Abstract
The use of ozone as a tool in the storage of some horticultural produces is recommended for all steps from harvest to consumption. However, little is known about its effects on the removal of pesticides and postharvest physiology of fresh peppers. In the present study, the effects of ozone treatment on the removal of pesticides, storage life and quality of green peppers were investigated. Malathion, emamectin benzoate and acetamiprid were applied to pepper plants before harvest. Residue contents of peppers were measured at harvest time and after all treatments to determine the effect of ozone on the removal of pesticide residues. Peppers were subjected to four treatments: immersion in ozonated water (2 ppm) and only tap water (control) for 10 min, exposure to 2 ppm ozone gas in air and only air (control) for 45 min. Treated peppers were stored at 20 °C and 60 ± 5% relative humidity for 8 days, and some quality analyses were performed during storage. Ozonated water decreased, remarkable, pesticide residues in peppers compared to harvest time, but there was no meaningful changes in the samples treated with ozone in air. Ozone treatments suppressed clearly respiration rates and decreased weight losses of peppers compared to control groups. Ozonated water also maintained green color of peppers, with minimum change in h° values. Additionally, sensory quality of peppers was retarded by ozone application during storage. These findings revealed that ozone could be an alternative treatment to extend storage life of green peppers and remove pesticide residues.
Keywords: Ozone, Pepper, Quality, Pesticide residue, Storage
Introduction
Pepper is one of the most widely grown and consumed vegetables in many countries because of consumer demand. Today, the pepper production of world is 36.771.482 tons (FAOSTAT 2020), and continues to increase due to being used in different recipes. Although the application of pesticides close to harvest time is banned in many countries, they have still been used by growers, especially in developing countries. Therefore, vegetables having pesticide residues higher than limit values have been exported and imported between countries. Consumers are becoming gradually concerned about the chemical residues of their food, because they affect human and environmental safety. On the other hand, some sanitizer such as sodium hypochlorite and hydrogen peroxide used widely in the sanitation of fruit and vegetables after postharvest, have also been threatening human health and environment. Beltran et al. (2005) reported that some products derived from the reaction of chlorine with organic residues continue to be threat for living organism. Prolonging the postharvest life of fruit and vegetables by harmful products is restricted owing to their undesirable effects on the natural environment. Therefore, some researches have focused on the alternative agents, safety materials and methods for extending the postharvest life of horticultural crops (Horvitz and Cantalejo 2014). To prevent these harmful effects caused by pesticides and chlorine-based sanitizers, environmentally friendly treatments such as ultraviolet light (UV), ozone (O3) and other alternative product to chlorine must be investigated detailed.
Ozone, confirmed as a disinfectant agent by U.S. Food and Drug Administration in 2001, has attracted much attention of scientists and people who work in different sectors (Karaca and Velioğlu 2014). Ozone, being used widely in food industry, is a non-toxic and strong antimicrobial agent (Whangchai et al. 2006), and can safely be applied to fruit and vegetables as gaseous or dissolved in water (Kuşçu and Pazır 2004), when the appropriate dose and duration are determined. In previous studies, it has been tested for postharvest treatments in fruit and vegetables, and found to have positive effect on the storage life and quality (Palou et al. 2002; Dilmaçünal et al. 2014; Luo et al. 2019; Bolel et al. 2019). Moreover, postharvest ozone treatments promote resistance of plants, kill pathogen spores (Smilanick et al. 1999) and reduce microbial population on produce (Zhang et al. 2005; Whangchai et al. 2006). On the other hand, ozone has been used for mycotoxin and pesticide degradation in fruit and vegetables. McKenzie et al. (1997) reported that ozone degraded aflatoxin B1 and G1 rapidly, while aflatoxin B2 and G2 required higher ozone dose due to their resistance against oxidation. Patulin was completely degraded by using ozone within 15 s (Mckenzie et al. 1997) and 3 min (Karaca and Velioğlu 2007). Similarly, some pesticides such as malathion, diazinon, atrazine, bromoxynil and trifluralin were oxidized by ozone for decomposition or degradation in aqueous solution (Ku et al. 1998; Ma and Graham 2000; Masten et al. 2001). Furthermore, it was reported that some residual pesticides on the surface of fruit and vegetables were degraded by ozone treatment (Ong et al. 1996; Hwang et al. 2001; Wu et al. 2007; Kuşvuran et al. 2012). In these studies, pesticide residues were removed (changing levels between 46.0 and 98.6%) from produce depending on atmosphere temperature, application dose, type and duration. Ikeura et al. (2011) reported that pesticide residues on fruit could be removed efficiently by washing with water, in which ozone was dissolved using microbubbles system. Authors recommended this method, because the microbubbles, bigger than 50 µm, rise slowly to the surface of water and the gas inside is dissolved in water completely. Therefore, we used microbubbles system to dissolve ozone in cold water in the present study.
In previous studies, however, some physiological disorders and undesirable results in sensory quality of horticultural produce were determined depending on ozone dose, application duration and type (Smilanick 2003; Beltran et al. 2005). Moreover, the reactions between ozone and pesticides on the fruit surface may produce by-products during ozonation. Therefore, there is still need detailed investigation for possible effects of different ozone treatments on postharvest quality of fruit and vegetables.
This study aimed to determine the effects of ozonated water on the shelf life of peppers and removal of residual pesticides from the surface of peppers.
Materials and methods
Plant material, pesticide and ozone treatments
The study was conducted with green pepper (Capsicum annuum L. cv. BT Burdem 016). Three insecticides, which are used widely in peppers, were investigated to determine the effects of ozone on the removal of them from pepper surface. Active ingredients used in this experiment were malathion (65% EM), emamectin benzoate (5% SG) and acetamiprid (20% SP). Acetamiprid (60 g 100 L−1 water-3 days before commercial harvest) malathion (500 mL 100 L−1 water-7 days before commercial harvest) and emamectin benzoate (100 g 100 L−1 water-7 days before commercial harvest) were applied to pepper plants using hand sprayer. Application time and doses were determined according to practical (commercial) applications in the field. Peppers, picked up at commercial harvest time in Antalya, were transferred to laboratory immediately by refrigerated vehicle (4 °C). Foreign parts and injured plant materials were removed as well as yellow and withered leaves. After homogenization and visual examination, peppers were divided into two lots, and first group peppers were analyzed immediately for determination of insecticide residues before ozone treatments.
