Abstract
Background
Healthcare workers (HCWs) are at the front line of the ongoing coronavirus 2019 (COVID-19) pandemic. Comprehensive evaluation of the seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) among HCWs in a large healthcare system could help to identify the impact of epidemiological factors and the presence of symptoms on the immune response to the infection over time.
Aim
To determine the seroprevalence of SARS-CoV-2-specific antibodies among HCWs, identify associated epidemiological factors and study antibody kinetics.
Methods
A longitudinal evaluation of the seroprevalence and epidemiology of SARS-CoV-2-specific antibodies was undertaken in approximately 30,000 HCWs in the largest healthcare system in Connecticut, USA.
Findings
At baseline, the prevalence of SARS-CoV-2 antibody among 6863 HCWs was 6.3% [95% confidence interval (CI) 5.7–6.9%], and was highest among patient care support (16.7%), medical assistants (9.1%) and nurses (8.2%), and lower for physicians (3.8%) and advanced practice providers (4.5%). Seroprevalence was significantly higher among African Americans [odds ratio (OR) 3.26 compared with Caucasians, 95% CI 1.77–5.99], in participants with at least one symptom of COVID-19 (OR 3.00, 95% CI 1.92–4.68), and in those reporting prior quarantine (OR 3.83, 95% CI 2.57–5.70). No symptoms were reported in 24% of seropositive participants. Among the 47% of participants who returned for a follow-up serological test, the seroreversion rate was 39.5% and the seroconversion rate was 2.2%. The incidence of re-infection in the seropositive group was zero.
Conclusion
Although there is a decline in the immunoglobulin G antibody signal over time, 60.5% of seropositive HCWs had maintained their seroconversion status after a median of 5.5 months.
Keywords: SARS-CoV-2, Seroprevalence, Healthcare workers, IgG, Antibodies, Kinetics
Introduction
Healthcare workers (HCWs) are at the front line of the ongoing coronavirus-19 (COVID-19) pandemic [1]. Assessing the antibody response could provide a snapshot of the burden of infection among HCWs [2]. Studies of immune responses to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) showed the presence of antibody titres 7–14 days following symptom onset [[3], [4], [5]]. These antibodies decline over time but may remain detectable for several months following infection [2,6,7]. SARS-CoV-2 antibodies have been shown to have neutralizing capacity in vitro and to confer protection against re-infection in challenge models [[8], [9], [10], [11]].
Anti-SARS-CoV-2 seroprevalence among HCWs ranges from 0% to 45.3% [12]. A comprehensive study of seroprevalence among HCWs in a large healthcare system could help to identify the impact of epidemiological factors and the presence of symptoms on the immune response to the infection over time. The authors' healthcare system is the largest in Connecticut with over 30,000 employees, seven acute care hospitals, the state's largest behavioural health network, rehabilitation services, a large physician group, skilled nursing and home health services, and senior living facilities. The baseline prevalence of SARS-CoV-2 immunoglobulin G (IgG) antibodies and associated epidemiological factors were determined. This is one of the largest seroprevalence studies focusing on HCWs in a US healthcare system with longitudinal follow-up to investigate antibody kinetics.
Methods
Approximately 30,000 HCWs and affiliated medical staff were invited to participate via electronic communication. Due to the complexity of the healthcare system, recruitment and testing of subjects occurred in a staggered fashion; testing occurred from 11th May to 22nd August 2020 at eight testing sites (Table I ). Inclusion criteria were: current employment or affiliated medical staff and age ≥18 years. The only exclusion criterion was symptoms suspicious of active SARS-CoV-2 infection at the time of testing. HCWs on home quarantine, with or without a reverse transcriptase polymerase chain reaction (PCR) assay diagnosis of SARS-CoV-2 infection, could participate in the study upon return to work based on the guidelines of the Centers for Disease Control and Prevention (CDC) at the time of sampling.
Table I.
Testing schedule
| Test site | Date range | Tested | Percentage |
|---|---|---|---|
| Site 1 | 11 May–2 July | 3491 | 51% |
| Site 2 | 26 May–2 July | 891 | 13% |
| Site 3 | 8 June–2 July | 396 | 6% |
| Site 4 | 15 June–24 July | 998 | 15% |
| Site 5 | 22 June–10 July | 237 | 3% |
| Site 6 | 29 June–24 July | 424 | 6% |
| Site 7 | 27 July–31 July | 143 | 2% |
| Site 8 | 27 July–16 August | 283 | 4% |
| Total | 11 May–16 August | 6863 | 100% |
HCWs included all staff providing any level of direct or indirect care to patients. This included healthcare professionals, allied health workers, auxiliary health workers, cleaning and laundry personnel, radiology staff, clerks, phlebotomists, respiratory therapists, nutritionists, social workers, physical therapists, laboratory personnel, administrators, patient transporters and food service staff.
