Abstract
Objective:
Mentorship for Addiction Problems (MAP) is a new behavioral treatment formalizing client-to-client mentorship relationships as an adjunct to standard outpatient substance use disorder treatment. We tested the preliminary efficacy of MAP in reducing substance use and associated barriers to successful treatment outcomes.
Method:
A total of 65 participants (17 later recovery participants [LRPs] and 48 early recovery participants [ERPs]) with substance use disorders were randomized to MAP + Treatment as Usual (TAU) or TAU alone. Within MAP, for each cohort, a pool of 4–5 mentors (LRPs) was formed and engaged in mentoring activities for 24 weeks until 12–13 mentees (ERPs), newly admitted, had participated in MAP for 12 weeks. Behavioral and biological measures were conducted at baseline, weekly, monthly, and termination for all participants and during the 12-week follow-up for ERPs.
Results:
Substance use declined across both conditions for ERPs (N = 48) during treatment, Weeks 0–12 (p = .001); however, on average, ERPs in the MAP intervention used significantly fewer days than controls during Treatment Weeks 1–12 (p = .013) and during Follow-Up Weeks 13–24 (p = .043). Addiction Severity Index alcohol and drug use scores increased in TAU and decreased in MAP during Follow-Up Weeks 13–24 for ERPs, alcohol: b = -0.08, SE = 0.03, t(47) = -2.97, p = .005; drug use: b = -0.02, SE = 0.01, t(47) = -2.36, p = .023. In addition, there was high patient interest in MAP and good fidelity to delivery of treatment.
Conclusions:
MAP shows promise assisting in the reduction of substance use early in treatment when vulnerability and risk for relapse is high and has a positive impact on serious problems undercutting addiction treatment efficacy.
Substance use disorders and their consequences present a significant and ongoing public health burden within the United States. Alcohol and tobacco are the leading causes of preventable death in our nation (Centers for Disease Control and Prevention [CDC], 2012; Kochanek et al., 2019). In addition, illicit substance use is a significant contributor to the opioid overdose and HIV epidemics (CDC, 2012; De Cock et al., 2012; Substance Abuse and Mental Health Services Administration [SAMHSA], 2019). Further, the economic costs associated with alcohol, illicit/ nonmedical drug use, and tobacco are estimated to be more than $700 billion annually, including costs associated with criminal activity, issues in work productivity, and medical expenses excluding substance use treatments (National Institute on Drug Abuse, 2016). Yet, despite this astounding figure, only 10% of the more than 20 million adult Americans who have alcohol or drug use disorders are receiving treatment (Center for Behavioral Health Statistics and Quality, 2014; SAMSHA, 2019).
Creating treatments that not only reduce substance use but have appeal to individuals with substance use disorders and can be adopted or translated into practice in diverse settings could never be more critical. When individuals can relate to or identify with those who may provide an intervention because of shared life experiences, they are more likely to seek and engage in treatment (Kelly et al., 2019; Naslund et al., 2016). In addition, treatments that draw from elements that occur naturally within environments may also increase the likelihood of adoption and appeal. Mentorship is one such naturally occurring exchange in which individuals desire to seek guidance or deliver guidance that also joins people based on shared experiences (Birtel et al., 2017; Darling et al., 2002; DuBois & Silverthorn, 2005; Heaney & Israel, 2002). Mentoring as a concept can be traced back to ancient Greece (Freedman, 1991); however, the formal investigation of mentorship to treat substance use disorders has only been relatively recent.
Because of its appeal and high patient acceptance, mentorship has been a key component of several existing treatment and recovery approaches such as Therapeutic Communities (TCs) (Galanter et al., 1998; Guida et al., 2002; Kazdin, 2019),Twelve Step–oriented treatments (Huselid et al., 1991; Project MATCH Research Group, 1997), and the Community ReinforcementApproach (Azrin, 1976; Campbell et al., 2015; Higgins et al., 2000; Kazdin, 2019; Meyers & Miller, 2001; Meyers et al., 2002; Miller et al., 1999a, 1999b; Silverman et al., 2001; Stitzer et al., 1986).
Although Twelve Step programs and TCs have shown comparable results to other forms of behavioral treatments for substance use disorders (Hai et al., 2019; Project MATCH Research Group, 1997; Westreich et al., 1996), the sponsorship or mentorship components of these treatments have not been standardized for implementation or specifically designed to be managed by professional treatment personnel. In addition, alternatives to Twelve Step approaches are needed to more closely integrate peer mentorship with treatment and to provide an option to the religious nature of Twelve Step approaches, which can be a deterrent to certain participants and providers (Hai et al., 2019; Harris et al., 2003; Tonigan et al., 2002; Walters, 2002).
Recently, there has been a dramatic rise in the adoption of alternative forms of peer mentorship programs to assist recovery from substance use disorders because of the potential benefits offered to patients (Helseth et al., 2018; Kazdin, 2019). Although these programs provide access to treatment and nonusing peers, often the roles are not clearly defined, with limited research to support efficacy and sustained abstinence for individuals (Mericle et al., 2015; White, 2004, 2006). This has led to a lack in clarity in roles and outcome (Jenkins, 2015; Rebeiro Gruhl et al., 2015; White et al., 2014).
To address these needs, within Stage Ia and Stage Ib behavioral therapies development projects (Tracy et al., 2010, 2012, 2018), we developed an innovative intervention, Mentorship for Addiction Problems (MAP). MAP aims to reduce substance use through the novel application of peerdriven Goal Attainment Scaling specific to each individual’s needs, creating high appeal while being administered starting during the critical first month of treatment when relapse and attrition are high, and support is needed most.
Within our previously published Stage Ia project, there were significant reductions in both drug and alcohol use from baseline to Week 12 associated with the MAP treatment (Tracy et al., 2010,2012). Participants also reported high satisfaction with MAP, and treatment staff reported that it helped in allowing their patients to meet goals without interfering with clinic operations or their own treatment goals for the patient (Tracy et al., 2010, 2012). In addition, the Stage Ia pilot/feasibility study also demonstrated high rates of patient retention, good mentor adherence to MAP treatment delivery guidelines, and significant reductions in HIV/ID risk behavior (Tracy et al., 2010, 2012).
