Abstract
Evidences have suggested that Sjogren's syndrome (SS) is associated with viral infection. The aim of this study was to investigate the involvement of respiratory viral poly(I:C) in the pathogenesis of SS and potential mechanisms using a SS-like NOD/ShiLtJ (NOD) mouse model. 5-week female NOD mice were intratracheally administered poly(I:C) every other day for 5 times to mimic viral infection. Pilocarpine induced saliva secretion was determined every 8 days. Submandibular glands (SMG) and lungs were harvested for the detection of pathological changes. We found that intratracheal administration of poly(I:C) significantly advanced and enhanced the reduction of saliva flow rate in NOD mice. Furthermore, poly(I:C) treatment aggravated the histopathological lesions and inflammatory cells infiltration in SMG. Accompanied by elevated expression of IFN cytokines and IL-33, Th1 activation was enhanced in SMG of poly(I:C)-treated NOD mice, but Th17 cells activation was unchanged among the groups. In addition, intratracheal poly(I:C) exposure promoted the expression of IL-33 and increased T cells proportion in the lung, which were consistent with the change in SMG. Therefore, intratracheal poly(I:C) exposure aggravated the immunological and function disorder of SMG in NOD mice.
Keywords: Sjogren's syndrome, poly(I:C), salivary gland, immune response, IL-33
Introduction
Sjogren's syndrome (SS) is one of the most common rheumatic diseases characterized by chronic inflammation of the exocrine glands, especially salivary and lacrimal glands. Lymphocytic infiltration in the salivary glands usually leads to defective glandular function (1, 2). The prevalence of primary SS is 0.29 to 0.77% in Chinese population (3). Systemic manifestations involving the lung, kidneys, skin and blood systems (4), and the increased risk of B-cell lymphoma (5) are the main causes of poor prognosis and death.
In susceptible individuals, environmental triggers activate the innate immune system (mainly type I interferon (IFN) signature) representing the first stage of SS pathogenesis (6). The stimulus for the activation of type I IFN system in the salivary glands of SS has long been researched (7). Epstein-Barr virus (EBV) encoded small RNA combined with La/SSB from apoptotic salivary gland epithelial cell led to the type I IFN expression via the endosomal RNA sensor TLR3 (5, 8). Numerous independent studies have tested the infections of hepatitis C virus (9), retroviruses and respiratory tract virus [Coxsackie A virus (10, 11), H1N1 vaccination (12), split-virion influenza viral antigens (13)] in patients with SS. Animal research showed an upregulated expression of TLR3 and type I IFN in submandibular glands and SS-like sialadenitis in NZB/WF1 mice after intraperitoneal administration of poly(I:C) (14, 15). Zhou et al. found that intraperitoneal poly(I:C) treatment resulted in pathology of SS-like dacryoadenitis in non-autoimmune-prone C57BL/6 mice (16). These data suggest that SS is associated with viral infection. However, it is unclear the role and potential mechanisms of respiratory tract virus infection in the alteration of glandular function.
Polyinosinic: polycytidylic acid [poly(I:C)], a synthetic double stranded RNA, sensed by TLR3, has been widely used to mimic virus infection (17). Poly(I:C) stimulation could induce the release of IL-33 in other conditions (18, 19), which has been reported to be increased and acts with IL-12 and IL-23 to favor the secretion of IFN-γ in SS (20). The NOD/ShiLtJ (NOD) mouse model, spontaneously developing SS-like symptoms, is widely used for investigating SS (21). The earliest incidence of sialadenitis in submandibular glands (SMG) of NOD mice occurs at 6 to 7 weeks, while elevated blood glucose mainly occurs after 15 weeks (22). In this study, we found that intratracheal stimulation of poly(I:C) aggravated salivary gland dysfunction in spontaneous SS-like NOD mice. IFN cytokines and T cell chemokines were upregulated, along with an increased expression of IL-33 in salivary gland. Interestingly, poly(I:C) exposure also increased the IL-33 expression and T cells proportion in the lung. These data suggest that intratracheal poly(I:C) exposure aggravated the immunological and function disorder of SMG to promote SS-like progression.
Materials and Methods
Mice
This study was performed in compliance with the guidelines of Institutional Animal Care and Use Committee (IACUC) at Tongji Hospital (Wuhan, China). Five-week-old female NOD/ShiLtj (NOD) mice were purchased from Hua Fu Kang Bioscience company (Beijing, China) and allowed to maintain in the specific pathogen-free facility. The anesthetized NOD mice were in a hypsokinesis of head and vertical position, the tongue of mice was gently fixed to expose the root, 20 μl sterile PBS or poly (I:C) (1 mg/ml) was inhaled into the lung through the airway with a micropipette auxiliary. Poly(I:C) (InvivoGen Corp, San Diego, CA, USA) was administered each time with 20 μg on day 0, 2, 4, 6, 8. The sterile PBS-treated mice and untreated mice were used as controls. Pilocarpine (Abcam Corp, Cambridge, UK) (1 mg/ml) induced saliva volume was determined every 8 days on day 0, 9, 16, 24 and 32. SMG and lungs were harvested on the 52th day for further detection.
