Abstract
Grapes are known to synthesize resveratrol, a stilbene phytoalexin, associated with cancer chemopreventive activity and cardioprotection. The effect of ultrasound (US) abiotic elicitor treatments on trans-resveratrol content in Kalecik Karası fresh and frozen grape skin was determined. D-optimal point change design was used under RSM for the experimental design of US treatment. The optimization was solved with the help of the Pareto areas and the optimal input variable values were determined by the desirability function with fuzzy similar perceivable ratio method. The optimum conditions of US treatment for fresh grape skin were determined as follows: incubation time—24 h, US application method-(P01), US frequency—20 kHz, US treatment time—60 min and ultrasonic intensity (UI)—1.15 W cm−2. The trans-resveratrol content (0.18 ± 0.01 mg/g) in the untreated grape skin significantly increased with optimum US treatment (3.58 ± 0.08 mg/g), increasing production to 19.9 times.
Keywords: Grape skin, Trans-resveratrol, D-optimal design, Desirability function optimization, Abiotic elicitor, Ultrasound
Introduction
Phytochemicals in the chemical, cosmetic and agricultural sectors, especially in the pharmaceutical industry, are irreplaceable and economically important. In recent years, the number of groups actively working on natural products has increased dramatically in the pharmaceutical industry, and natural products still hold out the best options for finding new agents/active templates. This offers us the potential to discover novel structures that can lead to effective agents that can be used in the treatment of various human diseases. (Newman and Cragg 2020).
Resveratrol (3,5,4-trihydroxystilbene) which is a phytoalexin in the stilbene group, is present in glycosylated form as well as cis- and trans-isomers. It is used to produce a resistance mechanism against abiotic/biotic stress factors (etc. microbial infection, temperature fluctuations, injury, UV radiation, exposure to ozone effect and especially against animal and pathogen attacks) (Corre et al. 2005; Hasan and Bae 2017).
A small number of plant species such as grapes, peanuts, mulberries, blueberries, pine trees, and tomatoes produce resveratrol. However, grape contains more resveratrol than all other natural sources and resveratrol are the highest in the seed, skin, leaf, and support tissue organs of coloured grapes (Corre et al. 2005). In grapes, the skin contains 5–7 ppm, the seed contains 1 ppm and the pulp contains less than 0.1 ppm resveratrol (Counet et al. 2006).
Trans-resveratrol is a natural component of Vitis vinifera L. which is abundant in the grape skin, leaf epidermis, and red wines. As known, trans-resveratrol is found in the skin but not in the flesh (Fan et al. 2011). cis-resveratrol is not present in any significant amount in grape skin or grape juice (Wang et al. 2013a), but it appears to be formed by hydrolysis of pieced or resveratrol polymers (vinifera) or possibly by light-induced or enzymatic isomerization of trans-resveratrol during skin fermentation (Orallo 2006).
Plants mostly contain a glycoside form of resveratrol (3-O-D-glucoside), which protects resveratrol against oxidative degradation (Ather et al. 2007). Glycoside-resveratrol is very stable and soluble in water and can easily and highly be absorbed from the gastrointestinal tract (Signorelli and Ghidoni 2005).
In many studies investigating the relationship of resveratrol on human health, resveratrol has been reported to be a potent antioxidant, an anti-inflammatory that reduces the risk of coronary heart disease (Dong 2003). In addition to coronary heart disease, its estrogenic activity is known to be effective in cancer prevention and neurodegenerative diseases (Hao and He 2004; Corre et al. 2005; Delmas et al. 2005). Resveratrol has also been shown to display anti-initiation, anti-promotion and anti-progression activities in breast cancer cells (Corre et al. 2005).
With the understanding of the positive effects of resveratrol on human health, it has become important to use as a food supplement. In recent years, the global demand for resveratrol has led to an increase in the natural demand of resveratrol in grape and grape products. However, natural synthesis and accumulation of resveratrol are very low in grapes. Therefore, there are continued efforts to induce resveratrol accumulation in grape skin (Hasan and Bae 2017).
Resveratrol can be induced in grapes by both biotic and abiotic factors, including fungi (Schmidlin et al. 2008), UV-C irradiation, jasmonic acid (JA), salicylic acid (SA), H2O2, AlCl3 and US (Wang et al. 2013b; Hasan et al. 2014). Today, the effect of abiotic factors such as UV light, US and chemical substances (AlCl3, CuSO4, AgNO3, etc.) on resveratrol production under controlled conditions is investigated (Hasan and Bae 2017).
Although the application of ultrasound to biotechnology is relatively new, many processes in the presence of cells or enzymes are activated by ultrasonic waves. While high-intensity ultrasonic waves break down cells and denature enzymes, low-intensity ultrasonic waves can change the path of cellular metabolism or improve the mass transfer of reagents and products. In the case of enzymes, the increase in the mass transfer rate of the reagents appears to be the most important factor (Sinisterra 1992). Briefly, ultrasound (US) is a special type of physical elicitor with various biological effects. Although high-density US can generally damage biological substances, the light-density US can elicitor biological activities such as enzymatic and microbial biological transformations and cellular biosynthesis.
Although there are quite a few studies examining the effect of ultrasonic elicitor on resveratrol accumulation, the studies have emphasized that US treatment has a positive effect (Bremner 1986; Cordemans 1991; Barton et al. 1996; Wu and Lin 2002; Rudolf and Resurreccion 2005; Potrebko and Resurreccion 2009; Sales and Resurreccion 2009; Hasan and Baek 2013). In the first studies examining the effect of ultrasound on resveratrol production, it was found that resveratrol increased 8–143 times in peanut kernels after ultrasound treatment (Rudolf and Resurreccion 2005; Potrebko and Resurreccion 2009; Sales and Resurreccion 2009). Then, the ultrasound technique, which increases the accumulation of resveratrol in grape skin and leaves, was applied for the first time in the study of Hasan and Baek 2013.
