Abstract
This is the first original study on chemical, thermal, antimicrobial, and antioxidant activity of the leaf and gum hydroethanolic (20:80 v/v) extracts of Ferula asafoetida endemic for Iran. The ratio of solvent to the dried matters was 3:1 (w/w) and after filtering, the solvent was evaporated under vacuum (at 40 °C). Leaf extract contained greater phenolic and flavonoid compounds and thus higher DPPH scavenging and ferric reducing power. Leaf extract constituted high levels of carvacrol (15.40%) and α-bisabolol (9.75%) while in gum extract contained high levels of (Z)-b-ocimene (20.91%) and (E)-1-propenyl-sec-butyl-disulfide (17.62%). Umbelliprenin and ferulic acid were the major phenolic compounds in both extracts. Results of TGA and DSC revealed temperatures below and upper 100 °C and 160 °C as dominant regions of weight loss for gum and leaf extracts, respectively. Minimal inhibitory concentration for Escherichia coli, Staphylococcus aureus, Aspergillus niger, and Saccharomyces cerevisiae growth were 62.5, 62.5, 125, 125 mg/l and 400, 300, 50, 300 mg/l of leaf and gum extracts, respectively. Ferula asafoetida extracts can have particular applications in the food industry due to beneficial biological activity.
Keywords: Polyphenol, Flavonoids, DSC, Extract, Gum, Leaf, MIC
Introduction
The preservation of both the nutritional value and sensory attributes of food products comprises a major industrial target given the current consumer demands. Furthermore, the ever-growing competition between producers had made cost-minimization vital across the food industry (Clemente et al. 2016). In recent years, given the globalized nature of the food industry as well as the distant distribution of products, research and development have focused on safe, innovative technologies that prolong a food product’s shelf-life while keeping it ‘fresh’ in terms of quality (Becerril et al. 2013). At the same time, researchers have aimed to find alternatives to replace synthetic antimicrobials and antioxidants, with natural substances asserting their value as historically-accepted therapeutical agents (Wu et al. 2009). In fact, a major portion of the commonly used medical drugs is originally derived from natural substances (Shrivastava et al. 2012).
Extensive research has been done in the past to identify or extract medicinal and beneficial properties from different parts of plants, and this research is ongoing; for example antioxidant activities of Bletilla striata fibrous roots (Chen et al. 2020), Crocus sativus petals of saffron (Ahmadian-Kouchaksaraie and Niazmand 2017), black cumin seed oil and extract (Soleimanifar et al. 2019), and Portulaca oleracea L. Seed hydro-alcoholic extract (Jalali Mousavi et al. 2015) as well as antinociceptive, antioxidant and antimicrobial of essential oil from Hymenaea cangaceira (de Veras et al. 2020), mitodepressive, antioxidant, antifungal and anti-inflammatory of Romanian native Arctium lappa L. (Asteraceae) and Veronica persica Poiret (Fierascu et al. 2018), antimicrobial, antioxidant activity ginger, turmeric rhizome and dukung anak crude extract (Bordoh et al. 2020) and antimicrobial activity of ethyl acetate and chloroform fractions Zanthoxylum zanthoxyloides and Gongronema latifolium (Adeeyo et al. 2020) were investigated and reported.
One of the wild native Iranian plants is Ferula asafoetida; this herbaceous plant is also grown in other countries, including India, Afghanistan, Kyrgyzstan, Uzbekistan, and Turkmenistan. The plant has various names in different regions of Iran, most commonly being referred to as “Anghouzeh”, “Khorakoma” or “Anguzakoma” (Iranshahy and Iranshahi 2011). The Ferula genus of the Umbelliferae family comprises 140 different species spreading wide from the Mediterranean area to the central parts of Asia (Ur Rahman et al. 2008). In Iran, the gum extract of F. asafoetida has traditionally been utilized in the treatment of abdominal pain, constipation, diarrhea and helminth infections (Dehpour et al. 2009). Furthermore, recent studies have revealed other medicinal benefits of this plant including antioxidant, antimicrobial, antiviral, antifungal, cancer chemopreventive, anti-diabetic, anti-hypertensive, anticarcinogenic, and antispasmodic activity, as well as a relaxant, intestinal parasites, neuroprotective and molluscicidal properties (Amalraj and Gopi 2017; Mala et al. 2018; Tavassoli et al. 2018). However, a stable formulation appropriate for human consumption is yet to be derived pharmacologically, mostly because of the gummy nature and poor taste and odor of asafoetida gum; these unpleasant sensory attributes are due to a high content of volatile oils rich in sulfurous compounds (Vijayasteltar et al. 2017).
To date, many researches were done on essential oil extracted from Ferula genus. For example, Iranshahy and Iranshahi (2011) reviewed coumarins and sesquiterpene coumarins, Sulfur-containing compounds, diterpenes, phenolics, sesquiterpenes, and other miscellaneous compounds in Ferula asafoetida oleo-gum-resin. Prabaharan et al. (2016) produce antioxidant peptides from Ferula asafoetida root protein using gastrointestinal enzymes. Labed-Zouad et al. (2015) found that the main components of the Ferula vesceritensis essential oils were α-pinene, β-pinene, α-phellandrene, fenchylacetate, elixene, aristolene, caryophyllene oxide, and carotol. Further, they reported that the best antimicrobial activity was exhibited against Staphylococcus aureus, Pseudomonas aerugina, Escherichia coli, Morganella morganii, and Klebsiella pneumoniae strains. Kose et al. (2010) identified the chemical composition of Ferula lycia and revealed that the most prominent component of essential oil was α-pinene (59.89%). β-Pinene (19.01%), limonene (3.21%) and bornyl acetate (2.10%). They also described that the essential oil exhibited weak antioxidant activity.
Though a large literature described the chemical composition and antioxidative and antimicrobial effects of essential oil extracted from F. asafoetida gum, according to our knowledge, there is no information about leaf and gum hydroalcoholic extracts of F. asafoetida. However, due to the volatility of these compounds and possible evaporation during the various processes, the application of hydroalcoholic extracts of leaf and gum can be an alternative. Therefore, the objective of the present study was to investigate the chemical composition, antioxidant, antimicrobial, and thermal properties of F. asafoetida leaf and gum hydroalcoholic extracts.
Materials and methods
Chemicals and reagents
Folin–Ciocalteu reagent, sodium bicarbonate, gallic acid, quercetin, aluminum chloride, 1,1-diphenyl-2-picryl hydrazyl (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ), ferric chloride, sodium nitrite, potassium persulfate, and culture media were purchased from Sigma Aldrich (USA) and Merck (Germany). Ethanol with a purity of 99% bought from Hamoon Co. (Iran). All other chemicals were of analytical grade or purer.
Plant material
Leaves of F. asafoetida were obtained from the mountains of Sabzevar (Khorasan Razavi province, Iran) toward the end of April. A senior plant taxonomist from Ferdowsi University’s Department of Biology (Mashhad, Iran) identified the leaves taxonomically. Next, the samples were cleaned, washed, and air-dried at environmental temperature (25 ± 3 °C), before being pulverized to 40 mesh and stored at 4 °C until experimentation.
In June, the root of the F. asafoetida plant was scraped to collect its gum exudate. Ahead of experimentation, the samples were frozen and stored at − 18 °C.
