Abstract
Bingol is a famous region for honey in Turkey. The amount of phenolic substance is also considered important for the anticancer, antioxidant and antimicrobial properties of honey. Anticancer activity of honey extract was determined as the most effective dose of 1 mg/mL using the WST1 anti-proliferation kit in the PC-3 cell line. Total phenol content were found between 476.09 ± 1.67 and 865.22 ± 3.57 mg GAE/100 g honey, total flavonoid content 41.67 ± 0.25 and 1249.74 ± 0.85 mg QE/100 g honey, total phenolic acid content 0.74 ± 0.21 and 58.35 ± 1.56 mg SA/100 g honey, β-carotene 1.71 ± 0.06–3.61 ± 0.08 mg/kg honey, lycopene content 0.89 ± 0.03 and 3.41 ± 0.08 mg/kg honey, respectively. Percent removal of H2O2 was determined in the range of 69.79 ± 1.24 and 75.37 ± 1.72 at 10 mg/mL. DPPH percentage removal for 200 mg/mL was between 69.79 ± 1.24 and 75.37 ± 1.72. Phenolic compounds in honey extract were determined as gallic acid, caffeic acid, syringe acid, chlorogenic acid, p-coumaric acid, ferulic acid, catechin, quercetin, chrysin using HPLC analysis. The honey extracts were tested on Gram(+) and Gram(−) bacteria and yeast and their antimicrobial effects were determined. As a result, phenolic honey extraction, performed from five loci from the region of interest, showed anticancer, antioxidant and antimicrobial properties, and can be used as a functional food additive to replace synthetic counterparts.
Keywords: Anticancer, Antimicrobial, Antioxidant, Biological activity, Honey, Phenolic compound
Introduction
Honey is a product created by bees that collect nectar from several plants. It has been a natural nutrient for ages due to its high nutritional value and contribution to human health. Bees collect nectar from flowering plants and secretions from certain creatures living on the plants (Hemiptera), mix those with unique substances in their body, and store the transformed product in honeycombs, where the honey ripens into a sweet-tasting nutrient. The most prevalent honeybee is Apis mellifera, which is commonly found in Asia and Europe (Guerrini et al. 2009; Ismail et al. 2016).
Phenolic compounds, especially phenolic acids and flavonoids, are considered to be the most important components of honey (Prasain et al. 2004). The concentration of these components determines the colour of the honey. Furthermore, they are responsible for sensory features that reflect the specific quality of the honey and antioxidant characteristics (Alvarez-Suarez et al. 2010; Bogdanov et al. 2004; Sergiel et al. 2014). These compounds are also used as anticancer and antimicrobial agents and in the treatment of degenerative diseases.
Reactive oxygen types (ROS) are produced by several physiological and biochemical processes in the human body. Overproduction of these types can cause oxidative damage in cellular biomolecules and biopolymers (lipids, proteins, and DNA). These damages can result in several chronic ageing and degenerative diseases such as arteriosclerosis, cancer, diabetes and nervous transmission system disorders. Honey has potential therapeutic properties in the treatment of heart diseases, cancers, cataracts and various inflammatory diseases (Al-Mamary et al. 2002; Tsiapara et al. 2009).
The antioxidant activity of honey varies extensively based on the flower source (Alvarez-Suarez et al. 2010). Several studies demonstrated that most aromatic and medicinal plants have antioxidant and antimicrobial properties due to secondary metabolites they produce in their foliage, flowers, and fruits via biosynthesis. As a result of these secondary metabolites, most of these plants contain attractive colours and scents for the bees, enticing them to collect the plants’ nectars. Thus, bioactive secondary metabolites produced by the plant are transferred into the honey by honeybees. Studies conducted showed that the antioxidant properties of honey demonstrate differences based on environmental factors, geographical location, flora sources and elevation and temperature changes in honey production areas (Al-Mamary et al. 2002; Baltrušaitytė et al. 2007; Gheldof et al. 2002; Gośliński et al. 2019; Sagdic et al. 2010).
Characteristics such as environmental factors, geography and flora sources promote apiculture as a general livelihood in region with bees. Apiculture is common in Turkey, and particularly around Bingöl, due to these features. Every year, the variety of nectar types and honey production increase due to the unifloral and multifloral characteristics of the Bingöl region. However, there are limited studies analysing the specifications and quality of the honey produced in this region. The objective of this study is to determine the anticancer, antioxidant and antimicrobial effects of the phenolic extracts in honey collected in the Bingöl region, and to research whether these products and their derivatives could be used as functional protective nutrients to substitute for synthetic substances.
Materials and methods
Materials
Collection and storage of honey samples
Sampling was conducted in different areas of the Bingöl Province. Sampled honeys were stored in dark-coloured glass jars kept at room temperature in an environment without direct sunlight until the study commenced.
Chemical substances used
All utilised chemicals and reactive material were procured according to analytical or HPLC class pureness. Amberlite XAD-2 (polymeric adsorbent) was supplied for phenolic extraction. To determine and identify phenolic acid and flavonoid content in phenolic compounds in the honey, dichlorobenzoic acid, caffeic acid, syringic acid, chlorogenic acid, cinnamic acid, p-coumaric acid, gallic acid, sinapic acid, ascorbic acid, p-hydroxybenzoic acid, sinapic acid, hydrochloric acid, galangin, kaemferol, quercetin, myricetin, apigenin, luteolin, chrysin, hesperetin, naringenin, 2,2-diphenyl-picrylhydrazyl (DPPH) radical, ferrozine, linoleic acid, α-tocopherol, polyoxyethylene sorbitan monolaurate (tween-20), trichloroacetic acid (TCA), Folin–Ciocalteu reagent, sodium carbonate, sodium phosphate, aluminium chloride, sodium hydroxide, sodium nitrate, sodium molybdate, ammonium molybdate, methanol, dimethyl sulfoxide (DMSO), ethanol, butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), trolox, tocopherol, ferrous chloride, ferrous cyanate, hydrogen peroxide, acetone, hexane and phenanthroline were provided from Sigma-Aldrich (Germany). RPMI 1640, including foetal bovine serum, and 1% penicillin–streptomycin, for cell cultures, was purchased from Biological Industries (Israel).
