Abstract
The appearance of the virus SARS-CoV-2 at the end of 2019 and its spreading all over the world has caused global panic and increase of personal protection equipment usage to protect people against infection. Increased usage of disposable protective gloves, their discarding to random spots and getting to landfills may result in significant environmental pollution. The knowledge concerning possible influence of gloves and potential of gloves debris on the environment (water, soil, etc.), wildlife and humans is crucial to predict future consequences of disposable gloves usage caused by the pandemic. This review focuses on the possibility of chemical release (heavy metals and organic pollutants) from gloves and gloves materials, their adsorptive properties in terms of contaminants accumulation and effects of gloves degradation under environmental conditions.
Abbreviations: A-PE-MPs, Microplastics exposed to air; AAS, Atomic absorption spectroscopy; AHTN, Tonalide; AMWPE, Average molecular weight medium density polyethylene; AMX, Amoxicillin; BBP, Benzyl butyl phthalates; BET, Brunner-Emmet-Teller method; DIFE, Difenoconazole; BPA, Bisphenol A; DBP, Dibutyl phthalates; CAR, Carbendazim; CBZ, Carbamazepine; CIP, Ciprofloxacin; DEHP, di-(2-ethylhexyl) phthalate; DEP, Diethyl phthalate; DFC, Diclofenac; DIF, Diflubenzuron; DIP, Dipterex; DPG, Disposable protective glove; EE2, 17α-ethinyl estradiol; GC, Gas chromatography; GC-MS, Gas chromatography equipped with mass spectrometer; HDPE, High-density polyethylene; HHCB, Galaxolide; HPLC, High-performance liquid chromatography; IBU, Ibuprofen; ICP-MS, Inductively coupled plasma mass spectrometry; ICP-OES, Inductively coupled plasma optical emission spectrometry; LDPE, Low-density polyethylene; MAL, Malathion; MK, Musk ketone; MO, Musk odors; MPs, Microplastics; MX, Musk xylene; NBR, Nitrile butadiene rubber; NPX, Naproxen; NRL, Natural rubber latex; NSAIDs, Non-steroidal anti-inflammatory drugs; PA, Polyamide; PAHs, Polycyclic aromatic hydrocarbons; PCBs, Polychlorinated biphenyls; PE, Polyethylene; PMMA, Polymethyl methacrylate; PP, Polypropylene; PPE, Personal protective equipment; PRP, Propranolol; PS, Polystyrene; PVA, Polyvinyl alcohol; PVC, Polyvinyl chloride; S-PE-MPs, Microplastics exposed to soil; SBR, Styrene butadiene rubber; SDBS, Sodium dodecyl benzene sulfonate; SDZ, Sulfadiazine; SER, Sertraline; SMX, Sulfamethoxazole; TC, Tetracycline; TCEP, tris(2-chloroethyl) phosphate; TCS, Triclosan; TMP, Trimethoprim; TnBP, tri-n-butyl phosphate; TPP, Triphenylphosphine; UHMWPE, Ultra-high molecular weight polyethylene; UV-EMPs, UV aged etched-microplastics; UV-MPs, UV aged microplastics; VOCs, Volatile organic compounds; W-PE-MPs, Microplastics exposed to water; XRF, X-ray fluorescence spectrometer
Keywords: Pollution, Disposable gloves, COVID-19, Pandemic, Plastics, Risk
Graphical Abstract
1. Introduction
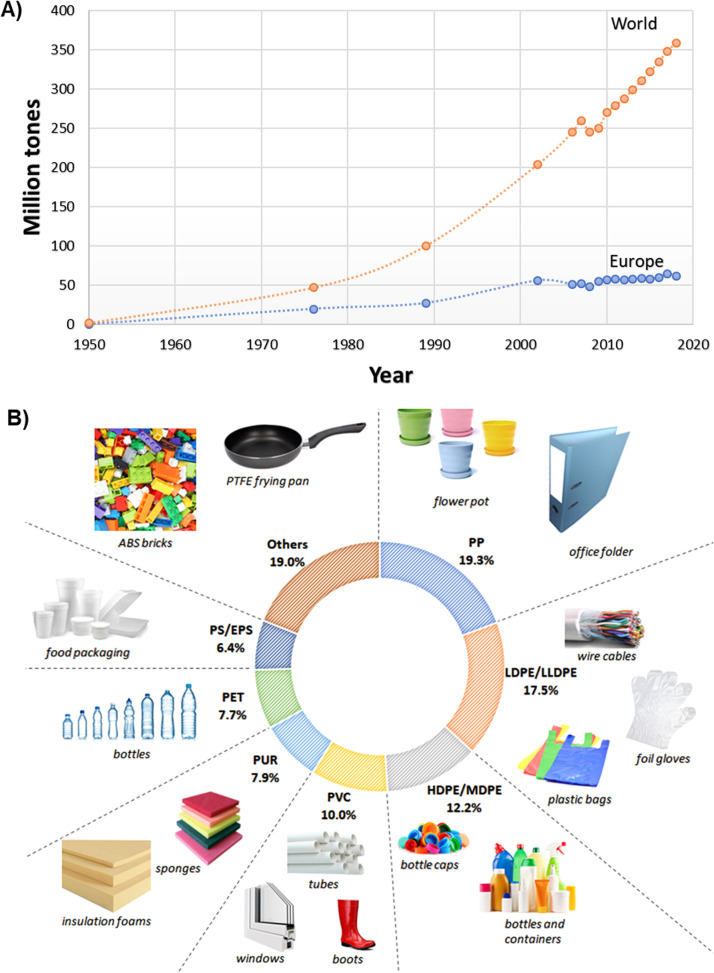
The production of global plastics in 2018 almost reached 360 million tons, while in Europe it was almost 62 million tons (Geyer et al., 2017, Plastics Europe, EPRO, 2019). The growth of global and Europe production of plastics from 1950 to 2018 presents in Fig. 1A. The most commonly used polymers are polyethylene (PE), polyvinyl chloride (PVC), polypropylene (PP) and polystyrene (PS), which are about 65.4% of global plastic requirement (Fig. 1B) (Wilkes and Aristilde, 2017). Plastics are used to produce everyday products (Fig. 1B). The increasing production and consumption of plastic products became a major problem due to growth of wastes and environmental pollution (Soares et al., 2021).
Fig. 1.
(A) World and Europe production of plastics (Geyer et al., 2017); (B) Plastics demand distribution by their types in 2018 and examples of particular plastics application (ABS – acrylonitrile butadiene styrene, HDPE/MDPE – high density polyethylene/medium density polyethylene, LDPE/LLDPE – low density polyethylene/linear low density polyethylene, PET – polyethylene terephthalate, PP – polypropylene, PS/EPS – polystyrene/expanded polystyrene, PTFE – polytetrafluoroethylene, PUR – polyurethane, PVC – polyvinylchloride).
The appearance of COVID-19 pandemic has caused global panic because of the disease spreading rate and mortality. Fast spreading of the virus required restrictions to reduce a number of infections. The main restriction was isolation, the mass gathering prohibition, physical distancing, strict travel control (cancellation of international flights, limitation of movement between cities), closure of schools, businesses, restaurants, shopping centers and other public places (Gostin and Wiley, 2020, Guan et al., 2020). The prevention strategies include recommendations of frequent hand washing, avoiding the face touching, social distancing (about 2 m), and many more (Acter et al., 2020). The most popular means of infection prevention are disposable protective gloves (DPGs) and protective masks. People are ordered to use DPGs during shopping, and to cover mouth and nose in public places. This fact caused the necessity of buying, possessing and daily usage of gloves. Gradually opening of public places has contributed to additional increase of the gloves usage due to maintaining hygiene standards when performing services in shopping centers, barbers, cosmetics, restaurants and offices.
Some of plastics (PE or PVC) are commonly applied for disposable protective gloves (DPGs) production. Additionally, the production of DPGs involves natural and synthetic rubbers, also made from polymers (Aldiyarov et al., 2020). Because of the material, DPGs are categorized into latex (natural rubber) , nitrile, foil, vinyl, neoprene and others. The required use of DPGs during the pandemic caused their increased production at the same time. Before the pandemic, production of disposable gloves by Polish companies usually showed about 6% increase every year (from 5.8% to 6.3% in years 2014–2019). However, during the pandemic period, the DPGs production only in March 2020 increased by 30% compared to the same time in 2019 (Ta polska spółka zarobiła krocie na pandemii wirusa. Są najnowsze szacunki, 2020). The price of the company stocks increased from $1.71 to $88.53 within a year (data from 20th August 2019 and 20th August 2020, respectively) (Frączyk, 2020). In the 2019, global DPGs market was valued at $7.6 billion and it is predicted to reach $11.8 billion by 2025 (World Market Outlook for the Disposable Gloves and Materials Markets, 2020–2025: The World Health Organisation Estimates Manufacturing Should be Increased by 40% to Meet Demand, 2020). The Malaysian Rubber Glove Manufacturers Association estimated that global production of DPGs will reach 420 billion pieces in 2021, up from 380 billion in 2020 (Phoonphongphiphat, 2021). Another projections for 2021 indicate the growth of demand for gloves at the level of 15–20% (Hutchinson and Bhattacharya, 2021).
The common use of DPGs by the entire community has caused increased quantities of gloves in landfills and also their presence in random spots such as parks, lawns, parking spaces and many others ( Fig. 2) (Ammendolia et al., 2021, Boyle, 2020b, Nowakowski et al., 2020). The customers of local shops, large supermarkets and shopping centers, despite placing containers for collecting used gloves, very often dispose of them randomly (Fig. 2). It generates a massive amount of used gloves, which creates a serious problem for the environment. Especially, the urban and suburban areas are significantly more polluted than before the pandemic. The different kinds of DPGs may be observed everywhere (Fig. 2).
Fig. 2.
The examples of discarded DPGs in random spots in Lublin and Poznań Cites (Poland).
Personal protective equipment (PPE) commonly used during the pandemic became a new waste category (Nowakowski et al., 2020). According to the Directive 2008/98/EC of European Union, this type of debris are classified as mixed or medical, separated wastes (European Commission, 2008). The appearance of SARS-CoV-2 virus caused introduction of new handle of wastes rules both for healthy and infected or sent to quarantine (Liang et al., 2021). It includes collecting of PPE in separate bags and then thrown into mixed waste container, disinfection of reusable containers and means of transport of waste, spraying the bag with a viricidal preparation (concerns the waste of people in isolation) and many others (Ministry of Climate and Environment, 2020). The medical COVID-wastes are classified as a hazardous bio-medical wastes. They must be separately stored, disinfected and properly transported because the SARS CoV-2 virus can survive on materials even 9 days (Nzediegwu and Chang, 2020). The hospital wastes can be neutralized in various technologies such as pyrolysis vaporization incinerator, rotary kiln incinerator, plasma incinerator, chemical disinfection or high temperature steam disinfection (Ilyas et al., 2020, Wang et al., 2020d). Unfortunately, beside the proper hospital wastes treatment, a lot of citizens do not follow rules, which causes significant pollution, especially in urban areas not only in Poland but also over the World (Ammendolia et al., 2021, Ardusso et al., 2021, Boyle, 2020b, Kalina and Tilley, 2020). Besides benefits resulting from protection equipment use, increased usage of plastics, from which gloves are made and their release to water and soil can adversely affect the environment. In the long-term it may pose a threat to living organisms including humans.
Plastic debris degraded under environmental conditions to microparticles can be ingested by marine organisms and get to the human food chain (Mattsson et al., 2017). Due to the fact that various substances/components are added to polymeric materials during the manufacturing process ( Table 1), a possibility of release of these substances/components during contact with water and/or soil may appear (Meng et al., 2020, Thompson et al., 2009). There is not covalent bonding between additives and polymers and their leaching is possible and easy (Bagel-Boithias et al., 2005, Gewert et al., 2015). These leachable substances may get to the water and soil, circulate in the environment and in consequence contaminate drinking water and human food chain (Bank et al., 2020, Waring et al., 2018). Data collected in Table 1 shows that commonly used plastic additives may variously affect living organisms health and contribute to the development of numerous disorders. Additionally, microplastics may play a role of contaminants carrier due to the ability of adsorption of various inorganic and organic pollutants (Bouwmeester et al., 2015). This review summarizes predicted risk of environmental pollution resulting from increased usage of disposable protective gloves (DPGs) during pandemic COVID-19 associated with possibility of contaminants release from gloves materials, pollutants accumulation on their surface and the effects of gloves materials aging contributing to changes of their properties.
Table 1.
The examples of organic components commonly added during plastics manufacturing, their role and influence on living organisms (chemical structure figures were taken from ChemSpider: http://www.chemspider.com).
| Compound | Structure | Negative effect | Organism | Ref. |
|---|---|---|---|---|
| Plasticizers | ||||
| dimethyl phthalate (DMP) | 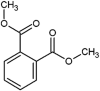 |
Oxidative damages, disturb of the gene expression levels | Zebrafish (Danio rerio) | (Cong et al., 2020) |
| inhibited growth, cell inactivation, cell membranes damage | Escherichia coli K12 | (Wang et al., 2019b) | ||
| prenatal exposure affects reproductive development | Human males | (Suzuki et al., 2012) | ||
| diethyl phthalate (DEP) | 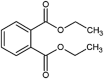 |
Reduced the average lifespan, decreased reproduction | Caenorhabditis elegans | (Pradhan et al., 2018) |
| histological structures damages of liver and kidneys, inhibition of cell proliferation | Flounder (Paralichthys olivaceus) | (Xiao et al., 2018) | ||
| diethylhexyl phthalate (DEHP) | 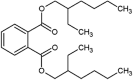 |
Liver damage, ROS generation, lipid peroxidation, immunosuppression | Catfish (Pelteobagrus fulvidraco) | (Mo et al., 2019) |
| reduced the average lifespan, decreased reproduction | Caenorhabditis elegans | (Pradhan et al., 2018) | ||
| disruption of female fertility, hormones and ovarian folliculogenesis | Mice | (Chiang et al., 2020, Chiang and Flaws, 2019) | ||
| utero exposure decreases anogenital distance and reproductive, poses reproductive hazard | Human males | (Dorman et al., 2019) | ||
| diisodecyl phthalate (DIDP) | 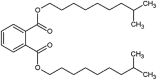 |
Behavioral, enzymological, and oxidative stress, disruption in circadian rhythm | Zebrafish (Danio rerio) | (Poopal et al., 2020) |
| liver and kidney damage, increase in levels of ROS | Balb/c mice | (Chen et al., 2019b) | ||
| diheptyl phthalate (DHP) | 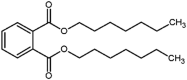 |
Behavioral, enzymological, and oxidative stress, disruption in circadian rhythm | Zebrafish (Danio rerio) | (Poopal et al., 2020) |
| diisononyl phthalate (DINP) | 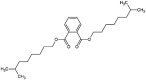 |
Accumulation of ROS, damage and inflammatory responses in liver and kidney tissues, neuroinflammation in the brain, disruption of female fertility, disruption of hormones and ovarian folliculogenesis | Mice | (Chiang et al., 2020, Duan et al., 2018, Ma et al., 2014) |
| decrease of testosterone level in male | Human | (Henrotin et al., 2020) | ||
| adversely affect oocytes growth and maturation, abnormal gonadal development and reproduction, disruption of the endocannabinoid system | Zebrafish (Danio rerio) | (Forner-Piquer et al., 2018, Santangeli et al., 2017) | ||
| Flame retardants | ||||
| tris(2-chloroethyl) phosphate (TCEP) | 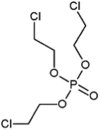 |
Influence on biochemical and electrolyte levels of fish, histopathological anomalies in the gills, liver, and kidney tissues | Freshwater fish (Cirrhinus mrigala) | (Sutha et al., 2020) |
| neurotoxicity by increasing thyroid hormones, induce of oxidative damage | Kunming mice | (Wang et al., 2020a) | ||
| down regulation of the expression of selected genes and proteins related to neurodevelopment of zebrafish embryos/larvae | Zebrafish | (Li et al., 2019) | ||
| tris(1-chloro-2-propyl)phosphate (TCPP) | 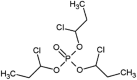 |
Significant alterations of proteins associated with: neurotransmission, neurodevelopment, signal transduction, transport, metabolism, and detoxification | Rockfish (Sebastes schlegeli) | (Ji et al., 2020) |
| tetrabromobisphenol A (TBBPA) | 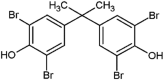 |
Affects the exploratory and anxiety-related behavior | Wistar rats | (Rock et al., 2019) |
| changes in thyroid receptor and deiodinase enzyme expression | Zebrafish (Danio rerio) | (Parsons et al., 2019) | ||
| Decabromodiphenyl ethane (DBDPE) | 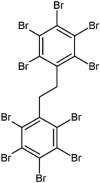 |
Induced oxidative stress, lipid membrane peroxidation, DNA damage | Earthworms (Eisenia fetida) | (Zhao et al., 2020) |
| induction of oxidative stress and an inflammation response resulted in endothelial dysfunction and cardiovascular disorders, could impair liver structure and function | Rats | (Jing et al., 2019, Sun et al., 2020) | ||
| Decabromodiphenyl ether (DBDE) | 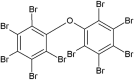 |
Disruption of molting, neurotoxicity (disruption of neurotransmitter signaling pathways) | Folsomia candida | (Zhang and Qiao, 2020) |
| adverse effect on phagocytosis of haemocytes, histopathological effects in gills | Marine scallop (Chlamys farreri) | (Xia et al., 2020) | ||
| Hexabromocyclododecane (HBCDD) | 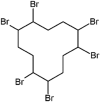 |
Developmental neurotoxicant, impairments in motor maturation of pups | Wistar rats | (Maurice et al., 2015) |
| induction of nuclear abnormalities | Benthic clams Macoma balthica (L.) | (Smolarz and Berger, 2009) | ||
| Antioxidants | ||||
| 3-t-butyl-4-hydroxyanisole (BHA) | 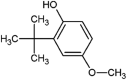 |
Stimulating action on ROS production | Rat liver | (de Oliveira Pateis et al., 2018) |
| butylated hydroxytoluene (BHT) | 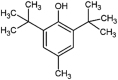 |
Cardiotoxicity, developmental toxicity | Zebrafish (Danio rerio) | (Sarmah et al., 2020) |
| Stabilizers | ||||
| bisphenol A (BPA) | 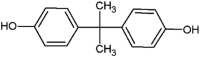 |
Developmental exposure lead to acute metabolic effects in larvae, affected hatchability and heart rates, craniofacial deformity, elongation of head length | Zebrafish (Danio rerio) | (Huang et al., 2020b, Martínez et al., 2020) |
| physiological and biochemical alterations, decrease in growth and vitality, increase in intracellular ROS level | Chlamydomonas reinhardtii Corbicula fluminea | (Esperanza et al., 2020) | ||
| Nonylphenol | 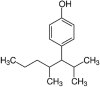 |
Impairing of germ cell development and maintenance the induction of the ROS leading to disruption of cell membrane and damages of cellular metabolism perinatally exposition causes myelination in the cerebellum of offspring | Mice | (Park et al., 2020) |
| Mullets (Liza klunzingeri) | (Salamat and Derakhshesh, 2020) | |||
| Wistar rats | (Jiang et al., 2020) | |||
ROS – reactive oxygen species.
2. Disposable protective gloves (DPGs)
DPGs are generally used in medical applications (during surgery, examinations) and less often in home, industry and food service. Due to the appearance of the SARS-CoV-2 and precautions consequently introduced by heads of states, gloves have begun to be used by the entire community. PPE, especially gloves and masks play a significant role during COVID-19 pandemic. Health care workers and presently the rest of the society use them to minimize the infection risk. Additionally, PPE may improve psychological comfort through the feeling of being more protected than in the reality (Mahmood et al., 2020, You et al., 2020).
