Abstract
Objectives
Studies on coronavirus disease 2019 (COVID-19) have mainly focused on hospitalized patients or those with severe disease. We aim to assess the clinical characteristics, outcomes and factors associated with hospital admission or death in adult outpatients with COVID-19.
Methods
This is a prospective cohort of outpatients with suspected or confirmed COVID-19, registered in the Covidom telesurveillance solution for home monitoring of patients with COVID-19 in the Greater Paris area, from March to August 2020. The primary outcome was clinical worsening, defined as hospitalization or death within 1 month after symptom onset.
Results
Among 43 103 patients, mean age was 42.9 years (SD 14.3 years); 93.0% (n = 40 081) of patients were <65 years old and 61.9% (n = 26 688) were women. Of these 43 103 patients, 67.5% (n = 29 104) completed a medical questionnaire on co-morbidities and symptoms. The main reported co-morbidities were asthma (12.8%; n = 3685), hypertension (12.3%; n = 3546) and diabetes (4.8%; n = 1385). A small proportion of all eligible patients (4.1%, 95% CI 3.9–4.2; 1751/43 103) experienced clinical worsening. The rate of hospitalization was 4.0% (95% CI 3.8%–4.2%; n = 1728) and 0.1% (95% CI 0.1%–0.2%; n = 64) died. Factors associated with clinical worsening were male sex, older age, obesity and co-morbidities such as chronic renal disease or cancer under treatment. Probability of worsening was reduced with anosmia/ageusia.
Conclusions
Clinical worsening was rare among outpatients. Male sex, older age and co-morbidities such as chronic renal disease, active cancers or obesity were independently associated with clinical worsening. However, our cohort may include patients younger and healthier than the general population.
Keywords: Community, Coronavirus disease 2019, Death, Hospitalization, Outpatients, Risk factors
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection spread rapidly from a cluster of cases in China to become a pandemic with more than 130 million cases and almost 3 million deaths worldwide [1,2].
Clinical characteristics of coronavirus disease 2019 (COVID-19) as well as factors associated with increased risk of poor outcome have been described [[3], [4], [5], [6], [7], [8]]. Age, sex, hypertension, diabetes, cardiovascular disease, chronic respiratory disease, cancer and other chronic co-morbidities such as obesity are associated with clinical worsening, treatment escalation and death [[3], [4], [5],[7], [8], [9]]. These evaluations mainly focused on hospitalized patients [7,8]. However, more than 80% of patients initially present a mild form of the disease, some even being asymptomatic [[10], [11], [12], [13]]. It was initially estimated that 10%–15% of these patients would experience a more severe disease [14,15], but we currently lack precise estimates of the rate of clinical worsening in this population. Patients at risk of worsening must be quickly identified to adapt surveillance and propose prompt clinical management when the first signs of worsening occur. Only a few studies have investigated the clinical features of outpatients with SARS-CoV-2 infection or the factors associated with hospital admission or death among these patients [[16], [17], [18]].
This study aimed to evaluate the rate of clinical worsening, defined as hospitalization or death, in adult COVID-19 outpatients managed with the Covidom telesurveillance solution, and to evaluate factors associated with clinical worsening.
Materials and methods
Study design and setting
This study is based on the Covidom cohort [19], an ongoing prospective cohort of patients in the Greater Paris area using the Covidom telesurveillance solution. This is a Web application for home monitoring of patients with COVID-19 as part of initial outpatient management or at hospital discharge after a COVID-19-related hospitalization. Patients are registered in Covidom by a physician, at the end of a medical encounter for COVID-19-related symptoms, after supplying brief information and obtaining oral consent. After completing registration online, patients answer a medical questionnaire on co-morbidities and symptoms, and they receive daily monitoring questionnaires for 30 days after symptom onset. The questionnaire answers can trigger alerts, managed in a single regional control centre that can conduct a remote medical assessment, address the patient to a hospital or send mobile emergency services to the patient's home if necessary [19]. Patients were informed of the potential use of their anonymized data for research purposes. This study was approved by the Scientific and Ethical Committee of AP-HP (IRB00011591).
Participants
We included all adults aged ≥18 years with suspected or proven COVID-19 as evaluated by a physician, who completed registration, who were registered as outpatients, and who had a date of COVID-19 symptom onset earlier than 11 August 2020. We excluded patients included in Covidom at hospital discharge.
Data
We collected patient characteristics recorded by the including physicians: means of inclusion (general practitioners, hospital, emergency medical services medical dispatcher), age, sex, date of first symptoms, postal code and risk profile (low or high risk). Patients were considered at high risk if they had cardiovascular disease, diabetes, chronic lung disease, immunodeficiency, were in the third trimester of pregnancy or were over 65 years old. The remaining characteristics were recorded in the self-reported medical questionnaire, generally completed at inclusion:
-
-
weight and height, from which we calculated body mass index
-
-
co-morbidities
-
-
current tobacco use
-
-
symptoms
-
-
diagnosis: whether the infection has been confirmed by a molecular test (RT-PCR).
The diagnosis information was collected from the including physicians and self-reported medical questionnaires, and was cross-checked with the biological databases of the AP-HP hospital network. AP-HP is a network of 39 university hospitals in the Greater Paris area covering a large part of this area's population (12 million inhabitants). We considered that a patient was positive if a positive test was self-reported or available in the biological databases during the 30-day follow up.
By using data from the French Institute for Statistics and Economic Studies (INSEE) overlaid with the patient's area of residence, we also collected the local median income as a proxy of the patient's socio-economic status [20].