Following the Stage Model of Behavior Therapy Development, these promising results led to the next step in treatment development, a small-scale Stage Ib randomized pilot study (Rounsaville et al., 2001). The Stage Ib study focused on continued testing of MAP to further expand our understanding of its feasibility, applicability, and initial/ preliminary efficacy to determine power for a future large scope Stage II efficacy trial (Rounsaville et al., 2001).
The current report presents our promising primary outcome of substance use and treatment delivery results from our Stage Ib initial efficacy testing of MAP in a randomized pilot study that evaluated the following hypothesis: Early recovery participants (ERPs)/mentees in MAP+Treatment As Usual (TAU) will show a greater reduction in drug and alcohol use than ERPs in TAU alone during active treatment and also the follow-up phase. The primary outcome presented augments our recently reported positive secondary Stage Ib outcomes, where MAP was associated with significant improvements in psychiatric symptoms, quality of life, and global clinical functioning (Tracy et al., 2018).
Method
This study received full approval from both the affiliated academic institution, New York University School of Medicine (i12-03054 CR5), and hospital, Bellevue Hospital Center (12-03054), Institutional Review Boards for Human Subject Safety. All human subject safety guidelines were followed.
Both ERPs and later recovery participants (LRPs) were recruited from Bellevue Hospital Center’s Chemical Dependency Clinic by clinician referral and self-referral. LRPs met lifetime diagnosis for a substance use disorder and were at least 6 months abstinent from drugs/alcohol. ERPs met current diagnosis for a substance use disorder and were actively using substances. Those LRPs assigned to MAP acted as mentors, working with ERPs assigned to MAP as the mentees. Interested participants who appeared to meet preliminary eligibility criteria were invited to participate in the study by the research assistants. Individuals who agreed to participate and signed informed consent were screened for entry into the study with the Structured Clinical Interview (SCID-I/P) for the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR), Axis I diagnoses (First et al., 2002); Substance Use Report (Robinson et al., 2014); clinician verification of abstinence or use; and biological verification of use/abstinence using urine toxicology and breathalyzer tests.
Sixty-five eligible participants (17 LRPs and 48 ERPs) were randomized to MAP+TAU or TAU using a computerized Urn Randomization Program, which balanced on the following characteristics: primary substance drug versus alcohol, gender, and presence of court-ordered treatment. ERPs were randomized during the first 30 days of treatment when vulnerability to relapse and attrition are high and continued their participation for the 24-week study period. Within MAP, for each cohort, a pool of 4–5 mentors (LRPs who were randomly assigned to MAP) was formed and engaged in mentoring activities for 24 weeks until 12–13 mentees (ERPs who were randomly assigned to MAP) who were newly admitted participated in MAP for 12 weeks.
Measures
Reliable and valid behavioral/biological measures were conducted at screen, baseline, weekly, monthly, and termination for all participants and during the 12-week follow-up for ERPs. The following measures were included:
Substance use—diagnostic and primary outcome measures.
The Structured Clinical Interview (SCID-I/P) for the DSM-IV-TR, Axis I diagnoses (First et al., 2002), is a semistructured interview used to establish diagnoses of psychiatric and substance use disorders for participants. The reliability and validity of the SCID diagnoses exceed those obtained from a clinical interview (First et al., 2015), and inter-rater reliability has been reported with κs ranging from .70 to 1.00 (Lobbestael et al., 2011; Segal et al., 1994).
The Substance Use Report (Robinson et al., 2014) provides a daily record of substance use by patient self-report. It assesses for the following substances: alcohol, amphetamines, barbiturates, benzodiazepines, cocaine, marijuana (cannabis), methadone, methamphetamines, heroin, and PCP. Using a 7-Day Timeline Followback styled format, the Substance Use Report tracks the use of each substance for the preceding week.
The Urine Toxicology Drug Test used screening of a Triage Panel of Drugs of Abuse (Model TDOA-1144A3; Drug Tests in Bulk, 2012). The detection of the following agents of abuse was of interest: cocaine, amphetamines, barbiturates, benzodiazepines, THC, methadone, heroin, and PCP. Agreement between objective Urine Toxicology Tests and the Substance Use Report was above 90%. As a result, Substance Use Report data were used as a reliable and more comprehensive assessment of participant use.
The Addiction Severity Index (ASI; McLellan et al., 1985) is a standardized semistructured clinical interview that provides problem severity profiles in six domains commonly affected in those who abuse substances. The domains covered within this article are alcohol and drug chemical abuse. Composite scores for each problem domain are derived mathematically by summing the questions within the domain. ASI alcohol and drug composite scores have good internal reliability consistency (alcohol: Cronbach’s α = .76, drug: Cronbach’s α = .89). Higher composite scores indicate greater substance use severity.
Treatment delivery measures.
The Program and Client Cost–Substance Abuse Treatment (Jofre-Bonet et al., 2004) assesses program and client costs of drug/alcohol abuse treatments. The scale obtains information on the total number of times/sessions that the participant received substance abuse and psychological services as well as indicates what was covered in the sessions.
The Medication Adherence Scale (Wu et al., 2008) asks the participant to list current medications for mental health and drug/alcohol use disorder treatment. The participants indicate how regularly they have been taking their medication as prescribed in the last week using a 5-point Likert scale from 1 (rarely or never) to 5 (always). These ratings are done separately for both mental health and drug/alcohol use disorder medication.
The Mentorship Adherence and Competence Scale (Tracy et al., 2000, 2012, 2018) is used to rate the adherence and competence of the mentor’s delivery of MAP during the audio-recorded Introductory Mentorship Pair Meetings. There are 14 items in the scale resulting from the rating of 7 items for both adherence and competence of the mentor’s delivery of the MAP treatment using a Likert scale from 1 (not at all) to 5 (extensively). We conducted Cronbach’s alpha reliability coefficients for the scale in a pilot which indicated good internal consistency of the items. The coefficient α for the full scale of 14 items is .98, 7 adherence items alone .96, and 7 competence items .95.
