Abstract
Background
Gains in muscle mass and strength have been documented in exercise training with blood flow restriction (BFR). However, the impact of retrograde blood flow during BFR training on vascular health remains unclear. The present study designed a protocol to evaluate the acute effects of exercise performed with different levels of BFR on vascular reactivity and biomarkers of endothelial function, oxidative stress, and muscle hypertrophy in young and older individuals.
Methods and study design
Sixty-eight physically inactive eutrophic men [34 young (18–25-yrs old) and 34 elderly (≥65-yrs old)] will be included in the study. Subjects will undergo three experimental protocols: a) control (ExCON) – handgrip exercise with intensity of 30% of the maximum voluntary contraction (MVC); b) blood flow restriction (ExBFR) – handgrip exercise with a resistance of 30% of the MVC with low level of BFR [80% of arterial occlusion pressure at rest (rAOP)]; and c) arterial occlusion pressure (ExAOP) – handgrip exercise with a resistance of 30% of the MVC with high level of BFR (120% of rAOP). Primary outcomes will be: a) vascular reactivity assessed by venous occlusion plethysmography; b) endothelial function (nitric oxide and apoptotic endothelial micro particles; c) oxidative stress (thiobarbituric acid reactive substances). Growth hormone and lactate concentration will be measured as secondary outcomes reflecting the hypertrophic drive and metabolic stress, respectively.
Discussion
The findings of the present study may help to elucidate the age-related impacts of BFR training on the vascular health.
Keywords: Aging, Muscle mass, Muscle strength, Blood pressure, Health
Trial registration
This trial is registered in the Thai Clinical Trials Registry office (registration number: TCTR20191219002, date of registration: December 17, 2019).
1. Introduction
Prior research has shown that exercise with loads corresponding to 20–40% of maximal strength combined with blood flow restriction (BFR) is capable to promote increases in skeletal muscle mass and strength, even when compared to high intensity strength exercises [1,2]. The results of these studies also suggest that different levels of BFR can promote positive muscle hypertrophy and strength adaptations in older adults, suggesting that this type of intervention may be a training alternative for individuals who are not tolerant to high intensity exercise protocols [3].
Although potentially relevant due to its clinical applications in the elderly within preventive or rehabilitation settings [[2], [3], [4]], few studies have evaluated the effects of this mode of intervention on the vascular health [[5], [6], [7]]. Moreover, studies with different age groups produced conflicting results. In young individuals, BFR training seems to compromise the vascular function due to retrograde flow, with increased endothelial cell apoptosis eliciting pro-inflammatory and pro-atherogenic endothelial phenotype [8] or reductions in flow-mediated dilation [6,7,9]. In contrast, some prior studies reported unaltered [10] and even improved [5,11] endothelial function in the elderly.
In brief, data from studies investigating the age-related effects of exercise performed with BFR on endothelial function are limited and mixed. The wide methodological variability, particularly in which concerns the mechanical restriction of blood flow makes difficult to compare results among trials. The optimal level of BFR to produce gains in muscle mass and strength, while avoiding adverse effects in endothelial function in young and older individuals remains undefined. In addition, although recent evidence suggested that low-intensity RT with different levels of BFR may be effective to induce hypertrophy [3,[12], [13], [14]], age-related dose-response relationships regarding the level of BFR occlusion vs. adaptations in muscle function and endothelial dysfunction have not been clearly established.
Given the exposed, the present study intends to investigate the acute effects of physical exercise performed with different levels of BFR on the vascular reactivity of young and old men. In addition, potential mechanisms of muscular and vascular responses across the age groups will be analyzed through biomarkers of muscle hypertrophy, endothelial function, and oxidative stress. We will test the hypothesis that a session of physical exercise performed with different levels of BFR would be able to increase the blood concentration of markers related to hypertrophic and strength gains, while preserving the vascular function in both young and older individuals.
2. - Methods/design
2.1. Study design
This randomized controlled trial will be conducted at the Laboratory of Physical Activity and Health Promotion (LABSAU) and Laboratory for Clinical and Experimental Research on Vascular Biology (BIOVASC), both located in the University of Rio de Janeiro State (UERJ), Rio de Janeiro, Brazil. It has been registered at Thai Clinical Trials Registry (registration number: TCTR20191219002, date of registration: December 17, 2019) and approved by local Ethics Committee (CAAE: 69072916.8.0000.5282, opinion number 2.166.110, approved in July 10, 2017).
The participants will receive oral and written instructions about study risks and benefits and will be included in the study only after giving their written informed consent. The present study protocol complies with standards of the SPIRIT 2013 checklist (Standard Protocol Items: Recommendations for International Trials) [15].