In our study, the US was thought to act as an abiotic elicitor in the plant defence mechanism to stimulate the secondary metabolite biosynthesis of plant cells. Trans-resveratrol, which was a valuable secondary product for the food and health sector, was intended to be produced in grape skin using the elicitor effect of the US application. For this purpose, trans-resveratrol production capacity was determined under the effect of ultrasound as an abiotic elicitor in fresh and frozen grape skins of Kalecik Karası (Vitis vinifera L.), one of the most important varieties in Turkey. Only a low-intensity ultrasound effect combined with heat and/or pressure can reduced thermal resistance to ensure the desired enzyme inactivity. Therefore, conditions that provide the low ultrasonic intensity working range was preferred in our study. D-optimal experimental design and desirable function approach were used to determine the best operational conditions in abiotic elicitor US application.
The US effect depends on a large number of external and internal operating parameters. Among these parameters, it is difficult to compare different studies for the US study parameters given in the literature due to the variety of operating frequency and power level values and lack of standardization. For this reason, there is a need for studies where operating parameters are evaluated simultaneously and optimum conditions are determined. For this purpose, we believe that our study will contribute to the solution to this problem and the knowledge of the literature.
Materials and methods
Material
Vitis vinifera L. cv. Kalecik Karası is an indigenous, high-quality red wine variety originally grown in Kalecik, in the Ankara province of Turkey. In this study, the grapes of Kalecik Karası clone 15 that were harvested from the vineyards of the Department of Horticulture, Faculty of Agriculture, Ankara University were used. Similar-sized fresh and frozen grapes samples were selected for treatment with or without ultrasonication. The grape samples were cleaned by deionized water, and following treatment, the grape samples were dried using a paper towel. The cleaned and dried grapes were stored in two conditions such as fresh grapes (25 °C) and frozen grapes (− 80 °C).
Chemicals
Standard trans-resveratrol (≥ 99% (HPLC)) was obtained from Sigma–Aldrich (St. Louis, MO, USA), and the stock trans-resveratrol solution was kept in the dark at − 20 °C. The chemicals and solvents used for analysis in this study were of analytical-grade and purchased from Merck (Darmstadt, Germany).
Ultrasonication treatment
Ultrasound (US) treatment was performed in an ultrasonic probe system Hielscher (UP100H, Germany), with 14 × 10 cm internal dimensions and maximal capacity of 1 L, equipped with a transducer at the base of jug operating at frequencies of 20 and 30 kHz. Cooling from the reactor jacket with a coolant (ethylene glycol–water) allowed the control of reaction temperature. Considering the actual input power from the device converted to heat which is dissipated in the medium, calorimetric measurements were performed to assess actual ultrasound power (P), calculated as shown in the Eq. (1) below (Toma et al. 2011),
| 1 |
where Cp is the heat capacity of the solution at constant pressure (J g−1oC−1), m is the mass of solution (g) and dT/dt is temperature rise per second. Then, the applied ultrasonic intensity (UI) was calculated using the calculated power as shown in the Eq. (2) ((Toma et al. 2011).
| 2 |
where UI is the ultrasonic intensity (W cm−2), P is the ultrasound power (W) as calculated by Eq. 1, and D is the internal diameter (cm) of the ultrasound reactor. The determined ultrasonic actual power was given in Table 1 as ultrasonic intensity (UI) (W cm−2).
Table 1.
Experimental design and observed responses for trans-resveratrol production in grape skin obtained by US abiotic treatment
| Run | Factor 1 X1: Physical characteristics of the grape |
Factor 2 X2: Incubation time (h) |
Factor 3 X3: US Application method |
Factor 4 X4:US Frequency (kHz) |
Factor 5 X5:US Treatment time (min) |
Ultrasonic intensity (UI) (W cm−2) |
Y1 trans-resveratrol (mg/g) |
|---|---|---|---|---|---|---|---|
| 1 | Fresh | 48 | Steady | 30 | 60 | 7.24 | 0.63 ± 0.02 |
| 2 | Fresh | 48 | Periodic (P05) | 30 | 60 | 3.65 | 1.91 ± 0.10 |
| 3 | Fresh | 72 | Periodic (P01) | 20 | 60 | 1.15 | 0.21 ± 0.01 |
| 4 | Fresh | 72 | Periodic (P05) | 30 | 10 | 0.75 | 0.21 ± 0.02 |
| 5 | Frozen | 24 | Periodic (P05) | 30 | 10 | 0.75 | 0.83 ± 0.05 |
| 6 | Frozen | 48 | Steady | 20 | 10 | 0.95 | 0.51 ± 0.03 |
| 7 | Frozen | 48 | Periodic (P05) | 20 | 10 | 0.54 | 0.60 ± 0.03 |
| 8 | Frozen | 72 | Periodic (P01) | 20 | 10 | 0.34 | 0.26 ± 0.02 |
| 9 | Fresh | 24 | Periodic (P05) | 30 | 10 | 0.75 | 2.73 ± 0.11 |
| 10 | Fresh | 48 | Periodic (P01) | 20 | 10 | 0.34 | 0.28 ± 0.00 |
| 11 | Frozen | 72 | Periodic (P05) | 30 | 60 | 3.65 | 0.56 ± 0.02 |
| 12 | Frozen | 48 | Steady | 30 | 60 | 7.24 | 0.63 ± 0.02 |
| 13 | Frozen | 48 | Steady | 30 | 10 | 1.45 | 0.65 ± 0.02 |
| 14 | Frozen | 24 | Periodic (P01) | 20 | 10 | 0.34 | 0.17 ± 0.00 |
| 15 | Frozen | 48 | Periodic (P01) | 20 | 60 | 1.15 | 1.33 ± 0.05 |
| 16 | Frozen | 24 | Steady | 20 | 10 | 0.95 | 0.19 ± 0.01 |