Saliva Flow Rate Measurement
Saliva flow rate was detected as previously described (23). Briefly, NOD mice were anesthetized and then intraperitoneally injected with pilocarpine (Sigma-Aldrich) of 5 mg/kg body weight. Total saliva was collected from the oral cavity for 10 min after pilocarpine stimulation, and then quantified as the volume. The saliva flow rate was presented as saliva volume to body weight for each individual.
Gene Expression Analysis
Quantitative real-time PCR was used to determine the gene expression levels in SMG and lung. Total RNA was obtained from tissues and then reverse transcribed to cDNA by RevertAid First Strand cDNA Synthesis Kit (Thermo Corp, Waltham, MA, USA) according to the manufacturer's instruction. The expression of IFN-α, IFN-β, IFN-γ, IFN-λ, TNF-α, IL-33, IL-17A, CXCL9, CXCL10, CXCL11, CXCL13 and β-actin (Primer from TsingKe Corp, Wuhan, China) (Table 1) was determined by SYBRGreen quantitative real-time PCR (TOYOBO Corp, Osaka-Shi, Japan). β-actin housekeeping gene was used as a control. The relative gene expression was calculated by using the 2∧ (−ΔΔCt) method.
Table 1.
The primer sequences of the genes.
| Gene | Primer sequences | Tm values |
|---|---|---|
| IL-33 | Forward TTCCAACTCCAAGATTTCCCC Reverse CAGAACGGAGTCTCATGCAG |
57.83 58.36 |
| IFN-α | Forward AGCGCCGTCTAAGATTCATGT Reverse CGTCGTGGCAATTCTAGTGG |
59.86 59.00 |
| IFN-β | Forward TCAGAATGAGTGGTGGTTGC Reverse GACCTTTCAAATGCAGTAGATTCA |
58.10 57.50 |
| IFN-γ | Forward CTTTGGACCCTCTGACTTGAG Reverse TCAATGACTGTGCCGTGG |
57.95 57.62 |
| IFN-λ | Forward AGCTGCAGGCCTTCAAAAAG Reverse TGGGAGTGAATGTGGCTCAG |
59.32 59.67 |
| CXCL9 | Forward AGTCCGCTGTTCTTTTCCTC Reverse TGAGGTCTTTGAGGGATTTGTAG | 57.83 57.83 |
| CXCL10 | Forward TCAGCACCATGAACCCAAG Reverse CTATGGCCCTCATTCTCACTG |
57.66 57.88 |
| CXCL11 | Forward ATGGCAGAGATCGAGAAAGC Reverse TGCATTATGAGGCGAGCTTG |
57.76 58.70 |
| CXCL13 | Forward AGATCGGATTCAAGTTACGCC Reverse ACAGACTTTTGCTTTGGACATG |
58.18 57.69 |
| IL-17A | Forward AGGCCCTCAGACTACCTCAACC Reverse GCCTCTGAATCCACATTCCTT |
63.16 57.99 |
| TNF-α | Forward CATCTTCTCAAAATTCGAGTGACAA Reverse TGGGAGTAGACAAGGTACAACCC |
58.11 61.33 |
| β-actin | Forward GGTCAGAAGGACTCCTATGTGG Reverse TGTCGTCCCAGTTGGTAACA |
59.57 58.88 |
Histological Analysis and Immunohistochemistry
SMG and lung tissues were collected in 4% paraformaldehyde and embedded in paraffin. Paraffin-embedded tissue sections (5 μm) were stained with hematoxylin and eosin (H&E) staining, an aggregation of inflammatory cells >50 was considered as a foci in SMG. The cross-sectional characteristics of SMG, quantified by focus scores and the proportion of inflammatory cells aggregation area to total SMG area, were evaluated. The severity of tissue damage was evaluated by a scoring system based on the degree of lymphoepithelial lesions (LELs) (24). Standard immunohistochemical (IHC) staining was conducted to evaluate the expression of CD3 in the lung tissues with anti-CD3 monoclonal antibody (1:200 dilution, Servicebio Corp, Wuhan, China), and IL-33 in SMG and lung tissues with anti-IL-33 polyclonal antibody (1:800 dilution, R&D systems, Minneapolis, MN, USA). The sections were analyzed by ImageJ software. Three slides from each tissue were screened by two pathologists who were blind to the group inflammation.
Statistical Analysis
All data were presented as mean ± standard error of the mean (SEM). One-way ANOVA analysis was used for multi-group test after passing the normality tests (Shapiro-Wilk tests). SPSS software (version 19.0) was used for statistic analysis, and a value of p < 0.05 was considered as statistically significant.
