Abstract
Healthy food trend is becoming popular these days fueling search for ingredients empowered by pharma-nutritional benefits. In contrast, numerous wild-growing mushrooms are traditionally cherished as health promoting gastronomies in India; although credibility of their effects has so far been limited. Hence the present study aimed to unveil a unique tribal cuisine, Russula alatoreticula, with nutritional, chemical and pharmacological relevance. The outcome demonstrated an excellent alimentary composition with carbohydrate and protein as prominent macronutrients in contrast to fat providing oleic acid (36.66%), linoleic acid (16.84%), palmitic acid (16.01%) and stearic acid (15.31%) indicative of profitable nutritive account. Conversely, ethanolic fraction enriched with phenolics (pyrogallol > cinnamic acid) presented effective antioxidant property in terms of radical scavenging, Fe2+ chelating and reducing power with EC50 ranging from 785 to 2500 μg/ml. Remarkable antibacterial activity was also noted against the tested microorganisms (MIC of 72.5–1560 μg/ml) preferentially targeting Gram-positive one. Besides treatment of the preparation rendered Hep3B proliferation as evident by phenotypic changes, cell cycle interference, reactive oxygen species generation, mitochondrial membrane potential reduction, DNA fragmentation, change in Bax/Bcl2 ratio and activation of caspase9 signifying induction of intrinsic mitochondrial pathway. Thus the study represents R. alatoreticula as a value-added bio-resource that could be featured in food and pharmaceutical industries for betterment of humankind.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04723-9) contains supplementary material, which is available to authorized users.
Keywords: Fatty acid composition, Hep3B human liver cancer, High performance thin layer chromatography (HPLC), Intrinsic mitochondrial pathway, Newly-found ethnic macrofungus
Introduction
Today more and more people are leaning towards healthy meals that are not only appealing to taste buds but also good for body (Wahl et al. 2017). In that essence, naturally grown mushrooms are long being appraised for aroma, taste, flavor, nutritional and nutraceutical effects particularly by indigenous people; even though research on them is virtually scarce. One of such matrices has recently been introduced in our previous publication as Russula alatoreticula K. Acharya, S. Khatua, A. K. Dutta & S. Paloi, sp. nov. that grows at the forest floor of lateritic region in West Bengal, India (Khatua et al. 2017a). During rainy season, the taxon produces fairly large basidiocarps with intriguing red coloured pileus making them easily recognizable in natural habitat. Indeed, the species is renowned among local people in the name of “Murgi Chhatu” (“Murgi” means hen, “Chhatu” depicts mushrooms) as shade of cap resembles comb of hen. In this connection it is worth mentioning that the mushroom has been a perennial component of indigenous diet for eons due to traditionally known health benefits (Khatua and Acharya 2019). However, such ancestral mycophagy communities spend their entire lifetime mainly in the jungle territory isolated from mainstream societies. As a result, ethnically gathered aesthetic value of the natural resource remains confined to the inhabiting rural areas (Khatua and Acharya 2019). Hence, consumption of R. alatoreticula is very limited in elite societies being neither prized in urban cuisine nor commercially exploited as food (Khatua et al. 2018, 2019). Intense scientific exploration on the macrofungus is thus highly needed not just to reduce gap between our traditional practice and contemporary life-style but also to convey clear-cut information regarding edibility of the mushroom to civilized folk. The effort to raise awareness may leave a permanent mark on economic activities as edible wild mushrooms are retailed with higher prices in market than the cultivated taxa due to their unique aroma and texture. In that scenario, investigation on nutrient and therapeutic status of R. alatoreticula would be very relevant as the attempt might enhance its scientific concern and societal use.
Research conducted during the last decade has indicated culinary macromycetes, particularly wild-growing ones, as “powerhouse of nutrition” being enriched in carbohydrate, good quality protein and crude fiber (Dimitrijevic et al. 2018). However macrofungi provide minute amount of fat making them low-energy functional foods which could definitely contribute to healthy diet. The profile can be compared with egg, meat and milk suggesting intake of mushrooms in regular basis not only satisfy hunger but also provide required nutrients (Wang et al. 2014).
Along with the immense alimentary supremacy, wild basidiomycetes are also considered as reservoir of many different nutraceuticals such as phenolic compounds possessing multi-functional, health advantageous properties (Huang et al. 2009). Phenolics can efficiently be isolated from natural bio-resources using polar solvents as “like dissolves like”. Amongst them, ethanol is considered as a conventional medium owing to its easy accessibility, safety for consumption and high affinity for bioactivity compared with other organic solvents. A large body of evidence conclusively supports that ethyl alcohol extracted low molecular weight compounds possess a number of therapeutic effects including antioxidant, antibacterial and anticancer capacities (Tungmunnithum et al. 2018; Shomali et al. 2019). Indeed researchers are looking for bio-based ingredients to overlap disadvantages of existing synthetic compounds which are considered to be hazardous not only to human health but also environment. Consequently wild and unexplored myco-resources may offer new avenues providing either stand-alone or combinatorial alternatives (Khatua et al. 2017c; Shomali et al. 2019).
In this backdrop, our research team has developed several works to draw attention on traditionally cherished yet neglected macrofungi by documenting their ethnic importance, basic composition and pharmacological properties (Khatua and Acharya 2019; Khatua et al 2019). Likewise, the present work also aimed to contribute above mentioned goal since it explored nutritional traits of a newly discovered mushroom, R. alatoreticula, to validate its ethnic consumption. In addition to that, an ethanol extract was prepared from dried basidiocarps and characterized in terms of bioactive compounds as well as medicinal effects.
Materials and methods
Collection of basidiocarps and authentication
Fruit bodies of R. alatoreticula were collected under Sal tree in lateritic areas of West Bengal during monsoon season. Identification of the gathered taxon was accomplished after thorough characterization based on morphology and phylogenetic position. Subsequently, representative voucher specimen (Accession No: CUH AM 114) was deposited in Calcutta University Herbarium (CUH) (Khatua et al. 2017a).
Proximate composition
The specimen was analyzed for its basic composition including moisture, ash, protein, fat and carbohydrate, according to AOAC (1995). The moisture content was determined by further heating of dried sample at 105 °C overnight until constant weight. Ash content was estimated by weighing the incinerated residue obtained at 550 °C for 24 h. Amount of crude protein (N × 4.38) was quantified with the help of KEL-PLUS autokjeldahl analyzer (Pelican Eqipments, Model number: Distyl-EM (VA), Chennai, India), by employing converting factor 4.38. While fat content was enumerated by extracting a known weight of powdered sample with petroleum ether. Further, extent of total carbohydrate was calculated by difference: total carbohydrate (g) = 100 − (g of protein + g of fat + g of ash). Energy was computed according to the following equation: total energy (kcal) = 4 × (g crude protein + g total carbohydrate) + 9 × (g crude fat).