Treatment conditions
Mentorship for Addiction Problems.
MAP had 4 key components:
(A) Mentorship training: Mentors participated in a 4-week training that met for 1 hour two times per week before providing mentorship. At each session, a urine toxicology drug screen was conducted to ensure abstinence.
The following topics were covered in the 4-week training period: (1) overview of MAP, (2) understanding policy and procedures (e.g., interacting with system), (3) your role as a mentor, (e.g., boundaries), (4) helping your mentee maintain sobriety/modified Goal Attainment Scaling, (5) maintaining your own sobriety in the process, (6) what to do in a crisis (e.g., suicidal ideation, homicidal ideation), (7) managing mental health issues, (8) HIV/ID risk reduction (e.g., learning and teaching mentee risk-reduction skills), and (9) being sensitive to diversity.
(B) Individual pair contact: Mentors provided mentorship to the mentee outside of group for approximately 1–4 hours per week either in person or by phone/text.
Individual pair contact was scheduled by the mentor– mentee pairs directly to meet each of their preferences and availability. The focus of the mentoring is the development of a relationship based on abstinence and the mentor helping the mentee to develop and achieve abstinence goals using harm-reduction strategies that are monitored through modified Goal Attainment Scaling. Abstinence goals may include a wide range of goals (e.g., reduction in substance use, changing social networks, resolving housing issues) seen as contributing to the mentee’s ability to remain abstinent, and achievements/setbacks are recognized incrementally corresponding to the Goal Attainment Scaling ratings.
(C) Mentorship group: Mentors, mentees, and the supervisory clinician participated in a weekly 1-hour group.
The groups are designed to provide a framework for the mentors and mentees to further work on issues outside of group in the Individual Mentorship Contact. Entailed in the group are discussions of development of Goal Attainment Scaling recovery plans, monthly formal mentee presentations of the progress on these plans, and weekly discussions to receive guidance and support from mentors and the other members of the group to achieve goals of these plans. These groups are facilitated by the supervisory clinician and mentors and provide an additional venue for mentors to offer support to mentees as well as another structure for the supervisory clinician to oversee the mentorship process, which is provided directly by the mentors.
(D) Supervision: Supervision was provided by the supervisory clinician on an ongoing basis during the weekly Mentor Supervision Group, which was 1 hour before the Mentorship Group and ad hoc if needed.
The groups are designed to provide an additional venue for mentors and the supervisory clinician to discuss any issues surrounding the mentorship relationship, mentor, or mentee that may require guidance. The supervisory clinician reviews the contact logs to understand the types of activities that are occurring during the individual mentoring contacts and oversees the mentorship relationship during these group meetings to address any areas to improve on.
Treatment as Usual (TAU).
We used Mohr and colleagues’ (2009) recommendations for selection and design of control conditions in randomized trials of psychological interventions. MAP is not designed to replace treatment, but to augment treatment. As a result, we compared TAU to MAP+TAU to see if the addition of MAP improves treatment outcomes. The Bellevue Chemical Dependency Clinic, where the pilot was conducted, offers both intensive treatment (4 or more hours per day 5 days per week) and less intensive treatment (4 or more hours per day 1–3 days per week), as is common in other substance use disorder outpatient clinics across the United States. Patients may upgrade or downgrade to more or less intensive treatment based on their functioning including relapse to use or sustained abstinence. Patients in similar stages of their recovery receive similar daily treatment. Analogous to the preliminary studies and common across substance use disorder clinics, a full range of treatments are offered at the clinic including individual counseling, group counseling, and pharmacotherapy.
To show that TAU was comparable in both treatment conditions, we ran statistical analyses and controlled for any differences (e.g., number of onsite groups attended) should they have arisen in the analyses.
Statistical analysis
This study used intent-to-treat (ITT) analysis. Categorical variables were described using frequencies and percentages, continuous variables using means and standard deviations. Baseline characteristics, including demographic/clinical characteristics and days of drug or alcohol use in the 30 days before the intervention, were compared between treatment conditions using chi-square or Fisher exact tests for categorical variables and t tests or Wilcoxon rank sum test for continuous variables, depending on their distribution. Mixed-effects models were used to assess the difference in substance use and ASI alcohol and drug composite scores over time and between conditions. Substance use was also compared between LRP treatment conditions using mixed-effects models adjusting for age, given the difference in mean age noted between these conditions. However, this analysis was exploratory because of the anticipated limited use by LRPs as a result of their later stage in recovery and smaller sample size (n = 17). In addition, overall days of substance use during the study period (Weeks 1–12) and follow-up period (Weeks 13–24) were compared between conditions using Wilcoxon rank sum tests. Standardized mean differences between treatment conditions were calculated and presented as Glass’s delta, to account for heterogeneity in variance estimates between treatment conditions. All analyses used SAS Version 9.4 (SAS Institute Inc., Cary, NC), and significance was indicated where p < .05.
Results
Demographics
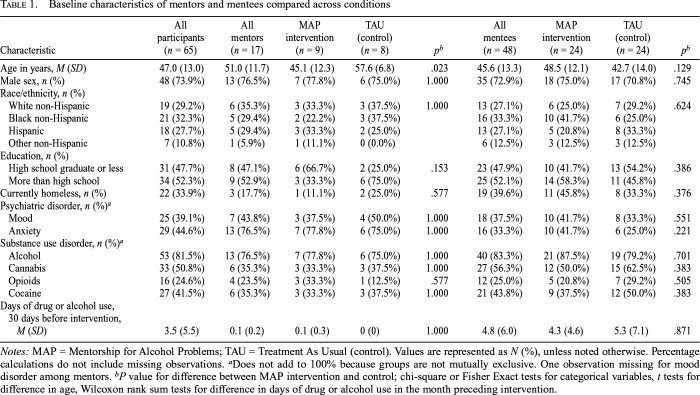
Among ERPs and LRPs combined (n = 65), mean age was 47.0 years (SD = 13.0). The majority were male 48 (73.9%); 21 (32.3%) were Black non-Hispanic, 19 (29.2%) were White non-Hispanic, 18 (27.7%) were Hispanic, and 7 (10.8%) were non-Hispanic other race. There was a broad range of substance use disorders in the overall sample: 53 (81.5%) had alcohol use disorder, 33 (50.8%) cannabis, 27 (41.5%) cocaine, and 16 (24.6%) opioid use disorder. See Table 1 for baseline characteristics among LRPs (n = 17) and ERPs (n = 48) by treatment condition. The distribution of these characteristics, including days of substance use in the month preceding intervention, was similar between conditions for both LRPs and ERPs, with the exception of age among LRPs (MAP intervention: M = 45.1, SD = 12.3; TAU (control condition): M = 57.6, SD = 6.8, p = .023).
