Abstract
Objectives
Cytokine release syndrome with elevated interleukin-6 (IL-6) levels is associated with multiorgan damage and death in severe coronavirus disease 2019 (COVID-19). Our objective was to update the data in a living systematic review of the literature concerning the efficacy and toxicity of the IL-6 receptor antagonist tocilizumab in COVID-19 patients.
Methods
Data sources were Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, Scopus up, preprint servers and Google from 8th October 2020 till 24th February 2021. Eligible studies were randomized controlled trials (RCTs) and observational studies at low or moderate risk of bias. The participants were hospitalized COVID-19 patients, and intervention was tocilizumab versus placebo or standard of care. We pooled crude risk ratios (RRs) of RCTs with a random effects model and evaluated inconsistency between studies with I2. We assessed the certainty of evidence using the GRADE approach.
Results
Of 1600 citations, eight RCTs and 28 cohorts were eligible. The eight RCTs had low risk of bias, and with 6311 patients they examined the effect of tocilizumab on short-term mortality; pooled RR was 0.91 (95%CI 0.78–1.07, I2 25%). Only the REMAP-CAP and RECOVERY trials, with the majority of their patients on concomitant corticosteroids, showed lower 30-day mortality with tocilizumab use: RR 0.74 (95%CI 0.59–0.93) and 0.89 (95%CI 0.81–0.97), respectively. Seven RCTs, with 5391 patients, examined the effect of tocilizumab on risk of mechanical ventilation; pooled RR was 0.84 (95%CI 0.76–0.93), I2 0%, with a corresponding number needed to treat of 20 (95%CI 14.3–33.3). Eight RCTs, with 5340 patients, examined the effect of tocilizumab on a composite of poor outcome; pooled RR was 0.82 (95%CI 0.76–0.90, I2 3%). Data from the RCTs showed a lower risk of infections and no higher risk of serious adverse events with tocilizumab: pooled RR 0.67 (95%CI 0.45–0.99, eight RCTs) and 0.85 (95%CI 0.63–1.16, seven RCTs), respectively. Among 28 cohorts with 15 484 patients, the pooled adjusted RR for mortality was 0.53 (95%CI 0.43–0.67, I2 76%).
Conclusions
Cumulative high-certainty evidence shows that tocilizumab reduces the risk of mechanical ventilation in hospitalized patients with severe COVID-19. Moderate-certainty evidence shows that tocilizumab reduces the risk of poor outcome and the risk of secondary infections in hospitalized COVID-19 patients. This review will continuously evaluate the role of tocilizumab in COVID-19 treatment.
Keywords: COVID, Meta-analysis, Outcome, Systematic review, Tocilizumab
Introduction
Cytokine release syndrome with elevated levels of interleukin-6 (IL-6) is associated with multiorgan damage and death in severe coronavirus disease 2019 (COVID-19). As of October 2020, cumulative moderate-certainty evidence from randomized controlled trials (RCTs) showed that the IL-6 receptor antagonist tocilizumab reduces the risk of mechanical ventilation in hospitalized COVID-19 patients, without affecting short-term mortality [1]. This report is the first update of our living systematic review assessing the efficacy and safety of tocilizumab in COVID-19 patients [1].
Methods
Using the same methodology and search databases detailed in our previous review [1], we searched for new studies published through 24th February 2021 and updated our analysis using the same quality assessment tools and analytical methods. As several RCTs have been published since our initial review, and as per peer reviewers' and editor's recommendations, this update includes observational studies up to January 2021, and future updates will include only data from RCTs. We also contacted all first authors of eligible RCTs for additional data for subgroup analyses.
Results
We evaluated an additional 1600 abstracts from peer-reviewed publications. We also searched for unpublished manuscripts using the medRxiv services and Researchsquare.com, Google, and the references of eligible studies and review articles. Only one author responded to our queries for additional data for subgroup analyses.
