Abstract
Background
The Centers for Medicare and Medicaid Services removed total hip arthroplasty (THA) from the inpatient-only list. This has created significant confusion regarding which patients qualify for an inpatient designation. The purpose of this study is to develop and validate a novel predictive tool for preoperatively objectively determining “outpatient” vs “inpatient” status for THA in the Medicare population.
Methods
A cohort of Medicare patients undergoing primary THA between January 2017 and September 2019 was retrospectively reviewed. A machine learning model was trained using 80% of the THA patients, and the remaining 20% was used for testing the model performance in terms of accuracy and the average area under the receiver operating characteristic curve. Feature importance was obtained for each feature used in the model.
Results
One thousand ninety-one patients had outpatient stays, and 318 qualified for inpatient designation. Significant associations were demonstrated between inpatient designations and the following: higher BMI, increased patient age, better preoperative functional scores, higher American Society of Anesthesiologist Physical Status Classification, higher Modified Frailty Index, higher Charlson Comorbidity Index, female gender, and numerous comorbidities. The XGBoost model for predicting an inpatient or outpatient stay was 78.7% accurate with the area under the receiver operating characteristic curve to be 81.5%.
Conclusions
Using readily available key baseline characteristics, functional scores and comorbidities, this machine-learning model accurately predicts an “outpatient” or “inpatient” stay after THA in the Medicare population. BMI, age, functional scores, and American Society of Anesthesiologist Physical Status Classification had the highest influence on this predictive model.
Keywords: Total hip arthroplasty, Medicare total hip, Medicare bundle payment, Medicare inpatient only list, Arthroplasty inpatient only, Predictive model
Introduction
Nearly 30 million adults in the United States have been diagnosed with osteoarthritis [1]. One-third of these patients are older than 65 years [1,2]. Most patients undergoing total hip arthroplasty (THA) are older than 65 years, hence this procedure represents a large proportion of Medicare expenditures in the United States [[3], [4], [5], [6]]. Patients of older age and those with higher measures of frailty account for a significantly increased cost after total joint arthroplasty (TJA), which may subsequently deincentivize care to older and higher risk patients [[5], [6], [7]].
In 2018, spending on health care grew 4.6%, reaching $3.6 trillion and accounting for 17.7% of America’s gross domestic product [7]. The Center for Medicare and Medicaid services (CMS) seeks to further lower costs, by removing TJA from the inpatient-only list [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. CMS has removed total knee arthroplasty (TKA) from the inpatient-only list in 2018, and THA in January 2020 [11,12]. CMS defines an inpatient stay when the patient spends 2 midnights at the hospital after a procedure [[11], [12], [13], [14]]. CMS Rule 42 C.F.R. §412.3(e) states that in addition to a 2-night hospital stay, if a procedure is on the inpatient-only list, no matter what the stay is, the admission is classified as inpatient [[13], [14], [15]]. In 2016, a revision was made to the two-midnight rule, stating that a hospital stay less than 2 midnights may fall under Medicare Part A if appropriate supporting documents are provided on the decision to keep a patient in the hospital [14,16].
The length of stay (LOS) after THA has decreased over the years, with many institutions and practices offering this procedure in the outpatient setting [[18], [19], [20]]. Therefore, many argue that Medicare beneficiaries can undergo THA at an ambulatory center or in the outpatient hospital setting, which will subsequently result in substantial savings for the CMS [9]. However, many Medicare patients, especially the elderly and those with more comorbidities and decreased preoperative functional status, require an inpatient admission after TJA [5,9,12,13,17]. Therefore, with THA taken off the inpatient-only list, providers and hospitals have added pressure in classifying most THAs as an outpatient procedure, subsequently resulting in increased costs for physicians and health-care systems. Ultimately, this may beget a bias in care toward the elderly and frail patients, who are more likely to require an inpatient admission after TJA [5,6,9,10,12,13].