| 17 | Fresh | 48 | Steady | 30 | 10 | 1.45 | 1.30 ± 0.04 |
| 18 | Frozen | 48 | Periodic (P05) | 30 | 60 | 3.65 | 1.09 ± 0.01 |
| 19 | Fresh | 72 | Periodic (P05) | 30 | 60 | 3.65 | 0.29 ± 0.00 |
| 20 | Fresh | 48 | Steady | 20 | 10 | 0.95 | 0.51 ± 0.02 |
| 21 | Fresh | 72 | Steady | 20 | 10 | 0.95 | 0.24 ± 0.01 |
| 22 | Frozen | 72 | Steady | 20 | 60 | 4.48 | 0.37 ± 0.02 |
| 23 | Fresh | 24 | Periodic (P01) | 20 | 10 | 0.34 | 1.74 ± 0.04 |
| 24 | Frozen | 72 | Periodic (P05) | 30 | 10 | 0.75 | 0.85 ± 0.01 |
| 25 | FRESH | 72 | Periodic (P05) | 20 | 10 | 0.54 | 0.24 ± 0.00 |
| 26 | Fresh | 72 | Periodic (P05) | 30 | 60 | 3.65 | 0.29 ± 0.00 |
| 27 | Fresh | 24 | Periodic (P01) | 20 | 60 | 1.15 | 3.62 ± 0.10 |
| 28 | Fresh | 24 | Periodic (P05) | 30 | 60 | 3.65 | 2.92 ± 0.08 |
| 29 | Frozen | 24 | Steady | 30 | 60 | 7.24 | 0.35 ± 0.00 |
| 30 | Fresh | 24 | Steady | 20 | 60 | 4.48 | 0.33 ± 0.00 |
| 31 | Frozen | 72 | Periodic (P05) | 20 | 60 | 1.89 | 0.56 ± 0.01 |
| 32 | Frozen | 24 | Periodic (P05) | 30 | 60 | 3.65 | 0.22 ± 0.00 |
Analytical results are the average of triplicates (mean ± SD)
Before US treatment, the skins of fresh and frozen grapes were separated from the pulp and the seed. After taking 10 grams of the skins and adding 10 mL of ultrapure water, it was mixed in the mechanical homogenizer (MICCRA D-9, Germany) at 15000 rpm for 5 min. to reduce the skin size and homogenize the medium. Samples of grape skin were then added to 20 mL of ultra-pure water and placed in a 100 mL volume, an amber glass reactor with a cooling jacket. The outer wall of the glass reactor was coated to prevent the adverse effects of sunlight. The working amplitude was fixed at 200 mm. The ultrasonic homogenizer with a constant frequency of 20 and 30 kHz (13 mm or 19 mm diameter probe) was used for continuous (C) and periodic (P). Different diameters were used to apply ultrasound at a different power to the unit area. At the same time, 0.1 s open 0.9 s off (P01) and 0.5 s open 0.5 s off (P05) in two conditions periodic application effect was examined for 20 and 30 kHz fixed frequency value. US treatment time was applied as 10 min. and 1 h. A cooled circulator was used to keep the reaction medium at the desired temperature (30 °C) and the temperature was measured digitally. The samples taken from the reactor at the end of US treatments were kept in the dark and at 25 °C condition to produced trans-resveratrol and to prevent isomerization of trans-resveratrol to cis-resveratrol (Trela and Waterhouse 1996) during the incubation time of 0, 24, 48 and 72 h.
The experimental design was made for extraction and 32 experiment points were specified. Stat-Ease Design-Expert 7.0.0 (Stat-Ease Inc., Minneapolis, USA) software program was used for experimental design and optimization study. The physical characteristics of the grape, incubation time, US application method, US frequency and US treatment time were determined as independent variables for the US abiotic effect. The measurement range for each variable was based on preliminary studies. The amount of trans-resveratrol (by HPLC analysis) was used as a dependent variable (response).
Extraction of trans-resveratrol
The extraction of trans-resveratrol produced by incubating over fresh and frozen grape skin for different incubation times was performed as described previously, with minor modifications (Keller et al. 2000). Briefly, one gram grape skin was homogenized with 10 mL of cold (− 20 °C) acetone by mechanical homogenizer (about 1 min) and was kept in a shaker water bath for 30 min. Then, it was centrifuged at 3000g for 10 min and the supernatant was transferred to a test tube. Also, 5 mL of acetone was added to 5 mL of methanol over the remaining skin pieces and centrifuged again under the same conditions. The supernatant was combined with the first filtrate. It was evaporated until dryness in a rotary evaporator under vacuum (Heidolph LABOROTA 4000, Germany) at 60 °C, 2 mL of analytical purity methanol was added to the remaining residue and stored at +4 °C for HPLC analysis. The same procedures were followed for the control samples, excluding the US treatment.
Analysis of trans-resveratrol
Quantification of trans-resveratrol in grape skin extracts was determined by HPLC (Jeandet et al. 1997). The equipment utilized was a Shimadzu LC10A (Kyoto, Japan) series coupled to SIL-10AXL an autosampler, SPD-M10AVP photodiode array detector, CTO-10 column oven, LC-10 AD pump, CBM-10A control unit and controlled by Shimadzu LC solution software. Reversed-phase HPLC (High-Performance Liquid Chromatography) method was used with column C18 (250x4mm; 5 µm). PDA detector was used at a wavelength range of 190–550 nm. This range was screened and 330 nm wavelength was used for quantitative measurements.
The high-pressure gradient method with two solvents (A: acetonitrile, B: water (H2O)) was used. The flow rate was 1.0 mL/min and the elution program was as follows: 0–18 min from 10 to 85% of A; 18–23 min. steady 85% A; 23–30 min from 85 to 10% of A; 30–35 min. from steady 10% of eluent A. The elute was monitored at 330 nm for trans-resveratrol. The injection volume of samples and standard solutions were 5 μL.