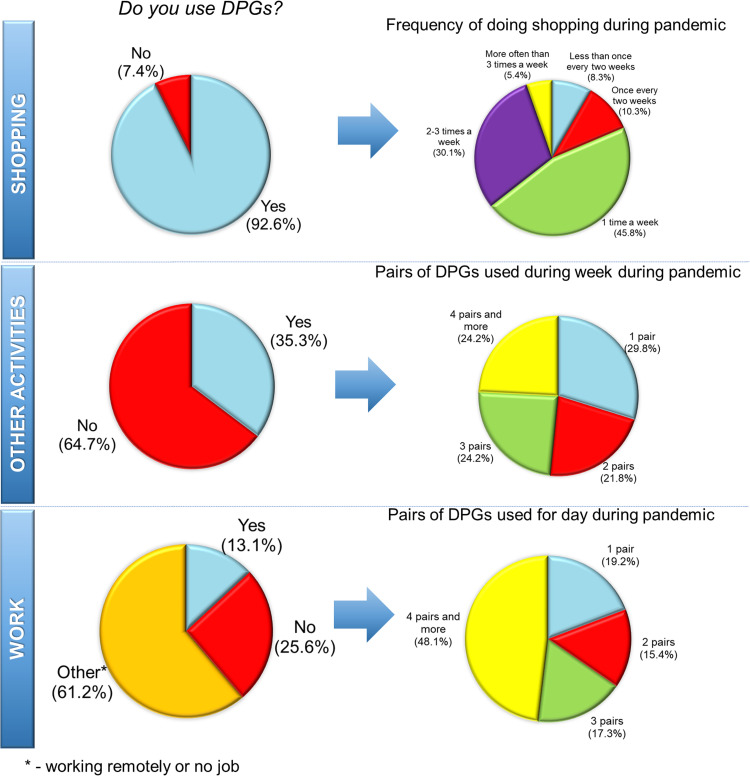
Preliminary insight based on questionnaire revealed that the daily usage of DPGs during pandemic have greatly increased in comparison to the pre-pandemic period. The survey was performed for group of 312 people (85.9% of women and 14.1% of men) about 2 months after the occurrence of first case of COVID-19 in Poland (on the beginning of May 2020). Most of the answers (55.1%) came from people at the age from 20 to 29. Less than half of respondents declared that before pandemic they had been using disposable gloves (e.g. during cleaning or working). During that time, calculated usage of gloves pairs equaled 0.58 per person per week. After 2 months of pandemic duration, up to 93.6% of respondent declared using of the DPGs during shopping and from this group, 24.3% used four or more pairs of DPGs a week. Calculated usage of DPGs at that moment equaled 5.4 pairs of gloves/person/week. These preliminary estimations revealed that after 2 months of the pandemic, the usage of DPG have increased about ten times (936% increase). Detailed results obtained from the survey are shown in Fig. 3.
Fig. 3.
Diagrams presenting the usage of DPGs during pandemic (data come from the survey performed at the beginning of May 2020).
The survey was performed during the period when the most restrictions were introduced. Gradual opening of public places and maintaining the order of gloves wearing additionally affected the rapid increase of DPGs use at numerous places. For instance, hairdressers and cosmetics are obliged to use a fresh pair of gloves every new client. For the mentioned reason, the duration of pandemic COVID-19 generates still increasing amounts of DPGs being then introduced to the environment.
3. Materials for DPGs production
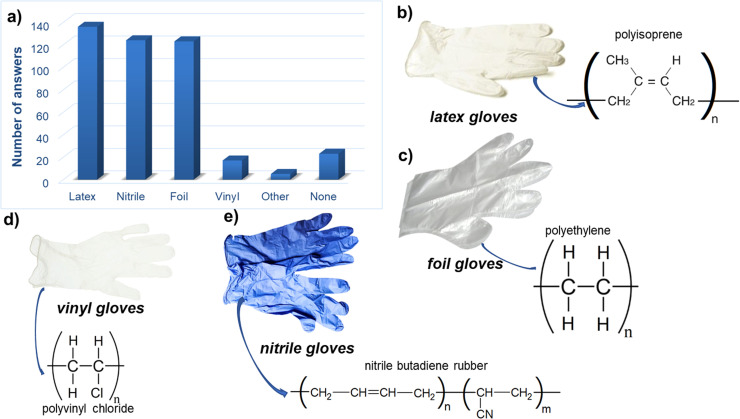
Gloves properties are dependent on the applied material for their production, additives and manufacturing process. Following materials are used for the gloves production: natural rubber latex (NRL), nitrile butadiene rubber (NBR), PVC, PE, styrene butadiene rubber (SBR), neoprene, polyvinyl alcohol (PVA) and few others (Preece et al., 2020, Yew et al., 2019). Based on the performed survey, during the pandemic three types of DPGs are mainly used: latex, nitrile and folic, which make up almost 94.6% of all gloves used, and less often used are vinyl gloves ( Fig. 4).
Fig. 4.
The survey summary concerning the DPGs types usage during a pandemic (n = 312) (a) and types of mainly used gloves: latex (b), foil (c), vinyl (d), and nitrile (e).
The most commonly rubber used for the production of gloves (mainly for medical application) is natural rubber, called latex (Fig. 4b) (Preece et al., 2020). Natural rubber is obtained from Amazonian rubber tree (Hevea brasiliensis) by the tapping of the tree bark. Milky fluid (fresh latex) is collected into containers and afterwards processed into raw rubber in the factory (Bang-iam et al., 2013, Yip and Cacioli, 2002). Latex generally consists of cis-1,4-polyisoprene, has a white color and good physical properties (Agrawal and Konno, 2009). Solid natural rubber also contains natural lipids, phospho- and glycolipids, proteins, and others (Sakdapipanich and Rojruthai, 2012). Latex contain only small percentage of nonrubber substances. However, the improvement of material’s properties can be achieved through the additional processes such as chlorination or polymer coating resulting in introducing of additional elements to the latex structure (Yip and Cacioli, 2002). The main disadvantage of latex gloves is the fact of provoking allergic reaction even in 6% of the worldwide population (Preece et al., 2020). For this reason, other types of materials were proposed and used for gloves production.
Nitrile gloves are a good alternative to other types of gloves. They characterize resistance to chemicals, low allergic risk and good dexterity (Yew et al., 2019). The cost of nitrile rubber is the lowest for the gloves production and this material characterizes suitable mechanical and chemical performance quality (Yew et al., 2020). Nitrile rubber is obtained as a result of a polymerization process of butadiene and acetonitrile (Fig. 4e) and hence is known as acetonitrile butadiene rubber (NBR) (Yew et al., 2019). The content of acetonitrile varies from 18% to 50% depending on targeted properties of the material. Increased concentration of acetonitrile in rubber results in higher strength, hardness, heat resistance and low-temperature flexibility (McKeen, 2012). During the manufacturing process such additives as emulsifiers, activators and catalyst are commonly used (Why Nitrile Gloves Is a Better Alternative Compared To Latex, n.d.). The improvement of nitrile material’s properties can be gained through the addition of substances and chemical reaction (carboxylation or hydrogenation) (Yew et al., 2019). Nishioka et al. (2020) proved that additives in nitrile gloves can, rarely but also, cause allergic reaction.
Other gloves commonly used during the pandemic are made from PE foil gloves (Fig. 4c). Polyethylene is not suitable for medical gloves production and it is also toxic. This type of gloves is mainly used in non-medical applications, such as food service lines, deli counters, petrol stations and others. During pandemic, this type of gloves is often used during shopping (shops are obliged to provide DPGs for clients and this type of gloves are selected in majority). PE is widely used due to their properties such as good chemical stability, resistance, odourlessness and cost-effectiveness. Disadvantages of this material are low dexterity, poor physical and mechanical properties and toxicity (Yew et al., 2019). PE may be produced as a high-density polyethylene (HDPE), which is harder and more flexible and less rigid low-density polyethylene (LDPE). Apart from the glove’s material, PE is commonly applied for the production of other products e.g. packaging, laboratory equipment and some medical applications. Nowadays, PE is the most abundant plastic in the aquatic environment (Crawford and Quinn, 2017). Increased consumption of PE poses a serious threat for the environment (Zhang et al., 2016b).
PVC is a material used for vinyl gloves production (Fig. 4d). This type of gloves is rarely used during pandemic. Based on the survey, only 4.1% of respondents use vinyl DPGs. PVC is a polymer with high chlorine content. Due to the high content of chlorine (57% of total weight), settling of PVC in soil and water may lead to introducing of harmful chlorinated substances, HCl and chlorinated dioxins to the environment (Glas et al., 2014). During the manufacturing process, the degradation of PVC (dehydrochlorination process) often occurs, which requires addition of heat stabilizers (Zhang et al., 2016a). Additionally, plasticizers and other additives (heavy metals, phthalates) are applied providing better physical and chemical properties of the material (Giacomucci et al., 2020, Prata et al., 2020). It was proved that vinyl gloves contain plasticizers at level upwards 30% of the glove’s weight (Arbor et al., 2019).
4. DPGs influence on the environment
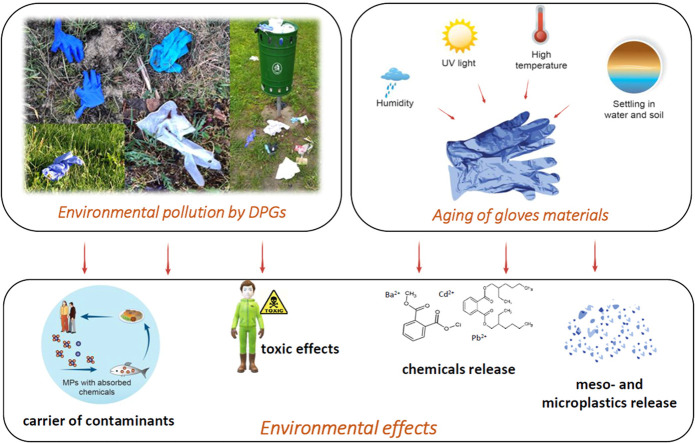
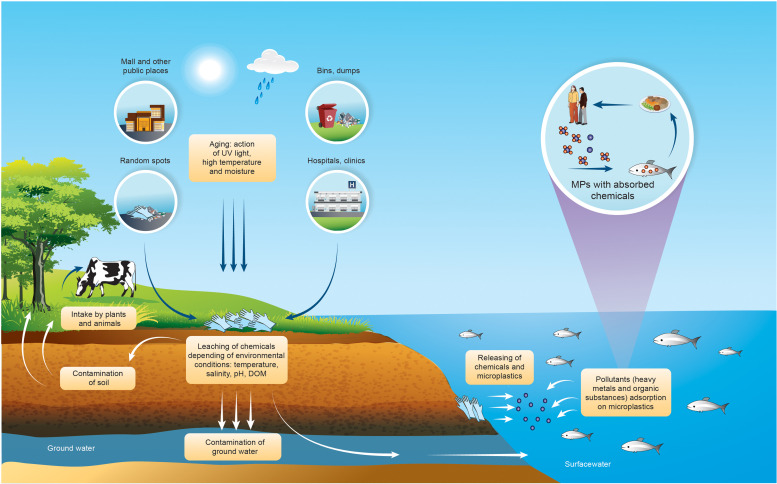
Increased use of DPGs have generated a significant amount of gloves waste in both landfills and random places. Plastics, poorly-degradable materials are not environmentally inert (Elizalde-Velázquez et al., 2020). The components of DPGs such as heavy metals and organic chemicals may be easily released from gloves and may contaminate water and soil (Garçon et al., 2017, Hospital et al., 2007, Kawamura et al., 2002, Kawamura et al., 2000, Reichel, 2012, Sugiura et al., 2002, Wakui et al., 2001). Gradual release of contaminants locally influences wildlife by disturbing their development and leads to various disorders. Additionally, plastic materials are characterized by adsorptive properties by which different inorganic (heavy metals) (Besson et al., 2020, Zhang et al., 2020a) and organic contaminants (e.g. polycyclic aromatic hydrocarbons (PAHs)) (Lei et al., 2020, Rochman et al., 2013), pharmaceuticals (Elizalde-Velázquez et al., 2020, Wu et al., 2016) and personal care products (Dong et al., 2019) may be adsorbed, accumulated and transported ( Fig. 5). Fragmentation of gloves is other important aspect. The fragmentation may make the release of contaminants easier, but also the surface area of disintegrated gloves may increase, which make this fragments a good adsorbent. The longer the pandemic lasts, the more DPG-waste will get to the environment and more serious effects may cause. Discarding gloves in a long-term perspective may come out to be a real threat for present and future generations.
Fig. 5.
The routes of possible environmental pollution by DPGs.
4.1. Pollutants in DPGs and gloves materials
4.1.1. Heavy metals
Metals can be introduced to plastics during the gloves manufacturing process. Metal ions are applied as cross-linkers which speed up the sulfur vulcanization. As crosslinkers divalent: Zn(II) or Mg(II) or polyvalent metal ions: Zr(IV), Cr(III), Fe(III), Al(III) may be used (Yew et al., 2020, Yew et al., 2019). Additionally, divalent metal ions influence the quality of rubber, e.g. storage characteristics or plasticity (Zhao et al., 2018). Performed experiments revealed that a wide range of metals can occur and be leached from commercially available DPGs.
The potential of metals release from DPGs was evaluated by Garçon et al. (2017). Metals were leached from 15 different types of gloves provided by different suppliers, of different colors and materials (vinyl, nitrile, latex, and neoprene). Extracts of gloves were prepared by their soaking in 0.4 mol/L HNO3 + 0.05 mol/L HF at room temperature for 40 h. Performed measurements revealed that the most of 60 elements occurred in the extracts. Zn was present in the highest concentration (from 0.6 to 863.8 mg/L). The amount of Fe, Hf, Mg, Mn, Pb, Rb, Sr, Sn, Ti and Zr released from gloves to the solution was above of 50 µg/L, while the concentration of Ag, As, Ba, Ce, Cr, Cu, Ga, La, Li, Nd, Ni, Sc, Se, Th, Tl, V and Y in extract ranged from about 5–50 µg/L. From nitrile, latex and neoprene gloves the highest amounts of elements were released, while latex gloves released the most rare elements. No differences were observed depending on the glove’s color (Garçon et al., 2017).
Wakui et al. (2001) determined selected metals (Al, Ca, Cd, Cu, Mg, Pb, S, Si, Zn) in 4% of acetic acid extracts from 16 disposable gloves made of PVC, PE, NRL and NBR. The content of Pb and Cd in extracts were below the detection limit (0.10 mg/L) in all samples. The lowest concentration was noted for Al and it ranged from 0.11 to 0.55 mg/L (detected in 10 samples), while the highest concentration was observed for Ca, which ranged from 0.14 to 23.9 mg/L and was detected in almost all investigated samples. Mg and Cu were identified in the smallest number of samples (2 and 5, respectively) and the concentration ranged from 0.76 to 1.56 mg/L for Mg and from 0.18 to 2.29 mg/L for Cu (Wakui et al., 2001). Due to the insufficient data available in literature concerning heavy metal content in various types of DPGs, it is difficult to estimate potential risk of heavy metals release after getting DPGs to the environment. Studies concerning concentration of metals in materials from which gloves are made (NRL, NBR, PE, PVC) can provide more data and be a base to evaluate the potential risk of environmental pollution by DPGs.
Except of gloves, trace elements were detected in raw PE (Munier and Bendell, 2018), particles of plastic bags (Alam et al., 2019, Alam et al., 2018), litters (Nakashima et al., 2011, Turner, 2017), micro- and macro plastics (Munier and Bendell, 2018, Turner et al., 2019), and debris (Prunier et al., 2019) made of PE (both LDPE and HDPE). Ba, Cd, Cr, Cu, Pb and Zn concentrations were studied most often and the obtained results are collected in Table 2. Among the analyzed elements, the highest content of Cd, Cr and Pb was noted in PE debris (Prunier et al., 2019). In comparison to a new packaging or pristine pellets their level is several times higher. The concentration of Ba was the highest in beached bio-beads (Turner et al., 2019). Only Cu content was higher in a new packaging (Prunier et al., 2019) in comparison to debris and other PE materials. Both pristine PE and PE debris may be relevant sources of metals in the environment. Among the other elements, which were determined in PE, Fe content was noted on the high level, i.e. in the range from 92.5 to 29,200 µg/g in beached filamentous plastic litters (Turner, 2017). Mn was determined in PE, HDPE and LDPE bags, but their content varied from below detection limit to 25.1 µg/g (Alam et al., 2019, Alam et al., 2018). Other heavy metals (Bi, Hg, Ni, Sn, Sr, Ti, and V) rarely occurred in PE particles or their concentration was relatively low (Prunier et al., 2019, Turner, 2017, Turner et al., 2019).
Table 2.
The content of selected heavy metals in daily use products made from plastics usually used for gloves production (PE, LDPE, HDPE and PVC).