Participants provided electronic consent and completed a questionnaire in REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN, USA). Information on demographics, occupation, pre-existing health problems, recent history of COVID-19-associated symptoms, exposure to individuals with COVID-19 outside work, prior PCR testing and high-risk exposure at work [defined as contact with a patient with COVID-19 without proper personal protective equipment (PPE)] was collected. Proper PPE included use of an N-95 mask, face shield, gown and gloves when caring for a patient under investigation, or testing positive, for SARS-CoV-2. Exposure to patients diagnosed with COVID-19 was graded as high (daily or almost daily), intermediate (occasional) or low (no known contact), according to exposure to body fluids, potentially contaminated items and/or environmental surfaces.
Consented study participants were invited to schedule three appointments for blood draws at the initial visit, and approximately 2–4 weeks and 3–6 months after the initial visit. Results were made accessible to participants within 48 h. Educational materials on how to interpret results, encouragement on continued use of PPE, and caveats with regards to the uncertainty of the correlation between positive antibodies and protection against re-infection were also provided. All study activities were approved by the hospital's institutional review board (HHC-2020-0103).
Anti-SARS-CoV-2 IgG antibody detection
Samples were analysed in the ancillary microbiology laboratory using the Abbott Architect i2000 platform. Clinical assay performance was determined by the manufacturer but also validated by the ancillary laboratory in accordance with clinical laboratory regulations. The assay was performed in accordance with the manufacturer's instructions [13]. The assay is an automated, two-step chemiluminescent microparticle immunoassay for the qualitative detection of IgG antibodies to the nucleocapsid protein of SARS-CoV-2. Seropositivity was defined as IgG index [signal/cut-off (S/C)] ≥1.4; this index provides a semi-quantitative concentration of IgG antibody to SARS-CoV-2. According to the manufacturer's package insert, the positive percentage agreement was 100% [95% confidence interval (CI) 95.89–100.00] in molecular positive patients (N=31) tested after 14 days of symptom onset, and the negative percentage agreement was 99.6% (95% CI 99.05–99.90).
Statistical analysis
Descriptive statistics comprise means and standard deviations (SD) for normally distributed continuous variables, and medians and interquartile ranges for non-normally distributed continuous variables. Categorical variables are reported as frequencies, using percentages. Inferential statistics comprised Student's t-test or Mann–Whitney U-test, depending on distribution, for two-group comparisons of continuous variables. Categorical variables were evaluated using Chi-squared test. A forward stepwise logistic regression model was constructed to evaluate factors that might be significantly associated with baseline seropositivity among the entire sample. Factors that showed univariate differences at P≤0.10 were included in the model, which was iterated until no significant change in the model's overall Wald score was observed. Odds ratios (OR) and 95% CI were calculated.
All statistics were analysed using SPSS v. 26 (IBM Corp., Armonk, NY, USA) using an a priori alpha level of 0.05, such that all results yielding P<0.05 were deemed significant. No power analysis was conducted, as the study was open to all respondents meeting the enrolment criteria.
Results
In total, 8663 HCWs provided electronic consent and 6863 (23% of the entire employee population) provided an initial sample. The mean age of participants was 43.2 (SD 12.9) years (median 43, range 18–81 years). Of the 6811 participants who reported their gender, there were 5387 females (79.1%).
Based on initial testing, 433 (6.3%; 95% CI 5.7–6.9%) participants were seropositive. Demographic data and responses to COVID-19 exposure questions are presented in Table II . African Americans had a higher rate of seropositivity than Caucasians (11.7% vs 5.5%; P<0.001), and Hispanics had higher rate of seropositivity than non-Hispanics (10.2% vs 5.9%; P<0.001).