Second group pepper samples were washed by potable tap water to remove dirt and divided into four groups. The first group was immersed in cold water (8 ± 1 °C) containing 2 ppm dissolved ozone for 10 min. Second group was exposed to 2 ppm ozone gas for 45 min in air using a glass cabined manufactured for ozone applications. Third group (control for water treatment) samples were only immersed in cold water (8 ± 1 °C) for 10 min. The last group (control for air treatment) peppers were only exposed air in the same glass cabined (8 ± 1 °C) for 45 min. The application dose and type were determined based on our previous studies (Çakır et al. 2014; Uner 2018; Bolel et al. 2019). After treatments, residue analyses were repeated in samples taken from each treatment to determine the effect of ozone on the removal of pesticides.
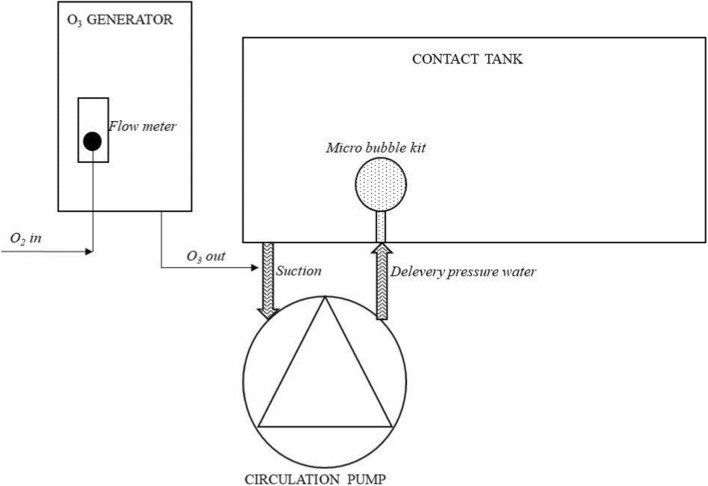
Ozone gas was generated by using a lab-scale ozone generator (corona discharge, Ozonoks System-Antalya, Turkey). Ozone gas was dissolved in cold water using by a system containing water pump, micro bubble apparatus and contact tank (Fig. 1). The ozone doses were measured automatically by a sensor (Ozone Sensor, OZ7MA5, German) placed on ozone generating device. The gas flow and the dose of ozone in water were controlled automatically by a control unit (JUMO- AQUIS 500- German) of ozone generator during treatments. The temperature of room during treatments was set to 5 ± 1 °C.
Fig. 1.
Ozonation of water using an ozone dissolving system combined with ozone generator
Evaluation of pesticide removal
Residue contents measured at harvest time and after all treatments were evaluated comparing with each other.
Determination of pesticide residues: A 15 g homogenized pepper sample was weighted into a 50 mL centrifuge tube to which 15 mL acetonitrile containing 1% HOAc is added along with 6 g MgSO4 and 1.5 g NaOAc. Then the tube was shaken and centrifuged during 1 min. A portion of the extract was mixed with 3 + 1 (w/w) MgSO4 –primary secondary amine sorbent (PSA) and centrifuged during 1 min again. The final extract was analyzed by LC–MS/MS (Lehotay 2007), and results were given as mg kg−1. The method was validated as per the single laboratory validation approach of Thompson et al. (2002) (Table 1).
Table 1.
Some validation parameters of pesticide residues analysis
| Acetamiprid | Emamectin benzoate | Malathion | ||||
|---|---|---|---|---|---|---|
| 10 ppb | 50 ppb | 10 ppb | 50 ppb | 10 ppb | 50 ppb | |
| RSDr (%) | 3.96 ± 0.37 | 1.57 ± 0.77 | 2.28 ± 0.22 | 1.10 ± 0.52 | 8.91 ± 0.74 | 7.11 ± 3.68 |
| RSDwR (%) | 4.14 ± 0.42 | 4.69 ± 2.56 | 2.65 ± 0.26 | 3.48 ± 2.81 | 13.90 ± 1.39 | 4.43 ± 2.46 |
| Recoveryr (%) | 94.0 | 98.7 | 94.4 | 94.8 | 82.8 | 100.32 |
| RecoverywR (%) | 101.85 | 109.27 | 99.75 | 104.31 | 99.71 | 111.26 |
Mean ± standard deviation (n = 10); RSDr = Repeatability; RSDwR = Within-laboratory reproducibility
Packaging and storage
Treated peppers were dried under a ventilator at room condition (20 °C and 55 ± 5% relative humidity) for 15 min. After drying, peppers were packaged in polystyrene trays (each containing 250 g samples) covered with stretch film (12 µ) and stored at 20 °C and % 60 ± 5% relative humidity for 8 days. The following chemical and physical analyses were performed at two day-intervals during shelf life.
Chemical and physical analysis
Respiration rate: Respiration rate was determined using a sample of 125 ± 15 g. Samples were weighed in 3.0 L airtight jars at room condition (20 °C). After 2 h the gas sample was taken from the jars using a gastight syringe and injected into loop of gas chromatography (GC). Gas measurements were performed in split/splitless (S/SL) of inlet in split mode with valve and fused silica capilar column (GS-GASPRO, 30 m × 0.32 mm I.D., U.S.A). Respiration rate was measured by Agilent model (6890 N) GC using thermal conductivity detector (TCD). The carrier gas flow was 1.7 mL min−1. The temperature of oven was chosen as 40 °C (isothermal). The temperature of the TCD was 250 °C. Results were calculated as mL CO2 kg−1 h−1.