| Determined element | Plastic type | Plastic origin | Concentration range (µg/g) | Sample preparation | Used technique | Ref. |
|---|---|---|---|---|---|---|
| Ba | PE | New bio-beads | 300–606 | – | XRF | (Turner et al., 2019) |
| Used bio-beads | 318–5500 | – | ||||
| Beached bio-beads | 342–8020 | – | ||||
| New packaging | 0.01–1528 | Acid digestion with bi-distilled HNO3; multistep procedure using microwave oven (110 °C - 3 min, 160 °C - 3 min, 200 °C - 15 min) | ICP-MS | (Prunier et al., 2019) | ||
| Virgin pellets | nd–0.5 | |||||
| Debris | 0.1–371.7 | |||||
| HDPE | Bags | 0.01–318.4 | Digestion with 65% HNO3 (30 min, RT), 90 min in 175 °C, 30% H2O2 added and heated 60 min | ICP-OES | (Alam et al., 2019) | |
| LDPE | 33.6 ± 0.8 | (Alam et al., 2018) | ||||
| PVC | 16.9–250.7 | (Alam et al., 2019) | ||||
| 110.1 ± 1.0 | (Alam et al., 2018) | |||||
| 1.12–197.3 | (Alam et al., 2019) | |||||
| 197.3 ± 4.0 | (Alam et al., 2018) | |||||
| 0.2–295.4 | (Alam et al., 2019) | |||||
| 62.0 ± 5.6 | (Alam et al., 2018) | |||||
| Toys | 3.1–71.3 | Incubation in 0.07 M HCl for 2 h (37 °C) | XRF | (Turner, 2018) | ||
| Cd | PE | Beached bio-beads | 35.1–312 | – | XRF | (Turner et al., 2019) |
| New packaging | nd – 0.83 | Acid digestion with bi-distilled HNO3; multistep procedure using microwave oven (110 °C - 3 min, 160 °C - 3 min, 200 °C - 15 min) | ICP-MS | (Prunier et al., 2019) | ||
| Virgin pellets | nd – 0.02 | |||||
| Debris | nd – 4285 | |||||
| HDPE | Bags | nd – 17.5 | Digestion with 65% HNO3 (30 min, RT), 90 min in 175 °C, 30% H2O2 added and heated 60 min | ICP-OES | (Alam et al., 2019) | |
| LDPE | nd – 34.2 | (Alam et al., 2018) | ||||
| PVC | nd – 23.1 | |||||
| nd – 473.8 | ||||||
| 135.0 ± 3.08 | ||||||
| LDPE | “Recently manufactured” polymers | 0.4 ± 0.1 | Washing in 10% HNO3 at 30 °C for 2 h | AAS | (Munier and Bendell, 2018) | |
| 1.8 ± 0.6 | ||||||
| PVC | Toys | nd – 274.0 | Incubation in 0.07 M HCl at 37 °C for 2 h | XRF | (Turner, 2018) | |
| Cr | PE | Litters | 14.0 ± 6.0 | – | XRF | (Nakashima et al., 2011) |
| 23.1–909.0 | – | (Turner, 2017) | ||||
| Used bio-beads | 24.9–62.4 | – | (Turner et al., 2019) | |||
| Beached bio-beads | 17.4–1400 | |||||
| New packaging | 0.1–2.40 | Acid digestion with bi-distilled HNO3; multistep procedure using microwave oven (110 °C - 3 min, 160 °C - 3 min, 200 °C - 15 min) | ICP-MS | (Prunier et al., 2019) | ||
| Virgin pellets | 0.1–10.0 | |||||
| Debris | nd – 2541 | |||||
| HDPE | Bags | nd – 75.1 | Digestion with 65% HNO3 (30 min, RT), 90 min in 175 °C, 30% H2O2 added and heated 60 min | ICP-OES | (Alam et al., 2019) | |
| LDPE | nd – 73.6 | (Alam et al., 2018) | ||||
| PVC | 1.6 ± 0.04 | (Alam et al., 2019) | ||||
| nd – 78.4 | (Alam et al., 2018) | |||||
| nd – 23.2 | ||||||
| 0.2 ± 0.1 | ||||||
| Toys | nd – 18.6 | Incubation in 0.07 M HCl for 2 h (37 °C) | XRF | (Turner, 2018) | ||
| Cu | PE | Litters | 11.6–808 | – | XRF | (Turner, 2017) |
| Used bio-beads | 14.3–83.5 | – | (Turner et al., 2019) | |||
| Beached bio-beads | 13.5–363 | – | ||||
| New packaging | nd – 1684 | Acid digestion with bi-distilled HNO3; multistep procedure using microwave oven (110 °C - 3 min, 160 °C - 3 min, 200 °C - 15 min) | ICP-MS | (Prunier et al., 2019) | ||
| Virgin pellets | nd – 0.21 | |||||
| Debris | 0.05–11.9 | |||||
| HDPE | Bags | nd – 95.6 | Digestion with 65% HNO3 (30 min, RT), 90 min in 175 °C, 30% H2O2 added and heated 60 min | ICP-MS | (Alam et al., 2019) | |
| LDPE | 95.6 ± 2.33 | (Alam et al., 2018) | ||||
| PVC | 1.17–259 | (Alam et al., 2019) | ||||
| 429.7 ± 1.42 | (Alam et al., 2018) | |||||
| nd – 157.9 | (Alam et al., 2019) | |||||
| 157.9 ± 11.9 | (Alam et al., 2018) | |||||
| 0.3–45.4 | (Alam et al., 2019) | |||||
| 4.21 ± 0.59 | (Alam et al., 2018) | |||||
| Pb | PE | Litters | 45.0 ± 14.0 | – | XRF | (Nakashima et al., 2011) |
| 2.8–3770 | – | (Turner, 2017) | ||||
| Used bio-beads | 9.4–69.9 | – | (Turner et al., 2019) | |||
| Beached bio-beads | 8.1–5380 | – | ||||
| New packaging | nd – 2.26 | Acid digestion with bi-distilled HNO3; multistep procedure using microwave oven (110 °C - 3 min, 160 °C - 3 min, 200 °C - 15 min) | ICP-MS | (Prunier et al., 2019) | ||
| Virgin pellets | nd – 0.15 | |||||
| Debris | 0.01–8314 | |||||
| HDPE | Bags | nd – 65.8 | Digestion with 65% HNO3 (30 min, RT), 90 min in 175 °C, 30% H2O2 added and heated 60 min | ICP-OES | (Alam et al., 2019) | |
| LDPE | 4.8 ± 0.2 | (Alam et al., 2018) | ||||
| PVC | nd – 71.2 | (Alam et al., 2019) | ||||
| 3.6 ± 0.6 | (Alam et al., 2018) | |||||
| nd – 12.4 | (Alam et al., 2019) | |||||
| 4.1 ± 0.1 | (Alam et al., 2018) | |||||
| nd – 15.7 | (Alam et al., 2019) | |||||
| MPs | 6691 ± 388 | Incubation in 2% HNO3 | ICP-MS | (Boyle et al., 2020) | ||
| for 1 h | ||||||
| Toys | nd – 163.0 | Incubation in 0.07 M HCl for 2 h (37 °C) | XRF | (Turner, 2018) | ||
| “Recently manufactured” polymers | 2.7 ± 1.5 | Washing in 10% nitric acid at 30 °C for 2 h | AAS | (Munier and Bendell, 2018) | ||
| LDPE | 52.2 ± 17.7 | |||||
| Zn | PE | Litters | 10.9–588 | – | XRF | (Turner, 2017) |
| New bio-beads | 420–588 | – | (Turner et al., 2019) | |||
| Used bio-beads | 58.6–854 | – | ||||
| Beached bio-beads | 16.3–1590 | – | ||||
| New packaging | 0.10–331 | Acid digestion with bi-distilled HNO3; multistep procedure using microwave oven (110 °C - 3 min, 160 °C - 3 min, 200 °C - 15 min) | ICP-MS | (Prunier et al., 2019) | ||
| Virgin pellets | nd – 0.19 | |||||
| Debris | 1.17–327 | |||||
| HDPE | Bags | nd – 153 | Digestion with 65% HNO3 (30 min, RT), 90 min in 175 °C, 30% H2O2 added and heated 60 min | ICP-MS | (Alam et al., 2019) | |
| LDPE | 107 ± 4.68 | (Alam et al., 2018) | ||||
| PVC | nd – 212 | (Alam et al., 2019) | ||||
| 130 ± 12.9 | (Alam et al., 2018) | |||||
| nd – 65.2 | (Alam et al., 2019) | |||||
| 54.0 ± 5.03 | (Alam et al., 2018) | |||||
| nd – 90.4 | (Alam et al., 2019) | |||||
| 8.75 ± 0.26 | (Alam et al., 2018) |
nd – not detected; AAS – atomic absorption spectroscopy; ICP-OES – inductively coupled plasma optical emission spectrometry; ICP-MS – inductively coupled plasma mass spectrometry; XRF – X-ray fluorescence spectroscopy.
Due to the limited data on the content of contaminants in PVC-based gloves, some information about the existing problem and a potential risk may be provided by analysis of data of other PVC products. Heavy metals were detected in raw PVC (Munier and Bendell, 2018), PVC microplastics (Boyle et al., 2020), and PVC products such as toys (Turner, 2018) or bags (Alam et al., 2019, Alam et al., 2018). Among the analyzed PVC materials, PVC bags (Alam et al., 2019, Alam et al., 2018) showed the highest content of the most determined heavy metals (Ba, Cd, Cr, Cu and Zn, Table 2). In case of Pb, the PVC microplastics (MPs) contained the highest on performed experiments on zebrafish, authors concluded that the amount of Pb released from PVC MPs may cause a negative response in zebrafish, while PVC may pose a long-term environmental Pb reservoir available for aquatic animals (Boyle et al., 2020). Toys made of PVC are a frequent subject of this kind of analysis and can be a good example of potential risk. Turner (2018) tested children’s toys to evaluate the occurrence of heavy metals and the possibility of their release under simulated stomach conditions. After extraction, the solution was analyzed in terms of Ba, Cd, Cr, Hg, and Pb determination. Author of the work confirmed the occurrence of Ba, Cd, Cr and Pb in extracts, where the Pb concentrations exceeded acceptable content of Pb regarding EU Toy Safety Directive (allowable concentration of Pb equals 23 μg/g) (Turner, 2018).
Depending on the material used, the concentration of metals in different plastics including gloves can be very high. However, additional research is needed for better understanding. The potential risk is not only related to the concentration of metals in DPGs but also, from the environmental point of view. Water and soil moisture probably are the most important factor, which causes the release of different contaminants from gloves. However, the others factors such as temperature, UV light or microorganisms can enhances the leaching process. This knowledge is necessary to estimate the environmental risk of pollution resulting from DPGs frequent usage and disposal.
4.1.2. Organic contaminants
As was mentioned before, many of different additives are applied during gloves manufacturing to obtain a material with desirable properties. Beside polymers, plastics and rubbers may contain plasticisers, reinforcements, toughners and/or colorants (Crawford and Quinn, 2017). Several reports indicate the problem of organic contaminants released from plastics DPGs (Hospital et al., 2007, Kawamura et al., 2002, Kawamura et al., 2000, Reichel, 2012, Sugiura et al., 2002), but information concerning the level of pollutants leached from DPGs in realistic environmental conditions are still scanty. Most of the experiments concerning organic contaminants release from DPGs are carried out beyond the actual environmental conditions by using harsh solvents such as n-heptane (Kawamura et al., 2002) or the content of organic additives has not been quantified (Reichel, 2012, Sugiura et al., 2002). In Table 3 the occurrence and possibility of various additives leaching from DPGs are presented.
Table 3.
The examples of organic substances occurred in various types of DPGs.
| Type of gloves | Leached substance | Function | Concentration range (µg/g) | Sample preparation | Used technique | Ref. |
|---|---|---|---|---|---|---|
| Vinyl | 1,2-benzisothiazolin-3-one | Antimicrobial agent | nd – 26 | Extraction with a mixture of water and methanol (3:1) overnight, RT | HPLC | (Hospital et al., 2007) |
| Diethyleneglycol dibenzoate | Plasticizer | nd – 350 | Extraction with n-heptane, 60 min, 25 °C | GC-MS | (Kawamura et al., 2002) | |
| Dipropyleneglycol dibenzoate | Plasticizer | nd – 520 | ||||
| Diisononyl adipate | Plasticizer | nd – 750 | ||||
| Alkylsulfonic acid phenyl ester | Plasticizer | nd – 1.39 | ||||
| di(2-ethylhexyl) phthalate | Plasticizer | 1.41–2.50a | Extraction with n-heptane, 60 min, 25 °C | GC-MS | (Kawamura et al., 2000) | |
| Diisononyl phthalate | Plasticizer | 720a | ||||
| di(2-ethylhexyl) adipate | Plasticizer | 137–841a | ||||
| 4-nonylphenol | Plasticizer | 2.72–36.4a | ||||
| Alcohol ethoxylates | Emulsifiers | – | Water extraction without shaking, 30 min | LC-MS/MS (LTQ-Orbitrap) | (Reichel, 2012) | |
| Foil | Octadecanoic acid amide | Antioxidant | – | Extraction with acetone, 30 min, 150 °C, again extraction with methanol, 30 min | FT-IR and GC-MS | (Sugiura et al., 2002) |
| 2,6-di-tert-butylphenol | Antioxidant | – | ||||
| di(2-ethylhexyl) phthalate | Antioxidant | – | ||||
| cis-13-docosenoic acid amide | Antioxidant | – | ||||
| octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate | Antioxidant | – | ||||
| Nitrile | Sodium dodecylbenzene sulfonate | Emulsifier | – | Water extraction without shaking, 30 min | LC-MS/MS (LTQ-Orbitrap) | (Reichel, 2012) |
| Polyethylene glycols | Non-aqueous solvents | – | ||||
| Alcohol ethoxylates | Emulsifiers | – | ||||
| Alkylphenol ethoxylates | Emulsifiers | – | ||||
| phthalates | Plasticizers | – | ||||
| Latex | Alcohol ethoxylates | Emulsifiers | – |
nd – not detected, below the detection limit; RT – room temperature; FT-IR – Fourier infrared absorption spectrophotometry; GC-MS – gas chromatography with mass spectrometry; HPLC – high performance liquid chromatography; LC-MS/MS (LTQ-Orbitrap) – liquid chromatography equipped with orbitrap mass spectrometer.
in µg/cm2.
Up to now, there are only a few works related to different organic contaminants content in pristine gloves (Hospital et al., 2007, Kawamura et al., 2002, Kawamura et al., 2000, Reichel, 2012, Sugiura et al., 2002) (Table 3). It can be found that latex, nitrile, vinyl and foil DPGs may release various organic substances, which are plasticizers, emulsifiers, antioxidants or antimicrobial agents. The content of additives released from gloves may even reach almost 800 µg/g diisononyl adipate (extracted from vinyl gloves) (Kawamura et al., 2002). There is an urgent need to estimate (also in environmental conditions) other additives, which may occur in DPGs and verify the environmental factors facilitating their leaching when DPGs get to the soil and/or water. Nowadays, the pollution risk from discarded gloves may be determined on the basis of experiments with the use of polymers of which gloves are made.
PE or PVC, which are used during DPGs production are also applied for production of other kinds of goods. Thus, even if we do not have information about the concentration of different compounds in gloves, it is possible to estimate the risk on the basis of other products. Akhbarizadeh et al. (2020) collected data concerning the content of selected pollutants in commercially available bottled water made of various. For instance, bisphenol A (BPA), an essential component of many plastic items (Osman et al., 2021), was detected in PE bottled water and BPA concentration equaled to 20.7 ng/L (Chailurkit et al., 2017). Other, very common components of plastics that may be released during plastics utilization are lubricants. Farajzadeh et al. (2006) determined the content of commercial lubricants (stearamide, oleamide and erucamide) in 12 samples of PE (granule or sheet forms) complete dissolving of PE in xylene ( Table 4) (Farajzadeh et al., 2006). Lv et al. (2009) quantified the content of fatty acid amides such as hexadecanoamide, octadecanamide, oleamide, erucamide in PE packaging material samples. The highest amount was noted for oleamide and it ranged from 243 to 969 µg/g. The possibility of selected phthalates: di-(2-ethylhexyl) phthalate (DEHP), diethyl phthalate (DEP), benzyl butyl phthalates (BBP), and dibutyl phthalates (DBP) releasing from PE materials to food was investigated by Ayamba et al. (2020). The authors confirmed that increasing contact time and temperature intensifies phthalates leaching process. Concentrations of the phthalates in the olive oil after 4 h of contact (at 80 °C) occasionally exceeded the specific migration limit according to the EU regulation 10/2011 (for DEHP and DBP) (Ayamba et al., 2020, Commission Regulation (EU) No 10/2011, 2011).
Table 4.
The content of selected organic chemicals in PE and PVC products.
| Quantified compound | Plastic type | Plastic origin | Concentration range (µg/g) | Extraction conditions | Used technique | Ref. |
|---|---|---|---|---|---|---|
| Stearamide | PE | Granule and sheet forms | 504–1244 | Dissolved in xylene by heating on hot plate | HPLC and GC | (Farajzadeh et al., 2006) |
| Oleamide | 91–858 | |||||
| Packaging | 243–969 | 1:1 of isopropanol in chloroform (30 min, 90 °C, 100 bar) | GC-MS | (Lv et al., 2009) | ||
| Erucamide | Granule and sheet forms | 343–1485 | Dissolved in xylene by heating on hot plate | HPLC and GC | (Farajzadeh et al., 2006) | |
| Packaging | 201–337 | 1:1 of isopropanol in chloroform (30 min, 90 °C, 100 bar) | GC-MS | (Lv et al., 2009) | ||
| Hexadecanoamide | nd – 116 | |||||
| Octadecanamide | nd – 327 | |||||
| ΣPAHsa | Synthetic rope | 0.18 | Extraction in sweater for 24 h and 120 h under artificial light (400–750 nm) at 28 °C on a magnetic agitator 600 rotations per minute (rpm). | GC-MS | (Gardon et al., 2020) | |
| ΣAdditivesb | 281.7 | |||||
| DEHP | PFC | 1.54 ± 0.06 | Extraction with olive oil at 20 °C for 4 h | GC-MS | (Ayamba et al., 2020) | |
| DEP | TAB | 1.39 ± 0.04 | ||||
| DBP | PFC | 0.78 ± 0.19 | ||||
| TAB | 0.67 ± 0.06 | |||||
| PFC | 0.43 ± 0.10 | |||||
| TAB | 0.54 ± 0.18 | |||||
| 2,4-di-tert-butylphenol | Recycled HDPE | Packaging | nd – 1.7 | Extraction with isooctane at 20 °C for 2 days | GC-MS | (Dutra et al., 2011) |
| Limonene | nd – 1.6 | |||||
| Bisphenol A | PVC | Stretch films | nd – 98 | Extraction with acetonitrile for 24 h at 60 °C | HPLC | (López-Cervantes and Paseiro-Losada, 2003) |
| Benzyl butyl phthalate (DBP) | Toys | nd – 0.263c | Dissolution in THF (kept overnight), precipitation with n-hexane | GC-MS | (Oteef and Elhassan, 2020) | |
| di-(2-ethylhexyl) phthalate (DEHP) | <LOQ – 32.2c | |||||
| Diisononyl phthalate (DINP) | nd – 14.2c |
The sum of naphtalene, benzothiophene, biphenyl, acenaphtylene, acenaphtene, fluorene, phenanthrene, anthracene, dibenzothiophene, fluoranthene, pyrene, benzo[a]anthracene, chrysene, benzo[b+k]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, perylene, indeno(1,2,3-cd)pyrene, dibenzo(a,h)anthracene, and benzo(g,h,i)perylene.
The sum of dimethyl phthalate (DMP), diethyl phthalate (DEP), di-n-butyl phthalate (DBP), butyl benzyl phthalate (BBP), diethylhexyl adipate (DEHA), di(2-ethylhexyl) phthalate (DEHP), Irgafos 168® oxidized, and Irgafos 168® reduced.
Concentration in % wt/wt; <LOQ – below the quantification limit; PFC – polyethylene food containers; TAB – take away polyethylene bags.
Toys and children’s supplies made from PVC usually contain plasticizers, which may be released during handling or mouthing due to the fact that plasticizers are not covalently bonded (Steiner et al., 1998). The same can occur with gloves that can have direct contact with the human body, food or saliva. Recently, Babich et al. (2020), who was investigating potential migration of 38 different plasticizers from children’s toys, child care articles, art materials, and school supplies, identified the following chemicals: acetyl tributyl citrate (ATBC), di (2-ethylhexyl) terephthalate (DEHT), diisononyl 1,2-cyclohexanedicarboxylic acid (DINX), 2,2,4-trimethyl-1,3 pentanediol diisobutyrate (TPIB), diisononyl phthalate (DINP), DEHP and tributyl citrate (TBC) in solutions obtained through the samples extraction with simulated saliva. Some of toys contained two or even three plasticizers at the same time. The determination of plasticizers in PVC children’s toys and supplies was performed also by Oteef and Elhassan (2020). Three plasticizers were quantified: DBP, DEHP and DINP (Table 4). One or more regulated phthalates from analyzed products exceeding regulatory limit level (Oteef and Elhassan, 2020). The migration of DEHP from respiratory PVC medical devices dedicated to newborns was investigated by Bouattour et al. (2020). The level of DEHP in the air reaches a cumulative quantity of 123 mg. A humidifying of the air decreased the DEHP content, which is important especially in term of environmental conditions (Bouattour et al., 2020). Similarly as PE products, PVC also may contain BPA. López-Cervantes and Paseiro-Losada (2003) estimated content of BPA in PVC stretch films used for food packaging (Table 4) (López-Cervantes and Paseiro-Losada, 2003).
The release of organic substances in environmental conditions is important to find if increased usage of DPGs can lead to elevated pollution levels and threaten living organisms. It is necessary to examine all the materials, which are used for gloves production and produced gloves to test the ability of the pollutants releasing, especially in environmental conditions under the influence of settling in the soil, humidity or increased temperature.
4.2. Pollutants adsorption and accumulation
Beside the direct effects of DPGs on living organisms (release of inorganic and organic contaminants), there is an indirect effect related to plastic sorption capacity to different compounds (Crawford and Quinn, 2017). It has been shown that plastics and plastic particles that occur in the environment may adsorb various inorganic and organic pollutants (Zhang et al., 2020a). After adsorption, plastic particles act as a contaminants carrier and for this reason pose an increased ecotoxicological risk (Santos-Echeandía et al., 2020). Due to that, it is very important to extend the knowledge about the possibility of contaminants accumulation by DPGs in the context of their use and disposal. The most widely used gloves are made from natural or synthetic rubber as well as from such plastics as PVC, PE, SBR, neoprene or PVA. The lack of knowledge concerning adsorption capacity of natural and synthetic rubbers makes it impossible to assess the risk of pollutants accumulation by rubber gloves. Up to now, the adsorption capacity to heavy metals (Pb, Cd, Cr, Cs, Cu, Zn) and organic pollutants (polycyclic aromatic hydrocarbons, pharmaceuticals, pesticides and others) on PVC and PE (which are used for vinyl and foil gloves production, respectively) were investigated (Besson et al., 2020, Chen et al., 2019a, Dong et al., 2019, Li et al., 2018, Razanajatovo et al., 2018, Wang et al., 2020e, Wang et al., 2021, Yu et al., 2020, Zhang et al., 2020b, Zon et al., 2018, Zon et al., 2020). Up to now, most of the experiments related to adsorption of heavy metals by plastics applied pristine or aged particles and no research has been done regarding gloves.