Several studies have assessed patient factors that are more likely to result in an inpatient admission after THA [9,10,18]. These factors include comorbidities, bilateral procedures, increased age, increased BMI, minority ethnicity, and female gender. With THA taken off the inpatient-only list, a reliable model is needed to assess the multifactorial nature of inpatient vs outpatient designation after this procedure, to mitigate financial losses and fears of audits. Models predicting inpatient vs outpatient stay have been created using large-scale national files, which are inherently flawed because of the nature of the data [10,21,22]. To date, no highly accurate and easily reproducible model exists in assessing inpatient vs outpatient admissions after THA. Hence, the purpose of this study is to develop and validate a novel predictive model for providers to use when assessing outpatient vs inpatient designation after THA.
Material and methods
After obtaining institutional research ethics board approval, a retrospective review of prospectively collected data of Medicare patients undergoing primary THA at our institution under the bundled payments for care improvement (BPCI) initiative from 2017 to 2019 was performed using Diagnosis Related Groups 469 (major joint replacement or reattachment of lower extremity with major complications or comorbidities) and 470 (major joint replacement or reattachment of lower extremity without major complications or comorbidities). One thousand eight hundred thirty-one patients underwent THA at our institution between January 2017 and September 2019. Patients who were younger than 65 years or diagnosed of traumatic fractures, inflammatory arthritis, and for conversion THA were excluded from the study. After exclusion, 1409 Medicare patients were included in the study. Outpatient stays accounted for 1091 patients (77.4%) undergoing THA, while 318 patients (22.6%) qualified for inpatient designation. All patients’ surgeries were performed at a large urban academic institution. Inclusion criteria for this study were patients older than 65 years who underwent a THA and had a full set of demographics and documented LOS. LOS was considered to fall under the outpatient class if the patient spent less than 2 midnights in the hospital, per the CMS 2 mid-night rule. Therefore, if an individual’s hospital stay was 2 midnights or greater, they fell into the inpatient category. Those with a LOS less than 2 midnights were considered an outpatient. Patient demographics (age, gender, BMI), current diagnosis leading to joint pain (rheumatoid arthritis, osteoarthritis, avascular necrosis), past medical history (cardiac history, history of a venous thromboembolic event [VTE], diabetes mellitus [DM], and other rheumatologic disease), Charlson Comorbidity Index (CCI), American Society of Anesthesiologist Physical Status Classification (ASA), Revised Cardiac Risk Index, and Modified Frailty Index (mFI) scores were obtained through individual chart review. In addition, preoperative functional scores were used and included hip disability and osteoarthritis outcome score (HOOSJR), VR12 physical component (pcs), and VR12 mental component (mcs) scores. The aforementioned variables were used as the aim of the model is to preoperatively predict which patients will fall under the “inpatient” vs “outpatient” designation. Baseline variables' ability of prediction for outpatient vs inpatient stay was assessed through two-sample t test for continuous variables and chi-square test for categorical variables. Significance level was set at P < .05.
XGBoost (eXtreme Gradient Boosting) is a machine-learning tool, which provides gradient boosting framework to build predictive models. XGBoost uses training data to predict a target variable, which was the inpatient or outpatient setting in our case. It is a decision tree ensemble, which consists of a set of classification and regressions trees. Gradient boosting builds new models that predict residuals or errors of prior models, and then the new models are added together to make the final model prediction. Hence, the final predictive model is built in a stage-wise fashion. The XGBoost model was used for the present article, as it proved to be the most accurate model. The authors ran 3 other models—an L1 penalized logistic regression, a support vector machine model, and a random forest model. The accuracy and area under the receiver operating characteristic curve (AUC) of these models is demonstrated in Table 1.
Table 1.
Accuracy and area under the curve (AUC) for machine learning and regression models.
| Accuracy/AUC | XGBoost | L1-penalized logistic regression |
|---|---|---|
| Accuracy | 78.72% | 71.17% |
| AUC |
81.54% |
76.09% |
| Support vector machine |
Random forest |
|
| Accuracy | 75.53% | 74.38% |
| AUC | 78.29% | 79.26% |
XGBoost proved to be the most accurate model with the highest AUC.
While preserving the proportion of outpatient and inpatient classes in the whole study population, 80% of patients were randomly selected to be used for training the XGBoost model while the remaining 20% were later used for testing the model performance. Stratified 5-fold cross-validation was conducted on the training set. Together with the grid search, we also tuned the hyper-parameters of the XGBoost model. Class weight was used to handle the imbalanced outcome. Feature importance was obtained for each feature used in the model to see the relative importance of predicting the inpatient or outpatient setting.
