Abstract
Hot-melt extrusion (HME) technology was employed to improve water dispersibility of phytosterol (P) using glycerol (G), lecithin (L), and gum arabic (A) as emulsifiers and stabilizers. The structural properties and water dispersibility of HME products were investigated. In contrast to physical mixtures, better water dispersibility and storage stability were observed for HME products, especially P:L:G:A extrudate. These improvements may be mainly associated with decreased crystallinity of phytosterol due to the occurrence of co-crystallization of phytosterol with glycerol during HME process, as confirmed by DSC and XRD data. In addition, HME-induced lecithin-arabic gum reaction products effectively stabilize phytosterol microparticle in aqueous dispersion by providing a steric hindrance. These results suggest that HME could be an effectively and potentially solvent-free technique to produce water-dispersible phytosterol on a large scale.
Keywords: Phytosterol, Hot melt extrusion, Water-dispersibility, Co-crystallization
Introduction
Phytosterol as a series of plant-derived sterols have a similar structure to cholesterol. Phytosterol has been verified as functional ingredients fortified in food products due to its effective cholesterol-lowering efficacy (MacKay 2011). Phytosterol-enriched foods and supplements are the way to achieve intakes high enough to reduce blood LDL cholesterol. Products currently on the market (enriched spreads, fermented milk drinks, etc.) usually contain the proposed daily phytosterol dose of 2.1 g (range 0.2–9.0) g/d. PS intakes of 0.6–3.3 g/d were found to gradually reduce LDL-cholesterol concentrations by, on average, 6–12% (Ras et al. 2014). Due to water insolubility of free phytosterols, esterified material is usually incorporated into commercial oily or fatty foods. Moreover, the improvement of water solubility and dispersibility of free phytosterols have been greatly concerned owing to their limited applications in aqueous foods (Leong et al. 2009, 2011; Cao et al. 2016).
To improve dissolution properties of phytosterol, a key point is the transformation of crystalline state to amorphous one. Colloidal delivery systems such as nanodispersions and microemulsions, have been used to stabilize free phytosterol. Rossi et al. (2010) proposed a strategy to produce phytosterol colloidal particles using antisolvent precipitation in the presence of Tween 80. The emulsification-evaporation technique had also been applied to stabilize phytosterol nanodispersions with different emulsifiers including Tween 20, sucrose fatty acid esters, and sodium caseinate (Leong et al. 2009, 2011; Cao et al. 2016). However, these approaches inevitably introduce more or less organic solvent to colloidal systems and more importantly possess inherent scale-up limitations.
To outcome above disadvantages, developing organic solvent-free or even solvent-free techniques is challenging. Hot-melt extrusion (HME), as a continuous and scalable process, has been extensively used in food and pharmaceutical industries (Castro et al. 2016; Keen et al. 2013). HME could effectively improve the dissolution characteristics of hydrophobic drugs by producing amorphous solid dispersions, leading to the bioavailability enhancement (Keen et al. 2013). Co-crystalization phenomena during HME process have been reported for some hydrophobic drugs (e.g., carbamazepine and ibuprofen) (Dhumal et al. 2010; Liu et al. 2012). HME extrudate exhibited faster dissolution rate than pure carbamazepine due to partly disappearance of crystalline state (Liu et al.2012). In this study, HME technology was used to fabricate water-dispersible phytosterol using glycerol, lecithin, and gum arabic as emulsifiers and stabilizers. The structural properties and water dispersibility of HME products were also investigated.
Materials and methods
Materials
Phytosterol with a total content of > 95% (dry basis), including β-sitosterol(~ 40%), stigmasterol (15–30%), and brassicasterol (< 10%), was purchased from Xi’an Healthful Biotechnology Co., Ltd. (China). Glycerol (> 99%) was obtained from Damao Chemical Reagent Factory (Tianjin, China). Soy lecithin (> 95%) was purchased from Guangzhou Hisoya Biological Science &Technology Co., Ltd. (China). Gum arabic was obtained from Guangzhou Kaihong Flavor Co., Ltd. (China). All other chemicals used were of analytical grade.
