Abstract
Background
Minimizing direct patient contact among healthcare personnel is crucial for mitigating infectious risk during the coronavirus disease 2019 (COVID-19) pandemic. The use of remote cardiac telemetry as an alternative to 12‑lead electrocardiography (ECG) for continuous QTc monitoring may facilitate this strategy, but its application has not yet been validated or implemented.
Methods
In the validation component of this two-part prospective cohort study, a total of 65 hospitalized patients with simultaneous ECG and telemetry were identified. QTc obtained via remote telemetry as measured by 3 independent, blinded operators were compared with ECG as assessed by 2 board-certified electrophysiologists as the gold-standard. Pearson correlation coefficients were calculated to measure the strength of linear correlation between the two methods. In a separate cohort comprised of 68 COVID-19 patients treated with combined hydroxychloroquine and azithromycin, telemetry-based QTc values were compared at serial time points after medication administration using Friedman rank-sum test of repeated measures.
Results
Telemetry-based QTc measurements highly correlated with QTc values derived from ECG, with correlation coefficients of 0.74, 0.79, 0.85 (individual operators), and 0.84 (mean of all operators). Among the COVID-19 cohort, treatment led to a median QTc increase of 15 milliseconds between baseline and following the 9th dose (p = 0.002), with 8 (12%) patients exhibiting an increase in QTc ≥ 60 milliseconds and 4 (6%) developing QTc ≥ 500 milliseconds.
Conclusions
Cardiac telemetry is a validated clinical tool for QTc monitoring that may serve an expanding role during the COVID-19 pandemic strengthened by its remote and continuous monitoring capability and ubiquitous presence throughout hospitals.
Keywords: Cardiac telemetry, QTc, QT interval, Coronavirus 2019, COVID-19
Abbreviations: COVID-19, coronavirus disease 2019; PPE, personal protective equipment; ECG, electrocardiography; CI, confidence intervals; ICC, intraclass correlation coefficient
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has transformed individual lives and societal framework on a global scale, and in no other sector is this more evident than in healthcare. As healthcare personnel have faced a significantly higher risk of infection, particularly in the early stages of the outbreak [1,2], mitigating such susceptibility is vital for the overall health of the healthcare delivery system and to avoid nosocomial transmission. The use of personal protective equipment (PPE) by those with the greatest exposure risk, implementation of infection control strategies, and rigorous compliance with such guidelines have reduced rates of infection in healthcare personnel. Yet despite such measures, the relatively long incubation phase coupled with the variable virulence [[3], [4], [5]], speak to the insidious nature of SARS-CoV-2.
Delivering necessary medical care to all hospitalized patients while minimizing individual risk among healthcare personnel has been a major challenge during this time. Unprecedented multilevel disruption of supply chain networks has led to severe shortages of vital supplies such as PPE [6], in turn hampering efforts and transiently limiting the delivery of services only to those deemed medically essential. Physical distancing is a powerful protective mechanism to reduce the transmission of SARS-CoV-2 [7], and therefore, novel strategies to minimize contact between healthcare personnel and patients while still providing essential care universally must be implemented in order to reopen the healthcare system.
Opportunity for such an intervention presented early in the outbreak when small observational studies described the use of hydroxychloroquine and azithromycin to treat infected patients [[8], [9], [10]]. Both drugs are known to increase the risk of drug-induced Torsades de pointes and sudden cardiac death in at-risk patients [[11], [12], [13]], thereby necessitating monitoring of the QT interval [14]. While the use of these therapies has fallen out of favor, the significance of continuous QTc monitoring in COVID-19 patients remains paramount as prior work reveals that QTc strongly predicts in-hospital mortality in this cohort [15], and that irrespective of QTc-prolonging medications, infected patients have longer baseline QTc intervals than the general population [16].
The available strategies for in-hospital QTc monitoring are suboptimal for the current context. Although 12‑lead electrocardiography (ECG) has traditionally been used for this purpose, it requires direct contact between the caregiver and the patient, and obtaining multiple readings in high-risk patients is inefficient and labor intensive. While the first smartphone-enabled personal ECG, AliveCor's KardiaMobile 6 L, was issued allowance by the Food and Drug Administration for QTc monitoring in response to COVID-19, this technology relies on user involvement and routine use in hospitalized patients would necessitate considerable upfront expense [17]. In addition, though the use of a wearable remote monitoring system, the BodyGuardian™, has previously identified patients at risk for drug-induced long QT syndrome, this was only validated in the out-of-hospital setting [18]. Cardiac telemetry offers several advantages, including 1) data acquisition that demands no action on the part of the patient or the caregiver, 2) remote capability for data analysis, 3) continuous monitoring for immediate detection of cardiac arrhythmia, and 4) ubiquitous presence in all hospitals worldwide.