Quantitative and qualitative determination of lipid class
The lipid was extracted from grounded mass using petroleum ether at room temperature. The isolated extract was then fractionated in silica gel coated TLC plate with hexane:diethyl ether (90:10) as a developing solvent. The triglyceride and sterol fractions were quantitatively re-extracted from TLC plate with the help of hexane. Weight of the samples collected after solvent removal was measured gravimetrically and finally the percentage of collected samples was noted. Phospholipids content of sample was determined spectrophotometrically following the protocol as described by Chen et al. (1956).
Fatty acid profiling
About 200 mg of sample oil was hydrolyzed with 0.5 N NaOH in methanol (50 ml) at reflux temperature. Afterward, BF3-methanol reagent (5 ml) and hexane (2 ml) were poured sequentially followed by boiling for 1 min after each addition. The mixture was cooled and then 15 ml of saturated NaCl solution was added. Hexane solution of methyl-esters at the top was extracted and transferred into a test tube with anhydrous Na2SO4 (Pedneault et al. 2006). One µl hexane solution was injected in gas chromatograph (Agilent 6890n, USA), fitted with a DB-wax capillary column (30 m × 0.25 mm I.D.) and flame ionization detector (250 °C). N2, H2 and airflow rates were maintained at 1, 30 and 300 ml/min respectively.
Preparation of ethanol extract
Ten gm of the powdered fruit bodies were steeped in 200 ml of absolute ethanol under shaking condition of 150 rpm. Residue was then filtered through Whatman No. 4 and re-extracted with 100 ml of ethanol. The combined ethanolic fractions were diminished in volume using rotary evaporator (Rotavapor R-3, Butchi, Switzerland).
Chemical characterization of ethanol extract
Determination of total phenol, flavonoid, carotenoid and ascorbic acid content
To quantify total phenolic compounds, extract solution was mixed with Folin-Ciocalteu reagent and sodium carbonate. Amount of total flavonoid content was determined using the fraction, aluminium nitrate, potassium acetate and 80% aqueous ethanol. Content of carotenoids including β-carotene and lycopene were estimated by mixing the preparation with acetone:hexane mixture and then absorbance was measured. Presence of ascorbic acid in the formulation was determined following a titration method (Khatua et al. 2018).
HPLC analysis of phenolic compounds
Ten mg of dried fraction was dissolved in one ml of HPLC grade methanol, filtered and 20 μl of that filtrate was then analysed by HPLC (Agilent, USA). Separation was performed with Agilent Eclipse Plus C18 column (100 mm × 4.6 mm, 3.5 μm). Elution was carried out by using acetonitrile (eluent A) and 0.1% phosphoric acid solution (eluent B) in a gradient procedure: 0–2 min, 5% A; 5–10 min, 15% A; 10–15 min, 40% A; 15–20 min, 60% A; 20–22 min, 90% A (Khatua et al. 2018).
Evaluation of antioxidative property
To determine 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, reaction mixture was prepared in 96-well plate followed by detection of final colour at 595 nm using microplate reader (Bio-Rad iMarkTM Microplate Reader, USA). To estimate 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical quenching effect, freshly prepared ABTS·+ was allowed to react with the fraction in microtiter plate and optical density was noted. For metal ion chelating assay; ferrous chloride, the formulation and ferrozine were added in 96-well plate. Absorbance was estimated at 595 nm after 10 min incubation. To assess reducing power, the preparation at different concentrations was mixed with K3[Fe(CN)6, TCA and FeCl3 where absorbance was enumerated at 750 nm using microplate reader (Khatua et al. 2017b). Finally, total antioxidant capacity was determined as described in our previous publication (Khatua et al. 2017a).
Estimation of antibacterial potential
The six investigating bacteria namely Listeria monocytogenes ATCC® 19111™, Staphylococcus aureus ATCC® 700699™, Bacillus subtilis ATCC® 6633™, Klebsiella pneumoniae ATCC® 15380™, Salmonella typhimurium ATCC® 23564™ and Escherichia coli ATCC® 25922™ were purchased from MTCC, Chandigarh, India. Reactions were performed in 96 well plate and incubated for 24 h. Afterwards p-iodonitrotetrazolium chloride (INT) dye was added followed by measuring absorbance at 595 nm (Khatua et al. 2017a).
Determination of anticancer activity
Cell culture and maintenance
Hep3B (human hepatoma cell line) and BRL-3A (normal rat liver cell line), purchased from NCCS, Pune, India, were routinely maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum (FBS), 0.25% amphotericin B and 0.5% PenStrep.
Cytotoxicity, clonogenic cell survival and wound healing assays
Cells (1 × 103 cells/well) were treated with various concentrations of the extract for 24 h. After that, water soluble tetrazolium (WST) was added and finally optical density was measured at 450 nm. DMSO was used to dilute the ethanol fraction; however the solvent was added in each well at the concentration not more than 0.05% (v/v). For clonogenic protocol, cells were treated with the formulation for 24 h, then cells were placed in fresh media and plate was returned to the incubator for 5–6 doubling. For enumeration of colonies, fixed cells were stained by 0.1% crystal violet. In order to perform wound healing assay, Hep3B cells were scraped in straight line to create a scratch. The preparation was added at different dosages and healing of the wound was assessed after 24 h incubation (Khatua et al. 2019).
Detection of cell death by morphological analysis
The treated and untreated cells were permeabilized and incubated with one μg/ml of 4′,6-diamidino-2-phenylindole (DAPI) solution. Changes in nuclear morphology were photographed with the help of fluorescent microscope (FLoid® Cell Imaging Station, Life Technologies, Carlsbad, CA, USA). For acridine orange/ethidium bromide (AO/EB) dual staining method, cells were exposed to the fraction for 24 h and suspended with 100 μl of AO/EB staining solution (Khatua et al. 2019). Intensity of fluorescent staining was observed and images were captured.
Cell cycle analysis
After 24 h treatment, cells were centrifuged and pellet was then fixed in 75% ethanol. Next day, cells were resuspended in 0.2 mg/ml RNase for one h and then propidium iodide (PI) staining solution was added. Cell cycle distribution was monitored by flow cytometry (BD Bioscience, San Jose, CA, USA) and results were analyzed with BD CellQuest Pro software.
Detection of DNA fragmentation by terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay
Cells were incubated with the preparation for 24 h and then permeabilised with 70% ethanol. Following centrifugation, broken DNA fragments were labelled at per manufacturer’s instruction using TdT enzyme and FITC-dUTP (APO-DIRECT™ Kit, BD Pharmingen, San Diego, CA, USA). At the end of incubation time, rinse buffer was added and the cell pellet was suspended in PI/RNase staining buffer. Cells were incubated at room temperature for 30 min and immediately analyzed by a flow cytometer.
Measurement of intracellular ROS and MMP
Hep3B cells were incubated with the extract for 30 min and 2′,7′-dichlorofluorescin diacetate (DCFDA) dye was added to each experimental set. Finally intracellular ROS levels were measured using flow cytometry as mentioned above. The same method was repeated for estimation of MMP where cells were treated for 24 h. Following incubation, cells were suspended in PBS containing 5,5′,6,6′-tetrachloro1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) (10 μg/ml) for 15 min and the stained cells were detected by flow cytometry (Czarnomysy et al. 2016).
