Abstract
Shelf-life of paddy straw mushroom could be extended to 3 days by pre-cooling mushrooms in air at 14 °C for 2 h followed by packing in 75 µ thick high impact polystyrene punnets with 1.2% perforations as primary package and subsequently stored in expanded polystyrene (EPS) cabinet as secondary package. The EPS cabinet has been designed for transportation of mushroom with ice as cooling aid to maintain the optimum storage temperature. Temperature profile inside the cabinet was studied under no-load and full-load condition. The temperature inside the cabinet with 6 kg pre-cooled paddy straw mushroom (packed in 24 number of punnets @ 250 g mushroom per punnet having 1.2% perforations) and 6 kg ice in the partition chamber, was maintained at optimum storage temperature of 15 ± 2 °C (92 ± 1% RH) up to 18 h. Results of the study suggest that the technology could be successfully adopted by the paddy straw mushroom growers and traders for storage, transportation and marketing for loss reduction and higher return.
Keywords: Paddy straw mushroom, Pre-cooling, Storage, Transportation cabinet
Introduction
Paddy straw mushroom (Volvariela volvacea) is an edible fungus which ranks sixth among cultivated mushrooms of the world. It possesses unique taste, texture, aroma, nutritional and medicinal attributes (Johl et al. 1995; Verma, 2002) for which it is popularly used in several delicious dishes for culinary purposes. It has a short shelf life after harvest under ambient condition due to high moisture content, respiration rate, water loss, microbial and enzymatic activities (Amuthan et al. 1999; Martine et al. 2002). All these lead to the paddy straw mushroom becoming unattractive in their appearance and deteriorate their quality, which in turn decrease the economic value (Mercado and Alabastro 1989; Nur Sakinah et al. 2019). The colour turns black in the post-harvest period due to oxidation of phenolic compounds to quinones which subsequently combine with amino acid to produce dark compound, melanin. Peroxidase and polyphenol oxidase present in mushroom are largely responsible for the enzymatic discolouration (Chang and Quimio 1980; Nerya et al. 2006).
Softening of mushroom or loss of firmness during post-harvest storage has been ascribed to changes in membrane and these textural changes are also related to protein and polysaccharide degradation (Zivanovic et al. 2000). Many works have been reported on modified atmospheric packaging (Antmann et al. 2008; Jafri et al. 2013; Kuyper et al. 1993; Simon et al. 2005; Briones 1992; Roy et al. 1995; Nussinovitch and Kampf 1993), perforation mediated modified atmospheric packaging (Dhalsamant et al. 2015; Byrnes and Beirne 2007; Xiao et al. 2011; Jiang et al. 2010), controlled atmospheric packaging (Jamjumroon et al. 2012; Briones 1992; Mau et al. 1993) and freezing (Thiribhuvanamala et al. 2012; Jaworska and Bernas 2010; Czapski and Szudyga 2000) of mushroom to extend the shelf life. Different pretreatments and coating methods have also been studied by various researchers to extend the storage life of mushroom (Dhalsamant et al. 2018; Moon and Lo 2013; Minh and Hang 2019; Eissa 2007). As the respiration rate of mushroom is high, rapid forced air cooling might help in maintaining the fresh state quality (Wills et al. 2007; Jamjumroon et al. 2012).
Paddy straw mushroom undergoes quick colour and textural changes during storage due to which, the market is mainly domestic. So, there is problem of storage and transportation of this mushroom to distant market. Fresh mushroom market is largely catered by the seasonal growers who do not have cool-chain storage and transport facilities and sell the produce in highly localized markets. The common method of packing is small polyethylene or polypropylene pouches of about 100 gauge thickness with 5% perforations to retain the freshness and firmness (Kaur and Kappor 2016; Khan and Patel 2010). The primary small pack units with approximately 500 g mushrooms in each are carried together in a larger secondary HDPE (high density polyethylene) woven sack for transportation to distant markets. There are significant quantitative and qualitative losses of the paddy straw mushroom during transportation leading to economic loss to the traders and depriving consumers of getting quality product.
Pre-cooling, packing and subsequent low temperature storage and transportation are important to maintain the quality of mushroom. The polypacks of white button mushroom stacked in small wooden cases or boxes with sufficient crushed ice in polypacks are practiced to keep the mushroom cool during transport to short distances. Paddy straw mushrooms packed in bamboo baskets with an aeration channel at the center and dry ice wrapped in paper placed above the mushrooms, is in practice for transportation in Taiwan. Packing in wooden cases for transport by rail or boat is practiced in China (Wakchaure 2011).