In our two-part study, we sought to first validate a “no-touch” method using cardiac telemetry for serial tracking of QTc intervals, and second, implement telemetry in a clinical protocol designed specifically for QTc monitoring in COVID-19 patients treated with hydroxychloroquine and azithromycin, to determine its real-life application in the pandemic.
Material and methods
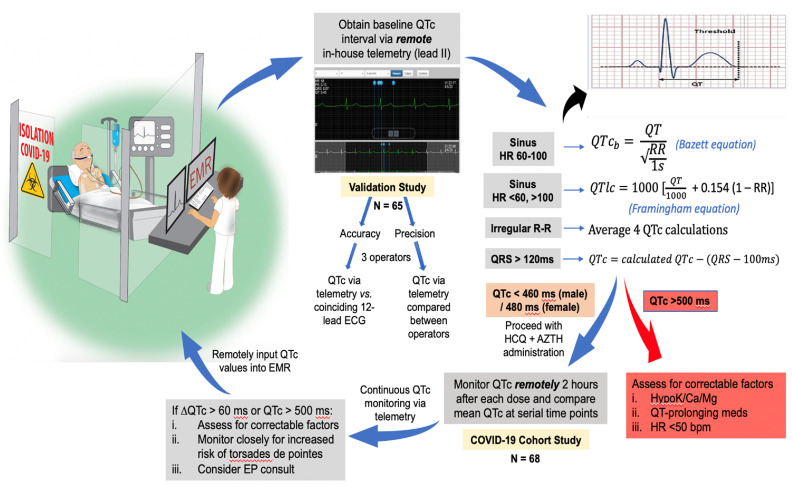
Study methodology is illustrated in Fig. 1 , and comprises two separate cohorts used to validate and clinically implement cardiac telemetry for remote and serial monitoring of QTc intervals in hospitalized patients. The protocol was approved by the Cleveland Clinic Institutional Review Board.
Fig. 1.
Stepwise flowchart of study methodology.
The study design includes both a validation component designed to assess the accuracy and precision of manually-calculated QTc intervals from lead II of remote telemetry, as well as a COVID-19 cohort study aimed at evaluating the clinical implementation of telemetry to assess the impact of hydroxychloroquine and azithromycin on QTc intervals at serial time points.
ECG indicates electrocardiography; HCQ, hydroxychloroquine; AZTH, azithromycin; HR, heart rate; EP, electrophysiology; EMR, electronic medical record.
Phillips real-time cardiac telemetry was used for QT interval measurement and compared with traditional standard 12‑lead ECG. The Philips real-time cardiac telemetry system digitized the signal at a sample rate of 500 samples/s and a high-pass frequency limit of 0.05 Hz. Utilizing 5 surface electrodes, the system provides 7 ECG waveform leads: limb (I, II, III), augmented (aVL, aVF, aVR), and precordial lead (V1). Enabled by the AirStrip ONE integrative platform, patient waveforms were accessed virtually, obviating the need for direct patient contact. Automated QTc intervals normalized for heart rate were not available via telemetry at the time of our study, and therefore, QTc values were calculated from measured QT intervals obtained using freeze capture of rhythm ECG strips. Using only lead II, QT intervals were manually measured from the onset of the Q wave to the offset of the T wave, defined by the threshold method as the point at which the T wave merges with the isoelectric baseline [[19], [20], [21]]. QTc intervals were then calculated using the following approaches: 1) Bazzett equation, correcting the QT with the RR interval of the preceding beat, if the heart rate was 60–100 bpm, 2) Framingham equation if the heart rate was <60 or > 100 bpm, 3) average of multiple QT measurements in the presence of atrial fibrillation, and 4) correction of the QTc, by subtracting the difference in QRS lengthening, in the presence of a wide QRS (>120 ms) [22].
Validation cohort
Sample size estimation was made prior to conducting the study to achieve a power of 0.9 with a one-sided alpha of 0.025. Accordingly, a total of 65 patients hospitalized at Cleveland Clinic Fairview Hospital between May 4–8, 2020, with a need for 12‑lead ECG for any indication and available concurrent cardiac telemetry, were identified at random. Using only lead II, three operators (TS, TF, CL) independently and in a blinded fashion, sampled three random QT interval measurements for each patient from a remotely accessed digital telemetry rhythm strip coinciding in time±) 60 s) with the time-date-stamped 12‑lead ECG. Telemetry waveforms were displayed at 25 mm/s sweep speed and 10 mm/mV voltage scale. QTc values were subsequently calculated from each of the three measured QT intervals and reported as a mean value per individual operator and collectively. QTc values were similarly derived from 12‑lead ECG. Manual measurements of QT intervals were independently performed by two board-certified electrophysiologists (RC, CT), using lead II displayed at 25 mm/s sweep speed and 10 mm/mV voltage scale. When the offset of the T wave could not be defined with certainty in lead II, an alternative ECG lead (V5 or V6) was used for QT interval measurement [23]. ECG-derived QTc values were calculated, reported as a mean, and used as a gold-standard for comparison.