2.2. Eligibility criteria
Two groups of physically inactive men will enroll in the study, 34 aged 18 to 25-yrs and 34 aged ≥65-yrs. Exclusion criteria include: smoking; mini-mental state exam score ≤13 points (only for the elderly); cardiovascular and metabolic disease; muscle skeletal disorders precluding handgrip exercise; and use of any medication interfering with the outcomes of the study or cardiovascular responses during exercise.
2.3. Recruitment of participants
Recruitment of volunteers will be carried out by the LABSAU and BIOVASC through institutional media and social networks from October 2019 to November 2020.
2.4. Sample size and randomization
The statistical power was calculated using the G*Power 3.1.9.4 (Universität Kiel, Kiel, Germany), considering the following parameters: a) statistical test: ANOVA – fixed effects (main effects and interactions); b) type of power analysis: a priori; c) effect size f = 0.5; d) α err prob. = 0.05; e) sample power of 0.80; f) numerator difference = 7; g) number of groups = 24, resulting in a total sample size of 68 individuals.
All participants will undergo the following experimental conditions, in a counter-balanced random order: a) ExCON – handgrip exercise with load corresponding to 30% of maximum voluntary contraction (MVC) without BFR; b) ExBFR – handgrip exercise with resistance of 30% of MVC and low-level BFR. In this protocol, vascular occlusion will be promoted by inflating an automatic pressure cuff to 80% of resting arterial occlusion pressure (rAOP); c) ExAOP – handgrip exercise with resistance of 30% of MVC and high-level BFR. In this condition, the automatic pressure cuff will be inflated up to 120% of rAOP.
2.5. Experimental design and sessions
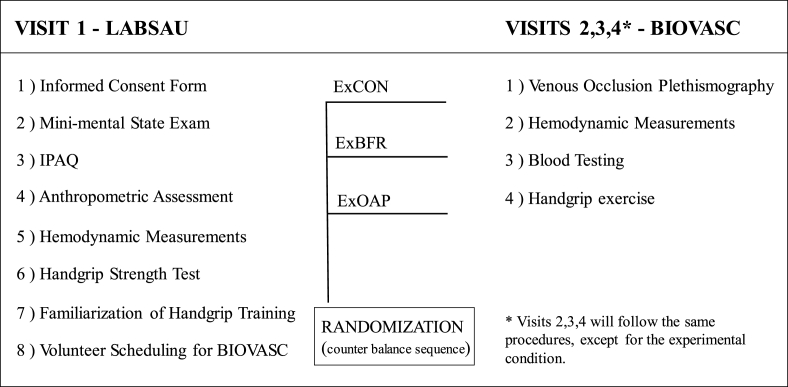
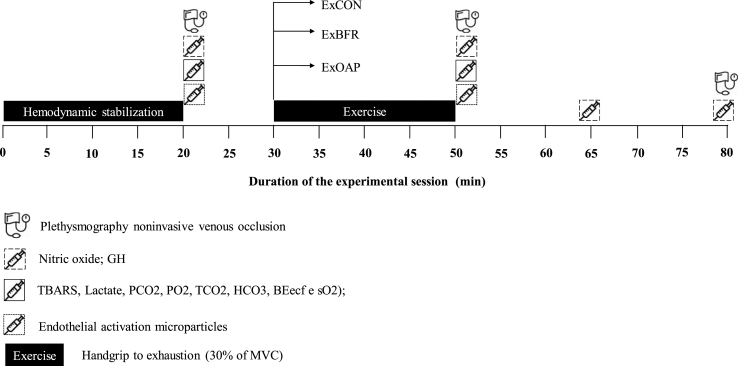
Fig. 1 depicts the overall study design, while Fig. 2 details the procedures applied in the experimental conditions (ExCON, ExBFR, and ExAOP). The participants will be instructed to fast for 8 h (for visit 2, 3 and 4), and to avoid alcohol or caffeinated beverages 24 h prior to experimental sessions. Visits 2, 3 and 4 will be interspersed by one week, all in the same time and conditions.
Fig. 1.
Flowchart of the experimental design.
ExCON – Control group; ExBFR – Blood flow restriction group; ExAOP – Arterial occlusion pressure; IPAQ – International physical activity questionnaires; LABSAU – Laboratory of Physical Activity and Health Promotion; BIOVASC – Laboratory for Clinical and Experimental Research on Vascular Biology.
Fig. 2.
Experimental sessions.
ExCON – Control group; ExBFR – Blood flow restriction group; ExAOP – Arterial occlusion pressure; GH – Growth Hormone; TBARS – Thiobarbituric acid reactive substances; PCO2 – Carbonic gas pressure; PO2 – Oxygen gas pressure; TCO2 – Total carbon dioxide; HCO3 – bicarbonate; BEecf – Base excess of the extracellular fluid; sO2 – saturation of oxygen; MVC - maximal voluntary contraction; *: The duration of the exercise will vary according to the time to exhaustion of volunteer in each protocol.