Table 1.
Baseline characteristics of mentors and mentees compared across conditions
| Characteristic | All participants (n = 65) | All mentor (n = 17) | MAP intervention (n = 9) | TAU (control) (n = 8) | pb | All mentees (n = 48) | MAP intervention (n = 24) | TAU (control) (n = 24) | pb |
| Age in years, M (SD) | 47.0 (13.0) | 51.0 (11.7) | 45.1 (12.3) | 57.6 (6.8) | .023 | 45.6 (13.3) | 48.5 (12.1) | 42.7 (14.0) | .129 |
| Male sex, n (%) | 48 (73.9%) | 13 (76.5%) | 7 (77.8%) | 6 (75.0%) | 1.000 | 35 (72.9%) | 18 (75.0%) | 17 (70.8%) | .745 |
| Race/ethnicity, n (%) | |||||||||
| White non-Hispanic | 19 (29.2%) | 6 (35.3%) | 3 (33.3%) | 3 (37.5%) | 1.000 | 13 (27.1%) | 6 (25.0%) | 7 (29.2%) | .624 |
| Black non-Hispanic | 21 (32.3%) | 5 (29.4%) | 2 (22.2%) | 3 (37.5%) | 16 (33.3%) | 10 (41.7%) | 6 (25.0%) | ||
| Hispanic | 18 (27.7%) | 5 (29.4%) | 3 (33.3%) | 2 (25.0%) | 13 (27.1%) | 5 (20.8%) | 8 (33.3%) | ||
| Other non-Hispanic | 7 (10.8%) | 1 (5.9%) | 1 (11.1%) | 0 (0.0%) | 6 (12.5%) | 3 (12.5%) | 3 (12.5%) | ||
| Education, n (%) | |||||||||
| High school graduate or less | 31 (47.7%) | 8 (47.1%) | 6 (66.7%) | 2 (25.0%) | .153 | 23 (47.9%) | 10 (41.7%) | 13 (54.2%) | .386 |
| More than high school | 34 (52.3%) | 9 (52.9%) | 3 (33.3%) | 6 (75.0%) | 25 (52.1%) | 14 (58.3%) | 11 (45.8%) | ||
| Currently homeless, n (%) | 22 (33.9%) | 3 (17.7%) | 1 (11.1%) | 2 (25.0%) | .577 | 19 (39.6%) | 11 (45.8%) | 8 (33.3%) | .376 |
| Psychiatric disorder, n (%)a | |||||||||
| Mood | 25 (39.1%) | 7 (43.8%) | 3 (37.5%) | 4 (50.0%) | 1.000 | 18 (37.5%) | 10 (41.7%) | 8 (33.3%) | .551 |
| Anxiety | 29 (44.6%) | 13 (76.5%) | 7 (77.8%) | 6 (75.0%) | 1.000 | 16 (33.3%) | 10 (41.7%) | 6 (25.0%) | .221 |
| Substance use disorder, n (%)a | |||||||||
| Alcohol | 53 (81.5%) | 13 (76.5%) | 7 (77.8%) | 6 (75.0%) | 1.000 | 40 (83.3%) | 21 (87.5%) | 19 (79.2%) | .701 |
| Cannabis | 33 (50.8%) | 6 (35.3%) | 3 (33.3%) | 3 (37.5%) | 1.000 | 27 (56.3%) | 12 (50.0%) | 15 (62.5%) | .383 |
| Opioids | 16 (24.6%) | 4 (23.5%) | 3 (33.3%) | 1 (12.5%) | .577 | 12 (25.0%) | 5 (20.8%) | 7 (29.2%) | .505 |
| Cocaine | 27 (41.5%) | 6 (35.3%) | 3 (33.3%) | 3 (37.5%) | 1.000 | 21 (43.8%) | 9 (37.5%) | 12 (50.0%) | .383 |
| Days of drug or alcohol use, 30 days before intervention, M (SD) | 3.5 (5.5) | 0.1 (0.2) | 0.1 (0.3) | 0 (0) | 1.000 | 4.8 (6.0) | 4.3 (4.6) | 5.3 (7.1) | .871 |
Notes: MAP = Mentorship for Alcohol Problems; TAU = Treatment As Usual (control). Values are represented as N (%), unless noted otherwise. Percentage calculations do not include missing observations.
Does not add to 100% because groups are not mutually exclusive. One observation missing for mood disorder among mentors.
P value for difference between MAP intervention and control; chi-square or Fisher Exact tests for categorical variables, t tests for difference in age, Wilcoxon rank sum tests for difference in days of drug or alcohol use in the month preceding intervention.
Participant flow/interest
Interest in MAP was high. Seventy-five patients were approached to participate in the MAP program, and 65 (87%) signed informed consent. Of the patients excluded from the study, 5 (7%) were lost to follow-up, 2 (3%) did not meet the required length of time of sobriety, 2 (3%) had conflicting medical conditions, and 1 (1%) displayed historic episodes of violence.
Attrition
Of the 65 participants who entered the study, only 9 dropped out, resulting in an overall retention rate of 86.2%, which is noteworthy because of the typically high rates of attrition in substance use disorder treatment. No LRPs dropped out of the study. If in active treatment as a mentor and relapse occurred, mentors were removed from mentoring and were given the opportunity to participate at mentee status. However, if this occurred, their relapse was still considered as a mentor relapse within the statistical analyses to be consistent with how they entered the study.