Tools and equipment used
High Performance Liquid Chromatography (Shimadzu LC 20AT), Microbiologic safety cabin (Bilser), UV–VIS spectrophotometer (Shimadzu/Jasco V650), UV-Spectrophotometer cell (3 cm3 quartz cell), magnetic mixer (Ika), heated water-bath (Wise Clean), automatic pipettes (Rainin), circular shaker (Gerhardt), ultrasonic bath (Wise Clean WUC-D06H), deep freeze (− 86 °C, Hettich/Nuair), rotating evaporator (Ika RV06-ML), pH-meter (Hanna Instrument), microbalance (Precisa/Denver), incubator (Elektro-Mag [0–300 °C]), vortex (Ika MS3 Basic), distilled water device (GFL 2004), dispenser (Isopenser), centrifuge (Hettich Universal 320), refrigerator (4 °C, Arçelik), autoclave (Hirayama), drying-oven (Memmert 100–800), and microplate reader (Molecular Devices) were used.
Selection of test microorganisms
To determine antimicrobial activity in Bingöl province honey phenolic extracts, three gram-positive (Bacillus subtilis, Staphylococcus aureus, Streptococcus mutans) and four gram-negative (Escherichia coli, Pseudomonas aeruginosa, Salmonella typhimurium, Klebsiella pneumonia) bacteria and two types of yeasts (Candida albicans, Saccharomyces cerevisiae), were used.
These microorganisms were procured from Ankara University, Faculty of Sciences Biology Department and Refik Saydam Public Hygiene Center.
Method
Preparation of honey extracts
Phenolic compounds (phenolic acid and flavonoids) were extracted from Bingöl honey samples. Approximately 100 g of honey was mixed each with 1 at 1 × 10−2 mol/dm3 HCl (300 mL). After the honey was dissolved completely, hard particles were removed by filtering through filter paper. Then, Amberlite was added to the filtrate mixture (pH 2 distilled water and honey). This was then mixed in the magnetic mixer for approximately one hour at room temperature, and the mixture was loaded into the glass chromatographic column and was washed with 1 × 10−2 mol/dm3 of HCl (300 mL) and then with deionised water to remove sugar and other honey ingredients. The phenolic components adsorbed in the colon were taken by laving with 1000 mL of methanol (pH:7), then the methanol was evaporated in a rotary evaporator under the combined conditions of 40 degrees and reduced pressure, and the phenolic extract was obtained.
Residues removed from the phenolic compounds were dissolved using water and diethyl ether. Combined extracts were weighed after exposing to evaporation. They were dissolved again in methanol for the residual piece and antioxidant test and with DMSO for anticancer and antimicrobial activity tests. To remove the phenolic compounds from water-dissolved residue, the residues were treated thrice with 30 mL of dimethyl ether. Combined cores were left for evaporation and then weighed (Estevinho et al. 2008).
HPLC analysis
For identification of flavonoid compounds and phenolic acids, HPLC reversed phase colon was used. The sample was loaded onto the C18 colon using an autoloader. For the mobile phase, 5% of pH 2.5 phosphoric acid (solvent A) and methanol (solvent B) were used. Loading volumes of all honey extract samples were 10 µL. The mobile phase was implemented with a gradient program with a flow rate of 0.5 mL/min as follows: 95–83% A (10 min), 83–74% A (10 min), 74–42% A (20 min), 42–5% A (10 min) and 5–95% A (10 min). The analyses of phenolic acid and flavonoids were conducted under 290 nm and by the comparison of retention times and spectra of specification peaks with commercial standards (Yao et al. 2003).
Determination of anticancer properties of the extracts
Cell Culture Human prostate cancer cells (PC-3) were reproduced in a 5% CO2 incubator with RPMI 1640 cell growth medium that contained 10% foetal bovine serum and 1% penicillin–streptomycin. The cells were checked every day and were passaged every 2 to 3 days. The number of passes of the cells to be tested was kept between 5 and 10. More passages will create stress in the cells. A more passaged cell response to the active substances given under stress will prevent getting the correct result. Therefore, the substances were applied by keeping the cells between 5 and 10 passages in the first active passage and the last active substance application.
Cell viability analysis
Cell viability analysis was conducted with the WST-1 cell reproduction kit (Clontech Laboratories). After PC-3 cells were reproduced, they were planted in a 96-well plate with 5 × 103 cells in each well. After 24 h, honey extracts were added to the cells in the well in 1000, 500, 250, 125, 60, 30, 15 and 7 µg/mL doses and incubated for 24 h. Only growth medium was added to control wells. At the end of incubation, 5 µL of WST-1 was added to each well and the cells were incubated for 4 h. The absorbance of each well at 450 nm was measured with a microplate reader (Molecular Devices). Reference wavelength was accepted as 630 nm.