Preparation of water-dispersible phytosterol using HME technology
Phytosterol (P), lecithin (L), glycerol (G), arabic gum(A) powers were blended in a mixer for 10 min at the ratio of 1:0.3: 0.2:0.3. In brief, the simple component of physical mixtures was weighed as the ratio firstly. Then these materials were transferred into the stirring cup. At last the mixtures were stirring at 600 rpm in the stirring cup for 10 min using IKA EUROSTAR 20 (IKA, Germany). The extrudate (P:L:G:A) was processed with a twin screw extruder (ZE-16, 25:1 L/D, ATS, China) equipped with a screw diameter of 16 mm. The feed rate is 10 g/min, and rotate speed is 50 rpm. The extruder has seven controllable temperature zones (including the die zone with die diameter of 5 mm), and the temperatures of barrels were set at 60, 110, 140, 140, 130, 110, 100 °C, respectively. The rectangular extrudates were grinded using an agate mortar and pestle after rapidly cooling down to ambient temperature, then passed through a 60-mesh sieve. Binary and ternary materials HME products (P:L = 1:0.3; P:L:G = 1:0.3:0.2) were also obtained, called as P:L, and P:L:G, respectively. The physical mixtures of different materials were used as control samples.
Scanning electron microscope (SEM)
The morphology of physical mixtures and HME products were observed using SEM (TM3000, Hitachi, Japan). Samples were deposited onto double adhesive carbon conductive tape, and coated with gold under vacuum in an argon atmosphere prior to observation (Ion sputter, E-1010, Hitachi, Japan). The morphology of the powders was studied at 25 °C and an accelerated voltage of 15 kV.
Differential scanning calorimetry (DSC)
DSC experiments were performed using a DSC Q100 (TA Instruments, USA). Samples (5-10 mg) were sealed in aluminum pans. DSC runs were carried out from 30 °C up to 200 °C at the heating rate of 10 °C/min. The employed nitrogen gas flow was 40 mL/min.
X-ray diffractometry (XRD)
XRD of extruded products were performed with a diffraction unit (polycrystal X-ray diffraction; Rigaku SmartLab SE, Japan) operating at 40 kV and 40 mA. The radiation was generated from a Cu-Kα (k = 1.5406 Å) source. The diffraction data were collected from 2θ values from 5° to 60°; step size, 0.01° and acquisition time of 1.0–2.0 s/step.
Water dispersibility
To test water dispersibility of HME products in aqueous solutions, the physical mixtures and HME products were dispersed in PBS buffer (10 mM, pH 7.0) at 10 mg/mL using a high-speed dispersing unit (model IKA-ULTRA-TURRAX T25basic, IKA Works, Inc., Wilmington, NC) at 15,000 rpm for 2 min. The size distribution of fresh dispersions was measured using a Mastersizer 3000 (Malvern Instruments Co. Ltd., Worcestershire, UK) at 25 °C. The refractive indexes of samples and PBS buffer were taken as 1.449 and 1.330, respectively (Rossi et al. 2010). The dispersions were placed in glass vials with caps and stored for 20 days at 25 °C to observe the physical stability of HME products.
Results and discussion
Structural properties of HME products
The photographs of physical mixtures and HME products are shown in Fig. 1a. There are no obvious differences between P:L mixture and extrudate. In the case of P:L:G and P:L:G:A, darker color was observed for extrudate compared to mixture samples, especially for P:L:G:A extrudate, probably meaning the occurrence of color reaction during HME process. The brown color of the product may be acceptable for brown drink. The potential reaction between lecithin and gum arabic may be mainly responsible for the color change of P:L:G:A group, as demonstrated by darker color of LA extrudate (Fig. 1a).The SEM images of physical mixtures and corresponding HME powders are shown in Fig. 1b. The crystalline phytosterol particles were observed clearly for physical mixture, especially P:L mixture. After hot-melt extrusion, the crystalline phytosterol particles of HME products seem to be partly disappeared. HME-induced disappearance of crystalline state of hydrophobic drugs carbamazepine also has been reported (Liu et al. 2012).
Fig.1.