TAU similarity
The standard treatment (TAU) was comparable in both conditions. There were no significant differences between conditions on the Program and Client Cost–Substance Abuse Treatment (Jofre-Bonet et al., 2004) or on the Medication Adherence Scale (Wu et al., 2008) to confound the outcomes.
MAP fidelity
The Mentorship Adherence and Competence Scale (Tracy et al., 2000, 2012, 2018) was used to rate the adherence and competence of the mentor’s delivery of MAP during the audio recorded Introductory Mentorship Pair Meetings. Mentors adhered to the delivery of the MAP treatment. On a Likert scale rating adherence and competence from 1 (not at all) to 5 (extensively), overall mean was M = 4.15, SD = 0.78, for adherence M = 4.06, SD = 0.75, and for competence M = 4.24, SD = 0.83.
Primary outcome: Substance use
Early recovery participants.
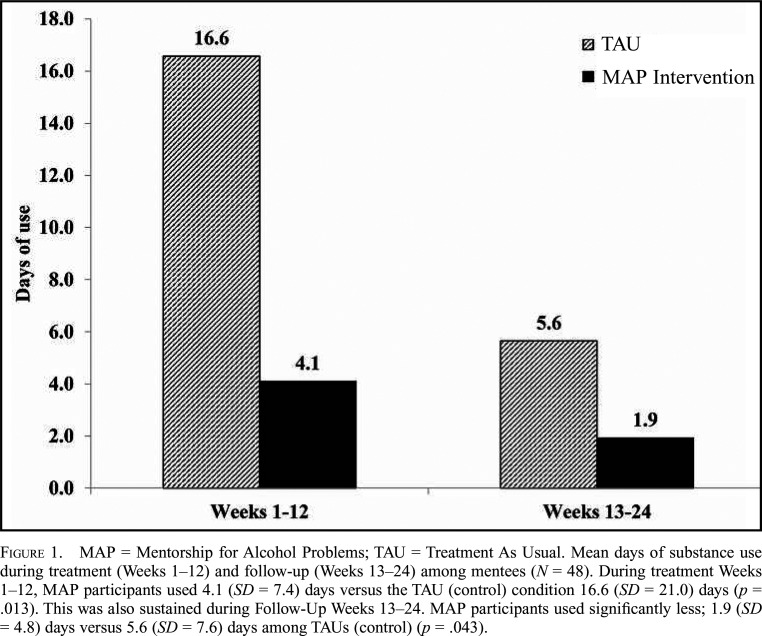
The primary outcome of weekly substance use (heroin, cocaine, or alcohol) declined across both conditions for ERPs (n = 48) over the 0- to 12-week study period, b = -0.06, SE = 0.02, t(413) = -3.42, p = .001. However, the difference in mean days weekly substance use was statistically significant during the 0- to 12-week study period, with significantly less weekly substance use on average among ERPs in the MAP+TAU intervention versus TAU, b = -0.88, SE = 0.41, t(425) = -2.11, p = .035. Looking more critically at days of substance use during Treatment Weeks 1–12, we found that MAP participants used 4.1 (SD = 7.3) days versus the TAU/control condition 16.6 (SD = 21.0) days, Z(1) = 2.50, p = .013; Glass’s D = .6. This was also sustained during Follow-Up Weeks 13–24; MAP participants used significantly less compared with control participants, 1.9 (SD = 4.8) days versus 5.6 (SD = 7.4) days, Z(1) =-2.03, p = .043; Glass’s Δ = .5. See Figure 1 for significant differences in mean days substance use during the study period and follow up period for ERPs.
Figure 1.
MAP = Mentorship for Alcohol Problems; TAU = Treatment As Usual. Mean days of substance use during treatment (Weeks 1–12) and follow-up (Weeks 13–24) among mentees (N = 48). During treatment Weeks 1–12, MAP participants used 4.1 (SD = 7.4) days versus the TAU (control) condition 16.6 (SD = 21.0) days (p = .013). This was also sustained during Follow-Up Weeks 13–24. MAP participants used significantly less; 1.9 (SD = 4.8) days versus 5.6 (SD = 7.6) days among TAUs (control) (p = .043).
In addition, ASI alcohol and drug use severity was examined as a primary outcome during active treatment and follow-up. Both the ASI alcohol use composite scores and drug use composite scores had different rates of change between treatment conditions during Follow-Up Weeks 13–24 (for interaction term between treatment condition and time; alcohol: b = -0.08, SE = 0.03, t(47) = -2.97, p = .005; drug use: b = -0.02, SE = 0.01, t(47) = -2.36, p = .023), with alcohol and drug use composite scores increasing in the TAU condition and decreasing in the MAP intervention condition for ERPs during that period.
Later recovery participants.
Given the relatively small sample size of LRPs due to the nature of the treatment, LRP analyses were only exploratory. There was no significant difference in substance use between LRP treatment conditions. However, mean weekly substance use was lower in the LRP MAP versus TAU condition during Study Weeks 0–12, b = -0.44, SE = 0.38, t(175) = -1.17, p = .245, and during Follow-Up Weeks 13–24, b = -0.44, SE = 0.42, t(155) = -1.05, p = .293, adjusting for age. The former reported substance use 0.3 (SD = 0.5) days versus the TAU condition use of 4.4 (SD = 10.4) days, Z(1) = 0.51, p = .609; Glass’s Δ = .4, during Weeks 1–12 and 0.3 (SD = 0.5) days versus 5.0 (SD = 10.7) days, Z(1) = 0.28, p = .782; Glass’s Δ = .4, during Weeks 13–24.
Discussion
Substance use disorders and their consequences remain a significant problem within our society, yet only a small percentage of those with substance use disorders seek treatment. Developing addiction treatments that are relatable for patients through identifiable shared experiences from those delivering the intervention that also build on natural occurrences in relationships, as in mentorship interventions, offers unique advantages to overcoming barriers to care.