Finally, the performance of the trained model was evaluated on the test set. The performance metrics were accuracy and the average AUC for outcome measures. The receiver operating characteristic curve illustrates the diagnostic ability of a binary classification system. This method was original developed for operators of military radar receivers. The curve is created through plotting the true positive rate against the false-positive rate, and the AUC represents the probability that a group of characteristics will accurately predict an outcome [23]. Using this method, 0.5 represents a random model without discrimination power while 1.0 represents a perfect predictive model. Stratification is considered excellent when the AUC is 90%-100%, good when the AUC is 80%-89%, fair when the AUC is 70%-80%, poor when the AUC is 60%-70%, and to have failed when the AUC is 50%-60% [24]. If the resultant AUC is 80% (0.8) or greater, the model is considered informed and can be regarded as a strong predictor of generating a decision based on a set of inputs. All statistical analyses and the modeling processes were conducted in Python, version 3.7.3, with the Jupyter Notebook interface.
Results
Patient baseline demographics and presurgery details
One thousand four hundred nine Medicare patients who underwent THA at our institution between January 2017 and September 2019 were included in the study. Outpatient stays accounted for 1091 patients (77.4%) undergoing THA, while 318 patients (22.6%) qualified for inpatient designation. Significant associations were demonstrated between inpatient visits and the following: higher BMI (P < .01), increased patient age (P < .01), lower HOOSJR scores (P = .03), lower VR12 pcs scores (P < .01), lower VR12 mcs scores (P < .01), higher ASA (P < .01), higher mFI (P < .01), higher CCI (P < .01), and female gender (P = .04). The mean and standard deviation for aforementioned variables in inpatient vs outpatient settings are further demonstrated in Table 2.
Table 2.
Patient demographics and presurgery details for the patients who had an outpatient THA and the patient who had an inpatient THA.
| Patient variables | Outpatient, N = 1091 | Inpatient, N = 318 | P value |
|---|---|---|---|
| Mean age (y) | 72.4 ± 5.5 | 75.5 ± 6.6 | <.01 |
| % Female | 682 (62.5%) | 219 (68.9%) | .04 |
| Mean BMI (kg/m2) | 27.9 ± 5.2 | 29.0 ± 6.0 | <.01 |
| Mean CCI | 3.3 ± 1.3 | 4.2 ± 2.0 | <.01 |
| Diagnosis | |||
| Rheumatoid arthritis | 507 (46.5%) | 129 (40.6%) | .17 |
| Osteoarthritis | 579 (53.1%) | 187 (58.8%) | |
| Avascular necrosis | 5 (0.4%) | 2 (0.6%) | |
| Mean ASA | 2.4 ± 0.6 | 2.7 ± 0.6 | <.01 |
| Mean HOOSJR | 52.7 ± 12.5 | 48.0 ± 14.1 | .03 |
| Mean VR12_pcs | 31.3 ± 7.8 | 27.2 ± 6.3 | <.01 |
| Mean VR12_mcs | 49.4 ± 11.9 | 44.0 ± 14.9 | <.01 |
| Mean mFI | 0.8 ± 0.9 | 1.4 ± 1.3 | <.01 |
| Mean RCRI | 0.1 ± 0.2 | 0.1 ± 0.3 | <.01 |
| Cardiac history | 171 (15.7%) | 94 (29.6%) | <.01 |
| Venous thromboembolism | 46 (4.2%) | 41 (12.9%) | <.01 |
| Diabetes mellitus | 64 (5.9%) | 46 (14.5%) | <.01 |
| Rheumatology | 165 (15.1%) | 90 (28.3%) | <.01 |
ASA, American Society of Anesthesiologist Physical Status Classification; CCI, Charlson Comorbidity Index; HOOSJR, hip disability and osteoarthritis outcome score; mFI, Modified Frailty Index; RCRI, Revised Cardiac Risk Index; THA, total hip arthroplasty; VR12_pcs, VR 12 physical component; VR12_mcs, VR12 mental component.
