Abstact
The objective of the present study is developing a new technique for the preservation of natural cheese by the use of an edible biofilm based on sodium alginate in order to evaluate the effect of the essential oils (O. basilicum L, R. officinalis L. A. herba alba Asso. M. pulegium L.) incorporated in the film on the oxidation stability, microbial spoilage, physicochemical characteristics and sensory criteria. The cheese samples coated with sodium alginate incorporated by the oils showed moderate stability in terms of oxidative stabilities of proteins and lipids during storage. In addition, poor microbial growth (total aerobic mesophilic flora, yeasts and fecal coliforms) was observed in cheese samples coated with biofilm, also, the growth of Staphylococci Salmonella and Molds for all types of cheese were completely inhibited. Additionally, it was observed that the biofilm coating reduced the weight loss and hardness of the cheese comparing with the uncoated sample. The results of sensory analysis revealed that uncoated cheese, coated with sodium alginate and sodium alginate composed of oil of O. basilicum were the most preferred by panelists, in comparison with others. Therefore, it was concluded that this technique of coating cheese with edible film activated with essential oils is preferred and favorable by virtue of the effect of oils preserving the cheese without seriously affecting their organoleptic properties.
Keywords: Home-made cheese, Preservation, Active film, Sodium alginate, Essential oils
Introduction
Cheese making is one of the oldest food processing technologies used to concentrate and store nutrients for consumption and extending shelf life. Nowadays, cheese is the most common dairy product in the world in terms of quantity and diversity. Consumption of dairy product also it is indispensable for human nutrition due to presence of essential nutrients with high values required for human body: the casein of milk (main component of the coagulum), lipids and unsaturated fatty acids, lactose and its derivatives (lactic acid), and a significant amount of mineral salts. Cheese can be classified according to its texture, style and flavor that are highly dependent to the origin of the milk. A fresh cheese is considered extremely perishable and highly susceptible to spoilage microbes due to its high moisture and fat content and is also very prone to oxidation (Gerschenson et al. 2018; Spehar et al. 2018). Recently, advances in dairy engineering and technology have made it possible to move from traditional cheese making operations to a modern industrial process in which research into increasing shelf life and promoting quality and the safety of cheese products. One of the main losses during the marketing of cheese occurs during storage where the cheese is generally contaminated with bacteria, molds and yeasts, so that the development of an unpleasant odor can occur, which leads to reduce the quality of the cheese, especially when it is stored without packaging. The oxidation phenomenon is particularly feared at the lipid level because oxidative degradations lead to decrease the product quality, and to the appearance of toxic substances. In addition, the high moisture loss of certain cheese types can be a problem due to the increase in hardness and the formation of undesirable organoleptic properties (Kampf 2000; Mihai and Popa 2013; Dai et al. 2013; Costa et al. 2018; Jeewanthi and Paik 2018). Taking into account natural preservatives in the food industry is essential to fight against the phenomena of oxidation and microbial resistance. For this purpose, the investigation of plants represents an invaluable potential for the discovery of new substances of antimicrobial and antioxidant character (Kirtiraj et al. 2017; Mahcene et al. 2020). Also, these medicinal and aromatic plants produce secondary metabolites (EO) as a means of defense against microorganisms, since they are considered as a source of food alteration. For this interest, essential oils are one of the most important examples of these natural preservatives which considered as natural bioactive substances occupy a good choice in the discovery of new preservateur and attract the interest of several researches. The essential oil has a strong antimicrobial and antioxidant activities which can be used as potential preservatives for perishable foods (G. cansu and fatih 2016). Although most EO are classified as generally recognized as food flavorings sure (IM Brasil et al. 2012; Costa et al. 2017; Fatih and Enes 2015; Cansu Feyzioglu and Tornuk 2016; Tornuk et al. 2011). As well, the development of new preservation techniques linked to materials with film-forming capacity and antimicrobial properties that help to improve food safety and to extend shelf life which is regarded a new trend in coatings for food technology (Alvarez et al. 2013; Di Maio et al. 2014; Mahcene et al. 2020). In a previous study, Kurt et al. (2017) showed that these materials have a capacity to transport bioactive components to produce an active antioxidant packaging. On the other hand, Perez et al (2011) stated that one of the edible films benefits is the reduction of microbial growth in food. It can extend the shelf life of products and make them safe for consumers. In addition, Mei et al. (2013), show that certain edible coatings have the potential to improve the appearance of food. According to, Kurt et al. (2017) one of these biopolymers, sodium alginate, is one of the most versatile biodegradable polymers which is a linear polysaccharide that is extracted from brown algae. The incorporation of bioactive substances such as essential oils that are antimicrobial or antioxidants agents in packaging materials occurred due to their availability, their effects, their lower toxicities and their better biodegradability in comparison with current preservatives. They are one of the most promising methods in barrier technologies that is used to improve food safety and quality by enhancing the antimicrobial and antioxidant activity of coatings (IM Brasil et al. 2012; Alvarez et al. 2013; Cristina Costa et al. 2017; Alexa et al. 2018; Kirtiraj 2017). Therefore, it has been recognized that the application of films for cheese coating is an important treatment to enhance the quality protection and the safety during processing and marketing. The product appearance is one of the critical factors which affect consumers perceptions and preferences that is highly associated with cheese marketability. In this regard, the choice of packaging and coating of cheese plays an important role in consumer preference for the product (Kampf and Nussinovitch 2000; Youssef et al. 2017). Production of active packaging films by incorporation of EO such as: Citral, Origanum vulgare, cinnamon, thyme and oregano, Zataria multiflora Boiss, Lemongrass and Ginger EO into the polymer matrix have also been well explained in the literature (Bermudez-Aguirre and Barbosa-Canovas 2010; Seyed Mahdi Ojagh et al. 2010; Monir-Sadat Shakeri et al. 2011; Miksusanti et al. 2013; Supardan et al. 2016; Gurdian et al. 2017; Valentina Siracusa et al. 2018). To the best of our knowledge, there is no literature related to incorporation of R. officinalis L, A. herba alba Asso, O. basilicum L and M. pulegium L EO from Algeria into the biodegradable packaging films and their application for cheese preservation (Mahcene et al. 2020). Therefore, in this study, it was aimed to develop a new technique for preserving natural cheese by applying an active edible film (sodium alginate) incorporated by some oils as bio-preservative and packaging.
Materials and methods
Essential oil analysis by gas chromatography–mass spectrometry (GC/MS)
The plant material that is used in this study is represented by the aerial part of M. pulegium L, A. herba alba Asso, O. basilicum L and leaves and flowers of R. officinalis L dried for 1 week, protected from light and, at room temperature. Extraction of the EO was carried out by hydrodistillation in a Clevenger apparatus where 100 g of plant material was immersed in a 1000 ml water flask for 3 h. Oils and water are separated by difference in density.
EO were analyzed using an Agilent 7890A gas chromatograph system (Agilent, Avondale, USA) coupled with a mass selective detector (Agilent Technologies, Agilent, Avondale, USA) with electron impact ionization (70 eV). Separation of oil constituents was performed on RXI-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm) in the split mode (1:50) at 250 °C. The oven temperature was programmed at 45 °C for 1 min, raised to 300 °C at 2 °C min−1 and finally held at this temperature for 10 min. Helium was used as carrier gas at a flow of 1 mL min−1. Also, linear retention indices (RI) for all compounds were determined using n-alkanes as standards. Qualitative analysis was based on the comparison of retention times and the computer mass spectra libraries using Wiley 7, Flavor 2 and HPCH 1607 GC/MS libraries. the percentage composition of the essential oils was obtained by dividing the area of each peak by the total area under all of the peaks.
Preparation of cheese and film solutions
Home-made cheese was made from cow’s milk in four stages: The curdling “coagulation”: This is the first stage of cheese making. For this, the enzyme “rennet” was added to the milk (37 °C), after which the whole was rested until the milk was completely coagulated (30 min). the second stage is drainage: during this phase, nearly 80% of the water contained in the curd is extracted by cutting, brewing and pressing. Salting: the cheese produced is salted by dipping in a brine bath 1% (w/v). Finally, molding: the cheese produced is put in mold.
The biodegradable and edible film that is used in this study was a biopolymer specifically made from a solution of sodium alginate (Sigma-Aldrich, Germany) (SAS). The later was prepared by mixing 3% (w/v) sodium alginate and 2.5% (w/v) glycerol in distilled water. This mixture was heated to 80 °C with continuous stirring for 2 h. On the other hand, the essential oil emulsion (EOE) was prepared in Tween 80 (1: 0.5 v: v) respectively. finally, the edible film used was obtained by mixing the both solutions SAS and EOE (10/0.1 v: v); the SAS without EOE was used as a control film.