Table II.
Characteristics associated with baseline severe acute respiratory syndrome coronavirus-2 immunoglobulin G (IgG) seropositivity
| Characteristic (N of responses) | IgG index (S/C) | Pa,b | ||
|---|---|---|---|---|
| <1.4 (N=6430, 93.7%) |
≥1.4 (N=433, 6.3%) |
|||
| Age, years (mean ± SD) (6649) |
43.2 ± 12.9 |
43.3 ± 12.8 |
42.0 ± 13.5 |
0.06 |
| All (%) |
N (%) |
N (%) |
||
| Gender (6811) | ||||
| Female | 5387 (79.1) | 5033 (93.4) | 354 (6.6) | 0.11 |
| Male | 1424 (20.9) | 1347 (94.6) | 77 (5.4) | |
| Race (6481) | <0.001 | |||
| African American | 351 (5.4) | 310 (88.3) | 41 (11.7) | |
| Asian | 365 (5.6) | 340 (93.2) | 25 (6.8) | |
| Caucasian | 5661 (87.3) | 5347 (94.5) | 314 (5.5) | |
| Hawaiian/Pacific Islander | 10 (0.2) | 7 (70.0) | 3 (30.0) | |
| Native American | 15 (0.2) | 12 (80.0) | 3 (20.0) | |
| Multi-racial | 79 (1.2) | 71 (89.9) | 8 (10.1) | |
| Ethnicity (6521) | <0.001 | |||
| Hispanic | 616 (9.4) | 553 (89.8) | 63 (10.2) | |
| Non-Hispanic | 5905 (90.6) | 5557 (94.1) | 348 (5.9) | |
| Prior PCR test (1317) | <0.001 | |||
| Positive | 223 (16.9) | 25 (11.2) | 198 (88.8) | |
| Negative | 1094 (83.1) | 1028 (94.0) | 66 (6.0) | |
| Exposure to patients with COVID-19 (6820) | <0.001 | |||
| Daily or almost daily contact | 2466 (36.2) | 2271 (92.1) | 195 (7.9) | |
| Occasional contact | 2887 (42.3) | 2712 (93.9) | 175 (6.1) | |
| No known contact | 1467 (21.5) | 1407 (95.9) | 60 (4.1) | |
| At least one symptom of COVID-19 (6863) | <0.001 | |||
| Yes | 2614 (38.0) | 2286 (87.5) | 328 (12.5) | |
| No | 4249 (62.0) | 4144 (97.5) | 105 (2.5) | |
| High-risk exposure to a COVID-19-positive patient (6821) | <0.001 | |||
| Yes | 880 (12.9) | 767 (87.2) | 113 (12.8) | |
| No | 5941 (87.1) | 5623 (94.6) | 318 (5.4) | |
| Exposure outside work (6819) | <0.001 | |||
| Yes | 439 (6.4) | 369 (84.1) | 70 (15.9) | |
| No | 6380 (93.6) | 6020 (94.4) | 360 (5.6) | |
| Prior quarantine (6822) | <0.001 | |||
| Yes | 791 (11.6) | 525 (66.4) | 266 (33.6) | |
| No | 6031 (88.4) | 5866 (97.3) | 165 (2.7) | |
| Medical conditions (responses) | condition present | |||
| Obesity (6777) | 1411 (20.8) | 1308 (92.7) | 103 (7.3) | 0.09 |
| Cancer (6749) | 107 (1.6) | 101 (94.4) | 6 (5.6) | 0.74 |
| Diabetes (6758) | 254 (3.8) | 234 (92.1) | 20 (7.9) | 0.31 |
| Pregnancy (women only; 5332) | 134 (2.5) | 126 (94.0) | 8 (6.0) | 0.77 |
| Immunodeficiency (6747) | 61 (0.9) | 59 (96.7) | 2 (3.3) | – |
| Heart disease (6747) | 171 (2.5) | 160 (93.6) | 11 (6.4) | 0.97 |
| Asthma requiring medication (6755) | 592 (8.8) | 557 (94.1) | 35 (5.9) | 0.65 |
| Chronic lung disease (6741) | 35 (0.5) | 34 (97.1) | 1 (2.9) | – |
| Liver disease (6748) | 18 (0.3) | 18 (100.0) | 0 (0.0) | – |
| Haematological disorder (6744) | 60 (0.9) | 58 (96.7) | 2 (3.3) | – |
| Chronic kidney disease (6743) | 21 (0.3) | 20 (95.2) | 1 (4.8) | – |
| Neurological disorder (6732) | 40 (0.6) | 40 (100.0) | 0 (0.0) | – |
| Organ/bone marrow recipient (6717) | 14 (0.2) | 14 (100.0) | 0 (0.0) | – |
| At least one medical condition (6799) | ||||
| Yes | 2242 (33.0) | 2095 (93.4) | 147 (6.6) | 0.61 |
| No | 4557 (67.0) | 4273 (93.8) | 284 (6.2) | |
PCR, polymerase chain reaction; COVID-19, coronavirus disease 2019; S/C, signal/cut-off; SD, standard deviation; –, too few cases for meaningful evaluation.
Values in bold denote statistical significance (P<0.05).
Age: Student's t-test; all others, Pearson's Chi-squared test.
Healthcare role
Seropositivity varied significantly (P<0.001) according to healthcare role (Table III ; responses from 6774/6863). When roles comprising at least 2% of the 6774 respondents were evaluated, the highest seropositivity was seen in patient care support (16.7%), medical assistants (9.1%), nurses (8.2%), physical/occupational therapists (7.4%) and administrative assistants (6.5%). Seropositivity was 3.8% for physicians and 4.5% for advanced practice providers.
Table III.