Results
Poly(I:C) Intratracheal Administration Enhanced the Reduction of Saliva Flow Rate in NOD Mice
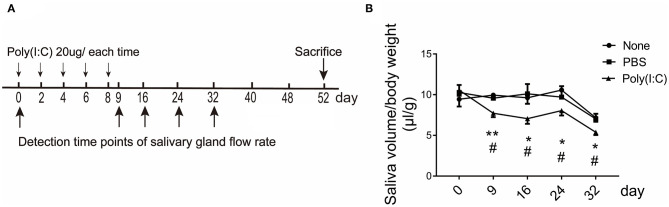
To examine whether intratracheally administered poly(I:C) has an effect on salivary glands function, NOD mice were repeatedly administered poly(I:C) intratracheally, and pilocarpine-induced saliva volume was determined at day 0 (5-week mice) and following every 8 days. The mice were sacrificed at day 52 (12-week mice), as SMG infiltration was obvious and without occurrence of diabetes at 12 weeks of age (Figure 1A and Supplementary Figure 1). As shown in Figure 1B, poly(I:C)-treated mice had a significant reduction in the saliva volume from the early stage until 32 days compared with the untreated and PBS-treated mice, the saliva flow rates of the control groups were normal until 24 days. There was no weight loss and mortality appeared among the groups. The above data suggest that poly(I:C) treatment leads to an advance of the onset of SS-like symptom.
Figure 1.
The saliva flow rate in NOD mice intratracheally treated with poly(I:C). (A) Administration regimen for the induction of poly(I:C)-treated mice. (B) Pilocarpine-induced saliva volume was determined every 8 days. The number of the three groups were 6, 6, and 8, respectively. Data were presented as mean ± SEM, *poly(I:C) vs. none, *p < 0.05, **p < 0.01; #poly(I:C) vs. PBS, #p < 0.05.
Treatment With Poly(I:C) Aggravated the Histopathological Lesions in Salivary Gland
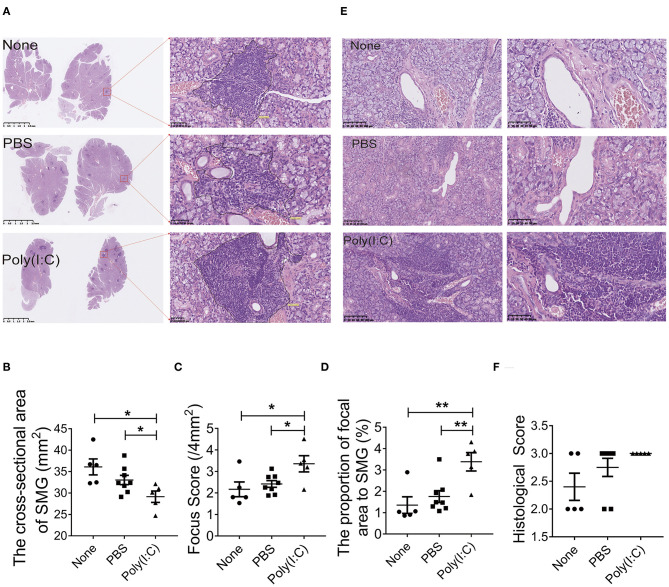
To determine the effect of poly(I:C) administration on the histopathological lesions of salivary gland in NOD mice, sections of salivary gland harvested at day 52 were stained with H&E staining. As shown in Figures 2A,B, the cross-sectional area of salivary gland in poly(I:C) treated group was smaller than that in PBS-treated or untreated group, indicating that the volume of gland was reduced after poly(I:C) administration. There were more inflammatory cells foci in salivary gland per 4 mm2 (focus score) in poly(I:C)-treated group (Figure 2C). The proportion of inflammatory cells aggregation area in total salivary gland cross-sectional area was increased after poly(I:C) administration (Figure 2D). Furthermore, we analyzed the histopathological change of another two sections every 10 μm interval in each salivary gland, and similar results were observed (data not shown). Poly(I:C) treatment increased CD3-positive T cells accumulation and unchanged the CD20-positive B cells accumulation in the salivary glands (Supplementary Figure 2). The lymphoepithelial lesions (LELs), evaluated by the proportion of the hyperplasia of epithelium resulted from infiltrated lymphocytes, was more serious in intratracheal poly(I:C)-treated group (Figures 2E,F). These data suggest that intratracheal poly(I:C) administration accelerated the histopathological changes of salivary glands in NOD mice.
Figure 2.
Treatment with poly(I:C) aggravated the histopathological lesions of salivary gland. (A) Infiltrated lymphocyte foci in the acinar tissue of salivary gland in different groups (HE, ×10, ×400) (n = 5–8 per group). (B) The analysis of the cross-sectional area of SMG in different groups. (C) The analysis of the focus score in different groups. (D) The analysis of the proportion of inflammatory cells area to the total salivary gland area in different groups. (E) Histological destruction of glandular duct (HE, ×100, ×200). (F) Histological destruction was assessed by a classification system with the proportion of the hyperplastic epithelium per square millimeter (mm2). Data were presented as mean ± SEM, *p < 0.05, **p < 0.01.