Analytical method validation
The calibration curve was prepared using the standard solution with 80% methanol/water solvent mixture from the range 0.10–40.00 mg L−1 with seven intervals to calculate the content of trans-resveratrol in the grape skin extracts (y = 158164x + 4266.8; R2 = 0.9991). Standard solutions were then filtered through a 0.22-μm syringe membrane filter, injected in volumes of 5μL in triplicate and analyzed using the previously mentioned chromatographic conditions.
Recovery was used to further evaluate the accuracy of the method. The spiking known quantities of the mixed standard solution were added to known amounts of samples. The identification and quantification of trans-resveratrol were carried out by comparison of the retention times and peak areas, with those of trans-resveratrol standard or by co-injection with the sample (spike test), respectively. Accuracy was evaluated by the mean percentage recoveries obtained. Repeatability was assessed through the relative standard deviation values. Precision was evaluated by the performance of intraday (repeatability) by three replicated injections of the same solution, same analyst within the same day and inter-day (reproducibility) was determined by analyzing the same solution on three different days (three injections a day). Satisfactory results were obtained for the used method.
Validation was performed according to International Conference on Harmonization (ICH) guidelines and the calculated validation parameters were shown in Table 2. The following parameters were determined: linearity, range, accuracy, precision, the limit of detection (LOD) and the limit of quantification (LOQ) (ICH 2005).
Table 2.
Assay validation parameters of the proposed HPLC method for determination of trans-resveratrol
| Parameter | Trans-resveratrol |
|---|---|
| Accuracya (mean recovery % ± SD) | 98.78 ± 0.45 |
| Precision | |
| Repeatabilityb | ±0.78 |
| Intermediate precisionc | ±1.79 |
| Linearity | |
| Slope | 158,164 |
| Intercept | +4266.8 |
| Correlation cofficient (r) | 0.9991 |
| Range (mg L−1) | 0.10–40 |
| LODd (mg L−1) | 0.03 |
| LOQd (mg L−1) | 0.10 |
aAverage of (n = 3), average five concentrations (0.5- 30 mg L−1) for trans-resveratrol
bThe intraday (n = 3), average of three concentrations (0.5, 10 and 30 mg L−1) for trans-resveratrol repeated three times within the day
cThe inter-day (n = 3), average of three concentrations (0.5, 10 and 30 mg L−1) for trans-resveratrol repeated three times in 3 days
dDetermined via calculations, LOD = 3.3 (SD of the response/slope), LOQ = 10 (SD of the response/slope)
Experimental design and statistical analysis
D-optimal experimental designs maximize the experimental space spanned by a selected number of experiments for a defined model matrix (Eriksson et al. 1998). The D-optimal design was selected to limit the number of resources (number of runs) required for analysis and investigation of the parameters. In the present study, the optimization of the effect of abiotic elicitor ultrasound application conditions on the yield of trans-resveratrol production in grape skin was performed using D-optimal point exchange design. A categorical, two and three-stage factorial design consisting of 32 experimental studies was used. The five independent variables and levels studied were: the physical characteristics of the grape (fresh or grape skin, frozen grape skin); incubation time (24 h, 48 h, 72 h); US application method (continuous, P01, P05), US frequency (20 kHz, 30 kHz) and US treatment time (10 min, 60 min). The corresponding response variable was trans-resveratrol in mg/g grape skin (Table 1).
The design matrix for the response factor and the fit of each term were analyzed by the analysis of variance (ANOVA) (Table 3). In this method, the statistical significance of the linear and interaction effects of each factor on the response variable was determined by applying the Fischer F test at 95% confidence level. The variance explained by the model was given by the multiple determination coefficients. The coefficients were used to show the effect of the independent variables on the response variable.
Table 3.
ANOVA results and predicted responce model with interaction terms for trans-resveratrol(HPLC) amount for US treatment (Backward Elimination Regression)
| Y1:trans-resveratrol (HPLC) | ||||
|---|---|---|---|---|
| Hierarchial Terms added after Backward Elimination Regression: β 1β 3, β 1β 5, β 2β 5, β 3β 4 | ||||
| SSE | df | F value | p value | |
| Model | 22.62 | 19 | 17.16 | < 0.0001 |
| x 1 | 1.56 | 1 | 22.52 | 0.0005 |
| x 2 | 5.93 | 2 | 42.74 | < 0.0001 |
| x 3 | 1.55 | 2 | 11.15 | 0.0018 |
| x 4 | 1.06 | 1 | 15.32 | 0.0021 |
| x 5 | 0.016 | 1 | 0.23 | 0.6374 |
| x 1 x 2 | 7.06 | 2 | 50.87 | < 0.0001 |
| x 1 x 3 | 0.1795 | |||
| x 1 x 4 | 0.67 | 1 | 9.66 | 0.0090 |
| x 1x 5 | 0.2952 | |||
| x 2 x 3 | 1.62 | 4 | 5.83 | 0.0076 |
| x 2 x 4 | 0.97 | 2 | 6.97 | 0.0098 |
| x 2 x5 | 0.9468 | |||
| x 3 x 4 | 0.5168 | |||
| x 3 x5 | 3.83 | 2 | 27.58 | < 0.0001 |
| x 4 x5 | 0.51 | 1 | 7.33 | 0.0191 |
| Residual | 0.83 | 12 | ||
| Lack of Fit | 0.83 | 11 | 1.96 | 0.069 |
| Pure Error | 0.07 | 1 | ||
| Cor Total | 23.45 | 31 | ||
| R2 | 0.9645 | |||
| R2 Adj | 0.9083 | |||
| Adeq Precision | 15.679 | |||
| Significant Model Terms | X1, X2, X3, X4, X1X2, X1X4, X2X3, X2X4, X3X5, X4X5 | |||
| The predicted responce model with interaction terms by D-optimal-after Backward Elimination | Ytrans-resveratrol=+0.90 − 0.23X1 + 0.59X2[1] − 0.054X2[2] − 0.35X3[1] + 0.49X3[2] + 0.44X4 + 0.084X5 − 0.76X1X2[1] + 0.061X1X2[2] − 0.15X1X4 − 0.15X2[1]X3[1] − 0.19X2[2]X3[1] + 0.55X2[1]X3[2] + 0.014X2[2] X3[2] +0.28 X2 [1]X4 + 0.10* X2 [2]X4 − 0.52 X3 [1]X5 + 0.84 X3 [2]X5 + 0.22 X4 | |||
Bold p values represents the statisticaly significant terms (p < 0.05)
A predicted response model with interaction terms was used as follows:
| 3 |
where y represents the response variable, β0 is a constant, βi, βij are the linear and interactive coefficients respectively. xi and xj are independent variables.
