Abstract
Objective
Flavopereirine has been identified to be a potential anti-cancer agent in several types of human cancer. This study aimed to investigate the anti-cancer activity of flavopereirine in oral cancer.
Methods
The effect of flavopereirine on cell viability of human oral cancer cell lines (BcaCD885 and Tca8113) was evaluated by MTT assay and colony formation assay. Cell apoptosis and cell cycle distribution were detected by flow cytometry. Cell invasion and migration were evaluated by Transwell assay. The expression of LASP1, JAK2, p-JAK2, STST3, p-STST3, STST5 and p-STST5 was evaluated by qRT-PCR and Western blot. In addition, the xenograft mouse model was constructed to determine the anti-cancer role of flavopereirine in vivo.
Results
Flavopereirine significantly inhibited cell proliferation, invasion, migration and EMT process of BcaCD885 and Tca8113 cells, while promoted cell apoptosis in vitro. Flavopereirine markedly decreased the expression levels of p-JAK2, p-STST3 and p-STST5, while increased the expression levels of LASP1. In addition, downregulation of LASP1 significantly increased the expression levels of p-JAK2, p-STAT3 and p-STAT5 compared with si-NC in BcaCD885 cells. Moreover, flavopereirine was found to decrease tumor weight and volume of xenograft tumors in vivo.
Conclusion
Flavopereirine inhibited the progression of oral cancer through inactivating the JAK/STAT signaling pathway by upregulating LASP1, suggesting that flavopereirine might be a potential anti-cancer agent for oral cancer.
Keywords: oral cancer, flavopereirine, LASP1, JAK/STAT signaling pathway, tumorigenicity
Introduction
Oral squamous cell carcinoma (OSCC) has become the sixth most common malignancy worldwide with an approximate incidence of over 300,000 newly diagnosed cases every year.1 Increasing evidence has revealed that smoking and alcohol consumption are the primary environmental factors resulting in the occurrence and development of OSCC.2 Although significant improvement in molecular diagnostics and therapeutics of OSCC has been made, the morbidity rate is still high and the 5-year survival of OSCC patients is low.3 The standard therapeutic strategy for OSCC is surgery, usually accompanied by radiotherapy and chemotherapy.4 Therefore, identification and development of effective therapeutic drugs against OSCC are urgently needed.
Flavopereirine (3-ethyl-12H-indolo [2,3-a]-quinolizinium perchlorate), a natural β-carboline alkaloid, is isolated from Geissospermum vellosii (Pao pereira).5 Previous studies have revealed that flavopereirine exhibits potent anti-cancer activities in various human cancers. For example, flavopereirine inhibits the growth of colorectal cancer cells via modulating P53 signaling dependence.6 Flavopereirine induces cell cycle arrest and apoptosis of human breast cancer cells by regulating the AKT/p38 MAPK/ERK1/2 signaling pathway.7 Flavopereirine has also been found to decrease cell viability of drug-resistant glioblastoma cells and inhibits IL-6-activated proliferation.8,9 In addition, the anticancer agent PB-100 that contains flavopereirine and dihydroflavopereirine can selectively suppress the proliferation of human melanoma cells but not their normal counterparts.10 All these studies suggest that flavopereirine has potential anti-cancer activities in cancers, therefore, this study was carried out to investigate the effect of flavopereirine in OSCC and the underlying mechanisms.
Activation of the JAK/STAT signaling pathway is observed in a variety of human tumors such as breast cancer,11 prostate cancer,12 non-small lung cancer,13 and OSCC.14 Increasing evidence has demonstrated that the constitutive activation of STAT contributes to the proliferation and oncogenesis through regulating the expression of a large number of genes required for the survival, proliferation, invasion and metastasis of tumor cells.15,16 Therefore, the JAK/STAT signaling plays a crucial role in tumorigenesis and is considered as a potential therapeutic target for novel drug development. In OSCC, astaxanthin, a non-provitamin A carotenoid that is mainly found in microalgae, fungi, plants, sea foods and some birds, can be potentially applied for the prevention and treatment of various diseases including OSCC.17,18
In the present study, we explored the role of flavopereirine in oral cancer. Our study demonstrated that flavopereirine could significantly inhibit proliferation, invasion, migration and EMT processes, while induced apoptosis of human oral cancer cells in vitro. Meanwhile, flavopereirine obviously inhibited tumor growth in a mouse model in vivo. Furthermore, flavopereirine inhibited the activation of the JAK/STAT signaling pathway by promoting the expression of LASP1. Our findings demonstrated that flavopereirine might be a potential anti-cancer agent for oral cancer.
Materials and Methods
Reagent Information
Flavopereirine (monohydrate, purity >98%) was purchased from Herbpurify (Chengdu, China). The structural formula is shown in Figure 1A. All other chemicals and reagents were commercially available and used as received. The pan-caspase inhibitor (Z-VAD-FMK) was purchased from Sigma Aldrich.
Figure 1.
Flavopereirine inhibited proliferation of human oral cancer cells. (A) The structural formula of flavopereirine. (B and C) The effect of different concentrations of flavopereirine on the proliferation of BcaCD885 (B) and Tca8113 cells (C) was evaluated by MTT assay. (D and E) BcaCD885, Tca8113 and HOES cells were treated with different concentrations of flavopereirine, and cell viability at 72 h (D) and 96 h (E) was detected by MTT assay. (F and G) The effect of different concentrations of flavopereirine on the colony formation ability of BcaCD885 (F) and Tca8113 cells (G). **P < 0.01, ***P < 0.001 vs 0 μmol/L flavopereirine group.