Poly(I:C) Treatment Increased IFN Cytokines and T Cells Chemokines Levels in Salivary Gland
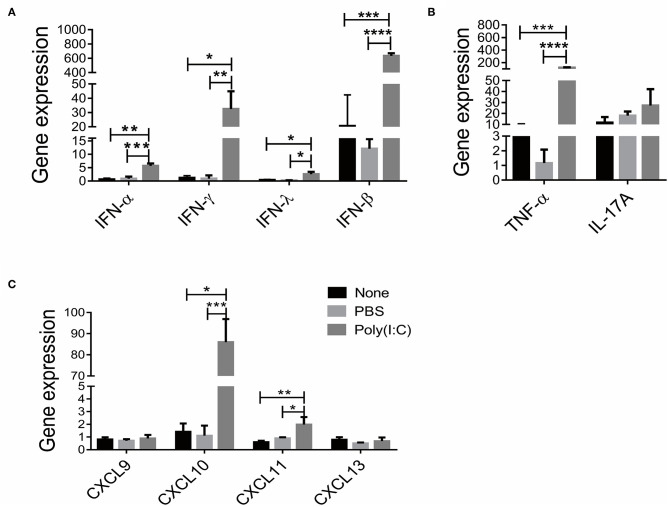
Previous studies have reported that IFN, Th1 and Th17 signaling participated in the development of SS (25–27). We then investigated the immune status in salivary gland after poly(I:C) administration. As shown in Figure 3A, significantly upregulated expressions of IFN-α, IFN-β, IFN-γ and IFN-λ, particularly IFN-β (72.3-fold) were observed in respiratory tract poly(I:C)-treated group. Furthermore, the expression of TNF-α, a Th1 cell associated cytokine, was increased compared with the control groups, though the expression of IL-17A, a Th17 cell associated cytokine, was unchanged after poly(I:C) treatment (Figure 3B). We further detected the expression of T cell chemotactic factors CXCL9, CXCL10, CXCL11 and B cells chemotactic factor CXCL13. As shown in Figure 3C, CXCL10 and CXCL11 expression were elevated in poly(I:C)-treated group, while CXCL9 and CXCL13 expression were not different from each group. The level of serum autoantibody ANA was comparable among the groups (Supplementary Figure 3). These data suggest that intratracheal poly(I:C) administration affected IFN signal and Th1 cell accumulation in salivary gland, which is consistent with virus infection-induced immune response.
Figure 3.
Poly(I:C) treatment increased IFN cytokines and T cell chemokines levels in salivary gland. (A) The mRNA levels of IFN (IFN-α, IFN-β, IFN-γ, IFN-λ) were determined by real-time PCR (n = 5, 5, 4). (B) The mRNA levels of T cell associated cytokines were determined by real-time PCR, TNF-α (Th1 cytokine) and IL-17A (Th17 cytokine). (C) The mRNA levels of CXCL9, CXCL10, CXCL11 and CXCL13 in salivary gland. Data were presented as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Poly(I:C) Intratracheal Stimulation Upregulated the Expression of IL-33 in Salivary Gland
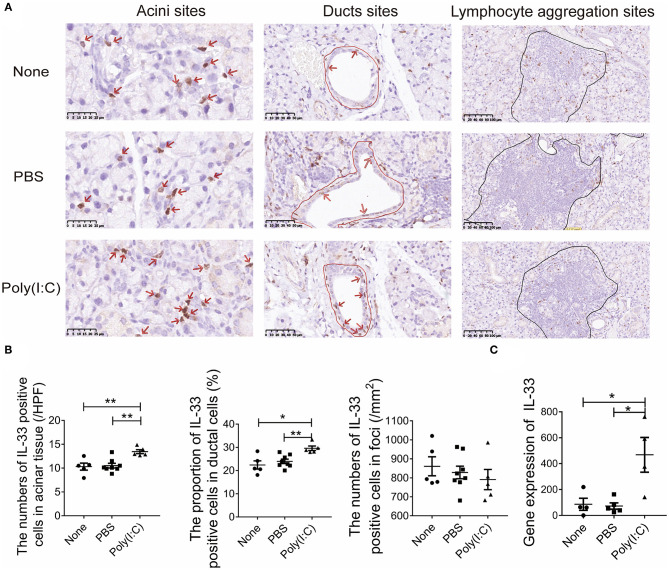
Poly(I:C) can induced the expression and release of IL-33, a damage associated molecular patterns (DAMP). Studies have shown that IL-33 is involved in Th1 cell response (28). The expression of IL-33 in salivary gland were detected by immunohistochemistry (Figure 4A). We found that the number of IL-33 positive cells per high power field (HPF) in acini sites was increased after poly(I:C) administration, the proportion of IL-33 positive cells to total ductal cells in ducts sites was also increased, though there was no difference in the lymphocyte aggregation sites among the three groups (Figure 4B). Meanwhile, the mRNA level of IL-33 expression in salivary gland was higher in poly(I:C)-treated mice (Figure 4C). Hence, intratracheal poly(I:C) treatment resulted in an upregulation of IL-33 expression, which might promote the Th1 cell response in salivary gland.
Figure 4.