It has been shown (Besson et al., 2020) that PE can adsorb heavy metals, such as Cd, Cs and Zn. Adsorption capacity of pristine microplastics (MPs) of PE (32–75 µm PE beads) was lower than natural sediment particles (35–91 µm). After 72 h of exposure, pristine PE MPs adsorbed less than 0.8% of Zn, Cs and Cd added to the solution, while for sediment particles the values ranged from 2.5% to 71.0%. It may suggest that the pristine PE MPs not efficiently adsorb and transport Zn, Cs and Cd in marine environment (Besson et al., 2020). LDPE microbeads (with size 200–300 µm) were used as plastic models to evaluate the adsorption kinetics of Cd (Zon et al., 2020). The maximum adsorption capacity was determined to be 10.1 µg/g based on the pseudo-first order model. Authors indicated that the adsorption process is reversible and the bonds between Cd and LDPE microbeads are relatively physically weak, however they suggested that microbeads may be a vector for heavy metals in aquatic life (Zon et al., 2020). PE beads (177–250 µm) were used for the evaluation of the Cr(VI) adsorption possibility (Zhang et al., 2020b). The experiments were designed to test the influence of pH and the presence of sodium dodecyl benzene sulfonate (SDBS) on the Cr adsorption capacity. The authors have found that the presence of SDBS can increase attractions between PE and Cr. The adsorption capability increased from 390 to 1360 µg/g after the addition of 2 mM SDBS. The pH was another factor causing changes in adsorption capability. Increasing pH decreased the adsorption capacity of PE beads to Cr (Zhang et al., 2020b). The adsorption capacity of Cr on pristine LDPE microbeads was also determined by Zon et al. (2018). The experiments were performed in artificial seawater. Based on the pseudo-first order model the maximum adsorption capability (qmax) of LDPE microbeads was calculated and equal 1.7 µg/g (Zon et al., 2018). Pristine PE MPs (75–140 µm) were tested to determine the adsorption capacity of Cu with and without the presence of tetracycline (Wang et al., 2021). Calculated qmax based on Langmuir model equaled 31.2 µg/g, while in the presence of 5 mg/L TC, qmax reached 42.6 µg/g. Additionally, authors of this work have compared adsorption capacity of pristine PE MPs with microplastics that were exposed to air (A-PE-MPs), water (W-PE-MPs) and soil (S-PE-MPs) and they found that the environment-exposed microplastics possessed higher adsorption capacity for Cu (Wang et al., 2021). Turner et al. (2020) suggested that due to the significant amount of metal additives in PE, they may be more harmful than the metals adsorbed on polymer surfaces. However, the release of metal which was preliminary adsorbed is higher than release of metal added during production of PE. Bioaccessibility of Pb is considerably larger when the metal is adsorbed on the plastic surface than when it is an additive consisting in the polymeric matrix. The authors evaluated that 0.1 µg/g of Pb was adsorbed on PE surface, while the concentration of Pb as an additive in PE was from a few thousand µg/g to about 40,000 µg/g. From the amount of Pb absorbed by PE, 60–70% was accessible, while only 16% was accessible from PE with Pb as a component of polymer structure (Turner et al., 2020).
Godoy et al. (2019) investigated the potential of PE and PVC as a carrier of Cr, Cu, Pb and Zn. Authors observed differentiated PE and PVC sorption capacity depending on the type of metal. PE more efficiently than PVC adsorbed Cr (4.7 and 2.4 mg/g on PE and PVC, respectively) and Pb (2.4 and 1.9 mg/g on PE and PVC, respectively), while Zn was slightly better adsorbed on PVC (0.5 and 0.6 mg/g on PE and PVC, respectively). The adsorption affinity of Cu was observed only for PE (0.3 mg/g). All adsorption capacities were calculated based on the Langmuir model (Godoy et al., 2019). The measurements performed using the Brunner-Emmet-Teller (BET) method confirmed more developed surface of PE than PVC (1.4 vs. 0.59 m2/g), which may suggests that surface area may be important for Cr and Pb, while other mechanisms are responsible for sorption of Zn. There are still very few studies relating to the sorption of heavy metals by plastics. However, the studies conducted so far allow us to believe that, depending on the type of material and the additives used in it, adsorption/desorption may have a significant impact on their distribution of metals in the environment.
The adsorption capacity of Cu and Zn on PVC was investigated by Brennecke et al. (2016). Aged PVC fragments collected from seawater showed very strong interactions with Cu and Zn leached from antifouling paint immersed in the seawater. The concentration of Cu and Zn reached 1320.7 ± 268.6 µg/g PVC and 102.1 ± 21.5 µg/g PVC, respectively. Authors concluded that high adsorption capacity of Cu and Zn to PVC allow to transport these pollutants over long distances in marine systems and provide a risk to water organisms (Brennecke et al., 2016). Wankasi and Dikio (2014) used PVC wastes to investigate Pb ions adsorption from the aqueous standard solutions. The adsorption capacity of PVC to Pb was estimated 1 mol/g (according to the Freundlish model), which needs to be a mistake as this value refers to 1 g of Pb on gram of polymer. An important issue regarding the potential risk related to heavy metals and plastic is the difference between the release of freshly adsorbed metals and the release of metals constituting a component of plastics (added during production).
The different anthropogenic organic contaminants, which are not constituents of plastics, are another group of substances, which may be adsorbed on plastics. The adsorption capacity of PE and PVC to PAHs (Frias et al., 2010, Hirai et al., 2011, Karapanagioti et al., 2010, Lei et al., 2020, Rochman et al., 2013), polychlorinated biphenyls (Camacho et al., 2019, Frias et al., 2010, Hirai et al., 2011, Mizukawa et al., 2013, Rochman et al., 2013), pharmaceuticals (Elizalde-Velázquez et al., 2020, Li et al., 2018, Razanajatovo et al., 2018, Wu et al., 2016, Yu et al., 2020), organophosphate esters (Chen et al., 2019a), and others (Dong et al., 2019, Wang et al., 2020c, Wang et al., 2020e) were investigated. PE has been characterized as a great sorbent of hydrophobic organic contaminants (Karapanagioti et al., 2010, Lei et al., 2020). Due to the fact that PAHs are one of the most common anthropogenic pollutants, the research on their adsorption by various plastics is best described in their case. Lei et al. (2020) determined the adsorption kinetics of 16 US EPA PAHs and 13 substituted PAHs (including 5 nitro-PAHs, 4 methyl-PAHs and 4 oxy-PAHs) on LDPE film. It was found that the equilibrium time was linearly dependent for PAHs and SPAHs rings number. Increasing the number of rings reduced the equilibrium time. Calculated log KLDPE (the coefficients of partitioning between LDPE and water) ranged between 4.45 and 6.25 (Lei et al., 2020). Rochman et al. (2013) investigated the adsorption capacity of 15 PAHs (US EPA) to pre-production plastic pellets made of HDPE, LDPE and PVC. The pellets were located during the long period (up to 12 months) in five spots in natural water. Authors observed that LDPE and HDPE more efficiently adsorb PAHs than PVC. The concentration of PAHs was about one order of magnitude higher on LDPE and HDPE than on PVC and likely that it is the result of greater permeability of LDPE and HDPE (Pascall et al., 2005, Rochman et al., 2013). After 12 months of exposure, the total PAHs concentration equaled 797 ng/g for HDPE, 722 ng/g for LDPE and 27 ng/g for PVC. In terms of environmental hazard, particles of PE may be a carrier for these compounds due to the fact that PE has a greater adsorption capacity than other plastics including PVC (Teuten et al., 2007) and pose a threat in case of PE ingestion by animals (Rochman et al., 2013). Karapanagioti et al. (2010) determined the adsorption kinetics of naphthalene, phenanthrene, and pyrene on eroded PE pellets collected from beaches. Phenanthrene and pyrene have similar kinetic behavior and equilibrium values, whereas naphthalene reaches equilibrium in shorter a time, but at lower equilibrium value (Karapanagioti et al., 2010). Wang et al. (2018) evaluated adsorption capacity of field sampled LDPE and nylon fibers toward phenanthrene. The lack of hydrophilic functional groups on LDPE fibers provided higher adsorption capacity of phenanthrene than more hydrophilic nylon. Based on the pseudo-second-order model, the amount of adsorbed phenanthrene equaled 147.1 µg/g on PE rope, and 135.1 µg/g on nylon rope (Wang et al., 2018). Fries and Zarfl (2012) investigated adsorption capacity of selected PAHs (acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene and fluoranthene) by low and high density PE. LDPE showed higher diffusivity and shorter equilibrium time compared to HDPE, which means that, LDPE may be considered as more hazardous in the environment and be a better carrier for PAHs than HDPE (Fries and Zarfl, 2012). Sørensen et al. (2020) have confirmed that beside the type and size of plastic particles, temperature and salinity of water may affect the sorption capacity of PAHs. In this work, the authors studied the sorption kinetics of two model PAHs (fluoranthene and phenanthrene) to PE particles (microbeads with mean diameters from 10 to 200 µm) in natural seawater at 10 and 20 °C. Additionally, the bioavailability of MPs-sorbed PAHs to marine copepods was investigated. Results indicated that adsorption dominated at lower temperatures and for smaller MPs (10 µm). The authors did not observe the accumulation of PAHs from plastics by copepods and concluded that free dissolved PAHs are only significantly bioavailable to organisms tested (Sørensen et al., 2020).
Polychlorinated biphenyls (PCBs) are the other very common group of organic contaminants. PCB were used in various commercial applications and it has been confirmed (Camacho et al., 2019, Frias et al., 2010, Hirai et al., 2011, Mizukawa et al., 2013, Rochman et al., 2013) that may be adsorbed by different kinds of plastics. Rochman et al. (2013) investigated the possibility of 27 PCBs adsorption on HDPE, LDPE and PVC over 12 months. During this long-term field experiment, sorption rates and concentrations of PCBs varied significantly among plastic types. Similarly like PAHs, PCBs were more efficiently adsorbed on HDPE and LDPE than on PVC (Rochman et al., 2013), however PVC reached equilibrium in the marine environment much faster than LDPE and HDPE. The authors concluded that products made from HDPE, or LDPE pose a greater risk than products made from PVC.
Besides very popular in environmental PAHs and PCBs, other emerging contaminants may adsorb on polymers. Razanajatovo et al. (2018) investigated the adsorption of three pharmaceuticals (sulfamethoxazole (SMX), propranolol (PRP) and sertraline (SER)) on PE microplastics in water. After 96 h of the experiment, the highest adsorption capacity was observed for SER (26.6%), slightly smaller for PRP (21.6%) and the lowest for SMX (15.3%). The hydrophobic interactions between polymers and pharmaceuticals were the main process responsible for adsorption. The authors also pointed out that the electrostatic interactions may affect SER, PRP and SMX adsorption by PE. Authors (Razanajatovo et al., 2018) proved that the SMX adsorption on PE microparticles is irreversible, while the PRP and SER adsorption may be reversible from the PE. In other research, carried out by Wu et al. (2016), the adsorption capacity of PE debris to carbamazepine (CBZ), triclosan (TCS), and 17α-ethinyl estradiol (EE2) was determined. The main role in the pharmaceutical adsorption (irrespective of the kind of pharmaceuticals), played hydrophobic interactions between pharmaceuticals and PE particles. The changes in water salinity (from 0.05% to 3.5%) have enhanced the adsorption capacity of PE regarding TCS, while in case of CBZ and EE2, salinity did not affect the adsorption process. Additionally, the decrease of EE2 and TCS adsorption on PE debris was noted with increase of dissolved organic matter content in the solution (Wu et al., 2016). The adsorption of commonly used non-steroidal anti-inflammatory drugs (NSAIDs): naproxen (NPX), diclofenac (DFC) and ibuprofen (IBU) on two types of PE: ultra-high molecular weight polyethylene (UHMWPE), and average molecular weight medium density polyethylene (AMWPE) was examined by Elizalde-Velázquez et al. (2020). The adsorption capacity was pH-depended. The adsorption of NAP, DFC and IBU was very slight under fresh water conditions (pH 6.9) and synthetic water conditions (pH 8.1. and 10.0). At pH 2.0 all pharmaceuticals were efficiently adsorbed on PE, mainly due to hydrophobic interactions (Elizalde-Velázquez et al., 2020). Among the examined compounds, DFC exhibited the highest, while NPX the lowest adsorption capacity to PE (Elizalde-Velázquez et al., 2020). In the work of Li et al. (2018) the possibility of 5 antibiotics adsorption on PE and PVC was examined. The following antibiotics were used: sulfadiazine (SDZ), amoxicillin (AMX), tetracycline (TC), ciprofloxacin (CIP), and trimethoprim (TMP). Amount of adsorbed antibiotics generally decreased in the following order: CIP > AMX > TMP > SDZ > TC. In the freshwater investigated antibiotics exhibited higher adsorption affinity to plastics than in seawater (Li et al., 2018). Adsorption capacity of levofloxacin (OFL) on PVC was determined by Yu et al. (2020). Based the Langmuir isotherm model, adsorption capacity of PVC was calculated to be about 2.5 mg/g at the temperature of 25 °C. Subsequently, the effect of Cu(II), Zn(II), Cr(III), Cd(II) and Pb(II) on the adsorption of OFL by PVC was investigated. The presence of Cd(II) and Pb(II) inhibited adsorption of OFL, while Cu(II), Zn(II) and Cr(III) significantly promoted this process (Yu et al., 2020).
The adsorption of two organophosphate esters (tri-n-butyl phosphate (TnBP) and tris(2-chloroethyl) phosphate (TCEP)) commonly used as flame retardants in plastic products on PE and PVC was investigated by Chen et al. (2019a). The adsorption capacity was dependent on the size of plastic particles and plastic type. TnBP and TCEP less efficiently adsorbed with increasing of PE and PVC particles size which was connected with decrease of the surface area of the polymers. PVC was more efficient in TCEP adsorption than PE while PVC was less effective in TnBP adsorption than PE ( Table 5). Authors suggested that adsorption process of both compounds (TnBP and TCEP) was dominated by pore-filling mechanism in the adsorption process on PVC MPs, while monolayer coverage was the predominant mechanism for PE MPs (Chen et al., 2019a).
Table 5.
The adsorption capacity of selected heavy metals and organic pollutants on PE and PVC.
| Chemical group | Sorbate | Adsorbent | Conditions | Adsorption capacity (µg/g) | Ref. |
|---|---|---|---|---|---|
| Heavy metals | Cd | LDPE microbeads | Artificial seawater, 96 h | 10.1a | (Zon et al., 2020) |
| Cr | LDPE microbeads | Artificial seawater, 180 h | 1.7a | (Zon et al., 2018) | |
| PE beads | pH 5, shaken in 25 °C, 12 h | 1360 | (Zhang et al., 2020b) | ||
| Cu | PE MPs | 0.01 mol/L NaNO3, shaken in dark, 25 ℃ | 31.2b | (Wang et al., 2021) | |
| 42.6b,c | |||||
| Cd | PE MPs | Filtered seawater, 72 h, 22 °C | < 0.8d | (Besson et al., 2020) | |
| Cs | < 0.8d | ||||
| Zn | < 0.8d | ||||
| PAHs | Phenanthrene | LDPE from mariculture farm | Ultrapure water (with < 0.1% of methanol), shaken orbitally at 150 rpm, 25.0 °C, 48 h | 146.4a | (Wang et al., 2018) |
| 147.1e | |||||
| PE | 25 mg/L NaN3, < 0.2% of methanol, orbital shaking of 200 rpm in the dark, 20 °C | 138.4a | (Wang and Wang, 2018) | ||
| 159.5e | |||||
| 69.8a | |||||
| PVC | 93.5e | ||||
| Pharmaceuticals | Sulfamethoxazole (SMX) | PE | 0.01 M CaCl2 and 0.02% (wt/vol) | 45.4a | (Razanajatovo et al., 2018) |
| 46.1e | |||||
| Propranolol (PRP) | NaN3 24 °C, 96 h | 60.4a | |||
| 64.4e | |||||
| Sertraline (SER) | 81.6a | ||||
| 88.8e | |||||
| Amoxicillin (AMX) | PE | Ultrapure water, 25 °C, shaking speed of 180 rpm, 4 d | 131b | (Li et al., 2018) | |
| PVC | 523b | ||||
| Ciprofloxacin (CIP) | PE | 200b | |||
| PVC | 453b | ||||
| Trimethoprim (TMP) | PE | 154b | |||
| PVC | 481b | ||||
| Levofloxacin | PVC | 25 °C, shaking speed of 150 rpm, 216 h | 290a | (Yu et al., 2020) | |
| 1740e | |||||
| Organophosphate esters | tri-n-butyl phosphate | PE | Seawater, 18 °C, 24 h, particles size 0.01–0.1 mm | 1.6 | (Chen et al., 2019a) |
| PVC | 0.6 | ||||
| tris (2-chloroethyl) phosphate | PE | 0.6 | |||
| PVC | 1.2 | ||||
| Pesticides | Carbendazim (CAR) | PE | pH 3.85, shaking 180 rpm, 25 °C | 10.6e | (Wang et al., 2020e) |
| 4.4b | |||||
| Dipterex (DIP) | 3.2e | ||||
| 2.9b | |||||
| Diflubenzuron (DIF) | 76.7e | ||||
| 74.1b | |||||
| Malathion (MAL) | 19.1e | ||||
| 25.9b | |||||
| Difenoconazole (DIFE) | 51.6e | ||||
| 273.2b | |||||
| Odors | Tonalide | PE | Simulated seawater (3.5% NaCl solution), 20 °C, 10 h | 0.9e | (Dong et al., 2019) |
| PVC | 1.5e | ||||
| Musk xylene | PE | 0.9e | |||
| PVC | 0.9e | ||||
| Galaxolide | PE | 1.0e | |||
| PVC | 1.6e | ||||
| Musk ketone | PE | 0.7e | |||
| PVC | 1.6e |
Pseudo first order model.
Langmuir model.
In the presence of 5 mg/L tetracycline.
Value in %.
Pseudo second order model.
Wang et al. (2020e) proved that commonly used in vegetable farmland pesticides like carbendazim (CAR), dipterex (DIP), diflubenzuron (DIF), malathion (MAL) and difenoconazole (DIFE) can be adsorbed onto PE agricultural soil film. According to the authors, hydrophobic interactions between PE and pesticides were the main mechanism involved in the adsorption and the process was very fast as the adsorption equilibrium was reached in 2 h. The adsorption capacity of investigated pesticides slightly increases with increase of temperature and equal from about 10 µg/g for CAR to 80 µg/g for DIF (Table 5) (Wang et al., 2020e). The adsorption capacity of virgin HDPE MPs regarding to a 8 pesticides (epoxiconazole, tebuconazole, myclobutanil, azoxystrobin, simazine, terbuthylazine, atrazine and metolachlor) was examined by Wang et al. (2020c) during 21 days of exposure. The adsorption ranged from 61 to 963 ng/g. HDPE MPs exposed higher adsorption capacity for epoxiconazole, tebuconazole, myclobutanil, terbuthylazine, and metolachlor ranging from 427 to 963 ng/g. The lowest affinity to HDPE MPs was observed for simazine (61 ng/g). Additionally, the presence of PE in the system decreased the pesticides degradation, increasing their persistence in the aquatic environment (Wang et al., 2020c).
Dong et al. (2019) investigated the adsorption capacity of PE and PVC to musk odors (MO): tonalide (AHTN), musk xylene (MX), galaxolide (HHCB), and musk ketone (MK). These synthetic compounds are commonly implemented in cosmetics, perfumes and other daily used chemicals. The experiments were performed in simulated seawater. The equilibrium adsorption capacity of MO was higher for PVC than PE. Authors of the work suggested that the difference results from the pore volume and specific surface area of polymers, which are larger for PVC than PE. Additionally, the influence of temperature on the adsorption capacity was investigated. Increasing of the temperature from 15° to 25°C resulted in improving the adsorption process. However, temperature increased above 25 °C deteriorated adsorption, as a result of desorption, which may have affected the adsorption process. The increase of NaCl content (up to 14%) in the background solution promoted adsorption of all compound tested. Presumably, the increase in NaCl concentration decreases the solubility of MO, which decreases the dissolved organic mass and results in the increase of the adsorption capacity (Dong et al., 2019). In Table 5, the adsorption capacities of selected organic pollutants are presented.