To assess the accuracy in the telemetry-based QTc acquisition method, we compared QTc values obtained simultaneously via remote telemetry and 12‑lead ECG. To evaluate the precision of the telemetry-based QTc approach, mean QTc values by each of the operators were compared for inter-rater reliability.
Implementation cohort
Utilizing telemetry-based remote monitoring, serial QTc intervals were compiled for analysis in a cohort of 68 symptomatic patients with COVID-19 treated with combined hydroxychloroquine and azithromycin. This population was identified by reviewing the electronic medical record within the Cleveland Clinic Health System. The diagnosis of COVID-19 was confirmed by a positive nasal swab test. The standard regimen included oral hydroxychloroquine 400 mg twice daily on day 1 followed by 200 mg twice daily on days 2–5 and intravenous azithromycin 500 mg daily on days 1–5, with drug administration timed to coincide (Supp. Fig. 1). For all patients, QTc values were recorded at baseline and 2 h after each drug dose administration. This timing was selected based upon previously reported peak plasma concentrations of hydroxychloroquine and azithromycin at 2–4 h [24,25]. QTc monitoring continued throughout hospitalization while being monitored via telemetry. To assess the ability of telemetry to detect the applied effect of hydroxychloroquine and azithromycin on QTc intervals, mean QTc values were compared at serial time points of drug administration.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Normal Q-Q plots and normality tests showed that QTc derived from both telemetry and 12‑lead ECG were normally distributed. A comparison of telemetry-based QTc measurements was made with the gold-standard QTc. Pearson correlation coefficient was calculated to measure the strength of linear correlation. Agreement was quantified utilizing Lin's concordance correlation coefficient. Concordance correlation coefficients and associated 95% confidence intervals (CI) were calculated, comparing standard 12‑lead ECG-based QTc with each of three telemetry operators individually and collectively, using the CCC function in the DescTools package or the epi.ccc function in the epiR package of R. Agreement was further assessed using an equivalence test for paired samples implemented with the TOST (−25, 25) option in the TTEST procedure of SAS, with equivalence margin (−25, 25 milliseconds), one-sided α of 0.025, and a (1 – α) CI. The intraclass correlation coefficient (ICC) was calculated to quantify the inter-rater reliability among the telemetry operators. The variance values were obtained from one-way analysis of variance implemented in the GLM procedure of SAS, where each subject was set both as a fixed effect and as a random effect. As a separate analysis, we used the Friedman rank-sum test of repeated measures to evaluate statistically significant differences between QTc with serial doses of hydroxychloroquine and azithromycin in the COVID-19 cohort. All data were de-identified for analysis. Statistical analysis was performed with the open-source R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) and the SAS software version 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Mean QTc measurements obtained by telemetry and 12‑lead ECG are reported in Table 1 . Telemetry-based QTc measurements highly correlated with gold-standard QTc values derived from 12‑lead ECG. Mean QTc reported by all 3 telemetry operators showed a strong linear correlation with the gold-standard, with Pearson's correlation coefficient calculated as 0.74 (operator: TS), 0.79 (operator: TF), 0.85 (operator: CL), and 0.84 (mean of all 3 operators). Lin's concordance correlation coefficients showed good overall agreement between the two methods, with concordance correlation coefficients (95% CI) of 0.67 (0.53–0.78), 0.74 (0.61–0.83), 0.80 (0.66–0.87) and 0.78 (0.68–0.86) for individual telemetry operators TS, TF, CL, and collectively averaged, respectively.
Table 1.
Assessment of the precision of QTc measurements obtained by remote cardiac telemetry and 12-lead electrocardiography.
| Mode of QTc measurement | Mean QTc ± Standard deviation (ms) | 95% Confidence interval (ms) |
|---|---|---|
| Telemetry (CL) | 432 ± 33 | (424, 440) |
| Telemetry (TF) | 432 ± 36 | (423, 441) |
| Telemetry (TS) | 430 ± 41 | (420, 440) |
| Telemetry (average) | 431 ± 34 | (422, 440) |
| 12-lead ECG (GS) | 443 ± 31 | (435, 450) |
n = 63. MS indicates millisecond; GS, gold-standard. Telemetry operators: CL, TF, TS.