Poly(I:C) intratracheal stimulation upregulated the expression of IL-33 in salivary gland. (A) Immunohistochemical staining for IL-33 expression in salivary gland (×400, ×400, ×200) (n = 5–8 per group). (B) The number of IL-33 positive cells per high power field (HPF) in acini sites, the proportion of IL-33 positive cells to total ductal cells in ducts sites, the number of IL-33 positive counts in lymphocyte aggregation sites per square millimeter (mm2). (C) The mRNA level of IL-33 in salivary gland (n = 5, 5, 4). Data were presented as mean ± SEM, *p < 0.05, **p < 0.01.
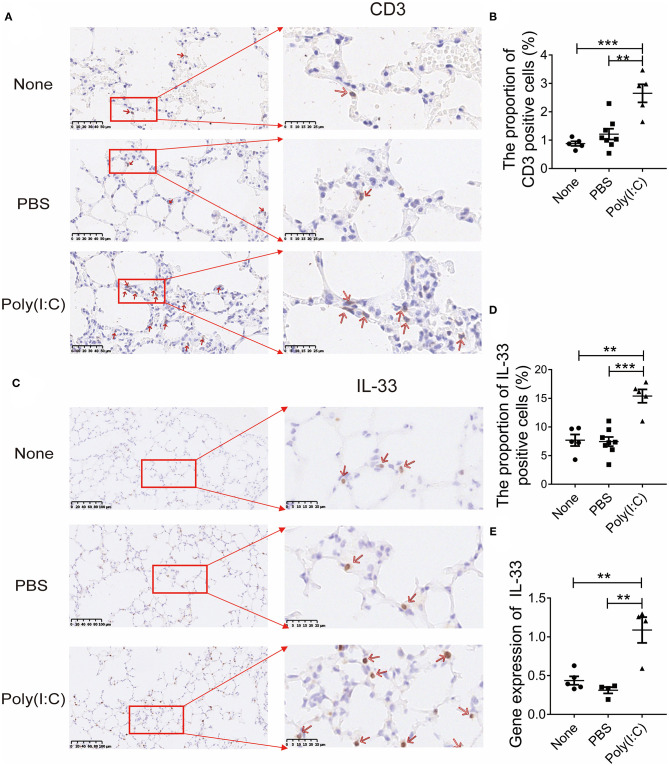
Poly(I:C) Treatment Increased IL-33 Expression and T Cells Proportion in the Lung
To observe the changes of lung which may be associated with salivary gland injury after poly(I:C) stimulation, we further explored the indicator in the lung. The sections of lung were stained with anti-CD3 antibody, the results showed the increased CD3-positive T cells accumulation in interstitium of lung (Figures 5A,B), in addition to aggravated mucus secretion and bronchial thickening compared with control groups (Supplementary Figure 4). We further detected the expression of IL-33 in the lung. The number of pulmonary IL-33 positive cells per HPF was increased obviously (Figures 5C,D). The expression of IL-33 in mRNA level in the lung was higher after poly(I:C) administration (Figure 5E). These data indicate that poly(I:C) stimulation increased IL-33 expression and T cells proportion in the lung, which are similar to the change in salivary gland.
Figure 5.
Poly(I:C) treatment increased IL-33 expression and T cells proportion in the lung. (A) Immunohistochemical staining for CD3 expression in lung (×400, ×800) (n = 5–8 per group). (B) The proportion of CD3 positive cells to total cells in lung per HPF. (C) Immunohistochemical staining for IL-33 expression in lung (×200, ×800) (n = 5–8 per group). (D) The proportion of IL-33 positive cells to total cells per HPF. (E) The mRNA levels of IL-33 in lung (n = 5, 5, 4). Data were presented as mean ± SEM, **p < 0.01 and ***p < 0.001.
Discussion
It is generally believed that viral infection may be an important environmental factor in genetically susceptible individuals of SS. Among them, respiratory virus infection including vaccinations and enterovirus may participate in the development of SS (10, 11). Studies have shown that poly(I:C) administration is a well-established model to mimic viral infection in systemic lupus erythematosus (29), type 1 diabetes (30), and arthritis (31) animal model. In this study, we found that repeatedly intratracheally administered poly(I:C) in susceptible NOD mice advanced the onset of sialadenitis, accelerated the histopathological lesions of SMG. Further analysis showed that the IFN signature and Th1 immune response were upregulated in the local of SMG. IL-33, which is participated in viral infection, was increased in SMG. Interestingly, the expression of IL-33 and T cells were also elevated in the lung, which is consistent with the change in SMG. Thus, respiratory tract viral infection might be involved in the etiopathogenesis of SS-like progression.