It has been showed above that polymeric materials of which the gloves are made (PE and PVC) may adsorb both heavy metals and organic substances, including persistent organic pollutants (Bakir et al., 2014). Unfortunately, no data has referred to DPGs and to other materials used in DPGs production (latex or nitrile rubber). The pristine materials usually used for adsorption capacity investigation may not contain specific additives (e.g. plasticizers, stabilizers, antioxidants and antimicrobial), which are used in gloves production. Presence of additives may increase the number of functional groups on the plastic surface and/or change hydrophilicity/hydrophobicity of plastics and in a consequence affect adsorption capacity. Lack of consideration of the functional groups potentially present on the surface of gloves during sorption capacity evaluation may lead to overestimating or underestimating the adsorption capacity of gloves. Therefore, it should be investigated if produced DPGs has adsorptive properties in natural conditions and how that properties change in relation to pristine polymers. Numerous experiments were carried out in laboratory environments, which may not be enough for reliable risk assessment. For instance, Magadini et al. (2020) suggest that the predominant factor influenced on adsorption capacity is rapid biofouling of plastics. Biofilms creation may significantly impact the pharmaceuticals adsorption on plastics (Magadini et al., 2020). Biofouling may alter polarity of plastic from hydrophobic to strong hydrophilic, which also changes their adsorptive properties and may facilitate pollutants transport (Van Melkebeke et al., 2020). Additionally, water salinity may affect the adsorption process, enhancing interactions between plastics and pollutants and increasing the potential of contaminants transported by plastics (Dong et al., 2019, Sørensen et al., 2020). Adsorptive properties of particular plastics and their settling in the environment may lead to accumulation of pollutants on their surface. This phenomenon was confirmed in several studies (Camacho et al., 2019, Fisner et al., 2013, Frias et al., 2010, Hirai et al., 2011, Karapanagioti et al., 2010, Wang et al., 2019a) through the collection of plastics debris and detection of the contaminants.
PE pellets were collected from sandy beaches of two locations in Saronikos Gulf to investigate the amount of PAHs adsorbed in real conditions. Extracted with hexane pellet released significant amount of PAHs (phenanthrene, anthracene, fluoranthene, pyrene, benzo[b] fluoranthene, benzo[a]pyrene). The concentration of desorbed PAHs varied between about 3 ng/g of benzo[a]pyrene to 86 ng/g of fluoranthene, which is the evidence of water pollution and accumulative properties of PE (Karapanagioti et al., 2010). Marine plastics (95% of plastics made of PE) examined by Camacho et al. (2019) contained PAHs (the sum of 16 US EPA PAHs) at the level of 52.1–17,023.6 ng/g for pooled pellets and 35.1–8725.8 ng/g for fragments. Concentration of 16 PAHs in extracts of PE microplastics collected from beaches of the Portuguese coasts was also determined by Frias et al. (2010). It has been noted that the highest concentration of adsorbed PAHs were pyrene, phenanthrene, chrysene and fluoranthene (Frias et al., 2010). The content of PAHs in all samples of plastic debris (made of PE and PP) collected from open ocean, remote and urbanized beaches was quantified by Hirai et al. (2011). The sum of 16 US EPA PAHs concentrations have ranged from 1 to 9300 ng/g, while those plastics originated from urbanized areas contained the highest amount of PAHs, which is associated with higher risk in urban beaches (Hirai et al., 2011). The sum of 16 PAHs adsorbed on PE originated from Feilaixia Reservoir (Guangdong Province, China) was determined in the work of Tan et al. (2019). The total concentration of PAHs on PE microplastics reached 364.2 ng/g, while the highest content was noted for chrysene (65.0 ng/g) and benzo[ghi]perylene (47.6 ng/g) (Tan et al., 2019). The total concentration of 33 quantified PAH species on PE pellet originated from 9 beaches of Portuguese coast determined by Mizukawa et al. (2013) ranged from 50 to 24,000 ng/g (Mizukawa et al., 2013). The dependence of PAHs concentration in plastic debris on the depth of the plastics occurrence (from 0 to 100 cm) in a sandy beach was analyzed by Fisner et al. (2013). The EPA PAHs, as well as alkyl-substituted PAHs, biphenyl, retene, benzo[c]phenanthrene, benzo[j]fluoranthene, benzo[e]pyrene, perylene, and benzo[b]chrysene were investigated. The concentration of contaminants differs and the highest was noted in the surface layer (Fisner et al., 2013).
The possibility of PCBs accumulation on PE MPs was confirmed based on determination of 17 PCBs in PE pellets collected from coast. The total content of PCBs equaled about 50 ng/g (Frias et al., 2010). PCBs were also determined in PE microplastic (pellets and fragments) collected from beaches. Their concentration ranged from 0.9 to 2285.8 and from 1.6 to 772.5 ng/g for pellets and fragments, respectively (Camacho et al., 2019). Plastic pellets from the Portuguese coast were also analyzed in terms of adsorbed PCBs amount by Muzukawa et al. (2013). Determined content of 13 PCBs ranged from 10 to 310 ng/g (Mizukawa et al., 2013). Plastic debris from marine environments made of PE was extracted and the amount of PCBs was quantified. The sum of PCBs ranged from 1 to 436 ng/g depending on the pellet origin (Hirai et al., 2011).
11 organophosphorus flame retardants (2-ethylhexyldiphenyl phosphate, tri (2-ethylhexyl)) phosphate, tributylphosphate, triethylphosphate, triisobutylphosphate, triphenylphosphate, tris ((2-chloro-1 chloromethyl)ethyl) phosphate, tris (2-butoxyethyl) phosphate, tris (2-chloroethyl) phosphate, tris (2-chloroisopropyl) phosphate, and tricresyl phosphate were determined in plastics debris (pellets and fragments) originated from Canary Islands beaches. More polluted pellets were from urbanized beach. The total content of organophosphorus flame retardants ranged from 20.0 to 378.0 ng/g for pellets and from 22.6 to 7013.9 ng/g for fragments (Camacho et al., 2019).
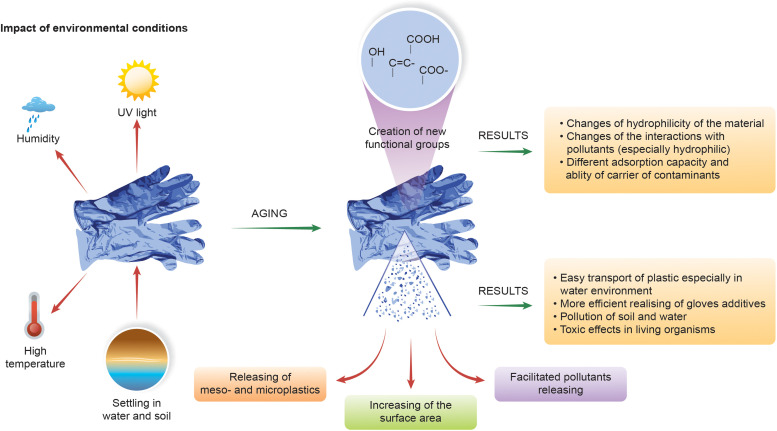
4.3. Degradation (aging) of glove’s materials
The increased risk of the plastic release to the environment can be associated with changes of the material properties as a result of the degradation process (aging). The environmental conditions affect plastics structure causing their degradation, decomposition or disintegration into smaller particles becoming micro- and even nano-plastics (Yu et al., 2019). The degradation of plastics is a result of breakage of large chains to shorter ones with a lower molecular weight. Additionally, the moisture and water chemistry, oxygen or/and UV light may change chemical structure of these materials, while microorganisms contribute to loss of the plastics mass and metabolize it (Lear et al., 2021, Singh and Sharma, 2008). It has been shown earlier that the large specific surface area of degraded polymeric materials has a strong ability to adsorb pollutants (Hüffer and Hofmann, 2016, Munier and Bendell, 2018). All processes changing the structure of plastics in the environment may influence their properties: adsorption capacity and a type of substances, which may be released from the plastic ( Fig. 6) (Müller et al., 2018, Wang et al., 2020b).
Fig. 6.
The impact of environmental conditions on gloves materials.
The degradation process of rubber is very slow in environmental conditions. Studies carried out by Gillen et al. (1996) suggest that elastomers (including nitrile rubber) may be degraded for several dozen of years. The degradation process of natural latex under various conditions (thermal, chemical, photochemical and others methods) was investigated by Ibrahim et al. (2016) and Fainleib et al. (2013). During the processes, chain breaking leaves different end groups depending on the degradation process and properties of the latex (e.g. additives used). The degradation leads to produce liquid natural rubber with various, mostly reactive functional groups such as carbonyl, hydrazine and hydroxyl, depending on the type of reagent used in a chemical method (degradation performed using sodium nitrite or hydrogen peroxide) (Ibrahim et al., 2014). Literature data reveal that thermal degradation of nitrile butadiene rubber (NBR) is a complex process, which affects physical properties of the material. Thermal aging leads to loss or migration of additives and fillers, extra crosslinking and chains oxidation results in loss of elastic properties and softening (Liu et al., 2016, Zaghdoudi et al., 2019). Liu et al. (2016) have performed accelerated thermal aging of nitrile-butadiene rubber using an air-circulating oven at a temperature of 65 °C for 90 days. Exposure of NBR on increased temperature has caused significant and inhomogeneous damages of the material surface. During the aging process, oxidation occurs on the NBR surface and extends to the inner structure. Additionally, on the surface hydroxyl groups were formed and additives migrated from inside of the NBR. These changes significantly influence both mechanical and chemical properties (Liu et al., 2016). Changes in mechanical properties of aged NBR was also tested by Hernández et al. (2018). Accelerated thermal aging was performed for up to 168 h at 100 °C. The degradation process has worsened mechanical properties of NBR (tear and tensile strength and elongation at break) (Hernández et al., 2018). In the literature, no available data regards changes of morphology and chemical properties of aged nitrile DPGs.
Polymers such as PVC or PE characterizes persistence and low degradability (Padervand et al., 2020). Chamas et al. (2020) estimated that a half-live (conversion of the 50% of the polymer mass) of PE may be from 4.6 (for low density PE) to even 5000 years (for high density PE). It is estimated that lifespan of PVC, used for industrial and consumer applications can be from 50 to 100 years (Boyle et al., 2020). The degradation of PE leads to the formation of low molecular weight fragments (alcohols, aldehydes, ketones and carboxylic acids). Additionally, numerous hydrocarbons may be formed (e.g. ethane, ethene, propane, propene or butene) (Gewert et al., 2015). Changes in morphology and chemical properties of aged PVC was investigated by Tang et al. (2018). As a result of heat, solar radiation and ultraviolet B action in a simulated aquatic environment the increased presence of functional groups on the PVC surface were confirmed. FTIR spectra have revealed that the degradation process led to formation of C˭C bonds, ester carbonyl (–COO–) and carboxylic (–COOH) groups. Furthermore, it is suggested that polymer irritation leads to dehydrochlorination and photooxidation. Surface area and pore volume also differ in relation to virgin PVC (Tang et al., 2018).
Degradation of plastics under various conditions causes changes in morphology of the material and their composition due to thermal, hydrolytic, photo-oxidative processes and degrading by microbes taking place in the environment. Changes in the structure of plastics may affect releasing of chemicals. Lomonaco et al. (2020) have investigated changes in volatile organic compounds (VOCs) released from pristine and aged plastics (LDPE and HDPE). Artificial aging was performed under exposure of xenon lamp radiation in temperature of 40 °C up to 4 weeks. Selected VOCs (24 compounds) with different functional groups and 27 other VOCs were determined before and after 1, 2, 3, and 4 weeks of accelerated aging. Performed experiments proved increasing release of ketones, lactones, esters, carboxylic acids, alcohols, ethers and total oxygenated VOCs from HDPE and LDPE with the aging time extension. Authors of the work have supported the hypothesis that microplastics represent an unrecognized source of environmental pollution (Lomonaco et al., 2020). The efficiency of VOCs emission from plastics including PVC and PE during thermal degradation was investigated by Noguchi and Yamasaki (2020). They proved emission of different aliphatic and aromatic hydrocarbons, oxygenated hydrocarbons, and additives. At temperature 50 °C PVC releases the highest amount of additives in comparison to the other plastics and it mainly emitted DEHP, triphenylphosphine (TPP), phenol, 2-ethylhexanol, and octadecene (Noguchi and Yamasaki, 2020).
Another effect, which can be a result of the aging process, is changes in adsorption capacity of plastics. As it was mentioned before, plastics may have the ability to bond different organic and inorganic contaminants. The binding efficiency depends on plastic properties (Bolan et al., 2020). If these properties change, the adsorption capacity of plastics also will change. Huang et al. (2020c) using pristine and aged LDPE observed that both plastics are able to adsorb heavy metals (Cu, Fe, Pb, Mn and Zn) on its surface. However aged LDPE had shown about 5-fold higher adsorptive properties than pristine LDPE. Aging processes have caused an increase of LDPE surface area, oxygen containing functional groups and hydrophilicity (Huang et al., 2020c), which affected sorption capacity of this polymer. Changes in adsorptive properties of Ag on pristine and aged microplastic originated from a common cosmetic product (body scrub), made of PE was evaluated by Kalčíková et al. (2020). Both adsorption and desorption was more efficient for aged microplastics (with the developed biofilm on the PE surface). After 7 days of adsorption experiment, the amount of adsorbed Ag was 3.51 and 2.44 mg/g for aged and pristine microplastics, respectively. Subsequently, after 168 h of that materials leaching into Steinberg medium (used for the cultivation of freshwater duckweeds), 30% of adsorbed Ag leached to medium from pristine microplastics, while aged microplastic desorbed 71% of Ag. Ecotoxicological tests carried out with duckweed (L. minor) revealed that pristine and aged PE MPs with adsorbed Ag affects the root cell viability and the root length of duckweed. Aged plastics more efficiently adsorbed Ag, which was more accessible. The formation of biofilm as a result of plastic aging changed the ecotoxicological potential of the material (Kalčíková et al., 2020). Based on experiments of Cu(II) adsorption, Yang et al. (2019) suggests that UV aging changes adsorption properties of microplastics due to the change of carboxyl index. The carboxyl index of UV-aged polyamide (PA) decreased in comparison to pristine PA, which affected at the same time the decrease of Cu adsorption. However, the carboxyl index of polymethyl methacrylate (PMMA) increased and the PMMA adsorption capacity of Cu also increased. Wang et al. (2021) exposed PE MPs on various environmental factors (obtaining specified aged materials): air (A-PE-MPs), water (W-PE-MPs) and soil (S-PE-MPs) and tested the adsorption capacity of that materials regarding to Cu(II) adsorption in comparison to pristine material. The calculated adsorption capacity of PE MPs based on the Langmuir model increased from 31.2 µg/g to 183, to 108 and to 318 µg/g for A-PE-MPs, W-PE-MPs and S-PE-MPs, respectively. The formation of C-O, H-O and C˭O groups on the surface of environment-exposed PE MPs were demonstrated by XPS spectra. The further studies indicated complexation between Cu(II) and oxygen-containing functional groups, which was responsible for the increasing Cu(II) adsorption efficiency. Additionally, the settling of PE MPs in water and soil caused the biofilms formations on their surface. The occurrence of microorganisms on the plastic surface may enhance the adsorption effectiveness due to the complexation and electrostatic interactions between metal ions and bacterial extracellular polymeric substances (Comte et al., 2008). The concentration of Zn, Cu, Cd and Pb may differ between pristine and aged plastics (Munier and Bendell, 2018). In the work of Munier and Bendell (2018) pristine plastics were purchased from hardware stores, while aged samples of PVC and LDPE were collected from several beaches. Differences between content of Cd, Cu, Pb and Zn were evaluated. It was noted that pristine LDPE contained higher content of Cd, Cu, Pb and Zn compared to aged LDPE, however the aged PVC was abundant in all metals compared to pristine PVC. It may suggest that the aging of PVC significantly changes the adsorption capacity of this polymer especially Cu, Pb and Zn (from about 100–7000 times higher amount of metal in aged PVC) (Munier and Bendell, 2018). The authors suggested that the change of PVC capacity to particular metals during aging may be related to electrostatic forces created between C, Al, Cl, Ca and O (attached to PVC or exposed as a result of aging) and Pb(II) ions. Boyle et al. (2020) have proved that fragmentation of PVC resulting from the PVC’s aging may lead to releasing of increased concentration of Pb to the environment and increase exposure on this element in aquatic life (Boyle et al., 2020).
The adsorption capacity of plastics to PAHs may be also affected by plastic aging. For example, Li et al. (2020) revealed that UV aging of HDPE MPs (UV-MPs) slightly enhanced 16 US EPA PAHs adsorption (log KMPs ranged from 3.80 to 4.95, while log KUV-MPs ranged from 3.71 to 4.98). The process of HDPE etching with chromic acid, simulating the natural oxidation also significantly altered MPs adsorption properties. For etched-MPs (both unfollowed (EMPs) and followed with UV aging (UV-EMPs)), adsorption capacity was higher than on the pristine MPs (log KEMPs in the range of 3.85–5.18 and log KUV-EMPs in the range of 3.90–5.28). Aged MPs characterizes higher specific surface area, pore volume and higher content of oxygen-containing groups in comparison to pristine MPs. According to authors, the mechanism of PAHs adsorption to MPs is based on partioning, while interactions between PAHs and EMPs or UV-EMPs are based on hydrogen bonding and π-π interactions (Li et al., 2020). Liu et al. (2019b) to simulate long-term aging of HDPE in water exposed this material on K2S2O8 and Fenton reagents for 30 days. Adsorptive properties of HDPE to ciprofloxacin (CIP, hydrophilic antibiotic) were examined. The meaningful enhancement of CIP adsorption by aged HDPE compared to pristine HDPE was observed (Liu et al., 2019b). Authors observed significant relationships between O/C ratio and adsorption capacity. The introduction of oxygen-containing groups and as a consequence increase of hydrophilicity and polarity of the material facilitates interactions between CIP and MPs (Liu et al., 2019b). Similarly, aged (in UV light for 96 h) PVC more efficiently adsorbs CIP than pristine PVC. The ability of CIP adsorption was about 20% higher on aged PVC (Liu et al., 2019a). In the case of pesticides adsorption, the aging process of PE microplastics had no significant effects (Wang et al., 2020c).
5. Future risk and challenges
Production of plastics in Europe in 2018 equaled 56.1 millions of tones and 4.9 millions of tones materials obtained from recycling (Tworzywa sztuczne w obiegu zamkniętym Analiza sytuacji w Europie, 2020). Despite increasing recycling, a large part of total plastic wastes gets to landfills. The unexpected appearance of the virus SARS-CoV-2 infections causing COVID-19 has contributed to make a new source of environmental pollution – PPE such as gloves, masks and coveralls. Besides the benefits resulting from gloves usage, which are the most PPE used by the community, the increased usage of plastics and rubbers, from which gloves are made, and their release to water and soil may have adverse effects on the environment. Furthermore, in a long-term perspective, it may pose a threat to living organisms including humans.
The influence of external conditions such as sunlight, temperature, moisture, settling in soil and water on DPGs contributes to material degradation mainly cracking, fracturing and in a consequence create small particles called meso- and microplastics (Giacomucci et al., 2019). These small parts can release or accumulate contaminants and migrate over long distances and transfer through the food chain (Wang et al., 2020c). Over time, properties of materials may change and the new functional groups may create as a result of external conditions. Possibility of long-term settling and degradation process extends the time of effecting on the environment. Due to this fact it should be considered to use easier degradable materials for DPGs productions (Misman and Azura, 2014). Additionally, it requires consideration of the need of such popular usage of DPGs in the case of future unpredictable pandemic appearance. The introduction of precautionary measures should not have serious environmental consequences.
The occurrence of plastics in the environment has wide negative effects on living organisms. Currently, additives leached from plastics are present in the environment (Sackaria and Elango, 2020, Schmidt et al., 2020). Beside the additives with toxic effects, which can be released and get to water or soil, microplastics can be ingested for instance by marine species and release toxins directly into the body (Alomar et al., 2017, Du et al., 2020, Koongolla et al., 2020, Ozturk and Altinok, 2020). Highly prone to pollutants accumulation are marine animals (Huang et al., 2020a). Plastic additives can also transfer to beverages from plastic packages (Gómez Ramos et al., 2019, Pelegrín et al., 2020). Numerous literature data report the presence of toxic chemicals in plastic food contact products (Ayamba et al., 2018, Qian et al., 2018) and beverages (Tian et al., 2020). Unfortunately, pregnant women (Zeman et al., 2013) and newborns (Socas-Rodríguez et al., 2018) are also exposed to plastics additives, which may pose a great danger. Adsorptive effects of microplastics allow to introduce more hazardous pollutants into the body of marine organisms and in a consequence with food to the human body. Based on the determined adsorption capacities of microplastics to PAHs, Sharma et al. (2020) suspect that the amount of PAHs adsorbed by microplastics in the water environment may be high enough to pose a cancer threat for human beings. Despite the fact that any microplastics were detected in gastrointestinal tract of commercial fish (Lepidopus caudatus), a high amount of phthalates, especially DIDP, DEHP, bis-benzyl ester phthalate, bis-butyl ester phthalate, mono-N-butyl ester phthalate and heavy metals in different tissues was determined (Salvaggio et al., 2019).