Coating application and storage
Cheese samples were immersed in the film-forming solutions for 1 min at room temperature. After edible coating application, the samples were dried at 30 °C and 60% relative humidity for 60 min in a controlled drying chamber (Pharma SCT, Argentina) to allow forming up a thin film layer on their surfaces. Control sample was immersed in distilled water and in sodium alginate solution without EO and was subjected to the same drying conditions specified above. After treatments, cheese samples (100 g) were stored in a refrigerated chamber at 5–7 °C for 10 days. Cheese samples were sampled immediately (day 0) and after 24 h, 5 and 10 d of the storage.
Microbiological analysis
For each sample, 10 g of the sample was added to a flask containing 90 ml of peptone water, thereafter the whole was well homogenized, so that the stock solution corresponding to the 10−1 dilution was obtained and decimal serial dilutions of 10−1–10−4 were prepared. Faecal coliforms counts were determined based on the method described by the NF V 08060 (2009). Violet Red Bile Lactose Agar (VRB-Agar) was inoculated with 1 ml of serial dilutions and incubated at 44 °C for 24 h. A method described by ISO 4833 (2003) was used to enumerate total mesophilic aerobic bacteria (TMAB), in Plate Count Agar (PCA) agar, after incubation at 30 °C for 72 h. In the case of enumeration of total yeasts and molds, 0.1 ml of fist dilution was inoculated by spreading on Dichloran Rose Bengal Chloramphenicol Agar (DRBC-Agar) and the plates were left for incubation at 25 °C for 5 days (ISO 21527-1 2008). In the enumeration of suspected pathogenic staphylococci, Baird Parker agar supplemented with egg yolk and potassium tellurite was used as culture medium the method described by standard ISO 6888-1 (2003) was used for enumeration. Presence of Salmonella was analyzed in three stages based on ISO 6579-1 (2017) method: A 25 g of the sample was taken and incorporated with 225 ml of peptone water. 1 ml of the culture obtained in the peptone water was transferred to a tube containing 10 ml of rappaport vassiliadis soya broth and presence of bacteria was performed on Salmonella-Schigella agar. All analyzes were carried out in three replicates.
pH, weight loss and hardness of cheese samples
For pH determination, 10 g of cheese was homogenized in 100 ml of distilled water and then filtered. In this stage, the pH of the samples was evaluated using a pH meter. All analyzes were carried out in three replicates.
To evaluate weight loss during the storage, separate samples in 3 replicates of each treatments were analyzed. The same samples were evaluated for weight loss each time at weekly intervals until the end of experiment. In this sense, weight loss was determined by the following formula (Eq. 1.):
| 1 |
where A indicates the initial cheese weight and B indicates the cheese weight after storage intervals (AOAC 1994).
Cheese hardness was determined in a Texture Analyzer, model TA. XT Plus Stable Micro Systems, Surrey, UK used to perform textural analysis, after data treatment by the Specific Expression PC Software. Cheese texture profile analysis (TPA) was performed with a penetration distance of 15 mm at 2 mm/s test speed, using an acrylic cylindrical probe with a diameter of 5 mm and 38.1 mm of height. Three penetrations were performed per cheese at distinct locations (Marta Henriques et al. 2014).
Optical proprieties
The color properties of the cheese samples were evaluated by using a Konica Minolta Chroma Meter (CR-400, Minolta Co. Ltd., Osaka, Japan). The L * (lightness), a * (redness), and b * (yellowness) color values were measured (Jafarzadeh et al. 2015).
Oxidative stability
Peroxide values of the cheese samples were determined according to AOAC 965.33 (1997). Briefly, about 1 g of sample was dissolved in chloroform and acetic acid (10: 15 v: v). The released iodine was titrated with sodium thiosulfate solution (0.02 N) by employing the starch as an indicator. The peroxide value (PV) was determined as follows (Eq. 2.):
| 2 |
where V0 is the volume of sodium thiosulfate (ml) required to titrate the blank, V is the volume of sodium thiosulfate (ml) required to titrate the test and N is the normality of the sodium thiosulfate solution.
In order to determine the effect of edible films on protein oxidation of the cheese (sulfhydryl group), the method describe by Ellman (1959) consist to, 0.2 mg of each sample was mixed with 4 ml of Tris-EDTA buffer (pH = 8). After that, 0.08 ml of DNTB (10 mM) was added, then, the mixture was incubated at room temperature for 20 min. After the centrifugation of the at 3000 g for 10 min the results were read at 412 nm using UV–Vis spectrophotometer (Shimadzu, UV – 1800, Japan). The concentration of the sulfhydryl group was calculated using the following equation (Eq. 3.):
| 3 |
where A and B are the absorbance values of the sample and the blank, respectively.
Sensory evaluation
The sensory acceptability of the cheese samples coated with the edible films containing different EO was determined at different storage times (0, 24 h and 5 days). The panel involved 47 untrained panelists including a male and a female genders This test was used to generally assess the degree of acceptance of cheese samples by performing sensory profiles for each product by assessing color, texture, flavor and taste.
Panelists were asked to reflect their degree of approval of the product based on color, texture, flavor and taste, and general acceptability based on a 6-level scale, in order to achieve sensory profiles.
Statistical analysis
Statistical analysis was carried out by a Windows based statistical analysis program (IMB SPSS 25). One-way analysis of variance (ANOVA) was performed and the statistical differences between the means were evaluated at the significance level of 95% by using the Tukey test. The analyses were performed in triplicate. Using XLSTAT 2016.02.28451 software, the Principal Component Analysis (PCA) and Hierarchical Ascending Classification (HAC) were used for individus and variables analysis for sensorial characterization. All analyzes were carried out in three replicates.
Results and discussion
Volatile profile
The yields of EO from A. herba alba Asso, O. basilicum L., M. pulegium L. and R. officinalis L were 0.57, 0.5, 1.10, 0.98%, respectively. Chromatographic analysis of EO illustrated that pulgone (38.02%), p-Menthone (25.56%), 1.8-cineole (12.04%) and linalool (27.74%). Anisole, p-allyl (17.15%), 1.8 cineole (7.86%), camphor (4.51%) and 1.8 cineol (18.85%) camphene (10.53%), 1,4 terpineol (14.34%)%, camphor (15.75%) and eucalyptol (8.32%), beta-thujone (8.34%), alpha-thujone (23.3%), camphor (20.84%) were the majority components of M. pulegium L, the O. basilicum L, R. officinalis L and A. herba-alba Asso respectively (Table 1).
Table 1.