Simultaneous QTc measurements derived from telemetry by three distinct operators were similar with very good inter-rater reliability observed by a calculated ICC of 0.81 (0.74–0.88). Of note, extreme QTc measures were observed in two patients as a result of telemetry operators' incorporation of the U wave into the QT interval; both data sets were removed from the analysis.
Equivalence tests for paired samples comparing mean differences in QTc values between the gold-standard and all telemetry operators, with predefined equivalence margins of −25 to 25 milliseconds, are shown in Table 2 . The null hypothesis of nonequivalence was rejected (p < 0.001) in all cases, indicating that mean QTc differences did not exceed the preset margin.
Table 2.
Evaluation of the accuracy of QTc measurements between paired methods.
| GS vs telemetry (CL) | GS vs telemetry (TF) | GS vs telemetry (TS) | GS vs telemetry (average) | |
|---|---|---|---|---|
| Mean difference (95% CI) (ms) | 10.9 (6.5, 15.4) | 10.9 (5.3, 16.6) | 12.8 (5.9, 19.7) | 11.6 (6.8, 16.3) |
| Test of lower bound (p)a | <0.001 | <0.001 | <0.001 | <0.001 |
| Test of upper bound (p)a | <0.001 | <0.001 | <0.001 | <0.001 |
n = 63. MS indicates millisecond; GS, gold-standard. Telemetry operators: CL, TF, TS.
P-values for comparisons of 95% confidence intervals of mean differences with a margin of (lower bound −25, upper bound +25).
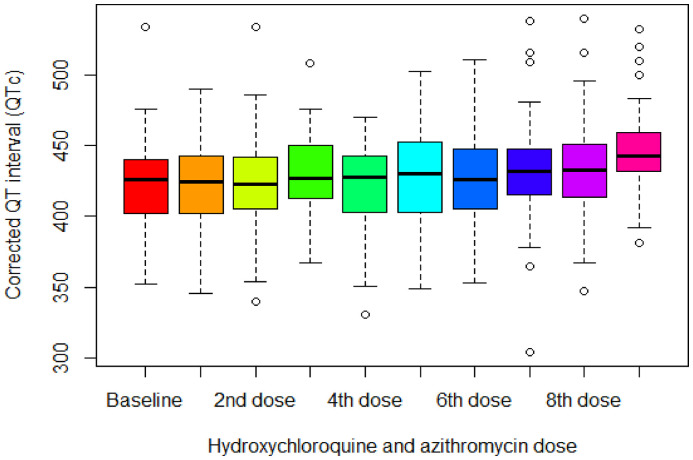
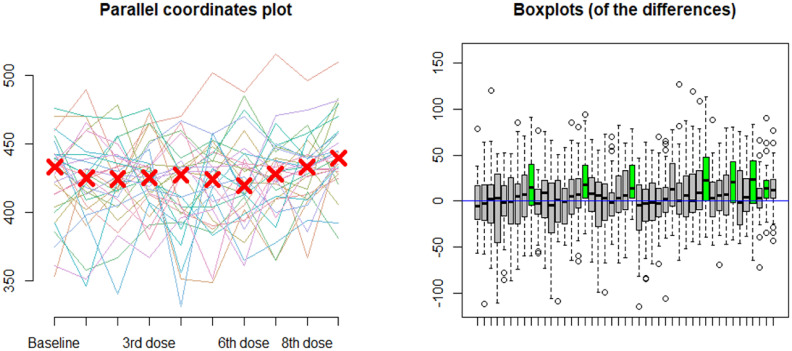
In the cohort of patients with COVID-19 treated with combination hydroxychloroquine and azithromycin and who underwent telemetry-based remote monitoring (n = 68), serial QTc intervals were compiled for analysis. The median QTc at baseline and following each dose of hydroxychloroquine and azithromycin or hydroxychloroquine alone are presented in Fig. 2 , with a statistically significant increase in QTc of 15 milliseconds [interquartile range (−2, 41)] between baseline and following the 9th dose (p = 0.002) by Friedman rank-sum tests of repeated measures. Fig. 3 reports both the parallel coordinates plot for telemetry-measured QTc at baseline and following each dose of hydroxychloroquine and azithromycin, as well as box plots indicating the distribution of differences between repeated measures of QTc with serial doses. Significant differences in QTc were found after the 9th drug dose administration compared with the QTc at baseline (p = 0.02) and following the 1st (p = 0.004), 2nd (p = 0.004), 4th (p = 0.006), 5th (p = 0.01), 6th (p = 0.02), and 7th doses (p = 0.02), separately. Additionally, eight (12%) patients showed an increase in QTc ≥ 60 milliseconds, and four (6%) developed QTc ≥ 500 milliseconds.