Cell Culture
Human oral cancer cell lines BcaCD885 and Tca8113, and normal oral epithelial cell line HOES were obtained from the American Type Tissue Culture Collection (ATCC). All cells were cultured in RPMI-1640 medium (Solarbio, Beijing, China) containing 10% fetal bovine serum (FBS, Gibco BRL, Grand Island, NY, USA), 100 unit/mL penicillin and 0.1 mg/mL streptomycin at 37°C with 5% CO2. Different concentrations of flavopereirine (25, 50, and 100 μmol/L) were added and incubated for 48 h. When needed, 20 μmol/L Z-VAD-FMK or 100 μmol/L flavopereirine was added to stimulate BcaCD885 and Tca8113 for 48 h.
MTT Assay
Cell proliferation was evaluated using MTT Cell Viability Assay Kit (Invitrogen; Carlsbad, CA, USA) following the manufacturer’s instructions. Briefly, approximately 1 × 104 BcaCD885 and Tca8113 cells were seeded into 96-well plates and treated with different concentrations of flavopereirine for 24, 48 and 72 h. Subsequently, 10 μL of MTT stock solution was added and incubated at 37°C for another 4 h. Then, 100 μL of dissolution reagent was added and incubated for 4 h. Finally, the absorbance at 490 nm was detected under a microplate reader (TECAN-infinite M200pro, Mannedorf, Switzerland).
Colony Formation Assay
Approximately 1000 BcaCD885 and Tca8113 cells were seeded into 6-well plates and cultured for 2 weeks. After washing with PBS, cells were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet for 15 min. Subsequently, cells were counted and photographed under a light microscope.
Transwell Assay
Cell invasion and migration were evaluated by Transwell assay as previously described.19 For invasion assay, 24-well Transwell™ plates with 8.0-μm-pore Matrigel™-coated membranes were applied (Corning, NY). Lower chambers were added with RPMI-1640 supplementing with 10% FBS, and the upper chambers were filled with 200 μL cell suspension. After 24 h of incubation, cells were fixed by 4% paraformaldehyde and stained with 0.1% crystal violet. The migration assay was performed similarly without coating the membranes with Matrigel™ plates. Ten random fields were photographed, and cells were counted under a microscope.
Hoechst33342 Staining Assay
Cells were plated into 6-well plates. After treating with different concentrations of flavopereirine for 48 h, cells were fixed with 4% paraformaldehyde at the room temperature for 10 min, and then stained with 1 μg/mL Hoechst33342 for 5 min. Subsequently, cells were washed with PBS and imaged using a fluorescence microscope.
Cell Transfection
To silence the expression of LASP1 in BcaCD885 and Tca8113 cells, siRNAs targeting LASP1 (si-LASP1) or negative control (si-NC) were transfected into cells using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Si-LASP1 and si-NC were purchased from RIBOBIO (China). After transfection for 48 h, cells were treated with or without different concentrations of flavopereirine and collected for subsequent experiments. The sequences for si-LASP1 and si-NC were listed as follows: si-LASP1: 5ʹ-UATUCGCTTCGUUGACGCUAA-3ʹ; si-NC: 5ʹ-AAGGTGAACTGTCTGGATAAG-3ʹ.
Cell Cycle and Apoptosis Assay
The distribution of the phases of cell cycle of BcaCD885 and Tca8113 cells was evaluated using a propidium iodide (PI) staining method. Briefly, cells were seeded into 6-well plates and treated with different concentrations of flavopereirine for 48 h, and then fixed with 75% ethanol at −20°C overnight. On the next day, cells were stained with PI for 20 min and analyzed by FACS Caliber (BD Company, USA). For apoptosis analysis, flavopereirine-treated cells were washed with PBS and re-suspended in the binding buffer (BD Bioscience, USA). Then, 100 μL cell suspension was incubated with 5 μL Annexin V-FITC (1:100) and 5 μL PI in the dark. The apoptotic cells were quantified by FACS Caliber (BD Company, USA).
RNA Extraction and qRT-PCR
Total RNAs were extracted from cultured cells using TRIzol reagent (Huamai, Beijing, China). RNA samples were reversely transcribed into cDNA by using a Reverse Transcription Kit (Takara). qRT-PCR analysis was performed using SYBR Green (Takara) on an ABI 7500 Detection System (Applied Biosystems, Foster City, CA, USA). The relative expression levels of targets were analyzed by 2−ΔΔCt method with GAPDH as the internal reference. The primers used in this study were listed as follows: LASP1 forward: 5′- AGGGAGTTGTGCCCATTTTG −3′, reverse: 5′- AGCAGATGCCCATGCTTTCT −3′; GAPDH forward: 5′- GAAGGTGAAGGTCGGAGTC −3′; reverse: 5′- GAAGATGGTGATGGGATTTC −3′.