Patients with specific comorbidities had significant associations with inpatient stays after THA. Cardiac history was noted in 29.6% of patients requiring inpatient admissions, while seen in only 15.7% of individuals with an outpatient admission (P < .01). In respect to proportions, 35.5% of patients with a documented cardiac history required an inpatient admission after THA. Rheumatologic disorders were noted in 28.3% of patients requiring inpatient admissions, while only seen in 15.1% of those classified as an outpatient (P < .01). A history of VTE was noted in 12.9% of patients requiring inpatient admissions, while only seen in 4.2% of those classified as an outpatient (P < .01). However, patients with a history of VTE required an inpatient admission in 47.1% of those with this documented history. DM accounted for 14.5% of the comorbidities requiring an inpatient stay. Similarly to VTE history, a significant proportion of those with DM (41.8%) required inpatient admissions after THA. The preoperative variables are further demonstrated in Table 1.
The XGBoost model
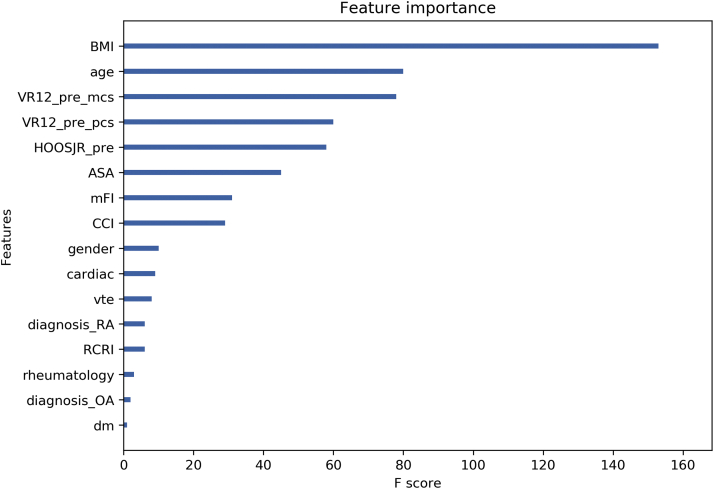
The trained XGBoost model for predicting an inpatient or outpatient stay was 78.7% accurate with the AUC to be 81.5%. Feature importance was represented by the number of times a feature was used to split the data across all trees, which demonstrated the contribution of each feature in discriminating inpatient vs outpatient stay. The importance of each feature in the trained XGBoost model can be seen in Figure 1. The higher value means greater influence in predicting the outcome. BMI, age, VR12 mcs, VR12 pcs, and HOOSJR scores are rather important in predicting the inpatient or outpatient setting. An example of the model is demonstrated in Figure 2.
Figure 1.
The feature importance of each variable is shown in the predictive XGBoost model for discriminating outpatient vs inpatient stay after THA. The feature with the highest importance was BMI while the diagnosis of AVN (not shown in the figure) did not play a role in predicting the outcome. Abbreviations: ASA, American Society of Anesthesiologist Physical Status Classification; CCI, Charlson Comorbidity Index; diagnosis_RA, diagnosis of Rheumatoid Arthritis; diagnosis_OA, diagnosis of Osteoarthritis; DM, diabetes mellitus; HOOSJR_pre, hip disability and osteoarthritis outcome score; mFI, Modified Frailty Index; RCRI, Revised Cardiac Risk Index; VR12_pre_mcs, VR12 mental component; VR12_pre_pcs, VR 12 physical component; VTE, venous thromboembolic event.
Figure 2.
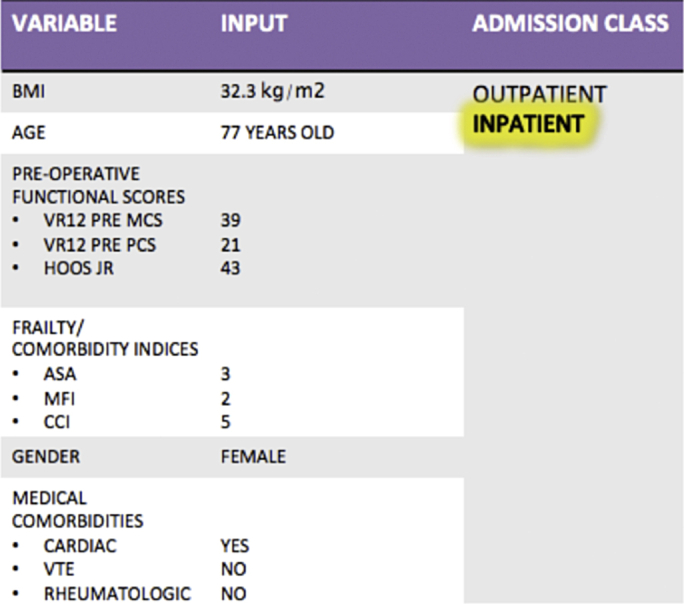
Case example of XGBoost predictive model using readily available preoperative data. AUC of this predictive model with the included variables is 81.5%. Variables are ordered in level of importance in predicting inpatient vs outpatient admission after THA.