Outcomes
Our primary outcome was clinical worsening, defined as hospitalization or death within 1 month after symptom onset. We used three complementary approaches to evaluate this outcome: (a) patient responses to follow-up questionnaires sent 15 and 30 days after symptom onset that asked patients whether they had been hospitalized during follow up; (b) responses reported by the regional control centre to the different types of alerts and the end of follow-up reasons in case of premature ending (the regional control centre called back all patients who did not answer the daily questionnaires or their relatives to check their status); and (c) data on patients hospitalized from the AP-HP warehouse (Entrepôt de données de santé de l’AP-HP). We evaluated hospitalization and death within 1 month after symptom onset, separately, as secondary outcomes.
Statistical analyses
We describe patient characteristics with frequencies (percentages) for categorical variables and mean (standard deviation) or median (quartile 1–quartile 3) for continuous variables. We used hierarchical clustering to identify clusters of symptoms based on the Jaccard index. We described the characteristics of all eligible patients, those with a completed medical questionnaire (overall and by PCR status: positive, negative, untested). We evaluated the primary outcome in these populations. Then, we used univariable logistic regression models followed by a multivariable logistic regression model including all relevant variables based on clinical likelihood and literature to evaluate factors associated with clinical worsening among patients with a completed medical questionnaire and a positive PCR test. Two sets of highly correlated symptoms (anosmia and ageusia, and fatigue, shivers and myalgia) were regrouped to avoid collinearity. We conducted three sensitivity analyses for the multivariable model to evaluate the consistency of results: (a) analysis based on all patients with a medical questionnaire regardless of the RT-PCR result, (b) analysis based on all eligible patients, and (c) analysis with inverse probability weighting to adjust for patients not answering the medical questionnaire. The propensity score is defined as the probability of answering given characteristics recorded at registration. Alpha risk was set at 5% for all analyses.
Results
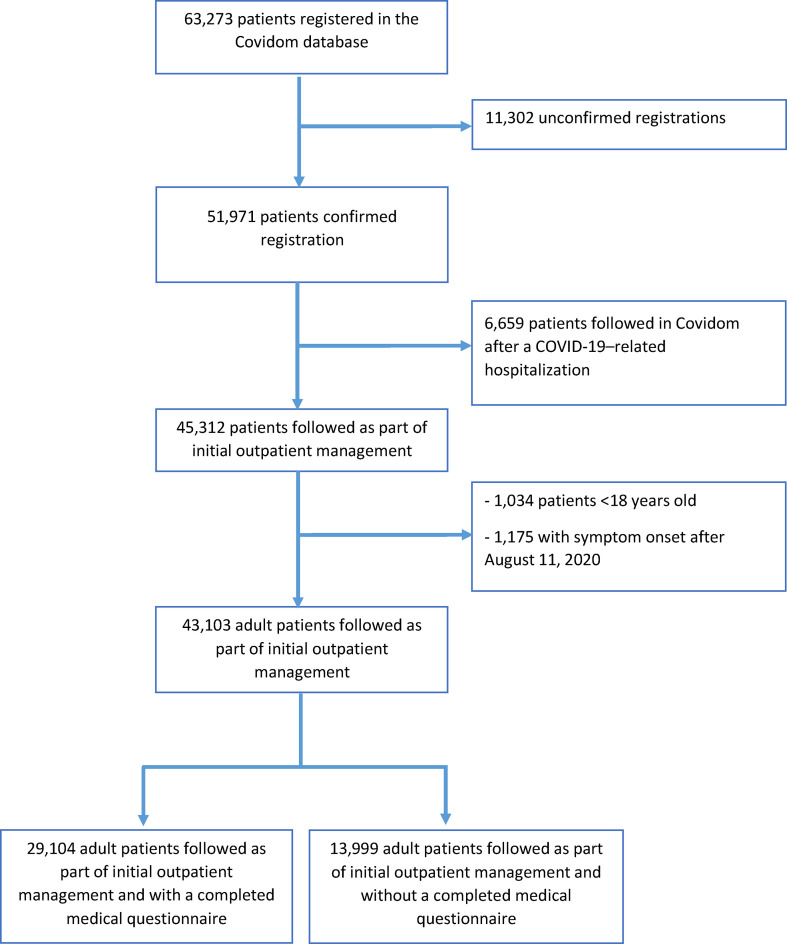
From 9 March 2020 to 11 August 2020, 63 273 patients with suspected or confirmed COVID-19 were registered in Covidom by more than 3800 physicians. Of these, 51 971 (82.1%) had confirmed registration and 43 103 (68.1%) met our inclusion criteria (Fig. 1 ). A total of 29 104 patients (67.5%) completed the medical questionnaire on co-morbidities and symptoms. Regarding follow up, 71% of patients (n = 20 647) had a follow up of at least 29 days. Among patients with a shorter follow up, 167 were hospitalized or deceased (median follow up 14 days, Q1–Q3 9–20), 3296 patients (11.3%) chose to interrupt follow up early (20 days, Q1–Q3 16–24) and 4988 (17.1%) stopped responding without formally ending follow up (23 days, Q1–Q3 18–26).
Fig. 1.
Flow chart of patients registered in Covidom from 9 March to 11 August 2020 and included in the study.