Fig. 2.
Telemetry-based QTc at baseline and following each dose of hydroxychloroquine and azithromycin in COVID-19 patients (n = 68).
The X-axis represents the number of doses (either hydroxychloroquine alone or hydroxychloroquine and azithromycin) and the Y-axis represents the median QTc interval in milliseconds as measured by remote telemetry. The QTc values measured at baseline and after each drug dose administration were not normally distributed. To account for within-subject variability in QTc measurements at baseline and after each dose, post-hoc analysis of Friedman rank-sum tests was employed.
Fig. 3.
Differences in telemetry-measured QTc values with serial doses of hydroxychloroquine and azithromycin in COVID-19 patients (n = 68).
Left) Parallel coordinates plot for telemetry-measured QTc at baseline and following each drug dose administration in COVID-19 patients. Right) Post-hoc analysis of Friedman rank sum tests of repeated measures of QTc with serial drug dose administration. The X-axis represents the dose considered and the Y-axis represents the difference between the measured QTc between the two considered doses. The box plot indicates the distribution of differences between repeated measures of QTc with serial doses of hydroxychloroquine and azithromycin. As indicated by the green box plots from left to right, there were statistically significant differences between QTc measurements after the 9th dose when compared with baseline (p = 0.02) and following the 1st (p = 0.004), 2nd (p = 0.004), 4th (p = 0.006), 5th (p = 0.01), 6th (p = 0.02), and 7th (p = 0.02) doses, separately.
Discussion
Using a novel “no-touch” telemetry-based approach to QTc monitoring, our primary findings are twofold: 1) telemetry-based QTc measurements are highly accurate relative to gold-standard 12‑lead ECG, and precise among three trained independent operators competent in measuring cardiac intervals; 2) initially validated in a general hospitalized population, this method can be successfully implemented in the clinical setting as demonstrated by its utilization in COVID-19 patients who experienced a significant increase in QTc after treatment with hydroxychloroquine and azithromycin. Our results lend credence to telemetry as a valid clinical tool for QTc monitoring in place of standard 12‑lead ECG, thereby minimizing direct patient contact and mitigating infectious risk among healthcare personnel and patients alike.
Notwithstanding perfect agreement, the mean QTc difference of 11.6 ± 19.3 milliseconds between telemetry and ECG in our study is inconsequential, coinciding with less than half of a millimeter measured on standard ECG paper speed (25 mm/s). Moreover, this discrepancy is in line with prior studies comparing QTc monitoring using wireless smartphone ECG acquisition technology (AliveCor KardiaMobile) and 12‑lead ECG [26,27]. Collectively, our findings support telemetry as a method for QTc monitoring in place of standard 12‑lead ECG.
In the separate prospective cohort analysis applying the validated method for temporal QTc monitoring in COVID-19 patients, our results are consistent with prior studies that demonstrate a significant increase in the QTc interval with treatment [[28], [29], [30], [31], [32]], further supporting the clinical application of telemetry-based monitoring, particularly in cohorts posing exposure risk and who warrant minimal direct contact.
COVID-19 has taken a dramatic toll on the health of societies worldwide. At the onset of the outbreak, the initial response by health systems was to reduce the intensity of care delivery. Although transient and necessary, an unintended outcome was subsequent deferment in health care needs. The Kaiser Family Foundation poll reported that 48% of Americans delayed medical care due to the pandemic, of whom 11% noted worsening in their medical condition [33]. In another survey by the American College of Emergency Physicians, 80% feared contracting COVID-19 from visiting the emergency room, while 29% avoided seeking medical care specifically because of these concerns [34]. Centers around the world observed a significant reduction in hospitalizations for acute myocardial infarction and ischemic stroke, and consequently reported adverse yet potentially avoidable consequences [[35], [36], [37], [38], [39]]. Thus, eliminating the provision of healthcare services is not a sustainable solution during the COVID-19 pandemic.
In its current state, the primary goal of the health system is to restore the public's confidence and sense of security in seeking medical care during the COVID-19 pandemic; prioritizing healthcare personnel safety is at the forefront of rebuilding this trust. The Centers for Disease Control and Prevention issued updated recommendations for infection prevention and control for healthcare personnel, with emphasis on universal screening, PPE use, and physical distancing [40]. While avoiding exposure entirely is the most effective means of mitigating risk, this is not practical, and potentially harmful in the clinical setting. Instead, it is incumbent upon the healthcare community to seek and maximize opportunities to limit non-essential physical contact between healthcare personnel and hospitalized patients. We have successfully demonstrated a novel “no-touch” cardiac telemetry-based approach for QTc monitoring in COVID-19-infected patients treated with drugs with arrhythmogenic potential. Importantly, we understood the sharp decline in the utility of these agents to treat the infection, and instead aimed to test the ability to clinically implement telemetry as QTc monitoring becomes increasingly critical [15,16] and for valid options in future unforeseen circumstances.