Chemical compounds of R. officinalis L, M. pulegium L, A. herba-alba Asso and O. basilicum L oil’s (Mahcene et al. 2020)
| Compounds | Rt | % |
|---|---|---|
| M. pulegium L | ||
| Alpha.-pinene (−)- | 13.822 | 1.93 |
| Camphene | 14.799 | 1.09 |
| Bicyclo[3.1.1]heptane, 6,6-dimethyl-2-methylene-, (1S)- | 17.071 | 4.06 |
| Beta.-myrcene | 18.489 | 1.99 |
| 1,8-Cineole | 21.301 | 12.04 |
| Gamma.-terpinene | 23.195 | 0.2 |
| Alpha.-terpinene | 25.198 | 0.19 |
| Bicyclo[3.1.0]hexan-3-one, 4-methyl-1-(1-methylethyl)- | 26.264 | 0.89 |
| Chrysanthenone | 27.605 | 0.59 |
| Camphor | 28.728 | 0.33 |
| P-Menthone | 30.088 | 25.56 |
| Isopulegone | 31.79 | 4.39 |
| Pulegone | 35.704 | 38.02 |
| Bicyclo[3.2.0]heptan-2-on, 5-formylmethyl-6-hydroxy-3,3-dimethyl-6-vinyl | 38.412 | 1.26 |
| Acetic acid, nonyl ester | 39.55 | 0.24 |
| 2-Cyclohexen-1-one, 3-methyl-6-(1-methylethylidene)- (CAS) Piperitenone | 41.203 | 1.97 |
| Neryl acetate | 43.424 | 0.29 |
| (-)-.Beta.-elemene | 43.759 | 0.15 |
| Spathulenol | 53.084 | 0.45 |
| Caryophyllene oxide | 55.938 | 1.22 |
| R. officinalis L | ||
| Alpha.-pinene, (-)- | 13.925 | 2.88 |
| L-Phellandrene | 14.415 | 1.19 |
| Tricyclene | 14.52 | 0.69 |
| Beta.-phellandrene | 14.57 | 0.25 |
| Camphene | 15.228 | 10.53 |
| L-.beta.-pinene | 18.516 | 4.17 |
| Delta.3-carene | 19.591 | 0.44 |
| 1-Octen-3-ol (CAS) Oct-1-en-3-ol | 20.015 | 1.54 |
| 1,8-cineole | 21.335 | 18.85 |
| Eucalyptol | 22.708 | 2.12 |
| Gamma.-terpinene | 23.684 | 1.5 |
| Alpha.-terpinene | 25.434 | 1.1 |
| Alpha.-thujone | 27.128 | 0.64 |
| Linalool | 27.667 | 1.61 |
| D-Fenchyl alcohol | 28.63 | 0.57 |
| Camphor | 29.366 | 15.75 |
| Borneol | 30.82 | 2.39 |
| L-4-Terpineol | 31.734 | 14.34 |
| Beta.-citronellol | 34.668 | 0.2 |
| Alpha.-fenchyl acetate | 37.945 | 2.3 |
| Alpha.-terpinenyl acetate | 41.37 | 0.32 |
| 3-Allyl-6-methoxyphenol | 41.808 | 0.29 |
| Alpha.-copaene | 42.774 | 0.35 |
| Alpha.-humulene | 46.87 | 0.29 |
| Delta.-cadinene | 50.52 | 0.48 |
| Caryophyllene oxide | 53.437 | 0.9 |
| Beta.-guaiene | 55.562 | 0.21 |
| Delta.-cadinol | 56.165 | 0.46 |
| Alpha.-bisabolol | 58.164 | 0.48 |
| O. basilicum L | ||
| Alpha.-pinene, (-)- | 13.836 | 1.9 |
| Camphene | 14.804 | 0.93 |
| 2-.beta.-pinene | 17.019 | 3.38 |
| 1-phellandrene | 19.084 | 0.16 |
| Delta.3-carene | 19.504 | 1.58 |
| Alpha. Terpinene | 20.043 | 0.21 |
| 1,8-cineole | 21.278 | 7.86 |
| Gamma.-terpinene | 23.23 | 0.27 |
| Beta. Terpineol | 24.33 | 0.19 |
| 1,3,7-octatriene, 2,7-dimethyl- | 25.195 | 2.11 |
| Linalool | 28.63 | 27.74 |
| Camphor | 30.11 | 4.51 |
| 1-borneol | 31.31 | 1.58 |
| Anisole, p-allyl- | 33.039 | 17.15 |
| Chavicol | 36.569 | 2.67 |
| Citral | 37.276 | 0.35 |
| Endobornyl acetate | 38.13 | 1.42 |
| Myrtenylacetate | 40.13 | 0.17 |
| Eugenol | 42.224 | 3.17 |
| Alpha.-copaene | 42.905 | 0.14 |
| Geranyl acetate | 43.378 | 0.19 |
| Germacrene-d | 43.683 | 0.21 |
| Alpha.-cubebene | 43.98 | 0.1 |
| Benzene, 1,2-dimethoxy-4-(2-propenyl)- | 44.705 | 2.14 |
| Alpha.-humulene | 47.023 | 0.5 |
| (-)-Isoledene | 48.022 | 0.11 |
| Germacrene-d | 48.472 | 1.55 |
| Bicyclogermacrene | 49.228 | 0.93 |
| Torreyol | 50.53 | 0.73 |
| Alpha.-santalol | 51.528 | 0.16 |
| Nerolidol | 52.522 | 0.29 |
| Spathulenol | 53.206 | 0.67 |
| Alpha.-cadinol | 56.506 | 3.59 |
| Beta.-eudesmol | 56.808 | 0.58 |
| Alpha.-bisabolol | 58.137 | 0.35 |
| A. herba-alba Asso | ||
| Tricyclene | 12.725 | 0.39 |
| Alpha.-pinene, (-)- | 13.827 | 1.75 |
| Camphene | 15.053 | 3.61 |
| 2,4(10)-thujadien | 15.304 | 0.2 |
| Sabinene | 16.881 | 1.44 |
| L-Phellandrene | 19.09 | 0.17 |
| Alpha.-terpinene | 20.032 | 0.25 |
| Eucalyptol | 21.366 | 8.32 |
| Gamma.-terpinene | 23.188 | 0.54 |
| Alpha.-terpinolene | 25.162 | 0.28 |
| Beta.-thujone | 26.84 | 8.34 |
| Alpha.-thujone | 28.302 | 23.5 |
| Chrysanthenone | 29.34 | 2.64 |
| Camphor | 29.75 | 20.84 |
| L-4-Terpineol | 31.971 | 2.03 |
| Myrtenol | 32.916 | 1.81 |
| Cis-Piperitol | 33.562 | 1.22 |
| Bornyl formate | 34.511 | 0.4 |
| Chrysanthenyl acetate | 36.59 | 1.29 |
| Apha sinensal | 37.44 | 0.28 |
| Endobornyl acetate | 37.93 | 0.95 |
| Gamma.-terpinene | 38.694 | 0.85 |
| Alpha.-copaene | 42.79 | 0.58 |
| Bicyclo [3.1.1]hept-3-en-2-one, 4,6,6-trimethyl- (CAS) Berbenone | 44.221 | 4.52 |
| Germacrene-d | 48.38 | 0.8 |
| Bicyclogermacrene | 49.14 | 0.22 |
| Nerolidol | 52.533 | 0.14 |
| Spathulenol | 53.229 | 0.79 |
| Caryophyllene oxide | 56.025 | 0.9 |
Bold values shows the main components of each oil
This result for chemical composition “chymotype” was found to be substantially similar to previous studies (Baghloul et al. 2017; Silva et al. 2017; Bertellaa et al. 2018). However, considerable differences were observed compared to other studies in terms of “chymotype” for the main constituents of R. officinalis L, M. pulegium L, A. herba-alba Asso and O. basilicum L. (Aurelie et al. 2016; Yann et al. 2018; Ayoub et al. 2018). Generally, it is concluded that the variation in the chemical composition of EO results from the geographical origin of the plant, the extraction technique, the time of harvest and climatic factors (Mahcene et al. 2020).
Microbiological properties
The research outcomes and the frequency of Total Mesophilic Aerobic Bacteria (TMAB), Fecal coliforms, Yeasts, Molds, Staphylococcus aureus, and Salmonella in cheese samples are represented in Fig. 1.
Fig. 1.
Total Mesophilic Aerobic Bacteria (TMAB), Faecal Coliforms and Yeasts count during the storage period of home-made cheese coated and uncoated. Uncoated: Cheese without film; Cheese C: Cheese coated with Sodium Alginate film; Cheese A: Cheese coated with Sodium Alginate film incorporated with Artemisia herba alba Asso EO; Cheese B: Cheese coated with Sodium Alginate film incorporated with Ocimum basilicum L EO; Cheese M: Cheese coated with Sodium Alginate film incorporated with Mentha pulegium L EO. and Cheese R: Cheese coated with Sodium Alginate film incorporated with Rosmarinus officinalis L EO. a–d Different letters in the column indicate a significant difference (P < 0.05) between the results of the same sample during the storage period; a–d different letters in the column indicate a significant difference (P < 0.05) between the results of different samples in the same day of analysis
Regarding the counting of TMAB in cheeses during their storage period, the Fig. 1 illustrates that during the first day of storage until the last day the microbiological analysis of the cheese revealed a significant increase of the TMAB population of coated and uncoated cheese samples (P < 0.05), where the control cheese coated with sodium alginate without EO represents the highest growth rate of 6.39 ± 0.015 and 6.618 ± 0.022 log CFU/g, respectively. However, the cheese coated with sodium alginate reinforced with EO of A. herba alba Asso (Cheese A) reveals less growth of TMAB in terms of 6.194 ± 0. 036 log CFU/g.
In addition, the yeast count results are described in Fig. 1 in which it is clearly shown that on the first day of analysis (T0) no yeast growth was noted in all the cheese samples. On the other hand, during the second day of storage, a significant large growth (P < 0.05) of yeast was found in the control cheese and the cheese coated with sodium alginate (2.095 ± 0.027 and 2.064 ± 0.047 log CFU/g, respectively). While a slight important growth (P < 0.05) was observed for cheese coated with sodium alginate incorporated with EO of A. herb alba Asso, M. pulegium L, R. officinalis L and O. basilicum L of 0.259 ± 0.241, 0.566 ± 0.23, 0.894 ± 0.272 and 0.159 ± 0.275 log CFU/g, respectively. Also, during the last day of analysis a significant growth between 1.662 ± 0.038 and 2.833± 0.017 log CFU/g of yeast was remarqued for the control cheese and the coated one with sodium alginate incorporated EO. Consequently, a fermentation of lactic acids promoted the growth of yeasts which led to a decrease in pH.
According to Fig. 1, a total absence of fecal coliform bacteria has been observed in all types of cheese (T0). After 24 h of storage, a significant proliferation (P < 0.05) of fecal coliforms was noted by the control cheese and coated with sodium alginate without EO of the order of 2.877 ± 0.056 and 2.939 ± 0.148 log CFU/g, respectively, where cheeses preserved by sodium alginate reinforced by different oils, show a lower proliferation between 0.534 ± 0.925 and 1.269 ± 1.099 log CFU/g. This significant proliferation (P < 0.05) was observed in the case of the control cheese and coated with sodium alginate without EO until the last day of analysis (3.939 ± 0.015 and 4.306 ± 0.057 log CFU/g, respectively).