Seropositivity rate based on healthcare role
| Role | N (%) | Seronegative | Seropositive |
|---|---|---|---|
| Nurse | 2129 (31.4%) | 1954 (91.8%) | 175 (8.2%) |
| Physician | 736 (10.9%) | 708 (96.2%) | 28 (3.8%) |
| Advanced practice providers | 572 (8.4%) | 546 (95.5%) | 26 (4.5%) |
| Radiology technician | 316 (4.7%) | 305 (96.5%) | 11 (3.5%) |
| Administrative assistant | 306 (4.5%) | 286 (93.5%) | 20 (6.5%) |
| Patient care support | 276 (4.1%) | 230 (83.3%) | 46 (16.7%) |
| Physical therapy/occupational therapy | 258 (3.8%) | 239 (92.6%) | 19 (7.4%) |
| Other – non-clinical | 222 (3.3%) | 213 (95.9%) | 9 (4.1%) |
| Patient care coordinator | 215 (3.2%) | 204 (94.9%) | 11 (5.1%) |
| Laboratory professional | 198 (2.9%) | 193 (97.5%) | 5 (2.5%) |
| Medical assistant | 175 (2.6%) | 159 (90.9%) | 16 (9.1%) |
| Pharmacist/pharmacy technician | 158 (2.3%) | 155 (98.1%) | 3 (1.9%) |
| Clerk | 149 (2.2%) | 141 (94.6%) | 8 (5.4%) |
| Hospital administrator | 137 (2.0%) | 136 (99.3%) | 1 (0.7%) |
| Manager/unit leader | 105 (1.6%) | 99 (94.3%) | 6 (5.7%) |
| Informatics | 102 (1.5%) | 96 (94.1%) | 6 (5.9%) |
| Technician – unspecified | 94 (1.4%) | 84 (89.4%) | 10 (10.6%) |
| Respiratory therapy practitioner | 92 (1.4%) | 86 (93.5%) | 6 (6.5%) |
| Phlebotomist | 64 (0.9%) | 62 (96.9%) | 2 (3.1%) |
| Dietary support | 62 (0.9%) | 60 (96.8%) | 2 (3.2%) |
| Surgical technician | 57 (0.8%) | 55 (96.5%) | 2 (3.5%) |
| Anaesthesiologist | 53 (0.8%) | 52 (98.1%) | 1 (1.9%) |
| Research | 40 (0.6%) | 38 (95.0%) | 2 (5.0%) |
| Operating room staff | 38 (0.6%) | 35 (92.1%) | 3 (7.9%) |
| Public safety officer | 35 (0.5%) | 33 (94.3%) | 2 (5.7%) |
| Environmental services support | 35 (0.5%) | 30 (85.7%) | 5 (14.3%) |
| Emergency room technician | 34 (0.5%) | 31 (91.2%) | 3 (8.8%) |
| Other | 116 (1.7%) | 111 (95.7%) | 5 (4.3%) |
| Total | 6774 | 6341 | 433 |
Symptoms
Of the seropositive participants, 328 (75.2%) reported symptoms suspicious for COVID-19 after 1st February 2020. The median number of symptoms was 4 [interquartile range (IQR) 1–6] for seropositive respondents, compared with 0 (IQR 0–2) for seronegative respondents. The symptoms reported by the seropositive participants were muscle aches (54.3%), loss of taste or smell (49.0%), cough (48.0%), extreme fatigue (48.0%), fever (42.3%), nasal congestion (37.1%), sore throat (31.6%), shortness of breath (31.1%), diarrhoea (28.2%), generalized abdominal pain (15.5%), phlegm or mucus production (15.2%), and vomiting (7.4%).
Exposure to SARS-CoV-2
Seropositivity rates differed significantly (P<0.001) based on self-reported exposure to SARS-CoV-2: 4.1%, 6.1% and 7.9% in those with no known contact, occasional contact and daily contact, respectively. Rates of seropositivity were higher in HCWs who were placed on home quarantine based on symptoms or a positive PCR test (33.6% vs 2.7%; P<0.0001) and those with exposure to COVID-19 outside of the hospital (15.9% vs 5.6%; P<0.0001). In addition, 880 of 6821 (12.9%) HCWs reported at least one high-risk exposure (i.e. contact with a COVID-19-positive patient without proper PPE) and were more than twice as likely (P<0.001) to test seropositive (12.8%) as those without such an exposure (5.4%).
PCR testing
Of the 6863 participants, 1317 (19.2%) had a SARS-CoV-2 PCR test prior to the serological test recorded in the laboratory information management system. Twenty-five (2.3%) of the 1053 seronegative HCWs had a positive PCR test at a median time of 55 days prior to the serological test (range 3–109 days). Sixty-six of the 264 seropositive HCWs (25%) had a negative SARS-CoV-2 PCR test at a median time of 35 days prior to the serological test (range 1–114 days).
Of the 6863 participants, 2222 (32.4%) had a PCR test after having a serological test. None of the 35 seropositive participants who had a subsequent PCR test at least 30 days following the positive serological test had a positive PCR test. One seropositive subject had a positive PCR test but only 14 days after the serological test. If one considers that a positive PCR test may persist for several weeks after the initial infection, the incidence of a positive PCR test in the seropositive group at least 30 days after the positive serology test was zero. This is in contrast with the 1.3% (29/2173) incidence of a positive PCR test in the seronegative group, which occurred after a median time of 72.4 days (range 7–135 days).
Logistic regression
When the baseline variables were evaluated in a multi-variate model, African American race (OR 3.26 compared with Caucasian, 95% CI 1.77–5.99), age 50–59 years (OR 1.91, 95% CI 1.19–3.08), prior positive PCR test (OR 37.83, 95% CI 24.77–57.77), presence of at least one symptom of COVID-19 (OR 3.00, 95% CI 1.92–4.68) and prior quarantine (OR 3.83, 95% CI 2.57–5.70) were significantly associated with baseline seropositivity (Table IV ).
Table IV.