Determination of chemical compositions
Initially, the chemical composition of all samples was determined. Moisture content was determined via drying for 24 h at 105 °C (AOAC 2006), whereas the Kjeldahl method was utilized to determine protein content (AOAC 2006). Calcination at 550 °C was employed to determine total ash content (AOAC 2006). For the determination of metal cations, HNO3 was used to dissolve the ashes; the resulting solution was heated using a water bath and filtered with Whatman filter paper (mesh 11 µm) before being injected into an inductively coupled plasma (ICP) machine (SPECTRO ARCOS ICP-OES System, Germany). A Cyclone spray chamber and a Mira-mist nebulizer were utilized. The operating parameters for the instrument are radio frequency power, 40 MHz; plasma gas 15.0 l/min; auxiliary gas, 0.20 l/min; carrier gas, 0.60 l/min; view direction, axial (Xiao et al. 2016). The Soxhlet method was used for fat analysis with hexane as the solvent (AOAC 2006). The contents of crude fibers and insoluble solids were evaluated using the AOAC’s (2006) methods.
Preparation of the extracts
The hydroethanolic extracts of leaf and crude F. asafoetida gum were prepared by adding 80% ethanol and stirring at 500 rpm for 3 h at ambient temperature (25 ± 3 °C). The ratio of solvent to the dried leaf or crude fresh gum was 3:1 (w/w) and the solvent was used in two cycles. After filtering, the solvent was evaporated under vacuum (at 40 °C) to obtain the residue. The residues were stored at − 18 °C until used.
Dried extracts characterizations
Total phenolic content (TPC) assay
The amount of total phenolic content in the leaf and gum extracts was determined using Folin–Ciocalteu reagent with gallic acid as a standard according to Kavoosi and Rowshan (2013) with some modifications. Briefly, 100 µl of each extract solution (diluted with distilled water) was added to 1 ml Folin–Ciocalteu reagent (diluted to 10%). After a 3 min 1.5 ml sodium bicarbonate solution (Na2CO3, 20%) was added and the mixture was allowed to stand in the dark for 90 min. Absorbance was read at 765 nm using a spectrophotometer (Pharmacia, Uppsala, Sweden). The results were expressed as milligram of gallic acid (GAE) equivalent per gram of dried extract.
Total flavonoids content (TFC) assay
The flavonoids contents in the extracts were determined spectrophotometrically using the method of Marinova et al. (2005) based on aluminum chloride colorimetric assay with quercetin as a standard. Each diluted solution of the extracts (0.1 ml) was mixed with 5% NaNO2 (0.3 ml). Dilution had been done beforehand using distilled water. When 5 min had passed, 10% AlCl3 (0.3 ml) was added to the mixture. Six minutes later, 1 M NaOH (2 ml) was poured into the mixture, then the total volume was made up to 10 ml using distilled water. After thorough mixing, the absorbance of each solution was read at 510 nm with a spectrophotometer (Pharmacia, Uppsala, Sweden). The total flavonoid content of each dried extract was reported in terms of milligrams of quercetin (QE) equivalents per gram of the sample.
DPPH radical scavenging assay
The bleaching of a purple colored methanol solution of 1,1-diphenyl-2-picryl hydrazyl radical (DPPH) was used for the determination of the free radical-scavenging activity of the extracts according to Kose et al. (2010) with some modifications. 200 µl of extract solution with proper dilution in ethanol was added to a 3 ml of DPPH radical solution in ethanol (0.1 mM). The mixture was shaken vigorously and allowed standing in dark for 30 min. the absorbance was read at 517 nm with a spectrophotometer (Shimadzu UV-1601, Kyoto, Japan). The percentage of radical scavenging capacity (RSCDPPH %) of DPPH scavenging that is calculated as follow:
where AControl and ASample are the absorbance of the control reaction and test compound, respectively.
Determination of ferric reducing/antioxidant power (FRAP assay)
FRAP assay was carried out by the method of Ahmadian-Kouchaksaraie et al. (2016) with minor modifications. The principle of this procedure is based on the reduction of ferric tripyridyltriazine complex to its ferrous, colored form in the presence of antioxidants. The stock solutions comprised 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl, and 20 mM FeCl3·6H2O solution. The working solution was made freshly by mixing 25 ml of acetate buffer, 2.5 ml of TPTZ solution, and 2.5 ml of FeCl3·6H2O solution. The mixed solution was incubated at 37 °C for 30 min and was referred to as FRAP solution. Sample (150 μl) was mixed with 3 ml of FRAP solution and kept for 30 min in dark. Readings of the colored products (ferrous tripyridyltriazine complex) were then taken at 593 nm. Ferrous sulfate was used as a reference standard, and the FRAP was expressed as µmol of Fe2+ equivalents (FE) per gram of dried extract (µmol FE/g dried extract).
Thermogravimetric analysis (TGA)
A TGA (Mettler Toledo, Model TGA1, Switzerland) instrument was used for the conduction of thermogravimetric analysis. Samples of dried leaf and gum extracts weighing 14.24 mg were placed separately on platinum pans before being heated from 50 to 500 °C at a rate of 10 °C/min under nitrogen atmosphere. To obtain a good understanding of the heat stability of these samples, the differential of the weight loss (% °C) was evaluated and reported.
Differential scanning calorimetry (DSC)
Thermal properties of dried leaf and gum extracts were measured by the DSC instrument (Mettler Toledo, Model DSCI, Switzerland). Samples weighing 15 mg were hermetically sealed in aluminum pans. Next, under a nitrogen atmosphere, the thermal behavior of the samples was evaluated between 0 and 500 °C with a 10 °C/min heat application. Universal Analysis 2000 software was used for data analysis.
Gas chromatography/mass spectrometry
GC analysis was carried out using Agilent-technology chromatograph. GC oven temperature was performed as follows: 60–210 °C at 3 °C/min; 210 °C to 240 °C at 20 °C/min and hold for 8.5 min, injector temperature 280 °C; detector temperature, 290 °C; carrier gas, N2 (1 ml/min); the split ratio of 1:50. The gum and leaf extracts from F. asafoetida were analyzed using an Agilent model 7890-A series gas chromatography and Agilent model 5975-C mass spectrometry. The HP-5 MS capillary column (phenylmethyl siloxane, 30 m × 0.25 mm i.d × 25 µm) was used with helium at 1 ml/min as the carrier gas. The split ratio was adjusted to 1:50 and the injection volume was 1 µl. The injector temperature was 280 °C. The quadrupole mass spectrometer was scanned over 40–550 amu with an ionizing voltage of 70 eV.
The standards were injected after the extracts under the same chromatographic conditions and subsequently, retention times (RT) were determined. The compounds were identified by comparison of retention indices (RI, HP-5) with those reported in the literature and by comparison of their mass spectra with the Wiley GC/MS Library, Adams Library, Mass Finder 2.1 Library data published mass spectra data (Adams 2007; McLafferty 2009).
HPLC analysis of phenolic compounds
A Shimadzu HPLC machine featuring a UV–Vis photodiode-array detector (DAD) was used to determine the phenolic compounds present in the samples both qualitatively and quantitatively. A control system (SCL-10A VP), featuring an LC pump (LC-10 AD VP) as well as an auto-injector (SIL-10AD VP), was used to control the apparatus. To obtain chromatographic isolation, a 5 mm ODS3 reversed-phase Prodigy column (250 mm × 4.6 mm; Phenomenex) was used with solvent A (water/acetic acid, 97/3, v/v) and solvent B (methanol); the following gradient conditions were applied: 0–3 min, 0% B; 3–9 min, 3% B; 9–24 min, 12% B; 24–30 min, 20% B; 30–33 min, 20% B; 33–43 min, 30% B; 43–63 min, 50% B; 63–66 min, 50% B; 66–76 min, 60% B; 76–81 min, 60% B; 81–86 min, 0% B. The temperature of the column was kept at 25 °C, while the flow rate was maintained at 1 ml/min. UV-detection was carried out at wavelengths measuring 260, 292 and 370 nm. For each compound, the UV-spectrum between 240, and 400 nm was recorded.