Lymphocytic infiltration of salivary glands is the hallmark of SS, and saliva volume is used to evaluate the function of salivary gland. In this study, we found that there was a significant reduction of saliva production in poly(I:C)-treated mice until day 32. The pathological lesion and lymphocyte infiltration in salivary glands in poly(I:C)-treated mice remained at the end of the study (52th day). We can see this phenomenon in other studies. Intraperitoneal administration of poly(I:C) in NZB/WF1 mice caused the reduction of saliva compared with untreated group (15), accompanied with more severe lymphocytic infiltration (14). Freund's incomplete adjuvant (IFA) stimulation resulted in the mild sialadenitis while significant glandular hypofunction (32). Even the exocrine gland dysfunction is considered as a process independent from inflammation in the pathogenesis of SS (33). These results indicate that the dysfunction of salivary glands presented with reduction of saliva results from mainly lymphocytic infiltration and other factors.
T lymphocytes play an important role in glandular damage and disease progression in SS (34), Activated CD4+T cells can mediate the local inflammatory responses and activate B cells to promote the production of plasma cells and autoantibodies (35). In the present study, the effects of intratracheal poly(I:C) administration on Th1-related chemokines and inflammatory cytokines production within the SMG were investigated. Poly(I:C) stimulation caused significant upregulation in the expression of Th1-related chemokines CXCL10 and CXCL11 genes that influence the inflammatory cell infiltration within the SMG. The expression levels of Th1-related cytokines TNF-α was also upregulated. The abnormality of IFN signature has been reported in the blood and salivary glands of patients with Sjogren syndrome (36). Poly(I:C) stimulation increased the expression levels of IFN genes, especially IFN-β, in SMG. The activation of IFN and Th1 response occurred in the condition of viral infection, which might explain the accelerated progression of salivary gland dysfunction in NOD mice after poly(I:C) stimulation.
Sjogren's syndrome is characterized by production of autoantibodies. We found that poly(I:C) stimulation unchanged the production of ANA compared with control groups in NOD mice. Accordingly, the B cell chemokine CXCL13 expression and CD20-positive B cells were unchanged in salivary glands after respiratory tract poly(I:C) stimulation. These factors may be associated with the unchanged production of ANA in poly(I:C)-treated NOD mice.
In the present study, intratracheal poly(I:C) stimulation increased IL-33 expression and T cells infiltration in the lung, which was similar to the changes observed in salivary glands. Therefore, we speculate that there was a link between lung and salivary glands, which resulted in the dysfunction of salivary glands in poly(I:C)-exposed NOD mice. IL-33, a DAMP, can be induced and released from epithelial or endothelial cells in respiratory virus infection (37, 38). Other studies have found that IL-33 promotes the differentiation and function of Th1 type cells, including the upregulated expression of IFN-γ, IL-18, t-bet and CXCR3 in influenza virus infection condition (37, 39). We found that IL-33 detected by IHC and real time-PCR both showed increased expression in lungs and salivary glands after poly(I:C) administration. Salivary glands and respiratory tract tissues belong to the mucosal immune system (40, 41). Thus, we speculate that the cytokine, such as IL-33, or activated T cells from lungs to salivary glands may result in the immune dysfunction and glandular destruction, which needs to be further investigated.
In conclusion, our results showed that intratracheal exposure of poly(I:C) results in a significant loss of glandular function, severe histopathological lesions and immune imbalance in salivary glands, indicating that intratracheal poly(I:C) exposure aggravated the immunological and function disorder of SMG to promote SS-like progression.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the guidelines of Tongji Hospital Animal Care and Use Committee.
Author Contributions
LD and FZ designed the study. PH and BM performed the experiments, analyzed the data, and wrote the paper. XW, SC, JT, YD, and TZ helped for bleeding the mice and samples acquired. ZT and JZ contributed to the interpretation of the data. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by grants from the National Natural Scientific Foundation of China (No. 81771754 and No. 81901586) and Tongji Hospital Clinical Research Flagship Program (No. 2019CR206).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.645816/full#supplementary-material
References