Patient general characteristics
Mean age was 42.9 years (SD 14.3), with 93.0% of patients <65 years old (n = 40 081), and 61.9% were women (n = 26 688). The median time from symptom onset to registration was 4 days (Q1–Q3 2–8 days). General characteristics of patients having completed the medical questionnaire did not appear different (Table 1 ). Among those patients, median body mass index was 24.8 kg/m2 (Q1–Q3 22.1–28.4), with 30.2% (n = 8568) being overweight and 18.3% (n = 5195) obese. Current tobacco use was reported by 5103 (17.7%) patients. Main co-morbidities were asthma, hypertension and diabetes: reported by 12.8% (n = 3685), 12.3% (n = 3546) and 4.8% (n = 1385) of patients, respectively.
Table 1.
Demographic characteristics, co-morbidities and symptoms of all eligible patients and in the cohort of patients having filled the medical questionnaire by type of PCR results (positive, negative, untested)
| All eligible patients (n = 43 103) | Eligible patients with a completed medical questionnaire (n = 29 104) | Medical questionnaire and positive PCR (n = 7320) | Medical questionnaire and negative PCR (n = 5281) | Medical questionnaire and no PCR result (n = 16 503) |
|
|---|---|---|---|---|---|
| General characteristics | |||||
| Age, mean (SD) | 42.9 ± 14.3 | 43.0 ± 14.0 | 43.0 ± 13.9 | 43.1 ± 14.3 | 42.9 ± 13.9 |
| Women | 26 668 (61.9%) | 18 329 (63.0%) | 5006 (68.5%) | 3440 (65.2%) | 9883 (59.9%) |
| Male | 16 385 (38.1%) | 10 743 (37.0%) | 2301 (31.5%) | 1835 (34.8%) | 6607 (40.1%) |
| Time to registration in Covidom | |||||
| After symptom onset, days, median (Q1–Q3) | 4.0 (2.0–8.0) | 4.0 (2.0–7.0) | 5.0 (3.0–8.0) | 4.0 (2.0–7.0) | 4.0 (2.0–7.0) |
| After physician referral, days, median (Q1–Q3) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–1.0) | 0.0 (0.0–0.0) |
| High-risk profile | 17 160 (39.8%) | 11 521 (39.6%) | 2710 (37.0%) | 2406 (45.6%) | 6405 (38.8%) |
| Mode of inclusion | |||||
| GP | 23 087 (53.6%) | 16 115 (55.4%) | 1781 (24.3%) | 2921 (55.3%) | 11 413 (69.2%) |
| Hospital | 11 977 (27.8%) | 8053 (27.7%) | 4475 (61.1%) | 1763 (33.4%) | 1815 (11.0%) |
| EMS | 8039 (18.7%) | 4936 (17.0%) | 1064 (14.5%) | 597 (11.3%) | 3275 (19.8%) |
| Socio-economic indicators | |||||
| District median income in euros, median (Q1–Q3) | 24 110.0 (20 320.0–27 990.0) | 24 110.0 (20 320.0–27 990.0) | 23 160.0 (19 720.0–27 700.0) | 24 110.0 (20 320.0–28 180.0) | 24 110.0 (20 320.0–28 180.0) |
| Lowest income districts (Q1) | 11 718 (27.5%) | 7759 (27.0%) | 2286 (31.7%) | 1357 (26.0%) | 4116 (25.3%) |
| Median income districts (Q2-Q3) | 20 313 (47.8%) | 13 862 (48.3%) | 3317 (46.0%) | 2535 (48.6%) | 8010 (49.2%) |
| Highest income districts (Q4) | 10 505 (24.7%) | 7103 (24.7%) | 1610 (22.3%) | 1322 (25.4%) | 4171 (25.6%) |
| Risk factors | |||||
| 18 ≤ Age ≤ 45 years | 25 455 (59.1%) | 17 049 (58.6%) | 4160 (56.9%) | 3096 (58.6%) | 9793 (59.4%) |
| 45 < Age ≤ 65 years | 14 626 (33.9%) | 10 204 (35.1%) | 2774 (37.9%) | 1806 (34.2%) | 5624 (34.1%) |
| Age > 65 years | 3016 (7.0%) | 1845 (6.3%) | 383 (5.2%) | 379 (7.2%) | 1083 (6.6%) |
| BMI (kg/m2), median (Q1–Q3) | 24.8 (22.1-28.4) | 25.3 (22.4-29.1) | 24.7 (21.9-28.4) | 24.7 (22.0-28.2) | |
| Healthy weight (BMI ≤25 kg/m2) | 14 621 (51.5%) | 3424 (47.9%) | 2700 (53.0%) | 8497 (52.6%) | |
| Overweight (BMI 25–30 kg/m2) | 8568 (30.2%) | 2185 (30.6%) | 1451 (28.5%) | 4932 (30.5%) | |
| Obesity (BMI >30 kg/m2) | 5195 (18.3%) | 1532 (21.5%) | 942 (18.5%) | 2721 (16.8%) | |
| Current tobacco use | 5103 (17.7%) | 790 (10.9%) | 1163 (22.5%) | 3150 (19.2%) | |
| Main co-morbidities | |||||
| Asthma | 3685 (12.8%) | 814 (11.2%) | 824 (16.0%) | 2047 (12.5%) | |