Gas composition of modified atmosphere package (MAP): Gas concentration (O2 and CO2) in the packages was measured by Gaspace 2 (Gas Headspace analyzer, Systech Instruments), and expressed as percentage (%).
Skin color: Skin color was evaluated on the surface of pepper with a colorimeter (Minolta CR 300, Ramsey, NJ, USA). The calibration of color measurement apparatus (Minolta) was performed using an original calibration plate (white). The values were evaluated according to CIE L* (represents brightness-darkness changing from 0 to 100), C* (represents vividity of color) and h° (represents perceived color) system. The chroma (C*) and hue angle (h°) values were calculated the following formulas (1);
| 1 |
weight loss: weight loss of peppers was measured based on the initial weight and calculated as percent (%).
Soluble solids content (SSC): The SSC of pepper juice was determined with a refractometer (Digital-Atago PAL-1) and expressed as percentage (%).
Sensory analysis: The sensory evaluation panel consisted of 7 members of the research staff (Horticulture Department) who were experienced in sensory analysis of horticultural crops. Pepper samples (coded with three-digit numbers) were served at room temperature and analyzed under fluorescent light in a sensory evaluation room. External appearance (visual quality) was used a key sensorial characteristic for fresh peppers by panelists. The hedonic scale was used for the evolution of external appearance of pepper samples.
External appearance (scale 1–9): poor quality:1–4; marketable quality: ≥ 5; good quality: 7–8; excellent quality: 9.
Statistical analysis
The completely randomized design was chosen for this experiment. Three replications, each containing 250 g samples of each experiment were carried out. Using software package (SPSS, v.18.0), the general linear model (GLM) was used for statistical analyses. The differences among means (at a significance level of 0.05) were analyzed using Tukey test.
Result and discussion
Removal of pesticides
The residual pesticide values in peppers at harvest and after ozone treatments are given in Table 2. Current MRL (maximum residue limits) values of European Commission in peppers are 0.3, 0.02 and 0.02 mg kg−1 for acetamiprid, malathion and emamectin benzoate, respectively. As can be seen in Table 2, acetamiprid and emamectin benzoate with 0.0635 and 0.0031 mg kg−1 remained under limit values, but malathion was measured as 0.125 mg kg−1 at harvest time. The removal efficiency of pesticides from the surface of peppers changed depending on ozone application type. While ozonated water and control (only dipping in water) treatments decreased pesticide residues in peppers compared to harvest time, there was no meaningful change in the samples treated with ozone in air. The removal percentages of acetamiprid, malathion and emamectin benzoate in peppers treated with ozonated water were detected as 70.08, 84.80 and 100%, respectively. The remaining residue values of all pesticides in control group (0.043, 0.021 and 0.0027 mg kg−1) were higher than those of ozonated water treatment as expected. The best results for the removal efficiency of pesticides were obtained from peppers dipped in ozonated water. For example, all of emamectin benzoate residues in peppers were completely removed by ozonated water. These results are agree with those of Ikeura et al. (2011) who reported that pesticide residues on fruit could be removed efficiently by immersing in ozonated water compared to exposure to ozone in air. These scientists found that pesticide residues were efficiently removed from lettuce, cherry tomatoes and strawberries by immersing them in ozonated water containing more than 1.0–2.0 ppm dissolved ozone. Similarly, it was indicated that the removal pesticides from horticultural produce was achieved meaningfully with dipping in water containing dissolved ozone (Wu et al. 2007; Kuşvuran et al. 2012; Khaled et al. 2017). In the present study, the removal percentage of acetamiprid was remarkable lower than malathion and emamectin benzoate in peppers dipped in ozonated water. Since acetamiprid is a systemic insecticide, the absorption rate of it by the peel tissues of peppers is expected to be higher than the others, which have contact effect. Therefore, it is thought that the degradation of systemic pesticide residues by ozone is difficult when compared to non-systemic ones. It is well known that ozone can only oxidize the residues on surface of produce. In other words, the remaining pesticides after ozonation, in general, are those that have penetrated into the crops.
Table 2.
The residual pesticide (mg kg−1) values in peppers at harvest and after ozone treatments
| Acetamiprid | Malathion | Emamectin benzoate | ||||
|---|---|---|---|---|---|---|
| Limit values (EC) | 0.3000 | 0.0200 | 0.0200 | |||
| At harvest | 0.0635 | 0.1250 | 0.0031 |
| Treatments | RR | RPP (%) | RR | RPP (%) | RR | RPP (%) |
|---|---|---|---|---|---|---|
| Ozonated water | 0.0190 ± 0.05 | 70.08 | 0.0190 ± 0.01 | 84.80 | – | 100 |
| Ozone gas | 0.2000 ± 0.06 | 0 | 0.8100 ± 0.30 | 0 | 0.0040 ± 0.00 | 0 |
| Control | 0.0430 ± 0.00 | 32.28 | 0.0210 ± 0.01 | 83.20 | 0.0027 ± 0.00 | 12.90 |
Values are mean ± standard error of triplicate determinations
EC European Commission, RR remaining residue, RPP removal percentage of pesticide
Respiration rate
Respiration rate is an important indicator for postharvest metabolic activity of horticultural produces. The factors that suppress respiration rate of fresh produces extend their postharvest life by delaying senescence and quality losses. In the present study, the effects of both ozone treatments and storage time on respiration rate of peppers were significant during storage (Table 6). Respiration rates fluctuated throughout storage and decreased at the end of experiment in all groups, changing between17.96 and 36.38 mLCO2 kg−1 h−1, compared to initial value (36.81 mLCO2 kg−1 h−1). Ozone treatments both in air (24.86 mLCO2 kg−1 h−1) and water (26.82 mLCO2 kg−1 h−1) suppressed clearly respiration rates of peppers compared to control groups (33.98, 27.35 mLCO2 kg−1 h−1, respectively) (Table 3). The suppressing effect of ozone treatments on respiration rate is accordance with the results of previous researches, in which ozone treatments decreased respiration rates by delaying metabolic activity and senescence processes of horticultural produces during storage (Zhang et al. 2005; Bolel et al. 2019). The decreasing effect of ozone on respiration rate can also be due to its ability to increase systemic resistance in fresh produces by increasing antioxidant capacity, which may suppress respiration processes in cell (Artes-Hernandez et al. 2007). It is known that the fruit skin that restricts gas permeability between fruit and atmosphere decreases respiration rate in cell. In our study, ozone treatment might have decreased respiration rate by preserving the structure of pepper skins. This idea is accordance with the findings of Han et al. (2017) who reported that ozone treatment significantly delayed the degradation of epidermal tissue by preserving morphological structure of fruit skin. On the other hand, Beltran et al. (2005) reported that there were no significant differences between ozonated fresh-cut lettuce and control sample for respiration rate. Therefore, the choice of ozone dose, treatment type and duration that affect skin structure of produce and physiological processes in fruit is crucial.