Due to the ecological detriment caused by polymeric materials it is recommended to limit their use and replacement by biodegradable materials (Panchal and Vasava, 2020). The pandemic COVID-19 has contributed to creation of another source of pollution – DPGs. The current possible risk for environment should be considered in detail: (1) how the increased DPGs use during pandemic will affect the emissions of various artificial substances into the environment and in combination with survey how much of these substances may be released into the environment locally and globally (in terms of country) (3) what effect the substances released from fresh and aged gloves will have on living organisms and what kind of compounds will be responsible for harmful effects (it may stimulate in the future consider use of more safe substances for gloves production) (4) how stimulated and natural environmental process may affect distribution of contaminants, bioavailability and their transport in the environment.
6. Conclusions
This work summarizes environmental effects which may occur as a result of increased disposable protective gloves usage and discarding during pandemic COVID-19. The main risk arising due to the fact that plastics materials are applied for gloves production. These materials can be directly ingested by organisms and/or release pollutants to the environment. As it was proved, these contaminants (both heavy metals and organic chemicals) accumulate in the tissues and organs and indicate various disorders and pose a threat to animals and human beings. That risk can be enhanced by pollutants adsorption on plastics, which may act as a pollutants carrier. As a conclusion it has been considered if ordering of DPGs usage should be introduced and that kind of protection will not have more far-reaching consequences for the environment and living organisms.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Dr. C. Baiyang
References
- Acter T., Uddin N., Das J., Akhter A., Choudhury T.R., Kim S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: a global health emergency. Sci. Total Environ. 2020;730 doi: 10.1016/j.scitotenv.2020.138996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A.A., Konno K. Latex: a model for understanding mechanisms, ecology, and evolution of plant defense against herbivory. Annu. Rev. Ecol. Evol. Syst. 2009;40:311–331. doi: 10.1146/annurev.ecolsys.110308.120307. [DOI] [Google Scholar]
- Akhbarizadeh R., Dobaradaran S., Schmidt T.C., Nabipour I., Spitz J. Worldwide bottled water occurrence of emerging contaminants: a review of the recent scientific literature. J. Hazard. Mater. 2020;392 doi: 10.1016/j.jhazmat.2020.122271. [DOI] [PubMed] [Google Scholar]
- Alam O., Wang S., Lu W. Heavy metals dispersion during thermal treatment of plastic bags and its recovery. J. Environ. Manag. 2018;212:367–374. doi: 10.1016/j.jenvman.2018.02.034. [DOI] [PubMed] [Google Scholar]
- Alam O., Yang L., Yanchun X. Determination of the selected heavy metal and metalloid contents in various types of plastic bags. J. Environ. Health Sci. Eng. 2019;17:161–170. doi: 10.1007/s40201-019-00337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldiyarov A., Sokolov D., Nurmukan A., Korshikov E. The study of thermophysical properties of rubber and plastic household waste to determine the temperature conditions of cryoprocessing. Appl. Surf. Sci. 2020;511 doi: 10.1016/j.apsusc.2020.145487. [DOI] [Google Scholar]
- Alomar C., Sureda A., Capó X., Guijarro B., Tejada S., Deudero S. Microplastic ingestion by Mullus surmuletus Linnaeus, 1758 fish and its potential for causing oxidative stress. Environ. Res. 2017;159:135–142. doi: 10.1016/j.envres.2017.07.043. [DOI] [PubMed] [Google Scholar]
- Ammendolia J., Saturno J., Brooks A.L., Jacobs S., Jambeck J.R. An emerging source of plastic pollution: environmental presence of plastic personal protective equipment (PPE) debris related to COVID-19 in a metropolitan city. Environ. Pollut. 2021;269 doi: 10.1016/j.envpol.2020.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbor, A., Olson, L., Miller, G.Z., Belliveau, M., 2019. Taking off the Toxic Gloves: An Investigation of Phthalates and Other Chemicals of Concern in Food-Handling Gloves Ecology Center’s Healthy Stuff program [WWW Document]. Ecology Center. URL https://www.ecocenter.org/healthy-stuff/reports/vinyl-gloves-study-2019.
- Ardusso M., Forero-López A.D., Buzzi N.S., Spetter C.V., Fernández-Severini M.D. COVID-19 pandemic repercussions on plastic and antiviral polymeric textile causing pollution on beaches and coasts of South America. Sci. Total Environ. 2021;763 doi: 10.1016/j.scitotenv.2020.144365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayamba A.A., Ali M., Carboo D., Awuku F.J. Extraction and determination of phthalates content in polyethylene food contact materials on the Ghanaian market. J. Nat. Sci. Res. 2018;8:1–6. [Google Scholar]
- Ayamba A.A.-M., Agyekum A.A., Derick C., Dontoh D. Assessment of phthalate migration in polyethylene food contact materials sold on the Ghanaian market. Cogent Environ. Sci. 2020;6:1–11. doi: 10.1080/23311843.2020.1794242. [DOI] [Google Scholar]
- Babich M.A., Bevington C., Dreyfus M.A. Plasticizer migration from children's toys, child care articles, art materials, and school supplies. Regul. Toxicol. Pharmacol. 2020;111 doi: 10.1016/j.yrtph.2019.104574. [DOI] [PubMed] [Google Scholar]
- Bagel-Boithias S., Sautou-Miranda V., Bourdeaux D., Tramier V., Boyer A., Chopineau J. Leaching of diethylhexyl phthalate from multilayer tubing into etoposide infusion solutions. Am. J. Health Pharm. 2005;62:182–188. doi: 10.1093/ajhp/62.2.182. [DOI] [PubMed] [Google Scholar]
- Bakir A., Rowland S.J., Thompson R.C. Transport of persistent organic pollutants by microplastics in estuarine conditions. Estuar. Coast. Shelf Sci. 2014;140:14–21. doi: 10.1016/j.ecss.2014.01.004. [DOI] [Google Scholar]
- Bang-iam N., Udnan Y., Masawat P. Design and fabrication of artificial neural network-digital image-based colorimeter for protein assay in natural rubber latex and medical latex gloves. Microchem. J. 2013;106:270–275. doi: 10.1016/j.microc.2012.08.003. [DOI] [Google Scholar]
- Bank M.S., Ok Y.S., Swarzenski P. w. Microplastic's role in antibiotic resistance. Science. 2020;369:1315. doi: 10.1126/science.abd4842. [DOI] [PubMed] [Google Scholar]
- Besson M., Jacob H., Oberhaensli F., Taylor A., Swarzenski P.W., Metian M. Preferential adsorption of Cd, Cs and Zn onto virgin polyethylene microplastic versus sediment particles. Mar. Pollut. Bull. 2020;156 doi: 10.1016/j.marpolbul.2020.111223. [DOI] [PubMed] [Google Scholar]
- Bolan N.S., Kirkham M.B., Halsband C., Nugegoda D., Ok Y.S. first ed. CRC Press; Abingdon: 2020. Particulate Plastics in Terrestrial and Aquatic Environments. [DOI] [Google Scholar]
- Bouattour Y., Wasiak M., Bernard L., Pinguet J., Richard D., Le Rouzo-Grèves M., Dhifallah I., Lambert C., Pereira B., Chennell P., Sautou V. Quantification of bis(2-ethylhexyl) phthalate released by medical devices during respiratory assistance and estimation of patient exposure. Chemosphere. 2020;255 doi: 10.1016/j.chemosphere.2020.126978. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Hollman P.C.H., Peters R.J.B. Potential health impact of environmentally released micro- and nanoplastics in the human food production Chain: experiences from nanotoxicology. Environ. Sci. Technol. 2015;49:8932–8947. doi: 10.1021/acs.est.5b01090. [DOI] [PubMed] [Google Scholar]
- Boyle D., Catarino A.I., Clark N.J., Henry T.B. Polyvinyl chloride (PVC) plastic fragments release Pb additives that are bioavailable in zebrafish. Environ. Pollut. 2020;263 doi: 10.1016/j.envpol.2020.114422. [DOI] [PubMed] [Google Scholar]
- Boyle, L., 2020. Discarded coronavirus face masks and gloves rising threat to ocean life, conservationists warn. Indep.
- Brennecke D., Duarte B., Paiva F., Caçador I., Canning-Clode J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016;178:189–195. doi: 10.1016/j.ecss.2015.12.003. [DOI] [Google Scholar]
- Camacho M., Herrera A., Gómez M., Acosta-Dacal A., Martínez I., Henríquez-Hernández L.A., Luzardo O.P. Organic pollutants in marine plastic debris from Canary Islands beaches. Sci. Total Environ. 2019;662:22–31. doi: 10.1016/j.scitotenv.2018.12.422. [DOI] [PubMed] [Google Scholar]
- Chailurkit L., Srijaruskul K., Ongphiphadhanakul B. Bisphenol A in canned carbonated drinks and plastic-bottled water from supermarkets. Expo. Health. 2017;9:243–248. doi: 10.1007/s12403-016-0235-5. [DOI] [Google Scholar]
- Chamas A., Moon H., Zheng J., Qiu Y., Tabassum T., Jang J.H., Abu-Omar M., Scott S.L., Suh S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020;8:3494–3511. doi: 10.1021/acssuschemeng.9b06635. [DOI] [Google Scholar]
- Chen S., Tan Z., Qi Y., Ouyang C. Sorption of tri-n-butyl phosphate and tris(2-chloroethyl) phosphate on polyethylene and polyvinyl chloride microplastics in seawater. Mar. Pollut. Bull. 2019;149 doi: 10.1016/j.marpolbul.2019.110490. [DOI] [PubMed] [Google Scholar]
- Chen Y., Li C., Song P., Yan B., Yang X., Wu Y., Ma P. Hepatic and renal tissue damage in Balb/c mice exposed to diisodecyl phthalate: the role of oxidative stress pathways. Food Chem. Toxicol. 2019;132 doi: 10.1016/j.fct.2019.110600. [DOI] [PubMed] [Google Scholar]
- Chiang C., Flaws J.A. Subchronic exposure to Di(2-ethylhexyl) phthalate and diisononyl phthalate during adulthood Has immediate and long-term reproductive consequences in female mice. Toxicol. Sci. 2019;168:620–631. doi: 10.1093/toxsci/kfz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C., Lewis L.R., Borkowski G., Flaws J.A. Exposure to di(2-ethylhexyl) phthalate and diisononyl phthalate during adulthood disrupts hormones and ovarian folliculogenesis throughout the prime reproductive life of the mouse. Toxicol. Appl. Pharmacol. 2020;393 doi: 10.1016/j.taap.2020.114952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission Regulation (EU) No 10/2011, 2011. COMMISSION REGULATION (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food. Off. J. Eur. Union 77.
- Comte S., Guibaud G., Baudu M. Biosorption properties of extracellular polymeric substances (EPS) towards Cd, Cu and Pb for different pH values. J. Hazard. Mater. 2008;151:185–193. doi: 10.1016/j.jhazmat.2007.05.070. [DOI] [PubMed] [Google Scholar]
- Cong B., Liu C., Wang L., Chai Y. The impact on antioxidant enzyme activity and related gene expression following Adult Zebrafish (Danio rerio) exposure to dimethyl phthalate. Animals. 2020;10:717. doi: 10.3390/ani10040717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford C.B., Quinn B. Elsevier; 2017. Microplastic Pollutants. [DOI] [Google Scholar]
- Dong X., Zheng M., Qu L., Shi L., Wang L., Zhang Y., Liu X., Qiu Y., Zhu H. Sorption of Tonalide, Musk Xylene, Galaxolide, and Musk Ketone by microplastics of polyethylene and polyvinyl chloride. Mar. Pollut. Bull. 2019;144:129–133. doi: 10.1016/j.marpolbul.2019.04.046. [DOI] [PubMed] [Google Scholar]
- Dorman D.C., Chiu W., Hales B.F., Hauser R., Johnson K.J., Mantus E., Martel S., Robinson K.A., Rooney A.A., Rudel R., Sathyanarayana S., Schantz S.L., Waters K.M. Systematic reviews and meta-analyses of human and animal evidence of prenatal diethylhexyl phthalate exposure and changes in male anogenital distance. J. Toxicol. Environ. Health Part B Crit. Rev. 2019;21:207–226. doi: 10.1080/10937404.2018.1505354.Systematic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Zhou Q., Li H., Xu S., Wang C., Fu L., Tang J. Environmental distribution, transport and ecotoxicity of microplastics: a review. J. Appl. Toxicol. 2020:1–13. doi: 10.1002/jat.4034. [DOI] [PubMed] [Google Scholar]
- Duan J., Kang J., Qin W., Deng T., Liu H., Li B., Yu W., Gong S., Yang X., Chen M. Exposure to formaldehyde and diisononyl phthalate exacerbate neuroinflammation through NF-κB activation in a mouse asthma model. Ecotoxicol. Environ. Saf. 2018;163:356–364. doi: 10.1016/j.ecoenv.2018.07.089. [DOI] [PubMed] [Google Scholar]
- Dutra C., Pezo D., Freire M.T., de A., Nerín C., Reyes F.G.R. Determination of volatile organic compounds in recycled polyethylene terephthalate and high-density polyethylene by headspace solid phase microextraction gas chromatography mass spectrometry to evaluate the efficiency of recycling processes. J. Chromatogr. A. 2011;1218:1319–1330. doi: 10.1016/j.chroma.2010.12.099. [DOI] [PubMed] [Google Scholar]
- Elizalde-Velázquez A., Subbiah, S. of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics, Anderson T.A., Green M.J., Zhao X., Cañas-Carrell J.E. Sorption of three common nonsteroidal anti-inflammatory drugs (NSAIDs) to microplastics. Sci. Total Environ. 2020;715 doi: 10.1016/j.scitotenv.2020.136974. [DOI] [PubMed] [Google Scholar]
- Esperanza M., Seoane M., Servia M.J., Cid Á. Effects of Bisphenol A on the microalga Chlamydomonas reinhardtii and the clam Corbicula fluminea. Ecotoxicol. Environ. Saf. 2020;197 doi: 10.1016/j.ecoenv.2020.110609. [DOI] [PubMed] [Google Scholar]
- European Commission, 2008. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. EUR-Lex-32008L0098-EN-EUR-Lex. Eur. Comm. 3–30.
- Fainleib A., Pires R.V., Lucas E.F., Soares B.G. Degradation of non-vulcanized natural rubber - renewable resource for fine chemicals used in polymer synthesis. Polimeros. 2013;23:441–450. doi: 10.4322/polimeros.2013.070. [DOI] [Google Scholar]
- Farajzadeh M.A., Ebrahimi M., Ranji A., Feyz E., Bejani V., Matin A.A. HPLC and GC methods for determination of lubricants and their evaluation in analysis of real samples of polyethylene. Microchim. Acta. 2006;153:73–78. doi: 10.1007/s00604-005-0429-1. [DOI] [Google Scholar]
- Fisner M., Taniguchi S., Moreira F., Bícego M.C., Turra A. Polycyclic aromatic hydrocarbons (PAHs) in plastic pellets: variability in the concentration and composition at different sediment depths in a sandy beach. Mar. Pollut. Bull. 2013;70:219–226. doi: 10.1016/j.marpolbul.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Forner-Piquer I., Santangeli S., Maradonna F., Rabbito A., Piscitelli F., Habibi H.R., Di Marzo V., Carnevali O. Disruption of the gonadal endocannabinoid system in zebrafish exposed to diisononyl phthalate. Environ. Pollut. 2018;241:1–8. doi: 10.1016/j.envpol.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Frączyk, J., 2020. Rękawice ochronne i maseczki. Są firmy, które zarabiają na epidemii [WWW Document]. 〈https://businessinsider.com.pl/biznesvscovid/koronawirus-w-polsce-rekawice-ochronne-i-maseczki/evywdz1〉.
- Frias J.P.G.L., Sobral P., Ferreira A.M. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar. Pollut. Bull. 2010;60:1988–1992. doi: 10.1016/j.marpolbul.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Fries E., Zarfl C. Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE) Environ. Sci. Pollut. Res. 2012;19:1296–1304. doi: 10.1007/s11356-011-0655-5. [DOI] [PubMed] [Google Scholar]
- Garçon M., Sauzéat L., Carlson R.W., Shirey S.B., Simon M., Balter V., Boyet M. Nitrile, Latex, Neoprene and Vinyl Gloves: a primary source of contamination for trace element and Zn isotopic analyses in geological and biological samples. Geostand. Geoanal. Res. 2017;41:367–380. doi: 10.1111/ggr.12161. [DOI] [Google Scholar]
- Gardon T., Huvet A., Paul-Pont I., Cassone A.L., Sham Koua M., Soyez C., Jezequel R., Receveur J., Le Moullac G. Toxic effects of leachates from plastic pearl-farming gear on embryo-larval development in the pearl oyster Pinctada margaritifera. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115890. [DOI] [PubMed] [Google Scholar]
- Gewert B., Plassmann M.M., Macleod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts. 2015;17:1513–1521. doi: 10.1039/c5em00207a. [DOI] [PubMed] [Google Scholar]
- Geyer R., Jambeck J.R., Law K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3:25–29. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomucci L., Raddadi N., Soccio M., Lotti N., Fava F. Polyvinyl chloride biodegradation by Pseudomonas citronellolis and Bacillus flexus. New Biotechnol. 2019;52:35–41. doi: 10.1016/j.nbt.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Giacomucci L., Raddadi N., Soccio M., Lotti N., Fava F. Biodegradation of polyvinyl chloride plastic films by enriched anaerobic marine consortia. Mar. Environ. Res. 2020;158 doi: 10.1016/j.marenvres.2020.104949. [DOI] [PubMed] [Google Scholar]
- Gillen K.T., Clough R.L., Wise J. Prediction of elastomer lifetimes from accelerated thermal-aging experiments. Adv. Chem. Ser. 1996;249:553–554. doi: 10.1021/ba-1996-0249.ch034. [DOI] [Google Scholar]
- Glas D., Hulsbosch J., Dubois P., Binnemans K., Devos D.E. End-of-life treatment of poly(vinyl chloride) and chlorinated polyethylene by dehydrochlorination in ionic liquids. ChemSusChem. 2014;7:610–617. doi: 10.1002/cssc.201300970. [DOI] [PubMed] [Google Scholar]
- Godoy V., Blázquez G., Calero M., Quesada L., Martín-Lara M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019;255 doi: 10.1016/j.envpol.2019.113363. [DOI] [PubMed] [Google Scholar]
- Gómez Ramos M.J., Lozano A., Fernández-Alba A.R. High-resolution mass spectrometry with data independent acquisition for the comprehensive non-targeted analysis of migrating chemicals coming from multilayer plastic packaging materials used for fruit purée and juice. Talanta. 2019;191:180–192. doi: 10.1016/j.talanta.2018.08.023. [DOI] [PubMed] [Google Scholar]
- Gostin L.O., Wiley L.F. Governmental Public Health Powers during the COVID-19 pandemic: stay-at-home orders, business closures, and travel restrictions. JAMA J. Am. Med. Assoc. 2020:7–8. doi: 10.1001/jama.2020.5460. [DOI] [PubMed] [Google Scholar]
- Guan D., Wang D., Hallegatte S., Davis S.J., Huo J., Li S., Bai Y., Lei T., Xue Q., Coffman D.M., Cheng D., Chen P., Liang X., Xu B., Lu X., Wang S., Hubacek K., Gong P. Global supply-chain effects of COVID-19 control measures. Nat. Hum. Behav. 2020;4:577–587. doi: 10.1038/s41562-020-0896-8. [DOI] [PubMed] [Google Scholar]
- Henrotin J.-B., Feigerlova E., Robert A., Dziurla M., Burgart M., Lambert-Xolin A.-M., Jeandel F., Weryha G. Decrease in serum testosterone levels after short-term occupational exposure to diisononyl phthalate in male workers. Occup. Environ. Med. 2020;77:214–222. doi: 10.1136/oemed-2019-106261. [DOI] [PubMed] [Google Scholar]
- Hernández F.E., Medina C., Moraga G., Ramírez J., Jaramillo A.F., Báez-Cruz R., Meléndrez M.F., Flores P. Correlation between mechanical and microstructural properties of vulcanized polyisoprene, polychloroprene, and nitrile-butadiene rubber subjected to accelerated thermal aging. J. Elastomers Plast. 2018;51:493–511. doi: 10.1177/0095244318798171. [DOI] [Google Scholar]
- Hirai H., Takada H., Ogata Y., Yamashita R., Mizukawa K., Saha M., Kwan C., Moore C., Gray H., Laursen D., Zettler E.R., Farrington J.W., Reddy C.M., Peacock E.E., Ward M.W. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar. Pollut. Bull. 2011;62:1683–1692. doi: 10.1016/j.marpolbul.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Hospital A., Agents C., Munksgaard B. 1,2-Benzisothiazolin-3-one in disposable polyvinyl chloride gloves for medical use. Contact Dermat. 2007;57:365–370. doi: 10.1111/j.1600-0536.2007.01278.x. [DOI] [PubMed] [Google Scholar]
- Huang J.S., Koongolla J.B., Li H.X., Lin L., Pan Y.F., Liu S., He W.H., Maharana D., Xu X.R. Microplastic accumulation in fish from Zhanjiang mangrove wetland, South China. Sci. Total Environ. 2020;708 doi: 10.1016/j.scitotenv.2019.134839. [DOI] [PubMed] [Google Scholar]
- Huang W., Zheng S., Xiao J., Liu C., Du T., Wu K. Parental exposure to bisphenol A affects pharyngeal cartilage development and causes global transcriptomic changes in zebrafish (Danio rerio) offspring. Chemosphere. 2020;249 doi: 10.1016/j.chemosphere.2020.126537. [DOI] [PubMed] [Google Scholar]
- Huang X., Zemlyanov D.Y., Diaz-Amaya S., Salehi M., Stanciu L., Whelton A.J. Competitive heavy metal adsorption onto new and aged polyethylene under various drinking water conditions. J. Hazard. Mater. 2020;385 doi: 10.1016/j.jhazmat.2019.121585. [DOI] [PubMed] [Google Scholar]
- Hüffer T., Hofmann T. Sorption of non-polar organic compounds by micro-sized plastic particles in aqueous solution. Environ. Pollut. 2016;214:194–201. doi: 10.1016/j.envpol.2016.04.018. [DOI] [PubMed] [Google Scholar]
- Hutchinson, F.E., Bhattacharya, P., 2021. Malaysia’s Rubber Glove Industry – The Good, the Bad and the Ugly [WWW Document].