| Hypertension | 3546 (12.3%) | 978 (13.5%) | 706 (13.7%) | 1862 (11.4%) | |
| Diabetes | 1385 (4.8%) | 402 (5.6%) | 286 (5.5%) | 697 (4.2%) | |
| Heart failure | 557 (1.9%) | 118 (1.6%) | 138 (2.7%) | 301 (1.8%) | |
| Chronic obstructive pulmonary disease | 517 (1.8%) | 87 (1.2%) | 147 (2.8%) | 283 (1.7%) | |
| Coronary artery disease | 399 (1.4%) | 77 (1.1%) | 97 (1.9%) | 225 (1.4%) | |
| Cancer under treatment | 322 (1.1%) | 92 (1.3%) | 106 (2.1%) | 124 (0.8%) | |
| Chronic renal disease | 312 (1.1%) | 66 (0.9%) | 69 (1.3%) | 177 (1.1%) | |
| Multiple co-morbidities (>1) | 1881 (6.5%) | 465 (6.4%) | 459 (8.9%) | 957 (5.8%) | |
| None of the reported co-morbidities | 20 468 (71.1%) | 5180 (71.6%) | 3378 (65.5%) | 11 910 (72.6%) | |
| Symptoms | |||||
| General symptoms | |||||
| Fatigue | 25 014 (85.9%) | 6592 (90.1%) | 4330 (82.0%) | 14 092 (85.4%) | |
| Temperature ≥38.5°C | 14 124 (48.5%) | 4130 (56.4%) | 2160 (40.9%) | 7834 (47.5%) | |
| Shivers | 15 706 (54.0%) | 4162 (56.9%) | 2570 (48.7%) | 8974 (54.4%) | |
| Myalgia | 15 721 (54.0%) | 4443 (60.7%) | 2574 (48.7%) | 8704 (52.7%) | |
| Fatigue, shivers, or myalgia | 26 258 (90.2%) | 6819 (93.2%) | 4582 (86.8%) | 14 857 (90.0%) | |
| Respiratory symptoms | |||||
| Cough | 18 014 (61.9%) | 4910 (67.1%) | 2816 (53.3%) | 10 288 (62.3%) | |
| Shortness of breath | 14 358 (49.3%) | 3470 (47.4%) | 2606 (49.3%) | 8282 (50.2%) | |
| Chest pain | 7643 (26.4%) | 1587 (21.8%) | 1433 (27.5%) | 4623 (28.1%) | |
| Chest oppression | 7913 (27.2%) | 1713 (23.4%) | 1479 (28.0%) | 4721 (28.6%) | |
| Gastrointestinal symptoms | |||||
| Anorexia | 11 216 (38.5%) | 3528 (48.2%) | 1616 (30.6%) | 6072 (36.8%) | |
| Nausea/vomiting | 6478 (22.3%) | 1771 (24.2%) | 1292 (24.5%) | 3415 (20.7%) | |
| Diarrhoea | 10 483 (36.0%) | 2742 (37.5%) | 1848 (35.0%) | 5893 (35.7%) | |
| Neurological symptoms | |||||
| Anosmia | 9109 (31.3%) | 4039 (55.2%) | 644 (12.2%) | 4426 (26.8%) | |
| Ageusia | 9170 (31.5%) | 3859 (52.7%) | 760 (14.4%) | 4551 (27.6%) | |
| Cutaneous symptoms | |||||
| Rash | 2851 (9.8%) | 721 (9.8%) | 499 (9.4%) | 1631 (9.9%) | |
| Chilblains | 580 (2.0%) | 128 (1.8%) | 111 (2.1%) | 341 (2.1%) | |
| Conjunctivitis | 2222 (7.6%) | 530 (7.2%) | 408 (7.7%) | 1284 (7.8%) | |
| Diagnosis confirmation | |||||
| PCR Untested | 14 983 (54.3%) | 0 (0.0%) | 0 (0.0%) | 14 983 (100.0%) | |
| PCR Negative | 5281 (19.1%) | 0 (0.0%) | 5281 (100.0%) | 0 (0.0%) | |
| PCR Positive | 7320 (26.5%) | 7320 (100.0%) | 0 (0.0%) | 0 (0.0%) | |
Abbreviations: BMI, body mass index; EMS, emergency medical service; GP, general practitioner.
Values are number (percentage) unless stated otherwise.
The most common symptoms were fatigue (n = 25 014; 85.9%), cough (n = 18 014; 61.9%), shivers (n = 15 706; 54.0%), myalgia (n = 15 721; 54.0%), shortness of breath (n = 14 358; 49.3%) and fever (n = 14 124; 48.5%). Almost one-third of patients reported anosmia (n = 9109; 31.3%) or ageusia (n = 9170; 31.5%). Clusters of symptoms are reported in the Supplementary material (Fig. S1), showing a cluster with anosmia and ageusia symptoms, one with chest pain and chest oppression and a larger cluster with general symptoms such as fever, fatigue, shivers and myalgia associated with cough. In total, 45.7% (n = 12 601) patients had available RT-PCR results, 58.1% (n = 7320) being positive. Characteristics stratified by RT-PCR status are reported in Table 1. Age, sex ratio, co-morbidities and symptoms did not appear different from the complete cohort, whereas RT-PCR-positive patients appeared to be included in hospitals more often, and reported anosmia or ageusia more frequently (Table 1).