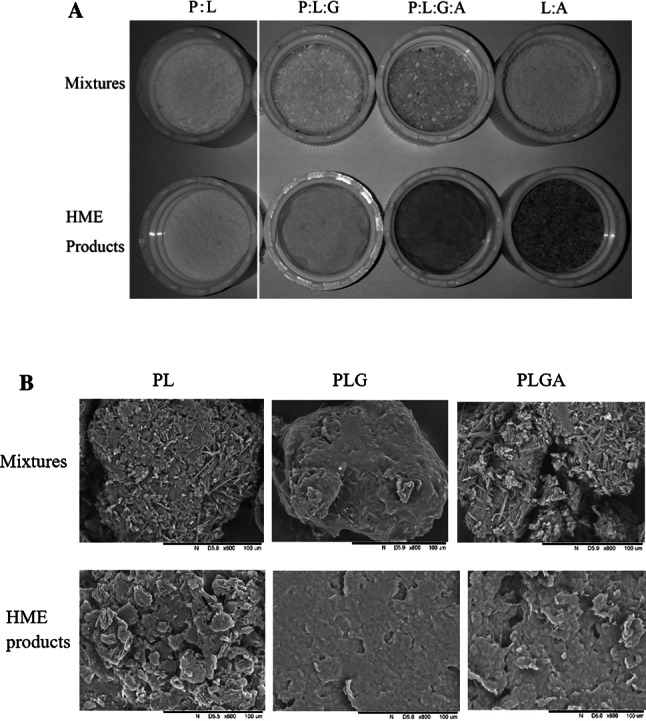
Photographs (a) and scanning electron microscopy images (b) of physical mixtures and HME products
Figure 2 shows DSC and XRD patterns of phytosterol and HME products. The phytosterol exhibited a sharp and narrow melting endotherm peak at 137.4 °C. Same DSC patterns were observed for binary, ternary, quaternary physical mixtures. For P:L products, the endotherm peak with lower heat enthalpy also appeared at 135.4 °C, meaning the existence of typical phytosterol crystal in the P:L extrudates. However, the disappearance of typical phytosterol endotherm, accompanied by the appearance of two other endothermic peaks, was observed for P:L:G (110.6 °C and 158.1 °C) and P:L:G:A (113.9 °C and 159.6 °C). These results may be mainly ascribed to the co-crystallization of phytosterol and glycerol and accompanying formation of phytosterol-glycerine complex during hot-melt extrusion when glycerol was added as a plasticizer and hydrogen-bonding agent (Liu et al. 2012; Padro et al. 1997; Qian et al. 2015). The phytosterol crystalline dissolved in the molten mixtures is followed by an immediate cocrystallization process. Glycerol has three hydroxyl groups per molecule, showing three-dimensional hydrogen-bonding patterns. It probably contribute to the cocrystallization process (Fig. 2a). In fact, glycerol could also act as a physical spacer molecule between neighboring phytosterol molecules, thereby altering the physical and chemical molecule associations and preventing aggregation and/or crystallization of phytosterol.
Fig.2.

DSC thermograms (a) and X-ray diffraction diffractograms (b) of free phytosterol and HME products
To further evaluate structural properties of phytosterols and HME products, the X-ray diffraction was carried out, as shown in Fig. 2b. The pure phytosterols exhibited the characteristic peaks around 2θ(°) = 14.9 and 2θ(°) = 18.4, similar to the observations reported by other authors (Cao et al. 2016). The diffractogram of P:L:G:A mixtures had shown the coincidence with the “fingerprint” of phytosterols, exhibiting peaks at 2θ(°) = 15.0 and 18.5. In the case of P:L:G:Aextrudate, the typical diffractogram peaks has almost disappeared, suggesting lower crystallinity degree of original phytosterol crystal compared to P:L:G:A mixture. Moreover, the appearance of two new diffractogram peaks at 2θ(°) = 4.8 and 41.2 were also observed, probably suggesting partly co-crystallization of phytosterol and glycerol during hot-melt extrusion (Bond. 2007; Qian et al. 2015). Upon heating and extrusion, a hydrogen bonding interaction occurred between a hydroxyl group on glycerol and the phytosterols hydroxyl (Qian et al. 2015). These results are in accordance with DSC results (Fig. 2a).