All procedures were conducted in duplicate; curves developed using commercially-obtained pure solutions of each compound (1–30 mg/ml) were utilized as points of comparison to determine the quantity of the identified phenols.
Antibacterial and antifungal assay
The Persian Type Culture Collection (PTCC; Tehran, Iran) was the place from which all microorganisms were obtained. Separate tests were conducted using the dried leaf and gum extracts of F. asafoetida to determine their activity against Escherichia coli [PTCC 1330 (ATCC 8739)], Staphylococcus aureus [PTCC 1112 (ATCC 6538)], Aspergillus niger [PTCC 5010 (ATCC 9142)] and Saccharomyces cerevisiae [PTCC 5051 (ATCC 9080)]. Serial dilutions between 0 and 600 mg/ml were used to identify the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC); the microdilution method of Borges et al. (2013) was slightly modified and applied for this purpose. Separate suspensions were prepared using strains of E. coli or S. aureus and Mueller–Hinton Broth (MHB); the bacterial concentration was initially set to 108 CFU/ml (0.5 McFarland standards at 640 nm) before dilution occurred using MHB and a final concentration of 105 CFU/ml was achieved. To culture S. cerevisiae and A. niger, yeast malt broth (YMB) and potato dextrose broth (PDB) were used, respectively. Next, sterile polystyrene microtiter plates featuring 96 wells were employed; a minimum of 16 wells was filled by 180 µl of bacterial suspension as well as 20 µl of leaf or gum extract. Incubation was subsequently conducted through a shaker device (150 rpm; Shaking Incubator, Shin Saeng, Fine Tech, Korea); plates containing bacteria were incubated at 37 °C for 24 h, whereas the conditions were 25 °C and 72 h for fungi-containing plates. For sterility control, the media were incubated without the addition of microbial species. For growth control, a blank preparation was made using the media and only the microbial species. Gentamicin and phenol (Padtan Teb, Iran, 0.01 mg/ml) was used in preparing positive controls for bacteria and fungi, respectively. A microplate reader (Spectramax M2e, Molecular Devices, Inc.) was utilized to read the absorbance of the preparations at 640 nm, facilitating the estimation of growth inhibition. The microdilution method was employed in the determination of the extracts’ minimum inhibitory concentration (MIC), i.e., the minimum concentration where growth could not be detected.
In order to determine MBC 100 ml of each suspension was transferred onto Mueller–Hinton agar (MHA) plates and incubated at 37 °C for bacteria and yeast malt agar (YMA) and Potato dextrose agar (PDA) plates used for S. cerevisiae and A. niger, respectively and incubated at 25 °C. Colony enumeration was carried out after 24 and 72 h for bacteria and fungi, respectively. Cell suspensions without phytochemicals were used as controls. The MBC was taken as the lowest concentration of phytochemicals at which no CFU was detected on solid medium. Each experimental procedure was conducted in triplicate.
Statistical analysis
All data are expressed as the means plus standard deviations. All measurements except chromatography analyses were taken in triplicate. The chromatography analyses including GC–MS and HPLC were done in duplicate. The significant differences between treatments were analyzed by one-way analysis of variance (ANOVA) test at P < 0.05 (Tukey test) using Minitab (version 16.2.4) software. Data were reported as mean ± standard deviation (SD).
Results and discussion
The chemical characteristics of plant materials
Table 1 shows some chemical composition of F. asafoetida’s dried leaf and fresh exudates (gum) which use for providing extracts. According to the results of ANOVA, there is a significant difference between them (P < 0.05). Clearly, the moisture content of gum significantly was more than leaves while the ash, protein and crude fiber contents were noticeably higher in leaves. The gum contained a little more fat content.
Table 1.
Initial chemical composition (mean ± SD) of F. asafoetida gum and dried leaf (%)
| Composition | Dried leaf | Fresh gum |
|---|---|---|
| Moisture | 6.03 ± 0.01B | 10.54 ± 0.75A |
| Ash | 13.78 ± 0.10A | 3.85 ± 0.11B |
| Fat | 1.29 ± 0.05B | 1.76 ± 0.16A |
| Protein | 21.85 ± 1.12A | 1.95 ± 0.23B |
| Crude fiber | 15.2 ± 1.32A | 0.42 ± 0.09B |
Non-similar letters in each row indicate a significant difference according Tukey test (P < 0.05)
Properties of leaf and gum extracts
Mineral content
The mineral content of ferula leaf and gum extracts are presented in Table 2. In general, the greatest mineral observed in ferula leaf and gum extracts was calcium (695 mg/kg dried extract and 860.57 mg/kg dried extract, respectively), followed by sulfur, phosphorus, and iron, respectively.
Table 2.
The minerals, total phenols content, total flavonoids content, The RSCDPPH and FRAP of F. asafoetida leaf and gum hydro-alcoholic extracts (mean ± SD)
| Parameter | Leaf extract | Gum extract |
|---|---|---|
| Minerals (mg/kg dried extract) | ||
| Barium | 1.91 ± 0.02A | 0.65 ± 0.00B |
| Calcium | 695.00 ± 13.92B | 860.57 ± 5.47A |
| Copper | 8.64 ± 0.075A | 3.55 ± 0.05B |
| Iron | 40.93 ± 1.31A | 13.81 ± 0.06B |
| Lithium | 2.75 ± 0.00A | 1.57 ± 0.01B |
| Manganese | 1.19 ± 0.04B | 1.60 ± 0.11A |
| Phosphorus | 62.81 ± 0.09A | 41.41 ± 0.63B |
| Selenium | 1.49 ± 0.16A | 0.23 ± 0.02B |
| Zinc | 6.73 ± 0.04B | 7.19 ± 0.10A |
| Sulfur | 111.01 ± 0.70A | 90.66 ± 1.62B |
| Total phenolic compounds (mg GAE/g dried extract) | 95.70 ± 2.69A | 9.67 ± 0.45B |
| Total Flavonoids compounds (mg QE/g dried extract) | 16.71 ± 0.54A | 0.11 ± 0.02B |
| RSCDPPH (%) | 84.00 ± 1.07A | 22.72 ± 0.35B |
| FRAP (µmol FE/g dried extract) | 2.57 ± 0.07A | 1.12 ± 0.02B |
Non-similar letters in each row indicate a significant difference according Tukey test (P < 0.05)
Achi et al. (2017) reported that the Ficus capensis leaves are contains considerable amount of Zinc (2.84 mg/100 g), Iron (1.89 mg/100 g), Calcium (1.86%), Magnesium (1.92%) and Potassium (0.72%). According to Yabalak and Gizir (2017) the maximum and minimum mineral concentration in methanolic extract from Allium Kharputense were related to calcium (5419.7 ppm) and barium (0.0017 ppm). It was reported that the artichoke extract is also an important source of calcium (386.9 mg Ca/100 g DM) and magnesium (Biel et al. 2020).
It was revealed that minerals play an important role in health. zinc is vital in protein synthesis, cellular differentiation and replication, immunity and sexual functions, Calcium is essential for blood clotting, bone, and teeth and as a co-factor in some enzyme catalysis, Iron facilitates the oxidation of biomolecules to control obesity, is necessary for hemoglobin, a constituent of certain enzymes and proteins and plays a role in energy transfer within the plant. Potassium is required for proper growth and plant reproduction. Phosphorous maintain blood sugar levels and normal heart contraction, is also important for normal cell growth and repair, bone growth and kidney function and maintaining the body’s acid-alkaline balance (Achi et al. 2017).
