Abstract
Objective
Qujing City, Yunnan Province, China, has a high incidence of lung cancer and related mortality. The etiology of NSCLC in Qujing area and distribution of associated molecular aberrations has not been fully elucidated. This study aimed to reveal the profile of driver gene mutations in patients with non-small-cell lung cancer (NSCLC) in Qujing and explore their relationships with clinicopathological characteristics.
Methods
In this study, the mutation profiles of NSCLC driver genes, including EGFR, ALK, ROS1, KRAS, BRAF, RET, MET, HER2, NRAS, and PIK3CA, were investigated in patients with NSCLC from Qujing and compared with those from other regions in Yunnan Province. The associations between molecular mutations and clinicopathological characteristics were further analyzed.
Results
A distinct profile of driver gene mutations was discovered in patients with NSCLC from Qujing. Interestingly, a higher proportion of EGFR compound mutations, including G719X + S768I (19.65% vs 3.38%, P < 0.0001) and G719X + L861Q (21.10% vs 2.82%, P < 0.0001), was observed in patients with NSCLC in Qujing compared with patients in non-Qujing area, besides significantly different distributions of EGFR (46.01% vs. 51.07%, P = 0.0125), ALK (3.17% vs. 6.97%, P = 0.0012), ROS1 (0.5% vs. 2.02%, P = 0.0113), and KRAS (23.02% vs. 7.85%, P < 0.0001). Further, EGFR compound mutations were more likely associated with the occupation of patients (living/working in rural areas, e.g., farmers). Moreover, KRAS G12C was the dominant subtype (51.11% vs 25.00%, P = 0.0275) among patients with NSCLC having KRAS mutations in Qujing.
Conclusions
Patients with NSCLC in Qujing displayed a unique profile of driver gene mutations, especially a higher prevalence of EGFR compound mutations and dominant KRAS G12C subtype, in this study, indicating a peculiar etiology of NSCLC in Qujing. Therefore, a different paradigm of therapeutic strategy might need to be considered for patients with NSCLC in Qujing.
Keywords: ALK, EGFR, KRAS, ROS1, mutation profile, non-small-cell lung cancer, Qujing
Introduction
Lung cancer has been the most common cancer globally for more than two decades (1). Every year, 1.8 million people are diagnosed with lung cancer and 1.6 million die of the disease (2). In China, lung cancer has the highest mortality and modality. Qujing City, located in Southwest China, is an area with an extremely high incidence of lung cancer, especially in Xuanwei County (3, 4). Previous studies showed several exposures contributing to a higher incidence of non-small-cell lung cancer (NSCLC), including the use of smoky coal in unvented stoves (5), tobacco smoking (6), food contamination (7), and arsenic and radon among tin miners (8) in this area. These external exposures might lead to a distinct profile of genetic alterations contributing to the occurrence and development of NSCLC. Studies with limited sample size indicated that patients with NSCLC in Xuanwei County had lower EGFR and ALK mutation rates and a higher rate of KRAS mutations (9–11). A higher proportion of EGFR exons 18 and 20 co-mutations were also reported (12). In recent years, the discovery of lung cancer driver genes has opened the door to individualized treatment of lung cancer and made the molecular typing of lung cancer more refined (13). Therefore, understanding the real-world driver gene mutation characteristics of patients with NSCLC in the Qujing area is of great importance in unrevealing the genetic etiology and optimizing therapeutic regimens for patients in this region. In this study, 2672 patients with NSCLC from Yunnan Province, including 946 patients from Qujing City, were retrospectively examined. The mutation statuses of lung cancer driver genes EGFR, ALK, ROS1, KRAS, BRAF, RET, MET, HER2, NRAS, and PIK3CA were detected. Also, the mutational characteristics of these driver genes were analyzed. A specific profile of mutations in these driver genes was revealed in patients from this region, which might lead to the development of more effective targeted therapeutic interventions for this disease.
Materials and Methods
Patients
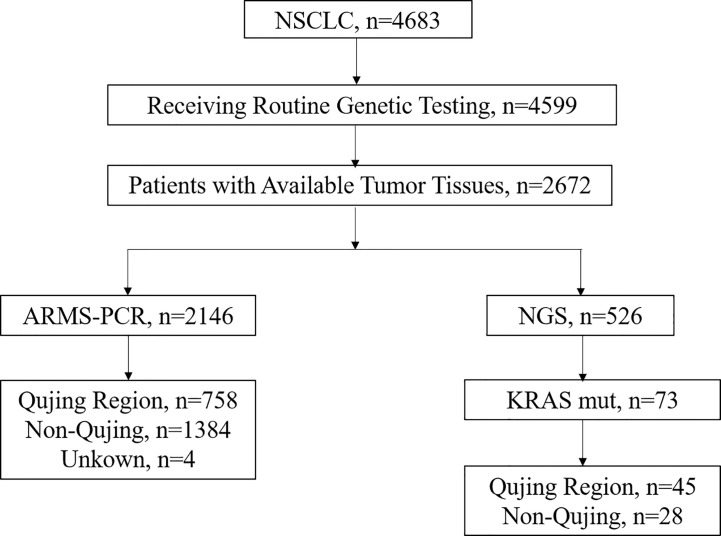
A total of 2672 patients with pathologically diagnosed NSCLC from various regions, including Qujing in Yunnan Province, who visited Yunnan Cancer Hospital between January 2016 and September 2019 were retrospectively recruited ( Figure 1 ). This study was conducted with approval from the Institutional Review Board of Yunnan Cancer Hospital. Informed consent was waived because of the retrospective nature of this study, and the de-sensitized clinical data were collected.
Figure 1.
Flowchart of participant selection in this study.