Suitable technology on packaging, storage and transportation of such perishable commodity is therefore of vital importance for marketing. Based upon the requirement, the present study was undertaken for development of suitable packaging, storage and transportation technology for better handling and transportation of paddy straw mushroom to distant markets.
Materials and methods
Procurement of raw material
Freshly harvested paddy straw mushrooms in the bud stage were procured from local farmers near Bhubaneswar in the early morning hours and were brought to the laboratory. The mushrooms were graded on the basis of size and maturity level (button and egg stage) discarding small and damaged ones (Nur Sakinah et al. 2019).
Measurement of physical properties of paddy straw mushroom
The bulk density, specific heat (Tansakul and Lumyong 2008), respiration rate and heat of respiration (Li and Zhang 2013) of paddy straw mushroom were measured using standard methods. These properties were useful for designing the transportation cabinet and to calculate the heat load.
Effect of storage temperature
In the first set of experiments, samples were kept at different storage temperatures of 10, 15 and 20 °C in cold room to find out the suitable storage temperature for paddy straw mushrooms.
Effect of perforation
As paddy straw mushroom is of low textural strength, bulk packaging leads to higher stress, respiration and quality loss. So rigid unit packs of 75 µ high impact polystyrene (HIPS) punnet was selected as the packaging material. Different levels of perforation i.e. 0.6, 0.9, 1.2 and 1.5% area were given on the HIPS punnet by making 16–40 numbers of holes of 0.5 cm diameter on all surfaces with a hot welding rod to study the effect of perforation on quality parameters of mushroom during storage.
Measurement of mushroom quality
The mushroom samples were analyzed for different quality parameters such as respiration rate, physiological loss in weight (PLW), texture (firmness), surface colour (Hunter ‘L’ value), and percent veil opening.
Respiration
About 20 g mushroom samples were put into gas tight containers with 20 ml of 0.4 N NaOH in a test tube, containing ambient air and initial atmosphere. Test tubes were taken out at 1 day interval and titrated with 0.2 N oxalic acid. The change in the concentration of CO2 was used to estimate respiration rate (Tienhua and Min 2013).
Physiological loss in weight
The weights of samples were recorded daily and mean PLW was reported as percentage loss in weight to the initial weight of the produce (Minh and Hang 2019).
Veil opening
Veil opening in each punnet was recorded by observing mushroom packages visually and counting the number of veils opened inside the package. The value was taken as the number of mushroom with veils opened to the total number of mushrooms present inside expressed in percentage.
Color
Color measurement was performed using Hunter Lab colorimeter (ColourFlex, Hunter Associates Laboratory, Inc., Virginia, USA) equipped with a 12 mm measuring head (Bal et al. 2011). Color was measured using the CIE L, a, b scale and illuminant D65. Since the mushroom did not cover the entire surface area, they were scanned at three different locations to determine the average ‘L value’, during the measurements. At each storage time, 10 measurements were carried out for each sample from the packages. Only L values were taken for determining the extent of darkening during the storage period.
Texture
The firmness of mushroom samples for puncture was measured with a texture analyzer (TA-XT Plus, Stable Microsystems Ltd., UK) using a 2 mm cylinder probe (Heavy Duty Platform, HDP/90). The experimental parameters were taken at pre-test speed (1.5 mm/s), test speed (1.5 mm/s), post-test speed (10.0 mm/s) and distance (5 mm). The cutting force was also measured with Warner/Blatzer (HDP/BS) blade set with experimental parameters at pre-test speed (1.5 mm/s), test speed (2.0 mm/s), post-test speed (10.0 mm/s) and distance (5 mm). 10 measurements were carried out for each pack-aging condition and average data has been obtained (Antmann et al. 2008; Xiao et al. 2011).