We identified eight multicentre RCTs [[2], [3], [4], [5], [6], [7], [8], [9]], of which one was stopped early for possible futility (RCT-TCZ-COVID-19) [9], one for possible benefit (REMAP-CAP) [8], and one for possible harm (TOCIBRAS) [7]. As of this update the results of the RECOVERY trial [6] have been published as a preprint only. Table 1 summarizes the characteristics of eligible RCTs and includes the design, setting, inclusion criteria, disease severity and definition of endpoints. Three RCTs [2,7,8] included a proportion of patients with critical COVID-19, while the others enrolled patients with moderate to severe COVID-19. Three trials (RECOVERY, RCT-TCZ-COVID-19 and TOCIBRAS) [6,7,9] required the presence of high inflammatory markers (such as C-reactive protein) for enrolment, although all trials reported a high range of inflammatory markers in their patient populations. All RCTs used the tocilizumab dose of 8 mg/kg, and six RCTs allowed a second dose if needed. The median time between the onset COVID-19 symptoms and the first dose of tocilizumab ranged from 7 to 11 days. The proportion of patients who received concomitant corticosteroid treatment was small (8–36%) in the RCT-TCZ-COVID-19 [9], CORIMUNO-TOCI [5], BACC Bay Tocilizumab [4] and COVACTA [2] trials. Most patients (>69%) in the RECOVERY [6], EMPACTA [3], REMAP-CAP [8] and TOCIBRAS [7] trials received concomitant corticosteroids therapy.
Table 1.
Characteristics of completed randomized controlled trials of tocilizumab for coronavirus disease 2019 (COVID-19) patients
| RCT | Design and setting | Enrolment dates | Inclusion criteriaa | MV (%) | Steroids (%) (TCZ) |
Steroids (%) (Control) |
Days to TCZ (TCZ)b Median (IQR) or mean (SD) |
Days to TCZ (control) Median (IQR) or mean (SD) |
Mortality | Composite of poor outcome | Follow-up duration for secondary infections |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT-TCZ-COVID-19 NCT04346355 | Open label: 60 TCZ versus 66 controls Italy, 24 centres |
31st March 2020 till 11th June 2020 | Severity of illness: severe High inflammatory marker: yes Progressive disease: no |
0% | 8% | 4.5% | 7 (4–11) | 8 (6–11) | 30 days | Death or continuous need for hospitalization at day 30 | Cutoff date was June 24, 2020 |
| CORIMUNO-TOCI NCT04331808 |
Open label: 64 TCZ versus 67 controls France, 9 centres |
31st March 2020 till 18th April 2020 | Severity of illness: moderate to severe High inflammatory marker: no Progressive disease: no |
0% | 33% | 61% | 10 (7–13) | 10 (8–13) | 28 days | Death or continuous need for hospitalization at day 28 | 28 days |
| BACC Bay Tocilizumab Trial NCT04356937 |
Double-blind, placebo-controlled: 161 TCZ versus 81 controls USA, 7 centres |
20th April 2020 till 15th June 2020 | Severity of illness: severe High inflammatory marker: yes Progressive disease: no |
0% | 11% | 6% | 9.0 (6.0–13.0) | 10.0 (7.0–13.0) | 28 days | Death or MV at day 28 | 28 days |
| COVACTA NCT04320615 |
Double-blind, placebo-controlled: 294 TCZ versus 144 controls 9 countries, 67 centres |
3rd April 2020 till 28th May 2020 | Severity of illness: severe to critical High inflammatory marker: no Progressive disease: no |
37.5% | 36.1% | 54.9% | 11.0 (1.0–49.0) | 10.0 (2.0–50.0) | 28 days | Death, withdrawal during hospitalization, transfer to ICU, or requirement for invasive MV within 28 days of baseline | 28 days |
| EMPACTA NCT04372186 |
Double-blind, placebo-controlled: 249 TCZ versus 128 controls 6 countries, 69 centres |
14th May 2020 till 18th August 2020 | Severity of illness: severe High inflammatory marker: no Progressive disease: no |
0% | 80.3% | 87.5% | 8.0 (0.0–31.0) | 8.0 (0.0–36.0) | 28 days | Death or MV by day 28 | 60 days |
| REMAP-CAP NCT02735707 |
Open label: 366 TCZ versus 412 controls 6 countries 113 sites |
19th April 2020 till 19th November 2020 | Severity of illness: critical High inflammatory marker: no Progressive disease: no |
29.4% | >80% | >80% | From hospital admission: 1.2 (0.8–2.8) |
From hospital admission: 1.2 (0.8–2.8) |
30 days | Death or MV or ECMO for non-intubated patients | 90 days |
| TOCIBRAS NCT04403685 |
Open label: 65 TCZ versus 64 controls Nine hospitals in Brazil |
8th May 2020 till 17th July 2020 | Severity of illness: severe to critical High inflammatory marker: yes Progressive disease: no |
16% | 69% | 73% | 10.0 (3.1) | 9.5 (3.0) | 29 days | Composite of death or MV at day 15 | 29 days |
| RECOVERY NCT04381936 |
Open label: 2022 TCZ versus 2094 controls 1 country 177 sites |
23rd April 2020 till 24th January 2021 | Severity of illness: severe and critical High inflammatory marker: yes Progressive disease: yes |
14% | 82% | 82% | 9 (7–13) | 10 (7–14) | 28 days | Death or MV by day 28 | 28 days |
MV, mechanical ventilation; ICU, intensive care unit; TCZ, tocilizumab; ECMO, extracorporeal membrane oxygenation.
