Abstract
Among the reported probiotic Bacillus strains, B. subtilis C-3102 has the unique potential to improve feed uptake under stress conditions in the broilers, piglets, and cows. In this study, we sought to evaluate the protective effect of feed additive probiotic Bacillus subtilis C-3102 against Salmonella enterica infection of specific pathogen-free (SPF) chicks in floor pens in two experiments. In the experiment-1, the chicks in the control group (n=32) were fed a basal diet and those in the C-3102 group (n=32) were fed a basal diet supplemented with 1×106 CFU/g of feed for 28 days. On day 7 post-challenge with S. enterica, there was no significant change in the body weight between both the groups throughout the test period, whereas detection rates of S. enterica in the C-3102 group were significantly lower in the cecum and liver on days 21 and 14 post-challenge, respectively. In the experiment-2, minimum dosage of C-3102 cells required to protect Salmonella infection was evaluated using 3 dosages. Chicks were divided into four groups, fed with different dosages of C-3102 (1×106, 5×105, 3×105, and 0 CFU/g of feed), and challenged with S. enterica (2.8×108 CFU/chicken). S. enterica infection was completed within 7 days post- challenge and was almost excluded from the liver and spleen on day 21 post- challenge in the control group. Average values showed a trend for higher infection rates in the control group >3×105>5×105>1×106 CFU/g on days 14 and 21 post-challenge. These results suggest that B. subtilis C-3102 supplementation has the potential to reduce S. enterica infection rates and/or to accelerate the exclusion of S. enterica from the chicks.
Keywords: chicken, detection rate, probiotic Bacillus subtilis C-3102, Salmonella enterica infection, specific pathogen-free (SPF)
Introduction
Prevention of pathogenic bacterial infection is the most important challenge for chicken production in the broiler industry. Salmonella species are often associated with poultry salmonellosis, which results in an acute inflammation of the intestines, severe morbidity, and mortality in the poultry (Foley et al., 2011; Leeson, 2012). Most Salmonella serovars are considered to be transferable from broilers to other livestock animals, resulting in the development of food-borne diseases and diarrhea in humans (Matulova et al., 2013; Videnska et al., 2013). Poultry-derived products can be contaminated with many serovars of Salmonella enterica, including Salmonella typhimurium, Salmonella typhi, Salmonella pullorum, and Salmonella gallinarum (Leeson, 2012). The serovar S. gallinarum has a restricted host range and is usually associated only with the poultry; however, it can cause significant losses in the profit and low production yields. Under unhygienic conditions, poultry is susceptible to upper respiratory tract infections and gastrointestinal (GI) diseases, such as diarrhea.
Probiotics can enhance the immune system and protect against the pathogenic bacterial infections (Fang et al., 2000; Corthesy et al., 2007). Probiotics are live microorganisms that confer a wide range of benefits to the animals, such as stimulation of immune responses, maintenance of gut barrier function, and prevention of pathogen invasion into gut tissues (Leeson, 2012; Yeoman et al., 2012). Accordingly, they were found to be suitable for the chickens for improving mucosal and general immunity (Cox et al., 2010; Gleeson et al., 2012). Gram-positive probiotic Lactobacillus and Bacillus strains have been used for immunostimulation and prevention of Salmonella infection in the broiler chickens (Park and Kim, 2015; Oh et al., 2017; Nakphaichit et al., 2018; Liu et al., 2018; Zhen et al., 2018). Among the reported probiotic Bacillus strains, B. subtilis C-3102 is used in the commercial product Calsporin®, which has the unique potential to improve feed uptake under stress conditions in the broilers, piglets, and cows (Silley, 2006). Enhancement of the eggshell quality was achieved by B. subtilis C-3102 supplementation (Nishiyama et al., 2020). Possible reasons for the probiotic effects of C-3102 are considered to be the protected gut barrier function by increasing bifidobacteria and lactic acid bacteria and preventive effect against the pathogenic bacterial infections (Maruta et al., 1996; Hooge et al., 2004; Jeong and Kim, 2014). Recent clinical studies with B. subtilis C-3102 spores revealed an increase in the bone mineral density in the postmenopausal women by inhibiting bone resorption (Takimoto et al., 2018), and improved stool frequency (Hatanaka et al., 2018) due to control of intestinal microbiota and increased germination in the gut (Hatanaka et al., 2012).
