. Impact of ultrasound and conventional extraction techniques on bioactive compounds and biological activities of blue butterfly pea flower (Clitoria ternatea L.) Ultrason Sonochem. 2019;51:12–19. doi: 10.1016/j.ultsonch.2018.10.013. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, Kumar V, Kumar NS, et al. The Ayurvedic medicine Clitoria ternatea-From traditional use to scientific assessment. J Ethnopharmacol. 2008;120:291–301. doi: 10.1016/j.jep.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Nair V, Bang WY, Schreckinger E, et al. Protective role of ternatin anthocyanins and quercetin glycosides from butterfly pea (Clitoria ternatea Leguminosae) blue flower petals against lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J Agric Food Chem. 2015;63:6355–6365. doi: 10.1021/acs.jafc.5b00928. [DOI] [PubMed] [Google Scholar]
- Neda GD, Rabeta MS, Ong MT. Chemical composition and anti-proliferative properties of flowers of Clitoria ternatea. Int Food Res J. 2013;20:1229–1234. [Google Scholar]
- Nguyen GKT, Zhang S, Nguyen NTK, et al. Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the Fabaceae family. J Biol Chem. 2011;286:24275–24287. doi: 10.1074/jbc.m111.229922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmal SA, Bhalke RD, Jadhav RS, et al. Anthelmintic activity of Clitoria ternatea. Pharmacologyonline. 2008;1:114–119. [Google Scholar]
- Nithianantham K, Ping KY, Latha LY, et al. Evaluation of hepatoprotective effect of methanolic extract of Clitoria ternatea (Linn.) flower against acetaminophen-induced liver damage. Asian Pac J Trop Dis. 2013;3:314–319. doi: 10.1016/s2222-1808(13)60075-4. [DOI] [Google Scholar]
- Oguis GK, Gilding EK, Jackson MA, et al. Butterfly pea (Clitoria ternatea), a cyclotide-bearing plant with applications in agriculture and medicine. Front Plant Sci. 2019;10:645. doi: 10.3389/fpls.2019.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect JR. Is there an emerging need for new antifungals? Expert Opin Emerg Drugs. 2016;21:129–131. doi: 10.1517/14728214.2016.1155554. [DOI] [PubMed] [Google Scholar]
- Pham TN, Nguyen DC, Lam TD, et al. Extraction of anthocyanins from Butterfly pea (Clitoria ternatea L. flowers) in Southern Vietnam: response surface modeling for optimization of the operation conditions. IOP Conf Ser Mater Sci Eng. 2019;542:012032. doi: 10.1088/1757-899x/542/1/012032. [DOI] [Google Scholar]
- Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4:89–96. [PMC free article] [PubMed] [Google Scholar]
- Phrueksanan W, Yibchok-anun S, Adisakwattana S. Protection of Clitoria ternatea flower petal extract against free radical-induced hemolysis and oxidative damage in canine erythrocytes. Res Vet Sci. 2014;97:357–363. doi: 10.1016/j.rvsc.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Rabeta MS, An Nabil Z. Total phenolic compounds and scavenging activity in Clitoria ternatea and Vitex negundo linn. Int Food Res J. 2013;20:495–500. [Google Scholar]
- Rajamanickam M, Kalaivanan P, Sivagnanam I. Evaluation of anti-oxidant and anti-diabetic activity of flower extract of Clitoria ternatea L. J Appl Pharm Sci. 2015;5:131–138. doi: 10.7324/japs.2015.50820. [DOI] [Google Scholar]
- Ranaganayaki S, Singh AK. Isolation and identification of pigments of the flowers of Clitoria ternatea. J Indian Chem Soc. 1979;56:1037–1038. [Google Scholar]
- Reid R, Sinclair DF (1980) An evaluation of a collection of Clitoria ternatea for forage and grain production. CSIRO, Division of Tropical Crops & Pastures; 1980. ISSN: 01596071
- Ruenroengklin N, Zhong J, Duan X, et al. Effects of various temperatures and pH values on the extraction yield of phenolics from litchi fruit pericarp tissue and the antioxidant activity of the extracted anthocyanins. Int J Mol. 2008;9:1333–1341. doi: 10.3390/ijms9071333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N, Abe K, Honda T, et al. Acylated delphinidin glucosides and flavonols from Clitoria ternatea. Phytochemistry. 1985;24:1583–1586. [Google Scholar]
- Scheffler RJ, Colmer S, Tynan H, et al. Antimicrobials, drug discovery, and genome mining. Appl Microbiol Biotechnol. 2013;97:969–978. doi: 10.1007/s00253-012-4609-8. [DOI] [PubMed] [Google Scholar]