Mentorship for Addiction Problems (MAP) is a recently developed intervention that aims to reduce substance use through the novel application of peer-driven Goal Attainment Scaling specific to each individual’s needs, creating high appeal by starting during the critical first month of treatment when relapse and attrition are high, and support is needed most. This article presented our primary outcomes related to substance use for our Stage Ib pilot study. MAP was found not only to be associated with significantly less substance use compared with standard treatment as usual alone, but also to have a positive impact on patient interest and retention, known to be serious problems undercutting addiction treatment efficacy. Patient interest and retention are necessities for Stage Ib successful treatment development, in addition to the high acceptance for its delivery and integration within the clinic from staff, which was consistent with our promising Stage Ia results previously published (Tracy et al., 2012). Also similar to our Stage Ia results, there were high levels of treatment fidelity in delivering MAP.
LRPs are uniquely qualified to enhance recovery through the provided social support and connections. The shared life experiences make them valuable resources for those starting the recovery journey (Bassuk et al., 2016; Resnick & Rosenheck, 2008; Tracy & Wallace, 2016). Mentorship training provides skills enabling mentors to feel comfortable and knowledgeable on troubleshooting possible psychiatric symptoms, substance use, and other issues in recovery, with added benefit of techniques learned through similar experiences.
MAP is an innovative approach drawing on natural experiences of relationship building and social support to achieve positive outcomes. The manualized treatment is accessible and easily implemented within a wide variety of treatment settings. The training elements allow for mentorship delivery with little modification applicable to most established treatment programs through its flexibility to engage patients at all levels of recovery and a focus on achieving individually designed goals. In addition, MAP has promising advantages for treating drug and alcohol use disorders whereby support may be provided by mentors who are not paid but participating as a way to sustain their own recovery by helping others, which is relatively cost efficient and requires minimal involvement from supervisory staff.
Similar to other Stage I studies, there are inherent limitations because of sample size, which may affect ability to detect statistically significant differences between groups. Additional studies can also be beneficial in understanding the impact of MAP as compared with other adjunctive treatments, as the presence of other interventions may similarly improve treatment outcomes. Based on our promising results, future sufficiently powered Stage II efficacy studies are recommended to substantiate these findings.
Conflict-of-Interest Statement
There are no conflicts of interest within this study.
Acknowledgments
We thank the patients and staff at Bellevue Hospital Center who participated in the running of this project. In addition, we thank the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism, because without their support for the Stage Ia (R01AA016160) and this Stage Ib (R34DA034898) studies this work would not have been made possible. We also acknowledge the loss of Bruce Rounsaville, M.D., who was a part of the initial development of this treatment. Although time has passed since his departing, he remains present in our memories as a mentor, colleague, and friend.
Footnotes
This work was supported by the National Institute on Drug Abuse Grant R34DA034898 and National Institute on Alcohol Abuse and Alcoholism Grant R01AA016160.
References
- Azrin N. H. Improvements in the community-reinforcement approach to alcoholism. Behaviour Research and Therapy. 1976;14:339–348. doi: 10.1016/0005-7967(76)90021-8. doi:10.1016/0005-7967(76)90021-8. [DOI] [PubMed] [Google Scholar]
- Bassuk E. L., Hanson J., Greene R. N., Richard M., Laudet A. Peer-delivered recovery support services for addictions in the United States: A systematic review. Journal of Substance Abuse Treatment. 2016;63:1–9. doi: 10.1016/j.jsat.2016.01.003. doi:10.1016/j.jsat.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Birtel M. D., Wood L., Kempa N. J. Stigma and social support in substance abuse: Implications for mental health and well-being. Psychiatry Research. 2017;252:1–8. doi: 10.1016/j.psychres.2017.01.097. doi:10.1016/j.psychres.2017.01.097. [DOI] [PubMed] [Google Scholar]
- Campbell A. N. C., Turrigiano E., Moore M., Miele G. M., Rieckmann T., Hu M.-C., Nunes E. V. Acceptability of a web-based community reinforcement approach for substance use disorders with treatment-seeking American Indians/Alaska Natives. Community Mental Health Journal. 2015;51:393–403. doi: 10.1007/s10597-014-9764-1. doi:10.1007/s10597-014-9764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health: Detailed tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- Centers for Disease Control and Prevention. Prevention. CDC grand rounds: Prescription drug overdoses - a U.S. epidemic. Morbidity and Mortality Weekly Report. 2012;61:10–13. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6101a3.htm. [PubMed] [Google Scholar]
- Darling N., Hamilton S., Toyokawa T., Matsuda S. Naturally occurring mentoring in Japan and the United States: Social roles and correlates. American Journal of Community Psychology. 2002;30:245–270. doi: 10.1023/A:1014684928461. doi:10.1023/A:1014684928461. [DOI] [PubMed] [Google Scholar]
- De Cock K. M., Jaffe H. W., Curran J. W. The evolving epidemiology of HIV/AIDS. AIDS. 2012;26:1205–1213. doi: 10.1097/QAD.0b013e328354622a. doi:10.1097/QAD.0b013e328354622a. [DOI] [PubMed] [Google Scholar]