With regard to the other bacteria, we note the total absence of molds, staphylococci and salmonella in all the cheese samples during their storage period.
Similar to the present results, Kuorwel et al (2011) reported that thermoplastic starch films containing carvacrol, linalol and thymol effectively inhibited the growth of S. aureus on the surface of Cheddar cheese. Also, G. KAVAS et al (2015) that they worke on semi-hard kashar cheese preservation using films based on sorbitol-modified whey isolate reinforced with EO of thyme and cloves (1.5%). Based on the results, it was found that the levels of E. coli O157: H7, L. monocytogenes and S. aureus all increased in the uncoated control samples but decreased in the samples covered with biofilms.
It can be emphasized that this reduction in the microbial population observed in the case of cheese preserved by the edible film based on sodium alginate enriched with the various oils studied is related to the oil’s chemical composition (terpene and phenolic components). As well, we can say that these oils are transported through the film to the cheese that has allowed their microbial preservation. The EO antimicrobial efficacy in dairy products can be influenced by several factors, such as the chemical composition of these products, the oils concentration used and the microorganisms to reduce or eliminate (M. Laranjo et al. 2017). CAILLET et LACROIX (2007), confirmed that the effectiveness of the oil increases with the pH reduction of the food, the storage temperature, and he oxygen quantity in the packaging. It is also proven that at low pH, the oils hydrophobicity increases which allows them to dissolve easily in the lipid phase of the bacterial membrane (Holley and Patel 2005). This observation concerning the pH decrease is confirmed by our result that the cheese preserved by the film added with the oils shows a low pH during the last day of storage compared to the controls.
pH, weight loss and hardness of cheese samples
Table 2 showed the change of the pH during 10 days of storage. A slight significant (P < 0.05) decrease was observed in the samples during the storage period. pH values of samples were ranged between 5.83 and 6.397 depending on the type of oil incorporated. This drop in pH could be due to possible due to peptization and bacterial fermentation. A study conducted by Kampf and Nussinovitch (2000) illustrated that the pH value of uncoated cheese was decreased to 5.7 as pH of the alginate-coated cheese as found to be 5.9 after 18 days of storage,
Table 2.
pH, weight loss and Hardness values of coated and uncoated cheese samples
| T0 | 24 h | 5 Days | 10 Days | |
|---|---|---|---|---|
| pH | ||||
| Uncoated | 6.3 ± 0.01(bcA) | 6.17 ± 0.01(bB) | 6.08 ± 0.02(bC) | 6.017 ± 0.042(aD) |
| Cheese C | 6.243 ± 0.006(dA) | 6.087 ± 0.006(cB) | 6 ± 0.03(cC) | 5.83 ± 0.035(bD) |
| Cheese A | 6.277 ± 0.006(cA) | 6.247 ± 0.025(aA) | 6.133 ± 0.02(aB) | 6.02 ± 0.01(aC) |
| Cheese B | 6.397 ± 0.015(aA) | 6.237 ± 0.006(aB) | 5.99 ± 0.017(cC) | 5.987 ± 0.015(aC) |
| Cheese M | 6.3 ± 0.01(bcA) | 6.19 ± 0.03(bB) | 5.96 ± 0.01(cC) | 5.877 ± 0.012(bD) |
| Cheese R | 6.327 ± 0.015(bA) | 6.17 ± 0.01(bB) | 6.07 ± 0.01(bC) | 5.96 ± 0.026(aD) |
| Weight loss (%) | ||||
| Uncoated | 0(C) | 2.524 ± 0.441(aC) | 11.205 ± 0.925(aB) | 19.318 ± 1.807(aA) |
| Cheese C | 0(C) | 3.562 ± 0.396(aC) | 10.144 ± 2.853(aB) | 18.862 ± 1.615(aA) |
| Cheese A | 0(B) | 2.126 ± 0.608(aB) | 10.05 ± 3.902(aA) | 15.354 ± 4.082(aA) |
| Cheese B | 0(C) | 1.976 ± 0.615(aC) | 9.074 ± 1.406(aB) | 15.226 ± 3.105(aA) |
| Cheese M | 0(B) | 2.651 ± 0.758(aB) | 9.93 ± 1.89(aA) | 15.274 ± 4.649(aA) |
| Cheese R | 0(C) | 2.497 ± 0.899(aC) | 9.138 ± 2.292(aB) | 15.365 ± 1.453(aA) |
| Hardness (N) | ||||
| Uncoated | 870.483 ± 421.385(aB) | 1173.39 ± 227.918(aAB) | 1249.303 ± 104.837(aAB) | 1589.897 ± 157.465(aA) |
| Cheese C | 443.003 ± 14.937(abB) | 551.763 ± 19.019(bB) | 752.887 ± 115.953(bA) | 870.19 ± 49.937(bA) |
| Cheese A | 381.13 ± 58.679(abC) | 580.843 ± 35.968(bBC) | 842.59 ± 127.46(bAB) | 1011.427 ± 150.716(bA) |
| Cheese B | 344.133 ± 69.061(aC) | 634.783 ± 128.034(bB) | 808.11 ± 19.2(bAB) | 1035.893 ± 115.034(bA) |
| Cheese M | 474.35 ± 76.164(abC) | 564.597 ± 31.53(bBC) | 636.163 ± 63.375(bAB) | 776.89 ± 50.972(bA) |
| Cheese R | 388.857 ± 67.271(abC) | 550.627 ± 54.664(bBC) | 671.49 ± 23.036(bAB) | 821.537 ± 119.091(bA) |
A−D: Different letters in the same line indicate significant difference (P < 0.05) between the results of same sample during storage period; a–d: Different letters in the column indicate significant difference (P < 0.05) between the results of different samples. Uncoated: Cheese without film; Cheese C: Cheese coated with Sodium Alginate film; Cheese A: Cheese coated with Sodium Alginate film incorporated with Artemisia herba alba Asso EO; Cheese B: Cheese coated with Sodium Alginate film incorporated with Ocimum basilicum L EO; Cheese M: Cheese coated with Sodium Alginate film incorporated with Mentha pulegium L EO. and Cheese R: Cheese coated with Sodium Alginate film incorporated with Rosmarinus officinalis L EO
It was observed that there was not a significant difference (P > 0.05) in term of the weight loss between the samples but a significant (P < 0.05) increase was found for all the types of the cheese during 10 days. As indicated in Table 2, cheese samples coated with sodium alginate film (cheese C) and uncoated cheese possess the highest weight loss (18.862 ± 1.615, 19.318 ± 1.807) respectively. The results also revealed that edible films, especially those incorporated in essential oils, had a positive influence on cheese by slightly reducing weight loss during storage. These results were due to the fact that these polymers acted as natural barrier, which then reduced the gas and water exchanges between the coated cheese and the environment. The similar results were reported by Kampf and Nussinovitch (2000). weight loss of cheese coating with alginate was 5.2% while a weight loss of uncoated cheese sample was found to be 6.2%. Mei et al. (2013) stated the highest weight loss was observed in Mongolian cheese without coating film after 30 days of storage.
According to Table 2, a significant increase (P < 0.05) in hardness is recorded for all cheese types where uncoated cheese has the highest hardness values during all storage days than these values ranging from 870.483 ± 421.385 to 1589.897 ± 157.465 (N). In addition, a significant increase (P < 0.05) in hardness was also noted between the cheese types themselves during their storage period. According to the results obtained concerning the weight loss and the measurement of the hardness, it has been observed that the greater the weight loss, the more the hardness is increased. Then, it was noted that the film based on sodium alginate also that it reinforced by the oils react positively with the cheese by the decrease in their hardness. The results obtained by Ramos et al (2012) on the evaluation of edible antimicrobial coatings from a base of whey protein isolate to improve the shelf life of cheese give statistically significant differences (P < 0.05) were observed between coated and coated cheeses where the coated cheeses had lower hardness values. However, no significant difference was found between cheeses bearing one or the other type of coating. In addition, a significant increase (P < 0.05) was obtained over 20 days of storage, corresponding to the period during which the greatest loss of water was observed. The study of the relationships between pH and hardness of cheese by Monteiro et al. (2009) noted that the hardness decreased non-linearly with increasing pH. The melting increased linearly with increasing pH. As a result, the cream cheese samples became softer and more fluid when melted. The hardness values of the cheese samples throughout the storage showing a significant increase (P < 0.05) in all cases. It was observed that the presence of coating, its nature and the method of polymerization considerably influenced this parameter (Marta Henriques et al. 2014).