Severity of illness based on NIH classification for the majority of patients.
From symptom onset.
We identified ten additional cohort studies published since our first review [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19]]. A total of 28 cohort studies, at moderate risk of bias, reported on adjusted effect estimates of mortality. Supplementary Material Table S1 illustrates the general characteristics of the included cohort studies. All studies reported on patients hospitalized with COVID-19 with varying degrees of disease severity.
Updated data synthesis
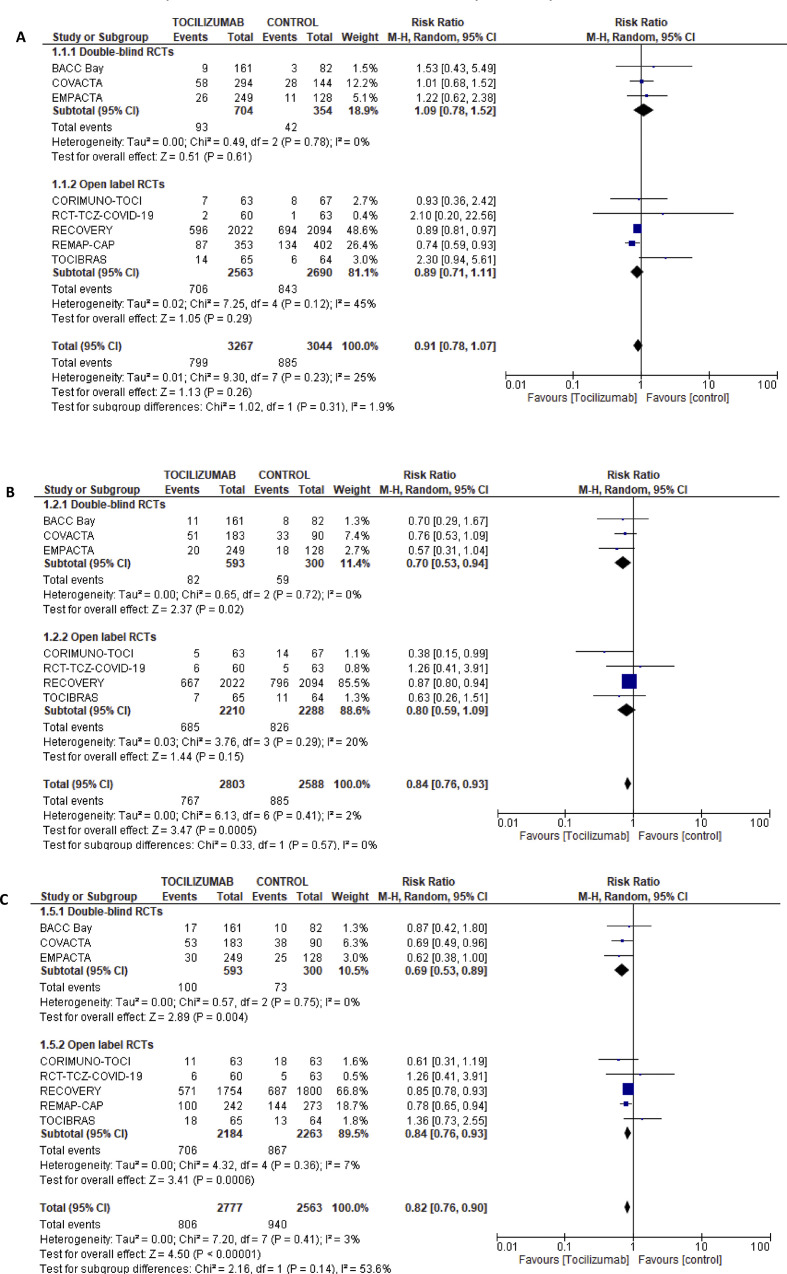
In total, 36 studies were eligible (eight RCTs and 28 cohorts). Eight RCTs were deemed at low risk of bias (Supplementary Material Figs S1 and S2). Although five RCTs were open-label studies [[5], [6], [7], [8], [9]], there was no evidence of performance bias or outcome assessment bias (Supplementary Material Figs S1 and S2). Only the REMAP-CAP [8] and RECOVERY [6] trials, with the majority of their patients on concomitant corticosteroids (>80%), showed lower 30-day mortality with tocilizumab use, RR 0.74 (95%CI 0.59–0.93) and 0.89 (95%CI 0.81–0.97), respectively. Eight RCTs [[2], [3], [4], [5], [6], [7], [8], [9]], with 6311 patients, examined the effect of tocilizumab on short-term mortality (Fig. 1 A); pooled RR was 0.91 (95%CI 0.72–1.07, I2 25%). Seven RCTs (Fig. 1B) [[2], [3], [4], [5], [6], [7],9], with 1274 patients, examined the effect of tocilizumab on risk of mechanical ventilation; pooled RR was 0.84 (95%CI 0.76–0.93), I2 0%), with a corresponding number needed to treat of 20 (95%CI 14.3–33.3).
Fig. 1.
(A) Forest plot for the effect of tocilizumab on 28–30 days mortality in randomized controlled trials. (B) Forest plot for the effect of tocilizumab on risk for mechanical ventilation in randomized controlled trials. (C) Forest plot for the effect of tocilizumab on 28–30 days composite of poor outcome in randomized controlled trials. Definitions of composite outcomes for each trial are listed in Table 1.