Western Blot
Cells were lysed using the RIPA Lysis Buffer (Beyotime, Beijing, China) following the manufacturer’s instructions. Protein concentration was determined using the bicinchoninic acid (BCA) colorimetric assay. The equal amount of protein samples were separated by SDS-PAGE and then transferred to PVDF membranes (Millipore). After blocking with 5% skim milk, the membranes were incubated with primary antibodies against Cyclin D1 (1:1000), CDK1 (1:1000), CDC2 (1:1000), Vimentin (1:1000), snail (1:1000), slug (1:1000), E-cadherin (1:1000), E-cadherin (1:1000), Cleaved-Caspase3 (1:1000), Cleaved-Caspase3 (1:1000), Bcl-2 (1:1000), Bax (1:1000), JAK2 (1:1000), p-JAK2 (1:1000), STAT3 (1:1000), p-STAT3 (1:1000), STAT5 (1:1000), p-STAT5 (1:1000) and β-actin (1:1000, Ab8227, Cambridge, MA) at 4°C overnight. All primary antibodies were purchased from Abcam. Subsequently, the membranes were exposed to horseradish peroxidase (HRP) conjugated secondary antibodies (1:1000) for 1 h. Finally, the protein bands were visualized by an enhanced chemiluminescence (ECL) reagent with Bio-Rad imaging system.
Xenograft Model
BALB/c nude mice (male, 4 weeks old) were purchased from Beijing HFK Bioscience Co. and kept under standard conditions. The xenograft model was constructed as previously described with minor modification.20 In brief, approximately 1 × 106 BcaCD885 cells were subcutaneously inoculated into the flanks of each mouse. When tumor volume reached ~50 mm3, all mice were randomly divided into two groups (n = 8): vehicle group and control group. Mice in model group were daily treated with flavopereirine (5 mg/kg body weight, prepared in 25% PEG) for 5 d per week, while mice in the control group were simultaneously treated with normal saline of the same concentration. Meanwhile, tumor volume was evaluated twice every week. After 5 weeks, tumor volume (V) was calculated using the following formula: V = 0.524 × length × width.2 Subsequently, mice were sacrificed by cervical dislocation, and then tumors were removed and weighted. All animal experiments were approved by the Animal Research Ethics Committee of Fujian Provincial Hospital, and were performed according to the Guide for the Care and Use of Laboratory Animals published by the National Research Council in China.
Immunohistochemistry (IHC) Staining
Xenograft tumors were removed and fixed in 10% neutral buffered formalin, embedded in paraffin, and cut into sections with 4 μm in thickness. Then the sections were immunohistochemically stained with anti-Ki67 antibody (Cell Signaling Technology) as previously described.21
Statistical Analysis
All data were presented as mean ± standard deviation (SD) and analyzed by GraphPad Prism 6.0 (San Diego, CA). Difference between the two groups was determined by LSD-t-test and two-way ANOVA was used for multiple comparisons. P < 0.05 was considered statistically significant. All experiments were repeated at least 3 times.
Results
Flavopereirine Inhibited Proliferation of Human Oral Cancer Cells in vitro
To explore the effect of flavopereirine, BcaCD885 and Tca8113 cells were treated with different concentrations of flavopereirine (25, 50, and 100 μmol/L). Cell viability was evaluated by MTT assay and the results showed that flavopereirine significantly reduced the viability of both BcaCD885 and Tca8113 cells at 72 h (p < 0.01) and 96 h (p < 0.001) compared with negative control (Figure 1B and C). Meanwhile, relative to human normal oral epithelial cell line (HOES), flavopereirine could selectively inhibit the proliferation of BcaCD885 and Tca8113 cells in a dose-dependent manner at both 72 h (p < 0.01) (Figure 1D) and 96 h (p < 0.01) (Figure 1E). In addition, colony formation assay results indicated that flavopereirine significantly decreased the number of colonies of BcaCD885 (p < 0.01) (Figure 1F) and Tca8113 cells (p < 0.01) (Figure 1G) in a dose-dependent manner. These results indicated that flavopereirine could specifically inhibit the proliferation of human oral cancer cells (BcaCD885 and Tca8113) with no obvious toxicity to normal epithelial cells.
Flavopereirine Induced Apoptosis of Human Oral Cancer Cells in vitro
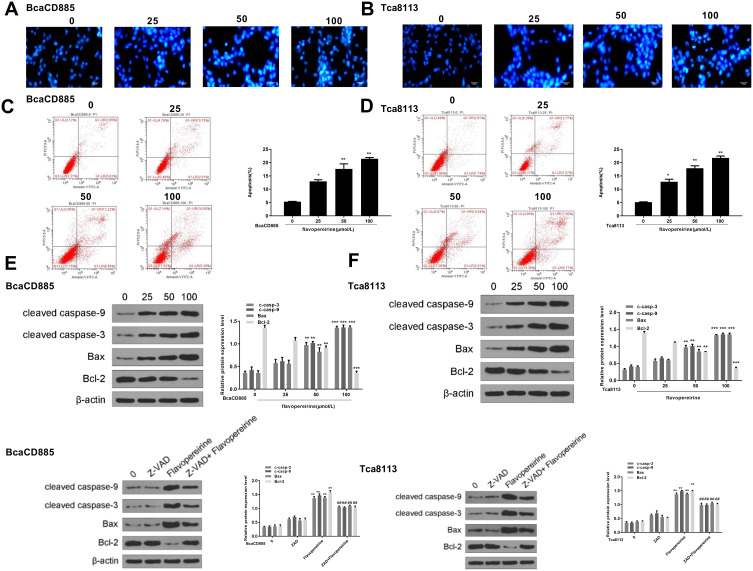
We then analyzed the effect of flavopereirine on cell apoptosis. Hoechst 33342 staining results showed that the apoptotic features of BcaCD885 cells (Figure 2A) and Tca8113 cells (Figure 2B) were obvious in flavopereirine-treated group, including cell shrinkage, rounding up and enhanced blue fluorescence intensity, compared with that in the control group (0 μmol/L). Flow cytometry results showed that flavopereirine significantly promoted cell apoptosis of both BcaCD885 (p < 0.05) (Figure 2C) and Tca8113 cells (p < 0.05) (Figure 2D) in a dose-dependent manner. In addition, the expression of apoptosis-related makers was detected by Western blot, and the results indicated that in both BcaCD885 (Figure 2E) and Tca8113 cells (Figure 2F), 50 and 100 μmol/L flavopereirine both increased the expression levels of pro-apoptotic factors including cleaved caspase-9 (p < 0.01), cleaved caspase-3 (p < 0.01) and Bax (p < 0.01), and decreased the expression levels of anti-apoptotic factor Bcl-2 (p < 0.001), while 25 μmol/L flavopereirine had no effect on the expression of these apoptosis-related proteins. Meanwhile, pan-caspase inhibitor (Z-VAD-FMK) exhibited no obvious effect on the expression of cleaved caspase-9, cleaved caspase-3, Bax and anti-apoptotic factor Bcl-2. The treatment of Z-VAD-FMK and flavopereirine significantly attenuated flavopereirine-induced increase on the expression levels of cleaved caspase-9 (p < 0.01), cleaved caspase-3 (p < 0.01) and Bax (p < 0.01), as well as flavopereirine-induced decrease on the expression levels of Bcl-2 (p < 0.01) (Figure 2E and F). These results indicated that flavopereirine could significantly induce apoptosis of human oral cancer cells, and Z-VAD-FMK could attenuate the effect of flavopereirine on cell apoptosis.