Table 6.
Anova for dependent variables for treatments, storage period and their interactions for peppers
| Quality parameters | Storage days (SD) | Treatments (T) | SD × T |
|---|---|---|---|
| Respiration rate (mLCO2 kg−1 h−1) | ** | ** | NS |
| Oksijen rate (%) | ** | NS | NS |
| Carbondioxide rate (%) | ** | NS | NS |
| L* value | ** | NS | ** |
| C* value | ** | NS | ** |
| Hue angle value | ** | NS | * |
| Weight loss (%) | ** | ** | NS |
| Soluble solids content (%) | ** | NS | NS |
| External appearance | ** | NS | NS |
NSrepresents non-significance at p < 0.05; **represents significance at the 0.01 level; *represents significance at the 0.05 level
Table 3.
The effects of different ozone treatments on the respiration rate (mLCO2 kg−1 h−1) of peppers and gas composition in MAP during storage
| Treatments | Storage days | Means | ||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | ||
| Respiration rate (mLCO2 kg−1 h−1) | ||||||
| Control (water) | 36.81 ± 4.52 | 22.01 ± 2.17 | 24.76 ± 3.69 | 28.18 ± 2.87 | 25.01 ± 1.85 | 27.35 |
| Ozonated water | 36.81 ± 4.52 | 23.36 ± 2.34 | 21.60 ± 1.38 | 25.26 ± 2.50 | 27.07 ± 0.95 | 26.82 |
| Control (air) | 36.81 ± 4.52 | 33.20 ± 3.02 | 28.98 ± 1.66 | 34.53 ± 4.59 | 36.38 ± 2.84 | 33.98 |
| Ozone gas | 36.81 ± 4.52 | 26.04 ± 3.24 | 18.65 ± 0.48 | 24.83 ± 1.61 | 17.96 ± 1.41 | 24.86 |
| Means | 36.81 | 26.15 | 23.50 | 28.20 | 26.60 | |
| O2 rate (%) | ||||||
| Control (water) | 21.00 ± 0.00 | 18.67 ± 2.08 | 17.67 ± 0.84 | 18.10 ± 0.47 | 18.33 ± 0.54 | 18.75 |
| Ozonated water | 21.00 ± 0.00 | 18.70 ± 1.64 | 17.67 ± 0.42 | 20.57 ± 0.18 | 18.73 ± 1.12 | 19.33 |
| Control (air) | 21.00 ± 0.00 | 18.10 ± 1.16 | 16.30 ± 0.67 | 16.67 ± 2.18 | 17.73 ± 1.07 | 17.96 |
| Ozone gas | 21.00 ± 0.00 | 15.23 ± 0.86 | 16.33 ± 1.39 | 18.10 ± 0.75 | 18.93 ± 0.97 | 17.92 |
| Means | 21.00 | 17.68 | 16.99 | 18.36 | 18.43 | |
| CO2 rate (%) | ||||||
| Control (water) | 0.03 ± 0.00 | 1.53 ± 0.28 | 1.50 ± 0.26 | 1.70 ± 0.15 | 1.27 ± 0.29 | 1.21 |
| Ozonated water | 0.03 ± 0.00 | 1.47 ± 0.44 | 1.90 ± 0.10 | 1.03 ± 0.09 | 1.30 ± 0.21 | 1.15 |
| Control (air) | 0.03 ± 0.00 | 1.87 ± 0.22 | 1.90 ± 0.06 | 1.87 ± 0.37 | 1.67 ± 0.17 | 1.47 |
| Ozone gas | 0.03 ± 0.00 | 2.37 ± 0.24 | 1.90 ± 0.17 | 1.53 ± 0.07 | 1.13 ± 0.09 | 1.39 |
| Means | 0.03 | 1.81 | 1.80 | 1.53 | 1.34 | |
Values are mean ± standard error of triplicate determinations
Gas composition of MAP
The gas composition (O2 and CO2) in MAP is affected by the respiration rate of produce, the amount of fruit in the package and the gas permeability of package materials. In the present study, O2 and CO2 concentrations in MAP were changed by only respiration rate of peppers since the packaging material and the fruit weight inside were the same for all treatments. As can be seen Table 3, although there were no statistical differences among treatments (Table 6), ozone treatments both in water and air suppressed respiration rate of peppers. The CO2 concentrations (1.15 and 1.39%) in MAP containing ozone treated peppers were lower than those of control groups (1.21 and 1.47%). It is known that, the CO2 content of MAP is an indicator of the respiration rate of produce inside the package. The lower O2 and the higher CO2 concentrations in MAP, which contain ozone treated peppers, represent suppressed respiration rate compared to control groups. The findings related to gas compositions of MAP were confirmed by respiration rate results that were detected by gas chromatography (Table 3). As mentioned above, ozone treatments might have decreased respiration rate of peppers by affecting their post-harvest physiology related to senescence and skin structure. Han et al. (2017) indicated that ozone treatment reduced water vapor transpiration by shrinkage of stoma on fruit surface, and delayed degradation of cell walls and epidermal tissues. In our study, restricted gas exchange, which affect respiration metabolism, between ozone treated fruit and atmosphere can be one of the reasons for decreased respiration rate in peppers. On the other hand, Artes-Hernandez et al. (2007) reported an increase in systemic resistance of ozone treated fresh produces, which could delay senescence processes. Similar results were also obtained from previous studies carried out by Çakır (2010) and Bolel et al. (2019) in ozone treated table grape and pomegranate during storage.