Determination of the antioxidant activity of the extracts
Total phenolic substance amount
Total phenol amount in extracts was determined by the Folin-Ciocalteu assay (Al-Mamary et al. 2002). Standard gallic acid samples were prepared in 70% methanol. 150 µL of the sample, 150 µL of Folin-Ciocalteu reagent (50%, h/h, in water) and 3 mL of sodium carbonate solution (2%, a/h, in water) were mixed in a flask and kept at room temperature for 30 min. After that, solution absorbance was measured in UV spectrophotometer at 760 nm, and the total phenol amount was calculated using the calibration curve plotted with gallic acid as the equivalent of mg gallic acid.
Total flavonoid content
To determine the total flavonoid amount, 150 µL of honey extract was transferred into 600 µL of distilled water, and 90 µL of 15% NaNO2 solution was added to this mixture and stirred. After 6 min, 10% AlCl3H2O solution was added to the mixture and stirred. At the end of following 7 min. 600 μL of 1 M NaOH and 1500 μL of distilled water were added, and the mixture was stirred gently until well-mixed. The resulting pink coloured mixture was read at 510–415 nm based on the previously prepared quercetin standard calibration graph, and total flavonoid content in mg/kg honey was calculated as quercetin equivalent (Barros et al. 2007a).
Total phenolic acid (TPA) content
The solvent (2.0 mL, prepared by dissolving 0.5 M of hydrochloric acid (2.0 mL), 10 g of sodium nitrite and 10 g of sodium molybdate in 100 mL of water, was added to the freshly prepared honey extract (1.0 mL). Then, 2.0 mL (8.5% w/w) of sodium hydroxide was added. Absorbance was read at 505 nm. 10 mL of water was used control. A standard calibration curve was plotted using total phenolic acid and sinapic acid. The total phenolic acid content was calculated using this calibration curve as sinapic acid equivalent (mg/g). (European Pharmacopoeia 2009).
Total β-carotene and Lycopene content
To determine carotenoid content spectrophotometrically based on the method developed by Barros et al. (2007b), 100 mg of honey extract samples were placed in 10 mL of acetone-hexane (4:6) mixture, stirred well for 1 min, and then filtered using Whatman No. 4 filter paper. Filtrate absorbance values were measured at 543, 505 and 663 nm wavelengths. β–carotene and lycopene contents were assessed with standard graphs and carotenoid amount per kg honey was calculated in milligrams (Barros et al. 2007b).
Removal of DPPH radical effect
In DPPH free radical capture method, this stable and synthetic radical is used, and antioxidant activity is identified by measuring the ability of the antioxidant to capture this free radical. 2.7 mL of methanol mixture containing DPPH radical, prepared as 6 × 10−5 mol/litre of watered honey phenolic extracts (0.3 mL), was prepared. This mixture was shaken well and stored in a dark area for 60 min. Absorption was measured at 517 nm to determine the decrease in the DPPH radical. Spectrophotometric reading was conducted by mixing the extract obtained from the honey and the radical to determine radical activity. It was calculated by opening the colour of DPPH (Barros et al. 2007b).
Measurement of reduction potential property
2.5 mL of honey’s extracts samples, diluted in different concentrations, and phenolic extracts were mixed with 2.5 mL of 200 mol/l sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferric cyanide, and incubated for 20 min at 50 °C. Then, 2.5 mL of 10% trichloroacetic acid was added, and the final mixture was centrifuged for 8 min at 1000 rpm. After the centrifuge, the supernatant was removed and 5 mL of deionised water and 1 mL of 0.1% ferrous chloride (FeCl3.6H2O) were added to approximately 5 mL of supernatant, and spectrophotometric reading on the final product was conducted at 700 nm. It was determined that high absorbance demonstrated high reduction property. The reduction potential of honey samples was calculated against α-tocopherol standard. (Barros et al. 2007b).
Hydrogen peroxide removal activity
Hydrogen peroxide removal activities for the honey extracts and synthetic antioxidant substances prepared at different concentrations were determined using Ruch’s (1989) method. For this purpose, a 43 mM hydrogen peroxide solution was prepared in a phosphate buffer with a pH value of 7.4. H2O2 concentration was determined spectrophotometrically with H2O2 absorbance reading at 230 nm. Honey extracts in 5, 10, 15, and 20 μg/mL concentrations were completed to 4 mL volume with buffer solution. Then, 0.6 mL of hydrogen peroxide (43 mM) solution was added. The solution was incubated for 10 min at room temperature, and the decrease in hydrogen peroxide amount was recorded as decreased absorbance at 230 nm. A buffer solution containing hydrogen peroxide was utilised as a control (Ruch et al. 1989).
Total antioxidant capacity
Antioxidant activities in the samples were assessed with phosphomolybdenum method and expressed as α-tocopherol equivalent. In short, the extracts dissolved in 0.4 mL methanol was mixed with 4 mL reagent solution (0.6 M sulfuric acid, 26 mM sodium phosphate and 4 mM ammonium molybdate). As a blind, the honey extract was substituted with methanol. The reaction solution was mixed with vortex and remained for 90 min in a water bath 95 °C. Absorbance was measured at 695 nm wavelength. Antioxidant activity was calculated as α-tocopherol equivalent (α-TE mg/g extract) (Prieto et al. 1999).
Determination of antimicrobial activity of the extracts
Extracts of honey samples, from which solvents were evaporated, were dissolved in dimethyl sulfoxide (DMSO) in a 25 mg/mL ratio. Extracts were filtered with by 0.22 μm nylon membrane filters. Later on, 17 g of Mueller–Hinton Agar was mixed with 500 mL of distilled water, and the prepared medium was transferred to petri dishes. Petri dishes containing hardened media were stored in the refrigerator at +4 °C until they were used. Microorganism culture suspensions used in the test, each 200 μl (includes approximately 106 colonies based on McFarland 0.5 equation), were transferred into petri dishes containing Mueller–Hinton Agar and spread on the surface using a swab. After that, discs that were saturated with 40 μl (20 μl + 20 μl) of extract each were placed in the media located in the petri dishes, in which bacteria were planted with a sterile plier. Discs that were saturated with 40 μl of DMSO were used as negative control. Streptomycin was utilised as the reference antimicrobial. Petri dishes were incubated for 1 h at 4 °C, and then for 24 h at 37 °C. Antimicrobial activity was determined by the measurement of the diameter of the zones around the discs saturated with the extract (Berghe and Vlietinck 1991).