In this study, the anti-pathogenic effect of B. subtilis C-3102 on chicks was evaluated, with the expected enhancement of immunostimulation and gut barrier function. For the evaluation of a preventive effect of C-3102 against S. enterica, the specific pathogen-free (SPF) chicks were challenged with Salmonella to eliminate the possibility of different microbial backgrounds interfering with the experiment.
Materials and Methods
Management of Birds and Diet
Hatching SPF eggs (vaccination-free) were obtained from a vaccine company (Nisseiken Co. Ltd., Japan) and hatched in an incubator P-05 (Showa Furanki Co. Ltd., Japan). All the animal studies were conducted from November 2016 to August 2017. In experiment-1, total 64 0-d-old SPF chicks (Lohmann valo, Germany) were randomly divided into two groups on day 0 (Fig. 1). The chicks in the control group (n=32) were fed a basal diet (Table 1) and those in the C-3102 group (n=32) were fed a basal diet supplemented with 1×106 CFU/g feed of B. subtilis C-3102 (commercially available as Calsporin®) (Silley, 2006) for 28 d. In experiment-2, total 120 0-d-old SPF chicks (Lohmann valo, Germany) were randomly divided into five groups (24 chicks each) on day 0 (Fig. 1), and the trials lasted for 28 d. The infection control and untreated groups were fed a basal diet (Table 1; Control and Normal, respectively). The third group was supplemented with a low dosage of C-3102 in the basal diet (3×105 CFU/g) (Low), the fourth group with a medium dosage (5×105 CFU/g) (Mid), and the fifth group with a high dosage (1×106 CFU/g) (High). All the chicks were housed in separate isolators of identical size (1.00 m×0.75 m) for each treatment group, and allowed ad libitum access to water and feed. The temperature at hatching was 32°C, which was reduced to 25°C until the end of the trial. All the chicks were exposed to 12 h cycles of light and dark. The experiments were approved by the animal welfare committee of Shoku-kan-ken, Inc.
Fig. 1.
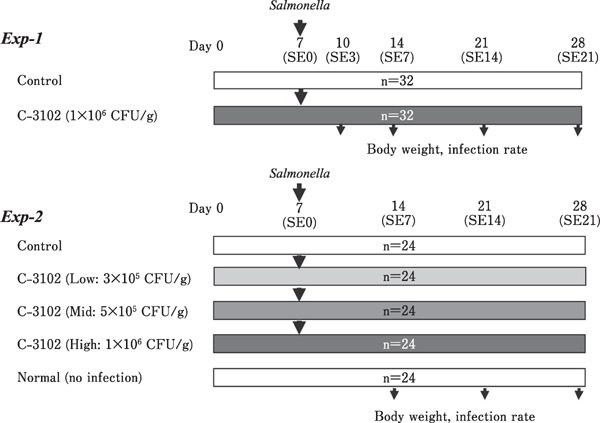
Treatment schedule for the challenge study with Salmonella enterica on the specific pathogen-free chicks in experiment-1 and -2. After feeding the Bacillus subtilis C-3102-containing feeds on Day 0, S. enterica was administered to the chicks on day 7 (SE0). Body weights, infection rates, and histology of the liver, spleen, and cecum were subsequently analyzed according to the scheme.
Table 1. Nutrient composition of the basal diet used in the present experimant.
| Ingredient (%) | Composition |
|---|---|
| Corn | 67.00 |
| Soybean meal | 22.00 |
| Fish meal | 5.00 |
| Other1 | 6.00 |
| Analyses | |
| Metabolisable energy (kcal/kg) | 2,850 |
| Crude protein (%) | 19.00 |
| Crude fat (%) | 2.50 |
| Crude fiber (%) | 5.00 |
| Crude ash (%) | 8.00 |
| Calcium (%) | 0.70 |
| Phosphorus, available (%) | 0.55 |
Other ingredients were composed of Alfalfa meal, Calcium carbonate, Salt, Vitamin premix, Mineral premix and Methionine. Vitamine premix: vitamine A, vitamine D3, vitamine E, vitamine K3, vitamine B1, vitamine B2, vitamine B6, vitamine B12, nicotinic acid, d-pantothenic acid, choline chloride and biotin. Mineral premix: manganese sulfate, zinc carbonate, iron sulfate, copper sulphate, cobalt sulfate and calcium iodate.