Skin color
The effect of treatments on L*, C*and h° values were not significant during storage. However these color values were statically affected by storage time and interactions between treatment and storage periods (Table 6). Color changes (L*, C*, h°) of peppers during storage are presented in Table 4. Skin color of fresh fruit and vegetables is considered one of the most important quality parameters because it affects the commercial value of crops. The L* values of peppers fluctuated during storage and increased at the 8th day of storage except for control in water compared to initial value (52.82). Although there was not much variation between ozone treated and un-treated samples, L* values of ozone treated peppers at the last day of storage both in water (54.22) and air (55.50) were higher than those of control samples (52.48 and 53.18, respectively). The relatively higher L* values can be attributed to the decrease in green color intensity of peppers with oxidizing of ozone, and therefore the increase in whiteness (Fig. 2). These results are similar to those reported by Şengün and Kendirci (2018), who indicated that ozone treatment in water did not affect statistically the L* value of minimally processed lettuce. Similar findings were also reported by Glowacz et al. (2015a, b) in different horticultural crops.
Table 4.
The effects of different ozone treatments on the skin color of peppers during storage
| Parameters | Treatments | Storage days | Means | ||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | |||
| L* | Control (water) | 52.82 ± 0.58 | 51.46 ± 1.04 | 51.58 ± 0.98 | 53.30 ± 0.73 | 52.48 ± 0.42 | 52.33 |
| Ozonated water | 52.82 ± 0.58 | 53.57 ± 0.74 | 49.68 ± 1.58 | 53.74 ± 0.81 | 54.22 ± 0.66 | 52.80 | |
| Control (air) | 52.82 ± 0.58 | 51.64 ± 0.59 | 53.31 ± 1.40 | 51.69 ± 0.65 | 53.18 ± 0.65 | 52.53 | |
| Ozone gas | 52.82 ± 0.58 | 53.90 ± 0.91 | 48.97 ± 0.83 | 51.65 ± 1.10 | 55.50 ± 0.81 | 52.57 | |
| Means | 52.82 | 52.64 | 50.89 | 52.59 | 53.84 | ||
| Chroma | Control (water) | 49.08 ± 1.75 | 48.11 ± 3.33 | 50.66 ± 2.57 | 50.43 ± 2.12 | 51.61 ± 1.52 | 49.98 |
| Ozonated water | 49.08 ± 1.75 | 50.81 ± 2.25 | 47.28 ± 4.85 | 51.14 ± 2.19 | 51.46 ± 1.45 | 49.95 | |
| Control (air) | 49.08 ± 1.75 | 50.35 ± 1.89 | 50.61 ± 5.32 | 50.17 ± 1.53 | 50.74 ± 1.73 | 50.19 | |
| Ozone gas | 49.08 ± 1.75 | 51.56 ± 1.96 | 44.49 ± 1.58 | 49.59 ± 1.83 | 52.32 ± 1.66 | 49.41 | |
| Means | 49.08 | 50.21 | 48.26 | 50.33 | 51.53 | ||
| Hue angle | Control (water) | 117.96 ± 0.67 | 117.81 ± 2.83 | 116.24 ± 0.92 | 114.92 ± 3.13 | 116.93 ± 0.89 | 116.77 |
| Ozonated water | 117.96 ± 0.67 | 117.49 ± 1.26 | 115.88 ± 2.82 | 117.00 ± 1.50 | 118.42 ± 0.47 | 117.35 | |
| Control (air) | 117.96 ± 0.67 | 117.13 ± 0.25 | 113.68 ± 2.16 | 114.68 ± 1.30 | 116.31 ± 0.78 | 115.95 | |
| Ozone gas | 117.96 ± 0.67 | 117.98 ± 1.11 | 111.50 ± 1.13 | 116.29 ± 1.73 | 117.04 ± 4.03 | 116.15 | |
| Means | 117.96 | 117.57 | 115.34 | 115.94 | 116.83 | ||
Values are mean ± standard error of triplicate determinations
Fig. 2.
Ozonated peppers in water (a) and control group (b) at the end of the storage
The C* values tended to rise with the increasing storage period in all treatments during storage (Table 4). Similar trend was also observed by Uner (2018) in ozone treated parsley during storage in room condition. The highest C* values (49.98, 50.19) were obtained from control samples, but there were no meaningful differences among the treatments. Şengün and Kendirci (2018) reported that the C* values of the minimally processed lettuce were not affected both ozone treatment and storage temperature. Similarly, ozone (0.7 μmol mol−1) exposure to the minimally processed peppers had no effect on L* value (Horvitz and Cantalejo 2012).
The h° angle values, which indicate the perceived color values, show the change from yellow to green as the angle value increases after 90°. In the present study, the color of peppers was relatively dark green corresponding to a hue angle (h°) value of 117.96 at beginning of the storage. This value, relatively, decreased with increasing storage time in all treatments (except for ozonated water) depending on increasing yellowness tone on the surface of peppers. The highest h° values, represent dark green in pepper, were obtained from ozone treated samples (117.35, 116.15) in water and air, respectively, while the lowest ones (116.77, 115.95, respectively) were determined in control samples (Table 4). These results showed that ozone treatments, especially in water, maintained green color of peppers, with minimum change in h° values, when compared to control. The positive results obtained from ozone treatments, at the present study, are in accordance with previous researches (Horvitz and Cantalejo 2012; Glowacz et al. 2015b) in which low doses of ozone did not bleach the pigments on the skin of intact and minimally processed peppers.