Analysis of apoptosis related gene expression by reverse transcription polymerase chain reaction (RT-PCR)
Reverse transcription was executed with RNA with the help of RT-and GO Mastermix (Two-Step Reverse Transcriptase-PCR Kit from MP Biomedicals, Santa Ana, CA, USA) at per manufacturer’s instruction. To analyze the expression of specific gene, PCR was performed as described by Khatua et al. (2019). The sequences of PCR primers are enlisted in Supplementary Table 1. The PCR cycle conditions were as follows: 95 °C for 4 min, 35 cycles of 94 °C for 20 s, annealing temperature for specific primer for 30 s and 72 °C for 45 s with 7 min final extension step at 72 °C using thermal cycler (Applied Biosystems, Foster City, CA, USA). For each gel, ImageJ software v. 1.46r was applied for numerical assessment of band strength.
Statistical analysis
All results presented herein are expressed as mean ± SD from three experiments (n = 3). Differences in mean values between the groups were analyzed by one-way analysis of variance (ANOVA) with post-hoc Tukey HSD test using IBM SPSS statistics for Windows, v. 23.0. (IBM Corp., Armonk, NY, USA).
Results
Proximate composition
The analysis of nutrient profile (Table 1) enumerated high level of water content in R. alatoreticula indicating perishable nature of the specimen. Carbohydrate was detected as the predominate macronutrient followed by protein. In contrast total fat was enumerated in trace; as a whole, low energy was attributed pointing apparent consideration of the studied specimen as dietetic food. Nevertheless sufficient amount of crude fibre was also determined which was at per the ash quantity. Overall the indigenous myco-food under investigation could be considered as an ideal dietary supplement for a broad range of consumers as it takes a position of nutritional excellence.
Table 1.
Proximate composition of Russula alatoreticula (mean ± SD)
| Name of mushroom | Moisture (g/100 g dry weight) | Carbohydrate (g/100 g dry weight) | Protein (g/100 g dry weight) | Fat (g/100 g dry weight) | Crude fibre (g/100 g dry weight) | Ash (g/100 g dry weight) | Energy (Kcal/100 g dry weight energy) | References |
|---|---|---|---|---|---|---|---|---|
| Russula alatoreticula | 91.33 ± 1.17 | 63.56 ± 1.32 | 22.34 ± 0.97 | 2.11 ± 0.12 | 10.06 ± 1.45 | 11.99 ± 0.17 | 362.59 ± 1.97 | _ |
| Russula brevipes | 7.67 | 40.81 | 31.63 | 3.46 | ND | 16.43 | 253.84 | Sharma et al. (2017) |
| Russula cyanoxantha | 7.39 | 63.24 | 19.84 | 1.7 | ND | 7.83 | 286.28 | Sharma et al. (2017) |
| Russula heterophylla | 6.89 | 46.11 | 26.36 | 5.44 | ND | 15.2 | 275.05 | Sharma et al. (2017) |
| Russula lepida | ND | 34.15 | 12.1 | 0.28 | 1.19 | 0.17 | 188.72 | Sharma and Gautam (2015) |
| Russula mairei | ND | 36.4 | 11.03 | 0.22 | 1.38 | 0.13 | 191.7 | Sharma and Gautam (2015) |
| Russula virescens | 92.49* | 62.27 | 21.85 | 1.85 | ND | 11.04 | 365.09 | Sharma et al. (2017) |
ND Not detected
*g/100 g fresh weight
Fatty acid profiling
Crude fat in macrofungi includes several kinds of lipid compounds like sterol, free fatty acid, sterol ester, mono, di and triglyceride as well as phospholipid (Pedneault et al. 2006). Thus the present study also aimed to characterize lipid profile of R. alatoreticula where triglyceride, sterol, phospholipid and fatty acids were detected in the amount of 33.9 ± 2.35%, 5.6 ± 1.26%, 43.3 ± 3.08% and 17.2 ± 2.3% w/w respectively. Further research illustrated presence of approximately 20 different fatty acids in the mushroom where carbon chain lengths varied from 6 to 24 (Supplementary Table 2). Out of them, oleic acid was quantified in the major proportion followed by linoleic acid, in sum accounting 53.5 ± 0.18% of total fatty acid. Conversely, palmitic and stearic acids were also encountered representing a major proportion of saturated fatty acids. Besides, it would be worth to note that both omega 3 and 6 types were identified in the profile; alongside the level of unsaturated fatty acid was detected in superior extent satisfying fat intake regulations.
Chemical characterization of ethanol extract
Determination of total phenol, flavonoid, carotenoid and ascorbic acid content
To predict downstream application of R. alatoreticula, an ethanol fraction was extracted that appeared light yellow in colour with sufficient recovery percentage (Table 2). Further, extent of major bioactive compounds was measured (Table 2) and studies on quantitative composition revealed phenolic substances as the most prevailing constituent. Among the cluster, total flavonoid content in the formulation was found to be nearly half of the phenolics. On the other hand, modest amount of vitamin C was identified in the formulation. Considering total carotenoid content, only traces of β-carotene and lycopene were detected.
Table 2.
Extractive yield and chemical characterization of ethanol fraction isolated from Russula alatoreticula
| Parameters | Russula alatoreticula |
|---|---|
| Extractive yield (%) | 5 ± 1 |
| Phenol (μg gallic acid equivalent/mg of dry extract) | 6.63 ± 0.49 |
| Flavonoid (μg quercetin equivalent/mg of dry extract) | 3.45 ± 2.6 |
| β-carotene (μg/mg of dry extract) | 0.05 ± 0.02 |
| Lycopene (μg/mg of dry extract) | 0.04 ± 0.01 |
| Ascorbic acid (μg/mg of dry extract) | 2.44 ± 0.05 |
HPLC analysis of phenolic compounds
Beside quantification of phenol by spectrophotometry, the present study also aimed for HPLC profiling to procure phenolic fingerprint and predict the molecular constituents. The chromatogram of R. alatoreticula fraction indicated existence of at least twelve compounds of which two were tentatively recognized (Supplementary Fig. 1). Quantification analysis indicated pyrogallol as the most dominant component (4 ± 0.09 μg/mg of extract) manifested in the extract followed by cinnamic acid (0.15 ± 0.01 μg/mg of extract).