- Ibrahim S., Mustafa A., Chemicals F., Programme B. Effect of reagents concentration and ratio on degradation of natural rubber latex in acidic medium. Malays. J. Anal. Sci. 2014;18:405–414. [Google Scholar]
- Ibrahim, S., Othman, N., Ismail, H., 2016. Degradation of natural rubber latex. In: Natural Rubber: Properties, Behavior and Applications, pp. 105–136.
- Ilyas S., Ranjan R., Kim H. Disinfection technology and strategies for COVID-19 hospital and bio-medical waste management. Sci. Total Environ. 2020;749 doi: 10.1016/j.scitotenv.2020.141652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C., Lu Z., Xu L., Li F., Cong M., Shan X., Wu H. Global responses to tris(1-chloro-2-propyl)phosphate (TCPP) in rockfish Sebastes schlegeli using integrated proteomic and metabolomic approach. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138307. [DOI] [PubMed] [Google Scholar]
- Jiang Y., You M., Li S., Xu Y., Wang Y. Perinatal exposure to nonylphenol delayed myelination in offspring cerebellum. Biochem. Pharmacol. 2020;178 doi: 10.1016/j.bcp.2020.114120. [DOI] [PubMed] [Google Scholar]
- Jing L., Sun Y., Wang Y., Liang B., Chen T., Zheng D., Zhao X., Zhou X., Sun Z., Shi Z. Cardiovascular toxicity of decabrominated diphenyl ethers (BDE-209) and decabromodiphenyl ethane (DBDPE) in rats. Chemosphere. 2019;223:675–685. doi: 10.1016/j.chemosphere.2019.02.115. [DOI] [PubMed] [Google Scholar]
- Kalčíková G., Skalar T., Marolt G., Jemec Kokalj A. An environmental concentration of aged microplastics with adsorbed silver significantly affects aquatic organisms. Water Res. 2020;175 doi: 10.1016/j.watres.2020.115644. [DOI] [PubMed] [Google Scholar]
- Kalina M., Tilley E. “This is our next problem”: cleaning up from the COVID-19 response. Waste Manag. 2020;108:202–205. doi: 10.1016/j.wasman.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapanagioti H.K., Ogata Y., Takada H. Eroded plastic pellets as monitoring tools for polycyclic aromatic hydrocarbons (PAH):Laboratory and field studies. Glob. Nest J. 2010;12:327–334. doi: 10.30955/gnj.000675. [DOI] [Google Scholar]
- Kawamura, Y., Maehara, T., Wakui, C., Yamada, T., 2000. Migration of Plasticizers and Nonylphenol from Polyvinyl Chloride Gloves. 食品衛生学雑誌.
- Kawamura Y., Mutsuga M., Wakui C., Maitani T. Identification of unknown substances in polyvinyl chloride gloves containing non-phthalate plasticizers. 食品衛生学雑誌. 2002;43:215–220. doi: 10.3358/shokueishi.43.215. [DOI] [PubMed] [Google Scholar]
- Koongolla J.B., Lin L., Pan Y.F., Yang C.P., Sun D.R., Liu S., Xu X.R., Maharana D., Huang J.S., Li H.X. Occurrence of microplastics in gastrointestinal tracts and gills of fish from Beibu Gulf, South China Sea. Environ. Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113734. [DOI] [PubMed] [Google Scholar]
- Lear G., Kingsbury J.M., Franchini S., Gambarini V., Maday S.D.M., Wallbank J.A., Weaver L., Pantos O. Plastics and the microbiome: impacts and solutions. Environ. Microbiomes. 2021;16:1–19. doi: 10.1186/s40793-020-00371-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P., Zhu J., Pan K., Zhang H. Sorption kinetics of parent and substituted PAHs for low-density polyethylene (LDPE): determining their partition coefficients between LDPE and water (KLDPE) for passive sampling. J. Environ. Sci. 2020;87:349–360. doi: 10.1016/j.jes.2019.07.021. [DOI] [PubMed] [Google Scholar]
- Li J., Zhang K., Zhang H. Adsorption of antibiotics on microplastics. Environ. Pollut. 2018;237:460–467. doi: 10.1016/j.envpol.2018.02.050. [DOI] [PubMed] [Google Scholar]
- Li R., Wang H., Mi C., Feng C., Zhang L., Yang L., Zhou B. The adverse effect of TCIPP and TCEP on neurodevelopment of zebrafish embryos/larvae. Chemosphere. 2019;220:811–817. doi: 10.1016/j.chemosphere.2018.12.198. [DOI] [PubMed] [Google Scholar]
- Li Z., Hu X., Qin L., Yin D. Evaluating the effect of different modified microplastics on the availability of polycyclic aromatic hydrocarbons. Water Res. 2020;170 doi: 10.1016/j.watres.2019.115290. [DOI] [PubMed] [Google Scholar]
- Liang Y., Song Q., Wu N., Li J., Zhong Y., Zeng W. Repercussions of COVID-19 pandemic on solid waste generation and management strategies. Front. Environ. Sci. Eng. 2021;15:115. doi: 10.1007/s11783-021-1407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Zhu Z., Yang Y., Sun Y., Yu F., Ma J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019;246:26–33. doi: 10.1016/j.envpol.2018.11.100. [DOI] [PubMed] [Google Scholar]
- Liu J., Li X., Xu L., Zhang P. Investigation of aging behavior and mechanism of nitrile-butadiene rubber (NBR) in the accelerated thermal aging environment. Polym. Test. 2016;54:59–66. doi: 10.1016/j.polymertesting.2016.06.010. [DOI] [Google Scholar]
- Liu P., Qian L., Wang H., Zhan X., Lu K., Gu C., Gao S. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ. Sci. Technol. 2019;53:3579–3588. doi: 10.1021/acs.est.9b00493. [DOI] [PubMed] [Google Scholar]
- Lomonaco T., Manco E., Corti A., La Nasa J., Ghimenti S., Biagini D., Di Francesco F., Modugno F., Ceccarini A., Fuoco R., Castelvetro V. Release of harmful volatile organic compounds (VOCs) from photo-degraded plastic debris: a neglected source of environmental pollution. J. Hazard. Mater. 2020;394 doi: 10.1016/j.jhazmat.2020.122596. [DOI] [PubMed] [Google Scholar]
- López-Cervantes J., Paseiro-Losada P. Determination of bisphenol A in, and its migration from, PVC stretch film used for food packaging. Food Addit. Contam. 2003;20:596–606. doi: 10.1080/0265203031000109495. [DOI] [PubMed] [Google Scholar]
- Lv G., Wang L., Liu J., Li S. Method for determination of fatty acid amides in polyethylene packaging materials-gas chromatography/mass spectrometry. J. Chromatogr. A. 2009;1216:8545–8548. doi: 10.1016/j.chroma.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Ma P., Yan B., Zeng Q., Liu X., Wu Y., Jiao M., Liu C., Wu J., Yang X. Oral exposure of Kunming mice to diisononyl phthalate induces hepatic and renal tissue injury through the accumulation of ROS. Protective effect of melatonin. Food Chem. Toxicol. 2014;68:247–256. doi: 10.1016/j.fct.2014.03.027. [DOI] [PubMed] [Google Scholar]
- Magadini D.L., Goes J.I., Ortiz S., Lipscomb J., Pitiranggon M., Yan B. Assessing the sorption of pharmaceuticals to microplastics through in-situ experiments in New York City waterways. Sci. Total Environ. 2020;729 doi: 10.1016/j.scitotenv.2020.138766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S.U., Crimbly F., Khan S., Choudry E., Mehwish S. Strategies for rational use of personal protective equipment (PPE) among healthcare providers during the COVID-19 crisis. Cureus. 2020;12:8248. doi: 10.7759/cureus.8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez R., Tu W., Eng T., Allaire-Leung M., Piña B., Navarro-Martín L., Mennigen J.A. Acute and long-term metabolic consequences of early developmental Bisphenol A exposure in zebrafish (Danio rerio) Chemosphere. 2020;256 doi: 10.1016/j.chemosphere.2020.127080. [DOI] [PubMed] [Google Scholar]
- Mattsson K., Johnson E.V., Malmendal A., Linse S., Hansson L.A., Cedervall T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017;7:1–7. doi: 10.1038/s41598-017-10813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N., Olry J.C., Cariou R., Dervilly-Pinel G., Le Bizec B., Travel A., Jondreville C., Schroeder H. Short-term effects of a perinatal exposure to the HBCDD α-isomer in rats: assessment of early motor and sensory development, spontaneous locomotor activity and anxiety in pups. Neurotoxicol. Teratol. 2015;52:170–180. doi: 10.1016/j.ntt.2015.08.005. [DOI] [PubMed] [Google Scholar]
- McKeen, L.W., 2012. Elastomers and Rubbers, Permeability Properties of Plastics and Elastomers, pp. 339–352. https://doi.org/10.1016/b978-1-4377-3469-0.10012-8.
- Meng X., Zhang N., Sun X., Niu Z., Deng Y., Xu J., Bai H., Ma Q. Suspect screening of 200 hazardous substances in plastic toys using ultra-high-performance liquid chromatography-hybrid quadrupole time-of-flight mass spectrometry. J. Chromatogr. A. 2020;1617:460830–460842. doi: 10.1016/j.chroma.2019.460830. [DOI] [PubMed] [Google Scholar]
- Ministry of Climate and Environment, 2020. Ministry of Climate. Guidelines of Ministry of Climate and Head of Public Sanitary Office Concerning Handling the Waste during SARS-CoV-2 Epidemics and Illness COVID-19.
- Misman M.A., Azura A.R. Overview on the potential of biodegradable natural Rubber Latex gloves for commercialization. Adv. Mater. Res. 2014;844:486–489. doi: 10.4028/www.scientific.net/AMR.844.486. [DOI] [Google Scholar]
- Mizukawa K., Takada H., Ito M., Geok Y.B., Hosoda J., Yamashita R., Saha M., Suzuki S., Miguez C., Frias J., Antunes J.C., Sobral P., Santos I., Micaelo C., Ferreira A.M. Monitoring of a wide range of organic micropollutants on the Portuguese coast using plastic resin pellets. Mar. Pollut. Bull. 2013;70:296–302. doi: 10.1016/j.marpolbul.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Mo N., Zhang M., Wang R., Xia S., Meng F., Qian Y., Li M. Effects of α-ethinyl estradiol (EE2) and diethylhexyl phthalate (DEHP) on growth performance, antioxidant status and immune response of juvenile yellow catfish Pelteobagrus fulvidraco. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019;226 doi: 10.1016/j.cbpc.2019.108615. [DOI] [PubMed] [Google Scholar]
- Müller A., Becker R., Dorgerloh U., Simon F.G., Braun U. The effect of polymer aging on the uptake of fuel aromatics and ethers by microplastics. Environ. Pollut. 2018;240:639–646. doi: 10.1016/j.envpol.2018.04.127. [DOI] [PubMed] [Google Scholar]
- Munier B., Bendell L.I. Macro and micro plastics sorb and desorb metals and act as a point source of trace metals to coastal ecosystems. PLoS One. 2018;13:1–13. doi: 10.1371/journal.pone.0191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima, E., Isobe, A., Kako, S., Magome, S., Deki, N., Itai, T., Takahashi, S., 2011. Toxic metals in polyethylene plastic litter. In: Interdisciplinary Studies on Environmental Chemistry—Marine Environmental Modeling & Analysis, pp. 271–277.
- Nishioka K., Koizumi A., Takita Y., Sasaki K., Numata M. Contact dermatitis due to 2,2,4-trimethyl 1,3-pentanediol diisobutyrate contained in latex-free, accelerator-free nitrile rubber gloves. Contact Dermat. 2020;82:255–257. doi: 10.1111/cod.13455. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Yamasaki A. Volatile and semivolatile organic compound emissions from polymers used in commercial products during thermal degradation. Heliyon. 2020;6:03314. doi: 10.1016/j.heliyon.2020.e03314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski P., Kuśnierz S., Sosna P., Mauer J., Maj D. Disposal of personal protective equipment during the covid-19 pandemic is a challenge for waste collection companies and society: a case study in Poland. Resources. 2020;9:1–11. doi: 10.3390/resources9100116. [DOI] [Google Scholar]
- Nzediegwu C., Chang S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour. Conserv. Recycl. 2020;161 doi: 10.1016/j.resconrec.2020.104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Pateis V., Bracht L., dos Santos Castro L., Bueno Franco Salla G., Comar J.F., Valderrama Parizotto A., Peralta R.M., Bracht A. The food additive BHA modifies energy metabolism in the perfused rat liver. Toxicol. Lett. 2018;299:191–200. doi: 10.1016/j.toxlet.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Osman M.A., Mahmoud G.I., Elgammal M.H., Hasan R.S. Bisphenol a hormonal disrupture and preventive effect of rose water and clove oil. Biointerface Res. Appl. Chem. 2021;11:8780–8803. doi: 10.33263/BRIAC112.87808803. [DOI] [Google Scholar]
- Oteef M.D.Y., Elhassan M.S. Plastic toys and child care articles as a source of children exposure to phthalates and other plasticisers in Saudi Arabia. Int. J. Environ. Anal. Chem. 2020;00:1–15. doi: 10.1080/03067319.2020.1784407. [DOI] [Google Scholar]
- Ozturk R.C., Altinok I. Interaction of plastics with marine species. Turk. J. Fish. Aquat. Sci. 2020;20:647–658. doi: 10.4194/1303-2712-v20_8_07. [DOI] [Google Scholar]
- Padervand M., Lichtfouse E., Robert D., Wang C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020;18:807–828. doi: 10.1007/s10311-020-00983-1. [DOI] [Google Scholar]
- Panchal S.S., Vasava D.V. Biodegradable polymeric materials: synthetic approach. ACS Omega. 2020;5:4370–4379. doi: 10.1021/acsomega.9b04422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.J., Zhang M., Lee W.Y., Hong K.H., Do J.T., Park C., Song H. Toxic effects of nonylphenol on neonatal testicular development in mouse organ culture. Int. J. Mol. Sci. 2020;21:3491–3505. doi: 10.3390/ijms21103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons A., Lange A., Hutchinson T.H., Miyagawa S., Iguchi T., Kudoh T., Tyler C.R. Molecular mechanisms and tissue targets of brominated flame retardants, BDE-47 and TBBPA, in embryo-larval life stages of zebrafish (Danio rerio) Aquat. Toxicol. 2019;209:99–112. doi: 10.1016/j.aquatox.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Pascall M.A., Zabik M.E., Zabik M.J., Hernandez R.J. Uptake of polychlorinated biphenyls (PCBs) from an aqueous medium by polyethylene, polyvinyl chloride, and polystyrene films. J. Agric. Food Chem. 2005;53:164–169. doi: 10.1021/jf048978t. [DOI] [PubMed] [Google Scholar]
- Pelegrín C.J., Flores Y., Jiménez A., Garrigós M.C. Recent trends in the analysis of chemical contaminants in beverages. Beverages. 2020;6:32. doi: 10.3390/beverages6020032. [DOI] [Google Scholar]
- Phoonphongphiphat, A., 2021. Thailand’s PTT to compete in rubber gloves as demand surges [WWW Document]. URL 〈https://asia.nikkei.com/Business/Health-Care/Thailand-s-PTT-to-compete-in-rubber-gloves-as-demand-surges〉.
- Plastics Europe, EPRO, 2019. Plastics – the Facts 2019. An analysis of European plastics production, demand and waste data.