Optical properties
As illustrated in Table 3, a significant difference (P < 0.05) in L *, a * and b * values was found between all of the coated and uncoated cheese samples during their storage period. It was also observed that there was a significant difference (P < 0.05) between the samples themselves during the storage period, with the exception of cheese. In addition, it was observed that the L * values of all the film samples were greater than 70% between 85.69 ± 0.311 and 91.157 ± 0.049 depending on the cheese types. The increase in the L * values of the films means that their colors approach white and the color film is therefore lighter. As well, it was noted that all of the samples gave negative a * values representing the color green (− 1.4 ± 0.3 to − 2.727 ± 0.086 depending on the cheese types). Although, the highest b * value was found on the last day of storage for all cheese types between 12.477 ± 0.228 and 16.38 ± 0.639.
Table 3.
Optical properties of cheese samples
| Color | T0 | 24 h | 5 Days | 10 Days | |
|---|---|---|---|---|---|
| L* | Uncoated | 89.86 ± 0.261(aA) | 88.367 ± 0.341(abA) | 89.78 ± 0.901(abcA) | 90.313 ± 1.507(aA) |
| Cheese C | 86.003 ± 0.885(bC) | 88.797 ± 1.419(aAB) | 87.463 ± 0.698(abBC) | 90.857 ± 0.973(aA) | |
| Cheese A | 86.387 ± 0.841(bC) | 86.55 ± 0.641(bC) | 88.073 ± 0.971(cdAB) | 90.733 ± 1.704(aA) | |
| Cheese B | 87.877 ± 0.434(abB) | 88.25 ± 0.792(abB) | 86.497 ± 0.497(bcdC) | 90.157 ± 0.667(aA) | |
| Cheese M | 86.563 ± 0.678(bAC) | 86.48 ± 0.685(bA) | 85.69 ± 0.311(cA) | 86.593 ± 0.716(bA) | |
| Cheese R | 86.973 ± 1.53(bC) | 89.19 ± 0.165(aAB) | 88.51 ± 0.374(aBC) | 91.157 ± 0.049(aA) | |
| a* | Uncoated | − 1.57 ± 0.37(aAB) | − 1.4 ± 0.3(aA) | − 2.07 ± 0.044(bcB) | − 2.063 ± 0.023(aB) |
| Cheese C | − 2.32 ± 0.184(bC) | − 2.15 ± 0.092(bBC) | − 1.663 ± 0.029(aA) | − 1.883 ± 0.04(aAB) | |
| Cheese A | − 2.413 ± 0.248(bB) | − 2.397 ± 0.154(bB) | − 1.893 ± 0.081(bA) | − 2.65 ± 0.121(bcB) | |
| Cheese B | − 2.433 ± 0.275(bBC) | − 2.09 ± 0.035(bAB) | − 1.9 ± 0.056(bA) | − 2.727 ± 0.086(cC) | |
| Cheese M | − 2.147 ± 0.038(abA) | − 2.087 ± 0.031(bA) | − 1.97 ± 0.017(bA) | − 3.297 ± 0.135(dB) | |
| Cheese R | − 2.26 ± 0.128(bA) | − 2.33 ± 0.137(bA) | − 2.2 ± 0.161(cA) | − 2.43 ± 0.132(bA) | |
| b* | Uncoated | 10.862 ± 1.324(aAB) | 10.64 ± 0.306(bB) | 10.6 ± 0.135(abB) | 12.477 ± 0.228(cA) |
| Cheese C | 10.172 ± 0.864(bC) | 10.473 ± 0.225(bBC) | 9.56 ± 0.288(bCB) | 12.85 ± 0.395(cdA) | |
| Cheese A | 10.782 ± 0.702(bB) | 10.49 ± 0.269(bB) | 11.167 ± 0.595(abB) | 12.747 ± 0.544(cA) | |
| Cheese B | 11.314 ± 0.733(bBC) | 11.53 ± 0.579(aBC) | 10.513 ± 0.139(abC) | 14.547 ± 0.667(bA) | |
| Cheese M | 10.548 ± 0.537(abA) | 10.697 ± 0.123(abB) | 10.53 ± 0.405(abB) | 16.38 ± 0.639(aA) | |
| Cheese R | 10.804 ± 0.287(bA) | 11.19 ± 0.191(abBC) | 11.463 ± 0.284(aB) | 14.140 ± 0.132(bcA) |
A–DDifferent letters in the same line indicate significant difference (P < 0.05) between the results of same sample during storage period; a–d: Different letters in the column indicate significant difference (P < 0.05) between the results of different samples. Uncoated: Cheese without film; Cheese C: Cheese coated with Sodium Alginate film; Cheese A: Cheese coated with Sodium Alginate film incorporated with Artemisia herba alba Asso EO; Cheese B: Cheese coated with Sodium Alginate film incorporated with Ocimum basilicum L. EO; Cheese M: Cheese coated with Sodium Alginate film incorporated with Mentha pulegium L. EO. and Cheese R: Cheese coated with Sodium Alginate film incorporated with Rosmarinus officinalis L. EO
According to Brasil et al (2012), slight changes in the a * and b * values were a good indicator of the absence of oxidative browning. This suggestion allows us to say that the films recessed by the oils leads to better preservation of cheese by the stability of their color which is also confirmed subsequently by their oxidative stability. Cerqueira et al (2009), said that this color change for uncoated cheese can be partly attributed to oxygen and light oxidation, which is lower in coated cheeses due to the reduction in permeability to oxygen and higher opacity. Color analysis based on L *, a *, b * coordinates by Jun et al (2015) confirmed that all samples of Bod Ljong cheese coated with the edible starch-chitosan film have changed in color. color throughout storage, with statistically significant differences (P < 0.05) recorded between them, too, the brightness of the cheese decreased significantly (P < 0.05) during storage while at the end of storage, the brightness uncoated cheese decreased by 21.8% and the decreases in luminosity observed in the coated cheese were less than 20.7%, thus, a * and b * (P> 0.05) increased and then decreased during storage. Bermudez-Aguirre and Barbosa-Canovas (2010), attributed that the decrease in luminosity due to microbial growth on the surface of the cheese. However, the decrease in a * and b *, reflecting progressive discoloration in the samples, probably due to the migration of fats to the surface (Romani et al. 2002). The discoloration was perceived as a loss of yellowing and lightness (Jun et al. 2015). According to, Gurdian et al. (2017), the color change results indicated that queso blanco cheeses without linseed oil (QB) wrapped in films of whey protein isolate (QBFO-WF) and with a film of whey protein isolate containing oregano essential oil (QBFO-WOF) had L * values significantly lower than queso blanco cheeses with linseed oil cheese (QBFO) throughout storage, also, the a valeurs values of cheeses decreased slightly during storage. However, QBFO-WF and QBFO-WOF (packaged cheese) showed significantly higher b* values than those of QB and QBFO (unpackaged cheese). Color changes may be due to the growth of yeast and mold on the surface of the cheese.
Oxidative stability
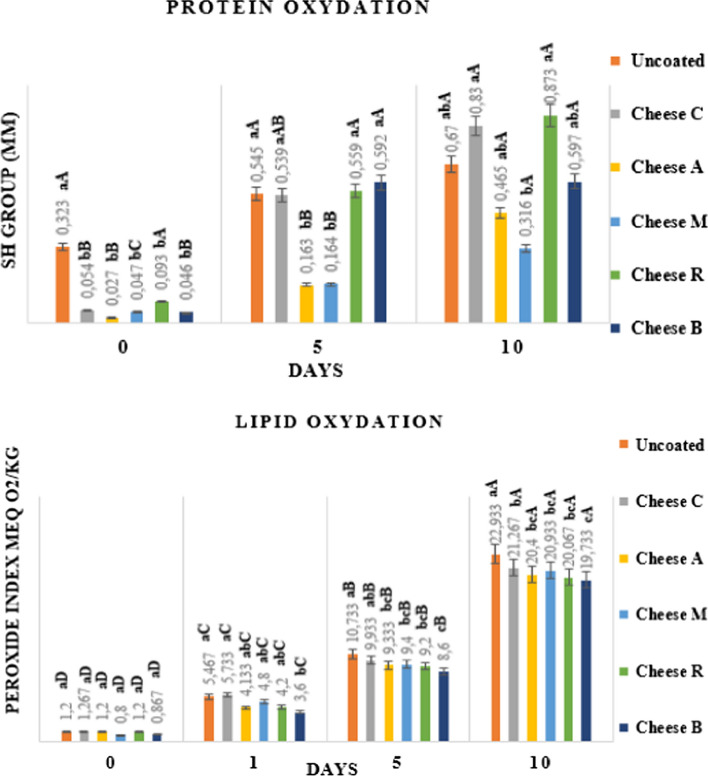
The coated and uncoated cheese samples were subjected to oxidation at storage temperature (4 °C) for 10 days. Oxidative changes can lead to unpleasant flavors, the destruction of valuable nutrients, and the production of toxic compounds. The modification of the content of sulfhydryl group (SH) and of lipid peroxide index has been illustrated in Fig. 2.
Fig. 2.