Evaluation of antioxidative property
A number of methods based on different principles have been used in the present work to determine antioxidant effect of ethanol fraction from R. alatoreticula where the outcome has been demonstrated in Table 3. Firstly, DPPH· scavenging assay was performed being the most easy method for estimation of antioxidant activity. At the level of 100, 500 and 1000 μg/ml, the extract quenched radicals at the rate of 2.17 ± 0.09%, 25.34 ± 1.05% and 63.14 ± 2.73% respectively (Supplementary Fig. 2a). Further to that, ABTS·+ scavenging potential was also evaluated for assessment of antioxidant activity of the studied extract. The fraction quenched 11.63 ± 0.87% and 47.13 ± 1.57% radicals at concentrations of 100 and 1000 μg/ml respectively. When the level was alleviated to 2000 μg/ml, 65.97 ± 1.98% radical inhibition was found (Supplementary Fig. 2b). Another popular method for determination of antioxidant activity is Fe2+ chelating assay that illuminated affinity towards metal ion of the investigated drug. At the dosages of 100, 1000, and 2000 μg/ml, R. alatoreticula preparation was able to bind with 1.16 ± 0.02%, 29.92 ± 1.16% and 68.78 ± 2.81% of ferrous ions respectively (Supplementary Fig. 2c). Conferring to outcome of ferricyanide/prussian blue assay, the fraction from R. alatoreticula demonstrated reasonable reducing power which increased with escalation of concentrations. At the level of 1500, 2000 and 2500 μg/ml, the extract exhibited reducing power of 0.26 ± 0.01, 0.43 ± 0.02 and 0.5 ± 0.01 respectively (Supplementary Fig. 2d). Finally, phosphomolybdenum method was performed for evaluation of total antioxidant capacity where the preparation exhibited effective activity.
Table 3.
Antioxidant and antibacterial activities of ethanol fraction isolated from Russula alatoreticula as represented by EC50 and MIC values (μg/ml) respectively
| Parameters to detect bioactivity | Russula alatoreticula | Standard | |
|---|---|---|---|
| Antioxidant activity | |||
| EC50 value | Scavenging ability of DPPH radicals | 785 ± 40a | 7.45 ± 0.02b |
| Scavenging ability of ABTS radicals | 1300 ± 32a | 3.65 ± 0.02b | |
| Chelating ability of ferrous ion | 1500 ± 27a | 2.54 ± 0.52b | |
| Reducing power | 2500 ± 37a | 32.21 ± 6.91b | |
| Total antioxidant activity (μg ascorbic acid equivalent/mg of dry extract) | 5.45 ± 0.12 | Not applicable | |
| Antibacterial effect | |||
| Gram positive | Listeria monocytogenes | 226.5 ± 58.5a | 4.68 ± 0.17b |
| Staphylococcus aureus | 88.7 ± 20.22a | 6.29 ± 0.16b | |
| Bacillus subtilis | 72.5 ± 23.33a | 5.61 ± 0.01b | |
| Gram negative | Escherichia coli | 156.23 ± 25.13a | 5.41 ± 0.11b |
| Salmonella typhimurium | 289 ± 15.36a | 5.09 ± 0.03b | |
| Klebsiella pneumoniae | 1560 ± 45.68a | 5.29 ± 0.14b | |
BHA was used as a standard in all antioxidant assays except chelating ability of ferrous ion and total antioxidant capacity methods where EDTA and ascorbic acid were considered as positive controls respectively. Streptomycin was used as a reference in antibacterial methods. In each row, dissimilar letters denote significant alterations between sample and standard (p < 0.05)
Estimation of antibacterial potential
Following the microplate protocol, pathogen susceptibility test was carried out in the present study using three Gram positive microbes namely L. monocytogenes, S. aureus and B. subtilis along with three Gram negative bacteria such as E. coli, S. typhimurium and K. pneumoniae. These microorganisms are known as causal agents for respiratory and intestinal infections in humans, aside from concern with food quality degradation. Results showed that the ethanol fraction from R. alatoreticula inhibited all the tested pathogens, suggesting a broad antimicrobial activity in a concentration-dependent manner. According to Table 3, it could be said that S. aureus, B. subtilis, S. typhimurium and E. coli were quite vulnerable, as the MIC values were lower than 300 μg/ml. Whilst, K. pneumoniae appeared as more resistant bacteria as relatively higher concentration of the extract was required to inhibit its growth. Overall, the formulation under investigation presented a pronounced activity with special affinity towards Gram-positive bacteria.
Determination of anticancer activity
Cytotoxicity, clonogenic cell survival and wound healing assays
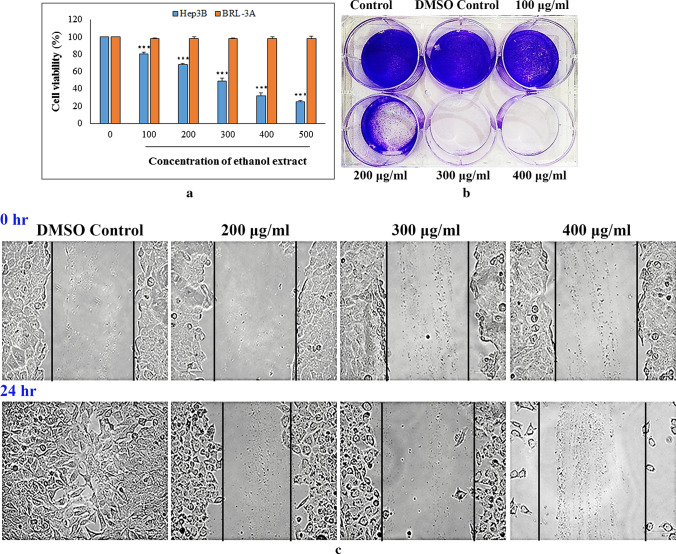
Cell viability study is a valuable tool for testing sensitivity of chemicals that also provides preliminary information for in vivo research. In this context, WST-8 was used in the present work to determine effect of ethanol extract from R. alatoreticula on Hep3B liver cancer cells. As portrayed in Fig. 1a, the evaluating formulation showed remarkable reduction in cellular growth within 24 h; however did not impose any toxic effect on BRL-3A cells indicating its selectivity for cancer. Considering Hep3B, treatment with 100, 300 and 500 μg/ml of the preparation dramatically reduced cell number by 19.5 ± 2.01%, 51.06 ± 3.2%, 75 ± 2% with a half-maximal inhibitory concentration of 300.6 ± 2.2 μg/ml. The value was found to be lower than the published GI50 data of methanol extract from R. alatoreticula (Khatua et al. 2019) indicating superior activity of the preparation under investigation. The observation was in accordance to other researchers conveying that absolute ethyl alcohol based formulations from mushrooms find the most extensive application in cancer intervention (Basli et al. 2017).
Fig. 1.
Effect of ethanol extract from Russula alatoreticula on Hep3B cell proliferation was estimated by a WST (***p < 0.001 vs. control) b colony-formation and c wound healing assays
Ability of liver hepatoma to survive after treatment with the studied fraction was further estimated using clonogenic survival or colony formation method. Result showed that there was a significant reduction of colonies in exposure with increasing concentration of the fraction (100–400 μg/ml), as compared to untreated cells. Indeed, in control sets cells divided many times and formed large colony, while cells gradually lost ability to divide in presence of the preparation from R. alatoreticula (Fig. 1b). Based on these outcomes, dosages of 200, 300 and 400 μg/ml of the formulation were selected for further experimentation.
Given that cell migration is a crucial step during angiogenesis, the wound healing assay was performed to monitor effect of the extract on hepatoma motility. As demonstrated in Fig. 1c, control cells proliferated to the opening of scratch completely and closed the area within a day. In contrast, a dramatic inhibition of wound closure was observed when cells were treated with the extract. Results thus indicated that the ethanol fraction was effective enough in restraining mobility of Hep3B cells in a dose-dependent manner, which might make it a potential drug for reverse of tumor metastasis.