Salmonella Infection
For the evaluation of a preventive effect of C-3102 against S. enterica, the specific pathogen-free (SPF) chicks were administrated with Salmonella by a single dosage study with a diet containing 1×106 CFU/g of diet (Experiment-1). Then, a study with three different dosages of C-3102 was conducted on SPF chicks for 28 d (Experiment-2). Salmonella enterica serovar enteritidis LM-7 (nalidixic acid-resistant) were pre-cultured on nalidixic acid agar plates at 37°C for 24 h, followed by sub-culturing in the heart infusion broth at 37°C with shaking for 5 h. To determine the concentration of S. enterica in the infection solution, the inoculum was diluted with PBS, plated on a nalidixic acid agar plate, and incubated at 37°C for 24 h. The colonies grown were counted as CFUs. In experiment-1, 64 SPF chicks were divided into two groups on day 0, i.e., the control group (n=32) and the C-3102 group (n=32) supplemented with 1×106 CFU of C-3102/g of feed. All the chickens in both the groups were challenged with S. enterica (1.5×107 CFU/chicken) on day 7, and body weight and detection rate of S. enterica in the cecum, liver, and spleen were measured on days 3, 7, 14, and 21 post-challenge (Fig. 1). In experiment-2, 120 chicks (SPF) were divided into five groups (24 chicks/group) on day 0, and chicks of four groups were fed different dosages of C-3102 (1×106: High, 5×105: Med, 3×105: Low, and 0: Control, CFU/g of feed). On day 7, chickens in the C-3102 groups were challenged with S. enterica (2.8×108 CFU/chicken) by an oral gavage; however, the fifth group was not treated with S. enterica (Normal group) (Fig. 1).
Body Weight Measurement and Sample Collection
On days 3, 7, 14, and 21 of experiment-1 and on days 7, 14, and 21 of experiment-2 post-challenge of S. enterica (SE3, SE7, and SE14 in experiment-1, SE7, SE14, and SE21 in experiment-2), eight chicks per group were randomly selected, and samples (liver, spleen, and cecum content) were isolated for Salmonella analysis. All the collected samples were stored at 4°C and Salmonella count was analyzed within the day of sampling. All the chicks were weighed on day 0 and 7, and the eight selected chicks were weighed on days 3, 7, 14, and 21 in experiment-1, and on days 7, 14, and 21 in experiment-2 post-challenge.
Salmonella Measurement
Intestinal contents were collected from the cecum, serially diluted with PBS, and plated on a nalidixic acid agar plate. Then, the number of viable Salmonella cells on the agar plate was counted after incubation at 37°C for 24 h, followed by calculation of CFU/g. Residual cecum contents were diluted 10 times in nalidixic acid-containing broth, incubated at 37°C for 24 h, and subsequently inoculated in Hajna tetrathionate broth, followed by incubation at 42°C for 24 h. For S. enterica detection, each culture was plated on a nalidixic acid agar plate, and incubated at 37°C for 24 h. The collected liver and spleen tissues were chopped, diluted 10 times in nalidixic acid-containing broth, and homogenized by a stomacher (Seword, UK). Then, the homogenized samples were incubated at 37°C for 24 h, followed by inoculation and incubation in Hajna tetrathionate broth at 42°C for 24 h. For S. enterica detection, each culture was plated on a nalidixic acid agar plate and incubated at 37°C for 24 h. The detection rate of S. enterica was calculated as [the number of positive chick/8 chicks].
Statistical Analysis
In experiment-1, Student's t-test (equal variance) or Welch's test (unequal variance) was performed for the significant effects of B. subtilis C-3102 on body weight. In experiment-2, analysis of variance and Tukey's test were performed for the significant effects of B. subtilis C-3102 on body weight. Chi-square test was used for the effects on S. enterica-positive ratios in both the experiments. An alpha (α) level of 0.05 was used as threshold for statistical significance, and a P-value of 0.10 was considered to represent a trend. All the analyses were conducted using the statistical software “Statistix 10” (Analytical Software, USA).
Results
General Observations
To eliminate the influence of microbial background on the S. enterica infection, SPF chickens in floor pens were used to evaluate the protective effect of probiotic B. subtilis C-3102 against S. enterica infection. There were no significant differences in feed intake, performance, and fecal appearance between SPF chickens of the C-3102 and control groups throughout the experimental period (data not shown).