Protein Oxydation and Lipid Oxydation of home-made cheese coated and uncoated. Uncoated: Cheese without film; Cheese C: Cheese coated with Sodium Alginate film; Cheese A: Cheese coated with Sodium Alginate film incorporated with Artemisia herba alba Asso EO; Cheese B: Cheese coated with Sodium Alginate film incorporated with Ocimum basilicum L EO; Cheese M: Cheese coated with Sodium Alginate film incorporated with Mentha pulegium L EO. and Cheese R: Cheese coated with Sodium Alginate film incorporated with Rosmarinus officinalis L EO. a–d Different letters in the column indicate a significant difference (P < 0.05) between the results of the same sample during the storage period; a–d: Different letters in the column indicate a significant difference (P < 0.05) between the results of different samples in the same day of analysis
The sulfhydryl group (SH) and the disulfide bonds (S–S) are important for maintaining the structure and functions of native proteins. According to Fig. 2, a significant increase (P < 0.05) in the SH group concentration was observed in all the products tested for 10 days, including the cheese preserved by the films reinforced with EO from A. herba alba Asso, M. pulegium L and O. basilicum L show the lowest SH group concentration during the storage period, which reaches 0.465 ± 0.184, 0.316 ± 0.034 and 0.597 ± 0.087 MM, respectively, during the last day of storage, which indicates poor protein degradation. Lipid oxidation can be initiated and accelerated by various mechanisms, including the production of singlet oxygen, the enzymatic and non-enzymatic generation of free radicals and active oxygen. According to Fig. 2, a significant increase (P < 0.05) in the peroxide index was noted in all the products tested for 10 days, including preserved cheese. It was also observed that the control and cheese coated with sodium alginate without EO had a higher peroxide index during all the measurement days, than the cheese preserved the films incorporated by the A. herba alba Asso, M. pulegium L, R. officinalis L and O. basilicum L EO show the lowest values of this index during the 10th day of 20.4 ± 0.4, 20.933 ± 0.306, 20.067 ± 0.503 and 19.733 ± 0.231, respectively.
It was also noted that the cheese coated with sodium alginate incorporated with the oils was more resistant to oxidation than the control having a high sensitivity to oxidation. Therefore, it could be said that phenolic groups have important antioxidant properties which prevent the peroxidation of fatty acids and delay the denaturation of proteins by reducing the release of SH. So, EO can increase the shelf life of dairy products, not only by eliminating unwanted microorganisms but also by decreasing the degree of chemical deterioration during storage. This conclusion is also confirmed by Sabahu Noor et al. (2018), that the decrease in the values of substances reacting to thiobarbituric acid could be attributed to A. racemosus present in the films used which contains large amounts of bioactive phytochemicals which inhibit chain reactions of lipid oxidation by directly neutralizing radicals by providing hydroxyl groups. Some reports have shown that gas barrier properties are crucial for extending the shelf life of food by being resistant to oxygen diffusion, so may have delayed lipid oxidation Song et al. (2011). Olmedo et al. (2013); Asensio et al. (2015), state that the rancid and fermented aromas that have determined a shorter shelf life of the product have been less pronounced in products containing additional EO of oregano and rosemary because the oxidative processes have been inhibited. Application of edible films of whey protein isolate containing oregano essential oil has been effective in delaying the lipid oxidation of queso blanco with linseed oil (Gurdian et al. 2017). According to Bartolomeu et al (2013), lipid peroxidation occurs due to a chain reaction led by O2 free radicals in which a radical induces the oxidation of a considerable number of molecules of substrate. This process is catalyzed by light and the coating can also act as a barrier to the incidence of light, further decreasing the lipid oxidation of the cheese which leads to the lower peroxidation value in the coated cheese compared to the uncoated cheese.
Sensory analysis
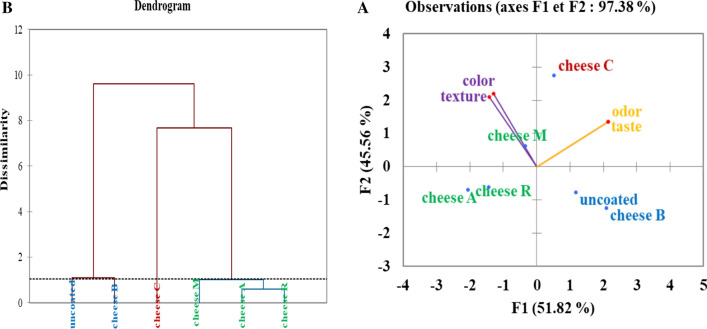
Principal Component Analysis (PCA) and Hierarchical Ascending Classification (HAC) was used for the analysis of individuals and variables for sensory characterization (color, odor, taste, appearance) and the determination of the degree of acceptance of the different types of cheese by the tasters.
For the analysis of the main components, it was noted that the observation quality equals 97.38% (> 50%) which shows that there is a difference between the cheese samples. Moreover, Fig. 3a clearly demonstrated that the dimension F1 opposes individuals the uncoated cheese and the cheese B with the cheese C which they appear on the left of the graph and characterized by a strongly negative coordinate on the axis of the individuals such as cheese C appearing at the top right of the graph and characterized by the highest odor, texture, taste and color scores compared to the others as well, it was noted that uncoated cheese and cheese B characterized by the lower values of color and texture. In addition, it was observed that the dimension F2 particularly distinguishes strongly negative individuals on the axis (uncoated cheese and cheese B) with cheese A, cheeses R and cheeses M which appeared to the right of the graph and characterized by a strongly positive coordinate on the axis, moreover, it has been observed that these latter types of cheese possess the lowest odor and taste values. Although, we noted from the variables correlation that the dimension F2 opposes the variables taste and odor which they appear at the top right of the graph with the variables color and texture whose they appear at the top left of the graph. Graphically, these variables are negatively correlated with each other where the increase in color and texture values lead to a decrease in taste and odor values.
Fig. 3.
Principal Component Analysis (PCA) of principal Sensory Characteristics (a) and Hierarchical Ascending Classification of Individuals (b). Uncoated: Cheese without film; Cheese C: Cheese coated with Sodium Alginate film; Cheese A: Cheese coated with Sodium Alginate film incorporated with Artemisia herba alba Asso EO; Cheese B: Cheese coated with Sodium Alginate film incorporated with Ocimum basilicum L EO; Cheese M: Cheese coated with Sodium Alginate film incorporated with Mentha pulegium L EO. and Cheese R: Cheese coated with Sodium Alginate film incorporated with Rosmarinus officinalis L EO
As shown in Fig. 3b, the ascending hierarchical classification of individuals gives the intra-class value equal to 16.20% which shows that the cheese groups are well differentiated and this classification reveals 3 classes: The first class consisted of individuals such as cheese A, cheese M and cheese R, this group being characterized by low taste values. Regarding the second class was represented by individuals of cheese C that this group is characterized by the strong taste value given by the panelists. Finally, the third class concerned individuals B cheese and uncoated cheese, this group being characterized by a low value for the color and texture variables. In addition, according to the result emerging from the statistical analysis and the scores given by the penalists of different types of cheese, it was concluded that the cheese coated with sodium alginate alone (cheese C) was the most preferred cheese by the penalists, followed by the cheese coated with the sodium alginate incorporated of O. basilium L EO (cheese B) and uncoated cheese on the other hand, cheese M, cheese R and cheese A which coated by sodium alginate incorporated of M. pulegium L, R. officinalis L and A. herba alba Asso EO, respectively, are however the least accepted.
The study carried out by Kampf and Nussinovitch (2000), on the sensory evaluation of hard cheeses coated with carrageenan, alginate and gellan were showed that no taste detected by the panel in cheese coated in comparison to the control cheese. Similarly, Altieri et al (2005) show that the use of chitosan did not affect the sensory characteristics of cheese. In addition, in Chitosan Mozzarella, the texture was better maintained, contributing to its acceptability. other study carried out by Conte et al (2013), reported that the overall cheese quality during the nine days of storage did not change and the control and the sample conditioned with the film CuNPs_PLA-B2 retained a similar trend and became unacceptable after three days. In contrast, the CuNPs_PLA-A2 sample retained its characteristics for nearly a week, demonstrating the potential of copper nanoparticles to control microbial growth and, consequently, the evolution of sensory attributes. According to Li et al. (2017), cottage cheese packaged in PLA, PLA/TiO2 and PLA/TiO21Ag films had significant (P < 0.05) odor, texture and overall acceptability scores greater than those packaged with LDPE film a 10-day of storage. The results suggested that PLA/TiO2 and PLA/TiO21Ag films could improve the quality of cottage cheese products during storage in the refrigerator.
Conclusion
Because of their edibility and biodegradability, natural edible films incorporated by EO are a promising alternative to petroleum based non-degradable films for food packaging applications. These films provided the cheese samples a good barrier that protected them against weight loss, hardness, discoloration, loss off-flavor and texture, etc.; and prevented microbial spoilage. Therefore, it is regarded as an important and durable alternative for the preservation of the quality and the safety of the cheese. On the other hand, it has been found that the EO reinforced into the film do not seriously affect the organoleptic characteristics of the cheese, as well, the sensory acceptance of the cheese is dependent on the type of oil used.