Development of mushroom transportation cabinet
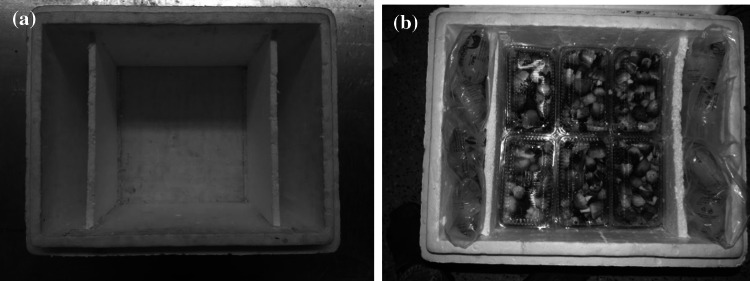
An insulated expanded polystyrene (EPS) box was selected for the transportation cabinet to keep the temperature inside the chamber around 15 °C by placing ice inside it. Further, the mushroom should not come in direct contact with ice as it promotes autolysis at low temperature. The EPS box with outside dimension of 57 cm × 45 cm × 38 cm and 0.0432 m3 volume was modified as mushroom transportation cabinet (Fig. 1a). The inside dimension of the box was 48 cm × 36 cm × 25 cm with sidewall thickness of 4.5 cm. Two EPS partition walls of 1.25 cm thickness were provided 5 cm away from the side wall along the width for keeping 250 g ice pouches and separating the mushroom from coming into direct contact of ice pouches to avoid autolysis and chilling injury. Nine holes (0.5 cm dia) were made in each partition wall for circulation of cool air. The empty weight of the box is 1.2 kg.
Fig. 1.
a Inside design of expanded polystyrene box as transportation cabinet, b mushroom transportation cabinet with pre-cooled mushroom packed in HIPS punnet and ice pouches
Thermal load calculation and requirement of quantity of ice
Outside dimension of the EPS cabinet is 57 cm × 45 cm × 38 cm.
Inside dimension of the EPS cabinet is 48 cm × 36 cm × 25 cm.
Thickness (t) of wall is 4.5 cm.
Assuming natural convective heat transfer (Datta 2001) if ice at 0 °C is placed between the wall and insulated partition (on both sides along width) and ambient temperature is 30 °C,
Air properties at 15 °C
Outside wall height = L = 0.38 m.
Volumetric co-efficient of expansion = = 0.00347.
Grashof Number (NGr) = = 2.45 × 108.
Prandlt Number (NPr) = = 0.741
For vertical planes with length less than 1 m and 104 < NGr NPr < 109.
Nusselt number = NNu = = 0.59 (NGr × NPr)0.25.
Natural convective heat transfer co-efficient = h = 4.533 W/m2K.
Assuming same inside and outside film heat transfer co-efficient to be same.
Overall heat transfer co-efficient = U = 0.567 W/m2K
Where,
Thermal conductivity (k) of EPS is 0.034 W/mK
Thickness (t) of EPS wall = 0.045 m
Assuming average temperature of 7.5 °C inside the EPS chamber (i.e. average of ice temperature 0 °C and inside temperature 15 °C) and calculating thermal load for 6 kg mushroom sample.
Total surface area of the chamber (A) = 2(lb + bh + lh) = 1.29 m2
Sensible heat of mushroom = (mcp∆t)mushroom = 6 × 3.98 x (25–15) = 239 kJ
Heat of respiration of mushroom = 0.806 × 6 kg × 24 h = 116 kJ
Total heat load = (1422 + 21.6 + 239 + 116) kJ = 1798.6 kJ
Amount of ice required = Total heat load / latent heat of fusion of ice = 1798.6 / 334 = 5.4 kg.
Assuming 10% loss at joint, amount of ice required = 1.1 × 5.4 = 6 kg.
Testing of the transportation cabinet
The temperature profile inside the EPS transportation cabinet (with 24 nos of ice pouches of 250 g i.e. 6 kg ice as cooling aid in the partition chamber) was recorded by a temperature data logger under no-load and with full load of 6 kg paddy straw mushroom (packed in 24 HIPS punnets @ 250 g mushroom). The effect of pre-cooling of mushroom on the temperature profile inside the EPS cabinet was also studied by pre-cooling mushroom at 14 °C for 2 h. The quality parameters of mushroom stored in the transportation cabinet under optimum packaging conditions and storage temperature was also determined. Fresh ice pouches were replaced after every 18 h to maintain the optimum storage temperature inside the EPS cabinet during the entire storage period of 3 days. The weight of box with load (6 kg of mushroom in 24 HIPS punnet @ 250 g and 6 kg of ice in 24 pouches @ 250 g) is 13.2 kg (Fig. 2b).
Fig. 2.