While the primary application of cardiac telemetry in current practice is arrhythmia detection in hospitalized patients, we believe it holds greater potential and expanding clinical use. Our focus on telemetry as a non-contact method for QTc monitoring in patients with COVID-19 treated with proarrhythmic drugs stands as an important application. Similarly, telemetry may further replace serial 12‑lead ECGs used in antiarrhythmic drug loading protocols, allowing safe uninterrupted delivery of necessary elective services throughout the duration of the pandemic. Furthermore, telemetry holds great promise as a tool for predicting ventricular arrhythmias. Heightened QTc variability has been described in certain pathophysiologic states and shown to predict increased risk for potentially lethal arrhythmias and cardiovascular death [[41], [42], [43], [44], [45], [46], [47], [48], [49]]. The continuous recording nature of telemetry lends itself to advanced diagnostics that can provide granular insight beyond mere static measurement of QTc, and may offer automated serial analysis of simultaneous ECG leads as previously demonstrated by digital Holter recordings [50]. With increasing metadata that necessitates a standardized exchange between a growing number of ECG devices, the use of digital and continuous recordings is paramount for accurate and precise ECG measurements such as QTc. The advent of the internationally-recognized Standard Communication Protocol for Computer-Assistant ECG, for instance, has facilitated open, secure storage and exchange of full or select ECG sequences between operating systems, in turn optimizing the interpretation and serial analysis of large data, and ultimately allowing for more reliable and efficient diagnostics.51
Limitations
The main limitation of the method validated and employed in our study is the labor-intensive nature of manual QT interval measurements and QTc calculations. However, the hand-operated nature of our method with attention to use the most appropriate QT correction formula in each instance, resulted in highly accurate QTc values that were made readily available and easily applicable by all caregivers irrespective of experience or knowledge in the nuances of QTc determination. The future of automated QTc via telemetry holds great promise if algorithms consider the application of the most appropriate correction formula in a given circumstance.
Conclusions
Our prospective cohort study validates the use of remote cardiac telemetry as a novel “no-touch” approach to QTc monitoring. In light of its capability for remote continuous monitoring and ubiquitous presence throughout hospitals, our findings suggest that telemetry may replace 12‑lead ECG during the COVID-19 pandemic and in similar times that warrant avoidance of patient contact to mitigate infectious risk among healthcare personnel. The COVID-19 pandemic will undoubtedly reshape our society in lasting ways. Healthcare must embrace this time as an opportunity to revise existing practices and encourage innovation to build a more safe and resilient future.
Funding
This work was supported by unrestricted philanthropic support to the Heart, Vascular and Thoracic Institute Center for Healthcare Delivery Innovation, Cleveland Clinic. The funding source had no role in the design or conduct of the study; collection, management, analyses, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Acknowledgements
The authors wish to express our gratitude to Traci Sustersic RN, MSN, Carrie Lachner RN, BSN, RCES, and Todd Finau RN, RCES for their notable contributions with data collection to this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jelectrocard.2021.04.014.