The response which characterizes the system in the optimization study was solved with the help of Pareto zones. In addition to Pareto optimality, desirability function (Derringer and Suich 1980) optimization by fuzzy similar perceivable ratio method was also used to find optimum conditions for maximum trans-resveratrol (mg/g) amount response. Backward elimination regression was used to improve the statistics removing factors from the full model that were not significant in a stepwise manner.
For the comparison of the result of the experimental values of the response variables at optimum processing parameters, statistical analysis was done using the MiniTab 17 Statistical Programme. Two-way ANOVA and Tukey’s posthoc test was carried out (p < 0.05) and the results were presented in average values and standard deviations of the replicates.
Results and discussion
Fitting the model
In terms of coded factors, it was found that the equation can be used to make predictions for the response. By default, the levels of each factor were coded as +1, and the lower levels of the factors were coded as − 1. The coded equation was useful in determining the relative effect of factors by comparing factor coefficients.
ANOVA results for trans-resveratrol production in grape skin obtained by US abiotic treatment were shown in Table 3. The model was highly significant with low p values of < 0.0001 high F-value 17.16 and a lack of fit value (0.069) which confirmed the reliability of the model for the prediction of trans-resveratrol (mg/g). Also, the squared of the correlation coefficient (R2) value and the adjusted (R2) was 0.9645 and 0.9068 respectively which was very close to 1 (Table 3). The predicted response model with interaction terms for US abiotic treatment was expressed by the following equation;
| 4 |
Analysis of interaction and 3D Plot
The interaction plot was helped to compare the effects of all the factors at a particular point in the design space. The interaction plot was a good visual tool for showing the relative size of the effects. The response was plotted by changing only one factor over its range while holding all the other factors constant. By default, the reference point was set at a lower point (coded − 1) of all the factors. The assumed model was a two-factor interaction (2F1) model.
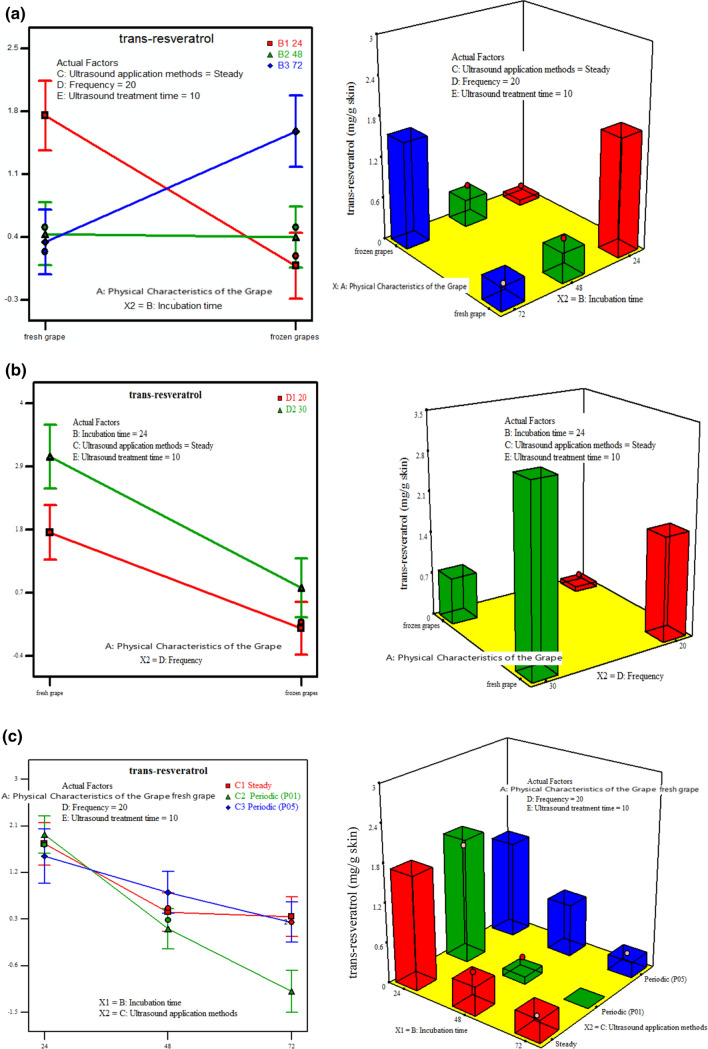
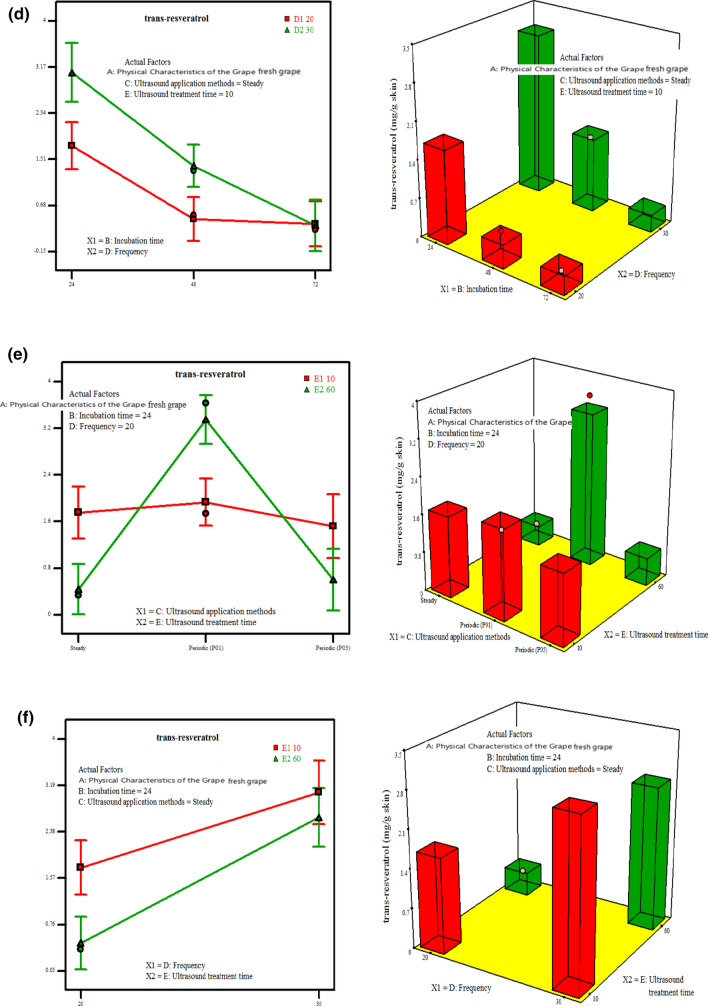
The effects of abiotic elicitor parameters (physical characteristics of the grape, incubation time, US application method, US frequency and US treatment time) on the maximum trans-resveratrol production were evaluated. Interaction and 3D plots have been created for the highest desirability of the solutions found through numerical optimization and they have been generated to express the effect of the independent variables on the trans-resveratrol amount (Fig. 1a–f).