Clinical worsening
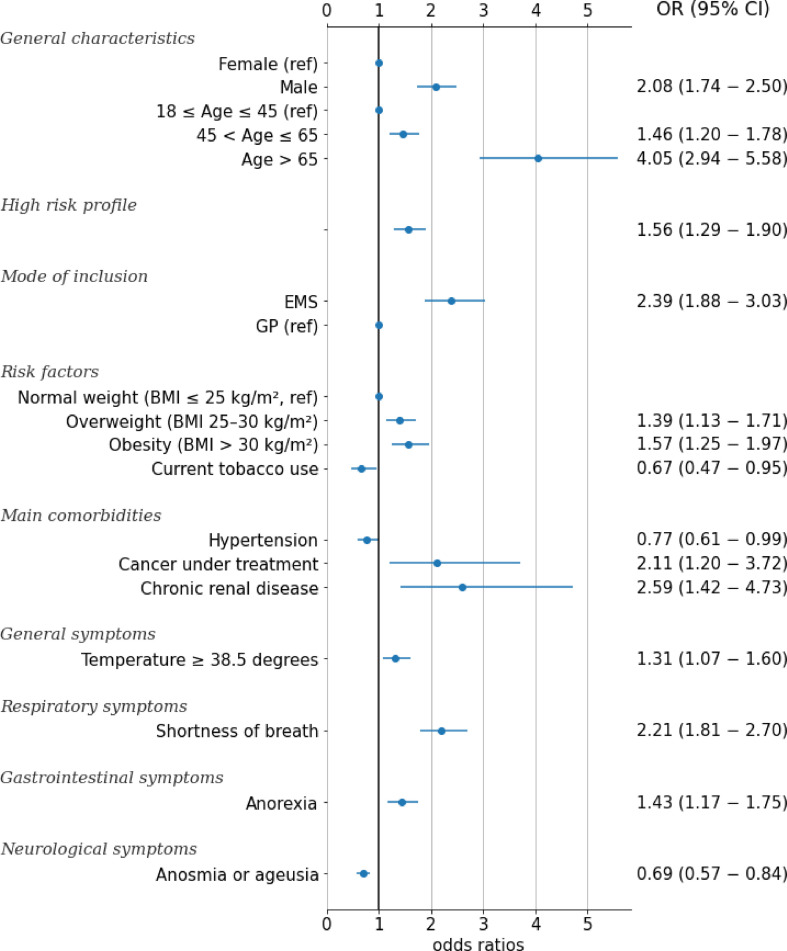
A small proportion of all eligible patients (4.1%; 95% CI 3.9%–4.2%; 1751/43 103) experienced clinical worsening. At 1 month after symptom onset, 4.0% (95% CI 3.8%–4.2%; n = 1728) required hospitalization and 0.1% died (95% CI 0.1%–0.2%; n = 64). Among the patients with a positive RT-PCR, 9.0% (95% CI 8.3%–9.7%; 659/7320) experienced clinical worsening, 9.0% (95% CI 8.3%–9.7%; n = 659) required hospitalization and 0.04% died (95% CI 0.01%–0.09%; n = 3) (Table 2 ). Patient characteristics by clinical outcome and by RT-PCR status are reported in the Supplementary material (Table S1). Independent factors associated with clinical worsening are reported in Fig. 2 and Table 3 . Both age >65 years and obesity were independent predictors of worsening (OR 4.05, 95% CI 2.94–5.58 and OR 1.57, 95% CI 1.25–1.97, respectively). Male sex was also associated with worsening (OR 2.08, 95% CI 1.74–2.50), as was chronic renal disease (OR 2.59, 95% CI 1.42–4.73) and cancer under treatment (OR 2.11, 95% CI 1.20–3.72). Temperature, shortness of breath and anorexia were associated with worsening, but patients appeared less prone to worsening if they presented anosmia or ageusia (OR 0.69, 95% CI 0.57–0.84). Current tobacco use was associated with a lower risk of worsening (OR 0.68, 95% CI 0.48–0.96).
Table 2.
Patient outcomes in all eligible patients and in the cohort of patients having filled the medical questionnaire by type of PCR results (positive, negative, untested)
| All eligible patients (n = 43 103) | Eligible patients with a completed medical questionnaire (n = 29 104) | Medical questionnaire and positive PCR (n = 7320) | Medical questionnaire and negative PCR (n = 5281) | Medical questionnaire and no PCR result (n = 16 503) | |
|---|---|---|---|---|---|
| Clinical worsening | 1751 (4.1%; 95% CI 3.9−4.2) | 1124 (3.9%; 95% CI 3.6−4.1) | 659 (9.0%; 95% CI 8.3−9.7) | 239 (4.5%; 95% CI 4.0−5.1) | 226 (1.4%; 95% CI 1.2−1.5) |
| Patient outcomea | |||||
| Hospitalized | 1728 (4.0%; 95% CI 3.8−4.2) | 1121 (3.9%; 95% CI 3.6−4.1) | 659 (9.0%; 95% CI 8.3−9.7) | 239 (4.5%; 95% CI 4.0−5.1) | 223 (1.4%; 95% CI 1.2−1.5) |
| Deceased | 64 (0.1%; 95% CI 0.1−0.2) | 6 (0.02%; 95% CI 0.00−0.04) | 3 (0.04%; 95% CI 0.01−0.09) | 0 (0.00%; 95% CI 0.00−0.00) | 3 (0.02%; 95% CI 0.00−0.04) |
The sum of the categories can exceed the total number of patients having experienced a clinical worsening as some were included in more than one category.
Fig. 2.
Independent factors associated with clinical worsening from a multivariate logistic regression model for the cohort of Covidom patients with a positive RT-PCR (n = 7320).
Table 3.