Figure 2.
Flavopereirine induced apoptosis of human oral cancer cells. (A and B) The morphological apoptosis of BcaCD885 (A) and Tca8113 cells (B) was evaluated by Hoechst33342 staining (blue). Magnification, × 200, scale bar = 100 μm. (C and D) The apoptotic rate of BcaCD885 (C) and Tca8113 cells (D) was evaluated by flow cytometry. (E and F) The expression of pro-apoptotic factors cleaved caspase-9, cleaved caspase-3, Bax and anti-apoptotic factor Bcl-2 in BcaCD885 (E) and Tca8113 cells (F) was detected by Western blot. *P < 0.05, **P < 0.01, ***P < 0.001 vs 0 μmol/L group. ##P < 0.01 vs 100 μmol/L flavopereirine group.
Flavopereirine Blocked the Cell Cycle at the G2/M Phase of Human Oral Cancer Cells in vitro
To explore the mechanism of anti-proliferative activity of flavopereirine, cell cycle distribution of BcaCD885 and Tca8113 cells after flavopereirine treatment was evaluated. The results showed that different concentrations of flavopereirine all decreased the proportion at S and G2/M phase of both BcaCD885 (Figure 3A, p < 0.01) and Tca8113 cells (Figure 3B, p < 0.01). Meanwhile, Western blot results showed that 50 and 100 μmol/L flavopereirine significantly decreased the expression levels of the G2/M-phase checkpoint proteins such as CDK1 (p < 0.01), CyclinB1 (p < 0.01) and CDC2 (p < 0.01) in BcaCD885 cells (Figure 3C) and Tca8113 cells (Figure 3D). These results indicated that flavopereirine inhibited the proliferation of oral cancer cells through blocking cell cycle at the G2/M phase.
Figure 3.
Flavopereirine block the cell cycle at the G2/M phase of human oral cancer cells. (A and B) Cell cycle of BcaCD885 (A) and Tca8113 cells (B) was detected by flow cytometry. (C and D) The protein expression levels of G2/M-phase checkpoint proteins were evaluated by Western blot. **P < 0.01.
Flavopereirine Inhibited Invasion, Migration and EMT Process of Human Oral Cancer Cells in vitro
To explore the effect of flavopereirine on cell invasion, migration and EMT process, Transwell assay was performed and the results showed that 50 and 100 μmol/L flavopereirine significantly reduced the migration (p < 0.01) (Figure 4A) and invasion (p < 0.01) (Figure 4B) capacity of BcaCD885 cells, as well as the migration (p < 0.01) (Figure 4C) and invasion (p < 0.01) (Figure 4D) capacity of Tca8113 cells compared with that in the control group, while 25 μmol/L flavopereirine exhibited no obvious effect on the migration and invasion of these two cell lines. In addition, 50 and 100 μmol/L flavopereirine also markedly decreased the expression levels of mesenchymal markers including Vimentin (p < 0.05), Snail (p < 0.05) and Slug (p < 0.05), while increased the epithelial marker E-cadherin (p < 0.05) in both BcaCD885 (Figure 4E) and Tca8113 cells (Figure 4F). These data suggested that flavopereirine could inhibit invasion, migration and EMT process of human oral cancer cells in vitro.
Figure 4.
Flavopereirine inhibited invasion, migration and EMT process of human oral cancer cells in vitro. (A and B) The effect of flavopereirine on the migration ability of BcaCD885 (A) and Tca8113 cells (B). Magnification, × 200, scale bar = 100 μm. (C and D) The effect of flavopereirine on the invasion ability of BcaCD885 (C) and Tca8113 cells (D). Magnification, × 200, scale bar = 100 μm. (E and F) The expression of EMT-related proteins in BcaCD885 (E) and Tca8113 cells (F) after flavopereirine treatment was evaluated by Western blot. *P < 0.05, **P < 0.01, ***P < 0.001 vs 0 μmol/L flavopereirine group.