2.6. Handgrip exercise
Prior to experimental sessions, a familiarization session with the handgrip exercise will be allowed. Three sets of 30-s contractions with load corresponding to 30% of MVC will be performed every 2 s. A computer screen will be used to allow individuals to control the contraction intensity and cadence.
The acute handgrip exercise (Hand Dynamometer TSD121C, Biopac™ Systems Inc., Holliston, MA, USA) will be performed in the supine position with arms along the body and elbows extended until volitional fatigue. The load and cadence will be of 30% of MVC and 30 contractions per min (one contraction every 2 s), respectively. A notebook screen with 17.3 inches (DELL Inspiron, Round Rock, Texas, USA) will help participants to keep constant the intensity and cadence of contractions.
The upper left arm will be positioned beside the trunk at heart level and comfortably supported on a platform, with a cuff placed on the proximal third of the arm (Hokanson™, Bellevue, WA, USA). Cuff pressures will be set at 0%, 80% and 120% of rAOP, depending on the experimental condition. Perceived effort will be assessed every 30 s using the 10-point Borg scale [16]. The transducer (10 MHz) of a portable vascular Doppler (model DV 610B, Medmega™, Franca, SP, Brazil) positioned on the medial biceps will be used to confirm vascular occlusion during the BFR protocol.
2.7. Blood flow restriction
The BFR and AOP will be applied using a nylon cuff size (11 × 85 cm) connected to a pneumatic cuff inflator (Hokanson™ TD312, Bellevue, WA, USA) placed over the left arm and inflated to 80 or 120% of rAOP (in ExBFR or ExAOP, respectively). The occlusion will comply to the latest recommendation regarding the procedure [17]. The duration of BFR will be monitored per exercise protocol (ExAOP and ExBFR), and the pressure will be maintained throughout the whole exercise.
2.8. Potential harms
The participants presenting any clinical complication during the experiment will be conducted to the University Hospital. Adverse clinical events will be evaluated by the research team to determine severity. Serious events will be reported to ethic committee to take appropriate action. In the case of harm resulting from the management, design or conduct of the research, University insurance policies will apply.
2.9. Withdrawals
Volunteers can leave study at any time without providing a reason, and may choose to remove their collected data. Participants will be considered to be withdrawn if they request to leave the trial or are lost to follow-up (absence in one of the experimental sessions). Only data from participants who complete all the experimental sessions (visits 2, 3 and 4) and baseline assessment (visit 1) will be included in analysis.
2.10. Dissemination policy
The results of the study will be publicized in scientific congresses and journals. Data (unnamed) will be deposited in an appropriate data archive for sharing purposes. All personal data will be confidentially stored in folders by the main investigator and will not be shared to any third party without consent from the participants.
2.11. Data collection and accessibility
The research results, outcomes, and forms related to data collection will be available in the cloud (Dropbox) and can be consulted after permission granted by the main investigator. Data from participants who discontinue the intervention protocol will also be accessible.
2.12. Primary, secondary and other outcome measures and description of assessment instruments
The primary outcomes will be: a) vascular reactivity reflected by forearm blood flow assessed through venous occlusion plethysmography; b) biochemical markers of endothelial function (nitric oxide; NO and apoptotic endothelial microparticles; EMP) and oxidative stress (thiobarbituric acid reactive substances; TBARS). As secondary outcomes, growth hormone and lactate will be also measured as surrogates of muscle responses and local metabolic milieu. Further outcomes will be maximum strength measured by handgrip test, anthropometry (including body mass, height and body mass index calculation). The blood pressure at rest will also be measured, being used to determine BFR and AOP.
2.12.1. Primary outcomes
2.12.1.1. Vascular reactivity
Vascular reactivity will be evaluated by venous occlusion plethysmography (Hokanson™ AI6, Bellevue, WA, USA), as previously described [18]. Four measurements will be performed: a) forearm blood flow (FBF) at baseline 1; b) peak FBF during reactive hyperemia, after 5 min of forearm arterial occlusion with pressure 50 mmHg above systolic blood pressure; c) FBF at baseline 2; and d) peak FBF after 5 min of 0.4 mg sublingual nitroglycerin administration (Nitrolingual Burns Adler Pharmaceuticals™ Inc, Charlotte, NC, USA). FBF will be measured for 2 min at each stage in ml/min/100 mL tissue. Each phase will be performed interspersed with 3-min intervals, except between reactive hyperemia and baseline 2, when a 15-min interval will be allowed. Also, relative increments in FBF will be calculated: % Hyper = peak FBF in hyperemia ÷ FBF baseline 1 and % Nitro = peak FBF nitroglycerin ÷ FBF baseline 2.