Samples and Mutation Detection
Formalin-fixed paraffin-embedded (FFPE) tumor tissues, or fine‐needle aspiration and/or core needle biopsies, were used to detect mutations in at least one of the following genes, EGFR, ALK, ROS1, KRAS, BRAF, RET, MET, HER2, NRAS, and PIK3CA. Genomic DNA and total RNA were extracted from FFPE samples using the AmoyDx FFPE DNA/RNA extraction kit (Amoy Diagnostics, Xiamen, China) following the manufacturer’s protocols. For other types of samples, an AmoyDx Tissue DNA/RNA extraction kit (Amoy Diagnostics) was used. An Amplification Refractory Mutation System Polymerase Chain Reaction (ARMS-PCR) and a Mutation Detection Kit (Amoy Diagnostics) were used to detect the mutations in driver genes (n = 2146). The other 526 specimens were captured using commercially available panels and subjected to next-generation sequencing (NGS) following manufacturer’s protocols ( Table 1 and Supplementary Table 1 ).
Table 1.
Characteristics of patients with NSCLC from Qujing and non-Qujing areas.
| Characteristic | All patients (n=2142) | Region | P-value | |
|---|---|---|---|---|
| Qujing (n=758) | Non-Qujing (n=1384) | |||
| Gender | ||||
| Male | 1026 (47.90%) | 357 (47.10%) | 669 (48.34%) | |
| Female | 1116 (52.10%) | 401 (52.90%) | 715 (51.66%) | 0.3901 |
| Age | ||||
| Median (range) | 53 (17-92) | 55 (24-89) | ||
| ≤40 | 109 (5.09%) | 42 (5.54%) | 67 (4.84%) | |
| >40 | 2033 (94.91%) | 716 (94.46%) | 1317 (95.16%) | 0.481 |
| Histopathology | ||||
| Adenocarcinoma | 1978 (92.34%) | 726 (95.78%) | 1252 (90.46%) | |
| Squamous carcinoma | 157 (7.33%) | 32 (4.22%) | 125 (9.03%) | <0.001 |
| Unknown (NSCLC) | 7 (0.33%) | 0 | 7 (0.51%) | |
| Smoking history | ||||
| Yes | 672 (31.37%) | 251 (33.11%) | 421 (30.42%) | |
| No | 1454 (67.88%) | 504 (66.49%) | 950 (68.64%) | 0.2285 |
| Unknown | 16 (0.75%) | 3 (0.40%) | 13 (0.94%) | |
| Family history | ||||
| Yes | 187 (8.73%) | 96 (12.66%) | 91 (6.58%) | |
| No | 1954 (91.22%) | 661 (87.20%) | 1293 (93.42%) | <0.001 |
| Unknown | 1 (0.05%) | 1 (0.13%) | 0 | |
| Staging | ||||
| I-IIIa | 988 (46.13%) | 411 (54.22%) | 577 (41.69%) | |
| IIIb-IV | 976 (45.56%) | 345 (45.51%) | 631 (45.59%) | 0.0044 |
| Unknown | 251 (11.72%) | 75 (9.89%) | 176 (12.72%) | |
| Lesion site | ||||
| Left | 769 (35.90%) | 229 (30.21%) | 540 (39.02%) | |
| Right | 1255 (58.59%) | 446 (58.84%) | 809 (58.45%) | 0.0076 |
| Unknown | 48 (2.24%) | 13 (1.72%) | 35 (2.53%) | |
| Occupation | ||||
| Farmer | 1005 (46.92%) | 460 (60.39%) | 545 (39.38%) | |
| Non-farmer/Unknown | 1137 (53.08) | 298 (39.31%) | 592 (42.77%) | <0.001 |
Statistical Analysis
SPSS23.0 (SPSS version 23.0 for Windows, IBM Inc., IL, USA) was used to analyze the relationship between gene mutations and clinicopathological characteristics with the help of χ 2 test, Fisher’s exact test, or binary logistic regression. The two‐sided significance level was set at P <0.05.
Results
Clinicopathological Characteristics of Patients With NSCLC in Qujing and Non-Qujing Regions
Among the 2146 patients with NSCLC tested by ARMS-PCR, 758 (35.25%) were from Qujing and 1384 (64.75%) were non-Qujing patients. Regional information was not available for the remaining four patients. The clinicopathological characteristics, including sex, age at diagnosis, smoking history, staging, histopathology, family history, ethnic, lesion site, and metastasis, are listed in Table 1 . No difference in baseline characteristics was found between these two patient groups. The clinicopathological characteristics of other 526 specimens tested using NGS are shown in Supplementary Table 2 .