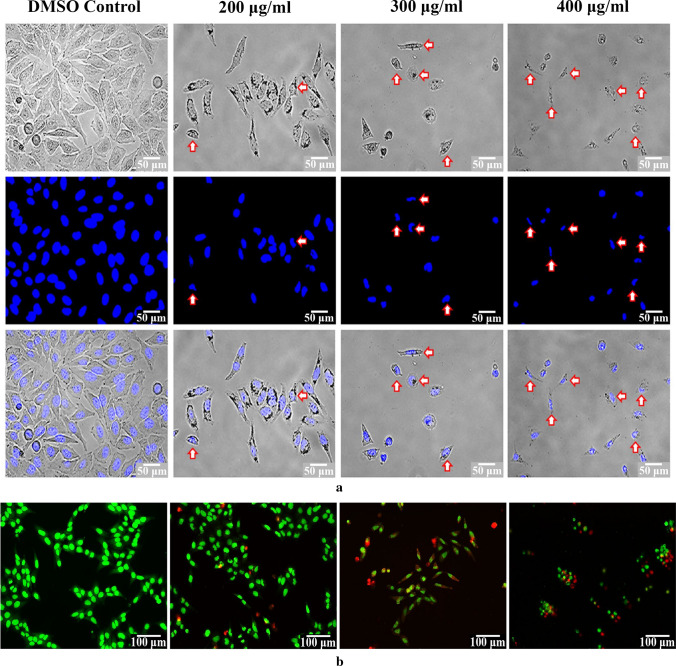
Detection of cell death by morphological analysis
In order to determine role of apoptosis in cell growth inhibition by the fraction, morphological changes in Hep3B cells were examined. Initial assessment by using inverted microscope illustrated vivid morphological changes in treated cells, like reduced cell density, cell shrinkage, roundness and irregular morphology in comparison with control (Fig. 2a). Fluorescent microscopy using DAPI also presented various alterations, such as nuclear fragmentation, membrane disruption and chromatin condensation indicating that the ethanol extract might have induced apoptotic cell death in Hep3B.
Fig. 2.
Effect of ethanol extract from Russula alatoreticula on cellular morphology of Hep3B cells was investigated using a DAPI and b acridine orange/ethidium bromide dual staining methods
Morphological changes of liver hepatoma incubated with the extract for 24 h at a range of concentrations were also analyzed by AO/EB double fluorescence staining. Microscopic scrutiny depicted that untreated cells were represented by round and intact nucleus with green fluorescence; whereas cells exposed to 200 and 300 µg/ml dosages of the preparation exhibited bright greenish yellow staining indicating early apoptotic phase. After treatment of the highest investigating level, morphology of cells was found to be transformed to cytoplasmic shrinkage and nuclear chromatin condensation. Presence of red coloured cells was also detected at these phases signifying late apoptotic condition (Fig. 2b). Thus, the sign of apoptosis was more prominent with increasing concentrations, where cell numbers were drastically reduced.
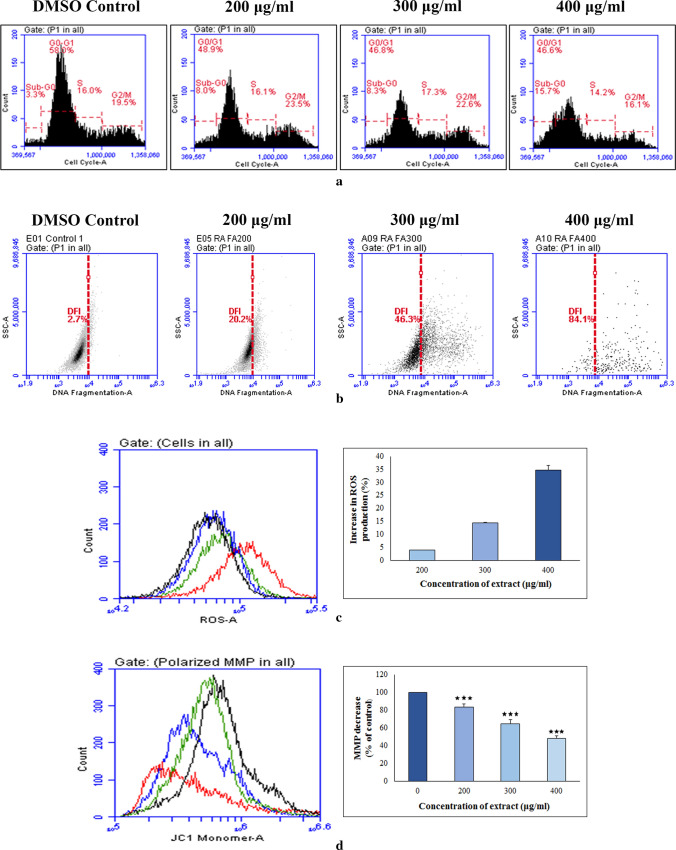
Cell cycle analysis
It is known that cells can be arrested at any stage of cycle in response to un-repairable damage and finally destined for death. The status of cell cycle arrest by ethanol extract on Hep3B was therefore monitored by flow cytometric study. The results showed that there were significant differences between treated and control groups with respect to percentage of cells at different phases (Fig. 3a). When cancer cells were exposed with the fraction, consequence was a marked accumulation of cells in sub-G0/G1 level. This finding thus let us assumed that the studied formulation from R. alatoreticula induced apoptosis by halting the cell cycle progression at subG0/G1 phase.
Fig. 3.
Effect of ethanol extract from Russula alatoreticula on a cell cycle distribution b DNA fragmentation c intracellular ROS generation (*p < 0.05, ***p < 0.001 vs. control) and d mitochondrial membrane depolarization (***p < 0.001 vs. control) on Hep3B cells was detected flow cytometrically
Detection of DNA fragmentation by TUNEL assay
One of the later step in apoptosis is DNA fragmentation that results due to activation of endogenous nucleases that cleave DNA pieces of around 50 bp with accessible 3’-OH. These free hydroxyl termini can be marked by incorporating labelled dUTP with the help of terminal deoxynucleotidyl and such staining is the principal of TUNEL assay (Kouassi et al. 2016). In the present study, no measurable effects on TUNEL staining were observed under normal growth condition. After incubation of Hep3B cells with the ethanol extract for 24 h, number of TUNEL-positive cells increased dose dependently (Fig. 3b), suggesting that the treatment resulted DNA disintegration in the cancer cells, which is a hallmark of apoptosis.
Measurement of intracellular ROS and MMP
ROS has been suggested as an important mediator for apoptosis and its elevated level is sufficient to trigger cell death. Thus to study impact of the fraction on oxidative stress, intracellular ROS production in Hep3B cells was measured by DCFDA probe as it produces 2′,7′-dichlorofluorescein (DCF) after reacting with ROS (Khatua and Acharya 2019). As illustrated in Fig. 3c, DCF fluorescence increased after exposure to the ethanol extract from R. alatoreticula suggesting induction of ROS production. Quantitative analysis revealed that the fraction at 200, 300 and 400 µg/ml concentrations elevated ROS generation by 3.97 ± 0.03%, 14.36 ± 0.87% and 34.62 ± 1.1% respectively in comparison with control cells.