Ferula is one of the few plants that contains high sulfur. The presence of high amounts of sulfur compounds in ferula asafeotida is associated with its antimicrobial properties. According to the results, the amount of minerals (except calcium, manganese, and zinc) in leaf extract was significantly higher than gum extract (P < 0.05).
Antioxidant activity
In comparison with synthesized antioxidants, natural alternatives have greater safety and fewer side effects. Hence, herbal compounds featuring high antioxidant activity can help prevent and treat conditions precipitated by oxidative stress, such as cardiovascular diseases and diabetes (Noroozi et al. 2009). Performing research and development on such naturally-derived antioxidants and investigating their efficacy relative to the synthetic agents, novel agents can be identified for use in both the food and pharmaceutical industries.
The antioxidant activity and total flavonoids content and total polyphenol content of hydroalcoholic extracts of leaf and gum extracts presented in Table 2. Antioxidant activities of the extracts were evaluated by DPPH and FRAP assay. The leaf extract showed more antioxidant activity than gum extract that it can be related to a significantly higher amount of phenolic and flavonoids compounds. The content of flavonoid compounds in leaf extract was 16.71 mg QE/g dried extract, while in gum extract it was only 0.11 mg QE/g dried extract. These results are in line with Ahmadvand et al. (2013). They reported that the flavonoid content in Ferula asafoetida leaf hydroalcoholic extract and Ferula asafoetida leaf essential oil were 12.53 mg/100 g and 0.015 mg/100 g, respectively. Martínez et al. (2019) explained that TPC content of hydroxytyrosol extract obtained from olive fruit, pomegranate extract, rosemary extract rich in rosmarinic acid, rosemary extract rich in diterpenes, and hydroxytyrosol extract obtained from olive leaf were 41.44, 40.74, 36.37, 36.49, and 36.34 mg GAE/g, respectively. They also reported that the DPPH scavenging of pomegranate extract, rosemary extract rich in rosmarinic acid, and hydroxytyrosol extract obtained from olive leaf were 92.55%, 81.29%, 77.96%, respectively (Martínez et al. 2019). According to Gedikoğlu et al. (2019) FRAP value of Thymus vulgaris and Thymbra spicata obtained by ethanolic extraction were 26.93 and 14.19 µM Fe+2/g, respectively which was more than Ferula asafoetida leaf and gum extracts. Further, they reported that the total flavonoid content of them were 6.17 and 3.24 mg QUE/g, respectively.
The redox characteristics of certain compounds result in their ability to scavenge free radicals; such ability is important in the neutralization of free radicals, the quenching of singlet and triplet oxygen, and the decomposition of peroxides. It was found that total phenol content significantly correlated with antioxidant ability in a linear pattern (Katalinic et al. 2006). We found that there is significant correlation between TPC content and RSCDPPH of Ferula leaf extract in a linear pattern (R2 = 99.1; P = 0.059) while there was no correlation between them in gum extract.
Identification of the leaf and gum extracts components
The leaf and gum extracts of the F. asafoetida are identified by the GC–MS analysis which presented in Table 3. We identified 29 compounds in leaf extract and 12 compounds in gum extract. The most identified compounds were terpenes. In accordance with data reported in Table 3, it is evident that between leaf and gum extracts, there are significant differences concerning not only the qualitative chemical profile but also the quantitative one. For example, (E)-1-propenyl sec-butyl disulfide wasn’t found in leaf extract while it was one of the abundant compounds found in gum extract (17.62%). In addition, there were no phellandrene compounds in leaf extract as well as carvacrol in gum extract.
Table 3.
Quantitative analysis (mean ± SD) and retention time (RT) of chemical composition of F. asafoetida leaf and gum extracts identified by GC–MS
| Compound | Leaf extract | Gum extract | ||
|---|---|---|---|---|
| RT (min) | Level (%) | RT (min) | Level (%) | |
| α-Pinene | 7.08 | 3.15 ± 1.12 | 7.12 | 6.68 ± 1.54 |
| β-Pinene | 9.10 | 2.03 ± 0.92 | 8.94 | 9.77 ± 2.11 |
| Myrcene | 9.34 | 1.90 ± 0.63 | 9.32 | 1.38 ± 0.17 |
| Decane | 9.42 | 1.49 ± 0.38 | – | ND |
| Benzene | 9.68 | 1.30 ± 0.51 | – | ND |
| α-Phellandrene | – | ND | 10.76 | 7.23 ± 0.97 |
| Limonene | 11.16 | 1.60 ± 0.52 | – | ND |
| β-Phellandrene | – | ND | 11.42 | 6.63 ± 1.40 |
| (Z)-β-ocimene | 11.72 | 1.70 ± 0.66 | 11.58 | 20.91 ± 3.33 |
| (E)-β-ocimene | 11.88 | 1.03 ± 0.34 | 11.74 | 17.27 ± 2.89 |
| Triethylarsine | 13.68 | 8.20 ± 1.78 | – | ND |
| (Z)-1-propenyl sec-butyl disulfide | – | ND | 14.02 | 5.80 ± 1.23 |
| (E)-1-propenyl sec-butyl disulfide | – | ND | 14.70 | 17.62 ± 3.21 |
| Fenchyl acetate | 15.30 | 4.56 ± 1.22 | – | ND |
| Bis (1-methyl thio) propyl disulfide | – | ND | 17.74 | 2.86 ± 0.63 |
| Phenol, 5-methyl-2-(1-methylethyl) | 19.86 | 1.95 ± 0.109 | – | ND |
| Phenol, 2-methyl-5-(1-methylethyl) | 20.04 | 15.40 ± 2.93 | 20.12 | 2.21 ± 0.75 |
| (+)-2-carene | 20.70 | 1.01 ± 0.17 | – | ND |
| Carvacrol acetate | 21.64 | 0.93 ± 0.14 | – | ND |
| β-Elemene | 22.26 | 0.97 ± 0.14 | – | ND |
| (+)-beta-selinene | 23.02 | 1.41 ± 0.57 | – | ND |
| 2,2-Dimethyl-3-methylenorbornane | 23.30 | 1.43 ± 0.39 | – | ND |
| β-Chamigrene | 24.90 | 2.30 ± 0.91 | – | ND |
| 4βH, 5α-eremophila-1(10),11-diene | 25.30 | 2.20 ± .0.85 | – | ND |
| β-Bisabolene | 26.04 | 3.76 ± 1.11 | – | ND |
| y-bisabolene | 26.5 | 5.98 ± 1.32 | – | ND |
| α-bisabolene | 26.92 | 3.18 ± 1.20 | – | ND |
| Elemol | 27.58 | 1.27 ± 0.75 | – | ND |
| β-Dihydroagarofurane | 29.08 | 1.33 ± 0.61 | – | ND |
| Guai-1(5)-en-11-ol | 32.12 | 3.92 ± 1.08 | – | ND |
| β-Calarene | 33.36 | 3.48 ± 0.99 | – | ND |
| 8-Oxo-neoisolongifolene | 37.08 | 8.32 ± 2.01 | – | ND |
| 1β,4βH,10βH-Guaia-5,11-diene; y-Gurjunene; 7- | ||||
| Isopropenyl-1,4-dimethyl-1,2,3,3a,4,5,6,7-0ctahydroazulene | 39.16 | 4.46 ± 1.05 | – | ND |
| α-Bisabolol | 41.9 | 9.75 ± 1.85 | 41.76 | 1.64 ± 0.88 |
ND not detected
As shown in Table 3, GC–MS analysis of the F. asafoetida leaf extract indicated that the main component was phenol, 2-methyl-5-(1-methyl-ethyl), or carvacrol (15.40%), followed by α-bisabolol (9.75%), 8-oxo-neoisolongifolene (8.32%) and triethylarsine (8.20%). Carvacrol is a compound that has been a key focus of research in recent years given its broad-spectrum antimicrobial activity as well as other beneficial properties. The compound features hydrophobic and hydrophilic properties simultaneously due to its substituted aromatic ring and phenolic OH group, respectively; a multitude of studies have reported its activity against oxidative stress, inflammation, bacteria, fungi, protozoa, carcinogens and nociception, as well as its cardioprotective, blood sugar lowering, and neuroprotective characteristics (Memar et al. 2017).