- 1.Lisi S, Sisto M, D'Amore M, Lofrumento DD. Co-culture system of human salivary gland epithelial cells and immune cells from primary Sjogren's syndrome patients: an in vitro approach to study the effects of Rituximab on the activation of the Raf-1/ERK1/2 pathway. Int Immunol. (2015) 27:183–94. 10.1093/intimm/dxu100 [DOI] [PubMed] [Google Scholar]
- 2.Fox RI. Sjogren's syndrome. Lancet. (2005) 366:321–31. 10.1016/S0140-6736(05)66990-5 [DOI] [PubMed] [Google Scholar]
- 3.Zhang NZ, Shi CS, Yao QP, Pan GX, Wang LL, Wen ZX, et al. Prevalence of primary Sjogren's syndrome in China. J Rheumatol. (1995) 22:659–61. [PubMed] [Google Scholar]
- 4.Brito-Zeron P, Acar-Denizli N, Zeher M, Rasmussen A, Seror R, Theander E, et al. Influence of geolocation and ethnicity on the phenotypic expression of primary Sjogren's syndrome at diagnosis in 8310 patients: a cross-sectional study from the Big Data Sjogren Project Consortium. Ann Rheum Dis. (2017) 76:1042–50. 10.1136/annrheumdis-2016-209952 [DOI] [PubMed] [Google Scholar]
- 5.Nocturne G, Mariette X. B cells in the pathogenesis of primary Sjogren syndrome. Nat Rev Rheumatol. (2018) 14:133–45. 10.1038/nrrheum.2018.1 [DOI] [PubMed] [Google Scholar]
- 6.Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren's syndrome. Nat Rev Rheumatol. (2010) 6:529–37. 10.1038/nrrheum.2010.118 [DOI] [PubMed] [Google Scholar]
- 7.Bodewes ILA, Versnel MA. Interferon activation in primary Sjogren's syndrome: recent insights and future perspective as novel treatment target. Expert Rev Clin Immunol. (2018) 14:817–29. 10.1080/1744666X.2018.1519396 [DOI] [PubMed] [Google Scholar]
- 8.Talotta R, Sarzi-Puttini P, Atzeni F. Microbial Agents as Putative Inducers of B Cell Lymphoma in Sjogren's Syndrome through an Impaired Epigenetic Control: the state-of-the-art. J Immunol Res. (2019) 219:8567364. 10.1155/2019/8567364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldeira-Dantas S, Furmanak T, Smith C, Quinn M, Teos LY, Ertel A, et al. The chemokine receptor CXCR3 promotes CD8(+) T cell accumulation in uninfected salivary glands but is not necessary after murine cytomegalovirus infection. J Immunol. (2018) 200:1133–45. 10.4049/jimmunol.1701272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triantafyllopoulou A, Tapinos N, Moutsopoulos HM. Evidence for coxsackievirus infection in primary Sjogren's syndrome. Arthritis Rheum. (2004) 50:2897–902. 10.1002/art.20463 [DOI] [PubMed] [Google Scholar]
- 11.Stathopoulou EA, Routsias JG, Stea EA, Moutsopoulos HM, Tzioufas AG. Cross-reaction between antibodies to the major epitope of Ro60 kD autoantigen and a homologous peptide of Coxsackie virus 2B protein. Clin Exp Immunol. (2005) 141:148–54. 10.1111/j.1365-2249.2005.02812.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brauner S, Folkersen L, Kvarnstrom M, Meisgen S, Petersen S, Franzen-Malmros M, et al. H1N1 vaccination in Sjogren's syndrome triggers polyclonal B cell activation and promotes autoantibody production. Ann Rheum Dis. (2017) 76:1755–63. 10.1136/annrheumdis-2016-210509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bjork A, Thorlacius GE, Mofors J, Richardsdotter Andersson E, Ivanchenko M, Tingstrom J, et al. Viral antigens elicit augmented immune responses in primary Sjogren's syndrome. Rheumatology. (2020) 59:1651–61. 10.1093/rheumatology/kez509 [DOI] [PubMed] [Google Scholar]
- 14.Deshmukh US, Nandula SR, Thimmalapura PR, Scindia YM, Bagavant H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med. (2009) 38:42–7. 10.1111/j.1600-0714.2008.00700.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nandula SR, Scindia YM, Dey P, Bagavant H, Deshmukh US. Activation of innate immunity accelerates sialoadenitis in a mouse model for Sjogren's syndrome-like disease. Oral Dis. (2011) 17:801–7. 10.1111/j.1601-0825.2011.01839.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Jin JO, Du J, Yu Q. Innate immune signaling induces IL-7 production, early inflammatory responses, and sjogren's-like dacryoadenitis in C57BL/6 mice. Invest Ophthalmol Vis Sci. (2015) 56:7831–8. 10.1167/iovs.15-17368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida I, Azuma M. An alternative receptor to poly I:C on cell surfaces for interferon induction. Microbiol Immunol. (2013) 57:329–33. 10.1111/1348-0421.12050 [DOI] [PubMed] [Google Scholar]
- 18.Arshad MI, Patrat-Delon S, Piquet-Pellorce C, L'Helgoualc'h A, Rauch M, Genet V, et al. Pathogenic mouse hepatitis virus or poly(I:C) induce IL-33 in hepatocytes in murine models of hepatitis. PLoS ONE. (2013) 8:e74278. 10.1371/journal.pone.0074278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Natarajan C, Yao SY, Sriram S. TLR3 Agonist poly-IC induces IL-33 and promotes myelin repair. PLoS ONE. (2016) 11:e0152163. 10.1371/journal.pone.0152163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Awada A, Nicaise C, Ena S, Schandene L, Rasschaert J, Popescu I, et al. Potential involvement of the IL-33-ST2 axis in the pathogenesis of primary Sjogren's syndrome. Ann Rheum Dis. (2014) 73:1259–63. 10.1136/annrheumdis-2012-203187 [DOI] [PubMed] [Google Scholar]