- Senica M, Stampar F, Petkovsek MM. Different extraction processes affect the metabolites in blue honeysuckle (Lonicera caerulea L. subsp. edulis) food products. Turk J Agric For. 2019;43:576–585. doi: 10.3906/tar-1907-48. [DOI] [Google Scholar]
- Senkal BC, Uskutoglu T, Cesur C, et al. Determination of essential oil components, mineral matter, and heavy metal content of Salvia virgata Jacq. grown in culture conditions. Turk J Agric For. 2019;43:395–404. doi: 10.3906/tar-1812-84. [DOI] [Google Scholar]
- Shen Y, Du L, Zeng H, et al. Butterfly pea (Clitoria ternatea) seed and petal extracts decreased Hep-2 carcinoma cell viability. Int J Food Sci Technol. 2016;51:1860–1868. doi: 10.1111/ijfs.13158. [DOI] [Google Scholar]
- Shyamkumar IB, Ishwar B. Anti-inflammatory, analgesic and phytochemical studies of Clitoria ternatea Linn flower extract. Int Res J Pharm. 2012;3:208–210. [Google Scholar]
- Singh NK, Garabadu D, Sharma P, et al. Anti-allergy and anti-tussive activity of Clitoria ternatea L. in experimental animals. J Ethnopharmacol. 2018;224:15–26. doi: 10.1016/j.jep.2018.05.026. [DOI] [PubMed] [Google Scholar]
- Sinha K, Saha PD, Ramya V, et al. Improved extraction of natural blue dye from butterfly pea using microwave assisted methodology to reduce the effect of synthetic blue dye. Int J Chem Technol. 2012;4:57–65. doi: 10.3923/ijct.2012.57.65. [DOI] [Google Scholar]
- Siti Azima AM, Noriham A, Manshoor N. Anthocyanin content in relation to the antioxidant activity and colour properties of Garcinia mangostana peel, Syzigium cumini and Clitoria ternatea extracts. Int Food Res J. 2014;21:2369–2375. [Google Scholar]
- Sivaranjan VV, Balachandran I. Ayurvedic drugs and their plant sources. New Delhi: Oxford & IBH Publishers Pvt., Ltd.; 1994. [Google Scholar]
- Sreejith S, Samant MP, Jakhar JK, et al. Modeling the impact of extraction conditions on functional properties of gelatin from scales of blackspotted croaker (Protonibea diacanthus) Proc Natl A Sci India B. 2014;84:1021–1029. doi: 10.1007/s40011-013-0259-6. [DOI] [Google Scholar]
- Srichaikul B. Ultrasonication extraction, bioactivity, antioxidant activity, total flavonoid, total phenolic and antioxidant of Clitoria ternatea linn flower extract for anti-aging drinks. Pharmacogn Mag. 2018;14:322. doi: 10.4103/pm.pm_206_17. [DOI] [Google Scholar]
- Subramanian MS, Prathyusha P. Pharmaco-phytochemical characterization of Clitoria ternatea Linn. Int J Pharmtech Res. 2011;3:606–612. [Google Scholar]
- Terahara N, Saito N, Honda T, et al. Structure of ternatin D1, an acylated anthocyanin from Clitoria ternatea flowers. Tetrahedron Lett. 1989;30:5305–5308. doi: 10.1016/s0040-4039(01)93771-2. [DOI] [Google Scholar]
- Terahara N, Oda M, Matsui T, et al. Five new anthocyanins, ternatins A3, B4, B3, B2, and D2, from Clitoria ternatea flowers. J Nat. 1996;59:139–144. doi: 10.1021/np960050a. [DOI] [PubMed] [Google Scholar]
- Terahara N, Toki K, Saito N, et al. Eight new anthocyanins, ternatins C1–C5 and D3 and preternatins A3 and C4 from young Clitoria ternatea flowers. J Nat. 1998;61:1361–1367. doi: 10.1021/np980160c. [DOI] [PubMed] [Google Scholar]
- Uma B, Prabhakar K, Rajendran S. Phytochemical analysis and antimicrobial activity of Clitorea ternatea Linn against extended spectrum beta lactamase producing enteric and urinary pathogens. Asian J Pharm Clin Res. 2009;2:94–96. [Google Scholar]
- Wen C, Zhang J, Zhang H, et al. Advances in ultrasound assisted extraction of bioactive compounds from cash crops: a review. Ultrason Sonochem. 2018;48:538–549. doi: 10.1016/j.ultsonch.2018.07.018. [DOI] [PubMed] [Google Scholar]
- Zakaria NNA, Okello EJ, Howes MJ, et al. In vitro protective effects of an aqueous extract of Clitoria ternatea L. flower against hydrogen peroxide-induced cytotoxicity and UV-induced mtDNA damage in human keratinocytes. Phytother Res. 2018;32:1064–1072. doi: 10.1002/ptr.6045. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Pang X, Ji Z, et al. Role of anthocyanin degradation in litchi pericarp browning. Food Chem. 2001;75:217–221. doi: 10.1016/s0308-8146(01)00202-3. [DOI] [Google Scholar]