Statistical analyses
All measurements were repeated 3 times, and the analyses of differences were conducted using Kruskal–Wallis ANOVA, multiple post hoc comparisons were made using Dunett’s and Tukey’s tests. Statistical significances were expressed at P ≤ 0,05. The statistical analysis was performed with Graphpad Prism 8.0.3 software.
Results and discussion
Phenolic compounds in honey
Analysis of phenolic compounds in honey samples revealed the presence of gallic acid, dichlorobenzoic acid, caffeic acid, syringic acid, chlorogenic acid, p-Coumaric acid, sinapic acid, ferulic acid and chrysin, quercetin, catechin as of flavonoids. HPLC chromatogram of phenolics for the Bingöl province are shown in Table 1; phenolic acids counts were higher than flavonoids. The chromatogram demonstrates that there were peaks related to other compounds as well. However, due to insufficient standard compounds, it was not possible to define these phenolic groups and compounds. Identified phenolic compounds were consistent with those determined in previous studies (Yao et al. 2003).
Table 1.
Phenolic substances determined in Bingöl honey phenolic extracts by HPLC chromatography
| Samples | Gallic acid |
Dichlorobenzoic acid | Caffeic acid | Syringic acid | Chlorogenic acid | Coumaric acid | Sinapic acid | Ferulic acid | Catechin | Quercetin | Chrysin |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Center Honey Extract | + | − | + | + | + | + | – | + | + | + | + |
| Solhan Honey Extract | + | + | − | + | − | + | + | + | − | + | + |
| Yedisu Honey Extract | + | − | + | + | + | + | − | + | + | + | + |
| Adaklı Honey Extract | + | − | + | + | + | + | − | + | + | + | + |
| Karlıova Honey Extract | + | + | + | + | + | + | + | + | + | + | + |
Anticancer research
Effects of phenolic extracts on the proliferation of PC-3 cells
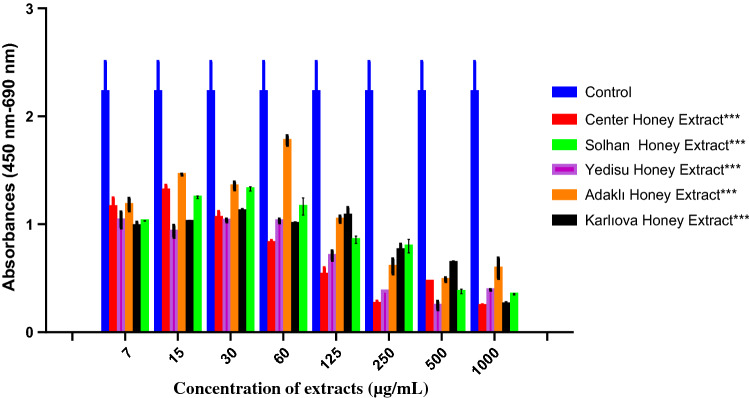
The effect of phenolic extracts of honey samples on the vitality of PC-3 cells was determined with the WST-1 method as in vitro, and shown in Fig. 1. It was determined. It was determined that honey phenolic extracts applied to PC-3 cancer cells during a 24 h period in different concentrations significantly decreased cell vitality in 500 and 1000 µg/mL concentrations compared to control cells. It showed an anticancer effect in these doses and significantly decreased cell reproduction. The significant decreases observed with other identified doses are presented in the bar chart displayed in Fig. 1.
Fig. 1.
The graph shows the cytotoxic effect of honey phenolic extract on PC-3 cells. Honey phenolic extract was applied on PC-3 cells for 24 h in specific doses. No agent was applied to control cells and only growth medium was added. Statistical analysis was performed with one-way ANOVA using the Dunnett’s multiple comparison test as a post test in the confidence interval of P < 0.001 (***). Values are expressed in mean ± SEM (n = 3)
As could be observed in Fig. 1, honey phenolic extracts dose application caused a significant cytotoxic effect in PC-3 cells compared to the control group.
Total phenolic content
Phenolic compounds in honeys were expressed as total phenolic substance, total flavonoid and total phenolic acid contents (Table 2). According to the results, the total phenolic content in honey extracts was found to be in the range of 476.08–865.21 as gallic acid equivalent by plotting gallic acid standard calibration curve (R2: 0.9905). Total phenolic content of various honey extracts has also been reported in several previous studies (Boussaid et al. 2014; Estevinho et al. 2008; Sergiel et al. 2014). In a study by Al-Mamary et al. (2002), phenolic content of various honey types was identified as 56.32–246.21 mg GAE/100 g. Ferreira et al. (2009) reported total phenolic content as 22.61–72.77 mg GAE/100 g in the honey of northern Portugal. Sagdic et al. (2010) determined total phenolic content of Vitellaria honey as 86.67 mg GAE/100 g. Can et al. (2015) determined total phenolic substance amount in various honey types from Turkey as 16.02–120.04 mg GAE/100 g. Özkök et al. (2010) reported phenolic content in pine honey samples from Muğla province in Turkey between 25 and 50.0 mg GAE/100 g. The results of the analysis in this section show that; The range of values (Table 2) found in this study was to be higher than those obtained from various regions in the literature.