Logistic regression of baseline characteristics associated with severe acute respiratory syndrome coronavirus-2 immunoglobulin G seropositivity
| Characteristic | OR (95% CI) | P (two-tailed)a |
|---|---|---|
| Age group, years | ||
| 18–39 | 1 Reference | |
| 40–49 | 1.17 (0.72–1.90) | 0.519 |
| 50–59 | 1.91 (1.19–3.08) | 0.007 |
| 60–69 | 0.90 (0.47–1.73) | 0.757 |
| ≥70 | 2.58 (0.31–21.58) | 0.383 |
| Gender | ||
| Female | 1 Reference | |
| Male | 1.04 (0.65–1.68) | 0.864 |
| Race | ||
| African American | 3.26 (1.77–5.99) | <0.001 |
| Asian | 1.13 (0.53–2.41) | 0.750 |
| Caucasian | 1 Reference | |
| Multi-racial | 1.19 (0.27–5.33) | 0.818 |
| Native American | 6.01 (0.70–51.29) | 0.101 |
| Ethnicity | ||
| Hispanic | 1.13 (0.57–2.22) | 0.733 |
| Non-Hispanic | 1 Reference | |
| Prior PCR test | ||
| Negative | 1 Reference | |
| Positive | 37.83 (24.77–57.77) | <0.001 |
| Exposure to patients with COVID-19 | ||
| Daily or almost daily contact | 1.59 (0.88–2.86) | 0.125 |
| Occasional contact | 1.36 (0.77–2.42) | 0.294 |
| No known contact | 1 Reference | |
| At least one symptom of COVID-19 | ||
| No | 1 Reference | |
| Yes | 3.00 (1.92–4.68) | <0.001 |
| High-risk exposure to a COVID-19-positive patient | ||
| No | 1 Reference | |
| Yes | 1.20 (0.78–1.87) | 0.409 |
| Exposure outside work | ||
| No | 1 Reference | |
| Yes | 1.61 (0.90–2.87) | 0.106 |
| Prior quarantine | ||
| No | 1 Reference | |
| Yes | 3.83 (2.57–5.70) | <0.001 |
| At least one medical condition | ||
| No | 1 Reference | |
| Yes | 0.87 (0.60–1.27) | 0.470 |
| Roleb | ||
| Nurse | 1.23 (0.81–1.87) | 0.335 |
| Physician | 0.92 (0.44–1.92) | 0.816 |
| Advanced practice provider | 0.63 (0.28–1.39) | 0.253 |
| Radiology technician | 0.54 (0.13–2.18) | 0.385 |
| Administrative assistant | 0.81 (0.29–2.23) | 0.679 |
| All other | 1 Reference |
PCR, polymerase chain reaction; COVID-19, coronavirus disease 2019; OR, odds ratio; CI, confidence interval.
Values in bold denote statistical significance (P<0.05).
Top five roles based on number of responses.
Second serological test
Of the 6863 participants, 2307 (33.6%) had a second serological test after a median of 25 days (range 10–40 days). Of these 2307 participants, 194 (8.4%) were seropositive on the first serological test. One hundred and eighty-five (95.3%) of those participant who were seropositive on the first serological test were also seropositive on the second serological test [nine (4.6%) seroreverted]. Eleven (0.5%) of those who were seronegative on the first serological test were seropositive on the second serological test. Eighty-seven participants who had the second serological test outside the 10–40 day time frame were excluded. Only one of the 87 subjects had a change in serological status (from initial positive to just below the threshold negative).
Third serological test
Of the 6863 participants, 3195 (46.6%) returned for a third serological test after a median of 164 days (range 90–196). Of the 444 seropositive subjects (combined first and second visit), 314 (70.7%) were tested; 190 (60.5%) subjects remained seropositive and 124 (39.5%) seroreverted. Of the 6419 seronegative subjects (combined first and second visit), 2881 (44.9%) were tested and 64 (2.2%) seroconverted.
Comparison with Connecticut State data
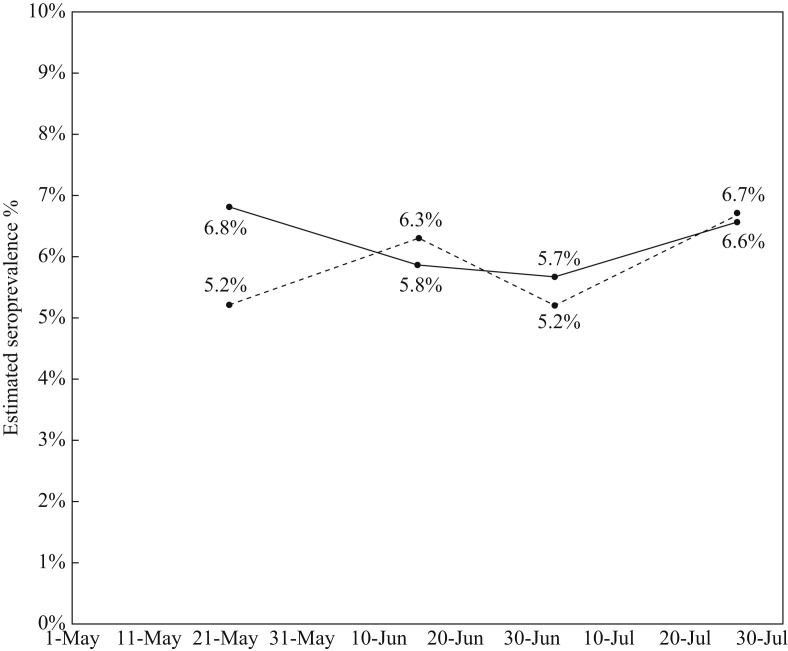
CDC conducted a nationwide commercial laboratory seroprevalence survey starting on 26th April 2020 [14]. Testing dates were matched with the CDC study, and seropositivity rates in Connecticut were compared with the seropositivity rates found in this study (Figure 1 ). Although the initial testing in May showed higher seroprevalence among HCWs compared with the public (6.8% vs 5.2%), subsequent testing revealed comparable rates of seropositivity. In a study of seroprevalence in the general population in Connecticut, conducted between 10th June and 29th July 2020, 567 participants were tested for the presence of IgG against SARS-CoV-2, resulting in a weighted seroprevalence of 4.0% (90% CI 2.0–6.0) [15]. During the same test period, seroprevalence among HCWs in this study was 6.2%.