Water dispersibility of HME products
The water dispersibility and storage stability of phytosterol physical mixtures and HME products was investigated, as shown in Fig. 3. The phytosterol dispersion stabilized by physical mixtures exhibited a large size distribution with the appearance of large peaks at a size of around 30 μm and 1000 μm, while a small size distribution was observed for HME products (P:L, P:L:G, and P:L:G:A) (Fig. 3), suggesting the improvement of water dispersibility, especially P:L:G and P:L:G:A. Compared to P:L extrudate, the P:L:G and P:L:G:A extrudates exhibited smaller size distribution (10 μm). This could be mainly attributed to the lower crystallinity degree of phytosterol crystal compared to P:L:G:A mixture, as evidenced by DSC and XRD data (Fig. 2). The formation of phytosterol-glycerol complex due to the co-crystallization of phytosterol and glycerol during hot-melt extrusion is a reasonable explanation for this structural change of phytosterol. In addition, lecithin and gum arabic as emulsifier and stabilizer could also further improve water dispersibility of phytosterol. It should be pointed out that HME products exhibit relatively poor water dispersibility compared to other products previously developed by other techniques (e.g. antisolvent precipitation) (Rossi et al. 2010). However, HME, as a continuous and scalable solvent-free process, has significant advantage in industrial production.
Fig.3.
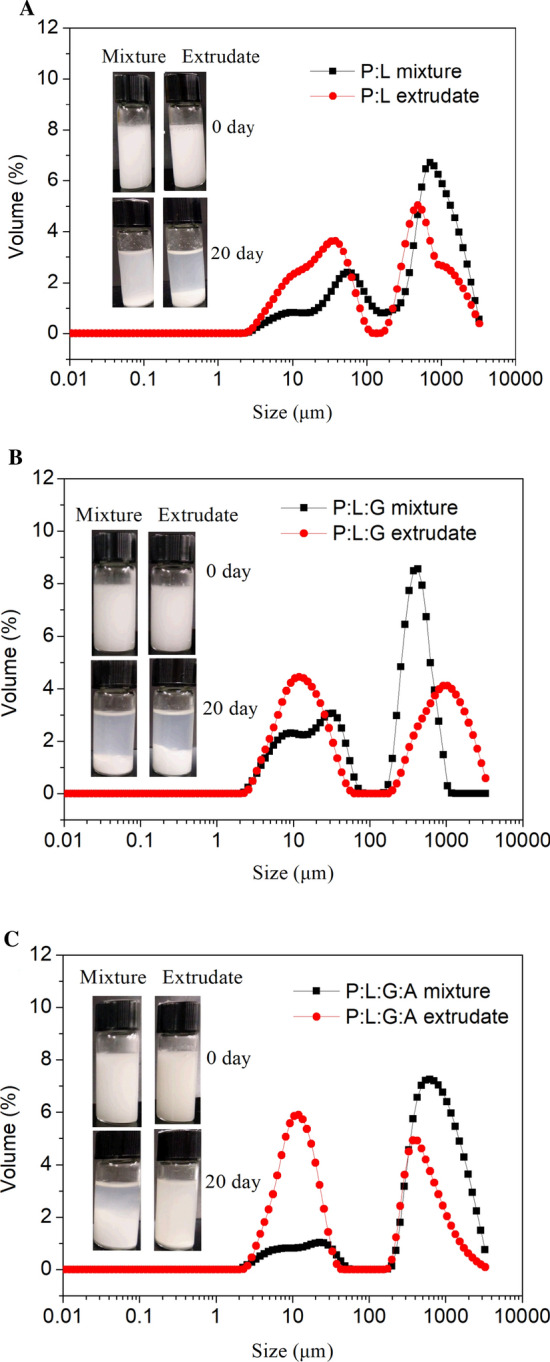
Size distribution of fresh phytosterol dispersion for physical mixtures and HME extrudates (1.0%, w/v). a P:L; b P:L:G; c P:L:G:A. The embedded images were visual observation of fresh phytosterol dispersion (1.0%, w/v) for fresh (0 d) and stored (20 d) samples
To evaluate the storage stability of phytosterol dispersion, fresh aqueous dispersion was stored for 20 days at 25 °C. After 20 days of storage, the large amounts of agglomeration in the bottom were observed for the dispersion of all physical mixtures (Fig. 3). Similar phenomena were observed for P:L and P:L:G extrudates. It is worth noting that, compared with P:L:G:A mixture, P:L:G:A extrudate had shown a smaller amount of agglomeration, suggesting better storage stability. This is probably related to the co-crystallization of phytosterols and glycerol as well as the steric hindrance provided by lecithin-gum arabic reaction products. The potential reaction between lecithin and gum arabic had been demonstrated by darker color of LA extrudate (Fig. 1a).