Univariable and multivariable analyses of factors associated with clinical worsening for the cohort of Covidom patients with a positive RT-PCR (n = 7320)
| Univariable OR (95% CI) | Multivariable OR (95% CI) | |
|---|---|---|
| General characteristics | ||
| Female (ref) | 0.43 (0.37−0.51) | 1 (1−1) |
| Male | 2.32 (1.97−2.72) | 2.08 (1.74−2.50) |
| High risk profile | 2.55 (2.17−3.00) | 1.56 (1.29−1.90) |
| Mode of inclusion | ||
| EMS | 0.99 (0.82−1.19) | 2.39 (1.88−3.03) |
| GP (ref) | 0.46 (0.40−0.55) | 1 (1−1) |
| Hospital | 3.18 (2.66−3.80) | 0.98 (0.79−1.22) |
| Socio-economic indicators | ||
| Lowest income districts (Q1) | 1.25 (1.06−1.48) | 1.16 (0.95−1.42) |
| Median income districts (Q2–Q3, ref) | 0.79 (0.67−0.93) | 1 (1−1) |
| Highest income districts (Q4) | 1.05 (0.87−1.27) | 1.07 (0.85−1.34) |
| Risk factors | ||
| 18 ≤ Age ≤ 45 years (ref) | 0.42 (0.35−0.49) | 1 (1−1) |
| 45 < Age ≤ 65 years | 1.48 (1.26−1.73) | 1.46 (1.20−1.78) |
| Age > 65 years | 4.42 (3.48−5.63) | 4.05 (2.94−5.58) |
| Normal weight (BMI ≤25 kg/m2, ref) | 0.51 (0.43−0.61) | 1 (1−1) |
| Overweight (BMI 25–30 kg/m2) | 1.45 (1.22−1.71) | 1.39 (1.13−1.71) |
| Obesity (BMI >30 kg/m2) | 1.53 (1.28−1.83) | 1.57 (1.25−1.97) |
| Current tobacco use | 0.48 (0.34−0.67) | 0.67 (0.47−0.95) |
| Main co-morbidities | ||
| Asthma | 1.32 (1.04−1.67) | 0.96 (0.74−1.24) |
| Hypertension | 1.76 (1.44−2.16) | 0.77 (0.61−0.99) |
| Diabetes | 2.49 (1.91−3.25) | 1.20 (0.88−1.64) |
| Heart failure | 2.10 (1.29−3.42) | 0.73 (0.41−1.30) |
| Chronic obstructive pulmonary disease | 1.47 (0.78−2.79) | 0.64 (0.32−1.28) |
| Coronary artery disease | 2.08 (1.14−3.79) | 1.18 (0.61−2.30) |
| Cancer under treatment | 2.87 (1.74−4.74) | 2.11 (1.20−3.72) |
| Chronic renal disease | 4.51 (2.65−7.67) | 2.59 (1.42−4.73) |
| Symptoms | ||
| General | ||
| Temperature ≥38.5°C | 1.97 (1.66−2.35) | 1.31 (1.07−1.60) |
| Fatigue, shivers, or myalgia | 1.22 (0.87−1.72) | 0.69 (0.47−1.02) |
| Respiratory | ||
| Cough | 1.55 (1.29−1.86) | 1.05 (0.85−1.29) |
| Shortness of breath | 2.35 (1.99−2.79) | 2.21 (1.81−2.70) |
| Chest pain | 1.50 (1.25−1.79) | 1.19 (0.96−1.46) |
| Chest oppression | 1.26 (1.05−1.51) | 1.03 (0.83−1.28) |
| Gastrointestinal | ||
| Anorexia | 1.68 (1.43−1.98) | 1.43 (1.17−1.75) |
| Nausea/vomiting | 1.41 (1.18−1.68) | 1.18 (0.96−1.46) |
| Diarrhoea | 1.57 (1.33−1.84) | 1.15 (0.95−1.39) |
| Neurological symptoms | ||
| Anosmia or ageusia | 0.63 (0.54−0.74) | 0.69 (0.57−0.84) |
| Cutaneous symptoms | ||
| Rash | 1.04 (0.80−1.36) | 1.05 (0.78−1.40) |
| Chilblains | 1.35 (0.78−2.32) | 1.02 (0.55−1.86) |
| Conjunctivitis | 0.88 (0.64−1.22) | 0.81 (0.58−1.15) |
Abbreviations: BMI, body mass index; EMS, emergency medical service; GP, general practitioner; OR, odds ratio.
The sensitivity analyses were consistent for factors associated with clinical worsening (see Supplementary material, Table S2 and Fig. S2).
Discussion
In this study, we describe the characteristics, outcomes and factors associated with disease worsening in a large population of adult outpatients with suspected or confirmed COVID-19 with mild symptoms, and followed by the Covidom telesurveillance programme. Only a small proportion of these patients experienced hospitalization or death, and the mortality rate was 0.1% (95% CI 0.1%–0.2%). Male sex, older age and co-morbidities such as chronic renal disease, active cancer or obesity were independently associated with clinical worsening.
Covidom represents the largest telesurveillance programme deployed in the context of COVID-19 and is a unique source of epidemiological data on outpatients with COVID-19, who represent most cases but are the least studied. Most of the literature focused on hospitalized patients or those with severe COVID-19 and reported a higher rate of clinical worsening with 5%–36.1% of patients needing admission to intensive care units, and an overall mortality ranging from 2.3% to 26.2% [4,7,8,14,21]. In a study describing outpatients, 6% of patients needed hospital referral after remote assessment by an emergency physician but they did not distinguish between patients attending the hospital for a consultation and those who were hospitalized [16]. The Chinese Centre for Disease Control and Prevention initially estimated that 10%–15% of patients with mild disease will worsen, with a final case-fatality rate of 2.3% [14]. However, most of these patients were probably hospitalized because immediate admission of all potential COVID-19 patients was recommended to control the pandemic in mainland China [22]. Therefore, our cohort provides a unique insight into the evolution of outpatients with mild COVID-19 symptoms.