Mutational Status of Driver Genes in Patients With NSCLC in Qujing
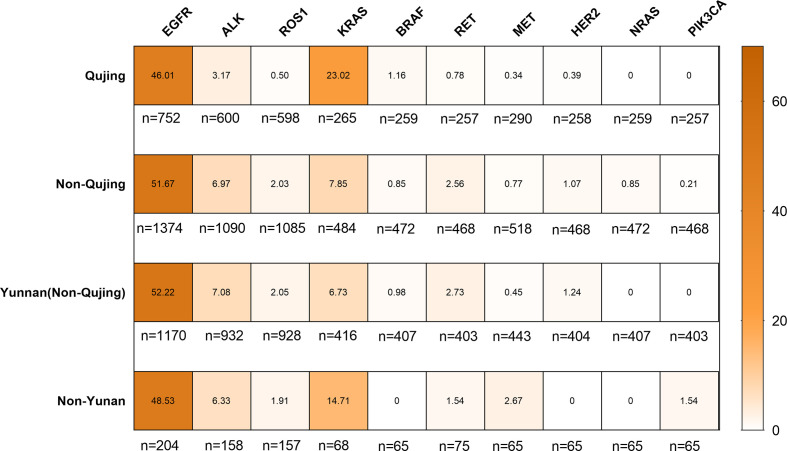
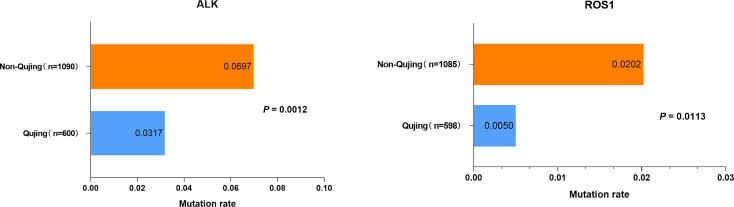
Among 2142 patients with NSCLC, 1978 were diagnosed with lung adenocarcinoma (92.34%) and 157 had lung squamous carcinoma (7.33%). The landscape of driver mutations in patients with NSCLC from Qujing, non-Qujing, Yunan (non-Qujing), and non-Yunnan regions displayed a region-specific mutational profile ( Figure 2 ). Especially, the prevalence of EGFR (46.01% vs 51.07%, P = 0.0125), ALK (3.17%% vs 6.97%%, P = 0.0012), and ROS1 (0.5% vs 2.02%, P = 0.0113) was significantly lower in patients from Qujing than in those from non-Qujing regions. On the contrary, the KRAS mutation rate was significantly higher in patients with NSCLC in Qujing compared with non-Qujing patients (23.0214% vs 7.85%, P < 0.0001) ( Figures 3 , 4A , and 5A ). Similar results were also obtained in patients with lung adenocarcinoma ( Supplementary Figure 1 ). In addition, fewer patients with NSCLC in Qujing had co-mutations of these 10 driver genes compared with those from non-Qujing areas ( Supplementary Figure 2 ). Among 526 specimens tested using NGS, the prevalence of ALK and KRAS mutations in NSCLC patients from Qujing and non-Qujing areas was as similar as the results by ARMS-PCR ( Supplementary Table 3 ).
Figure 2.
Mutation frequencies of 10 lung cancer driver genes in patients with NSCLC according to the regions in Yunnan Province.
Figure 3.
Mutation frequencies of ALK and ROS1 in patients with NSCLC from Qujing and non-Qujing regions.
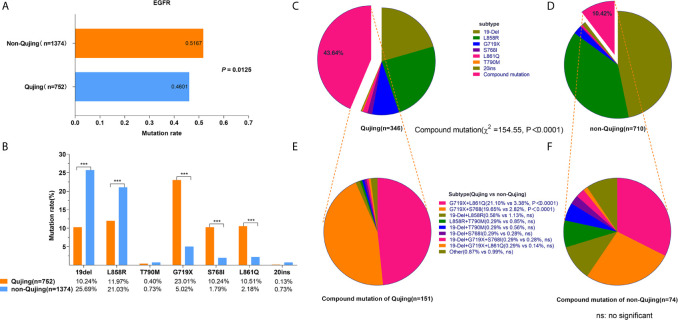
Figure 4.
Frequencies of EGFR mutations, EGFR subtypes, and compound mutations in patients with NSCLC from Qujing and non-Qujing areas. (A) the prevalence of EGFR mutation in Qujing patients was compared with non-Qujing patients. (B) the point mutation frequencies of G719X, S768I, and L861Q were higher in Qujing patients, and the point mutation of 19Del and L858R were lower in Qujing patients. (C, D) the distribution of EGFR mutation subtypes in Qujing and non-Qujing patients. (E, F) the distribution of EGFR compound mutation subtypes in Qujing and non-Qujing patients.
Figure 5.
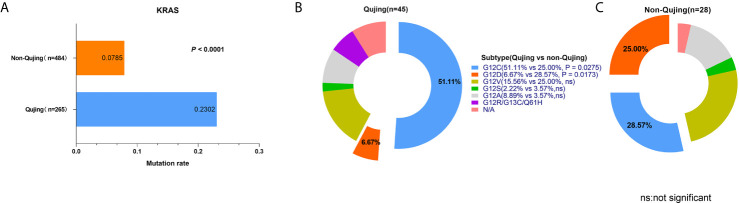
Profile of KRAS mutation and subtypes in patients with NSCLC from Qujing and non-Qujing areas. (A) the prevalence of KRAS mutation in Qujing patients was compared with non-Qujing patients. (B, C) the distribution of KRAS mutation subtypes in Qujing and non-Qujing patients.
Relationship Between Clinical Characteristics and EGFR, ALK/ROS1, and KRAS Mutation Statuses in Patients With NSCLC in Qujing
Further, the relationship between EGFR, KRAS, and ALK/ROS1 mutation statuses and clinical characteristics in patients with NSCLC in Qujing was analyzed. EGFR mutations were more common in female patients with adenocarcinoma, nonsmoker patients with NSCLC (P < 0.0001), and patients aged more than 40 years (P = 0.0196). ALK/ROS1 fusions occurred more in patients younger than 40 years old (P = 0.0002). However, KRAS mutations were more frequent in men (P = 0.0066) and smokers (P = 0.0084) ( Table 2 and Supplementary Table 4 ).
Table 2.