Acknowledgements
This study is for research only, and it is explained that it was not funded by any organizations. Also, we would like to thank Yeldiz Technical University, Istanbul, Turkey for its cooperation to complete this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ainane A, Benhima R, Khammour F, Elkouali M, Talbi M, Abba EH, Cherroud S, Ainane T (2018) Composition chimique et activité insecticide de cinq huiles essentielles: Cedrus atlantica, Citrus limonum, Eucalyptus globules, Rosmarinus officinalis et Syzygium aromaticum. In: Proceedings BIOSUNE, pp 67–79
- Alam T, Goyal GK (2011) Effect of MAP on microbiological quality of Mozzarella cheese stored in different packages at 7 ± 1 °C. J Food Sci Technol 48(1):120–123 [DOI] [PMC free article] [PubMed]
- Alexa E, Danciu C, Cocan I, Negrea M, Morar A, Obistioiu D, Dogaru D, Berbecea A, Radulov I (2018) Chemical composition and antimicrobial potential of Satureja hortensis L. in fresh cow cheese. J Food Qual. ID 8424035
- Altieri C, Scrocco C, Sinigaglia M, Del Nobile MA. Use of chitosan to prolong mozzarella cheese shelf life. J Dairy Sci. 2005;88:2683–2688. doi: 10.3168/jds.S0022-0302(05)72946-5. [DOI] [PubMed] [Google Scholar]
- Alvarez MV, Ponce AG, Moreira MR. Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT Food Sci Technol. 2013;50:78–87. doi: 10.1016/j.lwt.2012.06.021. [DOI] [Google Scholar]
- Amri Jalila EL, Khalid E, Touria Z, Hayate B, Chakir S, Lmolk A. Étude de l’activité antibactérienne des huiles essentielles de Teucrium capitatium L et l’extrait de Siléne vulgaris sur différentes souches testées. J Appl Biosci. 2014;82:7481–7492. doi: 10.4314/jab.v82i1.16. [DOI] [Google Scholar]
- AOAC (1994) Association of official analytical chemists. Official Methods of Analysis
- AOAC . Official methods of analysis. 15. Washington DC: Association of Official Analytical Chemistry; 1997. [Google Scholar]
- Asensio CM, Grosso, NR, Juliani HR. Quality preservation of organic cottage cheese using oregano essential oils. LWT Food Sci Technol. 2015 doi: 10.1016/j.lwt.2014.10.054. [DOI] [Google Scholar]
- Aurelie FDG, Ascension NM, Gabriel TH, Herman AAP, Pauline NM, Lebel TJ (2016) Chemical composition and ovicidal, larvicidal and pupicidal activity of Ocimum basilicum essential oil against Anopheles gambiae (Diptera: Culicidae). Eur J Med Plants 16(3):1–13
- Baghloul F, Mansori R, Djahoudi A (2017) In vitro antifungal effect of Rosmarinus officinalis essential oil on Aspergillus niger. Natl J Phys Pharm Pharmacol 7
- Bermudez-Aguirre D, Barbosa-Canovas GV. Processing of soft Hispanic cheese (‘‘queso fresco’’) using thermo-sonicated milk: a study of physicochemical characteristics and storage life. J Food Sci. 2010;75:S548–S558. doi: 10.1111/j.1750-3841.2010.01850.x. [DOI] [PubMed] [Google Scholar]
- Bertellaa A, Benlahcena K, Abouamamaa S, Pintob DCGA, Maamara K, Kihala M, Silvab AMS. Artemisia herba-alba Asso. essential oil antibacterial activity and acute Toxicity. Ind Crops Prod. 2018;116:137–143. doi: 10.1016/j.indcrop.2018.02.064. [DOI] [Google Scholar]
- Brasil IM, Gomes C, Puerta-Gomez A, Castell-Perez ME, Moreira RG. Polysaccharide-based multilayered antimicrobial edible coating enhances quality of fresh-cut papaya. LWT Food Sci Technol. 2012;47:39–45. doi: 10.1016/j.lwt.2012.01.005. [DOI] [Google Scholar]
- CAILLET Stéphane et LACROIX Monique (2007) Les huiles essentielles : leurs propriétés antimicrobiennes et leurs applications potentielles en alimentaire. Laboratoire de Recherche en Sciences Appliquées à l’Alimentation (RESALA)
- Cansu Feyzioglu G, Tornuk F (2016) Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT Food Sci Technol 28
- Cerqueira MA, Lima AM, Souza BWS, Teixeira JA, Moreira RA, Vicente AA. Functional polysaccharides as edible coatings for cheese. J Agric Food Chem. 2009;57:1456–1462. doi: 10.1021/jf802726d. [DOI] [PubMed] [Google Scholar]
- Chawla R, Patil GR, Singh AK. High hydrostatic pressure technology in dairy processing: a review. J Food Sci Technol. 2011;48(3):260–268. doi: 10.1007/s13197-010-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte A, Longano D, Costa C, Ditaranto N, Ancona A, Cioffi N, Scrocco C, Sabbatini L, Contò F, Del Nobile MA. Novel preservation technique applied to fiordilatte cheese. Innov Food Sci Emerg Technol. 2013;19:158–165. doi: 10.1016/j.ifset.2013.04.010. [DOI] [Google Scholar]
- Costa C, Lucera A, Conte A, Zambrini AV, Nobile MAD. Technological strategies to preserve burrata cheese quality. Coatings. 2017;7:97. doi: 10.3390/coatings7070097. [DOI] [Google Scholar]
- Costa MJ, Maciel LC, Teixeira JA, Vicente AA, Cerqueira MA (2018) Use of edible films and coatings in cheese preservation: opportunities and challenges. Food Res Int [DOI] [PubMed]
- Dai J, Liang Zhu LI, Yang JQ. Chemical composition, antioxidant and antimicrobial activities of essential oil from Wedelia prostrata. EXCLI J. 2013;12:479–490. [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeu G, Medeiros S, Souza MP, Pinheiro AC, Bourbon AI, Cerqueira MA, Vicente AA, Carneiro-da-Cunha MG. Physical Characterisation of an Alginate/Lysozyme Nano-Laminate Coating and Its Evaluation on ‘Coalho’ Cheese Shelf Life. Food Bioprocess Technol. 2013 doi: 10.1007/s11947-013-1097-5. [DOI] [Google Scholar]
- Di Maio L, Scarfato P, Milana MR, Felicini R, Denaro M, Padula G, Incarnato L (2014) Bionanocomposite polylactic acid/ organoclay films: functional properties and measurement of total and lactic acid specific migration. Pakag Technol Sci 27(7):535–547
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Faleiro ML (2011) The mode of antibacterial action of essential oils. Science against microbial pathogens: communicating current research and technological advances, pp 1143–1156
- Gaikwad KK, Lee YS. Effect of storage conditions on the absorption kinetics of nonmetallic oxygen scavenger suitable for moist food packaging. Food Meas. 2017;11:965–971. doi: 10.1007/s11694-017-9470-0. [DOI] [Google Scholar]
- Gaikwad KK, Lee JY, Lee YS. Development of polyvinyl alcohol and apple pomace bio-composite film with antioxidant properties for active food packaging application. J Food Sci Technol. 2016;53(3):1608–1619. doi: 10.1007/s13197-015-2104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenson LN, Jagus R, Resa CPO (2018) Biodegradable packaging applied to dairy products. Adv Dairy Prod
- Gurdian C, Chouljenko A, Mis Solval K, Boeneke C, King JM, Sathivel S (2017) Application of edible films containing oregano (Origanum vulgare) essential oil on queso blanco cheese prepared with flaxseed (Linum usitatissimum) Oil. J Food Sci [DOI] [PubMed]
- Hay Y-O, Abril-Sierra MA, Sequeda-Castañeda LG, Bonnafous C, Raynaud C. Evaluation of combinations of essential oils and essential oils with hydrosols on antimicrobial and antioxidant activities. J Pharm Pharm Res. 2018;6(3):216–230. [Google Scholar]
- Henriques M, Santos G, Rodrigues A, Gomes D, Pereira C, Gil M (2014) Replacement of conventional cheese coatings by natural whey protein edible coatings with antimicrobial activity. J Hyg Eng Des
- Holley RA, Patel D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005;22:273–292. doi: 10.1016/j.fm.2004.08.006. [DOI] [Google Scholar]
- ISO 11870 (2000) International standard. Milks and milk products—determination of fat content—general guidance on the use of butyrometric methods
- ISO 4883 (2003) International Norm. Microbiology of food and animal feeding stuffs—horizontal method for the enumeration of microorganisms—Colony—count technique at 30 °C