The major components in gum extract were (Z)-b-ocimene (20.91%), (E)-1-propenyl sec-butyl disulfide (17.62%), (E)-b-ocimene (17.27%) and β-pinene (9.77%). Başer et al. (2000) reported that the main constituents of the essential oil of Ferula elaeochytris collected from Turkey were nonane (27.1%), α-pinene (12.7%) and germacrene B (10.3%).
Ocimene has a defensive role for the plant due to its antifungal properties (Bisht et al. 2011). The effect of α-bisabolol has been proven in the treatment of bacterial infections. It is also an excellent antioxidant with anticoagulants and analgesic properties (Forrer et al. 2017).
The results of Kavoosi and Rowshan (2013) indicated that the main components for essential oil obtained from Ferula asafoetida oleo-gum-resins were; (E)-1-propenyl sec-butyl disulfide, 10-epi-c-eudesmol, (Z)-1-propenyl sec-butyl disulfide, b-pinene, and a-pinene.
Phenols are recognized as natural products with a multitude of biological properties including activity against bacteria, fungi, viruses, inflammation, allergens, thrombosis, and carcinogenesis, as well as hepatoprotective and vasodilatory action. Hence, phenolic compounds have immense potential for therapeutic use in a wide range of conditions such as neurodegenerative, cardiovascular, and inflammatory diseases, as well as cancer, diabetes, and aging (Soobrattee et al. 2005).
The phenolic profile of F. asafoetida leaf and gum extracts were characterized by HPLC analysis. We identified 7 phenolic compounds in both leaf and gum extracts including ferulic acid, vanilic acid, coumaric acid, umbelliprenin, galbanic acid, karatavicinol, and kamolonol. The amount (expressed as µg/mg extract) of each identified phenolic compound has been reported in Table 4. In particular, the content of these compounds appeared to be very higher in leaf extract than in gum extract. In accordance with data reported in Table 4, ferulic acid was the most abundant compound in both leaf and gum extracts followed by Umbelliprenin. The concentration of ferulic acid, vanilic acid, coumaric acid, Umbelliprenin, Galbanic acid, Karatavicinol, and Kamolonol in leaf extract were 1.24, 2.04, 1.07, 3.07, 3.66, 1.59 and 1.92 times greater than that of gum extract, respectively.
Table 4.
Quantitative analysis of phenolics (mean ± SD) in F. asafoetida leaf and gum extracts determined by HPLC
| Compound | Leaf extract (µg/mg) | Gum extract (µg/mg) |
|---|---|---|
| Ferulic acid | 4.62 ± 0.53 | 3.72 ± 1.09 |
| Vanilic acid | 0.99 ± 0.12 | 0.49 ± 0.13 |
| Coumaric acid | 0.31 ± 0.09 | 0.29 ± 0.07 |
| Umbelliprenin | 3.14 ± 1.14 | 1.02 ± 0.10 |
| Galbanic acid | 1.46 ± 0.39 | 0.40 ± 0.06 |
| Karatavicinol | 0.59 ± 0.11 | 0.37 ± 0.08 |
| Kamolonol | 0.93 ± 0.17 | 0.48 ± 0.11 |
A large number of species in the Ferula genus are able to carry out the synthesis of sesquiterpene coumarins, with umbelliprenin being the first to be synthesized. This coumarin compound highly resembles auraptene (found in Citrus species) in structure, distinguished solely due to having 15 carbons instead of 10 in its 7-prenyloxy chain. Studies conducted recently have revealed the promising activity of umbelliprenin in the inhibition of inflammation, carcinogenesis, genotoxicity, lipoxygenase, and acetylcholinesterase, also demonstrating its cytotoxic features (Shakeri et al. 2014).
Membrane characteristics including charge, permeability, and physiochemical properties were irreversibly modified by ferulic acid due to changes in hydrophobicity, generation of localized ruptures or pores, as well as a reduction in negative surface charge in the cellular membranes; essential intracellular contents consequently leaked out to the surrounding environment (Borges et al. 2013).
In terms of the underlying mechanism, ferulic acid’s antioxidative activity is attributed to the reaction of the antioxidant molecule with a radical to form a stable phenoxyl radical, which impedes the initiation of complex reaction cascades and consequent free radical formation. Another possible antioxidative mechanism is the direct donation of hydrogen to the radicals, which is crucial for protecting the lipid acids of cell membranes from autoxidation. Furthermore, secondary antioxidative activity results from the binding of iron, copper and other transition metals by ferulic acids, which prevents peroxidation of the cell membrane due to hydroxyl radical formation (Zduńska et al. 2018).
Thermal properties
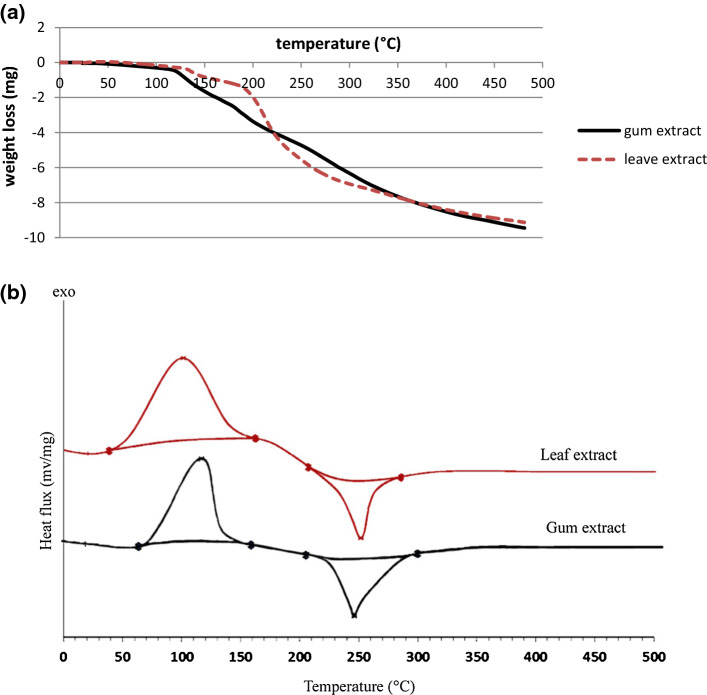
TGA thermograms of F. asafoetida leaf and gum extracts are given in Fig. 1. Thermal treatment caused the weight of the leaf extract to decrease in two main steps while gum extract weight decreased in one step. The leaf and gum extracts degradation process started at upper than 100 °C and 130 °C respectively, but the rate of weight loss showed a sharper peak in gum extract. The first one resulted from water desorption and volatile compounds. The second degradation in leaf extract occurred upper 160 °C due to the degradation of leaf structure. Moreover, in this respect, the rate of weight loss showed a higher weight reduction in gum extract. The main thermal degradation resulting from calcination of the sample. Up to temperature 160 °C, 7% of the leaf extract was degraded, while the amount of gum weight reduction was 14.2% which indicates greater thermal stability of leaf extract. Our data were in accordance with the findings of Saeidy et al. (2018) about oleo-gum-resin from the root of Ferula asafoetida that defined the temperatures upper than 200 °C as the main regions of weight decreasing phenomenon due to degradation of gum structure. According to TGA results of Trivedi et al. (2017), the ashwagandha root extract degraded in the three steps at temperatures of 185 °C, 485 °C and 895.88 °C, which weight losses in these temperatures were 9.04%, 60.94% and 9.79%, respectively which indicate more thermal stability than Ferula leaf and gum extract.