Figure 1.
Comparison of seroprevalence in Connecticut between the current study (solid line) and the Centers for Disease Control and Prevention study (dashed line).
Discussion
This study assessed the impact of the COVID-19 pandemic on the workforce at the study hospital and identified epidemiological factors associated with its distribution. The seroprevalence rate in the initial phase of this study was 6.3%, and the subsequent seroconversion rate was 0.5% at the second testing phase and 2.2% at the third testing phase. Throughout the duration of the study, the seropositivity rate was 7.4% (508/6863). Published studies of seroprevalence among HCWs in the USA report rates between 6% and 35.8% [[16], [17], [18], [19], [20], [21], [22]].
A recent meta-analysis of seroprevalence of SARS-CoV-2 antibodies in HCWs by Galanis et al. included 127,480 HCWs from 49 studies [12]. The estimated overall seroprevalence rate was 8.7% (95% CI 6.7–10.9%). Seroprevalence was higher in studies that were conducted in North America (12.7%). Factors associated with seropositivity included male gender; Black, Asian and Hispanic race/ethnicity; working in a COVID-19 unit; working in areas with a shortage of PPE; self-reported belief of previous SARS-CoV-2 infection; previous PCR test; and household contact with suspected or confirmed cases of COVID-19. The higher rate of seropositivity observed in females may be due to the fact that 35.8% of enrolled HCWs in this study were nurses and 91% of nurses were females. The racial and ethnic disparity observed in the seropositivity rates among the HCWs mirrors the findings of prior studies [18,22,23]. In Connecticut, the weighted seroprevalence was 6.4% among Blacks and 19.9% among Hispanics, compared with the overall weighted seroprevalence of 4.0% [15]. This difference may be partially explained by existing disparities in access to health care, financial inequality and high-density urban living. The authors' healthcare system has applied strategies to mitigate the effect of these socio-economic disparities on the welfare of minority employees.
Prior studies have shown inconsistent results about the effect of age on seroprevalence [[24], [25], [26]]. This is not surprising considering the geographic variations of the exposure of the population to SARS-CoV-2. In this study, the age group 50–59 years was associated with a higher seroprevalence rate.
In a large observational cohort study of 28,792 HCWs in Denmark, seroprevalence was higher in HCWs (4.04%) than in blood donors (3.04%) [24]. Seroprevalence was higher in male HCWs (risk ratio 1.49), front-line HCWs (risk ratio 1.38) and HCWs working on dedicated COVID-19 wards (risk ratio 1.65). As in the present study, a stepwise increase in seropositivity was observed with increase in job-related exposure.
A Swedish study reported an overall seropositivity rate of 19.1%, with nurses having a higher rate (35%) than physicians (21%) and assisting nurses (27%), in a sample of 2149 HCWs [27]. In a study of 40,329 HCWs in the Greater NYC area, the overall seroprevalence rate was 13.7%, with service/maintenance HCWs having the highest rate (20.9%), followed by nurses (13.1%), administrative and clerical (12.6%), allied health professionals (11.6%) and physicians (8.7%) [21]. In a study by Shields et al., seroprevalence was highest in HCWs working in housekeeping (34.5%), acute medicine (33.3%) and general internal medicine (30.3%), and lowest in HCWs working in intensive care (14.8%), emergency medicine (13.3%) and general surgery (13.0%) [28]. In the present study, the seropositivity rate of nurses was twice as high as that of physicians, and the healthcare role with the highest seroprevalence rate was patient care support (16.7%).
In a study by Moncunill et al., 578 HCWs had serial levels of IgM, IgA and IgG over the course of 3 months [29]. The initial combined seropositivity rate was 9.3% and increased to 14.5% at 1 month. The seroreversion rate at 3 months was 77.6% for IgM, 3.7% for IgG and 24.5% for IgA. Self et al. looked at 3248 front-line HCWs at 13 centres in 12 states who were tested for SARS-CoV-2 immunoglobulins (IgA, IgM or IgG) at baseline and 60 days later [30]. The initial seroprevalence was 6.0%, and the seroreversion rate at 60 days was 28.2%. In the present study, the seroreversion rate at 5.5 months (median time) was 39.5%. The authors concluded that a substantial number of HCWs infected with SARS-CoV-2 may have negative serological test results in the months following infection. This is an important consideration when serology is used as a marker of prior SARS-CoV-2 infection, and although humoral immunity may decline with time, protection against future SARS-CoV-2 infections may be rendered by memory B cells and T cells.