Discussion
To our knowledge, this is the most accurate and applicable predictive model to date, in determining inpatient vs outpatient designation after THA. When using the readily available variables of BMI, patient age, preoperative functional scores, ASA, mFI, CCI, gender, and comorbidities, the model was 78.7% accurate with an AUC of 81.5%. Only one other study has made a predictive model through a retrospective review of patients at their institution, to assess inpatient vs outpatient stay after THA [8]. Turcotte et al. reviewed 1415 patients undergoing THA at their institution and demonstrated increased age, female gender, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and number of comorbidities to be significantly associated with an inpatient stay [8]. Contrary to our study, the authors found increasing BMI to reduce the odds of staying in the hospital for 2 or more midnights. They also found primary hypertension (HTN) to reduce the odds of having an inpatient stay as well. The authors subsequently built a predictive model with an AUC of 0.731. Our study demonstrates a nearly 10% increase in the AUC when compared to the aforementioned predictive model. Furthermore, their model excluded patients who were discharged on the day of surgery, which does not accurately reflect all patients undergoing THA. Unlike our study, which performed an analysis based solely on Medicare patients, the abovementioned predictive model was built on patients with a mean age of 66 years (SD, ±10 years). Their younger non-Medicare patient population cannot adequately predict inpatient vs outpatient admissions after THA in the Medicare population. Hence, this article is the first and only predictive model for inpatient vs outpatient admissions after THA, using a single-institution’s Medicare-only data.
A predictive model was prepared by Gabriel et al. assessing which patients were at risk for a prolonged hospital stay after THA [21]. The authors defined prolonged LOS as greater than 3 days, which does not correspond with inpatient or outpatient cutoff of 2 midnights per the CMS. However, when assessing 960 patients at their institution who underwent THA from 2014 to 2016, the authors found increased age, preoperative opioid use, gender, preoperative anemia, COPD, HTN, obesity, and anesthesia type to predict prolonged LOS. Their model had an AUC of 0.735.
Other predictive models have been built assessing LOS or admission designation using large-scale databases [10,21]. Ramkumar et al. formed a predictive model, with an AUC of 82.0%, using 78,355 patients from national registries [22]. This model was able to accurately predict LOS, hospital encounter costs, and discharge disposition. Gronbeck et al. demonstrated CHF, bilateral THA, increased age, and female gender to predict inpatient admission after THA in 47,611 patients from the National Surgical Quality Improvement Program database [10]. Their model, with an AUC of 69%, was less accurate than the aforementioned model, which obtained a majority of their data from the National Inpatient Sample. The model built by Gronbeck et al. was formed using patients of the “Medicare age”. Both of these models obtained their information from large-scale publicly available databases, which is in contrast to the methods utilized in this study [8,10,21].
Removing TJA from the inpatient-only list has created significant confusion and concern as there are no accepted parameters that can be applied to determine which patients to classify as inpatient vs outpatient status [5,9,10,12,13]. Owing to the discontinuation of TJA from the inpatient-only list, a physician has the following 3 options when a patient has already spent one night at the hospital: 1) provide appropriate documentation on why a patient requires the extra nights stay; 2) discharge the patient as an outpatient hospitalization; 3) discharge the patient as a short-stay inpatient hospitalization [20]. However, these decisions are nearly impossible to make preoperatively without evidenced-based literature for guidance. Factors that are associated with increased LOS after THA has been identified; however, hospital administrators and financial directors may not be able to defend inpatient designations if exposed to audits. Schwartz et al. demonstrated that the percentage of Medicare short-stay inpatient hospitalizations after TJA had increased from 2.7% to 17.8%, from 2012 to 2016 [13].