Eight RCTs, with 5340 patients, examined the effect of tocilizumab on a composite of poor outcome; pooled RR was 0.82 (95%CI 0.76–0.90, I2 3%) (Fig. 1C). The definition of this composite outcome in each trial is summarized in Table 1.
Using available data from five studies [2,3,[5], [6], [7]], we assessed the effect of steroids on the composite of mortality or mechanical ventilation in patients taking tocilizumab versus controls. The risk ratios for patients receiving steroids and those not receiving steroids were 0.87 (95%CI 0.68–1.12) and 0.99 (95% 0.85–1.16) respectively. Additionally, there was no interaction between the use of mechanical ventilation and the effect of tocilizumab on mortality based on data from three studies [2,6,7]. The risk ratios for patients receiving and those not receiving mechanical ventilation are 1.02 (95%CI 0.79–1.31) and 0.98 (95%CI 0.89–1.07), respectively.
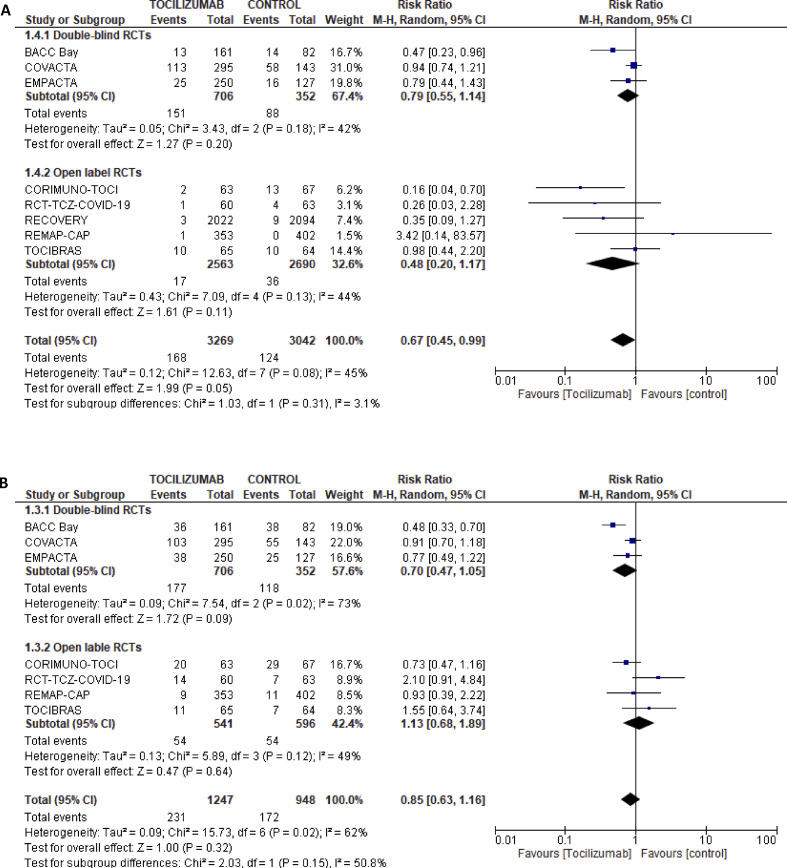
Data from the RCTs showed a lower risk of infections (Fig. 2 A) and no difference in the risk of developing serious adverse events (Fig. 2B) with tocilizumab: pooled RR 0.67 (95%CI 0.45–0.99, eight RCTs) and 0.85 (95%CI 0.63–1.16, seven RCTs), respectively. The follow-up period for infections was 28 days in all studies except for the EMAPCTA and REMAP-CAP trials. There were no significant differences in the infection rates that may be attributed to longer follow-up duration.
Fig. 2.
(A) Forest plot for relative risk of infections with tocilizumab versus control in randomized controlled trials. (B) Forest plot for relative risk of serious adverse events with tocilizumab versus control in randomized controlled trials.
Among 28 cohorts at moderate risk of bias (Supplementary Material Fig. S3), with 15 484 patients, the pooled adjusted RR for mortality was 0.53 (95%CI 0.43–0.67, I2 76%). This association was observed over all degrees of COVID-19 severity (Supplementary Material Fig. S4). Contour-enhanced funnel plot and Egger's test for small-study effects (p 0.27) did not show evidence of publication bias (Supplementary Material Fig. S5).