Flavopereirine Suppressed Proliferation and Induced Apoptosis of Human Oral Cancer Cells Through Inhibiting the Activation of JAK/STAT Signaling by Targeting LASP1
The effect of flavopereirine on the JAK/STAT signaling pathway, which is closely involved in the proliferation and apoptosis of oral cancer cells, was then explored. The results showed that different concentrations of flavopereirine had no obvious effect on the expression of JAK2, STAT3 and STAT5, while 50 and 100 μmol/L flavopereirine significantly decreased the expression levels of p-JAK2 (p < 0.01), p-STAT3 (p < 0.01) and p-STAT5 (p < 0.01) compared with that in the control group both in BcaCD885 (Figure 5A) and Tca8113 cells, and 25μmol/L flavopereirine exhibited no obvious effect (Figure 5B). To further explore the underlying molecular mechanisms of flavopereirine in oral cancer, the expression levels of LASP1 in BcaCD885 and Tca8113 cells were detected and the results showed that LASP1 was significantly downregulated at both mRNA and protein levels in BcaCD885 (p < 0.01) and Tca8113 cells (p < 0.001) compared with that in normal oral epithelial cell line (HOES) (Figure 6A). Moreover, we found that 50 and 100 μmol/L flavopereirine could significantly increase the expression levels of LASP1 compared with that in the control group (p < 0.01) (Figure 6B). In addition, silencing of LASP1 obviously decreased the expression levels of LASP1 compared with si-NC (p < 0.01) (Figure 6C). Meanwhile, silencing of LASP1 significantly promoted cell viability (p < 0.05) and inhibited apoptosis (p < 0.05) of BcaCD885 cells compared with si-NC, while flavopereirine obviously attenuated si-LASP1 induced cell viability (p < 0.01) and inhibited cell apoptosis (p < 0.01) of BcaCD885 cells, and there was no obvious change on the cell apoptosis between the control group and si-NC group (Figure 6D and E). These results suggested that flavopereirine might inhibit the proliferation and apoptosis of human oral cancer cells by upregulating LASP1 to inhibit the activation of JAK-STAT signaling pathway in vitro.
Figure 5.
Flavopereirine inhibited the activation of JAK/STAT signaling pathway in vitro. The expression of JAK2, p-JAK2, STAT3, p-STAT3, STAT5 and p-STAT5 in BcaCD885 (A) and Tca8113 cells (B) after treatment with different concentrations of flavopereirine for 48 h was evaluated by Western blot. *P < 0.05, **P < 0.01 vs 0 μmol/L flavopereirine group.
Figure 6.
Flavopereirine inhibited the progression of human oral cancer cells by regulating LASP1 in vitro. (A) The expression of LASP1 in BcaCD885 and Tca8113 cells was evaluated by qRT-PCR and Western blot. (B) BcaCD885 cells were treated with different concentrations of flavopereirine for 48 h, and the expression levels of LASP1 were evaluated by Western blot. (C) BcaCD885 cells were transfected with si-LASP1 or si-NC, and the mRNA level of LASP1 was evaluated by qRT-PCR. (D and E) BcaCD885 cells were transfected with si-LASP1 or si-NC, and then treated with flavopereirine. (D) Cell viability was detected by MTT assay. (E) Cell apoptosis was evaluated by flow cytometry. **P < 0.01, ***P < 0.001 vs control group, ##P < 0.01 vs si-LASP1 group.
Flavopereirine Attenuated the Progression of Oral Cancer in vivo
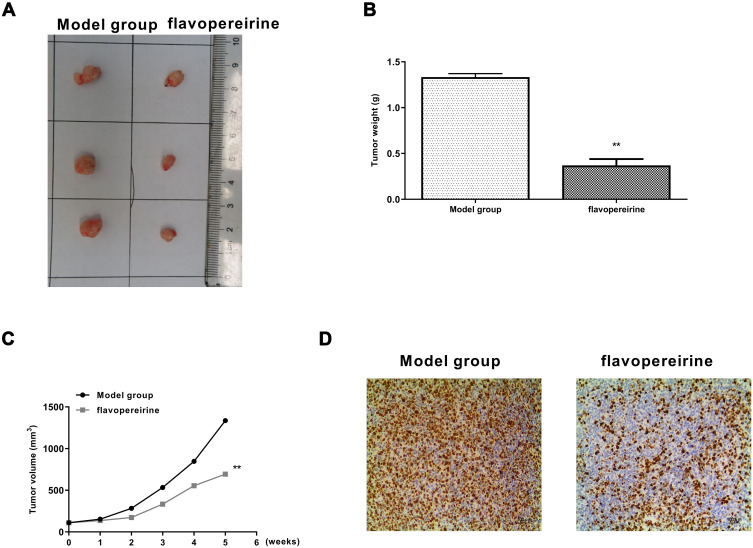
To determine the anti-tumor effect of flavopereirine, a xenograft model in rats was constructed. Flavopereirine treatment obviously reduced the tumor growth compared with that in model group (Figure 7A). Similar to the results in vitro, compared with the model group, flavopereirine treatment obviously decreased the tumor weight (p < 0.01) (Figure 7B) and tumor volume (p < 0.01) (Figure 7C). Meanwhile, IHC assay in tumor tissues showed that flavopereirine treatment obviously decreased the number of Ki-67 positive cells compared with that in model group (Figure 7D). These results suggested that flavopereirine could effectively attenuate the development of oral cancer in vivo.
Figure 7.
Flavopereirine inhibited the progression of oral cancer in vivo. (A) Representative images of tumors from model group and flavopereirine treatment group. (B) Tumor weight. (C) Tumor volume. (D) Cell proliferation in tumor tissues was evaluated by Ki-67 staining assay. Magnification, × 200, scale bar = 100 μm. **P < 0.01.