Table 2.
Honey phenolic extract amounts
| Samples | Total phenolic content (mg GAE/100 g extract) |
Total flavonoid content (mg QE/100 g extract) |
Total phenolic acid content (mg SA/100 g extract) |
β-carotene (mg/kg extract) |
Lycopene (mg/kg extract) |
|---|---|---|---|---|---|
| Center Honey Extract | 625.47 ± 1.97* | 65.41 ± 0.01* | 14.99 ± 0.85* | 1.49 ± 0.02* | 0.89 ± 0.03* |
| Solhan Honey Extract | 476.08 ± 1.65** | 41.67 ± 0.25** | 0.74 ± 0.21** | 2.40 ± 0.06** | 0.58 ± 0.04* |
| Yedisu Honey Extract | 699.06 ± 2.36*** | 101.29 ± 0.32*** | 14.77 ± 1.21* | 3.60 ± 0.08*** | 3.41 ± 0.07*** |
| Adaklı Honey Extract | 697.52 ± 3.66*** | 137.16 ± 0.23*** | 18.49 ± 0.99* | 3.07 ± 0.09*** | 2.48 ± 0.09** |
| Karlıova Honey Extract | 865.22 ± 3.57**** | 1249.74 ± 0.85**** | 58.35 ± 1.56*** | 1.70 ± 0.05**** | 1.68 ± 0.04**** |
Statistical analysis of phenolics contents in honey phenolic extracts was done according to was performed with one-way ANOVA using Kruskal–Wallis multiple comparison as a post test in the confidence interval of either P < 0.05 (*), P < 0.01 (**), P < 0.001 (***) or P < 0.0001(****).Values are expressed in mean ± SEM (n = 3)
Determination of total flavonoid content
Flavonoids are secondary metabolite phenolic components of honey with low molecular weight, which are responsible for antioxidant and aromatic functions. The total flavonoid content of honey extracts was calculated as the equivalent of quercetin in the range 41.67–1249.74 (mg QE/100 g) using a standard calibration curve (R2: 0.9705) plotted for quercetin (Table 2). Meda et al. (2005) found total flavonoid content in multifloral honey within 0.17–8.35 mg QE/100 g honey mg interval. In a study conducted with unifloral Bangladesh honey, In a study conducted with monofloral pine honey in Muğla, Turkey, Özkök et al. (2010) determined that total flavonoid content was between 4.80 and 54.78 mg QE/kg honey. In study conducted with unifloral various honey types from Turkey, Can et al. (2015) determined that total flavonoid content was between 1.65 and 8.10 mg QE/100 g honey. Total flavonoid amount found in Bingöl honey extracts was higher when compared to other studies.
Total phenolic acid (TPA) content
Methods to determine total phenolic acid content have not been used in previous studies. The total phenolic substance is given as a gallic acid equivalent, which is a phenolic acid itself. The phenolic acid amount in the extracts was calculated using the standard graph plotted with sinapic acid, which is a cinnamic acid derivative, and was found as 0.74–58.35 sinapic acid equivalent (R2: 0.9621) (Table 2).
Determination of total β-carotene and Lycopene content
Total carotene and total lycopene content were calculated as 1.70 ± 0.05–3.60 ± 0.08 mg/kg honey and 0.89 ± 0.03–3.41 ± 0.07 respectively (Table 2). In a study with Portuguese honey, Ferreira et al. (2009) reported that the total carotenoid content was between 8.64 ± 0.06 mg/kg and 9.49 ± 0.15 mg/kg. In a study with arid and unifloral honey, Habib et al. (2014) reported that the total carotenoid content was 38.89 ± 1.42 μg/100 g.
Antioxidant research findings
In several studies, antioxidant activity in honey has been found using different methods (Can et al. 2015; Gheldof et al. 2002). However, this study was the first to determine the antioxidant capacity of the honey extract using phenolic extraction in the whole Bingöl province.
Removal of the effect of DPPH radical
The radical removal effect of honey phenolic extracts is identified by removal of the dark violet colour of DDPH oxidant prepared in methanol solution spectrophotometrically at 517 nm. Removal of the colour provides for the removal of oxidant functional groups of DPPH and its transformation to its analogue, hydrazine. Working with this radical is considered to be more advantageous because this minimizes secondary reactions (Amarowicz et al. 2004). Furthermore, it can be used to determine potential antioxidant effects of extracts in a short period of time. The results using DPPH percentage that includes extract against the control sample prepared using only DPPH, and are presented in Table 3. Analysis results are presented in Table 3. The results of the study conducted by Estevinho et al. (2008) found DPPH removal activity between 27.24 and 68.17 mg/mL as EC50 value. DPPH (EC50 value) removal activity of this study were between 85.25 and 98.28 μg/mL. Al-Mamary et al. (2002) found the antioxidant capacity within the 9.9–65.44 range in 200 µL/mL concentration in acacia honey, a monofloral honey.
Table 3.