2.12.1.2. Blood analysis
Blood samples will be harvested before exercise, immediately after, 15 min and 30 min postexercise. Data pre vs. immediately after will be used to estimate changes in metabolic stress (lactate), oxidative stress (TBARS), and endothelial function (EMP). Samples taken before exercise, immediately after, and following 15 min and 30 min postexercise will be used as markers of potential drive for muscle hypertrophy (GH) and endothelial function (NO).
The blood samples (100 μL of deproteinized serum) will be plated in 96-well microplates, and 100 μL of saturated solution of Vanadium (III) chloride (VCl3, Sigma-Aldrich, Saint Louis, MO, USA) at 8 mg/mL of 1 M HCl will be added. NO production will be measured by assaying for nitrite using the Griess method [19]. The two solutions of Griess reagent, Naftiletilenodiamine (Sigma-Aldrich, Saint Louis, MO, USA) at 0.1% in 5% phosphoric acid and p-sulfonamine aminobenzen (Sigma-Aldrich, Saint Louis, MO, USA) at 1% in 5% phosphoric acid (Sigma-Aldrich, Saint Louis, MO, USA) will be mixed at a ratio of 1:1 immediately prior to usage, after which 100 μL will be added to each sample; the samples will be then incubated for 15 min in the absence of light at room temperature. Next, the samples will be measured on a microplate reader (ELX 800, Bio Tek, Winooski, VT, USA) at 570 nm. NO will be calculated using a standard curve derived from solutions of sodium nitrite in the range of 10–200 μM [20].
The number of EMP released from endothelial apoptosis (EMP) will be determined by flow cytometry (Accuri C6 Plus, BD Biosciences, San Jose, CA, USA), as previously demonstrated [21]. Briefly, 100 μL of platelet poor plasma will be incubated with 8 μL of anti-CD31-Alexa 647 (BD Biosciences; Franklin Lakes, NJ, USA), 8 μL of anti-CD41a BB515 (BD Biosciences; Franklin Lakes, NJ, USA) monoclonal antibodies and 5 μL of Annexin V-PERCP-CY5.5 (BD Biosciences; Franklin Lakes, NJ, USA) for 30 min in the dark at 4 °C. Prior to flow cytometry analysis, each sample will be diluted with 900 μL of PBS. Microparticles CD41-/CD31+/Annexin V+ with diameter inferior than 1.1 μm.We will be consider apoptotic EMP.
The lipid peroxidation reflected by TBARS will be assessed as previously described [22]. Before and immediately after the exercise sessions, the blood will be collected into tubes with EDTA. It will be centrifuged for 10 min at 1000 and the plasma will be isolated to perform an assay for lipid peroxidation. The plasma (50 μL) will be mixed with 200 μL of 10% trichloroacetic acid (TCA, Sigma-Aldrich, Saint Louis, MO, USA) and 150 μL of potassium phosphate buffer (100 mM, pH 7.4) and incubated at room temperature for 10 min before centrifugation (2000 × g for 15 min). The supernatant will be collected and then, 500 μL of thiobarbituric acid (0.67%) will be added followed by an additional incubation at 95 °C for 60 min. The samples will be then cooled for 5 min and homogenized. Finally, the absorbance will be measured at 532 nm in a microplate reader, TP reader Thermo Plate. The MDA (malondialdehyde) concentrations will be evaluated using 1,1,3,3-Tetramethoxypropane (TMP, Sigma-Aldrich, Saint Louis, MO, USA) standard curve. The human plasma will be collected from the donor volunteers and stored at – 80 °C until analysis. For deproteinization, samples of 500 μL from each plasma sample will be centrifuged at 40,000 g during 1.5 h (4 °C) through a 30-kDa molecular weight filter (Amicon, Merck™, Darmstadt, Germany).
2.12.2. Secondary outcomes
2.12.2.1. Growth hormone
Assessment of serum GH will be performed using the Human Growth Hormone Immunoassay ELISA kit (R&D Systems™, Minneapolis, MN, USA). In short, concentration standards and samples (without prior dilution) will be added to the assay plate and during the incubation period the GH molecules will bind to specific monoclonal antibodies present at the bottom of the microplate wells. Then, a wash cycle will be performed to remove nonspecific binding and, at the end of this step, polyclonal antibodies conjugated to the peroxidase enzyme will be added to the plate, binding to the GH molecules trapped at the bottom of the plate.