In a survey of American Association of Hip and Knee Surgeon members, 59.5% of respondents reported that their hospitals have instructed them that all TKAs be scheduled as outpatient procedures, following the decision to take the procedure off the inpatient-only list [17]. Predesignation of THA as outpatient admissions for Medicare patients undergoing this procedure will beget significant hospital costs—our study has demonstrated nearly a quarter of those undergoing THA had an LOS greater than 2 hospital midnights. Not only is the confusion on inpatient vs outpatient designation a burden to hospitals and providers, but nearly a third of surgeons stated that their patients have had additional personal costs after their TKA because of the surgical classification being designated as an outpatient procedure [17]. Based on the literature reviewed, we expect that this will also occur in hospital systems across the United States for THA after its discontinuation from the inpatient-only list.
Furthermore, Iorio et al. assessed the impact of inpatient-only rules on TKA since its removal in 2018 [12]. The authors demonstrated that hospital reimbursement averages $10,122 in an outpatient setting (which does not include physician payment) and $11,760 in an inpatient setting, with physicians being compensated an average of $1403 per TKA. Substantial costs were seen for patients, as Medicare part B has an annual deductible of $185, a 20% copay. Individuals designated as having an outpatient procedure are subject to increased postoperative costs with respect to equipment and medications [12]. In an American Association of Hip and Knee Surgeon study surveying providers after removal of TKA from the inpatient-only list, 17.65% of patients were subject to a quality improvement opportunity audit [12]. After the discontinuation of TKA from the inpatient-only list, Iorio et al. reported that 68.1% of Medicare patients undergoing TKA at a large academic medical center did not have a 2 midnight stay—the institution was subjected to 2 quality improvement opportunity audits [12]. As THAs were taken off the inpatient-only list just a few months before the writing of this article, no studies have been published on the unintended consequences of its removal from the inpatient-only list. However, based on the literature discussed in respect to TKA, we will likely see increased hospital losses, financial consequences to patients, and audits after THA inpatient-only rules.
Before taking TJA off the inpatient-only list, the CMS alternative payment model of the BPCI has resulted in significant hospital losses after orthopedic procedures [6]. Petersen et al. demonstrated a loss of $1934 per patient, in those undergoing primary TJA who are between the ages of 85 and 99 years [6]. As this age group continues to increase in size, the current BPCI initiative in TJA is expected to result in declining profits by 2030 [6]. Pepper et al. similarly demonstrated an increase in cost of care for patients aged 72 years or older at the time of their TJA [5]. In addition to age, the authors found an increasing frailty score to significantly increase costs after TJA. This study has similar findings to the abovementioned literature, as increasing age and frailty are both significant predictors of requiring an inpatient stay after THA. In addition, our article demonstrated nearly 50% of those undergoing THA with a history of VTE, and around 41% of patients with a history of DM, required inpatient admissions. Those with a history of cardiac disease accounted for the highest number of inpatient admissions in respect to documented comorbidities. Hence, if orthopedic surgeons and hospital systems continue to classify all TJAs as outpatient procedures, the hospital losses will be significantly increased as the population continues to age and undergo TJA. Therefore, providers and institutions may be subsequently biased to performing arthroplasty procedures in patients of older age and increased frailty, to mitigate the projected loss in profits.
In an effort to ease potential hospital losses, because of classifying all patients undergoing THA as an outpatient, we have built an accurate predictive model for justifying inpatient designation, without fear of potentially destructive audits. Our model uses readily available preoperative characteristics based on factors that have significant associations with an inpatient stay after THA. These factors are a higher BMI, increased patient age, higher ASA, higher CCI, and female gender. Preoperative functional scores and mFI scores predicted admission designation; however, these may not be readily available factors. Therefore, it may be beneficial for institutions to include these measures during their preoperative assessment. To date, this is one of only 2 predictive models, which do not use large-scale databases, built on assessing inpatient vs outpatient designation after THA. Although large-scale databases may provide useful information, their biggest limitations are the failure to capture patient-reported data and a diminutive amount of diagnostic information [25]. To our knowledge, the model discussed in this article is slightly more accurate than previous models. Future studies should focus on validating the current model. Ultimately, the use of this model may influence a change in our current system, in which providers may easily be able to transition patients from outpatient to inpatient designation without additional costs to the hospitals or patient. When replicated, it is our goal that providers may be able to justify their preadmission decision for admission designation based on preoperative factors, without the fear of financially damaging audits, a repercussion from CMS. Subsequently, this will allow for the continuation of performing “the surgery of the century” on the increasing older and frail population, without bias and fear of economic losses.