Appendix A. Supplementary data
Supplementary material
References
- 1.Chou R., Dana T., Buckley D.I., Selph S., Fu R., Totten A.M. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173(2):120–136. doi: 10.7326/M20-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen L.H., Drew D.A., Joshi A.D., Guo C.G., Ma W., Mehta R.S., et al. Risk of COVID-19 among frontline healthcare workers and the general community: a prospective cohort study. medRxiv. 2020 doi: 10.1101/2020.04.29.20084111. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J. Pathogenicity and transmissibility of 2019-nCoV-A quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khot U. Navigating healthcare supply shortages during the COVID-19 pandemic: a cardiologist’s perspective. Circ Cardiovasc Qual Outcomes. 2020;13(6) doi: 10.1161/CIRCOUTCOMES.120.006801. [DOI] [PubMed] [Google Scholar]
- 7.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. On behalf of the COVID-19 systematic urgent review group effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ. 2020;49(2):215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z., Hui J., Zhang Z., Jiang S., Han S., Yan D., et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.22.20040758. Preprint. [DOI] [Google Scholar]
- 11.Chen C.Y., Wang F.L., Lin C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol. 2006;44(2):173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 12.O’Laughlin J.P., Mehta P., Wong B. Life threatening severe QTc prolongation in patient with systemic lupus erythematosus dues to hydroxychloroquine. Case Rep Cardiol. 2016 doi: 10.1155/2016/4626279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray W.A., Murray K.T., Hall K., Arbogast P.C., Stein C.M. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus Disease-19 (COVID-19) Mayo Clin Proc. 2020;95(6):1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicentini A., Masiello L., Amore S.D., Baldi E., Ghio S., Savastano S., et al. San Matteo COVID Cardiac Injury Task Force. QTc interval and mortality in a population of SARS-2-COV infected patients. Circ Arrhythm Electrophysiol. 2020:13. doi: 10.1161/CIRCEP.120.008890. [DOI] [PubMed] [Google Scholar]
- 16.Aromolaran A.S., Srivastava U., Alí A., Chahine M., Lazaro D., El-Sherif N., et al. Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haberman Z.C., Jahn R.T., Bose R., Tun H., Shinbane J.S., Doshi R.N., et al. Wireless smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol. 2015;26(5):520–526. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 18.Castelletti S., Dagradi F., Goulene K., Danza A.I., Baldi E., Stramba-Badiale M., et al. A wearable remote monitoring system for the identification of subjects with a prolonged QT interval or at risk for drug-induced long QT syndrome. Int J Cardiol. 2018;266:89–94. doi: 10.1016/j.ijcard.2018.03.097. [DOI] [PubMed] [Google Scholar]
- 19.Panicker G.K., Karnad D.P., Natekar M., Kothari S., Narula D., Lokhandwala Y. Intra- and interreader variability in QT interval measurement by tangent and threshold methods in a central electrocardiogram laboratory. J Electrocardiol. 2009;42(4):348–352. doi: 10.1016/j.jelectrocard.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Guidance for Industry: E4 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential of Non-Antiarrhythmic Drugs. 2005. https://www.fda.gov/media/71372/download Available from.
- 21.Al-Khatib S.M., LaPointe N.M.A., Kramer J.M., Califf R.M. What clinicians should know about the QT interval. JAMA. 2003;289(16):2120–2127. doi: 10.1001/jama.289.16.2120. [DOI] [PubMed] [Google Scholar]
- 22.Bogossian H., Frommeyer G., Ninios I., Hasan F., Nguyen Q.S., Karosiene Z., et al. New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm. 2014;11:2273–2277. doi: 10.1016/j.hrthm.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 23.Postema P.G., Wilde A.A.M. The measurement of the QT interval. Curr Cardiol Rev. 2014;10:287–294. doi: 10.2174/1573403X10666140514103612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plaquenil hydroxychloroquine sulfate tablets, USP. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf Available from. [Google Scholar]
- 25.Zithromax (azithromycin for injection) 2000. https://www.accessdata.fda.gov/drugsatfda_docs/label/2001/50733s5lbl.pdf Available from. [Google Scholar]
- 26.Haberman Z.C., Jahn R.T., Bose R., Tun H., Shinbane J.S., Doshi R.N., et al. Wireless smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol. 2015;26(5):520–526. doi: 10.1111/jce.12634. [DOI] [PubMed] [Google Scholar]
- 27.Garabelli P., Stavrakis S., Albert M., Koomson E., Parwani P., Chohan J., et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and in patients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol. 2016;27(7):827–832. doi: 10.1111/jce.12976. [DOI] [PubMed] [Google Scholar]
- 28.Mercurro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramireddy A., Chugh H., Reinier K., Ebinger J., Park E., Thompson M., et al. Experience with hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9(12) doi: 10.1161/JAHA.120.017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bessière F., Roccia H., Delinière Charrière R., Chevalier P., Argaud L., et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5(9):1067–1069. doi: 10.1001/jamacardio.2020.1787. doi: 10.100/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chorin E., Dai M., Shulman E., Wadhwani L., Bar-Cohen R., Barbhaiya C., et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat Med. 2020;26(6):808-809.Cipriani a, Zorzi a, Ceccato D, Capone F, Parolin M, Donato F, et al. arrhythmic profile and 24 hour QT interval variability in COVID-19 patients treated with hydroxychloroquine and azithromycin. Int J Cardiol. 2020;316:280–284. doi: 10.1016/j.ijcard.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamel L., Kearney A., Kirzinger A., Lopes L., Muñana C., Brodie M. KFF health tracking poll. May 2020. https://www.kff.org/report-section/kff-health-tracking-poll-may-2020-health-and-economic-impacts/ Available from:
- 33.American College of Emergency Physicians. Public Poll: Emergency Care Concerns Amidst COVID-19. 2020. https://www.emergencyphysicians.org/article/covid19/public-poll-emergency-care-concerns-amidst-covid-19 Available from.