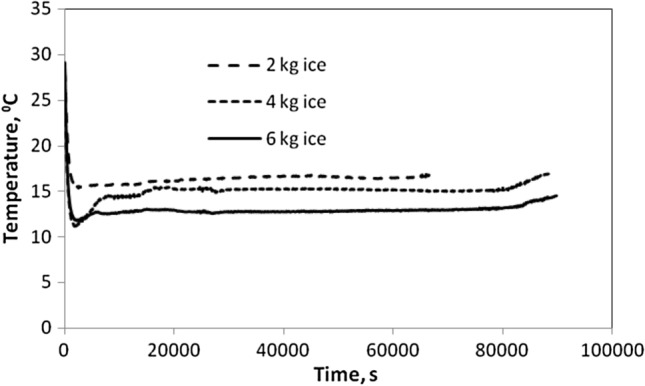
Temperature profile inside EPS box with different ice content under no load condition
Results and discussion
Physical properties of paddy straw mushroom
The physical properties of paddy straw mushroom were determined to design the transportation cabinet. The properties of paddy straw mushroom are given in Table 1. The average bulk density of paddy straw mushroom was found to be 401 kg/m3 and specific heat was 3.98 kJ/kg K. The rate of respiration and heat of respiration were found to be 75.5 mg CO2/kg h and 0.806 kJ/kg h respectively, at 15 °C. The high respiration rate is due to their thin and porous epidermal structure, which is also reported by Minh and Hang (2019).
Table 1.
Physical properties of paddy straw mushroom
| Average bulk density, kg/m3 | 401 |
| Specific heat, KJ/kg K | 3.98 |
| Rate of respiration at 15 °C, mgCO2/kg h | 75.5 |
| Heat of respiration at 15 °C, kJ/ kg h (1 mg of carbon dioxide yields 2.55 cal) | 0.806 |
| Firmness, g | 174.9 |
| Moisture content, % w.b | 92.1 |
Effect of temperature on storage of mushroom
Samples were kept in different storage temperatures of 10, 15 and 20 °C in cold store to find out the optimum storage temperature for paddy straw mushrooms. Physiological loss in weight, respiration rate and veil opening increased, whereas firmness and colour value ‘L’ decreased with increase in storage temperature and storage period (Table 2). Though the PLW, respiration rate and veil opening were less at 10 °C, autolysis of the samples was observed leading to poor sensory acceptance. Autolysis resulted in oozing out of cellular fluid and loss of texture which might be due to the chilling injury of the tropical mushroom. More veil openings at 20 °C was due to high respiration rate and moisture loss. It was inferred that 15 °C is the optimum temperature for storage of paddy straw mushroom up to 3 days. Similar results had also been reported by Kaur and Kappor (2016), Bao et al. (2013), Rai and Arumuganathan (2018) and Jamjumroon et al. (2012).
Table 2.
Effect of storage temperature on quality of paddy straw mushroom
| Temperature (°C) | Storage period (days) | PLW (%) | Respiration rate mgCO2/kgh−1 | Colour ‘L’ value | Firmness (g) | Veil opening (%) |
|---|---|---|---|---|---|---|
| 10 | 0 | – | 73.33 | 35.72 | 176.35 | – |
| 1 | 4.33 | 69.54 | 33.61 | 146.12 | 5.7 | |
| 2 | 8.50 | 70.18 | 21.03 | 111.36 | 11.8 | |
| 3 | 10.61 | 66.73 | 14.55 | 80.85 | 18.8 | |
| 15 | 0 | – | 73.33 | 37.52 | 176.35 | – |
| 1 | 5.17 | 75.50 | 32.16 | 138.90 | 9.8 | |
| 2 | 8.33 | 71.65 | 29.07 | 123.07 | 16.5 | |
| 3 | 12.68 | 68.18 | 26.34 | 106.73 | 28.3 | |
| 20 | 0 | – | 73.33 | 37.52 | 176.35 | – |
| 1 | 7.06 | 83.06 | 32.34 | 161.46 | 17.6 | |
| 2 | 9.45 | 76.15 | 28.99 | 137.80 | 28.1 | |
| 3 | 14.77 | 73.66 | 17.51 | 98.57 | 39.7 |
Effect of perforation on the physical parameters of mushroom
The Effect of perforations in the packaging material on different physical and chemical parameters of paddy straw mushroom is shown in Table 3. The PLW was found to be least in samples packed in HIPS punnet with 1.2% perforation area and maximum in punnet with 1.5% perforation area after 3 days of storage in cool chamber at 15 °C. Rate of respiration decreased with storage period. Colour value ‘L’ decreased with storage period turning in to blackish brown and it was comparatively less in samples packed in punnet with 1.2% perforation. Firmness decreased with time indicating softer texture. Percentage veil opening was observed to be maximum (26.75%) in samples stored in punnet with 1.5% perforation, whereas it was minimum (18.34%) in punnet with 1.2% perforation. Higher veil opening at 1.5% perforation might be due to higher respiration rate, whereas moisture condensation leading to fungal growth was observed in samples stored at lower perforations of 0.6 and 0.9%. It could be inferred from the results that 75 µ thick HIPS punnet with 1.2% holes as the best packaging material to store paddy straw mushrooms at 15 °C.