Factors independently associated with clinical worsening were comparable to those identified in hospitalized patients [[7], [8], [9]]. Age is a well-known risk factor that could be explained by the possibly stronger host innate responses to virus infection than in younger adults or by age-dependent defects in T- and B-cell function [23]. Obesity seems to worsen the effect of COVID-19; high body mass index was significantly correlated with young age in patients with COVID-19 requiring intensive care because of reduced respiratory function or susceptibility to trigger hyper-inflammation [3,9,24]. As others, we found a lower rate of worsening among patients who reported being current smokers [25,26], but further studies are needed to explore these results.
Our study has some limitations. Our population is not representative of all outpatients with COVID-19. Only those with initially mild symptoms and a smartphone, tablet or computer, at ease with these recent technologies and accepting the telesurveillance programme were included. Digital readiness of older adults has often been described as one of the causes of their lower engagement with electronic health or with mobile device-based monitoring. This could possibly explain that only 7.0% of the patients included in our study were over 65 years old. The low rate of clinical worsening should be understood in the context of this younger population. In addition, our data concern only patients included in the Greater Paris area, which is a high-density area and was a major epicentre during the outbreak. Our population is based on suspected or confirmed cases of COVID-19 following the definition of French public health authorities. Many patients were initially not tested in France because RT-PCR tests were mostly reserved for the most severe patients or those with co-morbidities. In the region of the study, 41 539 positive RT-PCR have been reported by public health authorities, whereas over the same time period 40 076 COVID-19 hospitalizations were reported. These numbers highlight the lack of RT-PCR test availability in France at this time [27]. In our study, among tested patients, 58.1% had positive results, which seems slightly lower than expected for RT-PCR false-negative rates, given that up to 33% of patients hospitalized with acute respiratory symptoms and typical radiological findings tested negative at least once on respiratory specimens, and RT-PCR false-negative rates could represent as much as 29%, depending on the assay used [28,29]. We cannot exclude that some of these patients did not have COVID-19. However, we believe that eliminating a possible SARS-CoV-2 infection in a population of patients presenting symptoms compatible with COVID-19 (i.e. with a high pre-test probability), in a region with a high infection incidence, and in a context of lack of PCR test availability, on the basis of a negative nasopharyngeal RT-PCR would possibly lead to underestimating the pandemic burden. We therefore believe that it is important to also consider the patients who were not tested in the first wave but who had suspected COVID-19 according to the assessing physician, to capture the overall picture of the disease and its evolution. Most data were self-reported by patients, with a potential risk of misclassification, recall bias or social desirability bias, but these data were previously shown to be reliable [30]. Not all patients completed the medical questionnaire because it was not initially available. Nevertheless, characteristics were not different and results were consistent in sensitivity analyses. The fact that the patients in our cohort have benefited from an initial medical evaluation could lead to a selection bias towards a healthier, better cared for, population. Finally, ethnicity was not recorded in Covidom, in accordance with French legislation.
In conclusion, the rate of clinical worsening in adult outpatients with COVID-19 was lower than expected, about 4%, with a mortality rate of 0.1%. Male sex, older age and co-morbidities such as chronic renal disease, active cancer and obesity were independently associated with worsening. As countries face a third wave of the COVID-19 pandemic, our results give a unique insight into the outcomes of patients with mild symptomatology.
Transparency declaration
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by the Programme Hospitalier de Recherche Clinique 2020 of the French Ministry of Health, by a research fund by APHP-Fondation de France. The Covidom platform received a funding from EIT Health specific Covid-19 fund.
Ethics approval
This study received the ethical approval of the ethics committee of APHP (IRB00011591).
Availability of data and materials
The data sets generated and analysed during the current study are not publicly available because of restrictions by the French data protection authority. However, all reasonable requests should be addressed to the corresponding author.
Authors' contributions
YY (guarantor), JL and DA were involved in the study conception, data extraction, data analysis, interpretation of results and drafting the manuscript. DiA, ED and JP were involved in the Covidom solution development, study conception, interpretation of results and critically revising the manuscript. BA was involved in the study conception, interpretation of results and critically revising the manuscript. MA was involved in the study conception, data extraction, data analysis and interpretation of results. LX was involved in the Covidom solution development, interpretation of results and critically revising the manuscript.
Acknowledgements
We thank A. Falzon, G. Fayolle, F. Laporte, Amélie Tortel and all the Nouveal-e Santé team for their help in the Web application and regional centre surveillance interface development. We also thank L Debastard, A. Grenier, J. Hody, T. Penn and the Paris region Union régionale des professionnels de santé (URPS) for their help in the development and spreading of the Covidom solution. We thank A. Banzet, J. Marchand-Arvier, N. Schmidt and P Villie from the AP-HP headquarters for their invaluable help. We would also like to thank @BioHospitalix.
Editor: J. Bielicki
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.04.010.
Appendix. AP-HP/Universities/Inserm COVID-19 research collaboration members
Writing committee: Youri Yordanov, Aurélien Dinh, Alexandre Bleibtreu, Arthur Mensch, François-Xavier Lescure, Erwan Debuc, Patrick Jourdain, Luc Jaulmes, Agnes Dechartres.
Data-sciences committee: Caroline Apra (AC), Charlotte Caucheteux (CC), Luc Jaulmes (JL), Alexandre Gramfort (GA), Jenny Mansour (MJ), Arthur Mensch (MA), Viannet Taquet (TV), Jill Jenn VIE (VL).
AC is affiliated with the Sorbonne Université, AP-HP, Hôpital Pitié Salpêtrière, Service de Neurochirurgie, Paris, France.
CC and GA are affiliated with Université Paris-Saclay, INRIA, CEA, Palaiseau, 91 120, France.