Weight loss
Weight loss of fresh vegetables is a very important commercial parameter after harvest because it directly refers to the decrease in product weight and quality, especially external appearance. Ozone treatments and storage time significantly affected the weight loss of peppers during storage (Table 6). As expected, weight loss of all treated peppers increased in parallel with increasing storage period, but ozone treatments in air and water delayed it compared to control. As can be seen in Table 5, weight losses of peppers treated with 2 ppm ozone in water (12.78%) and air (12.75%) were lower than those of control groups (13.05 and 14.75%, respectively). It is known that the weight loss of fresh fruit and vegetables is affected by transpiration from product surface, respiration rate and electrolyte leakage. Similarly, Keutgen and Pawelzik (2008) indicated that respiration rate and water loss affected weight losses of fruit, which exposed to ozone gas at pre-harvest stage, under storage condition. In the present study, it is thought that ozone treatment restricted water loss by preserving the structure of pepper skin and suppressing respiration rate of fruit. In fact, scanning electron microscopy (SEM) observations showed that ozone treatments suppressed water loss of black mulberry by maintaining shape and structure of stoma on the fruit skin (Han et al. 2017). The results of present research are accordance with the findings of previous researchers (Çakır et al. 2014; Tabakoğlu and Karaca 2018) who reported that ozone treatment decreased weight loss of different horticultural crops during storage. On the other hand, weight loss of ozone treated fruit was higher (Cayuela et al. 2009) or did not change (Palou et al. 2002) when compared to the control groups. These findings can be explained by the fact that the effects of ozone treatments on weight loss are different depending on dose, application time and type, variety or species of produce and storage conditions.
Table 5.
The effects of different ozone treatments on the weight loss, soluble solids content and external appearance of peppers during storage
| Treatments | Storage days | Means | ||||
|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | ||
| Weight loss (%) | ||||||
| Control (water) | – | 3.00 ± 0.15 | 14.44 ± 0.72 | 16.55 ± 0.83 | 18.20 ± 0.93 | 13.05 |
| Ozonated water | – | 1.77 ± 0.14 | 14.02 ± 0.79 | 16.26 ± 0.90 | 19.07 ± 2.01 | 12.78 |
| Control (air) | – | 3.47 ± 0.13 | 16.31 ± 0.62 | 18.68 ± 0.68 | 20.55 ± 0.70 | 14.75 |
| Ozone gas | – | 2.97 ± 0.17 | 14.04 ± 0.78 | 16.16 ± 0.93 | 17.84 ± 1.02 | 12.75 |
| Means | – | 2.80 | 14.70 | 16.91 | 18.91 | |
| SSC (%) | ||||||
| Control (water) | 5.03 ± 0.18 | 5.50 ± 0.31 | 7.67 ± 0.38 | 7.70 ± 0.21 | 8.53 ± 0.73 | 6.89 |
| Ozonated water | 5.03 ± 0.18 | 5.57 ± 0.38 | 7.40 ± 0.12 | 7.83 ± 0.17 | 7.73 ± 0.67 | 6.71 |
| Control (air) | 5.03 ± 0.18 | 5.17 ± 0.29 | 7.13 ± 0.12 | 9.13 ± 0.42 | 9.27 ± 0.03 | 7.15 |
| Ozone gas | 5.03 ± 0.18 | 5.23 ± 0.48 | 7.57 ± 0.69 | 7.60 ± 0.35 | 8.20 ± 0.06 | 6.73 |
| Means | 5.03 | 5.37 | 7.44 | 8.07 | 8.43 | |
| External appearance (1–9 score) | ||||||
| Control (water) | 9.00 ± 0.00 | 8.83 ± 0.17 | 6.83 ± 0.17 | 5.67 ± 0.51 | 4.25 ± 0.14 | 6.92 |
| Ozonated water | 9.00 ± 0.00 | 9.00 ± 0.00 | 5.00 ± 0.58 | 5.00 ± 0.38 | 5.17 ± 0.67 | 6.63 |
| Control (air) | 9.00 ± 0.00 | 8.83 ± 0.17 | 5.33 ± 0.60 | 5.06 ± 0.34 | 4.25 ± 0.14 | 6.49 |
| Ozone gas | 9.00 ± 0.00 | 9.00 ± 0.00 | 6.00 ± 0.29 | 5.75 ± 0.63 | 5.67 ± 0.67 | 7.08 |
| Means | 9.00 | 8.92 | 5.79 | 5.37 | 4.83 | |
Values are mean ± standard error of triplicate determinations
Soluble solids content
SSC, which represents soluble sugars in horticultural crop, is an indicator of fruit maturity, and it is used as a harvest criteria. There were no significant differences among the treatments (Table 6). The SSC of peppers increased with increasing storage period in all sample groups, but was delayed in ozone treated fruit. The average SSC of ozone treated peppers both in water (6.71%) and air (6.73%) were lower when compared to control samples (6.89 and 7.15%) (Table 5). The higher SSC of control groups can be attributed to the higher water loss (Table 5) and metabolic activity in maturity processes in control samples compared to ozone treated ones, as reported in previous studies (Çakır et al. 2014; Uner 2018). Ozone treatment maintained SSC of peppers with relatively lesser change during storage. These results were in accordance with the findings of Han et al. (2017).
Sensory analysis
Storage time and interactions between treatments and storage time affected significantly the external appearance of peppers during storage. The external appearance quality of peppers declined during storage as expected (Table 5). Similar results were also reported by Beltran et al. (2005) and Uner (2018) in ozonated leafy vegetables during storage. The highest score (5.67) for external appearance was determined in samples exposed to 2 ppm ozone in air followed by ozonated water treatment (5.17). Panelists gave lower external quality scores (4.25) for both control peppers at the last day of storage. Peppers with marketable quality (score ≥ 5.00) were only obtained from ozone treatments at the 8th day of storage. Control samples retained their marketable quality until 6th day of storage (Table 5). This affirmative effect of ozone can be explained by its suppressing effect on metabolic activity and water loss of peppers compared to control. As can be seen in Table 3 and Table 5, ozone treatments decreased respiration rate and weight loss, which effects external quality of fresh vegetables. In agreement with our findings, ozone treatment delayed sensory quality losses in different fruit and vegetables compared to control samples during storage (Beltran et al. 2005; Çakır et al. 2014; Uner 2018).