Fig. 1.
Thermal properties of F. asafoetida leaf and gum extracts; a TGA thermograms, b DSC thermogram
The results of the TGA analysis were confirmed by the obtained DSC thermogram, which is presented in Fig. 1. Heat flow in the leaf and gum extracts increased slightly when the temperature was increased to around 100 °C, and when the temperature reached approximately 132 °C and 117 °C, respectively, the first degradation and weight loss started. At the mentioned temperature levels, water desorption is responsible for the endothermic peak. The second stage of leaf extract degradation began at about 164 °C that accompanied by 7% weight loss while the weight loss of gum extract at this temperature was about 14.2%. The results also showed that the weight losses of leaf and gum extracts at 200 °C were 13.95% and 23.5%, respectively, indicating higher thermal stability of leaf extract. Dehydration, depolymerization, and pyrolytic decomposition can be responsible for the exothermic peaks. Saeidy et al. (2018) reported that around 90.4% weight reduction of F. asafoetida gum occurred at 200 °C. This contrast with our results can be attributed to the difference in the chemical composition of the F. asafoetida gum and its hydro-alcoholic extract as well as the cultivation and geographical region.
Extraction conditions as well as solvent type affect the type of plant-separated components and thermal stability of the final extract. Liao et al. (2017) stated that the onset temperature and conclusion temperature of mulberry leaf polysaccharides extracted with hot buffer, chelating agent, dilute alkali and concentrated alkali ranged from 108.58 to 197.22 °C, 79.03 to 172.55 °C, 154.51 to 173.24 °C and 99.51 to 203.66 °C, respectively.
According to our results, Ferula leaf and gum extracts have good thermal stability, and if the usual thermal processes (temperatures below 100 °C) are employed during processing, we can be sure that their bioactive compounds will be largely preserved. However, our results also showed that these compounds are resistant to temperatures above 100 °C so that they showed low weight loss at temperatures below 150 °C. It is worth noting that especially at temperatures above 100 °C, which the rate of degradation increases, other factors such as the type of compounds in the formulation, heating time, pH, heating method, etc. affect the thermal stability of extracts.
Antimicrobial activity
The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of F. asafoetida leaf and gum extracts are shown in Table 5. Regarding the MIC values, A. niger was the most sensitive microorganism, providing the lowest microorganism growth in the presence of gum extract. The antimicrobial activity of gum extract against S. aureus and S. cerevisiae was the same. Leaf extract possessed much stronger anti-E. coli and anti-S. aureus activity than gum extract, since MIC of leaf extract was 6.4 times higher than the MIC of gum extract. Both extracts had no bactericidal effect on E. coli. There was not significantly difference between MBC values against S. aureus and S. cerevisiae in the presence of gum extract. However, leaf extract provided the same MIC and MBC values against A.niger and S. cerevisiae. Cristofoli et al. (2019) reported that MIC values of Spondias mombin extracts against different microorganism depend on the method of extraction. They showed that the MIC value of extracts obtained by supercritical fluid extraction (CO2 + 2.5% ethanol + H2O) and ultrasound-assisted extraction (CO2 + 2.5% ethanol) was more than 1000 μg/ml against S. aureus and E. coli while they show no inhibition effect on S. cerevisiae. Nafis et al. (2019) stated that the MIC values of Moroccan Cannabis sativa essential oil against S. aureus and E. coli, were 4.7 and 1.2 mg/ml, respectively which was greater that our findings for Ferula asafoetida.
Table 5.
The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of F. asafoetida leaf and gum extracts
| Microorganism | Leaf extract (mg/l) | Gum extract (mg/l) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| E. coli | 62.5 ± 3 | – | 400 ± 12 | – |
| S. aureus | 62.5 ± 2 | 250 ± 6 | 300 ± 9 | 400 ± 10 |
| A. niger | 125 ± 5 | 250 ± 7 | 50 ± 2 | 100 ± 4 |
| S. cerevisiae | 125 ± 6 | 250 ± 9 | 300 ± 8 | 400 ± 8 |
Mean ± SD
Antimicrobial substances carry out different types of activity against microorganisms. Molecules with hydrophobic characteristics exert damage to both the structure and function of the cell membrane by aggregating in its lipid-rich environment, with toxicity being related to an optimum level of hydrophobicity (Becerril et al. 2007). Aqueous solubility has been proposed as a limiting factor to lethal-level hydrophobic compound aggregation (Goñi et al. 2009). Gram-negative bacteria (E. coli) have a thick layer of lipopolysaccharide outer membrane covering the cell wall. This structure have shown to be more resistant to hydrophobic substance compared with the gram-positive S. aureus (Zhang et al. 2016).
The antimicrobial activity of F. asafoetida can be related to the content of phenolic and flavonoid compounds such as carvacrol, (E)-1-propenyl sec-butyl disulfide, ferulic acid, Umbelliprenin and etc. The greater antimicrobial activity of leaf extract can be attributed to the higher content of these compounds than the gum extract.
Umbelliprenin reportedly has antimicrobial activity against Bacillus subtillis, Bacillus cereus, E. coli, Klebsiella pneumoniae, Salmonella typhi, S. aureus, and Staphylococcus epidermidis (Shakeri et al. 2014). Safdari et al. (2014) isolated auraptene, umbelliprenin, and galbanic acid from Ferula szowitsiana, and assessed these compounds for their ability in class A β-lactamase inhibition; the researchers found that these compounds potentiated the activity of penicillin against certain strains of penicillin-resistant S. aureus that had been isolated clinically. As enzymes linked to antibiotic resistance (particularly in S. aureus), class A β-lactamases degrade the β-lactam ring, which is a key structural feature in a variety of antibiotic compounds. Shrivastava et al. (2012) reported that the ethanolic extract of Asafoetida crude sample showed activity against S. aureus whereas there was no antibacterial activity against Bacillus subtilis and A. niger. It was also reported that Ferula asafoetida exhibit the antifungal and antiviral effects due to possessing of (E)-1-propenyl sec-butyl disulfide and galbanic acid (Dissanayake and Perera 2020).
Overall, the present study reinforces the immense potential of natural plant constituents as a novel, renewable, and environmentally-friendly sources of wide-spectrum antibiotics.
Conclusion
Through our research, specific traits of the hydro-alcoholic extracts of F. asafoetida leaf and gum were clarified. It was found that the leaf extract had excellent radical-scavenging activity, reducing power, and antioxidant activity, meaning that it can act as a potent inhibitor of lipid peroxidation. It is also worth noting that various bioactive compounds were identified for the first time as being constituents of the F. asafoetida leaf. Moreover, it was clearly demonstrated that the leaf extract had better antioxidant activity and thermal characteristics relative to the gum extract. It was revealed that leaf extract possesses stronger antimicrobial properties against E. coli, S. aureus, and S. cerevisiae than gum extract. TGA and DSC results leaf and gum extracts are stable up to 160 °C which is an advantage in the food thermal processing.