- ISO 21527-1 (2008) International Norm. Microbiology of food and animal feeding stuffs—Horizontal method for the enumeration of yeasts and molds
- ISO 6579-1 (2017) International Norm. Microbiology of food and animal feeding stuffs—Horizontal method for the detection, enumeration and serotyping of Salmonella
- ISO 6888-1 (2003) International Norm. Microbiology of food and animal feeding stuffs—horizontal method for the enumeration of coagulase—positive staphylococci (Staphylococcus aureus and other species)
- Jafarzadeh S, Alias AK, Ariffin F, Mahmud S, Najafi A (2015) Preparation and characterization of bionanocomposite films reinforced with nano kaolin. J Food Sci Technol 53(2):1111–1119. 10.1007/s13197-015-2017-7 [DOI] [PMC free article] [PubMed]
- Jeewanthi RKC, Paik H-D (2018) Modifications of nutritional, structural, and sensory characteristics of non-dairy soy cheese analogs to improve their quality attributes. J Food Sci Technol. 10.1007/s13197-018-3408-3 [DOI] [PMC free article] [PubMed]
- Kampf N, Nussinovitch A. Hydrocolloid coating of cheeses. Food Hydrocolloids. 2000;14:531–537. doi: 10.1016/S0268-005X(00)00033-3. [DOI] [Google Scholar]
- Kavas G, Kavas N, Saygili D. The effects of thyme and clove essential oil fortified edible films on the physical, chemical and microbiological characteristics of kashar cheese. J Food Qual. 2015;38:405–412. doi: 10.1111/jfq.12157. [DOI] [Google Scholar]
- Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Bigger SW (2011) Antimicrobial activity of natural agents coated on starch-based films against Staphylococcus aureus. J Food Sci 76 [DOI] [PubMed]
- Kurt A, Toker OS, Tornuk F. Effect of xanthan and locust bean gum synergistic interaction on characteristics of biodegradable edible film. Int J Biol Macromol V. 2017;102:1035–1044. doi: 10.1016/j.ijbiomac.2017.04.081. [DOI] [PubMed] [Google Scholar]
- Laranjo M, Fernández-Léon AM, Potes ME, Agulheiro-Santos AC, Elias M (2017) Antimicrobial research: Novel bioknowledge and educational programs (A. Méndez-Vilas, Ed)
- Li W, Li L, Zhang H, Yuan M, Qin Y (2017) Evaluation of PLA nanocomposite films on physicochemical and microbiological properties of refrigerated cottage cheese. J Food Process Preserv
- Lucera A, Mastromatteo M, Conte A, Zambrini AV, Faccia M, Del Nobile MA. Effect of active coating on microbiological and sensory properties of fresh mozzarella cheese. Food Pack Shelf Life. 2014;1(1):25–29. doi: 10.1016/j.fpsl.2013.10.002. [DOI] [Google Scholar]
- Mahcene Z, Khlil A, Hasni S, Akman PK, Bozkurt F, Birech K, Goudjil MB, Tornuk F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int J Biol Macromol. 2020;145:124–132. doi: 10.1016/j.ijbiomac.2019.12.093. [DOI] [PubMed] [Google Scholar]
- Mastromatteo M, Conte A, Lucera A, Saccotelli MA, Buonocore GG, Zambrini AV, Nobile MAD. Packaging solutions to prolong the shelf life of Fiordilatte cheese: Bio-based nanocomposite coating and modified atmosphere Packaging. LWT Food Sci Technol. 2015;60:230–237. doi: 10.1016/j.lwt.2014.08.013. [DOI] [Google Scholar]
- Mehrsorosh H, Gavanji S, Larki B, Mohammadi D, Karbasiun A, Hashemzadeh F, Mojiri A. Essential oil composition and antimicrobial screening of some Iranian herbal plants on Pectobacterium carotovorum. Glob NEST J. 2015;16(2):240–251. [Google Scholar]
- Mei J, Yuan Y, Yan W, Li Y. Characterization of edible starch–chitosan film and its application in the storage of Mongolian cheese. Int J Biol Macromol. 2013;57:17–21. doi: 10.1016/j.ijbiomac.2013.03.003. [DOI] [PubMed] [Google Scholar]
- MIHAI Adriana Laura et POPA Mona Elena Essential oils utilization in food industry. Lit Rev. 2013;6:187–192. [Google Scholar]
- Monteiro RR, Tavares DQ, Kindstedt PS, Gigante ML. Effect of pH on Microstructure and Characteristics of Cream Cheese. J Food Sci. 2009;74(2):C112–C117. doi: 10.1111/j.1750-3841.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- NFV 08060 (2009) Microbiology of food and animal feeding stuffs—enumeration of thermotolerant coliforms by colony——count technique at 44 °C
- Olmedo RH, Nepote V, Grosso NR. Preservation of sensory and chemical properties in flavoured cheese prepared with cream cheese base using oregano and rosemary essential oils. LWT Food Sci Technol. 2013;53:409–417. doi: 10.1016/j.lwt.2013.04.007. [DOI] [Google Scholar]
- Perez LM, Balague CE, Rubiolo AC, Verdini RA. Evaluation of the biocide properties of whey—protein edible films with potassium sorbate to control non-O 157 shiga toxin producing Escherichia coli. Proc Food Sci. 2011;1:287–293. doi: 10.1016/j.profoo.2011.09.032. [DOI] [Google Scholar]
- Rahmani B, Hsseini H, Khani MR, Farhoodi M, Hanarvar Z, Feizollahi E, Shokri B, Shojae S (2017) Development and characterization of chitosan or alginate—coated low density polyethylene films containing Satureja hortensis extract. Int J Biol Macromol [DOI] [PubMed]
- Ramos ÓL, Pereira JO, Silva SI, Fernandes JC, Franco MI, Lopes-da-Silva JA, Pintado ME, Malcata FX. Evaluation of antimicrobial edible coatings from a whey protein isolate base to improve the shelf life of cheese. J Dairy Sci. 2012;95:6282–6292. doi: 10.3168/jds.2012-5478. [DOI] [PubMed] [Google Scholar]
- Romani S, Sacchetti G, Pittia P, Pinnavaia G, Dalla Rosa M. Physical, chemical, textural and sensorial changes of portioned Parmigiano Reggiano cheese packed under different conditions. Food Sci Technol Int. 2002;8:203–211. doi: 10.1177/1082013202008004118. [DOI] [Google Scholar]
- Sabahu Noor ZF, Bhat SK, Mudiyanselage RJ. Preservative effect of Asparagus racemosus: a novel additive for bioactive edible films for improved lipid oxidative stability and storage quality of meat products. Meat Sci. 2018 doi: 10.1016/j.meatsci.2018.02.001. [DOI] [PubMed] [Google Scholar]
- Silva SM, Rodrigues JPA, da Cunha S, de Carvalho M, Zandonadi CHS, Martins RC, Chang R. Ocimum basilicum essential oil combined with deltamethrin to improve the management of Spodoptera frugiperda. Ciência e Agrotecnologia. 2017;41(6):665–675. doi: 10.1590/1413-70542017416016317. [DOI] [Google Scholar]
- Snoussi M, Dehmani A, Noumi E, Flamini G, Papetti A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microbial Pathogenesis. 2016;90:13–21. doi: 10.1016/j.micpath.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Song Y, Liu L, Shen H, You J, Luo Y. Effect of sodium alginate-based edible coating containing different anti-oxidants on quality and shelf life of refrigerated bream (Megalobrama amblycephala) Food Control. 2011;22:608–615. doi: 10.1016/j.foodcont.2010.10.012. [DOI] [Google Scholar]
- Spehar ID, Kalit MT, Kalit S. Characterization of Preveli cheese, traditional Croatian dried acid-coagulated cheese Karakterizacija prevelog sira, tradicionalnog hrvatskog sušenog kiselinskog sira. J Cent Eur Agric. 2018;19(4):810–822. doi: 10.5513/JCEA01/19.4.2202. [DOI] [Google Scholar]
- Törnük F, Dertli E (2015) Decontamination of Escherichia coli o157:h7 and staphylococcus aureus from fresh-cut parsley with natural plant hydrosols. J Food Proc Preserv ISSN 1745-4549
- Tornuk F, Cankurt H, Ozturk I, Sagdic O, Bayram O, Yetim H. Efficacy of various plant hydrosols as natural food sanitizers in reducing Escherichia coli O157:H7 and Salmonella Typhimurium on fresh cut carrots and apples. Int J Food Microbiol. 2011;148:30–35. doi: 10.1016/j.ijfoodmicro.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Youssef AM, Assem FM, El-Sayed SM, Salama H, Abd El-Salam MH. Utilization of edible films and coatings as packaging materials for preservation of cheeses. J Package Technol Res. 2017;1:87–99. doi: 10.1007/s41783-017-0012-3. [DOI] [Google Scholar]