Discussion
In the last decades, oral cancer has become a serious disease that brings a huge burden on people’s health.22 Therefore, it is urgently needed to identify and develop new and effective anti-cancer agents to prevent oral cancer progression. Migration and invasion of cancer cells into surrounding tissues is an essential step in cancer metastasis.23 Apoptosis is required to balance cell proliferation, and the two interdependent processes together regulate the growth of tissues.24 In this study, we found that flavopereirine significantly suppressed proliferation, invasion, migration, EMT processes, and promoted apoptosis of human oral cancer cell lines BcaCD885 and Tca8113 cells in vitro. Flavopereirine could also effectively inhibited tumor development in a xenograft model in vivo. In addition, our study revealed an underlying mechanism of flavopereirine: the potential anti-cancer of flavopereirine was mediated by inactivation of the JAK/STAT signaling pathway through upregulating LASP1. We for the first time demonstrated that flavopereirine might be used as a potential cancer agent for the treatment of oral cancer.
Studies have identified a series of anti-cancer agents against oral cancer. For example, eldecalcitol (ED-71), an analog of 1α,25-dihydroxyvitamin D3, inhibits the growth of cancer cell lines NA and UE in vitro, and reduces tumor formation in vivo through regulating the expression of CYP24A1.25 Honokiol (HK) has been revealed to inhibit proliferation and colony formation of oral cancer cells, as well as tumor growth in vivo by directly binding to ERp44.26 Curcumin, a phyto-compound isolated from turmeric (Curcuma longa), can significantly decreased OSCC cell viability, and supplement of copper obviously strengthens the inhibition of curcumin treatment on viability and migration of oral cancer cells.27 In addition, ABT-263, a potent BH3 mimetic, can significantly inhibit viability and induce apoptosis of oral cancer cell lines HSC-3 and HSC-4.28 Here, we found that flavopereirine could effectively and specifically suppress the growth, invasion, migration, and EMT processes, and induce apoptosis of human oral cancer cells in vitro, as well as tumor development in vivo. However, several agents that could potentially treat oral cancer have been reported to have certain side effects, such as gastrointestinal side effects and severe cutaneous manifestation.29 Although our results demonstrated that flavopereirine might function to prevent against the progression of oral cancer, the potential side effects of flavopereirine should be investigated in future studies.
LIM and SH3 protein 1 (LASP1), a member of the LIM protein family, is initially discovered through a cDNA library of human breast cancer tissues.30 Previous studies have demonstrated that LASP1 was expressed in all normal tissues and involved in a series of cellular processes.31 Increasing evidence has revealed that LASP1 is highly expressed in many malignant human tumors and closely related to poor clinical prognoses of patients with different types of human cancer such as ovarian cancer,32 colorectal cancer,33 and bladder cancer.34 In addition, high expression levels of LASP1 in patients with OSCC were observed compared with that in healthy subjects, and high expression levels of LASP1 are correlated with the primary tumor size.35 One study demonstrated that overexpression of LASP1 could result in accelerated G2/M phase transition that contributed to enhanced tumourigenesis in oral cancer.35 In this study, we found that LASP1 was markedly downregulated in human oral cancer cell lines BcaCD885 and Tca8113 cells compared with that in normal oral epithelial cell line HOES, and silencing of LASP1 promoted cell viability and inhibited apoptosis of BcaCD885 cells in vitro. Meanwhile, flavopereirine significantly increased the expression levels of LASP1, and could obviously attenuated si-LASP1 induced cell viability and apoptosis of BcaCD885 cells.
Constitutive activation of the JAK/STAT signaling pathway has been identified to be positively correlated with cell proliferation and metastasis as well as angiogenesis.36 Therefore, targeting the JAK/STAT signaling may be a potential therapeutic strategy against human cancer including oral cancer.18,37 Based on the important role of JAK/STAT signaling in oral cancer, many anti-cancer agents targeting JAK/STAT in oral cancer have been identified, and some of which are well studied under experimental conditions such as honokiol,37 astaxanthin,18 8αTGH,38 Blueberry and malvidin.39 Here, we demonstrated that flavopereirine obviously decreased the expression levels of p-JAK2, p-STAT3 and p-STAT5. And silencing of LASP1 significantly increased the expression levels of p-JAK2, p-STAT3 and p-STAT5 compared with si-NC in BcaCD885 and Tca8113 cells. Taken together, our results suggested that the anti-cancer activity of flavopereirine in oral cancer might be partially mediated by inactivation of the JAK/STAT signaling pathway via upregulating LASP1.
Meanwhile, the potential application value of flavopereirine on the treatment of oral cancer needs to be determined in clinical practice. In addition, it could be possible to quantitatively understand possible pharmacological events occurred in the patients by understanding pharmacokinetics of the corresponding agents.40 Hence, the pharmacokinetics of flavopereirine in human oral function should also be investigated in the future.