- 21.Xiao F, Han M, Wang X, Gong X, Huang E, Zhu Z, et al. Animal models of Sjogren's syndrome: an update. Clin Exp Rheumatol. (2019) 37(Suppl 118):209–16. [PubMed] [Google Scholar]
- 22.Scuron MD, Fay B, Oliver J, Smith P. Spontaneous Model of Sjogren's Syndrome in NOD Mice. Curr Protoc Pharmacol. (2019) 86:e65. 10.1002/cpph.65 [DOI] [PubMed] [Google Scholar]
- 23.Xiao F, Lin X, Tian J, Wang X, Chen Q, Rui K, et al. Proteasome inhibition suppresses Th17 cell generation and ameliorates autoimmune development in experimental Sjogren's syndrome. Cell Mol Immunol. (2017) 14:924–34. 10.1038/cmi.2017.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Ginkel MS, Haacke EA, Bootsma H, Arends S, van Nimwegen JF, Verstappen GM, et al. Presence of intraepithelial B-lymphocytes is associated with the formation of lymphoepithelial lesions in salivary glands of primary Sjogren's syndrome patients. Clin Exp Rheumatol. (2019) 37(Suppl 118):42–8. [PubMed] [Google Scholar]
- 25.Marketos N, Cinoku I, Rapti A, Mavragani CP. Type I interferon signature in Sjogren's syndrome: pathophysiological and clinical implications. Clin Exp Rheumatol. (2019) 37(Suppl 118):185–91. [PubMed] [Google Scholar]
- 26.Verstappen GM, Kroese FGM, Bootsma H. T cells in primary Sjogren's syndrome: targets for early intervention. Rheumatology. (2019) kez004. 10.1093/rheumatology/kez004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo J, Ming B, Zhang C, Deng X, Li P, Wei Z, et al. IL-2 Inhibition of Th17 generation rather than induction of treg cells is impaired in primary Sjogren's syndrome patients. Front Immunol. (2018) 9:1755. 10.3389/fimmu.2018.01755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komai-Koma M, Wang E, Kurowska-Stolarska M, Li D, McSharry C, Xu D. Interleukin-33 promoting Th1 lymphocyte differentiation dependents on IL-12. Immunobiology. (2016) 221:412–7. 10.1016/j.imbio.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steinberg AD, Baron S, Talal N. The pathogenesis of autoimmunity in New Zealand mice, I. Induction of antinucleic acid antibodies by polyinosinic-polycytidylic acid. Proc Natl Acad Sci USA. (1969) 63:1102–7. 10.1073/pnas.63.4.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devendra D, Eisenbarth GS. Interferon alpha—a potential link in the pathogenesis of viral-induced type 1 diabetes and autoimmunity. Clin Immunol. (2004) 111:225–33. 10.1016/j.clim.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 31.Yarilina A, DiCarlo E, Ivashkiv LB. Suppression of the effector phase of inflammatory arthritis by double-stranded RNA is mediated by type I IFNs. J Immunol. (2007) 178:2204–11. 10.4049/jimmunol.178.4.2204 [DOI] [PubMed] [Google Scholar]
- 32.Deshmukh US, Ohyama Y, Bagavant H, Guo X, Gaskin F, Fu SM. Inflammatory stimuli accelerate Sjogren's syndrome-like disease in (NZB x NZW)F1 mice. Arthritis Rheum. (2008) 58:1318–23. 10.1002/art.23368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi T. Dysfunction of lacrimal and salivary glands in Sjogren's syndrome: nonimmunologic injury in preinflammatory phase and mouse model. J Biomed Biotechnol. (2011) 2011:407031. 10.1155/2011/407031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karabiyik A, Peck AB, Nguyen CQ. The important role of T cells and receptor expression in Sjogren's syndrome. Scand J Immunol. (2013) 78:157–66. 10.1111/sji.12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. (2007) 25:821–52. 10.1146/annurev.immunol.25.022106.141557 [DOI] [PubMed] [Google Scholar]
- 36.Aota K, Ono S, Yamanoi T, Kani K, Momota Y, Azuma M. MMP-9 Inhibition suppresses interferon-gamma-induced CXCL10 production in human salivary gland ductal cells. Inflammation. (2019) 42:2148–58. 10.1007/s10753-019-01079-x [DOI] [PubMed] [Google Scholar]
- 37.Le Goffic R, Arshad MI, Rauch M, L'Helgoualc'h A, Delmas B, Piquet-Pellorce C, et al. Infection with influenza virus induces IL-33 in murine lungs. Am J Respir Cell Mol Biol. (2011) 45:1125–32. 10.1165/rcmb.2010-0516OC [DOI] [PubMed] [Google Scholar]
- 38.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. (2011) 186:4375–87. 10.4049/jimmunol.1003020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baumann C, Bonilla WV, Frohlich A, Helmstetter C, Peine M, Hegazy AN, et al. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci USA. (2015) 112:4056–61. 10.1073/pnas.1418549112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong BY, Sobue T, Choquette L, Dupuy AK, Thompson A, Burleson JA, et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome. (2019) 7:66. 10.1186/s40168-019-0679-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarro S, Cossalter G, Chiavaroli C, Kanda A, Fleury S, Lazzari A, et al. The oral administration of bacterial extracts prevents asthma via the recruitment of regulatory T cells to the airways. Mucosal Immunol. (2011) 4:53–65. 10.1038/mi.2010.51 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.