Experiment-1
In experiment-1, 64 SPF chicks were randomly divided into two groups on day 0, i.e., the control group (n=32) and the C-3102 group (n=32) supplemented with 1×106 CFU of C-3102/g of the feed (Table 1). All the chickens in both the groups were challenged with S. enterica (1.5×107 CFU/chicken) on day 7, and body weight and detection rate of S. enterica in the cecum, liver, and spleen were measured on days 3, 7, 14, and 21 post-challenge (Fig. 1). There was no significant difference in body weight between the control and C-3102 groups during the test period (Table 2). After challenging with S. enterica, high rates of infection were observed in the liver, spleen, and cecum of all the chickens in the control group on days 3, 7, 14, and 21 post-challenge (Fig. 2). These infection rates decreased to 12.5% in the liver and 50% in the spleen on day 21 post-challenge in the control group (Fig. 2). However, no infection in the liver was observed in the C-3102 group on day 14 post-challenge, and the infection rate was significantly lower than that of the control group. In the spleen, the infection rate on day 14 post-challenge showed a lower tendency than that of the control group. In the cecum, the infection rate observed in the C-3102 group was significantly lower than that of the control group on day 21 post-challenge. These results indicated that S. enterica was excluded from the liver within 3 weeks of infection, but these beneficial effects were delayed in the spleen and cecum. These results also revealed that the treatment with C-3102 might accelerate the exclusion of S. enterica from the liver, spleen, and cecum after an infection.
Table 2. Change of body weight of broiler chickens after challenging of Salmonella enteritidis in the 1st experiment.
| Body weight (g) |
P-value | ||
|---|---|---|---|
| Control | C-3102 | ||
| Day 0 | 39.2±2.1 | 39.2±2.4 | 0.98 |
| Day 7 | 58.2±3.4 | 60.2±6.0 | 0.11 |
| Day 3 after challenge | 75.2±4.0 | 77.0±7.2 | 0.55 |
| Day 7 after challenge | 111.2±7.5 | 113.3±7.2 | 0.59 |
| Day 14 after challenge | 187±17 | 194±17 | 0.47 |
| Day 21 after challenge | 257±24 | 266±31 | 0.51 |
Fig. 2.
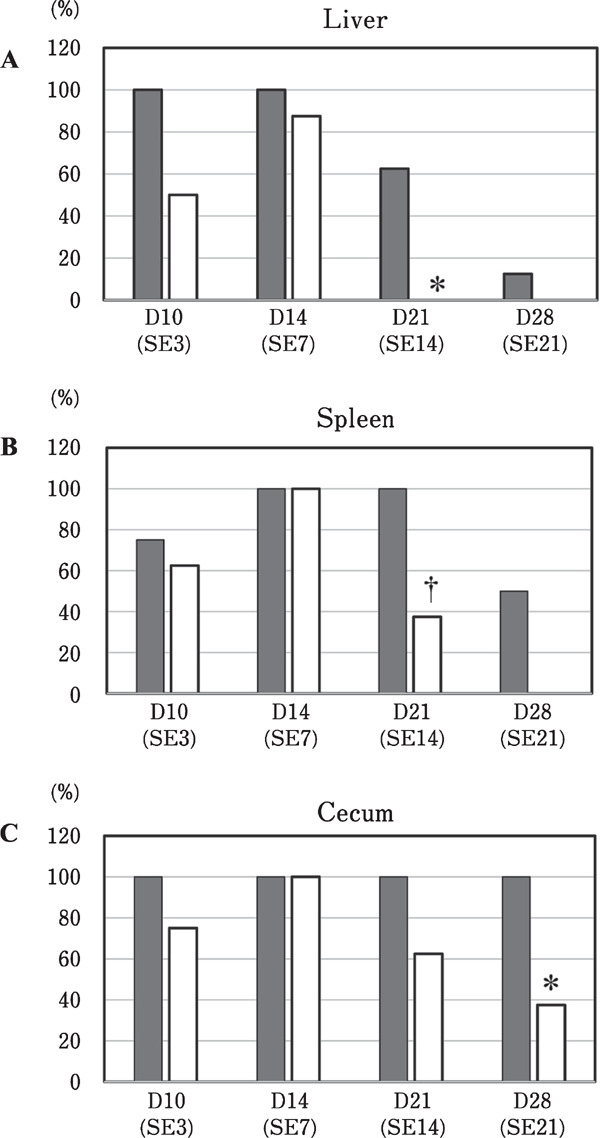
Changes in the infection rates in the liver (A), spleen (B), and cecum (C) of specific pathogen-free chicks at different time points after challenging (n=32/group) with Salmonella enterica on day 7. The experimental diet was either a basal diet (■) or a basal diet containing Bacillus subtilis C-3102 (□). Significant differences between the control and C-3102 groups are indicated as † P<0.10 and * P<0.05.