Fig. 1.
Interaction and 3D plots of trans-resveratrol accumulation of Kalecik Karası grape skin as affected by physical characteristic of the grape, incubation time, US application method, US frequency and US treatment time. a Physical characteristic of the grape and incubation time, b physical characteristic of the grape and US frequency, c incubation time and US application method, d incubation time and US frequency, e US application method and US treatment time, f US frequency and US treatment time
The effect of US treatment parameters on trans-resveratrol production
In our study, the trans-resveratrol content was 0.18 mg/g in the untreated grape skin. At optimum conditions of US treatment, the trans-resveratrol content increased by a substantial increase (19.9 fold) to 3.58 mg/g for the fresh grape skin. The ultrasonic intensity applied under these operating conditions was 1.15 W cm−2. Hasan and Baek 2013 study; a resveratrol accumulation trend similar to our study, it was found to be increased in grape skin by 7.7 folds, after treatment with 5 min US treatment, followed by 6 h incubation. However, the increase of resveratrol in leaves was quite less than that in grape skin.
In our study, the different physical characteristics of the grape, incubation time, US application method, US frequency and the interactions between, the physical characteristics of the grape and incubation time, the physical characteristics of the grape and US frequency, incubation time and US application method, incubation time and US frequency, US application method and US treatment time, US frequency and US treatment time, were the factors that significantly affected trans-resveratrol concentration in grape skin (Table 3). In our experimental design conditions, ultrasonic intensity ranged between 0.34 and 7.24 W cm−2, while the amount of trans-resveratrol varied in the range of 0.17–3.62 mg/g grape skin.
In this study, because of the different physical characteristics of the grape fresh and frozen grape skins were used. The trans-resveratrol was affected significantly by the change in the physical characteristics of the grape (mg/g) (p < 0.0005) (Table 3). Fresh grapes produced trans-resveratrol at higher concentrations than frozen grapes. In the production reaction of resveratrol, four different enzymes, especially CoA-ligase and resveratrol-synthase enzymes, were active (Becker et al. 2003). We think that these enzymes were more effective in fresh grape skins. There was no significant interaction between the physical characteristics of the grape and US application methods (p < 0.1795) for trans-resveratrol, additionally while the interaction between the physical characteristics of the grape and US treatment time was not significant (p < 0.2952). The value of β1β3 and β1β5 removed from backward elimination wasn’t used in the model equation and it was thought that it was not appropriate to discuss with interaction and 3D plots. The physical characteristics of the grape and incubation time were found as significant model parameters of the binary interaction (p < 0.0001). In interaction and 3D graphics, it was determined that the highest trans-resveratrol accumulation was obtained in the fresh grape skin at 24 h incubation time (Fig. 1a). The interaction between the physical characteristics of the grape and US frequency was found significant for the trans-resveratrol amount (p = 0.0090) and both US frequencies of 20 and 30 kHz showed the highest trans-resveratrol accumulation in fresh grape skin (Fig. 1b).
Figure 1c shows the effect of incubation time with US application methods (p = 0.0076) for trans-resveratrol. All three US application methods (continuous, P01, P05) gave high results at 24 h of incubation time. The highest amount of trans-resveratrol was obtained for 24 h incubation time and (P01) US application method. Additionally, the interaction between the incubation time and US frequency was found statistically significant (p = 0.0098). As the incubation time increased, the accumulation of trans-resveratrol was found to decrease. The different applications of US frequency did not change this result. (Figure 1d). However, the relationship between the incubation time and US treatment time was not statistically significant (p = 0.9468) and was not shown in the model equation (Backward elimination). But, the US treatment time was statistically significant in relation to both US application method (p < 0.0001) and US frequency (p = 0.0191). In the 60 min. US treatment time with (P01) US application method (Fig. 1e) and in the 10 min. US treatment time with 30 kHz US frequency (Fig. 1f) conditions, it was determined that there was a significant trans-resveratrol accumulation. Nevertheless, there was no significant interaction between US application method and US frequency (p = 0.5168). The value of β3β4 removed from backward elimination wasn’t used in the model equation.
In conclusion, US treatment was shown as a potential accumulation method of secondary metabolites and was a powerful abiotic elicitor for trans-resveratrol production of grape skins. It could be said that the most important stage of US treatment is the incubation time-β2 (p < 0.0001).