Relationship between clinical characteristics and EGFR, ALK/ROS1, and KRAS mutations in patients with NSCLC from Qujing.
| Characteristics | EGFR | ALK/ROS1 | KRAS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mut | WT | χ2 | p | Mut | WT | χ2 | p | Mut | WT | χ2 | p | ||
| Gender | |||||||||||||
| Male | 120 | 235 | 13 | 269 | 39 | 90 | |||||||
| Female | 226 | 171 | 40.34 | <0.0001 | 9 | 307 | 1.305 | 0.2532 | 22 | 114 | 7.381 | 0.0066 | |
| Age | |||||||||||||
| ≤40 | 12 | 30 | 5 | 27 | 4 | 7 | |||||||
| >40 | 334 | 376 | 5.446 | 0.0196 | 17 | 549 | 13.62 | 0.0002 | 57 | 197 | 1.153 | 0.2829 | |
| Histopathology | |||||||||||||
| AD | 343 | 374 | 21 | 554 | 59 | 196 | |||||||
| SCC | 3 | 29 | 18.23 | <0.0001 | 1 | 22 | 0.5847 | 2 | 8 | 0.05345 | 0.8172 | ||
| Smoking history | |||||||||||||
| Yes | 74 | 176 | 9 | 184 | 26 | 51 | |||||||
| No | 272 | 227 | 40.58 | <0.0001 | 12 | 391 | 1.091 | 0.2963 | 35 | 152 | 6.953 | 0.0084 | |
| Family history | |||||||||||||
| Yes | 45 | 50 | 4 | 76 | 10 | 34 | |||||||
| No | 301 | 335 | 5.67E-05 | 0.994 | 18 | 500 | 0.4549 | 0.5 | 51 | 170 | 0.0025 | 0.9599 | |
| Ethnic | |||||||||||||
| Han | 333 | 339 | 22 | 559 | 60 | 196 | |||||||
| Non-Han | 13 | 7 | 1.854 | 0.1734 | 0 | 17 | 1* | 1 | 8 | 0.6892* | |||
| *Staging | |||||||||||||
| I-IIIa | 196 | 213 | 10 | 328 | 40 | 127 | |||||||
| IIIb-IV | 116 | 153 | 1.504 | 0.22 | 9 | 189 | 0.9196 | 0.3376 | 18 | 51 | 0.1201 | 0.729 | |
| Lesion site | |||||||||||||
| Left | 138 | 158 | 9 | 217 | 26 | 73 | |||||||
| Right | 204 | 239 | 0.023 | 0.8786 | 13 | 354 | 0.07582 | 0.783 | 34 | 129 | 1.019 | 0.3128 | |
| Occupation | |||||||||||||
| Farmer | 214 | 242 | 10 | 341 | 35 | 112 | |||||||
| Non farmer/unknown | 132 | 164 | 0.394 | 0.5302 | 12 | 235 | 1.652 | 0.1987 | 26 | 92 | 0.1165 | 0.7329 | |
AD, adenocarcinoma; SCC, squamous cell carcinoma; *Fisher exact test.
Distribution of EGFR Mutation Subtypes in Patients With NSCLC in Qujing
The mutation frequencies of G719X (23.01% vs 5.02%, P < 0.0001), S768I (10.24% vs 1.79%, P < 0.0001), and L861Q point mutations (10.51% vs 2.18%, P < 0.0001) were significantly higher, while the prevalence of 19Del (10.24% vs 25.69%, P < 0.0001) and L858R point mutations (11.97% vs 21.03%, P < 0.0001) was significantly lower in patients with NSCLC from Qujing compared with those from non-Qujing areas ( Figure 4B ). In addition, a significantly higher proportion of EGFR compound mutations were detected (43.35% vs 10.12%, P < 0.0001, for all EGFR mutations) ( Figures 4C, D ), and the proportion of EGFR G719X + L861Q (21.10% vs 3.38%, P < 0.0001) and EGFR G719X + S768I (19.65% vs 2.82%, P < 0.0001) subtypes was significantly higher in patients with NSCLC from Qujing ( Figures 4E, F ). The multivariate analysis showed that the occupation of patients (living/working in the rural area, e.g., farmers) (odds ratio, 1.923; 95% confidence interval, 1.179–3.137) was independently associated with an increased rate of EGFR compound mutations ( Table 3 ).
Table 3.
Multivariate logistic regression analysis of correlations between baseline characteristics and EGFR compound mutations in patients with NSCLC from Qujing.
| Characteristics | Exp(B) | EXP(B) 95% CI | P-value | |
|---|---|---|---|---|
| upper | lower | |||
| Sex (Male vs Female*) | 0.986 | 0.499 | 1.948 | 0.969 |
| Age | 0.993 | 0.967 | 1.020 | 0.628 |
| Occupation (Farmer vs non-Farmer*) | 1.923 | 1.179 | 3.137 | 0.009 |
| Race (Han vs non-Han*) | 1.313 | 0.368 | 4.690 | 0.675 |
| Smoking (Yes vs No*) | 1.145 | 0.519 | 2.527 | 0.738 |
| Family history (Yes vs No*) | 1.223 | 0.622 | 2.403 | 0.560 |
| Lesion (Left vs Right*) | 0.991 | 0.621 | 1.581 | 0.969 |
| Stage (I-IIIa vs IIIb-IV*) | 0.779 | 0.484 | 1.255 | 0.305 |
| Histopathology (AD vs SCC*) | 1.502 | 0.121 | 18.679 | 0.752 |
*reference variable.
Mutational Profile of KRAS Subtypes in Patients With NSCLC From Qujing
A total of 73 patients harbored KRAS mutations (13.88%) among 526 patients receiving NGS testing, including 45 patients from Qujing and 28 patients from non-Qujing areas. The mutation frequency of KRAS G12C was significantly higher in patients with NSCLC from Qujing than in those from non-Qujing areas (51.11% vs 25.00%, P = 0.0275). However, the frequency of KRAS G12D was significantly lower in patients with NSCLC from Qujing than in those from non-Qujing areas (6.17% vs 28.57%, P = 0.0173) ( Figures 5B, C ).