- DuBois D. L., Silverthorn N. Natural mentoring relationships and adolescent health: Evidence from a national study. American Journal of Public Health. 2005;95:518–524. doi: 10.2105/AJPH.2003.031476. doi:10.2105/AJPH.2003.031476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. W. Structured Clinical Interview for DSM-IV-TR axis 1 disorders, research version. Patient Edition. SCID- I/P); New York, NY: Biometrics Research; 2002. [Google Scholar]
- First M. B., Williams J. B. W., Karg R. L., Spitzer R. L. Structured Clinical Interview for DSM-5- Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV) Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- Freedman M. The kindness of strangers: Reflections on the mentoring movement. Philadelphia, PA: Public/Private Ventures; 1991. [Google Scholar]
- Galanter M., Dermatis H., Egelko S., De Leon G. Homelessness and mental illness in a professional- and peer-led cocaine treatment clinic. Psychiatric Services. 1998;49:533–535. doi: 10.1176/ps.49.4.533. doi:10.1176/ps.49.4.533. [DOI] [PubMed] [Google Scholar]
- Guida F., De Leon G., Monahan K. Measuring peer interaction in the therapeutic community. Presentation at the American Psychological Association Convention; Chicago, Illinois: 2002. [Google Scholar]
- Hai A. H., Franklin C., Park S., DiNitto D. M., Aurelio N. The efficacy of spiritual/religious interventions for substance use problems: A systematic review and meta-analysis of randomized controlled trials. Drug and Alcohol Dependence. 2019;202:134–148. doi: 10.1016/j.drugalcdep.2019.04.045. doi:10.1016/j.drugalcdep.2019.04.045. [DOI] [PubMed] [Google Scholar]
- Harris J., Best D., Gossop M., Marshall J., Man L.-H., Manning V., Strang J. Prior Alcoholics Anonymous (AA) affiliation and the acceptability of the Twelve Steps to patients entering UK statutory addiction treatment. Journal of Studies on Alcohol. 2003;64:257–261. doi: 10.15288/jsa.2003.64.257. doi:10.15288/jsa.2003.64.257. [DOI] [PubMed] [Google Scholar]
- Heaney C. A., Israel B. A. Social networks and social support. In: Glanz K K., Rimer B. K., Lewis F. M., editors. Health behavior and health education: Theory, research, and practice. 3rd ed. San Francisco, CA: Jossey-Bass; 2002. pp. 185–209. [Google Scholar]
- Helseth S. A., Janssen T., Scott K., Squires D. D., Becker S. J. Training community-based treatment providers to implement contingency management for opioid addiction: Time to and frequency of adoption. Journal of Substance Abuse Treatment. 2018;95:26–34. doi: 10.1016/j.jsat.2018.09.004. doi:10.1016/j.jsat.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. T., Wong C. J., Badger G. J., Ogden D. E., Dantona R. L. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. doi:10.1037/0022-006X.68.1.64. [DOI] [PubMed] [Google Scholar]
- Huselid R. F., Self E. A., Gutierres S. E. Predictors of successful completion of a halfway-house program for chemically-dependent women. American Journal of Drug and Alcohol Abuse. 1991;17:89–101. doi: 10.3109/00952999108992812. doi:10.3109/00952999108992812. [DOI] [PubMed] [Google Scholar]
- Jenkins S. E. USFSP Honors Program Theses (Undergraduate) 2015. Are peer specialists happy? How training and role clarity affect job satisfaction; p. 213. Retrieved from http://digital.usfsp.edu/honorstheses/213. [Google Scholar]
- Jofre-Bonet M., Sindelar J. L., Petrakis I. L., Nich C., Frankforter T., Rounsaville B. J., Carroll K. M. Cost effectiveness of disulfiram: Treating cocaine use in methadone-maintained patients. Journal of Substance Abuse Treatment. 2004;26:225–232. doi: 10.1016/S0740-5472(04)00004-2. doi:10.1016/S0740-5472(04)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin A. E. Annual research review: Expanding mental health services through novel models of intervention delivery. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2019;60:455–472. doi: 10.1111/jcpp.12937. doi:10.1111/jcpp.12937. [DOI] [PubMed] [Google Scholar]
- Kelly J. F., Hoffman L., Vilsaint C., Weiss R., Nierenberg A., Hoeppner B. Peer support for mood disorder: Characteristics and benefits from attending the Depression and Bipolar Support Alliance mutual-help organization. Journal of Affective Disorders. 2019;255:127–135. doi: 10.1016/j.jad.2019.05.039. doi:10.1016/j.jad.2019.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek K. D., Murphy S. L., Xu J., Arias E. Deaths: Final data for 2017. National Vital Statistics Reports. 2019;68:1–77. [PubMed] [Google Scholar]
- Lobbestael J., Leurgans M., Arntz A. Inter-rater reliability of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID I) and Axis II Disorders (SCID II) Clinical Psychology & Psychotherapy. 2011;18:75–79. doi: 10.1002/cpp.693. doi:10.1002/cpp.693. [DOI] [PubMed] [Google Scholar]
- McLellan A. T., Luborsky L., Cacciola J., Griffith J., Evans F., Barr H. L., O’Brien C. P. New data from the Addiction Severity Index. Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. doi:10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Mericle A. A., Miles J., Way F. Recovery residences and providing safe and supportive housing for individuals overcoming addiction. Journal of Drug Issues. 2015;45:368–384. doi:10.1177/0022042615602924. [Google Scholar]
- Meyers R. J., Miller W. R., editors. A community reinforcement approach to addiction treatment. Cambridge, England: Cambridge University Press; 2001. [Google Scholar]
- Meyers R. J., Miller W. R., Smith J. E., Tonigan J. S. A randomized trial of two methods for engaging treatment-refusing drug users through concerned significant others. Journal of Consulting and Clinical Psychology. 2002;70:1182–1185. doi:10.1037/0022-006X.70.5.1182. [PubMed] [Google Scholar]
- Miller W. R., Meyers R. J., Hiller-Sturmhöfel S. The community-reinforcement approach. Alcohol Health and Research World. 1999a;23:116–121. [PMC free article] [PubMed] [Google Scholar]
- Miller W. R., Meyers R. J., Tonigan J. S. Engaging the unmotivated in treatment for alcohol problems: A comparison of three strategies for intervention through family members. Journal of Consulting and Clinical Psychology. 1999b;67:688–697. doi: 10.1037//0022-006x.67.5.688. doi:10.1037/0022-006X.67.5.688. [DOI] [PubMed] [Google Scholar]