Conclusion
Ozone application type (in water or air) affected the removal efficiency of pesticides from pepper surface. Ozonated water containing 2 ppm ozone was the best treatment for degradation of pesticides residues in green peppers. Weight losses of ozone treated peppers decreased compared to control samples during storage. Ozone treatment preserved green color of peppers, depending on application type, better than control. Ozonated water was the best treatment for maintaining vivid green color of pepper skin, which is represented with higher h°. Although no significant differences in the external appearance were observed among treatments, ozonated water (2 ppm) gave the highest scores at the end of storage. Ozone treatments decreased respiration rates by slowing down metabolic processes in peppers throughout storage. Peppers treated with 2 ppm ozone both in water and air could be stored with good quality in polystyrene trays covered with stretch film (12 µ) at 20 °C and 60 ± 5% relative humidity for 8 days. Control samples lost their marketable quality after 6 days in room condition. Ozone treatments, especially in water, can be an alternative tool for preserving postharvest quality of peppers during storage. However, it is needed detailed investigation to determine the appropriate dose and application type for removing pesticides and storage of peppers.
Acknowledgements
The authors gratefully acknowledge the financial support of the Scientific and Technical Research Council of Turkey.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tansu Özen, Email: tozen07@gmail.com.
Mehmet Ali Koyuncu, Email: koyuncu.ma@gmail.com.
Derya Erbaş, Email: deryabyndr@gmail.com.
References
- Artes-Hernandez F, Aguayo E, Artes F, Tomas-Barberan FA. Enriched ozone atmosphere enhances bioactive phenolics in seedless table grapes after prolonged shelf life. J Sci Food Agric. 2007;87(5):824–831. doi: 10.1002/jsfa.2780. [DOI] [Google Scholar]
- Beltran D, Selma MV, Marin A, Gil MI. Ozonated water extends the shelf life of fresh-cut lettuce. J Agric Food Chem. 2005;53(14):5654–5663. doi: 10.1021/jf050359c. [DOI] [PubMed] [Google Scholar]
- Bolel H, Koyuncu MA, Erbaş D. The effects of ozone and fungicide treatments on the fruit quality changes of pomegranate during cold storage. JIST. 2019;9(4):1841–1850. doi: 10.21597/jist.551675. [DOI] [Google Scholar]
- Çakır IO (2010) The storage of Red Globe grape variety at normal, modified and controlled atmosphere conditions. M.Sc. Thesis, Süleyman Demirel University, Turkey, 125 p
- Çakır IO, Koyuncu MA, Erbaş D (2014) The effect of ozone treatment on cold storage of Red Globe grapes. Oral presentation at the meeting of VI. Storage and Marketing Symposium in Horticultural Crops, Bursa, Turkey
- Cayuela JA, Vazquez A, Perez AG, Garcia JM. Control of table grapes postharvest decay by ozone treatment and resveratrol induction. Food Sci Technol Int. 2009;15(5):495–502. doi: 10.1177/1082013209350539. [DOI] [Google Scholar]
- Dilmaçünal T, Erbaş D, Koyuncu MA, Onursal CE, Kuleaşan H. Efficacy of some antimicrobial treatments compared to sodium hypochlorite on physical, physiological and microbial quality of fresh-cut melons (Cucumis melo L. var. inodorus) LWT Food Sci Technol. 2014;59(2):1146–1151. doi: 10.1016/j.lwt.2014.07.033. [DOI] [Google Scholar]
- Faostat (2020) Statistic: Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#data/QC. Accessed 16 June 2020
- Glowacz M, Colgan R, Rees D. The use of ozone to extend the shelf-life and maintain quality of fresh produce. J Sci Food Agric. 2015;95(4):662–671. doi: 10.1002/jsfa.6776. [DOI] [PubMed] [Google Scholar]
- Glowacz M, Colgan R, Rees D. Influence of continuous exposure to gaseous ozone on the quality of red bell peppers, cucumbers and zucchini. Postharvest Biol Technol. 2015;99:1–8. doi: 10.1016/j.postharvbio.2014.06.015. [DOI] [Google Scholar]
- Han Q, Gao H, Chen H, Fang X, Wu W. Precooling and ozone treatments affects postharvest quality of black mulberry (Morus nigra) fruits. Food Chem. 2017;221:1947–1953. doi: 10.1016/j.foodchem.2016.11.152. [DOI] [PubMed] [Google Scholar]
- Horvitz S, Cantalejo MJ. Effects of ozone and chlorine postharvest treatments on quality of fresh-cut red bell peppers. Int J Food Sci Technol. 2012;47(9):1935–1943. doi: 10.1111/j.1365-2621.2012.03053.x. [DOI] [Google Scholar]
- Horvitz S, Cantalejo MJ. Application of ozone for the postharvest treatment of fruits and vegetables. Crit Rev Food Sci Nutr. 2014;54(3):312–339. doi: 10.1080/10408398.2011.584353. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Cash JN, Zabik MJ. Postharvest treatments for the reduction of mancozeb in fresh apples. J Agric Food Chem. 2001;49(6):3127–3132. doi: 10.1021/jf010234h. [DOI] [PubMed] [Google Scholar]
- Ikeura H, Kobayashi F, Tamaki M. Removal of residual pesticides in vegetables using ozone microbubbles. J Hazard Mater. 2011;186(1):956–959. doi: 10.1016/j.jhazmat.2010.11.094. [DOI] [PubMed] [Google Scholar]