This study is not without limitations. The design is a retrospective review in nature, and the bias that comes with this type of data cannot be avoided. Furthermore, this study uses a predictive model, which has the limitations of uncertain projections in respect to future trends in patient characteristics and CMS initiatives. The data used in this study were from a single tertiary academic center, and our urban population may not adequately represent the population of the United States. This study was also based on a single surgery and did not assess predictors of complications, discharge disposition, or readmissions after THA. This study should not be used for patients ypunger than 65 years, as we excluded those who were not on Medicare. In addition, the data used were from 2017 to 2019, which is before THA’s official removal from the inpatient-only list, in 2020. This may alter hospital and physician behavior relative to the data used in this model. The aforementioned exclusion criteria were set, as the purpose of this article was to build a predictive model based on the Medicare population. The next step in moving forward with the potential for clinical application is for other centers to externally validate the model presented in this report.
Conclusions
Using readily available key baseline characteristics, functional scores, and comorbidities, this machine-learning model accurately predicts an “outpatient” or “inpatient” stay after THA in the Medicare population. BMI, age, functional scores, and ASA had the highest influence on this predictive model. Future research should be focused on validating these results at academic institutions around the country, using XGBoost machine learning and the aforementioned preoperative variables.
Conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Long disclosures to be a board or committee member in AAOS; a paid presenter or speaker for Convatec; a paid consultant for DePuy, A Johnson & Johnson Company, and TJO; to have received publishing royalties from Elsivier, financial or material support from Journal of Arthroplasty, and IP royalties from Editorial or governing board OrthoDevelopment; to be a paid consultant and paid presenter or speaker for Pacira and Think Surgical.
Footnotes
Availability: The trained XGBoost model and data processing code are available upon request from the authors.
Appendix A. Supplementary data
References
- 1.Lawrence R.C., Felson D.T., Helmick C.G., Arnold L.M., Choi H., Deyo R.A., Jordan J.M. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum. 2008;58(1):26. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtz S., Mowat F., Ong K., Chan N., Lau E., Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. JBJS. 2005;87(7):1487. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 4.Press M.J., Rajkumar R., Conway P.H. Medicare’s new bundled payments: design, strategy, and evolution. JAMA. 2016;315(2):131. doi: 10.1001/jama.2015.18161. [DOI] [PubMed] [Google Scholar]
- 5.Pepper A.M., Novikov D., Cizmic Z., Barrett J.T., Collins M., Iorio R., Long W.J. Age and frailty influence hip and knee arthroplasty reimbursement in a bundled payment care improvement initiative. J Arthroplasty. 2019;34(7):S80. doi: 10.1016/j.arth.2019.01.050. [DOI] [PubMed] [Google Scholar]
- 6.Petersen W.P., Jr., Teo G.M., Friedlander S., Schwarzkopf R., Long W.J. The implications of aging population demographics on the delivery of primary total joint arthroplasty in a bundled payment system. JBJS. 2020 doi: 10.2106/JBJS.19.01264. [DOI] [PubMed] [Google Scholar]
- 7.https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NationalHealthAccountsHistorical#:∼:text=U.S.%20health%20care%20spending%20grew,spending%20accounted%20for%2017.7%20percent
- 8.Turcotte J., Menon N., Aja J., Grover J., King P., Macdonald J. Preoperative predictors of patients requiring inpatient Admission for total hip arthroplasty following removal from the medicare inpatient only list. J Arthroplasty. 2020 doi: 10.1016/j.arth.2020.03.038. [DOI] [PubMed] [Google Scholar]