DPPH and reduction power inhibition and EC50 values of honey phenolic extracts and synthetic antioxidants
| Extracts and synthetic antioxidants | DPPH inhibition EC50 (µg/mL) |
% Inhibition of DPPH (200 µg/mL) |
Reduction power (200 µg/mL) (Abs) |
|---|---|---|---|
| Center Honey Extract | 97.26 ± 5.79* | 54.08 ± 0.26** | 0.19 ± 0.08* |
| Solhan Honey Extract | 98.29 ± 8.43* | 55.71 ± 0.33** | 0.18 ± 0.06* |
| Yedisu Honey Extract | 85.25 ± 6.56** | 64.00 ± 0.81* | 0.22 ± 0.03* |
| Adaklı Honey Extract | 97.46 ± 9.79* | 55.91 ± 0.47** | 0.22 ± 0.04* |
| Karlıova Honey Extract | 98.07 ± 7.89* | 56.60 ± 0.72** | 0.12 ± 0.08** |
| BHA | 80.31 ± 9.91 | 61.40 ± 039 | 2.44 ± 0.09 |
Statistical analysis of DPPH inhibition EC50 and Reducing Power in honey phenolic extracts was done according to BHA standard antioxidant. Statistical analysis was performed with one-way ANOVA using Dunnett’s multiple comparison as a post test in the confidence interval of either P < 0.05 (*) or P < 0.001 (**). Values are expressed in mean ± SEM (n = 3)
Measurement of reduction potential property
In a study conducted to determine the reduction capacity of phenolic compounds obtained from light and dark coloured honey from the Tras Montes region located in north-eastern Portugal, Estevinho et al. (2008) identified that absorbance value at 700 nm (0.23, 0.45, 0.79) increased with the increasing concentration (12.5 mg/mL, 25 mg/mL, 50 mg/mL) in dark-coloured honey. In the same study, it was determined that absorbance value at 700 nm (0.07, 0.12, 0.23) increased with the increasing concentration (12.5 mg/mL, 25 mg/mL, 50 mg/mL) in light-coloured honey. In this study 200 µg/mL, reduction capacity of phenolic extracts obtained from honey samples 0.12 ± 0.08–0.22 ± 0.03 of ferric ions (Fe+3) to ferrous ions (Fe+2) increased based on the concentration, demonstrating similar findings to the study conducted by Estevinho et al. (2008). However, when the ability of honey extracts used in our study was compared to synthetic antioxidant (Trolox), its reduction potential was lower, as displayed in Table 3.
Hydrogen peroxide removal activity
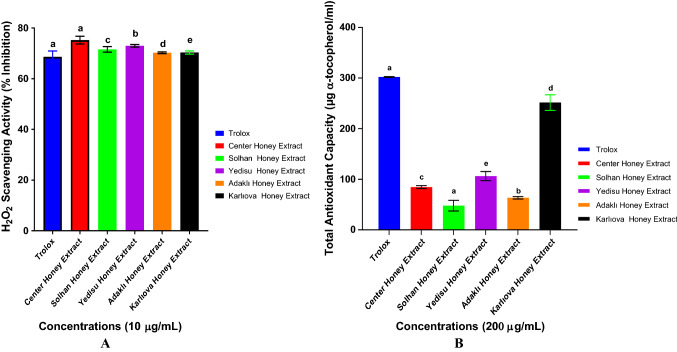
In this study, antioxidant tests were carried out to determine the effectiveness of phenolic extraction of honey. One of the antioxidant activity assessment analyzes is hydrogen peroxide removal activity. However, this analysis is not studied in honey samples since it contains hydrogen peroxide in honey. In this study, honey’s phenolic extraction was performed and the activity of phenolic substances to remove hydrogen peroxide agent was determined. The main aim of the study is to find out the answer to the question whether the phenolic content of such honey can be evaluated as a functional food additive. Many antioxidant determination methods have been used in the literature. In this study, DPPH removal activity and reduction power as well as hydrogen peroxide removal activity methods were used to determine antioxidant effects. Figure 2 demonstrates hydrogen peroxide removal activity of 10 μg/mL concentration of methanol extract obtained from the honey sample as compared to standard antioxidant Trolox. Percent removal of H2O2 was determined in the range of 69.79 ± 1.24–75.37 ± 1.72 at 10 μg/mL in this study.
Fig. 2.
A Statistical analysis of H2O2 removal activity of honey phenolic extracts and Trolox at a concentration of 10 μg/mL was performed with one-way ANOVA using the Kruskal–Wallis as a post test in the confidence interval of P < 0.05. B Total antioxidant activity in the honey phenolic extracts and Trolox was performed with one-way ANOVA using the Kruskal–Wallis as a post test in the confidence interval of P < 0.001. Values are expressed in mean ± SEM (n = 3)
Total antioxidant capacity
The antioxidant capacity of the phenolic honey extract was determined with the spectrophotometric phosphomolybdenum method. 3 parallel repetitions were conducted to determine the antioxidant capacity of the honey samples, and conducted measurements were calculated. El-Haskoury et al. (2018), the total antioxidant capacity in 8 different honeys of Morocco was defined as ascorbic acid equivalent in the range of 35.03 ± 0.61 to 60.94 ± 1.88 mg AAE/g honey. Sagdic et al. (2013), who performed a study regarding 35 different honey varieties of Turkey, found out total antioxidant capacity between 57.03 and 114.69 mg AAE/g honey. Imtara et al. (2018) determined the total antioxidant capacity in the 10 honey varieties of Palestine in the range of 81.18 ± 3.01 to 120.04 ± 1.59 mg AAE/g. Total antioxidant capacity was determined in the range of 37.24 ± 5.6–267.6 ± 9.2 mg α- TE/g extract in this study. There are total antioxidant studies generally done with honey itself in literature. There are not many studies regarding the phenolic extract of honey. Total antioxidant capacity was found out higher in this study than former studies due to concentrated phenolic substances in phenolic extract. Results are presented in Fig. 2 as mg α- TE/g extract. Antioxidant activity was calculated as α-tocopherol equivalent (R2 = 0.999) (α-TE mg/g extract).