JL is affiliated with the Centre de pharmaco-épidémiologie de l’AP-HP (Cephépi).
MJ is affiliated with Ecole Polytechnique, Paris, France.
MA is affiliated with Ecole Normale Supérieure, PSL University, CNRS, Départment de Mathématiques et Applications, 75 005 Paris, France.
TV and VL are affiliated with, INRIA Lille-Nord Europe, 59 650 Villeneuve-d’Ascq, France.
Scientific committee: Amélie Aime-Eusebi, Caroline Apra, Alexandre Bleibtreu, Erwan Debuc, Agnes Dechartres, Laurène Deconinck, Aurélien Dinh, Patrick Jourdain, Christine Katlama, Josselin Lebel, François-Xavier Lescure, Youri Yordanov.
Covidom regional centre steering commitee: Yves Artigou, Amélie Banzet, Elodie Boucheron, Christiane Boudier, Edouard Buzenac, Marie-Claire Chapron, Dalhia Chekaoui, Laurent De Bastard, Erwan Debuc, Aurélien Dinh, Alexandre Grenier, Pierre-Etienne Haas, Julien Hody, Michèle Jarraya, Patrick Jourdain, Louis Lacaille, Aurélie Le Guern, Jeremy Leclert, Fanny Male, Jean-Christophe Mercier, Emmanuel Martin-Blondet, Apolinne Nassour, Oussama Ourahou, Thomas Penn, Ambre Ribardiere, Nicolas Robin, Camille Rouge, Nicolas Schmidt, Pascaline Villie.
Centre de pharmaco-épidémiologie de l’AP-HP (Cephépi): Sofia Zemouri, Nessima Yelles.
Covidom including physicians, supervising physicians and remote monitoring responders: the complete list is available in the Supplementary material (Appendix S1).
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lu H., Stratton C.W., Tang Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus update (Live): 33,342,965 cases and 1,002,985 deaths from COVID-19 virus pandemic - worldometer. https://www.worldometers.info/coronavirus/ n.d. Available at:
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020:369. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020:369. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tartof S.Y., Qian L., Hong V., Wei R., Nadjafi R.F., Fischer H. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann Intern Med. 2020 doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gandhi R.T., Lynch J.B., Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 11.Wang X., Fang J., Zhu Y., Chen L., Ding F., Zhou R. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect. 2020;26(8):1063–1068. doi: 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byambasuren O., Cardona M., Bell K., Clark J., McLaws M.-L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. Infect Dis (Except HIV/AIDS) 2020 doi: 10.1101/2020.05.10.20097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 15.Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19 n.d. Available at:
- 16.Lapostolle F., Schneider E., Vianu I., Dollet G., Roche B., Berdah J. Clinical features of 1487 COVID-19 patients with outpatient management in the Greater Paris: the COVID-call study. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clift A.K., Coupland C.A.C., Keogh R.H., Diaz-Ordaz K., Williamson E., Harrison E.M. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020:371. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yordanov Y., Dechartres A., Lescure X., Apra C., Villie Pa, Marchand-Arvier J. Covidom, a telesurveillance solution for home monitoring of patients with Covid-19. (Preprint) J Med Internet Res. 2020 doi: 10.2196/20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Répartition des ménages, leurs revenus et niveau de vie moyen et médian en 2016 − Revenu, niveau de vie et pauvreté en 2016 | Insee. https://www.insee.fr/fr/statistiques/3650234?sommaire=3650460 n.d. Available at:
- 21.Goletti O., Castoldi M., Bombardieri E. Keep or release: experience on management of COVID-19 during maximum emergency in Bergamo and impact on patient outcomes. Eur J Emerg Med. 2020;27:309. doi: 10.1097/MEJ.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W., Wang Y., Xiao K., Zhang H., Tian Y., Clifford S.P. Establishing and managing a temporary coronavirus disease 2019 specialty hospital in Wuhan, China. Anesthesiology. 2020 doi: 10.1097/ALN.0000000000003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opal S.M., Girard T.D., Ely E.W. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(Suppl 7):S504–S512. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 24.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395:1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Zyl-Smit R.N., Richards G., Leone F.T. Tobacco smoking and COVID-19 infection. Lancet Respir Med. 2020;8:664–665. doi: 10.1016/S2213-2600(20)30239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polverino F. Cigarette smoking and COVID-19: a complex interaction. Am J Respir Crit Care Med. 2020;202:471–472. doi: 10.1164/rccm.202005-1646LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pullano G., Di Domenico L., Sabbatini C.E., Valdano E., Turbelin C., Debin M. Underdetection of cases of COVID-19 in France threatens epidemic control. Nature. 2021;590:134–139. doi: 10.1038/s41586-020-03095-6. [DOI] [PubMed] [Google Scholar]
- 28.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection — challenges and implications. N Engl J Med. 2020;383:e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 29.Lascarrou J.-B., Colin G., Le Thuaut A., Serck N., Ohana M., Sauneuf B. Predictors of negative first SARS-CoV-2 RT-PCR despite final diagnosis of COVID-19 and association with outcome. Sci Rep. 2021;11:2388. doi: 10.1038/s41598-021-82192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourgeois F.T., Porter S.C., Valim C., Jackson T., Cook E.F., Mandl K.D. The value of patient self-report for disease surveillance. J Am Med Inform Assoc. 2007;14:765–771. doi: 10.1197/jamia.M2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and analysed during the current study are not publicly available because of restrictions by the French data protection authority. However, all reasonable requests should be addressed to the corresponding author.