Experiment-2
In experiment-2, 120 SPF chicks were divided into five groups (24 chicks/group) on day 0, and chicks of four groups were fed different dosages of C-3102 (1×106: High, 5×105: Med, 3×105: Low, and 0: Control, CFU/g of feed). On day 7, chickens in the C-3102 groups were challenged with S. enterica (2.8×108 CFU/chicken), while the fifth group was kept untreated (Normal group) (Fig. 1). Interestingly, the C-3102 (High) group showed significant increases in the body weight compared to the control group on day 7 post-challenge (Table 3).
Table 3. Change of body weight of broiler chicken after challenging of Salmonella enteritidis in the 2nd experiment.
| Body weight (g) |
|||||
|---|---|---|---|---|---|
| Control | C-3102 (Low) | C-3102 (Med) | C-3102 (High) | Normal | |
| Day 0 | 42.36±2.5 | 42.3±2.7 | 42.4±2.6 | 42.4±2.6 | 42.4±2.5 |
| Day 7 | 59.33±4.9 | 61.2±6.1 | 59.5±4.6 | 60.9±4.5 | 60.2±4.5 |
| Day 7 after challenge | 108.42±4.6b | 113.0±3.5ab | 113.5±3.7ab | 114.4±2.7a | 110.0±4.9ab |
| Day 14 after challenge | 197.04±17 | 205±15 | 206.6±5.5 | 210.3±7.9 | 208.3±7.9 |
| Day 21 after challenge | 284.06±20 | 292±17 | 288±20 | 293±16 | 294±20 |
Within the same colum, means significant difference with different superscripts (P<0.05).
In experiment-2, S. enterica was detected in all the samples isolated from liver, spleen, and cecum from S. enterica-challenged groups on day 7 post-challenge (Fig. 3). On day 14 post-challenge, infection rates of S. enterica were significantly decreased in the liver. While the infection rate in the control group was 62.5%, S. enterica was completely excluded from the liver in the group treated with the highest dosage of C-3102 (1×106 CFU/g feed). The liver infection rate in the C-3102 (Med) group was insignificantly different from the control group on day 14 post-challenge, with the average rate of 50%. In the spleen, no significant differences were detected among all the groups in the infection rates on days 7, 14, and 21 post-challenge. However, averaged values showed a trend for higher infection rates in the control group (without C-3102 treatment) > Low group > Mid group > High group on days 14 and 21 post-challenge. In the cecum samples, a trend was observed for faster exclusion of S. enterica from the liver than from the cecum. Considering the above observations, S. enterica infection was completed within 7 days post-challenge, and was almost excluded from the liver and spleen on day 21 post-challenge in the control group. In contrast, supplementation of C-3102 was associated with shorter times for the exclusion of S. enterica from the chickens, in a dose dependent manner.
Fig. 3.
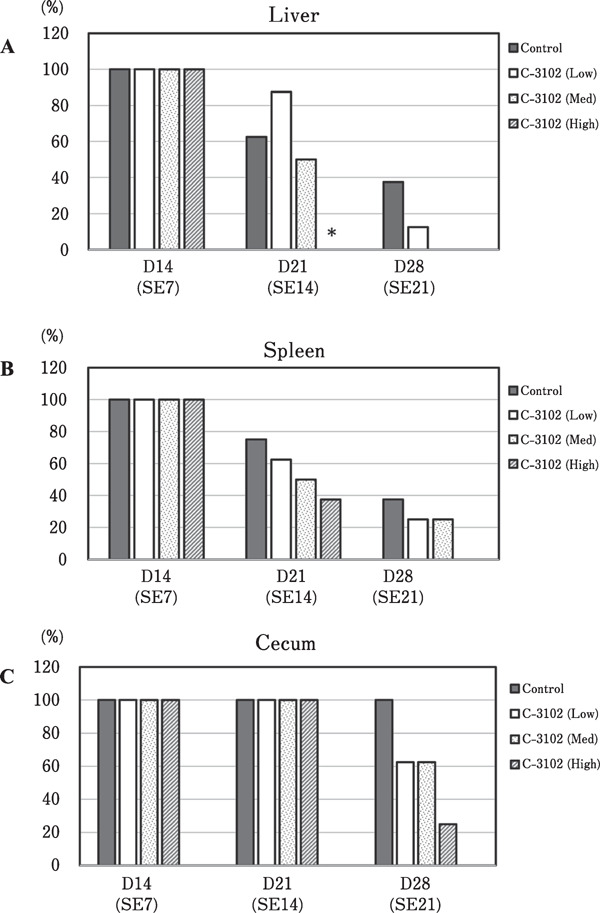
Changes in the infection rates in the liver (A), spleen (B), and cecum (C) of specific pathogen-free chicks at different time points after challenging (n=24/group) with Salmonella enterica on day 7. The experimental diet was either a basal diet or a basal diet containing low-dosage (3×105 CFU/g of diet), medium-dosage (5×105 CFU/g of diet), or high-dosage (1×106 CFU/g of diet) of C-3102. Significant differences between the control and C-3102 groups are indicated as * P<0.05.