Optimization and verification
The US treatment conditions were optimized for the maximum trans-resveratrol accumulation of grape skin. The response variable was optimized using the desirability function and the desirability value of 0.919 was observed for US treatment conditions. To verify the adequacy of the model, the experimental values and predicted values of the responses were compared in the D-optimal design method. There were no significant differences (p > 0.05) between the experimental and predicted values (Fig. 2). Therefore, it is understood that the predicted response model with interaction terms are correct and valid.
Fig. 2.
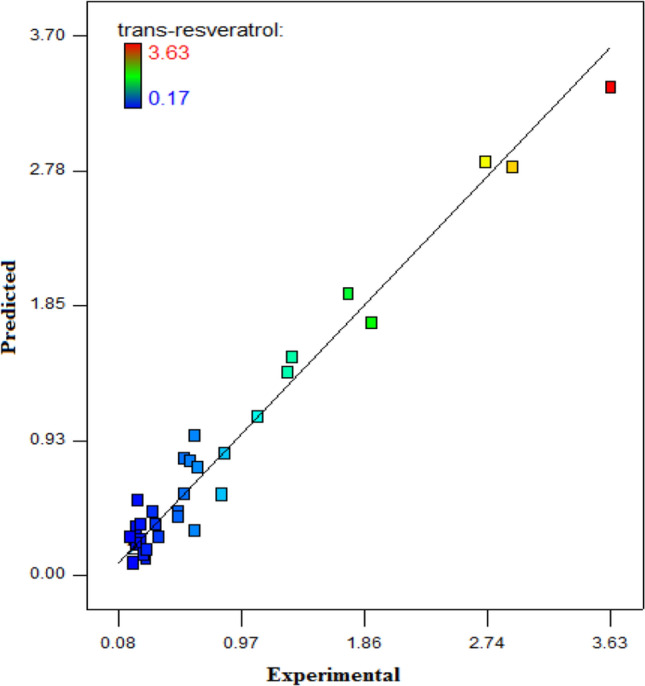
Comparison of experimental and predicted values of trans-resveratrol (HPLC) of US treatments
The adequacy of the mathematical model was verified (Eq. 4) to predict the optimum conditions. The model was tested (n = 3) using the optimum conditions identified as fresh grape skin, 24 h incubation time, (P01) US application method, 20 kHz US frequency, and 60 min. US treatment time and 1.15 W cm−2 of ultrasonic intensity (UI). Under these conditions, the experimental value for trans-resveratrol was (3.58 ± 0.08 mg/g). The predicted results from the mathematical model were found to be (3.63 mg/g). The trans-resveratrol content of (0.18 ± 0.01 mg/g) in the untreated grape skin increased 19.89 fold the production by significantly increasing to (3.58 ± 0.08 mg/g) with optimum US treatment.
Conclusion
In this study, a chemometric approach was designed and experimental design/optimization study was carried out due to its important advantages, such as easier to study to the combined effect of two or more factors simultaneously and analyze their interrelationships. The effect of abiotic elicitor on the production of resveratrol in the grape type of Kalecik Karası (Vitis vinifera L.) was investigated for their forms of fresh and frozen. US treatment was used as a potential accumulation method of secondary metabolites and trans-resveratrol content of in the untreated grape skin increased 19.89 fold the production by significantly increased with optimum US treatment. It was pointed out that a powerful abiotic elicitor for trans-resveratrol production from grape skins.
The optimum conditions were identified on fresh grape skin as 24 h incubation time, (P01) US application method, 20 kHz US frequency, and 60 min US treatment time and 1.15 W cm−2 of ultrasonic intensity (UI). It was observed that the most important stage of US treatment is the incubation time-β2 (p < 0.0001). This study concludes that the results obtained from experimental studies with the mathematical model generated were in agreement with each other. It is important to point out that experimental design and optimisation should be more widely used in literature.
Today, trans-resveratrol is one of the best sources for the food and nutraceutical industry, despite its low water solubility, low oral bioavailability, chemical instability, easy oxidation, extremely light-sensitive compound, short biological half-life, rapid metabolism and elimination properties. For this purpose, resveratrol in the skin of the grape can be the most important source to meet the increasing demand.
Ultrasonication baths and probes can usually be used to produce non-contamination products in combination with the induction of resveratrol in grapes. The US, together with UV and/or LED, can be an important way of increasing resveratrol deposition in grape skin. At the same time, the induction of trans-resveratrol in grape skin can have a significant practical effect in the grape juice and red wine industry. The application of combinations of different elicitors known as inducers of plant secondary metabolites for further studies may be another strategy for increasing resveratrol production with the US, LED and/or UV.
Funding
This research was supported by the Scientific Research Projects Coordination Unit of Ankara University Biotechnology Institute (Project number: 2005163).