Subsequently, a new wash cycle will be performed to remove nonspecific binding, and then the peroxidase enzyme substrate (hydrogen peroxide) and the chromogen (tetramethylbenzidine, TMB) will be added to the plate. At this stage, the peroxidase present in the sample wells or concentration patterns reacts with hydrogen peroxide and the reaction product oxidizes the TMB promoting the appearance of a blue color whose intensity is directly proportional to the concentration of the conjugated enzyme and consequently of GH.
At the end of the assay the enzymatic reaction will be inhibited with the addition of a sulfuric acid solution and then the optical density of each of the 96 wells of the microplate will be measured by a Universal Microplate Reader (ELX 800, Bio Tek™, Winooski, VT, USA). The GH concentration will be determined by correlating the optical density value of the samples with the optical density values of the standard curve generated by the 4-parameter logistic adjustment with the aid of an appropriate data analysis program (KC Junior, Bio Tek™, Winooski, VT, USA).
2.12.2.2. Lactate
After deproteinization using a 10 kDa MW cut-off spin filter (Amicon Ultra 0.5 Centrifugal Filter Unit 10 kDa, Merck™, Darmstadt, Germany), serum samples will be assayed using Lactate Assay kit II (Sigma-Aldrich, Saint Louis, MO, USA) according instructions provided by the kit manufacturer's. In brief, 46 μl deproteinized serum samples diluted 1:10 in lactate assay buffer will be mixed with 2 μL of lactate enzyme mix and 2 μL of lactate substrate constituting 50 μL of reaction mix per well. All wells will be homogenized by pipetting and then incubated at room temperature, protected from the light, during 30 min. Next, the absorbance of samples will be measured on a microplate reader (ELX 800, Bio Tek, Winooski, VT, USA) at 450 nm. Lactate concentration will be calculated using a standard curve ranging from 2 to 10 nmole/well.
2.12.3. Other outcomes
2.12.3.1. Handgrip strength
Exercise intensity will be fixed according to by MVC achieved during handgrip contraction (Hand Dynamometer TSD121C, Biopac™ Systems Inc., Holliston, MA, USA). Strength measurements will be performed with participants in the supine position, arms along the body, and elbows fully extended. Three MVC of 3 s will be performed interspersed with 1-min intervals. The highest value in kgf will be recorded as result.
2.12.3.2. Anthropometry
Body mass and height will be measured by means of a calibrated electronic scale (Filizola™, Sao Paulo, SP, Brazil) and wall stadiometer (Sanny™, Sao Paulo, SP, Brazil), respectively. The body mass index (BMI) will be calculated as the ratio between body mass and height squared (kg/m2).
2.12.3.4. Resting blood pressure
The blood pressure at rest will be measured in supine position, always in the morning (7–10 a.m.), in a controlled temperature room (22–25 °C) by semi-automated oscillometric device (LifeWindow LW6000, Digicare Biomedical Technology™, West Palm Beach, FL, USA), according to standard recommendations [23].
2.13. Data management
A research assistant will be responsible for filling a form including individual primary and secondary outcomes at each timeline point. The project manager will be responsible for checking the integrity of the completed printed form. The principal investigator will be responsible for initial data cleaning, identifying, and coding, as well as for converting the data into proper format for analysis.
2.14. Masking and blinding
In this study, it will not be possible to blind the participants and evaluators who will supervise the handgrip exercise sessions. On the other hand, researchers responsible for the analyses of primary and secondary outcomes, as well as for statistical calculations, will be blinded for the age-group and experimental conditions.
2.15. Statistical analysis
Data normality will be checked by the Shapiro Wilk test and data will be presented as mean and standard deviation or percentage, whenever appropriate. To compare the variables of interest, a 3-way ANOVA will be used (age-groups, exercise protocols, and time points), followed by the Tukey-Kramer Test verifications in the event of significant F ratios. In all cases, the significance level will be set at P ≤ 0.05 and calculations performed using the NCSS statistical software (LLC™, Kaysville, UT, USA).
3. Discussion
The BFR training is usually performed with low intensity (20–40% of repetition maximum) combined to the application of an external pressure on the upper or lower limbs through pneumatic cuff, resulting in reduction of arterial and venous blood flow [17]. This method has been shown to induce substantial gains of muscle mass and strength in healthy young [1] and older [2] adults with different clinical conditions [3,4,24,25]. However, the influence of age on vascular effects remains unclear, with some studies reporting deleterious responses in young subjects [6,8,9,26], while others observed preserved or improved responses in the elderly [5,10,11].
In the present protocol, the handgrip exercise will be used as model of resistance exercise. This type of exercise is well described in acute and chronic studies investigating the vascular responses associated with blood flow restriction [6,7], with intensities similar to ours, of approximately 30% of MVC [17]. Different levels of mechanical compression have been applied in BFR studies, with absolute pressures ranging from 100 to 200 mmHg) [12,14] or being relative to individual systolic BP [3,13,24,27]. In our study, we will apply pressures 20% above and below resting BP, as we understand that these levels are sufficient to engender relative vascular restriction effects with different magnitudes of retrograde blood flow. In addition, these selected levels of BFR have not been described in the literature as uncomfortable or painful.