Excessive ROS production can influence mitochondrial function and initiate that organelle intermediated cell death via disruption of MMP in cancer cells. According to the results, MMP reduction was encountered in presence of the ethanol extract dose dependently as evident by gradual decrease in fluorescence (Fig. 3d). Outcome presented that in case of 400 µg/ml concentration of the extract, MMP was lost by 48.01 ± 1.98% in respect to control cells. Overall, a parallel oscillation in radical production and MMP value recommended that the ethanol fraction induced intracellular ROS overproduction resulting mitochondrial dysfunction.
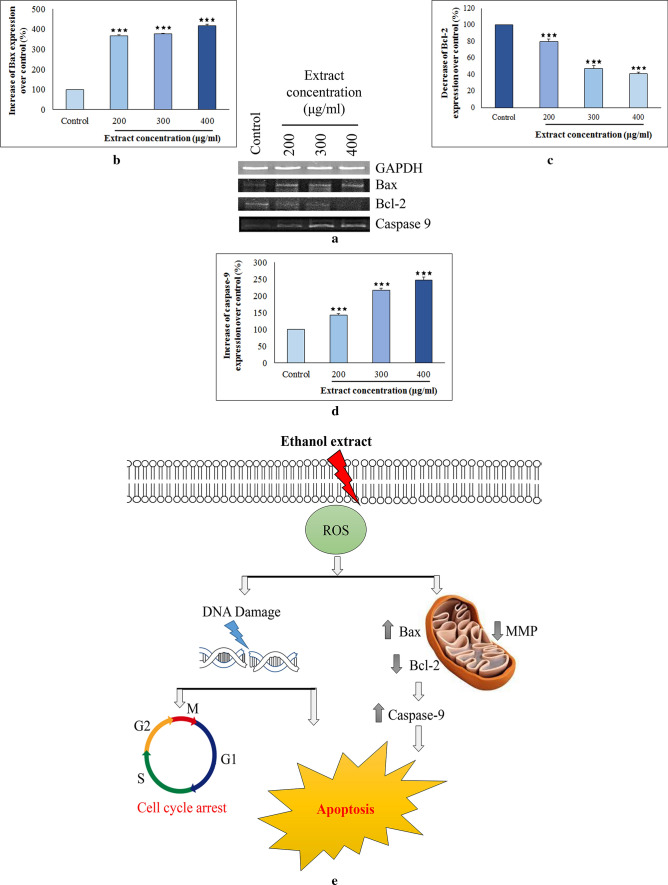
Analysis of apoptosis related gene expression and elucidation of mode of action
In general, intrinsic pathway is the most common mode of apoptosis that is activated within cell itself in response to stress initiated by damage-inducing agents like cytotoxic drugs. In this context, one of the foremost gene groups that control apoptosis is Bcl-2 family such as Bax and Bcl-2 which elicits contrasting effects on mitochondria. Enhancement of pro-apoptotic Bax over anti-apoptotic Bcl-2 can induce permeability of mitochondrial membrane. The phenomenon in turn activates caspases; a conserved group of enzymes that conclusively commits a cell to die (Basli et al. 2017). The current study as illustrated in Fig. 4a–d indicated that ethanol extract up-regulated mRNA expression of Bax and decreased level of Bcl-2, thus shifting Bax/Bcl-2 ratio in favour of apoptosis. Furthermore, pronounced stimulation of caspase-9 expression was also noticed indicating that the studied fraction induced apoptosis of Hep3B cells through intrinsic mitochondria-mediated pathway (Fig. 4e).
Fig. 4.
Influence of ethanol extract from Russula alatoreticula on Hep3B cells was determined with the help of a reverse transcriptase-PCR by assessing effect on transcription level of three different genes b Bax c Bcl-2 and d Caspase-9 (***p < 0.001 vs. control). e A diagrammatic representation
Discussion
The genus Russula is known as one of the most widely distributed ectomycorrhizal agaric group; of which many members have culinary use being packed with flavor enhancer, gourmet appeal and health promoting effects (Khatua et al. 2018). Despite longstanding use in tribal communities, they still remain an anonymous group, particularly in the nutraceutical aspect. Thus, it is not only important to recognize these functional foods but also to utilize the beneficial aspects for betterment of society, before they disappear from the Earth. In that essence our study attempted to record nutritional characterization of a novel mushroom, R. alatoreticula. The mushroom was found to contain high amount of moisture where the value fit well within the range of 80–95%, as reported in case of other edible macrofungi. Literature survey also imply that basidiomycetes consist carbohydrate as the major substance in fruiting bodies ranging between 50 and 65% followed by protein (12–29.3%) and fat (1–6.7%) (Wang et al. 2014). Accordingly, the study revealed carbohydrate as the main component in the studied specimen which was in higher extent than that of Russula delica, Russula brevipes, Russula heterophylla, Russula virescens and Russula vesca (Pushpa and Purushothoma 2010; Singdevsachan et al. 2014; Sharma and Gautam 2015). On the other hand, protein content in the taxon was found to be better than Russula aurea, Russula integra and Russula cyanoxantha (Agrahar-Murugkar and Subbulakshmi 2005; Leal et al. 2013; Sharma et al. 2017). Contrarily, the macrofungus under investigation consisted fat in lower amount than Russula lepida, Russula mustelina and Russula delica (Kouassi et al. 2016). Overall total energy content was found to be slightly lower than other wild growing mushrooms (Wang et al. 2014) suggesting profitable nutritive account of R. alatoreticula.
Apart from macronutrient profiling, interest in fatty acid composition is also currently expanding as macrofungi provide essential entities including n3 and n6 series of polyunsaturated one that affect growth, development and nutrition-related chronic diseases (Pedneault et al. 2006). In this context, limited research has been performed previously focusing on Russulaceous fungi where they have been reported to encompass high amount of unsaturated fatty acids in comparison with saturated type. Indeed, oleic acid has been enumerated as the chief constituent in Russula anthracina, R. aurea, R. cyanoxantha and R. virescens followed by linoleic acid (Ribeiro et al. 2009; Ergönül et al. 2013; Leal et al. 2013) which is in accord to our observation. Literature study suggests that ingestion of these valuable components is quite essential as they are associated with decreased intra-myocellular lipid level, reduction of low-density lipoprotein in blood, improvement of inflammatory disorder and blood pressure controlling (Pereira et al. 2019).