Discussion
Lung cancer in Qujing City (including Xuanwei County), Yunnan, has four remarkable features: higher incidence, higher mortality, adenocarcinoma as the main histological type, and similar incidence in men and women (5). However, the mutation status of lung cancer driver genes has not been thoroughly investigated among the populations in this region due to the lack of a large-sized study cohort.
Previous studies suggested that lung cancer in the Qujing area had unique epidemiological characteristics due to severe air pollution and the toxicology of indoor coal-fired particles (4, 14–16). This area had more female patients with lung cancer, indicating the presence of strong carcinogens in the body, which had different effects on men and women (17). This study showed that patients with NSCLC in Qujing had a unique driver gene mutation profile and significant differences in EGFR and KRAS mutation frequencies between men and women. The characteristics of gene mutations associated with patients with lung cancer in Qujing have been identified. However, the underlying molecular mechanisms of lung cancer in Qujing are complex and still not fully understood. On the contrary, a number of animal and in vitro studies showed that alveolar macrophages loaded with carbon particles from smoke led to an increased risk of respiratory tract infections. They also showed that the pathways involved in lung carcinogenesis induced by indoor coal-fired particles and that induced by tobacco smoke might be identical (18–20). However, no evidence directly demonstrated that abnormal driver gene profile was the cause of the high incidence of lung cancer in this region. Therefore, the molecular mechanism underlying the high incidence of lung cancer in the Qujing population remains to be further explored.
This study was based on the analysis of multi-gene mutations in a large number of patients in Qujing compared with patients from other regions in Yunnan Province. In the present study, a distinct profile of driver gene mutations was found in patients with NSCLC from the Qujing area. Except the differential distribution of EGFR, ALK, ROS1, and KRAS mutations, EGFR compound mutations as well as KRAS G12C and G12D also displayed a “Qujing”-specific spectrum in this study. Previous findings and the findings of this study suggested that the prevalence of common driver mutations in patients with NSCLC from Qujing was different from that in patients from other regions of China, but similar to that in Western populations (9, 10, 21–23). However, further studies are required to validate the findings.
The EGFR mutation rate in patients with lung cancer from Xuanwei is still controversial. Wei et al. reported that 57% (51/90) and 43% (73/168) of patients with lung cancer from Xuanwei and non-Xuanwei regions carried EGFR mutations, respectively (24). Hosgood et al. showed that the incidence of EGFR mutation was 35% in female patients (never smokers) with lung cancer in Xuanwei (9). The present study found that the EGFR mutation rate was 46.01% in patients with NSCLC from Qujing, which was lower than that reported by Wei et al., but higher than that reported by Hosgood et al. This inconsistency in results might be due to the differences in population selection. On the one hand, Qujing City includes Xuanwei and other counties in its administrative area, and therefore this study included patients who were not in Xuanwei but belonged to Qujing City. On the other hand, the patients were not selected according to specific clinical characteristics.
The most commonly known type of EGFR mutation is 19Del (accounting for ~45% of EGFR mutations), followed by L858R (accounting for ~40% of EGFR mutations). The remaining ~10% of EGFR mutations are defined as uncommon mutations, including exon 20 insertions (20ins), T790M, G719X, L861X, and S768I (25). However, the mutation frequencies of 19Del (20.52%) and L858R (24.28%) in patients with NSCLC from Qujing were lower than those reported in the literature. In addition, a higher proportion of EGFR G719X + L861Q (21.10%) and G719X + S768I (19.65%) mutation subtypes were found in patients with NSCLC from Qujing. Interestingly, EGFR compound mutations were more likely associated with epidemiological issues (living/working in the rural area, e.g., farmers). People who used to live or work in the rural areas of Qujing might have a higher chance of being exposed, for example, to coal-fired flue gas (4, 10). However, further large-scale investigations are warranted to confirm the correlation between EGFR compound mutations in NSCLC and environmental exposures in this region.
NSCLC with the coexistence of multiple EGFR mutations may have a unique oncogenic mechanism that may reflect the efficacy of EGFR-specific tyrosine kinase inhibitors. ERBB2 phosphorylation was markedly reduced in cells expressing L861Q plus G719X compared with lung cancer cells expressing L861Q alone. The viability assays revealed that lung cancer cells expressing L861Q + G719A showed decreased sensitivity (8- to 58-fold reduction) to EGFR-specific inhibitors, erlotinib and osimertinib, compared with cells expressing L861Q alone, but pan-ERBB inhibitors exerted superior growth-inhibitory effects on cells expressing compound L861Q/G719X mutations (26). Similarly, the cells co-expressing G719X and S768I also showed a good response to afatinib, a pan-ERBB inhibitor (27). In this study, a higher proportion of EGFR compound mutations were detected in patients with NSCLC from Qujing. Therefore, pan-ERBB inhibitors exerted superior tumor-growth-inhibitory effects in these patients compared with EGFR-specific inhibitors. Further clinical data should be collected to confirm these cell research based findings.
KRAS is the second most common driver gene in lung cancer, and the frequency of KRAS mutation is lower in Chinese patients than in Western populations. However, the mutation frequency of KRAS in Qujing (including Xuanwei) was inconsistent (6.3%–29.2%) in previous reports due to the limited number of patients (9–11, 28, 29). In this study based on a large number of patients with NSCLC, the frequency of KRAS mutation was significantly higher in patients with NSCLC from Qujing than in those from non-Qujing regions (23.02% vs 7.85%). Targeting KRAS protein has been one of the toughest challenges in cancer treatment research. A specific mutation known as KRAS G12C is a major driver of tumor growth, occurring broadly across solid-tumor indications. KRAS G12C mutation is found in about 13% of patients with NSCLC in the United States (30), and approximately 32.3% of patients with NSCLC in China (31). In this study, KRAS G12C was also the main mutant subtype of KRAS in patients with NSCLC from Qujing (51.11%). With the development of drugs inhibiting KRAS G12C, this study suggested that patients with KRAS G12C mutations in Qujing might benefit from targeted therapy, such as AMG510 (32).