As above mentioned, although muscle benefits seem to result from BFR training, the relationship between the level of cuff pressure to induce muscle gains vs. vascular health may be different across age groups [28]. To address this question, our study was designed to compare the after-effects of low-intensity resistance exercise performed with different levels of blood restriction or AOP (ExBFR or ExAOP protocols, respectively) on the vascular reactivity of young and older adults. We expect that markers of vascular function will remain unaltered, while the hypertrophic drive reflected by GH production will occur irrespective of the exercise intensity and level of BFR.
The outcomes will be assessed through venous occlusion plethysmography and biomarkers of endothelial function (NO and EMP), oxidative stress (TBARS), and muscle hypertrophy (GH). The venous occlusion plethysmography is extensively used to measure the vasodilation response during reactive hyperemia after 5-min arterial occlusion (reflected by higher endothelial dependent vasodilatation), and the integrity of smooth muscle cells (reflected by endothelial independent vasodilatation) in young and older individuals [18], as well as in response to physical exercise [3]. The blood biomarkers of endothelial function and oxidative stress are acknowledged to explain changes in vascular physiological mechanisms [11,29,30].
The GH will be measured as a surrogate of sufficient muscle stimulus capable to promote muscular gains, as a secondary outcome of the study. Albeit the focus of this trial is to investigate the effects of BFR training on vascular health, this information will allow to verify whether or not the selected levels of restriction are capable to stimulate hypertrophic responses. Additionally, the lactate concentration reflects local hypoxia and muscle metabolic milieu [31], and the assessment of this outcome will help to ensure that exercise sessions were similar in terms of metabolic stress.
All measurements will be performed before (baseline), immediately after, 15 min and 30 min after exercise, as literature has shown these moments would be appropriate to assess the dependent variables of interest [7,[29], [30], [31]]. The presence or absence of blood flow during the ExAOP and ExBFR protocols (interruption and maintenance of the auscultatory pulse of the brachial artery) will be controlled by a vascular Doppler. This strategy will assure that ExAPO and ExBRF provokes different blood flow patterns [24].
Our protocol has some limitations. Firstly, it is difficult to monitor sleep and diet of participants during the study. Although participants will be required to record their daily routines, this is perhaps not sufficient to control for potential confounding variables influenced by sleep and nutrition patterns, such as sympathetic drive and basal levels of inflammation. Moreover, it will be impossible to blind participants and evaluators for the type of exercise intervention. However, we will ensure that data managers and statisticians are not aware of treatment allocations. Finally, we will not be able to directly measure the blood flow. This would be useful to verify the magnitude of retrograde blood flow.
The findings of this study will be novel and might contribute with the current knowledge, by expanding the discussion about the effectiveness and safety of BFR training in young and older populations. Our data will possibly warrant further studies testing different combinations of load and occlusion within BFR training in populations with poor capacity to exercise with intense loads.
Trial status
Actively recruiting. Start date: November 2019. Expected completion date: November 2020.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
BFR: blood flow restriction; MVC: maximum voluntary contraction; rAOP: Arterial Occlusion Pressure at rest; CON: control; AOP: arterial occlusion pressure; mTOR: mammalian target of rapamycin; CSA: cross-sectional area; RT: resistance training; FMD: flow mediated dilatation; LABSAU: Laboratory of Physical Activity and Health Promotion; BIOVASC: Laboratory for Clinical and Experimental Research on Vascular Biology; UERJ: Rio de Janeiro State University; HUPE: Pedro Ernesto Hospital; UNATI: University of the Third Age; BMI: body mass index; NO: nitric oxide; TBARS: thiobarbituric acid reactive substances; GH: growth hormone; EMP: apoptotic endothelial microparticles.
Author contributions statement
All authors will contribute to the data analyses and interpretation, drafting and revising the final version of manuscript. The study design was made by GGC, KGL and RBO and discussed with all authors. GGC, KGL, PF, DAB, MGCS and RBO reviewed the versions of this study protocol. The principal investigator will be RBO. The project administration and supervision will be performed by RBO and DAB. The laboratories (BIOVASC and LABSAU) where the study will occur are coordinated by EB and PF, respectively.