There is much evidence that reveals natural plants have been used for therapeutic and medicinal purposes due to their good pharmacological and physiological properties. The F. asafoetida is significantly active and can be successfully employed in the food, cosmetic and pharmaceutical industries.
Acknowledgements
The authors acknowledge the Iran National Science Foundation (INSF) for financial support of this work (Grant No. 9411113).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Razieh Niazmand, Email: r.niazmand@rifst.ac.ir.
Bibi Marzieh Razavizadeh, Email: m.razavizadeh@rifst.ac.ir.
References
- Achi NK, Onyeabo C, Ekeleme-Egedigwe CA, Onyeanula JC. Phytochemical, proximate analysis, vitamin and mineral composition of aqueous extract of Ficus capensis leaves in South Eastern Nigeria. J Appl Pharm Sci. 2017;7(03):117–122. [Google Scholar]
- Adams RP. Identification of essential oil components by gas chromatography/mass spectrometery. 4. Illinois: Allured Publishing Corporation; 2007. p. 456. [Google Scholar]
- Adeeyo AO, Odelade K, Msagati TA, Odiyo JO. Antimicrobial potencies of selected native African herbs against water microbes. J King Saud Univ Sci. 2020 doi: 10.1016/j.jksus.2020.03.013. [DOI] [Google Scholar]
- Ahmadian-Kouchaksaraie Z, Niazmand R. Supercritical carbon dioxide extraction of antioxidants from Crocus sativus petals of saffron industry residues: optimization using response surface methodology. J Supercrit Fluids. 2017;121:19–31. [Google Scholar]
- Ahmadian-Kouchaksaraie Z, Niazmand R, Najaf NM. Optimization of the subcritical water extraction of phenolic antioxidants from Crocus sativus petals of saffron industry residues: Box–Behnken design and principal component analysis. Innov Food Sci Emerg Technol. 2016;36:234–244. [Google Scholar]
- Ahmadvand H, Amiri H, Dehghani Elmi Z, Bagheri SH. Chemical composition and antioxidant properties of Ferula asafoetida leave essential oil. Iran J Pharm Ther. 2013;12:52–57. [Google Scholar]
- Amalraj A, Gopi S. Biological activities and medicinal properties of Asafoetida: a review. J Tradit Complement Med. 2017;7:347–359. doi: 10.1016/j.jtcme.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC (2006) Official methods of analysis. Association of Official Analytical Chemists, Washington, DC.
- Başer KHC, Özek T, Demirci B, Kürkçüoğlu M, Aytaç Z, Duman H. Composition of the essential oils of Zosima absinthifolia (Vent.) Link and Ferula elaeochytris Korovin from Turkey. Flavour Fragr J. 2000;15:371–372. [Google Scholar]
- Becerril R, Gomez-Lus R, Goni P, Lopez P, Nerin C. Combination of analytical and microbiological techniques to study the antimicrobial activity of a new active food packaging containing cinnamon or oregano against E. coli and S. aureus. Anal Bioanal Chem. 2007;388(5–6):1003–1011. doi: 10.1007/s00216-007-1332-x. [DOI] [PubMed] [Google Scholar]
- Becerril R, Manso S, Nerin C, Gómez-Lus R. Antimicrobial activity of lauroyl arginate ethyl (LAE), against selected food-borne bacteria. Food Control. 2013;32:404–408. [Google Scholar]
- Biel W, Witkowicz R, Piątkowska E, Podsiadło C. Proximate composition, minerals and antioxidant activity of artichoke leaf extracts. Biol Trace Elem Res. 2020;194(2):589–595. doi: 10.1007/s12011-019-01806-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht D, Pal A, Chanotiya CS, Mishra D, Pandey KN. Terpenoid composition and antifungal activity of three commercially important essential oils against Aspergillus flavus and Aspergillus niger. Nat Prod Res. 2011;25(20):1993–1998. doi: 10.1080/14786419.2010.521926. [DOI] [PubMed] [Google Scholar]
- Bordoh PK, Ali A, Dickinson M, Siddiqui Y. Antimicrobial effect of rhizome and medicinal herb extract in controlling postharvest anthracnose of dragon fruit and their possible phytotoxicity. Sci Hortic. 2020;265:109249. [Google Scholar]
- Borges A, Ferreira C, Saavedra MJ, Simoes M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Res. 2013;19(4):256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhao Y, Zhang M, Yang X, Yue P, Tang D, Wei X. Structural characterization and antioxidant activity of a new polysaccharide from Bletilla striata fibrous roots. Carbohydr Polym. 2020;227:115362. doi: 10.1016/j.carbpol.2019.115362. [DOI] [PubMed] [Google Scholar]
- Clemente I, Aznar M, Silva F, Nerín C. Antimicrobial properties and mode of action of mustard and cinnamon essential oils and their combination against foodborne bacteria. Innov Food Sci Emerg Technol. 2016;36:26–33. [Google Scholar]
- Cristofoli NL, Lima CAR, Vieira MMC, Andrade KS, Ferreira SR. Antioxidant and antimicrobial potential of cajazeira leaves (Spondias mombin) extracts. Sep Sci Technol. 2019;54(4):580–590. [Google Scholar]
- de Veras BO, de Oliveira MBM, da Silva Oliveira FG, dos Santos YQ, de Oliveira JRS, de Menezes Lima VL, da Silva Almeida JRG, Navarro DMDAF, de Oliveira Farias JCR, dos Santos Aguiar J, Gorlach-Lira K. Chemical composition and evaluation of the antinociceptive, antioxidant and antimicrobial effects of essential oil from Hymenaea cangaceira (Pinto, Mansano & Azevedo) native to Brazil: a natural medicine. J Ethnopharmacol. 2020;247:112265. doi: 10.1016/j.jep.2019.112265. [DOI] [PubMed] [Google Scholar]
- Dehpour AA, Ebrahimzadeh MA, Fazel NS, Mohammad NS. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas Aceites. 2009;60:405–412. [Google Scholar]
- Dissanayake KGC, Perera WPRT. Medicinal importance of Ferula asafetida oligo-gum resins against infective diseases. J Med Plants. 2020;8(2):135–139. [Google Scholar]
- Fierascu RC, Georgiev MI, Fierascu I, Ungureanu C, Avramescu SM, Ortan A, Georgescu MI, Sutan AN, Zanfirescu A, Dinu-Pirvu CE, Velescu BS. Mitodepressive, antioxidant, antifungal and anti-inflammatory effects of wild-growing Romanian native Arctium lappa L. (Asteraceae) and Veronica persica Poiret (Plantaginaceae) Food Chem Toxicol. 2018;111:44–52. doi: 10.1016/j.fct.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Forrer M, Kulik EM, Filippi A, Waltimo T. The antimicrobial activity of alpha-bisabolol and tea tree oil against Solobacterium moorei, a Gram-positive bacterium associated with halitosis. Arch Oral Biol. 2017;58(1):10–16. doi: 10.1016/j.archoralbio.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Gedikoğlu A, Sökmen M, Çivit A. Evaluation of Thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci Nutr. 2019;7(5):1704–1714. doi: 10.1002/fsn3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goñi P, López P, Sánchez C, Gómez-Lus R, Becerril R, Nerín C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009;116:982–989. [Google Scholar]