Conclusion
In summary, our study demonstrated that flavopereirine could act as a potential anti-cancer agent in oral cancer to inhibit the progression of oral cancer both in vitro and vivo by modulating the LASP1-JAK/STAT signaling pathway.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Hussein AA, Helder MN, de Visscher JG, et al. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: a systematic review. Eur j Cancer. 2017;82:115–127. doi: 10.1016/j.ejca.2017.05.026 [DOI] [PubMed] [Google Scholar]
- 2.Kumar M, Nanavati R, Modi TG, Dobariya C. Oral cancer: etiology and risk factors: a review. J Cancer Res Ther. 2016;12(2):458–463. doi: 10.4103/0973-1482.186696 [DOI] [PubMed] [Google Scholar]
- 3.Geum DH, Roh YC, Yoon SY, et al. The impact factors on 5-year survival rate in patients operated with oral cancer. J Korean Assoc Oral Maxillofacial Surg. 2013;39(5):207–216. doi: 10.5125/jkaoms.2013.39.5.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glenny AM, Furness S, Worthington HV, et al. Interventions for the treatment of oral cavity and oropharyngeal cancer: radiotherapy. Cochrane Database Syst Rev. 2010;(12):Cd006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Silva ESJV, Cordovil Brigido HP, Oliveira de Albuquerque KC, et al. Flavopereirine-an alkaloid derived from geissospermum vellosii-presents leishmanicidal activity in vitro. Molecules (Basel, Switzerland). 2019;24:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li JM, Huang YC. Flavopereirine suppresses the growth of colorectal cancer cells through P53 signaling dependence. Cancers. 2019;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh HT, Tsai YS, Chen MS, et al. Flavopereirine induces cell cycle arrest and apoptosis via the AKT/p38 MAPK/ERK1/2 signaling pathway in human breast cancer cells. Eur J Pharmacol. 2019;863:172658. doi: 10.1016/j.ejphar.2019.172658 [DOI] [PubMed] [Google Scholar]
- 8.Beljanski M, Crochet S, Beljanski MS. PB-100: a potent and selective inhibitor of human BCNU resistant glioblastoma cell multiplication. Anticancer Res. 1993;13(6a):2301–2308. [PubMed] [Google Scholar]
- 9.Beljanski M, Crochet S. The selective anticancer agent pb-100 inhibits interleukin-6 induced enhancement of glioblastoma cell-proliferation in-vitro. Int J Oncol. 1994;5(4):873–879. doi: 10.3892/ijo.5.4.873 [DOI] [PubMed] [Google Scholar]
- 10.Beljanski M, Crochet S. The selective anticancer agents PB-100 and BG-8 are active against human melanoma cells, but do not affect non malignant fibroblasts. Int J Oncol. 1996;8(6):1143–1148. doi: 10.3892/ijo.8.6.1143 [DOI] [PubMed] [Google Scholar]
- 11.Samsonov A, Zenser N, Zhang F, Zhang H, Fetter J, Malkov D. Tagging of genomic STAT3 and STAT1 with fluorescent proteins and insertion of a luciferase reporter in the cyclin D1 gene provides a modified A549 cell line to screen for selective STAT3 inhibitors. PLoS One. 2013;8(7):e68391. doi: 10.1371/journal.pone.0068391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu F, Li Q, Wang Z, Cao X. Sinomenine inhibits proliferation, migration, invasion and promotes apoptosis of prostate cancer cells by regulation of miR-23a. Biomed Pharmacother. 2019;112:108592. doi: 10.1016/j.biopha.2019.01.053 [DOI] [PubMed] [Google Scholar]
- 13.Patel MR, Dash A. JAK/STAT inhibition with ruxolitinib enhances oncolytic virotherapy in non-small cell lung cancer models. Nov. 2019;26(11–12):411–418. [DOI] [PubMed] [Google Scholar]
- 14.Tan J, Xiang L, Xu G. LncRNA MEG3 suppresses migration and promotes apoptosis by sponging miR-548d-3p to modulate JAK-STAT pathway in oral squamous cell carcinoma. Jul. 2019;71(7):882–890. [DOI] [PubMed] [Google Scholar]
- 15.Subramaniam A, Shanmugam MK, Perumal E, et al. Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta. 2013;1835(1):46–60. [DOI] [PubMed] [Google Scholar]
- 16.Gurbuz V, Konac E, Varol N, et al. Effects of AG490 and S3I-201 on regulation of the JAK/STAT3 signaling pathway in relation to angiogenesis in TRAIL-resistant prostate cancer cells in vitro. Oncol Lett. 2014;7(3):755–763. doi: 10.3892/ol.2014.1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Sun Z, Sun P, Chen T, Chen F. Microalgal carotenoids: beneficial effects and potential in human health. Food Funct. 2014;5(3):413–425. doi: 10.1039/c3fo60607d [DOI] [PubMed] [Google Scholar]
- 18.Kowshik J, Baba AB, Giri H, Deepak Reddy G, Dixit M, Nagini S. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS One. 2014;9(10):e109114. doi: 10.1371/journal.pone.0109114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruan P, Tao Z, Tan A. Low expression of miR-30a-5p induced the proliferation and invasion of oral cancer via promoting the expression of FAP. Biosci Rep. 2018;38:1. doi: 10.1042/BSR20171027 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Wen Q, Luo K, Huang H, Liao W, Yang H. Xanthoxyletin inhibits proliferation of human oral squamous carcinoma cells and induces apoptosis, autophagy, and cell cycle arrest by modulation of the MEK/ERK signaling pathway. Med Sci Monitor. 2019;25:8025–8033. doi: 10.12659/MSM.911697 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Foschini MP, Gaiba A, Cocchi R, et al. Pattern of p63 expression in squamous cell carcinoma of the oral cavity. Virchows Archiv. 2004;444(4):332–339. doi: 10.1007/s00428-003-0969-x [DOI] [PubMed] [Google Scholar]