Sponsor
The project will be supported by grants from the Carlos Chagas Filho Foundation for the Research Support in the State of Rio de Janeiro (FAPERJ, processes E-26/010.002490/2016, recipient DAB and E-26/110.184/2013, recipient PF), and National Council for the Technological and Scientific Development (CNPq, process 304798/2016-9, recipient PF). These agencies do not play any role in the design of the study, data collection, analysis, or interpretation, and manuscript writing. FAPERJ and CNPq require to be mentioned as sponsors in any intellectual production resulting from their support, not imposing other requirements.
Ethics approval and consent to participate
The Ethical Board of the University of Rio de Janeiro State approved this study (CAAE: 69072916.8.0000.5282). Informed consent will be obtained from all participants.
Conflict of interest statement
The authors declare that the research is conducted in the absence of any commercial or financial relationships that could be considered a potential conflict of interest.
References
- 1.Lixandrão M.E., Ugrinowitsch C., Berton R., Vechin F.C., Conceição M.S., Damas F., Libardi C.A., Roschel H. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: a systematic review and meta-analysis. Sports Med. 2018;48:361–378. doi: 10.1007/s40279-017-0795-y. [DOI] [PubMed] [Google Scholar]
- 2.Centner C., Wiegel P., Gollhofer A., König D. Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: a systematic review and meta-analysis. Sports Med. 2019;49 doi: 10.1007/s40279-018-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes K.G., Alexandre Bottino D., Farinatti P., Coelho De Souza M. das G., Alves Maranhão P., De Araujo C.M.S., Bouskela E., Alves Lourenço R., De Oliveira R.B. Strength training with blood flow restriction – a novel therapeutic approach for older adults with sarcopenia? A case report. Clin. Interv. Aging. 2019;14:1461–1469. doi: 10.2147/CIA.S206522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes L., Paton B., Rosenblatt B., Gissane C., Patterson S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br. J. Sports Med. 2017;51:1003–1011. doi: 10.1136/bjsports-2016-097071. [DOI] [PubMed] [Google Scholar]
- 5.Schreuder T.H.A., Green D.J., Hopman M.T.E., Thijssen D.H.J. Impact of retrograde shear rate on brachial and super fi cial femoral artery fl ow-mediated dilation in older subjects. Atherosclerosis. 2015:1–6. doi: 10.1016/j.atherosclerosis.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Credeur D.P., Hollis B.C., a Welsch M. Effects of handgrip training with venous restriction on brachial artery vasodilation. Med. Sci. Sports Exerc. 2010;42:1296–1302. doi: 10.1249/MSS.0b013e3181ca7b06.Effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paiva F.M., Vianna L.C., Fernandes I.A., Nóbrega A.C., Lima R.M. Effects of disturbed blood fl ow during exercise on endothelial function : a time course analysis. Braz. J. Med. Biol. Res. 2016;49:1–9. doi: 10.1590/1414-431X20155100. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins N.T., Padilla J., Boyle L.J., Credeur D.P., Laughlin M.H., Fadel P.J. Disturbed blood flow acutely induces activation and apoptosis of the human vascular endothelium. Hypertension. 2013;61:615–621. doi: 10.1161/HYPERTENSIONAHA.111.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thijssen D.H.J., Dawson E.A., Tinken T.M., Cable N.T., Green D.J. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53:986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda T., Fukumura K., Fukuda T., Uchida Y., Iida H., Meguro M., Sato Y., Yamasoba T., Nakajima T. Muscle size and arterial stiffness after blood flow-restricted low-intensity resistance training in older adults. Scand. J. Med. Sci. Sports. 2014;24:799–806. doi: 10.1111/sms.12087. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu R., Hotta K., Yamamoto S., Matsumoto T. Low - intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur. J. Appl. Physiol. 2016 doi: 10.1007/s00421-016-3328-8. [DOI] [PubMed] [Google Scholar]
- 12.Fry C.S., Glynn E.L., Drummond M.J., Timmerman K.L., Fujita S., Abe T., Dhanani S., Volpi E., Rasmussen B.B., Cs F., El G., Mj D., Kl T., Fujita S. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J. Appl. Physiol. 2010:1199–1209. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J., Lang J.A., Pilania N., Franke W.D. Effects of blood flow restricted exercise training on muscular strength and blood flow in older adults. Exp. Gerontol. 2017;99:127–132. doi: 10.1016/j.exger.