Discussion
In this study, the anti-pathogenic effect of the spores formed by Bacillus subtilis C-3102 on Salmonella-challenged SPF chicks was examined. As a result, pre-treatment with C-3102 for 7 days induced increased shedding of S. enterica from the SPF chicks.
S. enterica detected in all the chickens 7 days post-challenge tended to be excluded within 3 weeks, probably due to the basic host immune response. However, shedding of S. enterica from the cecum, liver, and spleen was accelerated by pre-treatment with C-3102 (Figs. 2 and 3). Upon evaluating the treatment with probiotic Bacillus strains against Salmonella infection, supplementation of B. subtilis DSM17299 to the broiler chickens showed a reduction in the Salmonella counts in the cecum compared to that in the control group (Knarreborg et al., 2008). Salmonella infection in the broilers fed with B. subtilis DSM17299 diet was reduced to 58% compared to that in the positive control birds (100% infection), where a decrease in the intestinal pH by an increase of lactic acid bacteria provided by DSM17299 was a suggested reason (Knap et al., 2011). In our study, no change in the gut microflora was detected; however, an increase in the lactic acid bacteria and a decrease in the fecal pH in the broilers supplementation with C-3102 were observed in the previous study (Khaksefidi and Ghoorchi, 2006). Exclusion of S. enteritidis and Clostridium perfringens by the B. subtilis supplementation was considered to be caused by a change in the microflora composition and microbial competition (Videnska et al., 2013). Reduced lateral spread and improved shedding of S. enterica from the chicks by pre-treatment with B. subtilis PY79hr was also explained by competitive infection of S. enterica in the GI tract (La Ragione and Woodward, 2003). The idea that chicks pre-treated with the probiotics are potentially protected against pathogenic bacterial infections is an important concept to utilize the probiotic strains as feed additive ingredient.
For Salmonella infection in various tissues, the disruption of tight junctions between the epithelial cells in the GI tract may be a crucial event in the translocation and invasion of pathogenic bacteria into the blood stream. In the birds, the barrier function of the intestinal epithelium is considered to be modulated by the commensal microbiota (Van Deun et al., 2008; McCarville et al., 2016). Orally administered Bacillus licheniformis and Bacillus flexus spores induced the germinal centers of Peyer's patches (Xin et al., 2012). In another study, birds challenged with B. subtilis showed decreased crypt depth and increased villus height relative to control and Escherichia coli-challenged broilers (Manafi et al., 2017). Further, C-3102 supplementation showed a reduction in the bacterial translocation and protective gut barrier function in mice (Marubashi et al., 2001). In this study, S. enterica was detected in the cecum, liver, and spleen on day 3 post-challenge. This may have resulted from the damaged gut barrier function by S. enterica infection, its frequent invasion into the blood stream, and its delivery to the spleen and liver through the circulation. To understand the relationship between barrier function and Salmonella infection, histological analysis of the chicken guts will be addressed in the future studies.
Another possible explanation for reduced S. enterica counts in the SPF chickens upon C-3102 treatment, as observed in this study, is an immunostimulatory effect. In a study of laying breeders, the concentration of IgM and the level of influenza virus titer in the serum increased by dietary supplementation of B. subtilis C-3102 (Liu et al., 2019). Another study showed a stimulation of antigen presenting cells and T lymphocytes, which were markedly enhanced by a challenge with Bacillus species owing to enhanced expression of toll-like receptor (TLR) 2 and 4 genes (Xin et al., 2012). Pro-inflammatory cytokines, such as TNF-α, IFN-γ, IL-1β, IL-6, and IL-12 were induced by B. subtilis B4 treatment (Xi et al., 2012) through the stimulation of lipoteichoic acids (Opitz et al., 2001). However, the impact of C-3102 on the chicken immune system and the subsequent reduction of S. enterica infection in this study are unclear.