- 9.Greenky M.R., Wang W., Ponzio D.Y., Courtney P.M. Total hip arthroplasty and the Medicare inpatient-only list: an analysis of complications in medicare-aged patients undergoing outpatient surgery. J Arthroplasty. 2019;34(6):1250. doi: 10.1016/j.arth.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Gronbeck C.J., Cote M.P., Halawi M.J. Predicting inpatient status after total hip arthroplasty in Medicare-Aged patients. J Arthroplasty. 2019;34(2):249. doi: 10.1016/j.arth.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Medicare & Medicaid Services . Center for Medicare and Medicaid Services; Baltimore, MD: 2019. CY 2020 Medicare hospital outpatient prospective payment system and ambulatory surgical center payment system final rule (CMS-1717-FC). 2019. [Google Scholar]
- 12.Iorio R., Barnes C.L., Vitale M.P., Huddleston J.I., Haas D.A. Total knee replacement: the inpatient-only list and the two midnight rule, patient impact, length of stay, compliance solutions, audits, and economic consequences. J Arthroplasty. 2020 doi: 10.1016/j.arth.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz A.J., Clarke H.D., Sassoon A., Neville M.R., Etzioni D.A. The clinical and financial consequences of the Centers for Medicare and Medicaid Services’ Two-Midnight Rule in total joint arthroplasty. J Arthroplasty. 2020;35:1. doi: 10.1016/j.arth.2019.08.048. [DOI] [PubMed] [Google Scholar]
- 14.Center for Medicare and Medicaid Services Center for medicare and Medicaid innovation. https://innovation.cms.gov/initiatives/bpci-advanced [accessed 01.06.19]
- 15.CMS code of federal regulations. https://wwwgovinfogov/app/details/CFR-2015-title42-vol2/CFR-2015-title42-vol2-sec412-3
- 16.CMS-1633-FC; CMS-1607-F2. https://wwwcmsgov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/Hospital-Outpatient-Regulations-and-Notices-Items/CMS-1633-FChtml
- 17.Yates A.J., Kerr J.M., Froimson M.I., Della Valle C.J., Huddleston J.I. The unintended impact of the removal of total knee arthroplasty from the center for Medicare and Medicaid services inpatient-only list. J Arthroplasty. 2018;33:3602. doi: 10.1016/j.arth.2018.09.043. [DOI] [PubMed] [Google Scholar]
- 18.Aynardi M., Post Z., Ong A., Orozco F., Sukin D.C. Outpatient surgery as a means of cost reduction in total hip arthroplasty: a case-control study. HSS J. 2014;10(3):252. doi: 10.1007/s11420-014-9401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollock M., Lanting B., Somerville L., Firth A. Outpatient total hip arthroplasty, total knee arthroplasty, and unicompartmental knee arthroplasty–a systematic review of the literature. Osteoarthritis Cartilage. 2016;24:S433. doi: 10.2106/JBJS.RVW.16.00002. [DOI] [PubMed] [Google Scholar]
- 20.Weiser M.C., Kim K.Y., Anoushiravani A.A., Iorio R., Davidovitch R.I. Outpatient total hip arthroplasty has minimal short-term complications with the use of institutional protocols. J Arthroplasty. 2018;33(11):3502. doi: 10.1016/j.arth.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Gabriel R.A., Sharma B.S., Doan C.N., Jiang X., Schmidt U.H., Vaida F. A predictive model for determining patients not requiring prolonged hospital length of stay after elective primary total hip arthroplasty. Anesth Analg. 2019;129(1):43. doi: 10.1213/ANE.0000000000003798. [DOI] [PubMed] [Google Scholar]
- 22.Ramkumar P.N., Karnuta J.M., Navarro S.M. Preoperative prediction of value metrics and a patient-specific payment model for primary total hip arthroplasty: development and validation of a deep learning model. J Arthroplasty. 2019;34(10):2228. doi: 10.1016/j.arth.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 23.Zweig M.H., Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561. [PubMed] [Google Scholar]
- 24.Bradley A.E. The use of the area under the ROC curve in the evaluation of machine learning algorithms. Pattern Recognit. 1997;30:1145. [Google Scholar]
- 25.Grauer J.N., Leopold S.S. Large database studies—what they can do, what they cannot do, and which ones we will publish. Clin Orthop Relat Res. 2015;473:1537. doi: 10.1007/s11999-015-4223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.