Survivor bias was addressed in the analysis in 11 out of 28 studies only. The pooled RR of the 11 studies that adjusted for survivor bias was 0.48 (95%CI 0.35–0.67). To study the potential effect of survivor bias on the observed results, we multiplied the RR or HR of studies that did not adjust for survivor bias and their corresponding 95%CIs by 1.6 and pooled the new adjusted effect estimates. The corrected pooled adjusted RR for mortality from the 28 cohorts was 0.69 (95%CI 0.53–0.89, I2 83%) (Supplementary Material Fig. S6).
GRADE of evidence
Data from RCTs, at low risk of bias, showed with high certainty that in hospitalized COVID-19 patients tocilizumab reduces the risk of mechanical ventilation with a corresponding number needed to treat of 20 (95%CI 14.3–33.3). Additionally, moderate-certainty evidence also suggests that tocilizumab is associated with a reduced risk of secondary infections, and a composite of poor outcome. Certainty of evidence was downgraded due to a wide confidence interval in the RR of infection, and due to possible performance bias for outcomes such as ICU admission in the composite outcome. We observed with moderate certainty that tocilizumab has no significant effect on mortality. The pooled RR of 0.91 (95%CI 0.72–1.07) was imprecise, with a wide 95%CI, suggesting that more studies may be needed for a definitive answer.
For the cohort studies, the overall quality of evidence (classified as low in observational studies) remained low given the moderate risk of study bias, low risk of publication bias, direct evidence, low inconsistency, and precise estimate. Although I2 was high for the pooled mortality risk estimate, it was driven by the magnitude of the association rather than its direction.
Discussion
In this updated systematic review and meta-analysis of eight RCTs, as of February 2021, we observed with high certainty that tocilizumab reduces the risk of mechanical ventilation in hospitalized COVID-19 patients. We also observed, with moderate certainty, that tocilizumab reduces the risk of poor outcome and the risk of secondary infections in hospitalized COVID-19 patients. We discuss in our prior review [1] the possible molecular mechanisms for this observed effect.