Table 3.
Effect of perforation in 75 µ HIPS punnet packaging material of paddy straw mushroom at 15 °C
| Perforation area (%) | Storage period (days) | PLW (%) | Respiration rate mgCO2/kgh−1 | Color ‘L’ value | Firmness (g) | % Veil opening |
|---|---|---|---|---|---|---|
| 0.60 | 0 | – | 72.61 | 35.84 | 179.15 | – |
| 1 | 4.77 | 61.55 | 26.50 | 139.57 | 8.39 | |
| 2 | 9.76 | 53.43 | 21.91 | 112.64 | 18.42 | |
| 3 | 17.89 | 46.73 | 19.38 | 118.96 | 24.66 | |
| 0.90 | 0 | – | 72.61 | 35.84 | 179.15 | – |
| 1 | 2.56 | 64.10 | 30.67 | 167.82 | 6.67 | |
| 2 | 7.02 | 54.57 | 23.41 | 146.26 | 17.50 | |
| 3 | 15.97 | 45.33 | 21.68 | 137.39 | 22.31 | |
| 1.2 | 0 | – | 72.61 | 35.84 | 179.15 | – |
| 1 | 2.33 | 65.74 | 30.49 | 165.49 | 5.81 | |
| 2 | 6.51 | 49.31 | 25.62 | 148.74 | 15.67 | |
| 3 | 11.36 | 46.92 | 23.51 | 140.43 | 18.34 | |
| 1.5 | 0 | – | 72.61 | 35.84 | 179.15 | – |
| 1 | 5.63 | 67.75 | 26.21 | 150.23 | 8.30 | |
| 2 | 10.32 | 61.90 | 19.74 | 129.30 | 19.69 | |
| 3 | 16.68 | 52.33 | 16.55 | 112.23 | 26.75 |
Temperature profile inside EPS box under no load condition
The temperature profile inside the transportation cabinet with different quantities of ice as cooling aid in polypouches under no load is shown in Fig. 2b. It was observed that with initial temperature depression, the temperature increased slightly and was almost constant up to 20 h inside the EPS cabinet. The temperature inside the cabinet with 6 kg ice was maintained at 12 °C under no load condition which was lower than the optimum storage temperature of paddy straw mushroom. So it was recommended to place 6 kg of ice inside the partitioned side compartments of the chamber to maintain the desired temperature range for storage of paddy straw mushroom.
Temperature profile inside EPS box loaded with without-pre-cooled and pre-cooled samples
The temperature profile inside the transportation cabinet with 6 kg of without-pre-cooled and pre-cooled mushroom and 6 kg ice is shown in Fig. 3. It was observed that the temperature inside the cabinet with without-pre-cooled sample was shooting up to 32 °C from an initial temperature of 24 °C after 12 h. The increase in temperature was due to the heat evolved by respiration of mushroom. This necessitates pre-cooling to remove respiratory field heat from freshly harvested produce.
Fig. 3.