- Poopal R.K., Zhang J., Zhao R., Ramesh M., Ren Z. Biochemical and behavior effects induced by diheptyl phthalate (DHpP) and Diisodecyl phthalate (DIDP) exposed to zebrafish. Chemosphere. 2020;252 doi: 10.1016/j.chemosphere.2020.126498. [DOI] [PubMed] [Google Scholar]
- Pradhan A., Olsson P.E., Jass J. Di(2-ethylhexyl) phthalate and diethyl phthalate disrupt lipid metabolism, reduce fecundity and shortens lifespan of Caenorhabditis elegans. Chemosphere. 2018;190:375–382. doi: 10.1016/j.chemosphere.2017.09.123. [DOI] [PubMed] [Google Scholar]
- Prata J.C., Patrício Silva A.L., Walker T.R., Duarte A.C., Rocha Santos T. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 2020:0–28. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- Preece D., Lewis R., Carré M.J. A critical review of the assessment of medical gloves. Tribol. Mater. Surf. Interfaces. 2020;0:1–10. doi: 10.1080/17515831.2020.1730619. [DOI] [Google Scholar]
- Prunier J., Maurice L., Perez E., Gigault J., Pierson Wickmann A.C., Davranche M., Halle A. ter. Trace metals in polyethylene debris from the North Atlantic subtropical gyre. Environ. Pollut. 2019;245:371–379. doi: 10.1016/j.envpol.2018.10.043. [DOI] [PubMed] [Google Scholar]
- Qian S., Ji H., Wu X.X., Li N., Yang Y., Bu J., Zhang X., Qiao L., Yu H., Xu N., Zhang C. Detection and quantification analysis of chemical migrants in plastic food contact products. PLoS One. 2018;13:1–11. doi: 10.1371/journal.pone.0208467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razanajatovo R.M., Ding J., Zhang S., Jiang H., Zou H. Sorption and desorption of selected pharmaceuticals by polyethylene microplastics. Mar. Pollut. Bull. 2018;136:516–523. doi: 10.1016/j.marpolbul.2018.09.048. [DOI] [PubMed] [Google Scholar]
- Reichel C. Mass spectrometric analysis of EPO IEF-PAGE interfering substances in nitrile examination gloves. Drug Test. Anal. 2012;4:761–774. doi: 10.1002/dta.1424. [DOI] [PubMed] [Google Scholar]
- Rochman C.M., Hoh E., Hentschel B.T., Kaye S. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ. Sci. Technol. 2013;47:1646–1654. doi: 10.1021/es303700s. [DOI] [PubMed] [Google Scholar]
- Rock K.D., Gillera S.E.A., Devarasetty P., Horman B., Knudsen G., Birnbaum L.S., Fenton S.E., Patisaul H.B. Sex-specific behavioral effects following developmental exposure to tetrabromobisphenol A (TBBPA) in Wistar rats. Neurotoxicology. 2019;75:136–147. doi: 10.1016/j.neuro.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackaria M., Elango L. Organic micropollutants in groundwater of India—a review. Water Environ. Res. 2020;92:504–523. doi: 10.1002/wer.1243. [DOI] [PubMed] [Google Scholar]
- Sakdapipanich J.T., Rojruthai P. Molecular structure of natural rubber and its characteristics based on recent evidence. Intech. 2012;13:213–238. doi: 10.1016/j.colsurfa.2011.12.014. [DOI] [Google Scholar]
- Salamat N., Derakhshesh N. Oxidative stress in liver cell culture from mullet, Liza klunzingeri, induced by short-term exposure to benzo[a]pyrene and nonylphenol. Fish Physiol. Biochem. 2020;46:1183–1197. doi: 10.1007/s10695-020-00783-y. [DOI] [PubMed] [Google Scholar]
- Salvaggio A., Tiralongo F., Krasakopoulou E., Marmara D., Giovos I., Crupi R., Messina G., Lombardo B.M., Marzullo A., Pecoraro R., Scalisi E.M., Copat C., Zuccarello P., Ferrante M., Brundo M.V. Biomarkers of exposure to chemical contamination in the commercial fish species Lepidopus caudatus(Euphrasen, 1788): a particular focus on plastic additives. Front. Physiol. 2019;10:1–13. doi: 10.3389/fphys.2019.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangeli S., Maradonna F., Zanardini M., Notarstefano V., Gioacchini G., Forner-Piquer I., Habibi H., Carnevali O. Effects of diisononyl phthalate on Danio rerio reproduction. Environ. Pollut. 2017;231:1051–1062. doi: 10.1016/j.envpol.2017.08.060. [DOI] [PubMed] [Google Scholar]
- Santos-Echeandía J., Rivera-Hernández J.R., Rodrigues J.P., Moltó V. Interaction of mercury with beached plastics with special attention to zonation, degradation status and polymer type. Mar. Chem. 2020;222 doi: 10.1016/j.marchem.2020.103788. [DOI] [Google Scholar]
- Sarmah R., Kanta Bhagabati S., Dutta R., Nath D., Pokhrel H., Mudoi L.P., Sarmah N., Sarma J., Ahmed A.M., Jyoti Nath R., Ingtipi L., Kuotsu K. Toxicity of a synthetic phenolic antioxidant, butyl hydroxytoluene (BHT), in vertebrate model zebrafish embryo (Danio rerio) Aquac. Res. 2020;51:3839–3846. doi: 10.1111/are.14732. [DOI] [Google Scholar]
- Schmidt N., Castro-Jiménez J., Fauvelle V., Ourgaud M., Sempéré R. Occurrence of organic plastic additives in surface waters of the Rhône River (France) Environ. Pollut. 2020;257 doi: 10.1016/j.envpol.2019.113637. [DOI] [PubMed] [Google Scholar]
- Sharma M.D., Elanjickal A.I., Mankar J.S., Krupadam R.J. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2020;398 doi: 10.1016/j.jhazmat.2020.122994. [DOI] [PubMed] [Google Scholar]
- Singh B., Sharma N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008;93:561–584. doi: 10.1016/j.polymdegradstab.2007.11.008. [DOI] [Google Scholar]
- Smolarz K., Berger A. Long-term toxicity of hexabromocyclododecane (HBCDD) to the benthic clam Macoma balthica (L.) from the Baltic Sea. Aquat. Toxicol. 2009;95:239–247. doi: 10.1016/j.aquatox.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Soares J., Miguel I., Venâncio C., Lopes I., Oliveira M. Public views on plastic pollution: knowledge, perceived impacts, and pro-environmental behaviours. J. Hazard. Mater. 2021;412 doi: 10.1016/j.jhazmat.2021.125227. [DOI] [PubMed] [Google Scholar]
- Socas-Rodríguez B., González-Sálamo J., Herrera-Herrera A.V., Santana-Mayor Á., Hernández-Borges J. Determination of phthalic acid esters in different baby food samples by gas chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2018;410:5617–5628. doi: 10.1007/s00216-018-0977-y. [DOI] [PubMed] [Google Scholar]
- Sørensen L., Rogers E., Altin D., Salaberria I., Booth A.M. Sorption of PAHs to microplastic and their bioavailability and toxicity to marine copepods under co-exposure conditions. Environ. Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113844. [DOI] [PubMed] [Google Scholar]
- Steiner I., Scharf L., Fiala F., Washüttl J. Migration of di-(2-ethylhexyl) phthalate from PVC child articles into saliva and saliva simulant. Food Addit. Contam. 1998;15:812–817. doi: 10.1080/02652039809374715. [DOI] [PubMed] [Google Scholar]
- Sugiura K., Sugiura M., Shiraki R., Hayakawa R., Shamoto M., Sasaki K., Itoh A. Contact urticaria due to polyethylene gloves. Contact Dermat. 2002;46:262–266. doi: 10.1034/j.1600-0536.2002.460503.x. [DOI] [PubMed] [Google Scholar]
- Sun Y., Wang Y., Liang B., Chen T., Zheng D., Zhao X., Jing L., Zhou X., Sun Z., Shi Z. Hepatotoxicity of decabromodiphenyl ethane (DBDPE) and decabromodiphenyl ether (BDE-209) in 28-day exposed Sprague-Dawley rats. Sci. Total Environ. 2020;705 doi: 10.1016/j.scitotenv.2019.135783. [DOI] [PubMed] [Google Scholar]
- Sutha J., Anila P.A., Umamaheswari S., Ramesh M., Narayanasamy A., Poopal R.K., Ren Z. Biochemical responses of a freshwater fish Cirrhinus mrigala exposed to tris(2-chloroethyl) phosphate (TCEP) Environ. Sci. Pollut. Res. 2020;27:34369–34387. doi: 10.1007/s11356-020-09527-0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Yoshinaga J., Mizumoto Y., Serizawa S., Shiraishi H. Foetal exposure to phthalate esters and anogenital distance in male newborns. Int. J. Androl. 2012;35:236–244. doi: 10.1111/j.1365-2605.2011.01190.x. [DOI] [PubMed] [Google Scholar]
- Ta polska spółka zarobiła krocie na pandemii wirusa. Są najnowsze szacunki [WWW Document], 2020. Bus. Inisder Pol. URL 〈https://businessinsider.com.pl/gielda/wiadomosci/szacunki-mercator-medical-w-i-kw-2020-r/2rp1xhc〉.
- Tan X., Yu X., Cai L., Wang J., Peng J. Microplastics and associated PAHs in surface water from the Feilaixia Reservoir in the Beijiang River, China. Chemosphere. 2019;221:834–840. doi: 10.1016/j.chemosphere.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Tang C.C., Chen H.I., Brimblecombe P., Lee C.L. Textural, surface and chemical properties of polyvinyl chloride particles degraded in a simulated environment. Mar. Pollut. Bull. 2018;133:392–401. doi: 10.1016/j.marpolbul.2018.05.062. [DOI] [PubMed] [Google Scholar]
- Teuten E.L., Rowland S.J., Galloway T.S., Thompson R.C. Potential for plastics to transport hydrophobic contaminants. Environ. Sci. Technol. 2007;41:7759–7764. doi: 10.1021/es071737s. [DOI] [PubMed] [Google Scholar]
- Thompson R.C., Moore C.J., Saal F.S.V., Swan S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R Soc. B Biol. Sci. 2009;364:2153–2166. doi: 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., Zheng J., Goodyer C.G., Bayen S. Non-targeted screening of plastic-related chemicals in food collected in Montreal, Canada. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.126942. [DOI] [PubMed] [Google Scholar]
- Turner A. Trace elements in fragments of fishing net and other filamentous plastic litter from two beaches in SW England. Environ. Pollut. 2017;224:722–728. doi: 10.1016/j.envpol.2016.11.034. [DOI] [PubMed] [Google Scholar]
- Turner A. Concentrations and migratabilities of hazardous elements in second-hand children’s plastic toys. Environ. Sci. Technol. 2018;52:3110–3116. doi: 10.1021/acs.est.7b04685. [DOI] [PubMed] [Google Scholar]
- Turner A., Wallerstein C., Arnold R. Identification, origin and characteristics of bio-bead microplastics from beaches in western Europe. Sci. Total Environ. 2019;664:938–947. doi: 10.1016/j.scitotenv.2019.01.281. [DOI] [PubMed] [Google Scholar]
- Turner A., Holmes L., Thompson R.C., Fisher A.S. Metals and marine microplastics: adsorption from the environment versus addition during manufacture, exemplified with lead. Water Res. 2020;173 doi: 10.1016/j.watres.2020.115577. [DOI] [PubMed] [Google Scholar]
- Tworzywa sztuczne w obiegu zamkniętym Analiza sytuacji w Europie, 2020.
- Van Melkebeke M., Janssen C., De Meester S. Characteristics and sinking behavior of typical microplastics including the potential effect of biofouling: implications for remediation. Environ. Sci. Technol. 2020;54:8668–8680. doi: 10.1021/acs.est.9b07378. [DOI] [PubMed] [Google Scholar]
- Wakui C., Kawamura Y., Maitani T. Migrants from disposable gloves and residual acrylonitrile. J. Food Hyg. Soc. Jpn. 2001;42:322–328. doi: 10.3358/shokueishi.42.322. [DOI] [PubMed] [Google Scholar]
- Wang Chao, Xian Z., Jin X., Liang S., Chen Z., Pan B., Wu B., Ok Y.S., Gu C. Photo-aging of polyvinyl chloride microplastic in the presence of natural organic acids. Water Res. 2020;183 doi: 10.1016/j.watres.2020.116082. [DOI] [PubMed] [Google Scholar]
- Wang Chengqiang, Chen Z., Lu Y., Wang L., Zhang Y., Zhu X., Song J. Neurotoxicity and related mechanisms of flame retardant TCEP exposure in mice. Toxicol. Mech. Methods. 2020;30:490–496. doi: 10.1080/15376516.2020.1765060. [DOI] [PubMed] [Google Scholar]
- Wang F., Gao J., Zhai W., Liu D., Zhou Z., Wang P. The influence of polyethylene microplastics on pesticide residue and degradation in the aquatic environment. J. Hazard. Mater. 2020;394 doi: 10.1016/j.jhazmat.2020.122517. [DOI] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y., Yang W., Li X., Wang, Junqi, Zhang L., Pan L. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus Disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Yu C., Chu Q., Wang F., Lan T., Wang J. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere. 2020;244 doi: 10.1016/j.chemosphere.2019.125491. [DOI] [PubMed] [Google Scholar]
- Wang W., Wang J. Different partition of polycyclic aromatic hydrocarbon on environmental particulates in freshwater: microplastics in comparison to natural sediment. Ecotoxicol. Environ. Saf. 2018;147:648–655. doi: 10.1016/j.ecoenv.2017.09.029. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang X., Li Y., Li J., Liu Y., Xia S., Zhao J. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021;404 doi: 10.1016/j.cej.2020.126412. [DOI] [Google Scholar]
- Wang Z., Chen M., Zhang L., Wang K., Yu X., Zheng Z., Zheng R. Sorption behaviors of phenanthrene on the microplastics identified in a mariculture farm in Xiangshan Bay, southeastern China. Sci. Total Environ. 2018;628–629:1617–1626. doi: 10.1016/j.scitotenv.2018.02.146. [DOI] [PubMed] [Google Scholar]
- Wang Z., Sun P., Li Y., Meng T., Li Z., Zhang X., Zhang R., Jia H., Yao H. Reactive nitrogen species mediated degradation of estrogenic disrupting chemicals by biochar/monochloramine in buffered water and synthetic hydrolyzed urine. Environ. Sci. Technol. 2019;53:12688–12696. doi: 10.1021/acs.est.9b04704. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhu X., Su Y., Xu W., Liu H., Liu Z., Chen W., Wang J. Dimethyl phthalate damaged the cell membrane of Escherichia coli K12. Ecotoxicol. Environ. Saf. 2019;180:208–214. doi: 10.1016/j.ecoenv.2019.05.009. [DOI] [PubMed] [Google Scholar]
- Wankasi D., Dikio E.D. Polyvinyl chloride waste as an adsorbent for the sorption of Pb2+ from aqueous solution. J. Chem. 2014;2014:1–7. doi: 10.1155/2014/817527. [DOI] [Google Scholar]
- Waring R.H., Harris R.M., Mitchell S.C. Plastic contamination of the food chain: a threat to human health? Maturitas. 2018;115:64–68. doi: 10.1016/j.maturitas.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Why Nitrile Gloves Is a Better Alternative Compared To Latex [WWW Document], n.d. URL 〈http://www.solcrestmy.com/wp-content/uploads/Nitrile-better-than-latex-proposal-2.pdf〉.
- Wilkes R.A., Aristilde L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: capabilities and challenges. J. Appl. Microbiol. 2017;123:582–593. doi: 10.1111/jam.13472. [DOI] [PubMed] [Google Scholar]
- World Market Outlook for the Disposable Gloves and Materials Markets 2020–2025: The World Health Organisation Estimates Manufacturing Should be Increased by 40% to Meet Demand [WWW Document], 2020. Research and Markets. URL https://www.globenewswire.com/news-release/2020/09/23/2098003/0/en/World-Market-Outlook-for-the-Disposable-Gloves-and-Materials-Markets-2020–2025-The-World-Health-Organisation-Estimates-Manufacturing-Should-be-Increased-by-40-to-Meet-Demand.html.
- Wu C., Zhang K., Huang X., Liu J. Sorption of pharmaceuticals and personal care products to polyethylene debris. Environ. Sci. Pollut. Res. 2016;23:8819–8826. doi: 10.1007/s11356-016-6121-7. [DOI] [PubMed] [Google Scholar]
- Xia B., Zhang J., Zhao X., Feng J., Teng Y., Chen B., Sun Xuemei, Zhu L., Sun Xiaojie, Qu K. Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ. Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113657. [DOI] [PubMed] [Google Scholar]
- Xiao Q., Li D., Guo R., Zheng L., An X., Zeng Z. In vivo and in vitro toxicities of diethyl phthalate to flounder fish Paralichthys olivaceus and its gill cell line (FG cells) J. Environ. Biol. 2018;39:73–81. [Google Scholar]
- Yang J., Cang L., Sun Q., Dong G., Ata-Ul-Karim S.T., Zhou D. Effects of soil environmental factors and UV aging on Cu2+ adsorption on microplastics. Environ. Sci. Pollut. Res. 2019;26:23027–23036. doi: 10.1007/s11356-019-05643-8. [DOI] [PubMed] [Google Scholar]
- Yew G.Y., Tham T.C., Law C.L., Chu D.T., Ogino C., Show P.L. Emerging crosslinking techniques for glove manufacturers with improved nitrile glove properties and reduced allergic risks. Mater. Today Commun. 2019;19:39–50. doi: 10.1016/j.mtcomm.2018.12.014. [DOI] [Google Scholar]
- Yew G.Y., Tham T.C., Show P.L., Ho Y.C., Ong S.K., Law C.L., Song C., Chang J.S. Unlocking the secret of bio-additive components in rubber compounding in processing quality nitrile glove. Appl. Biochem. Biotechnol. 2020;191:1–28. doi: 10.1007/s12010-019-03207-7. [DOI] [PubMed] [Google Scholar]
- Yip E., Cacioli P. The manufacture of gloves from natural rubber latex. J. Allergy Clin. Immunol. 2002;110:S3–S14. doi: 10.1067/mai.2002.124499. [DOI] [PubMed] [Google Scholar]
- You S., Christian S., Ok Y.S. COVID-19's unsustainable waste management. Science. 2020;368:1438. doi: 10.1126/science.abc7778. [DOI] [PubMed] [Google Scholar]
- Yu F., Yang C., Zhu Z., Bai X., Ma J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019;694 doi: 10.1016/j.scitotenv.2019.133643. [DOI] [PubMed] [Google Scholar]
- Yu F., Li Y., Huang G., Yang C., Chen C., Zhou T., Zhao Y., Ma J. Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere. 2020;260 doi: 10.1016/j.chemosphere.2020.127650. [DOI] [PubMed] [Google Scholar]
- Zaghdoudi M., Kömmling A., Jaunich M., Wol D. Scission, cross-linking, and physical relaxation during thermal degradation of elastomers. Polymers. 2019;11:1280. doi: 10.3390/polym11081280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman F.A., Boudet C., Tack K., Floch Barneaud A., Brochot C., Péry A.R.R., Oleko A., Vandentorren S. Exposure assessment of phthalates in French pregnant women: results of the ELFE pilot study. Int. J. Hyg. Environ. Health. 2013;216:271–279. doi: 10.1016/j.ijheh.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Zhang H., Pap S., Taggart M.A., Boyd K.G., James N.A., Gibb S.W. A review of the potential utilisation of plastic waste as adsorbent for removal of hazardous priority contaminants from aqueous environments. Environ. Pollut. 2020;258 doi: 10.1016/j.envpol.2019.113698. [DOI] [PubMed] [Google Scholar]
- Zhang J., Li D., Fu M., Zhang Y., Zhang L., Zhao P. Synergistic effect of adipic acid pentaerythritol ester with calcium and zinc stearates on poly(vinyl chloride) thermal stability. J. Vinyl Addit. Technol. 2016;22:293–299. doi: 10.1002/vnl.21436. [DOI] [Google Scholar]
- Zhang Q., Cao Y., Liu N., Zhang W., Chen Y., Lin X., Wei Y., Feng L., Jiang L. Recycling of PE glove waste as highly valuable products for efficient separation of oil-based contaminants from water. J. Mater. Chem. A. 2016;4:18128–18133. doi: 10.1039/c6ta08749c. [DOI] [Google Scholar]
- Zhang Q.Q., Qiao M. Transcriptional response of springtail (Folsomia candida) exposed to decabromodiphenyl ether-contaminated soil. Sci. Total Environ. 2020;719 doi: 10.1016/j.scitotenv.2019.134859. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhang L., Hua T., Li Y., Zhou X., Wang W., You Z., Wang H., Li M. The mechanism for adsorption of Cr(VI) ions by PE microplastics in ternary system of natural water environment. Environ. Pollut. 2020;257 doi: 10.1016/j.envpol.2019.113440. [DOI] [PubMed] [Google Scholar]
- Zhao F., Guan J., Bai W., Gu T., Liu H., Liao S. Role of divalent metal ions on the basic properties and molecular chains of natural rubber. IOP Conf. Ser. Mater. Sci. Eng. 2018;452 doi: 10.1088/1757-899X/452/2/022057. [DOI] [Google Scholar]
- Zhao Y., Sun L., Li Q., Yan X., Li Z., Liu B., Li G. Use of integrated biomarker response for evaluating antioxidant stress and DNA damage of earthworms (Eisenia fetida) in decabromodiphenyl ethane-contaminated soil. Environ. Pollut. 2020;264 doi: 10.1016/j.envpol.2020.114706. [DOI] [PubMed] [Google Scholar]
- Zon, N.F., Iskendar, A., Azman, S., Sarijan, S., 2018. Sorptive behaviour of chromium on polyethylene microbeads in artificial seawater, 250, 06001. https://doi.org/ 10.1051/matecconf/201825006001. [DOI]
- Zon N.F., Azman S., Abdullah N.H., Supian N.S. Kinetics and isotherm of cadmium adsorption onto polyethylene microbeads in artificial seawater. IOP Conf. Ser. Earth Environ. Sci. 2020;476 doi: 10.1088/1755-1315/476/1/012130. [DOI] [Google Scholar]