In general, previous studies based on patients with lung cancer from Xuanwei/Qujing showed that a higher proportion of EGFR compound mutations and KRAS mutations were observed, although EGFR mutation rate in patients with lung cancer patients from Xuanwei was still controversial (9–12, 24, 28, 29, 33–35) ( Table 4 ). The cause of these specific genetic changes remains unclear. However, the main findings were as follows: people using smoky coal had an up to 30-fold higher risk of lung cancer compared with those using smokeless coal and wood (4); lung cancer patients with coal exposure history in Xuanwei had a higher KRAS mutation rate (11, 33) ( Table 4 ); and the occupation of patients (living/working in the rural area, e.g., farmers) was independently associated with an increased rate of EGFR compound mutations in this study. These findings suggested that environmental exposure might be an important reason for the specific mutation spectrum in this area. Hence, further large-scale investigations are warranted to confirm the correlation between the driver gene profile of patients with lung cancer and the environmental exposure in this region.
Table 4.
EGFR and KRAS mutation characteristics in patients with lung cancer from Xuanwei/Qujing in previous studies.
| Study | Gene | Patients | n | Mutation rate |
|---|---|---|---|---|
| Hosgood 3rd, et al. (9) | EGFR | NSCLC, female, non-smoking |
40 | 35% |
| KRAS | NSCLC, female non-smoking | 40 | 15% | |
| Ma et al. (34) | EGFR | NSCLC | 119 | EGFR: 39.45% G719X+S768I: 22.69% G719X+L861Q: 0.8% |
| KRAS | NSCLC | 119 | 23.53% | |
| DeMarini et al. (29) | KRAS | Lung tumors, female, non-smoking, smoky exposure, |
24 | 29.2% |
| Keohavong et al. (10) | KRAS | No evidence of lung cancer from XuanWei County | 92 | 2.2% |
| Keohavong et al. (11) | KRAS | Lung cancer | 41 | 22.9% |
| Yu et al. (33) | EGFR | NSCLC, smoky coal exposure | 79 | 37.97% |
| KRAS | NSCLC, smoky coal exposure | 79 | 29.11% | |
| Chen et al. (24) | EGFR | NSCLC patients | 90 | EGFR: 57% G719X+S768I: 25.56% G719X+L861Q: 1.0% |
| Yang et al. (28) | EGFR | NSCLC patients | 63 | 55.6% G719X+S768I: 17.1% |
| KRAS | NSCLC patients | 63 | 6.3% | |
| Zhou et al. (12) | EGFR | NSCLC patients | 447 | EGFR: 34.9% G719X+S768I: 4.5% G719X+L861Q: 0.6% |
| Zhou et al. (35) | EGFR | NSCLC patients | 212 | EGFR: 30.1% |
On the other hand, some studies showed that patients with EGFR mutations in Xuanwei had a poor prognosis after receiving EGFR-TKI treatment. This might be due to the high incidence of rare EGFR mutations in this area (12, 36). These studies also found that the incidence of uncommon EGFR mutations and EGFR compound mutations was high in patients with NSCLC from Qujing. Therefore, the information on the significance of these mutations in targeted treatment deserves further investigation due to the high incidence of NSCLC with the so-called uncommon EGFR mutations in the Qujing population. On the contrary, chemotherapy is usually less effective in patients with NSCLC having KRAS mutations (37). Many novel treatment strategies have been developed, including targeting downstream signaling pathways (38), directly targeting KRAS (39), and using immunotherapy (40). Of these, immunotherapy may be one of the most promising treatment strategies for patients with NSCLC having KRAS mutations. Thus, we hope that these treatment strategies will bring clinical benefits to patients with lung cancer having KRAS mutations in Qujing in the future.
The incidence of lung cancer in the Qujing City of China is very high, and the related mortality is also high. Therefore, a comprehensive understanding of the molecular characteristics of patients with lung cancer in this region may provide the basis for a precise diagnosis and treatment. A major strength of this study was the large number of patients with NSCLC included to estimate the prevalence of common actionable genomic alterations (involving EGFR, ALK, ROS1, KRAS, BRAF, RET, MET, HER2, NRAS, and PIK3CA) in Qujing. These estimates can serve as a reference for future research. However, this study also had several limitations. First, it was a retrospective analysis and included only a single institution. Second, not all patients underwent the same molecular testing. Furthermore, the data on the treatment and prognosis of these patients were not collected, and therefore whether these patients could benefit from targeted therapy was unclear.
Conclusion
In conclusion, this study displayed a unique profile of driver gene mutations in patients with NSCLC in Qujing. More patients with NSCLC in Qujing harbored EGFR G719X + S768I and EGFR G719X + L861Q compound mutations, besides 19DEL and L858R. Also, patients with NSCLC in Qujing had a higher proportion of KRAS (G12C) mutations. Therefore, these findings suggested that different treatment strategies should be adopted in patients with NSCLC in Qujing.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material .
Ethics Statement
This study was conducted with approval from the Institutional Review Board of Yunnan Cancer Hospital. Informed consent was waived because of the retrospective nature of this study, and the de-sensitized clinical data were collected.
Author Contributions
YH, YZ, and HS conceived and designed the experiments. FG, YD, QL, JC, XL, YG, ZS, and LD collected the data. YZ, ZH, FY, and CZ analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was partially supported by The National Natural Science Fund (No. 81860513, and No. 81960335).