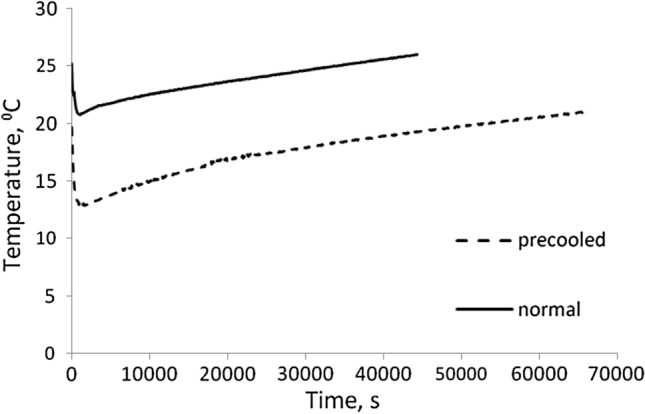
Temperature profile inside EPS box with 6 kg ice pouch storing normal (without-pre-cooled) and pre-cooled mushroom samples
Mushroom samples were pre-cooled at 14 °C and mushroom inside temperature reached 15 °C after 2 h. The pre-cooled samples were kept inside the EPS cabinet with 6 kg ice. It was observed that the temperature inside the cabinet was maintained at 15 ± 2 °C up to 18 h which was close to the optimum storage temperature of paddy straw mushroom. Thus, pre-cooling plays a vital role for maintaining the desired temperature i.e. around 15 °C inside the EPS cabinet for transportation purpose. Pre-cooling helped in slowing down respiratory metabolism and reduced deterioration prior to further transportation or storage.
Storage of pre-cooled mushroom under ambient and EPS cabinet storage conditions
Different physical parameters like physiological loss in weight (PLW%), colour ‘L’ value and percentage veil opening of pre-cooled mushroom samples stored under ambient and EPS transportation cabinet with ice were recorded (Table 4). Paddy straw mushrooms could stay up to 3 days with acceptable quality parameters in the transportation cabinet under controlled temperature condition of 15 °C, whereas the samples in ambient conditions spoiled after two days of storage and were discarded. The physiological loss in weight and veil opening were 12.65 and 13.09%, respectively in EPS transportation cabinet stored samples after 3 days with decrease in L value from 22.5 to 14.7.
Table 4.
Quality of pre-cooled mushroom stored under ambient and EPS cabinet
| Storage condition | Storage period (days) | PLW (%) | Color ‘L’ value | % Veil opening |
|---|---|---|---|---|
| Ambient (31 °C) | 0 | – | 22.5 | – |
| 1 | 2.5 | 18.7 | 9.21 | |
| 2 | 4.1 | 13.5 | 28.36 | |
| 3 | – | – | – | |
| Transportation cabinet (controlled temp. 15 °C) | 0 | – | 22.5 | – |
| 1 | 0.68 | 21.2 | 6.67 | |
| 2 | 9.5 | 18.8 | 9.53 | |
| 3 | 12.65 | 14.7 | 13.09 |
Conclusion
The above study revealed that the shelf-life of paddy straw mushroom could be extended to 3 days by pre-cooling mushroom at 14 °C for 2 h after harvest, packing in 75 µ thick HIPS punnet with 1.2% perforations and stored in cold storage at 15 ± 1 °C temperature. An EPS cabinet (57 × 45 × 38 cm) has been modified for transportation of HIPS punnet packed mushrooms to distant market with ice as a cooling aid. The technology could be successfully adopted by the paddy straw mushroom growers and traders for storage, transportation and marketing for loss reduction and higher return.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amuthan G, Viswanathan R, Kailappan R, Sreenarayanan VV. Studies on osmo-air drying of milky mushrooms Calocybe indica. Mush Res. 1999;8:49–52. [Google Scholar]
- Antmann G, Ares G, Lema P, Lareo C. Influence of modified atmosphere packaging on sensory quality of shiitake mushrooms. Postharvest Biol Techno. 2008;49:164–170. doi: 10.1016/j.postharvbio.2008.01.020. [DOI] [Google Scholar]
- Bal LM, Kar A, Satya S, Naik SN. Kinetics of color change of bamboo shoot during microwave drying. Int J Food Sci Technol. 2011;46:827–833. doi: 10.1111/j.1365-2621.2011.02553.x. [DOI] [Google Scholar]
- Bao D, Gong M, Zheng H, Chen M, Zhang L, Wang H, Tan Q. Sequencing and comparative analysis of the straw mushroom (Volvariella volvacea) genom. PLoS ONE. 2013;8(3):1–12. doi: 10.1371/journal.pone.0058294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones GL. Storage of common mushroom under controlled atmospheres. Int J Food Sci Technol. 1992;27:493–505. doi: 10.1111/j.1365-2621.1992.tb01216.x. [DOI] [Google Scholar]