Nevertheless, another main purpose of this work was to investigate effect of the prepared ethanolic extract from R. alatoreticula on bioactive status. For that, ethyl alcohol was used as an extractant solvent where the yield of the studied preparation was found to be superior to that of Hericium erinaceus and Cordyceps militaris (Phan et al. 2013). Quantitatively the fraction was detected to encompass different therapeutic substances presented in the order of phenol > flavonoid > ascorbic acid > β-carotene > lycopene. Comparison with previous studies revealed that the bioactive components were presented in greater amount in the formulation than that of Ganoderma lucidum (Rajasekaran and Kalaimagal 2011), R. lepida and R. mairei (Sharma and Gautam 2015; Khatua et al. 2019). Further to that, HPLC analysis was also performed where total eleven standard compounds were utilized that could be divided into three subclasses namely cinnamic acid and derivatives (ferulic, chlorogenic and p-coumaric acid), hydroxybenzoic acid and derivatives (gallic, salicylic, vanillic acid) and flavonoid (quercetin, myricetin). The fraction was purportedly consisted of pyrogallol and cinnamic acid which was in accord to earlier reports (Leal et al. 2013; Khatua et al. 2019; Khatua et al. 2018).
Interestingly the above-mentioned metabolites have been established to play enormous role behind therapeutic benefits of bio-resources, where the amounts are directly co-related to antioxidative properties. Indeed phenols of higher fungi are known to have outstanding property of delaying or suppressing spontaneous autoxidation of free radicals. On the other hand, β-carotene, lycopene and ascorbic acid have also been recognized as potent radical quenchers making them an extraordinary antioxidant compound (Carocho and Ferreira 2013). As a result, the studied formulation was found to be better radical quenchers than ethanolic fraction from Pleurotus flabellatus (Pumtes et al. 2016). Besides, the studied formulation also demonstrated a marked capacity for iron chelating ability which was superior to Pleurotus ostreatus (Jayakumar et al. 2009). On the other hand, total antioxidant capacity of ethanol fraction of R. alatoreticula was detected to be better than its methanol fraction (Khatua et al. 2019).
Besides antioxidant effect, such phenolic compounds have also been identified to possess strong antimicrobial power. The action results from an interaction between these constituents and cell membrane of targeted bacteria, possibly due to their ability to bind with soluble and extracellular proteins as well as cell walls (Araújo et al. 2012). In this context, previous studies revealed that the macrofungal secondary metabolites are able to control growth of several pathogens especially S. aureus, B. cereus, B. subtilis (among Gram-positive microbes), E. coli and K. pneumoniae (among Gram-negative bacteria) (Alves et al. 2012). Subsequently our finding is in accordance to the reports where the ethanolic fraction from R. alatoreticula presented efficient activity against all the tested microorganisms (B. subtilis > S. aureus > E. coli > L. monocytogenes > S. typhimurium > K. pneumoniae) with special affinity towards Gram-positive pathogens. This tendency could be clarified by the fact that Gram-negative bacteria retain outer membrane surrounding cell wall restricting diffusion of bioactive compounds (Araújo et al. 2012). Nevertheless comparative analysis with previous publication revealed that the studied preparation from R. alatoreticula was more aggressive against tested bacteria than ethyl alcohol extracts from Cordyceps sinensis, Laricifomes officinalis and Coprinus comatus (Hleba et al. 2016).
Natural products rich in antioxidative ingredients have great potential to prevent and treat various human degenerative diseases, including cancer. These secondary metabolites have been endorsed for antitumor, pro-apoptotic and antiangiogenic effects owing to the ability of suppression of cell proliferation and induction of apoptosis (Popović et al. 2013). Apoptosis or programmed cell death is demarcated by a set of typical morphological structures such as chromatin condensation, cell shrinkage and DNA destruction due to endonuclease activation, apoptotic body formation and loss of membrane integrity. Regulation system of apoptosis is also induced in mitochondria on the intrinsic pathway by several families of proteins, including Bcl-2 family, as well as membrane polarity and integrity. As a result, cell proliferation is suppressed which is a relevant strategy in current preventive approaches (Basli et al. 2017). Our study executed that treatment of the ethanol extract from R. alatoreticula augmented intracellular ROS generation resulting DNA damage and thus blocked cell cycle progression at sub G0/G1 phase. Conversely, the oxidative stress also induced overexpression of Bax altering balance between Bax and Bcl-2 that collapsed MMP. Further, activation of caspase-9 approved stimulation of the intrinsic pathway to trigger apoptosis and ultimately led to cell death. Thus in future, hope can come from R. alatoreticula which could be targeted of intense research for chemotherapeutic anticancer molecules.
Conclusion
Considering the results, R. alatoreticula could be acknowledged as a good source of nutrients where content of protein and carbohydrate stood out, in contrast to fat resulting low-caloric diet. The mushroom however showed healthier fatty acid profile with precious contribution of unsaturated substances including omega-3 and 6 types. Alongside the studied ethanol extract demonstrated excellent antioxidant and antibacterial efficacy. Of note, the formulation exhibited promising anticancer activity against Hep3B cells mediated through intrinsic mitochondrial pathway. Such remarkable multi-dimensional potency could be attributed to functional ingredients of the fraction such as phenolics, carotenoids and ascorbic acid. In sum, present study endorses immense health benefit and pharmaceutical potential of the novel myco-food recommending its use in wellness sectors so that such efficacious representative of traditional healthcare system no more remain abandoned.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors S.K. and K.A. would like to acknowledge the facilities provided by Department of Botany (UGC-CAS Phase VI, VII), University of Calcutta and DST-FIST for instrumental support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agrahar-Murugkar D, Subbulakshmi G. Nutritional value of edible wild mushrooms collected from The Khasi Hills of Meghalaya. Food Chem. 2005;89:599–603. doi: 10.1016/j.foodchem.2004.03.042. [DOI] [Google Scholar]
- Alves MJ, Ferreira IC, Dias J, Teixeira V, Martins A, Pintado M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012;78:1707–1718. doi: 10.1055/s-0032-1315370. [DOI] [PubMed] [Google Scholar]
- AOAC (1995) Official methods of analysis, 16th ed. Association of Official Analytical Chemists. Washington DC, USA