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was reported on behalf of all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ather M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton S, Bullock C, Weir D. The effects of ultrasound on the activities of some glycosidase enzymes of industrial importance. Enzyme Microbial Technol. 1996;18:190–194. doi: 10.1016/0141-0229(95)00092-5. [DOI] [Google Scholar]
- Becker JVW, Armstrong GO, Merwe MJ, Lambrechts MG, Vivier MA, Pretorius IS. Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine-related antioxidant resveratrol. FEMS Yeast Res. 2003;4:79–85. doi: 10.1016/S1567-1356(03)00157-0. [DOI] [PubMed] [Google Scholar]
- Bremner DH. Chemical ultrasonics. Chem Br. 1986;22:633. [Google Scholar]
- Cordemans E (1991) Ultrasound. A new performance tool for the chemical industry. Chimicaoggi, November 17–20
- Corre LL, Chalabi N, Delort L, Bingon YJ, Bernard-Gallon DJ. Resveratrol and breast cancer chemoprevention: molecular mechanisms. Mol Nutr Food Res. 2005;49:462–471. doi: 10.1002/mnfr.200400094. [DOI] [PubMed] [Google Scholar]
- Counet C, Callemien D, Collin S. Chocolate and cocoa: new sources of trans-resveratrol and trans-pieced. Food Chem. 2006;98:649–657. doi: 10.1016/j.foodchem.2005.06.030. [DOI] [Google Scholar]
- Delmas D, Jannin B, Latruffe N. Resveratrol: preventing properties against vascular alterations and aging. Mol Nutr Food Res. 2005;49:377–395. doi: 10.1002/mnfr.200400098. [DOI] [PubMed] [Google Scholar]
- Derringer G, Suich R. Simultaneous optimization of several response variables. J Qual Technol. 1980;12(4):214–219. doi: 10.1080/00224065.1980.11980968. [DOI] [Google Scholar]
- Dong Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat Res. 2003;523(524):145–150. doi: 10.1016/S0027-5107(02)00330-5. [DOI] [PubMed] [Google Scholar]
- Eriksson L, Johansson E, Wikstrom C. Mixture design–design generation PLS analysis, and model usage. Chemometr Intell Lab. 1998;43:1–24. doi: 10.1016/S0169-7439(98)00126-9. [DOI] [Google Scholar]
- Fan E, Lin S, Du D, Jia Y, Kang L, Zhang K. Current separative strategies used for resveratrol determination from natural sources. Anal Methods. 2011;3:2454–2463. doi: 10.1039/c1ay05353a. [DOI] [Google Scholar]
- Hao HD, He LR. Mechanisms of cardiovascular protection by resveratrol. J Med Food. 2004;7:290–298. doi: 10.1089/jmf.2004.7.290. [DOI] [PubMed] [Google Scholar]
- Hasan MM, Bae H. Molecules an overview of stress-induced resveratrol synthesis in grapes: perspectives for resveratrol-enriched grape products. Molecules. 2017 doi: 10.3390/molecules22020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan MM, Baek KH. Induction of resveratrol biosynthesis in grape skin and leaves by ultrasonication treatment. Korean J Hortic Sci Technol. 2013;31:496–502. doi: 10.7235/hort.2013.12229. [DOI] [Google Scholar]
- Hasan MM, Yun HK, Kwak EJ, Baek KH. Preparation of resveratrol-enriched grape juice from ultrasonication treated grape fruits. Ultrason Sonochem. 2014;21:729–734. doi: 10.1016/j.ultsonch.2013.08.008. [DOI] [PubMed] [Google Scholar]
- ICH (2005) Validation of analytical procedures: text and methodology Q2 (R1). In: International conference on harmonization, Geneva, Switzerland, pp 11–12
- Jeandet P, Breuil AC, Adrian M, Weston LA, Debord S, Meunier P, Maume G, Bessis R. HPLC analysis of grapevine phytoalexins coupling photodiode array detection and fluorimetry. Anal Chem. 1997;69(24):5172–5177. doi: 10.1021/ac970582b. [DOI] [Google Scholar]
- Keller M, Steel CC, Creasy GL (2000) Stilbene accumulation in grapevine tissues: Developmental and environmental effects. In: XXV international horticultural congress, part 4: culture techniques with special emphasis on environmental implications ISHS Acta Horticulturae 514: 275–286
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Orallo F. Comparative studies of the antioxidant effects of cis- and trans-resveratrol. Curr Med Chem. 2006;13(1):87–98. doi: 10.2174/092986706775197962. [DOI] [PubMed] [Google Scholar]
- Potrebko I, Resurreccion AVA. Effect of ultraviolet doses in combined ultraviolet-ultrasound treatments on trans-resveratrol and trans-pieced contents in sliced peanut kernels. J Agric Food Chem. 2009;57:7750–7756. doi: 10.1021/jf900667d. [DOI] [PubMed] [Google Scholar]
- Rudolf JR, Resurreccion AVA. Elicitation of resveratrol in peanut kernels by application of abiotic stresses. J Agric Food Chem. 2005;53(26):10186–10192. doi: 10.1021/jf0506737. [DOI] [PubMed] [Google Scholar]
- Sales JM, Resurreccion AVA. Maximising resveratrol and pieced contents in UV and ultrasound treated peanuts. Food Chem. 2009;11(7):674–680. doi: 10.1016/j.foodchem.2009.04.075. [DOI] [Google Scholar]
- Schmidlin L, Poutaraud A, Claudel P, Mestre P, Prado E, Santos-Rosa M. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008;148:1630. doi: 10.1104/pp.108.126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions, and promises. J Nutr Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Sinisterra JV. Application of ultrasound to biotechnology: an overview. Ultrasonics. 1992;30(3):180–185. doi: 10.1016/0041-624X(92)90070-3. [DOI] [PubMed] [Google Scholar]
- Toma M, Fukutomi S, AsakuraY Koda S. A calorimetric study of energy conversion efficiency of a sonochemical reactor at 500 kHz for organic solvents. Ultrason Sonochem. 2011;18(1):197–208. doi: 10.1016/j.ultsonch.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Trela BC, Waterhouse AL. Resveratrol: isomeric molar absorptivities and stability. J Agric Food Chem. 1996;44:1253–1257. doi: 10.1021/jf9504576. [DOI] [Google Scholar]
- Wang L, Xu M, Liu C, Wang J, Xi H, Wu B, Loescher W, Duan W, Fan P, Li S. Resveratrols in grape berry skins and leaves in vitis germplasm. PLoS ONE. 2013;8(4):e61642. doi: 10.1371/journalpone.0061642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Ma L, Xi HF, Duan W, Wang JF, Li SH. Individual and combined effects of CaCl2 and UV-C on the biosynthesis of resveratrols in grape leaves and berry skins. J Agric Food Chem. 2013;61:7135–7141. doi: 10.1021/jf401220m. [DOI] [PubMed] [Google Scholar]
- Wu J, Lin L. Ultrasound-induced stress responses of Panax ginseng cells: enzymatic browning and phenolics production. Biotechnol Prog. 2002;18:862–866. doi: 10.1021/bp0255210. [DOI] [PubMed] [Google Scholar]