Determination of antimicrobial activity
The antimicrobial effects of honey emerge as a result of acidity, osmolality, and the combination of hydrogen peroxide and phenolic substances. In this study, only the effect of phenolic substances was scrutinized. Several studies have demonstrated the antimicrobial effects of phenolic compounds. (Barros et al. 2007a; Estevinho et al. 2008).
The results of the study showed high antimicrobial properties against S. aureus, P. aeruginosa, S. typhimurium, and E. coli bacteria moderate antimicrobial properties against S. mutans and B. subtilis microorganisms and low antimicrobial properties against K. pneumoniae and C. albicans. The findings of the experiments conducted to determine the antimicrobial activity of the honey samples are given in Table 4. Results were similar to the findings of the study by Estevinho et al. (2008). Results demonstrated a higher sensitivity to the gram (+) bacteria. However, gram (−) ones exhibited findings were consistent with the results of other studies in the literature (Estevinho et al. 2008; Mundo et al. 2004).
Table 4.
Antimicrobial effect of honey phenolic extracts
| Microorganisms | Microorganisms international codes | Center honey extract | Solhan honey extract | Yedisu honey extract | Adaklı honey extract | Karlıova honey extract | Str. | DMSO |
|---|---|---|---|---|---|---|---|---|
| Inhibition zone, mm | ||||||||
| Bacillus subtilis(gram (+)) | IM622 | 9.2 ± 0.1*** | 8.1 ± 0.4** | 8.1 ± 0.1** | 8.2 ± 0.1** | 8.2 ± 0.1** | 25 | R |
| Candida albicans(yeast) | ATCC 96268 | 7.3 ± 0.2* | R* | R* | R* | R* | 25 | R |
| Escherichia coli(gram (−)) | ATCC 25922 | 10.2 ± 0.2*** | R* | 10.3 ± 0.1**** | 10.2 ± 0.5*** | R* | 26 | R |
| Klebsiella pneumoniae (gram (−)) | ATCC 13883 | 7.4 ± 0.3* | R* | 9.2 ± 0.3*** | R* | 9.1 ± 0.2*** | 20 | R |
| Pseudomonas aeruginosa (gram (−)) | DSM 50071 | 11.1 ± 0.2d | 9.1 ± 0.2*** | 8.1 ± 0.2** | 10.1 ± 0.3*** | 8 ± 0.1** | 26 | R |
| Salmonella typhimurium(gram (−)) | ATCC 13311 | 10.9 ± 0.1*** | 8.2 ± 0.1** | 11.2 ± 0.1**** | 10 ± 0.1*** | 7.2 ± 0.2** | 25 | R |
| Saccharomyces cerevisiae (yeast) | ATCC 76521 | 7.0 ± 0.5* | R* | R* | R* | 7.1 ± 0.1** | 22 | R |
| Staphylococcus aureus (gram (+)) | ATCC 6538P | 12.0 ± 0.1d | 10.2 ± 0.5*** | 10.1 ± 0.3*** | R* | 11 ± 0.1**** | 25 | R |
| Streptococcus mutans. (gram (+)) | ATCC 35668 | 8.0 ± 0.0** | R* | R* | R* | R* | 22 | R |
Statistical analysis was performed with one-way ANOVA using Dunnett’s multiple comparison as a post test in the confidence interval of either P < 0.05 (*), P < 0.01 (**), P < 0.001 (***) or P < 0.0001(****).Values are expressed in mean ± SEM (n = 3). Streptomycin (Str) discs were used as positive control. DMSO (Merck) was used as negative control. Each disc had a 6 mm diameter and each disc was saturated with 40 μl extract (1 mg/mL)
Conclusion
The results obtained from the study revealed that the Bingol Honey was represented with considerably high total phenolic and flavonoid content. Comparing to those recorded in the literature, this might be distinguishing feature of Bingöl Honey owing probably to the unique nature of regional geography. Accompanied with this, relatively higher β-carotene and lycopene content was also detected. Despite higher flavonoid and carotenoid content than those given in the literature, the antioxidant capacity and the ferric reducing capacity of the Bingöl Honey reflected higher values. This induced that further attempt on the application of other assays might be needed to make our results comparable to those of others or to assure if the measured low antioxidant activity was not due to the mechanism of the selected assays. On the other hand, the Bingöl Honey was proved compatible against its synthetic counterparts, tocoferol, BHT and BHA, in terms of antioxidant capacity and reducing power, values of which were determined similar or even better.
The findings of the present study demonstrated that floral properties of honey were active in correlation with the phenolic substances they contained with respect to their antibacterial properties. Also, the phenolic content of honey extract had anti-proliferation effects and prevents the reproduction of cancerous cells. These results implied that the Bingöl Honey has a great potential to use in preventive and rehabilitative utilization for the treatment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bülent Kaya, Email: b_kaya_tr@yahoo.com.
Adem Yıldırım, Email: adem_yildirim63@hotmail.com.