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Takarada Y., Takazawa H., Sato Y., Takebayashi S., Tanaka Y., Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000;88:2097–2106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 15.Chan A.W., Tetzlaff J.M., Altman D.G., Laupacis A., Gøtzsche P.C., Krleža-Jerić K., Hróbjartsson A., Mann H., Dickersin K., Berlin J.A., Doré C.J., Parulekar W.R., Summerskill W.S.M., Groves T., Schulz K.F., Sox H.C., Rockhold F.W., Rennie D., Moher D. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann. Intern. Med. 2013 doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borg G.A.V. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 17.Patterson S.D., Hughes L., Warmington S., Burr J., Scott B.R., Owens J., Abe T., Nielsen J.L., Libardi C.A., Laurentino G., Neto G.R., Brandner C., Martin-Hernandez J., Loenneke J. Blood flow restriction exercise position stand: considerations of methodology, application, and safety. Front. Physiol. 2019;10:1–15. doi: 10.3389/fphys.2019.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bottino D.A., Lopes F.G., de Oliveira F.J., Mecenas A. de S., Clapauch R., Bouskela E. Relationship between biomarkers of inflammation, oxidative stress and endothelial/microcirculatory function in successful aging versus healthy youth: a transversal study. BMC Geriatr. 2015;15:41. doi: 10.1186/s12877-015-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982 doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 20.Stuehr D.J., Marletta M.A. Proceedings of the National Academy of Sciences of the United States of America. 1985. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha H.N.M., Garcia V.P., Batista G.M.S., Silva G.M., Mattos J.D., Campos M.O., Nóbrega A.C.L., Fernandes I.A., Rocha N.G. Disturbed blood flow induces endothelial apoptosis without mobilizing repair mechanisms in hypertension. Life Sci. 2018;209:103–110. doi: 10.1016/j.lfs.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Keles M.S., Taysi S., Sen N., Aksoy H., Akçay F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can. J. Neurol. Sci. 2001 doi: 10.1017/S0317167100052823. [DOI] [PubMed] [Google Scholar]
- 23.Pickering T.G., Hall J.E., Appel L.J., Falkner B.E., Graves J., Hill M.N., Jones D.W., Kurtz T., Sheps S.G., Roccella E.J. Recommendations for blood pressure measurement in humans and experimental animals Part 1: blood pressure measurement in humans A statement for professionals from the subcommittee of professional and public education of the American heart association counc. Circulation. 2005;111:697–716. doi: 10.1161/01.hyp.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 24.Gualano B., Neves M., Lima F.R., Pinto A.L.D.S., Laurentino G., Borges C., Baptista L., Artioli G.G., Aoki M.S., Moriscot A., Lancha A.H., BonfÁ E., Ugrinowitsch C. Resistance training with vascular occlusion in inclusion body myositis: a case study. Med. Sci. Sports Exerc. 2010;42:250–254. doi: 10.1249/MSS.0b013e3181b18fb8. [DOI] [PubMed] [Google Scholar]
- 25.Kambic T., Novakovic M., Tomazin K., Strojnik V., Jug B. Blood flow restriction resistance exercise improves muscle strength and hemodynamics, but not vascular function in coronary artery disease patients: a pilot randomized controlled trial. Front. Physiol. 2019;10:1–11. doi: 10.3389/fphys.2019.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paiva F.M., Vianna L.C., Fernandes I.A., Nóbrega A.C., Lima R.M. Effects of disturbed blood flow during exercise on endothelial function: a time course analysis. Braz. J. Med. Biol. Res. 2016;49:1–9. doi: 10.1590/1414-431X20155100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takano H., Morita T., Iida H., Asada K.I., Kato M., Uno K., Hirose K., Matsumoto A., Takenaka K., Hirata Y., Eto F., Nagai R., Sato Y., Nakajima T. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur. J. Appl. Physiol. 2005;95:65–73. doi: 10.1007/s00421-005-1389-1. [DOI] [PubMed] [Google Scholar]
- 28.da Cunha Nascimento D., Schoenfeld B.J., Prestes J. Potential implications of blood flow restriction exercise on vascular health: a brief review. Sports Med. 2019 doi: 10.1007/s40279-019-01196-5. [DOI] [PubMed] [Google Scholar]
- 29.Vion A.C., Ramkhelawon B., Loyer X., Chironi G., Devue C., Loirand G., Tedgui A., Lehoux S., Boulanger C.M. Shear stress regulates endothelial microparticle release. Circ. Res. 2013;112:1323–1333. doi: 10.1161/CIRCRESAHA.112.300818. [DOI] [PubMed] [Google Scholar]
- 30.Eminice R.A.D., Icchieri T.I.S., Ialich M.I.S.M., Ilani F.R.M., Vidio P.A.P.O. Oxidative stress biomarker responses to an acute session of hypertrophy-resistance traditional interval training and circuit training. J. Strength Condit Res. 2011;25:798–804. doi: 10.1519/JSC.0b013e3181c7bac6. [DOI] [PubMed] [Google Scholar]
- 31.Takarada Y., Nakamura Y., Aruga S., Onda T., Miyazaki S., Ishii N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 2000;88:61–65. doi: 10.1152/jappl.2000.88.1.61. [DOI] [PubMed] [Google Scholar]