Conclusion
In this study, hot-melt extrusion effectively improved water dispersibility of phytosterol using glycerol, lecithin, and gum arabic as emulsifiers and stabilizers. Compared to physical mixtures, P:L:G:A extrudate exhibited better water dispersibility and storage stability, which may be mainly associated with the decrease of phytosterol crystallinity due to the occurrence of co-crystallization of phytosterol with glycerol during hot-melt extrusion process, as confirmed by DSC and XRD data. Moreover, lecithin-gum arabic reaction products possibly provided a steric hindrance to stabilize phytosterol microparticle in aqueous dispersion, leading to the improvement of water dispersibility of phytosterol. These findings provide food industry with a possibility for solvent-free production of water-dispersible phytosterol.
Acknowledgements
The work is supported by grants from the Fundamental Research Funds for the Central Universities (SCUT, 2019MS097) and Chinese National Natural Science Foundation (31801486).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bond AD. What is a co-crystal? Cryst Eng Comm. 2007;9:833–834. doi: 10.1039/b708112j. [DOI] [Google Scholar]
- Cao WJ, Ou SY, Lin WF, Tang CH. Food protein-based phytosterol nanoparticles: fabrication and characterization. Food Funct. 2016;7:3973–3980. doi: 10.1039/C6FO00861E. [DOI] [PubMed] [Google Scholar]
- Castro N, Durrieu V, Raynaud C, Rouilly A, Rigal L, Quellet C. Melt extrusion encapsulation of flavors: a review. Poly Rev. 2016;56:137–186. doi: 10.1080/15583724.2015.1091776. [DOI] [Google Scholar]
- Dhumal R, Kelly A, York P, Coates PD, Paradkar A. Cocrystalization and simultaneous agglomeration using hot melt extrusion. Phram Res. 2010;27:2725–2733. doi: 10.1007/s11095-010-0273-9. [DOI] [PubMed] [Google Scholar]
- Keen JM, McGinity JW, Williams RO. Enhancing bioavailability through thermal processing. Int J Pharm. 2013;450:185–196. doi: 10.1016/j.ijpharm.2013.04.042. [DOI] [PubMed] [Google Scholar]
- Leong WF, Man YBC, Lai OM, Long K, Misran M, Tan CP. Optimization of processing parameters for the preparation of phytosterol microemulsions by the solvent displacement method. J Agric Food Chem. 2009;57:8426–8433. doi: 10.1021/jf901853y. [DOI] [PubMed] [Google Scholar]
- Leong WF, Man YBC, Lai OM, Long K, Nakajima M, Tan CP. Effect of sucrose fatty acid esters on the particle characteristics and flow properties of phytosterol nanodispersions. J Food Eng. 2011;104(1):63–69. doi: 10.1016/j.jfoodeng.2010.11.028. [DOI] [Google Scholar]
- Liu X, Lu M, Guo ZF, Huang L, Feng X, Wu CB. Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion. Pharm Res. 2012;29:806–817. doi: 10.1007/s11095-011-0605-4. [DOI] [PubMed] [Google Scholar]
- MacKay D, Jones PJH. Phytosterols in human nutrition: type, formulation, delivery, and physiological function. Eur J Lipid Sci Tech. 2011;113:1427–1432. doi: 10.1002/ejlt.201100100. [DOI] [Google Scholar]
- Padro JA, Saiz L, Guardia E. Hydrogen bonding in liquid alcohols: a computer simulation study. J Mol Struct. 1997;416:243–248. doi: 10.1016/S0022-2860(97)00038-0. [DOI] [Google Scholar]
- Qian ZH, Yue X, Yi SJ, Li QT, Chen X. Unique lamellar lyotropic liquid crystal phases of nonionic phytosterol ethoxylates in glycerol. RSC Adv. 2015;5:101393–101400. doi: 10.1039/C5RA21446G. [DOI] [Google Scholar]
- Ras RT, Geleijnse JM, Trautwein EA. LDL-cholesterol-lowering effect of plant sterols and stanols across different dose ranges: a meta-analysis of randomised controlled studies. Br J Nutr. 2014;112:214–219. doi: 10.1017/S0007114514000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L, Seijen ten Hoorn JWM, Melnikov SM, Velilov KP. Colloidal phytosterols: synthesis, characterization and bioaccessibility. Soft Matter. 2010;6:928–936. doi: 10.1039/B911371A. [DOI] [Google Scholar]