References
- Al-Mamary M, Al-Meeri A, Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutr Res. 2002;22:1041–1047. doi: 10.1016/S0271-5317(02)00406-2. [DOI] [Google Scholar]
- Alvarez-Suarez JM, Tulipani S, Diaz D, Estevez Y, Romandini S, Giampieri F, Damiani E, Astolfi P, Bompadre S, Battino M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem Toxicol. 2010;48:2490–2499. doi: 10.1016/j.fct.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–562. doi: 10.1016/S0308-8146(03)00278-4. [DOI] [Google Scholar]
- Baltrušaitytė V, Venskutonis PR, Čeksterytė V. Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem. 2007;101:502–514. doi: 10.1016/j.foodchem.2006.02.007. [DOI] [Google Scholar]
- Barros L, Calhelha RC, Vaz JA, Ferreira ICFR, Baptista P, Estevinho LM. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur Food Res Technol. 2007;225:151–156. doi: 10.1007/s00217-006-0394-x. [DOI] [Google Scholar]
- Barros L, Ferreira M-J, Queirós B, Ferreira ICFR, Baptista P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007;103:413–419. doi: 10.1016/j.foodchem.2006.07.038. [DOI] [Google Scholar]
- Berghe VA, Vlietinck AJ. Screening methods for antibacterial and antiviral agents from higher plants. Methods Plant Biochem. 1991;6:47–68. [Google Scholar]
- Bogdanov S, Ruoff K, Oddo LP. Physico-chemical methods for the characterisation of unifloral honeys: a review. Apidologie. 2004;35:4–17. doi: 10.1051/apido:2004047. [DOI] [Google Scholar]
- Boussaid A, Chouaibi M, Rezig L, Hellal R, Donsì F, Ferrari G, Hamdi S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab J Chem. 2014;11(2):265–274. doi: 10.1016/j.arabjc.2014.08.011. [DOI] [Google Scholar]
- Can Z, Yildiz O, Sahin H, Akyuz Turumtay E, Silici S, Kolayli S. An investigation of Turkish honeys: their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015;180:133–141. doi: 10.1016/j.foodchem.2015.02.024. [DOI] [PubMed] [Google Scholar]
- El-Haskoury R, Kriaa W, Lyoussi B, Makni M. Ceratonia siliqua honeys from Morocco: physicochemical properties, mineral contents, and antioxidant activities. J Food Drug Anal. 2018;26(1):67–73. doi: 10.1016/j.jfda.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevinho L, Pereira AP, Moreira L, Dias LG, Pereira E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem Toxicol. 2008;46:3774–3779. doi: 10.1016/j.fct.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Ferreira ICFR, Aires E, Barreira JCM, Estevinho LM. Antioxidant activity of Portuguese honey samples: different contributions of the entire honey and phenolic extract. Food Chem. 2009;114:1438–1443. doi: 10.1016/j.foodchem.2008.11.028. [DOI] [Google Scholar]
- Gheldof N, Wang X-H, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 2002;50:5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- Gośliński M, Nowak D, Kłębukowska L. Antioxidant properties and antimicrobial activity of manuka honey versus Polish honeys. J Food Sci Technol. 2019;57(4):1269–1277. doi: 10.1007/s13197-019-04159-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini A, Bruni R, Maietti S, Poli F, Rossi D, Paganetto G, Muzzoli M, Scalvenzi L, Sacchetti G. Ecuadorian stingless bee (Meliponinae) honey: a chemical and functional profile of an ancient health product. Food Chem. 2009;114:1413–1420. doi: 10.1016/j.foodchem.2008.11.023. [DOI] [Google Scholar]
- Habib HM, Al Meqbali FT, Kamal H, Souka UD, Ibrahim WH. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014;153:35–43. doi: 10.1016/j.foodchem.2013.12.048. [DOI] [PubMed] [Google Scholar]
- Imtara H, Elamine Y, Lyoussi B. Physicochemical characterization and antioxidant activity of Palestinian honey samples. Food Sci Nutr. 2018;6(8):2056–2065. doi: 10.1002/fsn3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail NI, Abdul Kadir MR, Mahmood NH, Singh OP, Iqbal N, Zulkifli RM. Apini and Meliponini foraging activities influence the phenolic content of different types of Malaysian honey. J Api Res. 2016;55:137–150. doi: 10.1080/00218839.2016.1207388. [DOI] [Google Scholar]
- Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- Mundo MA, Padilla-Zakour OI, Worobo RW. Growth inhibition of foodborne pathogens and food spoilage organisms by select raw honeys. Int J Food Microbiol. 2004;97:1–8. doi: 10.1016/j.ijfoodmicro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Özkök A, D’Arcy B, Sorkun K. Total phenolic acid and total flavonoid content of Turkish Pine Honeydew honey. JAAS. 2010;2:65–71. doi: 10.3896/IBRA.4.02.2.01. [DOI] [Google Scholar]
- Prasain JK, Wang C-C, Barnes S. Mass spectrometric methods for the determination of flavonoids in biological samples. Free Radical Biol Med. 2004;37:1324–1350. doi: 10.1016/j.freeradbiomed.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Sagdic O, Silici S, Ekici L. Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem. 2010;121:238–243. doi: 10.1080/10942912.2011.561463. [DOI] [Google Scholar]
- Sagdic O, Silici S, Ekici L. Evaluation of the phenolic content, antiradical, antioxidant, and antimicrobial activity of different floral sources of honey. Int J Food Prop. 2013;16(3):658–666. doi: 10.1080/10942912.2011.561463. [DOI] [Google Scholar]
- Sergiel I, Pohl P, Biesaga M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014;145:404–408. doi: 10.1016/j.foodchem.2013.08.068. [DOI] [PubMed] [Google Scholar]
- Tsiapara AV, Jaakkola M, Chinou I, Graikou K, Tolonen T, Virtanen V, Moutsatsou P. Bioactivity of Greek honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and endometrial cancer (Ishikawa) cells: profile analysis of extracts. Food Chem. 2009;116:702–708. doi: 10.1016/j.foodchem.2009.03.024. [DOI] [Google Scholar]
- Yao L, Datta N, Tomás-Barberán FA, Ferreres F, Martos I, Singanusong R. Flavonoids, phenolic acids and abscisic acid in Australian and New Zealand Leptospermum honeys. Food Chem. 2003;81:159–168. doi: 10.1016/S0308-8146(02)00388-6. [DOI] [Google Scholar]