- Karaca H, Velioğlu YS. Ozone applications in fruit and vegetable processing. Food Rev Int. 2007;23(1):91–106. doi: 10.1080/87559120600998221. [DOI] [Google Scholar]
- Karaca H, Velioğlu YS. Effects of ozone treatments on microbial quality and some chemical properties of lettuce, spinach, and parsley. Postharvest Biol Technol. 2014;88:46–53. doi: 10.1016/j.postharvbio.2013.09.003. [DOI] [Google Scholar]
- Keutgen AJ, Pawelzik E. Influence of pre-harvest ozone exposure on quality of strawberry fruit under simulated retail conditions. Postharvest Biol Technol. 2008;49(1):10–18. doi: 10.1016/j.postharvbio.2007.12.003. [DOI] [Google Scholar]
- Khaled AO, Fahad B, Abdullah A. Ozone as a safety post-harvest treatment for chlorpyrifos removal from vegetables and its effects on vegetable quality. Int J Food Nutr Sci. 2017;4(1):38–48. doi: 10.15436/2377-0619.17.1319. [DOI] [Google Scholar]
- Ku Y, Chang JL, Shen YS, Lin SY. Decomposition of diazinon in aqueous solution by ozonation. Water Res. 1998;32(6):1957–1963. doi: 10.1016/S0043-1354(97)00353-9. [DOI] [Google Scholar]
- Kuşçu A, Pazır F. Ozone application in food industry. J Food. 2004;29(2):123–129. [Google Scholar]
- Kuşvuran E, Yıldırım D, Mavruk F, Ceyhan M. Removal of chloropyrifos ethyl, tetradifon and chlorothalonil pesticide residues from citrus by using ozone. J Hazard Mater. 2012;241–242:287–300. doi: 10.1016/j.jhazmat.2012.09.043. [DOI] [PubMed] [Google Scholar]
- Lehotay SJ. Determination of peticide residues in foods by acetonitrile extraction and partitioning with magnesium sulphate collaborative study. J AOAC Int. 2007;90(2):1–36. doi: 10.1093/jaoac/90.2.485. [DOI] [PubMed] [Google Scholar]
- Luo A, Bai J, Li R, Fang Y, Li L, Wang D, Zhang L, Liang J, Huang T, Kou L. Effects of ozone treatment on the quality of kiwifruit during postharvest storage affected by Botrytis cinerea and Penicillium expansum. J Phytopathol. 2019;167(7–8):470–478. doi: 10.1111/jph.12819. [DOI] [Google Scholar]
- Ma J, Graham NJD. Degradation of atrazine by manganese-catalysed ozonation-influence of radical scavenges. Water Res. 2000;34(15):3822–3828. doi: 10.1016/S0043-1354(00)00130-5. [DOI] [Google Scholar]
- Masten SJ, Tian M, Upham BL, Trosko JE. Effect of selected pesticides and their ozonation by products on gap junctional intercellular communication using rate liver epithelial cell lines. Chemosphere. 2001;44(3):457–465. doi: 10.1016/S0045-6535(00)00296-4. [DOI] [PubMed] [Google Scholar]
- Mckenzie KS, Sarr AB, Mayura AK, Bailey RH, Miller DR, Rogers TD, Norred WP, Voss KA, Platter RD, Kubena LF, Phillips TD. Oxidative degradation and detoxification of mycotoxins using a novel source of ozone. Food Chem Toxicol. 1997;35(8):807–820. doi: 10.1016/S0278-6915(97)00052-5. [DOI] [PubMed] [Google Scholar]
- Ong KJ, Cash JN, Zabik MJ. Chlorine and ozone washes for pesticide removal from apples and processed apple sauce. Food Chem. 1996;55(2):153–160. doi: 10.1016/0308-8146(95)00097-6. [DOI] [Google Scholar]
- Palou L, Crisosto CH, Smilanick JL, Adaskaveg JE, Zoffoli LP. Effects of continuous 0.3 ppm ozone exposure on decay development and physiological responses of peaches and table grapes in cold storage. Postharvest Biol Technol. 2002;24(1):39–48. doi: 10.1016/s0925-5214(01)00118-1. [DOI] [Google Scholar]
- Şengün IY, Kendirci P. Potential of ozonated water at different temperatures to improve safety and shelf-life of fresh cut lettuce. Ozone Sci Eng. 2018;40(3):216–227. doi: 10.1080/01919512.2017.1416284. [DOI] [Google Scholar]
- Smilanick JL. Postharvest use of ozone on citrus fruit. Packinghouse Newsl. 2003;199:1–6. [Google Scholar]
- Smilanick JL, Crisosto C, Mlikota F. Postharvest use of ozone on fresh fruit. Perishables Handl Q. 1999;99:10–14. [Google Scholar]
- Tabakoğlu N, Karaca H. Effects of ozone-enriched storage atmosphere on postharvest quality of black mulberry fruits (Morus nigra L.) LWT Food Sci Technol. 2018;92:276–281. doi: 10.1016/j.lwt.2018.02.044. [DOI] [Google Scholar]
- Thompson M, Ellison SL, Wood R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report) Pure Appl Chem. 2002;74(5):835–855. doi: 10.1351/pac200274050835. [DOI] [Google Scholar]
- Uner K (2018) Effect of postharvest ozone and salicylic acid treatments on parsley storage and quality. M.Sc. Thesis, Suleyman Demirel University, Turkey, 83 p
- Whangchai K, Saengnil K, Uthaibutra K. Effect of ozone in combination with some organic acids on the control of postharvest decay and pericarp browning of longan fruit. Crop Prot. 2006;25(8):821–825. doi: 10.1016/j.cropro.2005.11.003. [DOI] [Google Scholar]
- Wu JG, Luan TG, Lan CY, Lo TWH, Chan GYS. Removal of residual pesticides on vegetable using ozonated water. Food Cont. 2007;18(5):466–472. doi: 10.1016/j.foodcont.2005.12.011. [DOI] [Google Scholar]
- Zhang L, Lu Z, Yu Z, Gao X. Preservation fresh-cut celery by treatment of ozonated water. Food Control. 2005;16:279–283. doi: 10.1016/j.foodcont.2004.03.007. [DOI] [Google Scholar]