- Byrnes VC, Beirne D. Effects of gas atmosphere and temperature on the respiration rates of whole and sliced mushrooms (Agaricus bisporus)—implications for film permeability. J Food Sci. 2007;72:197–204. doi: 10.1111/j.1750-3841.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Chang ST, Quimio TH. Tropical mushroom biological nature and cultivation method. Hong Kong: The Chinese University Press; 1980. [Google Scholar]
- Czapski J, Szudyga K. Frozen mushrooms quality as affected by strain, flush, treatment before quality and time of storage. J Food Sci. 2000;65(4):722–725. doi: 10.1111/j.1365-2621.2000.tb16079.x. [DOI] [Google Scholar]
- Datta AK (2001) Natural convection in: transport phenomena in food process engineering. Himalaya Publishing House, Mumbai
- Dhalsamant K, Dash SK, Bal LM, Sahoo NR. Effect of natural antimicrobials (clove and garlic) on shelf life and quality of mushroom (Vovariella volvacea) under modified atmosphere. J Packag Technol Res. 2018;2:243–249. doi: 10.1007/s41783-018-0035-4. [DOI] [Google Scholar]
- Dhalsamant K, Dash SK, Bal LM, Panda M. Effect of perforation mediated MAP on shelf life of mushroom (Vovariella volvacea) Sci Hortic. 2015;189:41–50. doi: 10.1016/j.scienta.2015.03.027. [DOI] [Google Scholar]
- Eissa HA. Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. J Food Qual. 2007;30(5):623–645. doi: 10.1111/j.1745-4557.2007.00147.x. [DOI] [Google Scholar]
- Jafri M, Jha A, Bunkar S, Ram RC. Quality retention of oyster mush-rooms (Pleurotus florida) by a combination of chemical treatments and modified atmosphere packaging. Postharvest Biol Technol. 2013;76:112–118. doi: 10.1016/j.postharvbio.2012.10.002. [DOI] [Google Scholar]
- Jamjumroon S, Wongs-aree C, Mcglasson W, Srilaong V, Chalemklin P, Kanlayanarat S. Extending the shelf-life of straw mushroom with high carbon dioxide treatment. J Food Agric Environ. 2012;10:78–84. [Google Scholar]
- Jaworska G, Bernas E. Effects of pre-treatment, freezing and frozen storage on the texture of Boletus edulis (Bull: Fr) mushrooms. Int J Refrig. 2010;33:877–885. doi: 10.1016/j.ijrefrig.2009.12.031. [DOI] [Google Scholar]
- Jiang T, Jahangir MM, Jiang Z, Lu X, Ying T. Influence of UV-C treatment on antioxidant capacity, antioxidant enzyme activity and texture of postharvest shiitake (Lentinus edodes) mushrooms during storage. Postharvest Biol Technol. 2010;56:209–215. doi: 10.1016/j.postharvbio.2010.01.011. [DOI] [Google Scholar]
- Johl PP, Sodhi HS, Dhanda S, Kappor S. Mushroom as medicine—a review. J Plant Sci Res. 1995;11:73. [Google Scholar]
- Kaur K, Kappor S. Evaluation of different polymeric films for extending shelf life of Volvariella volvacea, the paddy straw mushroom. Agric Res J. 2016;53(4):548–552. doi: 10.5958/2395-146X.2016.00109.5. [DOI] [Google Scholar]
- Khan KA, Patel MB. Evaluation of physico-chemical and microbiological parameters of carrot stored under modified atmospheric packaging. SAARC J Agric. 2010;8:29–38. [Google Scholar]
- Kuyper L, Weinert IAG, McGill AEG. The effect of modified atmosphere packaging and addition of calcium hypochlorite on the atmosphere composition, colour and microbial quality of mushroom. Acad Press Ltd. 1993;26:14–20. [Google Scholar]
- Li T, Zhang M. The physiological and quality change of mushroom Agaricus bisporus stored in modified atmosphere packaging with various sizes of silicone gum film window. Food Sci Technol Res. 2013;19(4):569–576. doi: 10.3136/fstr.19.569. [DOI] [Google Scholar]
- Martine B, Gaelle LP, Ronan G. Post-harvest treatment with citric acid and hydrogen peroxide to extend the shelf life of fresh sliced mushrooms. Lebensm Wiss Technol. 2002;33:285–289. [Google Scholar]