- Drug Tests in Bulk. Model TDOA-1144A3, Fourteen panel All-In-One-T-Cup drug test.West Hills, California. 2012. [Google Scholar]
- Mohr D. C., Spring B., Freedland K. E., Beckner V., Arean P., Hollon S. D., Kaplan R. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychotherapy and Psychosomatics. 2009;78:275–284. doi: 10.1159/000228248. doi:10.1159/000228248. [DOI] [PubMed] [Google Scholar]
- Naslund J. A., Aschbrenner K. A., Marsch L. A., Bartels S. J. The future of mental health care: Peer-to-peer support and social media. Epidemiology and Psychiatric Sciences. 2016;25:113–122. doi: 10.1017/S2045796015001067. doi:10.1017/S2045796015001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (NIDA) Trends & statistics. 2016. Retrieved from http://www.drugabuse.gov/related-topics/trends-statistics#costs. [Google Scholar]
- Project MATCH Research Group. Project MATCH secondary a priori hypotheses. Addiction. 1997;92:1671–1698. doi:10.1111/j.1360-0443.1997.tb02889.x. [PubMed] [Google Scholar]
- Rebeiro Gruhl K. L., LaCarte S., Calixte S. Authentic peer support work: Challenges and opportunities for an evolving occupation. Journal of Mental Health. 2016;25:78–86. doi: 10.3109/09638237.2015.1057322. doi:10.3109/09638237.2015.1057322. [DOI] [PubMed] [Google Scholar]
- Resnick S. G., Rosenheck R. A. Integrating peer-provided services: A quasi-experimental study of recovery orientation, confidence, and empowerment. Psychiatric Services. 2008;59:1307–1314. doi: 10.1176/ps.2008.59.11.1307. doi:10.1176/ps.2008.59.11.1307. [DOI] [PubMed] [Google Scholar]
- Robinson S. M., Sobell L. C., Sobell M. B., Leo G. I. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors. 2014;28:154–162. doi: 10.1037/a0030992. doi:10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Rounsaville B. J., Carroll K. M., Onken L. S. A stage model of behavioral therapies research: Getting started and moving on from Stage 1. Clinical Psychology: Science and Practice. 2001;8:133–142. doi:10.1093/clipsy.8.2.133. [Google Scholar]
- Segal D. L., Hersen M., Van Hasselt V. B. Reliability of the Structured Clinical Interview for DSM-III-R: An evaluative review. Comprehensive Psychiatry. 1994;35:316–327. doi: 10.1016/0010-440x(94)90025-6. doi:10.1016/0010-440X(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Silverman K., Svikis D., Robles E., Stitzer M. L., Bigelow G. E. A reinforcement-based therapeutic workplace for the treatment of drug abuse: Six-month abstinence outcomes. Experimental and Clinical Psychopharmacology. 2001;9:14–23. doi: 10.1037/1064-1297.9.1.14. doi:10.1037/1064-1297.9.1.14. [DOI] [PubMed] [Google Scholar]
- Stitzer M. L., Bickel W. K., Bigelow G. E., Liebson I. A. Effect of methadone dose contingencies on urinalysis test results of polydrug-abusing methadone-maintenance patients. Drug and Alcohol Dependence. 1986;18:341–348. doi: 10.1016/0376-8716(86)90097-9. doi:10.1016/0376-8716(86)90097-9. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54) Rockville, MD: Author; 2019. Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- Tonigan J. S., Miller W. R., Schermer C. Atheists, agnostics and Alcoholics Anonymous. Journal of Studies on Alcohol. 2002;63:534–541. doi: 10.15288/jsa.2002.63.534. doi:10.15288/jsa.2002.63.534. [DOI] [PubMed] [Google Scholar]
- Tracy K., Baker S., LoCastro J., Mezinskis J., Simon S., Somoza E. National Institute on Drug Abuse Monograph 180, NIH Publication No. 00-4737. 2000. The Substance Clinical Global Impression (SCGI) Scale: Measuring global functioning in substance related clinical trials [Abstract] p. 169. Retrieved from https://archives.drugabuse.gov/sites/default/files/180.pdf. [Google Scholar]
- Tracy K., Burton M., Miescher A., Galanter M., Babuscio T., Frankforter T., Rounsaville B. Mentorship for Alcohol Problems (MAP): A peer to peer modular intervention for outpatients. Alcohol and Alcoholism. 2012;47:42–47. doi: 10.1093/alcalc/agr136. doi:10.1093/alcalc/agr136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy K., Burton M., Miescher A., Trujillo E., Galanter M., Meyers R., et al. It takes two: Teamwork set to improve recovery from alcohol abuse. International Innovation: Healthcare. 2010;4:94–96. [Google Scholar]
- Tracy K, Wachtel L.K, Goldmann E. Beyond substance use reduction: The positive impact of Mentorship for Addiction Problems (MAP) Journal of Alcoholism, Drug Abuse and Substance Dependence. 2018;4 doi:10.24966/ADSD-9594/100038. [Google Scholar]
- Tracy K., Wallace S. P. Benefits of peer support groups in the treatment of addiction. Substance Abuse and Rehabilitation. 2016;7:143–154. doi: 10.2147/SAR.S81535. doi:10.2147/SAR.S81535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters G. D. Lessons learned from Project MATCH. Addictive Disorders & Their Treatment. 2002;1:135–139. doi:10.1097/00132576200211000-00004. [Google Scholar]
- Westreich L., Galanter M., Lifshutz H., Metzger E. J., Silberstein C. A modified therapeutic community for the dually diagnosed Greenhouse Program at Bellevue Hospital. Journal of Substance Abuse Treatment. 1996;13:533–536. doi: 10.1016/s0740-5472(96)00110-9. doi:10.1016/S0740-5472(96)00110-9. [DOI] [PubMed] [Google Scholar]
- White W. L. Recovery coaching: A lost function of addiction counseling? Counselor. 2004;5:20–22. [Google Scholar]
- White W. L. Sponsor, recovery coach, addiction counselor: The importance of role clarity and role integrity. Philadelphia, PA: Philadelphia Department of Behavioral Health and Mental Retardation Services; 2006. [Google Scholar]
- White W. L., Evans A. C., Jr.2014The recovery agenda: The shared role of peers and professionals Public Health Reviews 351–15.25642015 [Google Scholar]
- Wu J.-R., Chung M., Lennie T. A., Hall L. A., Moser D. K. Testing the psychometric properties of the Medication Adherence Scale in patients with heart failure. Heart & Lung: The Journal of Critical Care. 2008;37:334–343. doi: 10.1016/j.hrtlng.2007.10.001. doi:10.1016/j.hrtlng.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]