- 22.Kakkar V, Verma MK, Saini K, Kaur IP. Nano drug delivery in treatment of oral cancer, a review of the literature. Curr Drug Targets. 2019;20(10):1008–1017. doi: 10.2174/1389450120666190319125734 [DOI] [PubMed] [Google Scholar]
- 23.Duff D, Long A. Roles for RACK1 in cancer cell migration and invasion. Cell Signal. 2017;35:250–255. doi: 10.1016/j.cellsig.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 24.Voss AK, Strasser A. The essentials of developmental apoptosis. F1000Research. 2020;9:148. doi: 10.12688/f1000research.21571.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shintani T, Rosli SNZ, Takatsu F, et al. Eldecalcitol (ED-71), an analog of 1α,25-dihydroxyvitamin D3 as a potential anti-cancer agent for oral squamous cell carcinomas. J Steroid Biochem Mol Biol. 2016;164:79–84. doi: 10.1016/j.jsbmb.2015.09.043 [DOI] [PubMed] [Google Scholar]
- 26.Cho JH, Jeon YJ, Park SM, et al. Multifunctional effects of honokiol as an anti-inflammatory and anti-cancer drug in human oral squamous cancer cells and xenograft. Biomaterials. 2015;53:274–284. doi: 10.1016/j.biomaterials.2015.02.091 [DOI] [PubMed] [Google Scholar]
- 27.Lee HM, Patel V, Shyur LF, Lee WL. Copper supplementation amplifies the anti-tumor effect of curcumin in oral cancer cells. Phytomedicine. 2016;23(12):1535–1544. doi: 10.1016/j.phymed.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 28.Yang IH, Jung JY, Kim SH, et al. ABT-263 exhibits apoptosis-inducing potential in oral cancer cells by targeting C/EBP-homologous protein. Jun. 2019;42(3):357–368. [DOI] [PubMed] [Google Scholar]
- 29.Harada K, Ferdous T, Ueyama Y. Therapeutic strategies with oral fluoropyrimidine anticancer agent, S-1 against oral cancer. Japanese Dental Sci Rev. 2017;53(3):61–77. doi: 10.1016/j.jdsr.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasetto C, Moog-Lutz C, Régnier CH, Schreiber V, Basset P, Rio MC. Lasp-1 (MLN 50) defines a new LIM protein subfamily characterized by the association of LIM and SH3 domains. FEBS Lett. 1995;373(3):245–249. doi: 10.1016/0014-5793(95)01040-L [DOI] [PubMed] [Google Scholar]
- 31.Orth MF, Cazes A, Butt E, Grunewald TG. An update on the LIM and SH3 domain protein 1 (LASP1): a versatile structural, signaling, and biomarker protein. Oncotarget. 2015;6(1):26–42. doi: 10.18632/oncotarget.3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunewald TG, Kammerer U, Winkler C, et al. Overexpression of LASP-1 mediates migration and proliferation of human ovarian cancer cells and influences zyxin localisation. Br J Cancer. 2007;96(2):296–305. doi: 10.1038/sj.bjc.6603545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L, Wang H, Liu C, et al. Promotion of colorectal cancer growth and metastasis by the LIM and SH3 domain protein 1. Gut. 2010;59(9):1226–1235. doi: 10.1136/gut.2009.202739 [DOI] [PubMed] [Google Scholar]
- 34.Chiyomaru T, Enokida H, Kawakami K, et al. Functional role of LASP1 in cell viability and its regulation by microRNAs in bladder cancer. Urol Oncol. 2012;30(4):434–443. doi: 10.1016/j.urolonc.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 35.Shimizu F, Shiiba M, Ogawara K, et al. Overexpression of LIM and SH3 Protein 1 leading to accelerated G2/M phase transition contributes to enhanced tumourigenesis in oral cancer. PLoS One. 2013;8(12):e83187. doi: 10.1371/journal.pone.0083187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao H, Bid HK, Jou D, et al. A novel small molecular STAT3 inhibitor, LY5, inhibits cell viability, cell migration, and angiogenesis in medulloblastoma cells. J Biol Chem. 2015;290(6):3418–3429. doi: 10.1074/jbc.M114.616748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang JS, Yao CJ, Chuang SE, et al. Honokiol inhibits sphere formation and xenograft growth of oral cancer side population cells accompanied with JAK/STAT signaling pathway suppression and apoptosis induction. BMC Cancer. 2016;16:245. doi: 10.1186/s12885-016-2265-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pouyfung P, Choonate S, Wongnoppavich A, Rongnoparut P, Chairatvit K. Anti-proliferative effect of 8α-tigloyloxyhirsutinolide-13-O-acetate (8αTGH) isolated from Vernonia cinerea on oral squamous cell carcinoma through inhibition of STAT3 and STAT2 phosphorylation. Phytomedicine. 2019;52:238–246. doi: 10.1016/j.phymed.2018.09.211 [DOI] [PubMed] [Google Scholar]
- 39.Baba AB, Nivetha R, Chattopadhyay I, Nagini S. Blueberry and malvidin inhibit cell cycle progression and induce mitochondrial-mediated apoptosis by abrogating the JAK/STAT-3 signalling pathway. Food Chem Toxicol. 2017;109(Pt 1):534–543. doi: 10.1016/j.fct.2017.09.054 [DOI] [PubMed] [Google Scholar]
- 40.Ishimoto T, Kato Y. Physiolgically-based pharmacokinetics: theory and examples. Clin Calcium. 2016;26(11):1529–1537. [PubMed] [Google Scholar]