Interestingly, this study found that only 75% of the seropositive HCWs had a prior positive PCR test and 24% reported no prior COVID-19 symptoms, which may suggest a significant rate of asymptomatic infection among HCWs. These results correspond with other reports of asymptomatic or mild infection among the general population in Iceland, among homeless shelter residents in Boston, and among PCR-positive HCWs in the Netherlands [7,31,32]. In a meta-analysis of asymptomatic patients as a source of COVID-19, the rate of asymptomatic cases was 24.2% [33]. In a study of HCWs in a paediatric dialysis unit, 11 of 25 (44%) HCWs seroconverted and 24 of 25 (96%) were asymptomatic [34].
Such a high rate of asymptomatic infections may pose a risk of transmission to other staff members and to the patients they serve. In the early phases of the pandemic, when PCR testing was not widely available, patients reporting symptoms suspicious for COVID-19 were asked to quarantine. As guidance evolved, the healthcare system mandated universal masking for HCWs on 27th March 2020 and for patients and visitors on 24th April 2020. Universal screening for symptoms was implemented in March 2020. Targeted PCR testing for SARS-CoV-2 became available to HCWs with suspicious symptoms or exposure without proper PPE in April 2020. Voluntary PCR testing for SARS-CoV-2 became available on 11th June 2020.
The presence of IgG antibodies against SARS-CoV-2 may have conferred immunity to the HCWs in this study considering the difference in the incidence of a positive PCR test between the seropositive group (0%; testing at least 30 days after the original serological test) and the seronegative group (1.3%; after a median follow-up of 72.4 days). Lumley et al. [35] noted that the incidence of a positive PCR test was 1.09 per 10,000 days at risk in the seronegative group vs 0.13 per 10,000 days at risk in the seropositive group. The authors concluded that the presence of IgG antibodies against SARS-CoV-2 was associated with a substantially reduced risk of re-infection in the ensuing 6 months.
Limitations
Recruitment was limited to employees who had access to communication via electronic devices. Only 23% of the 30,000 HCWs participated, and of those, 47% had a third test. Some HCWs may have limited understanding of English or may be wary of participation in an employer-sponsored research study. Enrolment was not random, and therefore motivation to participate may have been influenced by health-seeking behaviour and curiosity about serological status, especially among those with prior positive PCR tests or close contact with patients with COVID-19. Thus, these findings may not truly reflect the entire population of HCWs. Executing this study across a complex healthcare system of seven acute care hospitals, in the middle of the pandemic and with competing resources for testing was extremely challenging. The testing intervals were not as narrow as the authors would like, and not all participants were offered subsequent testing at the same time frames.
In conclusion, the seroprevalence rate of SARS-CoV-2-specific antibodies among the participating HCWs was comparable to the rate found in the general population of Connecticut. Seropositivity was associated with African American race, presence of at least one symptom of COVID-19, prior positive PCR result and prior quarantine. Twenty-five percent of the HCWs reported no prior symptoms suspicious for viral infection. At a median time of 5.5 months, the seroreversion rate was 39.5% and the seroconversion rate was 2.2%. The incidence of re-infection was 0% in the seropositive group, and the incidence of a positive PCR test was 1.2% in the seronegative group. Further studies are needed to determine the impact of PPE availability, severity of symptoms and degree of ongoing exposure on rates of seroconversion and duration of seropositivity among HCWs.
Acknowledgements
The authors wish to thank Jamie Fish-Furhman and the project management team for their assistance with study implementation. In addition, the authors wish to thank all staff for their courage and dedication to patient care.
Conflict of interest statement
None declared.
Funding sources
This work was funded internally by the authors' hospital.
References
- 1.Chou R., Dana T., Buckley D.I., Selph S., Rongwei F., Totten A. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173:120–136. doi: 10.7326/M20-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long Q.X., Liu B.Z., Deng H.J., Gui Chen W., Deng K., Yao-Chi C., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Zhang L., Sang L., Feng Y., Ruan S., Zhong B., et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest. 2020;130:5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ni L., Ye F., Cheng M.L., Feng y., Young-Qian D., Zhoa H., et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52 doi: 10.1016/j.immuni.2020.04.023. 971–7e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okba N.M.A., Muller M.A., Li W., Chunyan W., GeurtsvanKessel C., Corman V., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M., et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng W., Bao L., Liu J., Xiao C., Liu J., Xue J., et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020;369:818–823. doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K., et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wajnberg A., Amanat F., Firpo A., Altman D., Bailey M., Mansour M., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng C., Evans J.P., Pearson R., Qu P., Zheng Y., Robinson R., et al. Neutralizing antibody against SARS-CoV-2 spike in COVID-19 patients, health care workers, and convalescent plasma donors. JCI Insight. 2020;5 doi: 10.1172/jci.insight.143213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galanis P., Vraka I., Fragkou D., Bilali A., Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manufacturer package insert information . Abbott Laboratories; Abbott Park, IL: 2020. SARS-CoV-2 IgG G90418R01 06R8620. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Commercial laboratory seroprevalence survey data. Atlanta, GA: CDC; n.d. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/commercial-lab-surveys.html#connecticut [last accessed December 2020].