- Araújo MGF, Hilário F, Vilegas W, Santos LC, Brunetti IL, Sotomayor CE, Bauab TM. Correlation among antioxidant, antimicrobial, hemolytic, and antiproliferative properties of Leiothrix spiralis leaves extract. Int J Mol Sci. 2012;13:9260–9277. doi: 10.3390/ijms13079260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basli A, Belkacem N, Amrani I. Health benefits of phenolic compounds against cancers. In: Soto-Hernández M, Palma-Tenango M, Garcia-Mateos MR, editors. Phenolic compounds. London: IntechOpen; 2017. p. 752. [Google Scholar]
- Carocho M, Ferreira ICFR. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Chen PS, Toribari TY, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. doi: 10.1021/ac60119a033. [DOI] [Google Scholar]
- Czarnomysy R, Bielawski K, Muszynska A, Bielawska A, Gornowicz A. Biological evaluation of dimethylpyridine-platinum complexes with potent antiproliferative activity. J Enzyme Inhib Med Chem. 2016;31(3):150–165. doi: 10.1080/14756366.2016.1212191. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic MV, Mitic VD, Jovanovic OP, Stankov Jovanovic VP, Nikolic JS, Petrovic GM, Stojanovic GS. Comparative study of fatty acids profile in eleven wild mushrooms of Boletacea and Russulaceae families. Chem Biodivers. 2018;15(1):e1700434. doi: 10.1002/cbdv.201700434. [DOI] [PubMed] [Google Scholar]
- Ergönül PG, Akata I, Kalyoncu F, Ergönül B (2013) Fatty acid compositions of six wild edible mushroom species. Sci World J Article ID 163964 [DOI] [PMC free article] [PubMed]
- Hleba L, Kompas M, Hutková J, Rajtar M, Petrová J, Čuboň J, Kántor A, Kačániová M. Antimicrobial activity of crude ethanolic extracts from some medicinal mushrooms. J Microbiol Biotechnol Food Sci. 2016;5(special1):60–63. [Google Scholar]
- Huang W, Cai Y, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. 2009;62:1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- Jayakumar T, Thomas PA, Geraldine P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innov Food Sci Emerg Technol. 2009;10:228–234. doi: 10.1016/j.ifset.2008.07.002. [DOI] [Google Scholar]
- Khatua S, Acharya K (2019) Alkali treated antioxidative crude polysaccharide from Russula alatoreticula potentiates murine macrophages by tunning TLR/NF-κB pathway. Sci Rep 9:article number 1713 [DOI] [PMC free article] [PubMed]
- Khatua S, Dutta AK, Chandra S, Paloi S, Das K, Acharya K. Introducing a novel mushroom from mycophagy community with emphasis on biomedical potency. PLoS ONE. 2017;12:e0178050. doi: 10.1371/journal.pone.0178050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatua S, Ghosh S, Acharya K. A simplified method for microtiter based analysis of in vitro antioxidant activity. Asian J Pharma. 2017;11:S327–S335. [Google Scholar]
- Khatua S, Ghosh S, Acharya K. Laetiporus sulphureus (Bull.: Fr.) Murr. as food as medicine. Pharmacogn J. 2017;9:s1–s15. doi: 10.5530/pj.2017.6s.151. [DOI] [Google Scholar]
- Khatua S, Sikder R, Acharya K. Chemical and biological studies on a recently discovered edible mushroom: a report. FABAD J Pharm Sci. 2018;43:151–157. [Google Scholar]
- Khatua S, Chandra S, Acharya K. Exploration of a novel mushroom from tribal cuisines with chemical and medicinal relevance. Cytotechnology. 2019;71:245–259. doi: 10.1007/s10616-018-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouassi KA, Konan KH, Kouadio EJP, Due AE, Kouamé LP. Proximate composition, minerals and amino acids profiles of selected wild edible Russula Species from Côte d’Ivoire. Turk J Agric Food Sci Technol. 2016;4:882–886. [Google Scholar]
- Leal AR, Barros L, Barreira JCM, Sousa MJ, Martins A, Santos-Buelga C, Ferreira ICFR. Portuguese wild mushrooms at the “pharma-nutrition” interface: nutritional characterization and antioxidant properties. Food Res Int. 2013;50:1–9. doi: 10.1016/j.foodres.2012.10.012. [DOI] [Google Scholar]
- Pedneault K, Paul A, André G, Tweddell RJ. Fatty acid composition of lipids from mushrooms belonging to the family Boletaceae. Mycol Res. 2006;110:1179–1183. doi: 10.1016/j.mycres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Pereira E, Encina-Zelada C, Barros L, Gonzales-Barron U, Cadavez V, Ferreira ICFR. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: a good alternative to nutritious food. Food Chem. 2019;280:110–114. doi: 10.1016/j.foodchem.2018.12.068. [DOI] [PubMed] [Google Scholar]
- Phan C, David P, Naidu M, Wong K, Sabaratnam V. Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment using differentiating Neuro-2a and embryonic fibroblast BALB/3T3. BMC Complement Altern Med. 2013;13:261. doi: 10.1186/1472-6882-13-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popović V, Živković J, Davidović S, Stevanović M, Stojković D. Mycotherapy of cancer: an update on cytotoxic and antitumor activities of mushrooms, bioactive principles and molecular mechanisms of their action. Curr Top Med Chem. 2013;13(21):2791–2806. doi: 10.2174/15680266113136660198. [DOI] [PubMed] [Google Scholar]
- Pumtes P, Rojsuntornkitti K, Kongbangkerd T, Jittrepotch N. Effects of different extracting conditions on antioxidant activities of Pleurotus flabellatus. Int Food Res J. 2016;23(1):173–179. [Google Scholar]
- Pushpa H, Purushothoma KB. Nutritional analysis of wild and cultivated edible medicinal mushrooms. World J Dairy Food Sci. 2010;5:140–144. [Google Scholar]
- Rajasekaran M, Kalaimagal C. In vitro antioxidant activity of ethanolic extract of a medicinal mushroom, Ganoderma lucidum. J Pharm Sci Res. 2011;3(9):1427–1433. [Google Scholar]
- Ribeiro B, Pinho PG, Andrade PB, Baptista P, Valentão P. Fatty acid composition of wild edible mushroom species: a comparative study. Microchem J. 2009;93:29–35. doi: 10.1016/j.microc.2009.04.005. [DOI] [Google Scholar]
- Sharma SK, Gautam N (2015) Chemical, bioactive, and antioxidant potential of twenty wild culinary mushroom species. BioMed Res Int Article ID 346508 [DOI] [PMC free article] [PubMed]
- Sharma S, Atri NS, Kaur M, Verma B. Nutritional and neutraceutical potential of some wild edible Russulaceous mushrooms from North West Himalayas, India. Kavaka. 2017;48:41–46. [Google Scholar]
- Shomali N, Onar O, Karaca B, Demirtas N, Cihan AC, Akata I, Yildirim O. Antioxidant, anticancer, antimicrobial, and antibiofilm properties of the culinary-medicinal fairy ring mushroom, Marasmius oreades (Agaricomycetes) Int J Med Mushrooms. 2019;21(6):571–582. doi: 10.1615/IntJMedMushrooms.2019030874. [DOI] [PubMed] [Google Scholar]
- Singdevsachan SK, Patra JK, Tayung K, Sarangi K, Thatoi H. Evaluation of nutritional and nutraceutical potentials of three wild edible mushrooms from Similipal Biosphere Reserve, Odisha, India. J Verbrauch Lebensm. 2014;9:111–120. doi: 10.1007/s00003-014-0861-4. [DOI] [Google Scholar]
- Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5(3):93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl DR, Villinger K, König LM, Ziesemer K, Schupp HT, Renner B (2017) Healthy food choices are happy food choices: evidence from a real life sample using smartphone based assessments. Sci Rep 7:article number 17069 [DOI] [PMC free article] [PubMed]
- Wang X, Zhang J, Wu L, Zhao Y, Li T, Li J, Wang Y, Liu H. A mini-review of chemical composition and nutritional value of edible wild-grown mushroom from China. Food Chem. 2014;151:279–285. doi: 10.1016/j.foodchem.2013.11.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.