- 34.Zhao J., Hang L., Kung D., Fisher M., Shen Y., Liu R. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke. 2020;51(7):1996–2001. doi: 10.1161/STROKEAHA.120.030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kansagra A.P., Goyal M.S., Hamilton S., Albers G.W. Collateral effect of Covid-19 on stroke evaluation in the United States. N Engl J Med. 2020;383(4):400–401. doi: 10.1056/NEJMc2014816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeRosa S., Spaccarotella C., Basso C., Calabrò M.P., Curcio A., Filardi P.P., et al. Reduction of hospitalization for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41(22):2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon M.D., McNulty E.J., Rana J.S., Leong T.K., Lee C., Sung S.H., et al. The COVID-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. 2020;383(7):691–693. doi: 10.1056/NEJMc2015630. [DOI] [PubMed] [Google Scholar]
- 38.Baldi E., Sechi G., Mare C., Canevari F., Brancaglione A., Primi R., et al. Out-of-hospital cardiac arrest during the COVID-19 outbreak in Italy. N Engl J Med. 2020;383(5):496–498. doi: 10.1056/NEJMc2010418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention Interim Infection Prevention and Control Recommendations for Health Care Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. July 9, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html updated. Available from.
- 40.Berger R.D., Kasper E.K., Baughman K.L., Marban E., Calkins H., Tomaselli G.F. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96(5):1557–1565. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 41.Piccirillo G., Magnanti M., Matera S., Carlo S.D., Laurentis T.D., Torrini A., et al. Age and QT variability index during free breathing, controlled breathing and tilt in patients with chronic heart failure and healthy control subjects. Transl Res. 2006;148(2):72–78. doi: 10.1016/j.trsl.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Murabayashi T., Fetics B., Kass D., Nevo E., Gramatikov B., Berger R.D. Beat-to-beat QT interval variability associated with acute myocardial ischemia. J Electrocardiol. 2002;35(1):19–25. doi: 10.1054/jelc.2002.30250. [DOI] [PubMed] [Google Scholar]
- 43.Atiga W.L., Fananapazir L., McAreavey D., Calkins H., Berger R.D. Temporal repolarization lability in hypertrophic cardiomyopathy caused by beta-myosin heavy-chain gene mutations. Circulation. 2000;101(11):1237–1242. doi: 10.1161/01.cir.101.11.1237. [DOI] [PubMed] [Google Scholar]
- 44.Piccirillo G., Germanò G., Quaglione R., Nocco M., Lintas F., Lionetti M., et al. QT-interval variability and autonomic control in hypertensive subjects with left ventricular hypertrophy. Clin Sci (Lond) 2002;102(3):363–371. [PubMed] [Google Scholar]
- 45.Bilchick K., Viitasalo M., Oikarinen L., Fetics B., Tomaselli G., Swan H., et al. Temporal repolarization lability differences among genotyped patients with the long QT syndrome. Am J Cardiol. 2004;94(10):1312–1316. doi: 10.1016/j.amjcard.2004.07.123. [DOI] [PubMed] [Google Scholar]
- 46.Yeragani V.K., Pohl R., Jampala V.C., Balon R., Ramesh C., Srinivasan K. Increased QT variability in patients with panic disorder and depression. Psychiatry Res. 2000;93(3):225–235. doi: 10.1016/s0165-1781(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 47.Haigney M.C., Zareba W., Gentlesk P.J., Goldstein R.E., Illovsky M., McNitt S., et al. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the multicenter automatic defibrillator implantation trial (MADIT) II patients. J Am Coll Cardiol. 2004;44(7):1481–1487. doi: 10.1016/j.jacc.2004.06.063. [DOI] [PubMed] [Google Scholar]
- 48.Piccirillo G., Magrì D., Matera S., Magnanti M., Torrini A., Pasquazzi E., et al. QT variability strongly predicts sudden cardiac death in asymptomatic subjects with mild or moderate left ventricular systolic dysfunction: a prospective study. Eur Heart J. 2007;28(11):1344–1350. doi: 10.1093/eurheartj/ehl367. [DOI] [PubMed] [Google Scholar]
- 49.Sarapa N., Morganroth J., Couderc J.P., Francom S.F., Darpo B., Fleishaker J.C., et al. Electrocardiographic identification of drug-induced QT prolongation: assessment by different recording and measurement methods. Ann Noninvasive Electrocardiol. 2004;9(1):48–57. doi: 10.1111/j.1542-474X.2004.91546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rubel P., Pani D., Schloegl A., Fayn J., Badilini F., Macfarlane P.W., et al. SCP-ECG V3.0: an enhanced Standard Communication Protocol for Computer-assisted Electrocardiography. Comput Cardiol. 2016;43:309–312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material