- Mau JL, Miklus MB, Beelman RB. The shelf life of Agaricus mushrooms. In: Charalambous C, editor. The shelf life of foods and beverages. Amsterdam: Elsevier; 1993. pp. 255–288. [Google Scholar]
- Mercado LN, Alabastro EF. Effects of irradiation on the storage quality of fresh straw mushrooms (Volvariella volvacea) Food Qual Prefer. 1989;1(3):113–119. doi: 10.1016/0950-3293(89)90015-3. [DOI] [Google Scholar]
- Minh NP, Hang LP. Several factors affecting to shelf-life of paddy straw mushroom (Volvariella volvacea) in preservation. Plant Arch. 2019;19(2):444–448. [Google Scholar]
- Moon B, Lo YM. Conventional and novel applications of edible mushrooms in today’s food industry. J Food Process Preserv. 2013;38:2146–2153. doi: 10.1111/jfpp.12185. [DOI] [Google Scholar]
- Nerya O, Ben-Arie R, Luzzatto T, Musa R, Khativ S, Vaya J. Prevention of Agaricus bisporus postharvest browning with tyrosinase inhibitors. Postharvest Biol Technol. 2006;39:272–277. doi: 10.1016/j.postharvbio.2005.11.001. [DOI] [Google Scholar]
- Nussinovitch A, Kampf N. Shelf life extension and conserved texture ofalginate-coated mushrooms (Agaricus bisporus) Lebensm Wiss Technol. 1993;26:469–475. doi: 10.1006/fstl.1993.1092. [DOI] [Google Scholar]
- Nur Sakinah MJ, Misran A, Mahmud TM, Abdullah S. A review: production and post harvest management of Volvariella volvacea. Int Food Res J. 2019;26(2):367–376. [Google Scholar]
- Rai RD, Arumuganathan T (2018) Post harvest technology of mushrooms. National Research Centre for Mushroom (Indian Council of Agricultural Research). Technical Bulletin. Yugantar Prakashan Pvt. Ltd, New Delhi, pp 1–31
- Roy S, Anantheswaran RC, Beelman RB. Fresh mushroom quality as affected by modified atmosphere packaging. J Food Sci. 1995;60:334–340. doi: 10.1111/j.1365-2621.1995.tb05667.x. [DOI] [Google Scholar]
- Simon A, Fandos EG, Tobar V. The sensory and microbiological quality of fresh sliced mushroom (Agaricus bisporus L.) packaged in modified atmospheres. Int J Food Sci Technol. 2005;40:943–952. doi: 10.1111/j.1365-2621.2005.01028.x. [DOI] [Google Scholar]
- Tansakul A, Lumyong R. Thermal properties of straw mushroom. J Food Eng. 2008;87:91–98. doi: 10.1016/j.jfoodeng.2007.11.016. [DOI] [Google Scholar]
- Thiribhuvanamala G, Krishamoorthy S, Manoranjitham K, Praksam V, Krishnan S. Improved techniques to enhance the yield of paddy straw mushroom (Volvariella volvacea) for commercial cultivation. Afr J Biotechnol. 2012;64:12740–12748. [Google Scholar]
- Tienhua L, Min Z. The physiological and quality change of mushroom Agaricus bisporous stored in modified atmospheric packaging with various sizes of silicon gum film window. Food Sci Technol Res. 2013;19(4):569–576. doi: 10.3136/fstr.19.569. [DOI] [Google Scholar]
- Verma RN. Cultivation of paddy straw mushroom (Volvariella spp.) in recent advances cultivation of edible mushroom. Solan: National Research Institute of Mushroom; 2002. [Google Scholar]
- Wakchaure GC (2011) Postharvest handling of fresh mushrooms. https://www.researchgate.net/publication/235957041_Postharvest_Handling_of_Fresh_Mushrooms
- Wills RBH, McGlasson WB, Graham D, Joyce DC. Postharvest: an introduction to the physiology and handling of fruit, vegetables and ornamentals. 5. UK: UNSW Press; 2007. [Google Scholar]
- Xiao G, Zhang M, Shan L, You Y, Salokhe VM. Extension of the shelf-life of fresh oyster mushrooms (Pleurotus ostreatus) by modified atmosphere packaging with chemical treatments. Afr J Biotechnol. 2011;10:9509–9517. doi: 10.5897/AJB08.974. [DOI] [Google Scholar]
- Zivanovic S, Busher RW, Kim KS. Textural changes in mushrooms (Agaricus bisporus) associated with tissue ultra structure and composition. J Food Sci. 2000;65:1404–1408. doi: 10.1111/j.1365-2621.2000.tb10621.x. [DOI] [Google Scholar]