- 15.Mahajan S., Srinivasan R., Redlich C.A., Huston S., Anastasio K., Cashman L., et al. Seroprevalence of SARS-CoV-2-specific IgG antibodies among adults living in Connecticut: Post-Infection Prevalence (PIP) study. Am J Med. 2021;134:526–534. doi: 10.1016/j.amjmed.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeremias A., Nguyen J., Levine J., Pollack S., Engellenner W., Thakore A., et al. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Intern Med. 2020;180:1707–1709. doi: 10.1001/jamainternmed.2020.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mughal M.S., Kaur I.P., Patton C.D., Mikhail N., Vareechon C., Granet K. The prevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) IgG antibodies in intensive care unit (ICU) healthcare personnel (HCP) and its implications – a single-center, prospective, pilot study. Infect Control Hosp Epidemiol. 2020;12:1–2. doi: 10.1017/ice.2020.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Self W.H., Tenforde M.W., Stubblefield W.B., Feldstein L., Steingrub J., Shapiro N., et al. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network – 13 academic medical centers, April–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221–1226. doi: 10.15585/mmwr.mm6935e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stubblefield W.B., Talbot H.K., Feldstein L., Tenforde M., Rasheed M., Mills L., et al. Seroprevalence of SARS-CoV-2 among frontline healthcare personnel during the first month of caring for COVID-19 patients – Nashville, Tennessee. Clin Infect Dis. 2021;72:1645–1648. doi: 10.1093/cid/ciaa936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansour M., Leven E., Muellers K., Stone K., Mendu D., Wajnberg A. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J Gen Intern Med. 2020;35:2485–2486. doi: 10.1007/s11606-020-05926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moscola J., Sembajwe G., Jarrett M., Farber B., Chang T., McGinn T., et al. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York City area. JAMA. 2020;324:893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sydney E.R., Kishore P., Laniado I., Rucker L., Bajaj K., Zinaman M. Antibody evidence of SARS-CoV-2 infection in healthcare workers in the Bronx. Infect Control Hosp Epidemiol. 2020;41:1348–1349. doi: 10.1017/ice.2020.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg E.S., Tesoriero J.M., Rosenthal E.M., Chung R., Barranco M., Styer L., et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. 2020;48:23–9e4. doi: 10.1016/j.annepidem.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iversen K., Bundgaard H., Hasselbalch R.B., Kristensen J., Nielson P., Pries-Heje M., et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20:1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X., Sun J., Nie S., Li H., Kong Y., Liang M., et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med. 2020;26:1193–1195. doi: 10.1038/s41591-020-0949-6. [DOI] [PubMed] [Google Scholar]
- 26.Plebani M., Padoan A., Fedeli U., Schievano E., Vecchiato E., Lippi G., et al. SARS-CoV-2 serosurvey in health care workers of the Veneto Region. Clin Chem Lab Med. 2020;58:2107–2111. doi: 10.1515/cclm-2020-1236. [DOI] [PubMed] [Google Scholar]
- 27.Rudberg A.S., Havervall S., Manberg A., Falk A., Aguilera K., Ng H., et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J., et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moncunill G., Mayor A., Santano R., Jimenez A., Vidal M., Tortajada M., et al. SARS-CoV-2 seroprevalence and antibody kinetics among health care workers in a Spanish hospital after three months of follow-up. J Infect Dis. 2021;223:62–71. doi: 10.1093/infdis/jiaa696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Self W.H., Tenforde M.W., Stubblefield W.B., Feldstein L., Steingrub J., Shapiro N., et al. Decline in SARS-CoV-2 antibodies after mild infection among frontline health care personnel in a multistate hospital network – 12 states, April–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1762–1766. doi: 10.15585/mmwr.mm6947a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baggett T.P., Keyes H., Sporn N., Gaeta J.M. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323:2191–2192. doi: 10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kluytmans-van den Bergh M.F.Q., Buiting A.G.M., Pas S.D., Bentvelsen R., Bijllaardt W., van Oudheusden A., et al. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kronbichler A., Kresse D., Yoon S., Hwa Lee K., Effenberger M., Shin J. Asymptomatic patients as a source of COVID-19 infections: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:180–186. doi: 10.1016/j.ijid.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hains D.S., Schwaderer A.L., Carroll A.E., Starr M., Wilson A., Amanat F., et al. Asymptomatic seroconversion of immunoglobulins to SARS-CoV-2 in a pediatric dialysis unit. JAMA. 2020;323:2424–2425. doi: 10.1001/jama.2020.8438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lumley S.F., O’Donnell D., Stoesser N.E., Mattews P., Howarth A., Hatch S., et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]