In conclusion, B. subtilis C-3102 supplementation over 3 weeks accelerates the exclusion of S. enterica from the cecum, liver, and spleen of the chickens in a dose-dependent manner. Further studies are needed to investigate the relationship between gut barrier function and gut immune responses against Salmonella infection during C-3102-induced protection.
Conflicts of Interest
All authors declare no conflict of interest in this study.
References
- Corthesy B, Gaskins HR and Mercenier A. Cross-talk between probiotic bacteria and the host immune system. Journal of Nutrition, 137: 781S-790S. 2007. [DOI] [PubMed] [Google Scholar]
- Cox AJ, Pyne DB, Saunders PU and Fricker PA. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. British Journal of Sports Medicine, 4: 222-226. 2010. [DOI] [PubMed] [Google Scholar]
- Fang H, Elina T, Heikki A and Seppo S. Modulation of humoral immune response through probiotic intake. FEMS Immunology and Medical Microbiology, 29: 47-52. 2000. [DOI] [PubMed] [Google Scholar]
- Foley SL, Nayak R, Hanning IB, Johnson TJ, Han J and Ricke SC. Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Applied and Environmental Microbiology, 77: 4273-4279. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Oliveira M, Mccauley T, Tauler P and Lawrence C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. International Journal of Nutrition and Metabolism, 4: 235-242. 2012. [DOI] [PubMed] [Google Scholar]
- Hatanaka M, Nakamura Y, Maathuis AJ, Venema K, Murota I and Yamamoto N. Influence of Bacillus subtilis C-3102 on microbiota in a dynamic in vitro model of the gastrointestinal tract simulating human conditions. Beneficial Microbes, 3: 229-36. 2012. [DOI] [PubMed] [Google Scholar]
- Hatanaka M, Yamamoto K, Suzuki N, Iio S, Takara T, Morita H, Takimoto T and Nakamura T. Effect of Bacillus subtilis C-3102 on loose stools in healthy volunteers. Beneficial Microbes, 9: 357-365. 2018. [DOI] [PubMed] [Google Scholar]
- Hooge DM, Ishimaru H and Sims MD. Influence of dietary Bacillus subtilis C-3102 spores on live performance of broiler chickens in four controlled pen trials. Journal of Applied Poultry Research, 13: 222-228. 2004. [Google Scholar]
- Jeong JS and Kim IH. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, noxious gas emission, and intestinal microflora in broilers. Poultry Science, 93: 3097-3103. 2014. [DOI] [PubMed] [Google Scholar]
- Khaksefidi A and Ghoorchi T. Effect of probiotic on performance and immunocompetence in broiler chicks. Journal of Poultry Science, 43: 296-300. 2006. [Google Scholar]
- Knap I, Kehlet AB, Bennedsen M, Mathis GF, Hofacre CL, Lumpkins BS, Jensen MM, Raun M and Lay A. Bacillus subtilis (DSM 17299) significantly reduces Salmonella in broilers. Poultry Science, 90: 1690-1694. 2011. [DOI] [PubMed] [Google Scholar]
- Knarreborg A, Brockmann E, Høybye K, Knap I, Lund B, Milora N and Leser TD. Bacillus subtilis (DSM17299) modulates the ileal microbial communities and improves growth performance in broilers. International Journal of Probiotics and Prebiotics, 3: 83-88. 2008. [Google Scholar]
- La Ragione RM and Woodward MJ. Competitive exclusion by Bacillus subtilis spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in young chickens. Veterinary Microbiology, 94: 245-256. 2003. [DOI] [PubMed] [Google Scholar]
- Leeson S. Future considerations in poultry nutrition. Poultry Science, 91: 1281-1258. 2012. [DOI] [PubMed] [Google Scholar]
- Liu J, Hu D, Chen Y, Huang H, Zhang H, Zhao J, Gu Z and Chen W. Strain-specific properties of Lactobacillus plantarum for prevention of Salmonella infection. Food & Function, 9: 3673-3682. 2018. [DOI] [PubMed] [Google Scholar]
- Liu X, Peng C, Qu X, Guo S, Chen JF, He C, Zhou X and Zhu S. Effects of Bacillus subtilis C-3102 on production, hatching performance, egg quality, serum antioxidant capacity and immune response of laying breeders. Journal of Animal Physiology and Animal Nutrition, 103: 182-190. 2019. [DOI] [PubMed] [Google Scholar]
- Manafi M, Khalaji S, Hedayati M and Pirany N. Efficacy of Bacillus subtilis and bacitracin methylene disalicylate on growth performance, digestibility, blood metabolites, immunity, and intestinal microbiota after intramuscular inoculation with Escherichia coli in broilers. Poultry Science, 96: 1174-1183. 2017. [DOI] [PubMed] [Google Scholar]
- Marubashi T, Imabayashi H and Maruta K. Bacterial translocation inhibitor and method of inhibiting bacterial translocation. US patent US 2009/0022690 A1. 2001.