Conflict of Interest
ZH, FY, and CZ were employed by Amoy Diagnostics Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.644895/full#supplementary-material
Mutation frequencies of EGFR, ALK, ROS1, and KRAS in patients with lung adenocarcinoma from Qujing and non-Qujing areas.
Co-mutations of driver genes in patients with NSCLC from Qujing and non-Qujing areas.
Abbreviation
EGFR, Epidermal growth factor receptor; ALK, Anaplastic lymphoma kinase; ROS1, Proto-oncogene receptor tyrosine kinase; KRAS, Kirsten rat sarcoma 2 viral oncogene homolog; BRAF, V-raf murine sarcoma viral oncogene homolog B; RET, Ret Proto-Oncogene; MET, MET proto-oncogene receptor tyrosine kinase; HER2, Epidermal growth factor receptor 2; NRAS, Neuroblastoma RAS viral oncogene homolog; PIK3CA, Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin (2015) 65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136:E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 3. Xiao Y, Shao Y, Yu X, Zhou G. The epidemic status and risk factors of lung cancer in Xuanwei City, Yunnan Province, China. Front Med (2012) 6:388–94. 10.1007/s11684-012-0233-3 [DOI] [PubMed] [Google Scholar]
- 4. Barone-Adesi F, Chapman RS, Silverman DT, He X, Hu W, Vermeulen R, et al. Risk of lung cancer associated with domestic use of coal in Xuanwei, China: retrospective cohort study. BMJ (2012) 345:e5414. 10.1136/bmj.e5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lan Q, He X, Shen M, Tian L, Liu LZ, Lai H, et al. Variation in lung cancer risk by smoky coal subtype in Xuanwei, China. Int J Cancer (2008) 123:2164–9. 10.1002/ijc.23748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cai L, Wu X, Goyal A, Han Y, Cui W, Xiao X, et al. Patterns and socioeconomic influences of tobacco exposure in tobacco cultivating rural areas of Yunnan Province, China. BMC Public Health (2012) 12:842. 10.1186/1471-2458-12-842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shen M, Chapman RS, He X, Liu LZ, Lai H, Chen W, et al. Dietary factors, food contamination and lung cancer risk in Xuanwei, China. Lung Cancer (2008) 61:275–82. 10.1016/j.lungcan.2007.12.024 [DOI] [PubMed] [Google Scholar]
- 8. Taylor PR, Qiao YL, Schatzkin A, Yao SX, Lubin J, Mao BL, et al. Relation of arsenic exposure to lung cancer among tin miners in Yunnan Province, China. Br J Ind Med (1989) 46:881–6. 10.1136/oem.46.12.881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hosgood HD3, Pao W, Rothman N, Hu W, Pan YH, Kuchinsky K, et al. Driver mutations among never smoking female lung cancer tissues in China identify unique EGFR and KRAS mutation pattern associated with household coal burning. Respir Med (2013) 107:1755–62. 10.1016/j.rmed.2013.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keohavong P, Lan Q, Gao WM, Zheng KC, Mady HH, Melhem MF, et al. Detection of p53 and K-ras mutations in sputum of individuals exposed to smoky coal emissions in Xuan Wei County, China. Carcinogenesis (2005) 26:303–8. 10.1093/carcin/bgh328 [DOI] [PubMed] [Google Scholar]
- 11. Keohavong P, Lan Q, Gao WM, DeMarini DM, Mass MJ, Li XM, et al. K-ras mutations in lung carcinomas from nonsmoking women exposed to unvented coal smoke in China. Lung Cancer (2003) 41:21–7. 10.1016/s0169-5002(03)00125-9 [DOI] [PubMed] [Google Scholar]
- 12. Zhou Y, Yang Y, Yang C, Chen Y, Yang C, Du Y, et al. Epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer (NSCLC) of Yunnan in southwestern China. Oncotarget (2017) 8:15023–33. 10.18632/oncotarget.14706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Deiry WS, Goldberg RM, Lenz HJ, Shields AF, Gibney GT, Tan AR, et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J Clin (2019) 69:305–43. 10.3322/caac.21560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Guo W, Ran J, Tang R, Lin H, Chen X, et al. Five-year lung cancer mortality risk analysis and topography in Xuan Wei: a spatiotemporal correlation analysis. BMC Public Health (2019) 19:173. 10.1186/s12889-019-6490-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hosgood HD3, Sapkota AR, Rothman N, Rohan T, Hu W, Xu J, et al. The potential role of lung microbiota in lung cancer attributed to household coal burning exposures. Environ Mol Mutagen (2014) 55:643–51. 10.1002/em.21878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seow WJ, Hu W, Vermeulen R, Hosgood Iii HD, Downward GS, Chapman RS, et al. Household air pollution and lung cancer in China: a review of studies in Xuanwei. Chin J Cancer (2014) 33:471–5. 10.5732/cjc.014.10132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong J, Downward GS, Hu W, Portengen L, Seow WJ, Silverman DT, et al. Lung cancer risk by geologic coal deposits: A case-control study of female never-smokers from Xuanwei and Fuyuan, China. Int J Cancer (2019) 144:2918–27. 10.1002/ijc.32034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCarthy CE, Duffney PF, Gelein R, Thatcher TH, Elder A, Phipps RP, et al. Dung biomass smoke activates inflammatory signaling pathways in human small airway epithelial cells. Am J Physiol Lung Cell Mol Physiol (2016) 311:L1222–1222L1233. 10.1152/ajplung.00183.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehra D, Geraghty PM, Hardigan AA, Foronjy R. A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PloS One (2012) 7:e52889. 10.1371/journal.pone.0052889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mondal NK, Saha H, Mukherjee B, Tyagi N, Ray MR. Inflammation, oxidative stress, and higher expression levels of Nrf2 and NQO1 proteins in the airways of women chronically exposed to biomass fuel smoke. Mol Cell Biochem (2018) 447:63–76. 10.1007/s11010-018-3293-0 [DOI] [PubMed] [Google Scholar]