- Iranshahy M, Iranshahi M. Traditional uses, phytochemistry and pharmacology of asafoetida (Ferula assa-foetida oleo-gum-resin), a review. J Ethnopharmacol. 2011;134(1):1–10. doi: 10.1016/j.jep.2010.11.067. [DOI] [PubMed] [Google Scholar]
- Jalali Mousavi SR, Niazmand R, Shahidi Noghabi M. Antioxidant activity of purslane (Portulaca oleracea L.) seed hydro-alcoholic extract on the stability of soybean oil. J Agric Sci Technol. 2015;17:1473–1480. [Google Scholar]
- Katalinic V, Milos M, Kulisic T, Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–557. [Google Scholar]
- Kavoosi GH, Rowshan V. Chemical composition, antioxidant and antimicrobial activities of essential oil obtained from Ferula assafoetida oleo-gum-resin: effect of collection time. Food Chem. 2013;138:2180–2187. doi: 10.1016/j.foodchem.2012.11.131. [DOI] [PubMed] [Google Scholar]
- Kose EO, Aktaş O, Deniz G, Sarikürkçü C. Chemical composition, antimicrobial and antioxidant activity of essential oil of endemic Ferula lycia Boiss. J Med Plants Res. 2010;4:1698–1703. [Google Scholar]
- Labed-Zouad I, Labed A, Laggoune S, Zahia S, Kabouche A, Kabouche Z. Chemical compositions and antibacterial activity of four essential oils from Ferula vesceritensis Coss. & Dur. Against clinical isolated and food-borne pathogens. Rec Nat Prod. 2015;9(4):518–525. [Google Scholar]
- Liao BY, Zhu DY, Thakur K, Li L, Zhang JG, Wei ZJ. Thermal and antioxidant properties of polysaccharides sequentially extracted from mulberry leaves (Morus alba L.) Molecules. 2017;22(12):2271. doi: 10.3390/molecules22122271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mala KN, Thomas J, Syam DS, Maliakel B, Krishnakumar IM. Safety and efficacy of Ferula asafoetida in functional dyspepsia: a randomized, double-blinded, placebo-controlled study. Evid Based Complement Altern Med. 2018;2018:4813601. doi: 10.1155/2018/4813601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova D, Ribarova F, Atanasova M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metall. 2005;40:255–260. [Google Scholar]
- Martínez L, Castillo J, Ros G, Nieto G. Antioxidant and antimicrobial activity of rosemary, pomegranate and olive extracts in fish patties. Antioxidants. 2019;8(4):86. doi: 10.3390/antiox8040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLafferty FW. Wiley registry of mass spectral data. 9. Hoboken: Wiley; 2009. p. 662. [Google Scholar]
- Memar MY, Raei P, Alizadeh N, Akbari AM, Kafil HS. Carvacrol and thymol: strong antimicrobial agents against resistant isolates. Rev Med Microbiol. 2017;28(2):63–68. [Google Scholar]
- Nafis A, Kasrati A, Jamali CA, Mezrioui N, Setzer W, Abbad A, Hassani L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind Crops Prod. 2019;137:396–400. [Google Scholar]
- Noroozi S, Mosaffa F, Soltani F, Iranshahi M, Karimi G, Malekaneh M, Haghighi F, Behravan J. Antigenotoxic effects of the disulfide compound persicasulfide A (PSA) on rat lymphocytes exposed to oxidative stress. Planta Med. 2009;75:32–36. doi: 10.1055/s-0028-1088360. [DOI] [PubMed] [Google Scholar]
- Prabaharan C, Thirumavalavan M, Pachaiappan R. Production of antioxidant peptides from Ferula Asafoetida root protein. Int J Mol Biol. 2016;1(1):00003. [Google Scholar]
- Saeidy S, Nasirpour A, Keramat J, Desbrières J, Cerf DL, Pierre G, Delattre C, Laroche C, Baynast H, Ursu AV, Marcati A, Djelveh G, Michaud P. Structural characterization and thermal behavior of a gum extracted from Ferula assafoetida L. Carbohydr Polym. 2018;1(181):426–432. doi: 10.1016/j.carbpol.2017.10.096. [DOI] [PubMed] [Google Scholar]
- Safdari H, Neshani A, Sadeghian A, Ebrahimi M, Iranshahi M, Sadeghian H. Potent and selective inhibitors of class A β-lactamase: 7-prenyloxy coumarins. J Antibiot. 2014;67:373–377. doi: 10.1038/ja.2014.9. [DOI] [PubMed] [Google Scholar]
- Shakeri A, Iranshahy M, Iranshahi M. Biological properties and moleculartargets of umbelliprenin—a minireview. J Asian Nat Prod Res. 2014;16(8):884–889. doi: 10.1080/10286020.2014.917630. [DOI] [PubMed] [Google Scholar]
- Shrivastava V, Bhardwaj U, Sharma V, Mahajan N, Sharma V, Shrivastava G. Antimicrobial activities of Asafoetida resin extracts (A Potential Indian Spice) J Pharm Res. 2012;5(10):5022–5024. [Google Scholar]
- Soleimanifar M, Niazmand R, Jafari SM. Evaluation of oxidative stability, fatty acid profile, and antioxidant properties of black cumin seed oil and extract. J Food Meas Charact. 2019;13:383–389. [Google Scholar]
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Tavassoli M, Jalilzadeh-Amin G, Fard VRB, Esfandiarpour R. The in vitro effect of Ferula asafoetida and Allium sativum extracts on Strongylus spp. Ann Parasitol. 2018;64(1):59–63. doi: 10.17420/ap6401.133. [DOI] [PubMed] [Google Scholar]
- Trivedi MK, Branton A, Trivedi D, Nayak G, Afaganis AE, Bader BM, Weekes BA, Dumas DL, Fiedler DM, Smith DM, Pano D. Evaluation of the physico-chemical, thermal and behavioral properties of ashwagandha root extract: effects of consciousness energy healing treatment. Int J Pharm Chem. 2017;3(3):41–51. [Google Scholar]
- Ur Rahman M, Gul SH, Ali OE. Antimicrobial activities of ferula assafoetida oil against gram positive and gram negative bacteria. Am Eur J Agric Environ Sci. 2008;4(2):203–206. [Google Scholar]
- Vijayasteltar L, Jismy IJ, Joseph A, Maliakel B, Kuttana R, Krishnakumar IM. Beyond the flavor: a green formulation of Ferula asafoetida oleo-gum-resin with fenugreek dietary fibre and its gut health potential. Toxicol Rep. 2017;4:382–390. doi: 10.1016/j.toxrep.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Fu K, Fu YJ, Zu YG, Chang FR, Chen YH, Liu XL, Kong Y, Liu W, Gu CB. Antioxidant activities of extracts and main components of Pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Molecules. 2009;14:1032–1043. doi: 10.3390/molecules14031032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Codling EE, Luo Y, Nou X, Lester GE, Wang Q. Microgreens of Brassicaceae: mineral composition and content of 30 varieties. J Food Compos Anal. 2016;49:87–93. [Google Scholar]
- Yabalak E, Gizir AM. Evaluation of total polyphenol content, antioxidant activity and chemical composition of methanolic extract from Allium Kharputense Freyn et. Sint. and determination of mineral and trace elements. J Turk Chem Soci Sect A Chem. 2017;4(3):691–708. [Google Scholar]
- Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol. 2018;31:332–336. doi: 10.1159/000491755. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu X, Wang Y, Jiang P, Quek S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control. 2016;59:282–289. [Google Scholar]