- Maruta K, Miyazaki H, Masuda S, Takahashi M, Marubashi T, Tadano Y and Takahashi H. Feeding with Bacillus subtilis C-3102 and its influence on the intestinal microflora in broilers. Animal Science Technology (Jpn), 67: 273-280. 1996. [Google Scholar]
- Matulova M, Varmuzova K, Sisak F, Havlickova H, Babak V, Stejskal K and Rychlik I. Chicken innate immune response to oral infection with Salmonella enterica serovar Enteritidis. BMC Veterinary Research, 44: 37. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarville JL, Caminero A and Verdu EF. Novel perspectives on therapeutic modulation of the gut microbiota. Therapeutic Advances in Gastroenterology, 9: 580-593. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakphaichit M, Sobanbua S, Siemuang S, Vongsangnak W, Nakayama J and Nitisinprasert S. Protective effect of Lactobacillus reuteri KUB-AC5 against Salmonella Enteritidis challenge in chickens. Benefial Microbes, 8: 1-12. 2018. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Nakagawa K, Imabayashi T, Iwatani S, Yamamoto N and Tsushima N. Probiotic Bacillus subtilis C-3102 improves eggshell quality after forced molting in aged laying hens. Veterinary Medicine and Science, In press. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JK, Pajarillo EAB, Chae JP, Kim IH and Kang DK. Protective effects of Bacillus subtilis against Salmonella infection in the microbiome of Hy-Line Brown layers. Asian-Australasian Journal of Animal Sciences, 30: 1332-1339. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B, Schroder NW, Spreitzer I, Michelsen KS, Kirschning CJ, Hallatschek W and Schumann RR. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-kappa B translocation. Journal of Biological Chemistry, 276: 22041-22047. 2001. [DOI] [PubMed] [Google Scholar]
- Park JH and Kim IH. The effects of the supplementation of Bacillus subtilis RX7 and B2A strains on the performance, blood profiles, intestinal Salmonella concentration, noxious gas emission, organ weight and breast meat quality of broiler challenged with Salmonella typhimurium. Journal of Animal Physiology and Animal Nutrition, 99: 326-334. 2015. [DOI] [PubMed] [Google Scholar]
- Silley P. Do bacteria need to be regulated? Journal of Appled Microbiology, 101: 607-615. 2006. [DOI] [PubMed] [Google Scholar]
- Takimoto T, Hatanaka M, Hoshino T, Takara T, Tanaka K, Shimizu A, Morita H and Nakamura T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: a randomized, placebo-controlled, double-blind clinical trial. Bioscience of Microbiota, Food and Health, 37: 87-96. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoman CJ, Chia N, Jeraldo P, Sipos M, Goldenfeld ND and White BA. The microbiome of the chicken gastrointestinal tract. Animal Health Research Reviews, 13: 89-99. 2012. [DOI] [PubMed] [Google Scholar]
- Zhen W, Shao Y, Gong X, Wu Y, Geng Y, Wang Z and Guo Y. Effect of dietary Bacillus coagulans supplementation on growth performance and immune responses of broiler chickens challenged by Salmonella enteritidis. Poultry Science, 97: 2654-2666. 2018. [DOI] [PubMed] [Google Scholar]
- Van Deun K, Pasmansm F, Ducatelle R, Flahoum B, Vissenberg K, Martel A and Haesebrouck F. Colonization strategy of Campylobacter jejuni results in persistent infection of the chicken gut. Veterinary Microbiology, 130: 285-297 2008. [DOI] [PubMed] [Google Scholar]
- Videnska P, Sisak F, Havlickova H, Faldynova M and Rychlik I. Influence of Salmonella enterica serovar Enteritidis infection on the composition of chicken cecal microbiota. BMC Veterinary Research, 9: 140. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin X, Qin H, Yulong M, Zhiwen C, Yali L, Yi H, Imran RR, Dongyou Y and Weifen, L. Immunomodulatory effects of Bacillus subtilis (natto) B4 spores on murine macrophages. Microbiology and Immunology, 56: 817-824. 2012. [DOI] [PubMed] [Google Scholar]