We did not observe a significant effect of tocilizumab on the risk of mortality or adverse events. Only the two largest RCTs—the RECOVERY [6] and REMAP-CAP [8], in which >80% of the patients were treated concomitantly with corticosteroids—showed an improved survival in patients receiving tocilizumab. We could not examine the interaction between corticosteroid use, mechanical ventilation, timing of tocilizumab, inflammatory markers, and the effect of tocilizumab on different outcomes. We contacted all authors of the eight RCTs asking them for data for different subgroups; only one author provided us with the needed data.
Despite the eight published trials in the past year, it is still difficult to specify which population will benefit from tocilizumab in COVID-19. Ambiguity is compounded when taking into consideration the differences in trial designs, such as inclusion criteria of patients with different disease severity, and the temporal trends in standard of care (such as the use of steroids) (Table 1). There are many variables that may dictate who will benefit from tocilizumab treatment, such as patient characteristics, disease severity, timing of treatment, and duration of illness (Table 1). Data from the RECOVERY trial showed that the survival benefit was restricted to patients who received concomitant steroids rather than patients who did not: RR 0.80 (0.70−0.90) versus 1.16 (0.91−1.48) respectively. We concur with the recommendations by the expert panels of the Infectious Disease Society of America [20] and National Institute of Health COVID-19 Treatment Guidelines [21] that tocilizumab should be used in combination with dexamethasone in hospitalized adults with COVID-19 who: (a) within the prior 24–48 hours have required high-flow supplemental oxygen, non-invasive or mechanical ventilation, and (b) are receiving low-flow oxygen and have increasing oxygen requirements and elevated inflammatory markers despite dexamethasone.
Interestingly, we found a significantly lower risk of secondary infections in the tocilizumab group as compared to the treatment group. This finding goes against the notion that immunomodulators are generally associated with higher infection rates. A possible explanation for our finding is that tocilizumab may play a role in curbing immune exhaustion associated with sepsis in general and COVID-19 in particular. In one study of 499 COVID-19 patients [22], the numbers of total T cells, CD4+ and CD8+ T cells were significantly reduced, especially in patients requiring ICU care. In comparison to healthy controls, COVID-19 patients showed markedly higher percentages of PD-1/CD8+ and CD4+ T cells, a sign of T-cell exhaustion in COVID-19 patients. These findings were more pronounced in those requiring ICU care and suggest that the decrease in T cells seen in COVID-19 patients may be the result of high serum concentration of tumour necrosis factor α (TNF-α), IL-6, and IL-10 which negatively regulate T-cell survival or proliferation. On the other hand, patients with sepsis in general experience a high rate of secondary infections due to impairments in the adaptive immune response and immune exhaustion (reviewed by Brady et al.) [23]. Another possible explanation for the lower infection rate in the tocilizumab group is the lower risk of clinical deterioration and need for mechanical ventilation with tocilizumab use. ICU stay and mechanical ventilation are associated with high risk of nosocomial infections.
Conclusion
Cumulative high-certainty evidence shows that tocilizumab reduces the risk of mechanical ventilation in hospitalized patients, while moderate-certainty evidence shows that tocilizumab reduces the risk of poor outcome and the risk of secondary infections. This review will continuously evaluate the role of tocilizumab in COVID-19 treatment. It is crucial that all RCT teams urgently contribute their trials data for a meta-analysis that incorporates subgroup analyses, and that they explore any heterogeneous treatment effects of tocilizumab.
Author contributions
IT and ZK contributed equally to this work. IT: conception and design of the study. IT, ZK, LH, VV and TK: acquisition of the data. IT, ZK and MR: analysis of the data. IT, ZK, LH, VV, MR and TK: interpretation of data. IT and ZK: drafting of the article. All authors: critical revision of the article for important intellectual content and final approval of the version to be submitted.