- 21. Liu SY, Mok T, Wu YL. Novel targeted agents for the treatment of lung cancer in China. Cancer (2015) 121 Suppl 17:3089–96. 10.1002/cncr.29522 [DOI] [PubMed] [Google Scholar]
- 22. Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA (2014) 311:1998–2006. 10.1001/jama.2014.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res (2015) 5:2892–911. 10.18632/oncotarget.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen Y, Ye L, Stanford RR, Zhang D, Zhang X, Wei W. Distinct epithelial growth factor receptor mutation profile in non-small-cell lung cancer patients from the Xuanwei area of China. Mol Clin Oncol (2016) 4:749–55. 10.3892/mco.2016.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gristina V, Malapelle U, Galvano A, Pisapia P, Pepe F, Rolfo C, et al. The significance of epidermal growth factor receptor uncommon mutations in non-small cell lung cancer: A systematic review and critical appraisal. Cancer Treat Rev (2020) 85:101994. 10.1016/j.ctrv.2020.101994 [DOI] [PubMed] [Google Scholar]
- 26. Sato H, Offin M, Kubota D, Yu HA, Wilhelm C, Toyooka S, et al. Allele-specific role of ERBB2 in the oncogenic function of EGFR L861Q in EGFR-mutant lung cancers. J Thorac Oncol (2020) 16:113–26. 10.1016/j.jtho.2020.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kutsuzawa N, Takahashi F, Tomomatsu K, Obayashi S, Takeuchi T, Takihara T, et al. Successful Treatment of a Patient with Lung Adenocarcinoma Harboring Compound EGFR Gene Mutations, G719X and S768I, with Afatinib. Tokai J Exp Clin Med (2020) 45:113–6. [PubMed] [Google Scholar]
- 28. Yang CS, Pan XY, Feng Q, Wang YY, Xu WM, Jiang T, et al. [Mutation status of epidermal growth factor receptor and KRAS gene in non-small cell lung cancers at Xuanwei regions of Yunnan Province]. Zhonghua Bing Li Xue Za Zhi (2016) 45:226–30. 10.3760/cma.j.issn.0529-5807.2016.04.003 [DOI] [PubMed] [Google Scholar]
- 29. DeMarini DM, Landi S, Tian D, Hanley NM, Li X, Hu F, et al. and TP53 mutations in nonsmokers reflect exposure to PAH-rich coal combustion emissions. Cancer Res (2001) 61:6679–81. 10.1016/S0140-6701(02)86513-4 [DOI] [PubMed] [Google Scholar]
- 30. Biernacka A, Tsongalis PD, Peterson JD, de Abreu FB, Black CC, Gutmann EJ, et al. The potential utility of re-mining results of somatic mutation testing: KRAS status in lung adenocarcinoma. Cancer Genet (2016) 209:195–8. 10.1016/j.cancergen.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jia Y, Jiang T, Li X, Zhao C, Zhang L, Zhao S, et al. Characterization of distinct types of KRAS mutation and its impact on first-line platinum-based chemotherapy in Chinese patients with advanced non-small cell lung cancer. Oncol Lett (2017) 14:6525–32. 10.3892/ol.2017.7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu SY, Sun H, Zhou JY, Jie GL, Xie Z, Shao Y, et al. Clinical characteristics and prognostic value of the KRAS G12C mutation in Chinese non-small cell lung cancer patients. Biomark Res (2020) 8:22. 10.1186/s40364-020-00199-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yu XJ, Yang MJ, Zhou B, Wang GZ, Huang YC, Wu LC, et al. Characterization of Somatic Mutations in Air Pollution-Related Lung Cancer. EBioMedicine (2015) 2:583–90. 10.1016/j.ebiom.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma Y, Li Q, Du Y, Chen W, Zhao G, Liu X, et al. Oncogenic Genetic Alterations in Non-Small-Cell Lung Cancer (NSCLC) in Southwestern China. Cancer Manag Res (2020) 12:10861–74. 10.2147/CMAR.S266069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou Y, Ma Y, Shi H, Du Y, Huang Y. Epidermal growth factor receptor T790M mutations in non-small cell lung cancer (NSCLC) of Yunnan in southwestern China. Sci Rep (2018) 8:15426. 10.1038/s41598-018-33816-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lv L, Liu Z, Liu Y, Zhang W, Jiang L, Li T, et al. Distinct EGFR Mutation Pattern in Patients With Non-Small Cell Lung Cancer in Xuanwei Region of China: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10:519073. 10.3389/fonc.2020.519073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wood K, Hensing T, Malik R, Salgia R. Prognostic and Predictive Value in KRAS in Non-Small-Cell Lung Cancer: A Review. JAMA Oncol (2016) 2:805–12. 10.1001/jamaoncol.2016.0405 [DOI] [PubMed] [Google Scholar]
- 38. Tomasini P, Walia P, Labbe C, Jao K, Leighl NB. Targeting the KRAS Pathway in Non-Small Cell Lung Cancer. Oncologist (2016) 21:1450–60. 10.1634/theoncologist.2015-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature (2019) 575:217–23. 10.1038/s41586-019-1694-1 [DOI] [PubMed] [Google Scholar]
- 40. Liu C, Zheng S, Jin R, Wang X, Wang F, Zang R, et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett (2020) 470:95–105. 10.1016/j.canlet.2019.10.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mutation frequencies of EGFR, ALK, ROS1, and KRAS in patients with lung adenocarcinoma from Qujing and non-Qujing areas.
Co-mutations of driver genes in patients with NSCLC from Qujing and non-Qujing areas.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material .