Transparency declaration
The authors declare no conflicts of interest. No funding was received for this work.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.04.019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Tleyjeh I.M., Kashour Z., Damlaj M., Riaz M., Tlayjeh H., Altannir M., et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:215–227. doi: 10.1016/j.cmi.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosas I., Bräu N., Waters M., Go R.C., Hunter B.D., Bhagani S., et al. Tocilizumab in hospitalized patients with COVID-19 pneumonia. medRxiv. 2020:20183442. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salama C., Han J., Yau L., Reiss W.G., Kramer B., Neidhart J.D., et al. Tocilizumab in patients hospitalized with covid-19 pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/NEJMoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone J.H., Frigault M.J., Serling-Boyd N.J., Fernandes A.D., Harvey L., Foulkes A.S., et al. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med. 2020;383:2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermine O., Mariette X., Tharaux P., Resche-Rigon M., Porcher R., Ravaud P. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horby P.W., Pessoa-Amorim G., Peto L., Brightling C.E., Sarkar R., Thomas K., et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv. 2021:2021. 02.11.21249258. [Google Scholar]
- 7.Veiga V.C., Prats, João A.G.G., Farias D.L.C., Rosa R.G., Dourado L.K., et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M., et al. Interleukin-6 receptor antagonists in critically ill patients with covid-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salvarani C., Dolci G., Massari M., Merlo D.F., Cavuto S., Savoldi L., et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. doi: 10.1001/jamainternmed.2020.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canziani L.M., Trovati S., Brunetta E., Testa A., De Santis M., Bombardieri E., et al. Interleukin-6 receptor blocking with intravenous tocilizumab in COVID-19 severe acute respiratory distress syndrome: a retrospective case-control survival analysis of 128 patients. J Autoimmun. 2020;114:102511. doi: 10.1016/j.jaut.2020.102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Rossi N., Scarpazza C., Filippini C., Cordioli C., Rasia S., Mancinelli C.R., et al. Early use of low dose tocilizumab in patients with COVID-19: a retrospective cohort study with a complete follow-up. EClinicalMedicine. 2020;25:100459. doi: 10.1016/j.eclinm.2020.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher M.J., Marcos Raymundo L.A., Monteforte M., Taub E.M., Go R. Tocilizumab in the treatment of critical COVID-19 pneumonia: a retrospective cohort study of mechanically ventilated patients. Int J Infect Dis. 2020;103:536–539. doi: 10.1016/j.ijid.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guisado-Vasco P., Valderas-Ortega S., Carralon-Gonzalez M.M., Roda-Santacruz A., Gonzalez-Cortijo L., Sotres-Fernandez G., et al. Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: a retrospective observational study (COQUIMA cohort) EClinicalMedicine. 2020;28:100591. doi: 10.1016/j.eclinm.2020.100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis T.C., Adhikari S., Tatapudi V., Holub M., Kunichoff D., Troxel A.B., et al. A propensity-matched cohort study of tocilizumab in patients with coronavirus disease 2019. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Molinero A., Perez-Lopez C., Galvez-Barron C., Minarro A., Macho O., Lopez G.F., et al. Matched cohort study on the efficacy of tocilizumab in patients with COVID-19. One Health. 2021;12:100214. doi: 10.1016/j.onehlt.2021.100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Baño J., Pachón J., Carratalà J., Ryan P., Jarrín I., Yllescas M., et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clin Microbiol Infect. 2021;27:244–252. doi: 10.1016/j.cmi.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menzella F., Fontana M., Salvarani C., Massari M., Ruggiero P., Scelfo C., et al. Efficacy of tocilizumab in patients with COVID-19 ARDS undergoing noninvasive ventilation. Crit Care. 2020;24:589. doi: 10.1186/s13054-020-03306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruiz-Antorán B., Sancho-López A., Torres F., Moreno-Torres V., de Pablo-López I., García-López P., et al. Combination of tocilizumab and steroids to improve mortality in patients with severe COVID-19 infection: a Spanish, multicenter, cohort study. Infect Dis Ther. 2021;10:347–362. doi: 10.1007/s40121-020-00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian J., Zhang M., Jin M., Zhang F., Chu Q., Wang X., et al. Repurposed tocilizumab in patients with severe COVID-19. J Immunol. 2021;206:599–606. doi: 10.4049/jimmunol.2000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C., et al. Infectious Diseases Society of America; 2021. Infectious diseases society of America Guidelines on the treatment and management of patients with COVID-19.https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ Version 4.2.0. Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2021. Coronavirus disease 2019 (COVID-19) treatment Guidelines.https://www.covid19treatmentguidelines.nih.gov/ Available at: [PubMed] [Google Scholar]
- 22.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady J., Horie S., Laffey J.G. Role of the adaptive immune response in sepsis. Intensive Care Med Exp. 2020;8:20. doi: 10.1186/s40635-020-00309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.